Abstract
Aim
The aim of this article is to assess diffusion tensor imaging (DTI) metrics in differentiating low-grade from high-grade gliomas.
Patients and methods
A prospective study was conducted on 35 patients with gliomas who underwent DTI. Gliomas were classified into low-grade and high-grade gliomas. The fractional anisotropy (FA), mean diffusivity (MD), linear coefficient (CL), planar coefficient (CP) and spherical coefficient (CS) of the solid tumoral part and peri-tumoral regions were calculated.
Results
There was significant difference (p = 0.001) in MD of the solid tumoral part of low-grade (1.78 ± 0.33 × 10−3 mm2/s) and high-grade (1.16 ± 0.22 × 10−3 mm2/s) gliomas. The selection of 1.42 × 10−3 mm2/s as a cutoff value of MD of the tumoral part was used to differentiate low-grade and high-grade gliomas; the best results were obtained with area under the curve (AUC) of 0.957 and accuracy of 91.4%. There was a significant difference in FA, MD, CP and CS of peri-tumoral regions of both groups with p values of 0.006, 0.042, 0.030 and 0.037, respectively. The cutoff values of MD, FA, CS and CP of the peri-tumoral region used to differentiate low-grade from high-grade gliomas were 1.24, 0.315, 0.726 and 0.321 with AUC of 0.694, 0.773, 0.734 and 0.724 and accuracy of 68.6%, 80.0%, 74.3% and 74.3%, respectively. The combined MD of the solid tumoral part and FA of the peri-tumoral region used to differentiate low-grade from high-grade gliomas revealed AUC of 0.974 and accuracy of 88.6%.
Conclusion
We conclude that the combination of MD of the solid tumoral part and FA of the peri-tumoral region is a noninvasive method to differentiate low-grade from high-grade gliomas.
Keywords: Diffusion tensor imaging, glioma, grading
Introduction
Gliomas are the most common primary brain tumors, representing 80% of primary brain tumors. According to World Health Organization (WHO) classification of brain tumors, high-grade gliomas constitute grade III and grade IV glial pathologies, and high-grade astrocytomas are the most common primary brain malignancies in adults. On the other hand, low-grade gliomas consist of a heterogeneous group of glial tumors, representing 15% brain tumors in adults. Grading of gliomas is crucial for the appropriate therapeutic strategies, clinical outcome1–5 and in assessment of prognosis as survival rates differ significantly between the two groups.3,6 Histopathology is the gold standard for glioma grading, but it carries a sampling error because of a limited number of biopsy samples.7,8 Routine and advanced magnetic resonance (MR) imaging sequences are used for grading of gliomas, but their results are overlapping. Several studies have shown that approximately 20% of low-grade gliomas may show regions of contrast enhancement and that a third of non-enhancing gliomas are pathologically found to be high grade. There is inconsistency in perfusion imaging parameters due to different MR imaging protocols, pharmacokinetic models, or perfusion-analysis software. The integrity of metabolites measured by proton MR spectroscopy may be affected by heterogeneity of the tumor and the technical parameters of MR devices.8–15
Diffusion tensor imaging (DTI) takes advantage of the diffusion of water in brain tissue within three main directions, which is decreased perpendicularly to the myelin sheaths and cell membranes of white-matter axons.3–8 There are different metrics of DTI used as a quantitate analysis. The most common metrics of DTI used are mean diffusivity (MD) and fractional anisotropy (FA). MD reveals the rate of water molecules’ diffusional motion as the tumor cellularity is the main target of histologic tumor classification with DTI. There is an inverse relationship between the cellularity and the MD value of gliomas.14–17 The FA expresses the orientation of the tissue microstructure and is related to the structural orientation of different tissues.14–18 The other three basic metrics that expresses the shape of the DT are: linear anisotropy coefficient (CL), in which diffusion is mainly along the direction corresponding to the largest eigenvalue; planar anisotropy coefficient (CP), in which diffusion is restricted to the plane spanned by the two eigenvectors with the two largest eigenvalues; and spherical anisotropy coefficient (CS), which indicates the isotropic diffusion. Each anisotropy coefficient shows a unique measure in the different areas of the white matter. These differences are due to the impact of the linear, planar, and spherical shape components of the DTI.19–26 Few studies discuss the role of diffusion tensor MR imaging in grading of gliomas.27–31 The novelty and uniqueness of this study is assessing the combined DT indices of tumoral and peri-tumoral regions in differentiating low-grade from high-grade gliomas.
Material and methods
Patients
This prospective study was approved by the local ethics committee and informed consents were obtained from all patients prior to the examination. The study included 38 patients provisionally diagnosed to have untreated gliomas based on conventional MR imaging. We excluded three patients from the study because two patients proved to be metastasic and one patient had lymphoma. The final patients included in this study were 35 patients (22 male and 13 female). Their ages ranged from 52 to 72 years (mean age, 63 years). The final diagnosis was based on histopathological examinations. Gliomas were classified into low-grade gliomas (WHO grade I and II) in 16 patients and high-grade gliomas (WHO grade III and IV) in 19 patients. The histopathological subtypes of WHO grade I were diffuse astrocytoma (n = 5) and oligoastrocytoma (n = 1), grade II were astrocytomas (n = 8) and oligodendrogliomas (n = 2), grade III was anaplastic astrocytomas (n = 6) and grade IV was glioblastoma multiforms (n = 13).
MR imaging
The MR imaging of the brain was performed using a 1.5 Tesla scanner (Ingenia, Philips) using Stream Head Neck 20 channel coil. T1-weighted (repetition time (TR)/echo time (TE) = 600/25 ms), T2-weighted (TR/TE = 6000/90 ms) and fluid-attenuated inversion recovery (FLAIR) (TR/TE/inversion time (TI) = 10,000/115/2700 ms) sequences were obtained. The scanning parameters were matrix of 80 × 80, field of view (FOV) of 250 × 170 mm2 and slice thickness of 5 mm. Post-contrast T1-weighted images were obtained after intravenous administration of gadoterate meglumine, 0.5 ml/kg (0.1 mmol/kg) body weight with maximum dose of 10 ml using a 20–22 G venous cannula with a flow rate of 2 ml/second.
DTI
DTI was obtained using a single-shot echo-planar imaging sequence (TR/TE 3200/90 ms) with parallel imaging (SENSitivity Encoding (SENSE) reduction factor P 2). Diffusion gradients were applied along 32 axes, using a b value of 0 and 1000 s/mm2. FOV was 250 × 170 mm2, data matrix of 92 × 88 with voxel dimensions of 2.43 × 2.54 × 2.5 mm3. Forty-eight slices were obtained, with a thickness of 2.5 mm, with no gap and the total scan duration was seven to eight minutes.
Post-processing
Image analysis was performed by one radiologist (EA) expert in neuroradiology for 10 years who was blinded to the histopathological results. The digital imaging and communications in medicine (DICOM) images were transferred to a workstation (extended MR Workspace 2.6.3.5, Philips Medical Systems Nederland B.V). The images were loaded to DTI software provided by the vendor. Automated registration of the DTI data was performed to eliminate eddy current artifacts. Co-registration of the FA maps to contrast T1-weighted images was performed for accurate placement of regions of interest (ROIs). A well-defined circular ROI (0.4 cm2) was placed in the solid tumoral part and another ROI was placed in the peri-tumoral region (Figure 1). The ROI was placed in the tumoral part at the most enhanced solid region seen at contrast T1-weighted images, avoiding the cystic or necrotic region. The peri-tumoral ROI was located in the immediate peri-tumoral region that appears as a bright T2 hyperintensity with a fingerlike projection and of low signal on T1-weighted images. If a peri-tumoral high T2 signal was absent, the ROI was placed just immediately adjacent to the tumor margin. Placement of ROIs within the normal gray matter structures was avoided to avoid the inherent DTI differences between white matter and gray matter The eigenvalues (primary, secondary and tertiary) of these regions were estimated. FA, MD, CL, CP and CS were calculated using the following equations.23,24
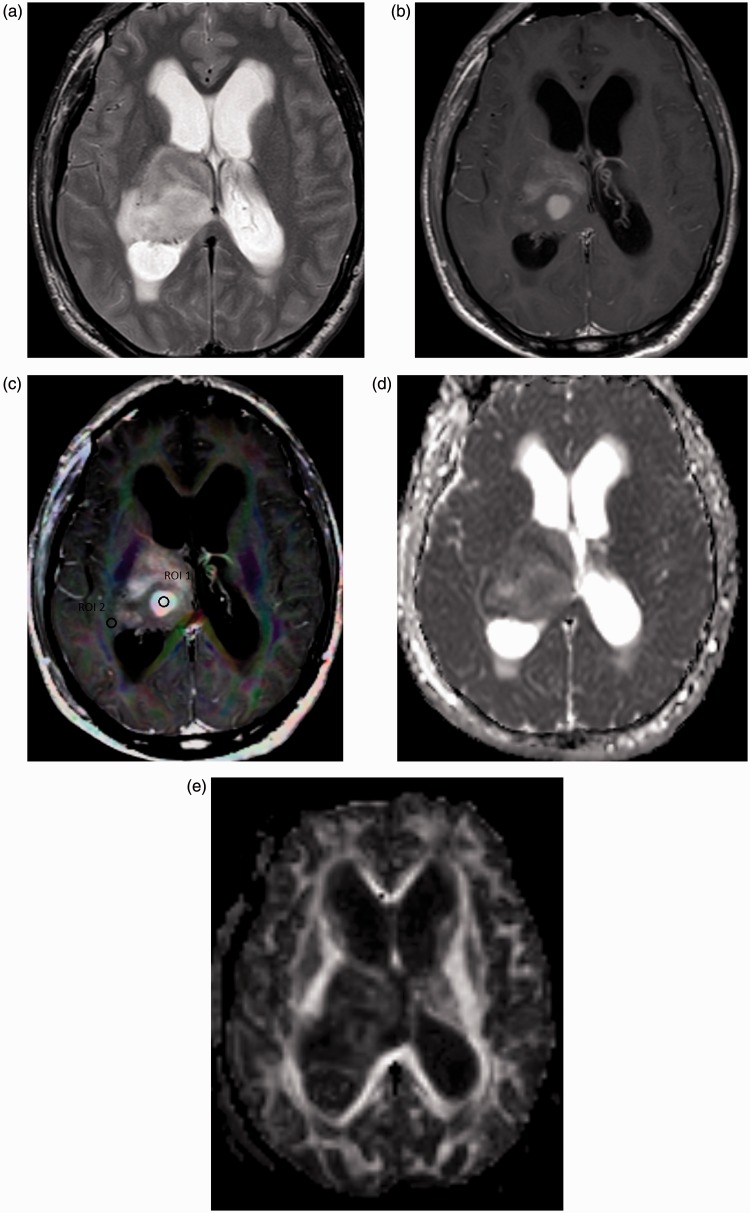
Figure 1.
Grade II astrocytoma. (a) Axial T2-weighted image shows an intra-axial space-occupying lesion of abnormal signal intensity seen in the right thalamic region with hyperintensity in the peri-tumoral region. (b) Axial contrast T1-weighted image shows multiple enhanced nodular areas within the lesion in T2 images. (c) Color FA map co-registered with contrast T1-weighted image shows ROI (1) located within the enhanced solid tumoral part and ROI (2) located in the peri-tumoral region. The MD of the tumoral part is 1.88. The MD, FA, CL, CS and CP of the peri-tumoral part are 1.21, 0.37, 0.41 and 0.78, respectively. (d) MD map shows relative iso-signal of the tumor parts. (e) FA map showing low signal of the solid tumor part. FA: fractional anisotropy; ROI: region of interest; MD: mean diffusivity; CL: linear coefficient; CS: spherical coefficient; CP: planar coefficient.
CL = (λ1 – λ2)/(λ1 + λ2 + λ3)
CP = 2 (λ2 – λ3)/(λ1 + λ2 + λ3)
CS = 3 λ3/(λ1 + λ2 + λ3) where denotes the mean of the three eigenvalues. The CL, CP, and CS values lie in the range from 0 to 1 and the sum of these three metrics is equal to 1.22
Statistical analysis
Statistical analyses were carried out using Statistical Package for Social Sciences version 20 (SPSS, Chicago, IL, USA). Quantitative data were presented as mean ± standard deviation (SD), median and range while qualitative data were presented as number (n) and percentage (%). Shapiro–Wilk test results, histograms and boxplots were used to determine the normality of the parameters (FA, MD, CL, CP and CS). Normally distributed data were compared between the two major groups using independent samples t test and between each WHO group. Data that violated the normality assumptions were compared using the Mann–Whitney test. Probability (p) values <0.05 were considered statistically significant. The receiver operating characteristic (ROC) curves of different matrices of the tumoral and peri-tumoral regions were obtained by calculating the area under the curve (AUC). The optimum cutoff values of different matrices of the tumoral and peri-tumoral regions with highest accuracy selected to differentiate low-grade from high-grade gliomas were calculation of sensitivity and specificity. The multivariate linear regression analysis was conducted for variables that reached a p value of 0.05 for best combination of DTI metrics in the tumoral and peri-tumoral parts used to differentiate low-grade from high-grade gliomas.
Results
Table 1 shows the mean, standard deviations, minimum and maximum DTI metrics of the tumoral part and peri-tumoral region in low-grade and high-grade gliomas. Table 2 shows the cutoff values of DT metrics in the tumoral and peri-tumoral regions of gliomas with area under the curve, accuracy, sensitivity, specificity, specificity, positive predictive value and negative predictive values.
Table 1.
Mean, standard deviations, minimum and maximum of DTI metrics in the solid tumoral part and peri-tumoral region of low-grade and high-grade gliomas.
| Mean ± SD (min/max) |
|||
|---|---|---|---|
| Metric | Low-grade gliomas (n = 16) | High-grade gliomas (N = 19) | p value |
| Tumor part | |||
| MD | 1.78 ± 0.33 (1.28–2.54) | 1.16 ± 0.22 (0.05–1.47) | 0.001 |
| FA | 0.16 ± 0.12 (0.05–0.49) | 0.12 ± 0.10 (0.61–0.49) | 0.272 |
| CL | 0.07 ± 0.07 (0.01–0.30) | 0.04 ± 0.04 (0.01–0.15) | 0.142 |
| CS | 0.82 ± 0.96 (0.62–0.95) | 0.87 ± 0.08 (0.59–0.98) | 0.133 |
| CP | 0.25 ± 0.16 (0.06–0.67) | 0.17 ± 0.12 (0.03–0.55) | 0.188 |
| Peri-tumoral region | |||
| MD | 1.11 ± 0.36 (0.62–2.06) | 1.39 ± 0.40 (0.74–2.06) | 0.042 |
| FA | 0.35 ± 0.17 (0.10–0.71) | 0.21 ± 0.10 (0.03–0.45) | 0.006 |
| CL | 0.13 ± 0.11 (0.02–0.47) | 0.07 ± 0.25 (0.01–0.19) | 0.056 |
| CS | 0.69 ± 0.12 (0.47–0.92) | 0.78 ± 0.11 (0.42–0.93) | 0.037 |
| CP | 0.43 ± 0.22 (0.12–0.96) | 0.28 ± 0.16 (0.08–0.74) | 0.030 |
MD: mean diffusivity; FA: fractional anisotropy; CL: linear coefficient; CS: spherical coefficient; CP: planar coefficient.
Table 2.
The cutoff values of diffusion tensor (DT) matrices in the tumoral part and peri-tumoral region of gliomas with area under the curve, sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV).
| Parameter | Cutoff | AUC | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Tumor MD | 1.42 | 0.957 | 94.7 | 87.5 | 91.4 | 90 | 93.3 |
| Peri-tumoral MD | 1.24 | 0.694 | 57.9 | 81.3 | 68.6 | 78.6 | 61.9 |
| Peri-tumoral FA | 0.31 | 0.773 | 89.5 | 68.8 | 80.0 | 77.3 | 84.6 |
| Peri-tumoral CP | 0.32 | 0.724 | 68.4 | 81.3 | 74.3 | 81.3 | 68.4 |
| Peri-tumoral CS | 0.72 | 0.734 | 78.9 | 68.8 | 74.3 | 75.0 | 73.3 |
AUC: area under the curve; MD: mean diffusivity; FA: fractional anisotropy; CL: linear coefficient; CP: planar coefficient; CS: spherical coefficient.
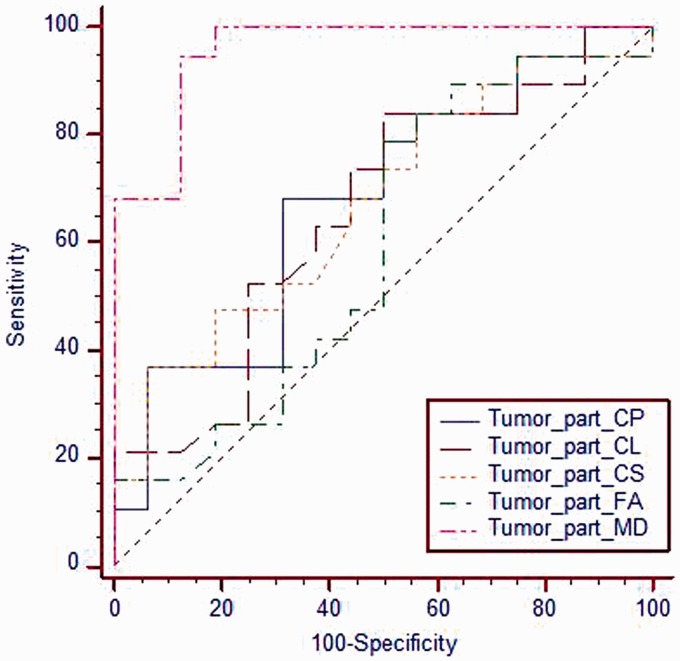
The mean MD (10−3 mm2/s) values of solid tumoral part of high-grade gliomas was 1.16 ± 0.22 (Figure 1) and of low-grade gliomas was 1.78 ± 0.33 (Figure 2) with significant difference (p = 0.001). At ROC curve, the AUC of MD of the tumoral region used to differentiate low-grade from high-grade gliomas was 0.957. The selection of 1.42 as a cutoff value of MD of the tumoral region to differentiate between the low-grade and high-grade gliomas revealed accuracy of 91.4%, sensitivity of 94.7% and specificity of 87.5% (Figure 3). There was insignificant difference of other parameters in the solid tumor parts between low-grade and high-grade gliomas (Table 1).
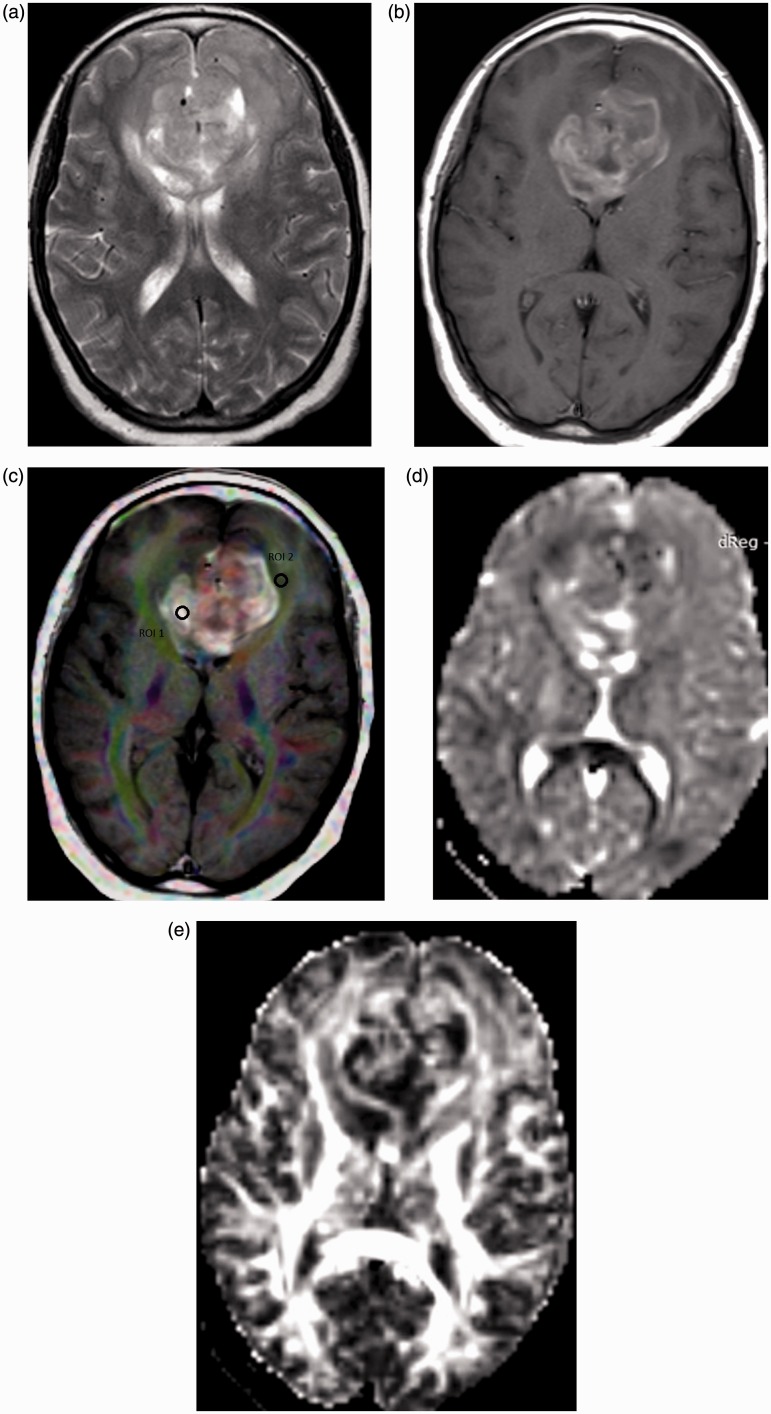
Figure 2.
Glioblastoma multiformis. (a) Axial T2-weighted image shows an intra-axial space-occupying lesion is seen in the genu of the corpus callosum crossing the midline with hyperintensity in the peri-tumoral region. (b) Axial contrast T1-weighted image shows heterogeneous enhancement of the tumor. (c) Color FA map co-registered with contrast T1-weighted image shows ROI (1) located in the enhanced solid tumoral part and ROI (2) located in the peri-tumoral region. The MD of the tumoral part is 1.22. The MD, FA, CL, CS and CP of the peri-tumoral region are 1.72, 0.23, 0.12, and 0.38, respectively. (d) MD map shows an iso-signal with relative low-signal linear areas of the solid tumoral parts. (e) FA map shows heterogeneous mixed mainly low signal of the solid tumoral part. FA: fractional anisotropy; ROI: region of interest; MD: mean diffusivity; CL: linear coefficient; CS: spherical coefficient; CP: planar coefficient.
Figure 3.
ROC curve for different metrics of the solid tumoral part. The selection of 1.42 as a cutoff value of MD of the solid tumoral part was used to differentiate low-grade from high-grade gliomas; the best results were obtained with AUC of 0.957, accuracy of 91.4%, sensitivity of 94.7% and specificity of 87.5%. ROC: receiver operating characteristic; MD: mean diffusivity; AUC: area under the curve.
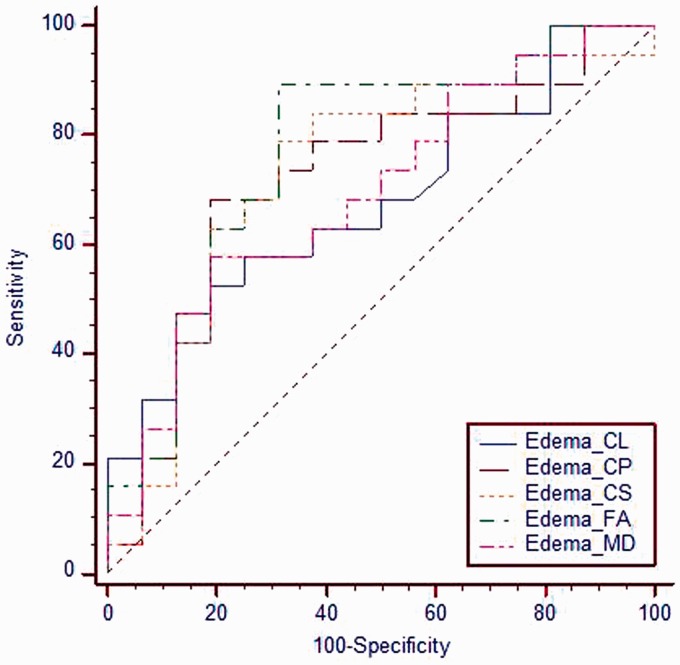
There was significant difference (p = 0.042) in MD of the peri-tumoral region between low-grade gliomas (1.11 ± 0.36) and high-grade gliomas (1.39 ± 0.40). There was significant difference (p = 0.006) in FA of the peri-tumoral region between low-grade gliomas (0.35 ± 0.17) and high-grade gliomas (0.21 ± 0.10). There was a significant difference (p = 0.037) in the CS of the peri-tumoral region between low-grade gliomas (0.6 ± 0.12) and high-grade gliomas (0.78 ± 0.11). There was an insignificant difference (p = 0.056) of CL of the peri-tumoral region between low-grade and high-grade gliomas. There was a significant difference (p = 0.030) in the CP of the peri-tumoral region between low-grade gliomas (0.43 ± 0.22) and high-grade gliomas (0.28 ± 0.16). At ROC curve, the AUC of MD, FA, CS and CP of the peri-tumoral region used to differentiate low-grade from high-grade gliomas were 0.694, 0.773, 0.734 and 0.724 respectively. The cutoff of MD, FA, CS and CP of the peri-tumoral region used to differentiate low-grade from high-grade gliomas were 1.24, 0.315, 0.726 and 0.321 with accuracy of 68.6%, 80.0%, 74.3% and 74.3% respectively (Table 2) (Figure 4). Combination of the tumoral MD and the peri-tumoral FA for differentiation of low-grade from high-grade gliomas revealed AUC of 0.974, accuracy of 88.6%, sensitivity of 89.5% and specificity of 87.5%.
Figure 4.
ROC curve for different metrics of the peri-tumoral region. The cutoff value of MD, FA, CS and CP of the peri-tumoral region used to differentiate low-grade gliomas from high-grade gliomas are 1.24, 0.315, 0.726 and 0.321 with areas under the curve of 0.694, 0.773, 0.734 and 0.724 and accuracy of 68.6%, 80.0%, 74.3% and 74.3%, respectively. ROC: receiver operating characteristic; MD: mean diffusivity; FA: fractional anisotropy; CL: linear coefficient; CS: spherical coefficient; CP: planar coefficient .
Discussion
In this study, there is significant difference between low-grade and high-grade gliomas using MD values in solid tumoral parts. The MD is equal to the apparent diffusion coefficient (ADC), which is a sensitive metric of the cellularity within solid tumor parts, so it shows significant decrease in high-grade gliomas.8,10,25,26 Another study reported there is significant difference with high CP value in the solid tumoral parts of high-grade gliomas and they explain that difference with higher cellularity; however, the CP values also are seen increasing in crossing fibers and in regions of edema.30 Another study added that higher anisotropic values within the solid parts of high-grade compared to low-grade gliomas may be attributed to the high cellularity of high-grade gliomas.3 Another study added that ADC, RD, and AD are useful DTI metrics for the differentiation between low-grade and high-grade gliomas with a diagnostic accuracy of more than 90%, and can be used as noninvasive reliable biomarkers in the grading of gliomas.8
The peri-tumoral region is a crucial site of differentiation between tumor types as regards to DTI metrics because of structural changes and edema grade differences between the low and high glioma grades. In peri-tumoral regions, the MD and anisotropic metrics show significant difference between low-grade and high-grade gliomas; the immediate peri-tumoral region of low-grade gliomas usually shows no or mild vasogenic edema compared to evident edema in the peri-tumoral region of the high-grade gliomas mixed with tumoral infiltrations that indicate increase cellularity. In peri-tumoral regions, there was as significant reduction of the FA values of high-grade gliomas compared to low-grade gliomas that may be attributed to tubular anisotropy changes.3,32 Another study added that FA is a useful diagnostic marker that could be used in distinguishing the low-grade from high-grade gliomas.17
In this study, CL didn’t show statistically a significant difference in peri-tumoral regions, indicating similar levels of single-orientated fibers. One study reported that there is significantly higher CL in the peri-tumoral region of high-grade gliomas. This is attributed to highly invasive tumor cells within high-grade glioma margins leading to decreased linearity in nearby space.33 Another study added that there is significant difference in the CS values between low-grade and high-grade gliomas in the peri-tumoral region.21
In this study, the combination of MD of the solid tumoral part and FA of the peri-tumoral region of gliomas revealed the highest accuracy in differentiating low-grade from high-grade gliomas. This is attributed to MD of the solid tumoral part representing the degree of cellularity of the gliomas with high cellularity in high-grade gliomas and FA of the peri-tumoral region expressing the orientation and infiltration of the peri-tumoral regions with tumor cells and associated edema.
There are a few limitations of this study. First, the small number of patients limits the statistical results of the cases. We recommend further studies including a large number of gliomas. Second, this study was performed using 1.5 Tesla. Further studies at higher 3 Telsa34 with application of other methods of fiber tracking are recommended to improve the results. Third, this study used DTI metrics. Further studies with application of advanced post-processing such as diffusion kurtosis, intravoxel incoherent motion diffusion MR imaging sequence with several b values and multi-parametric imaging with dynamic susceptibly perfusion-weighted MR imaging and MR spectroscopy35–41 will improve grading of gliomas and correlation with prognostic parameters.
Conclusion
We conclude that the combination of MD of the solid tumoral part and FA of the peri-tumoral region is a noninvasive method in differentiating between low-grade and high-grade gliomas.
Acknowledgments
We declare that this manuscript does not contain clinical studies or patient data.
Author contributions are as follows: L El-Serougy: writing of the manuscript and statistical analysis; A Abdel Razek: writing of the manuscript and data collection; A Ezzat: writing of the manuscript and data collection; H Eldawoody: clinical assessment and writing of the manuscript; and A El-Morsy: MR analysis, data collection, and writing of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Chung C, Metser U, Ménard C. Advances in magnetic resonance imaging and positron emission tomography imaging for grading and molecular characterization of glioma. Semin Radiat Oncol 2015; 25: 164–171. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papageorgiou TS, Chourmouzi D, Drevelengas A, et al. Diffusion tensor imaging in brain tumors: A study on gliomas and metastases. Phys Med 2015; 31: 767–773. [DOI] [PubMed] [Google Scholar]
- 4.El-Serougy LG, Abdel Razek AA, Mousa A, et al. Differentiation between high-grade gliomas and metastatic brain tumors using diffusion tensor imaging metrics. Egypt J Radiol Nuclear Med 2015; 46: 1099–1104. [Google Scholar]
- 5.Kang Y, Choi SH, Kim YJ, et al. Gliomas: Histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging—correlation with tumor grade. Radiology 2011; 261: 882–890. [DOI] [PubMed] [Google Scholar]
- 6.Zonari P, Baraldi P, Crisi G. Multimodal MRI in the characterization of glial neoplasms: The combined role of single-voxel MR spectroscopy, diffusion imaging and echo-planar perfusion imaging. Neuroradiology 2007; 49: 795–803. [DOI] [PubMed] [Google Scholar]
- 7.Essig M, Anzalone N, Combs SE, et al. MR imaging of neoplastic central nervous system lesions: Review and recommendations for current practice. AJNR Am J Neuroradiol 2012; 33: 803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Server A, Graff BA, Josefsen R, et al. Analysis of diffusion tensor imaging metrics for gliomas grading at 3 T. Eur J Radiol 2014; 83: e156–e165. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Shah A, Young RJ, et al. Imaging of brain tumors: Functional magnetic resonance imaging and diffusion tensor imaging. Neuroimag Clin North Am 2010; 20: 379–400. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Liu M, Bao J, et al. The correlation between apparent diffusion coefficient and tumor cellularity in patients: A meta-analysis. PloS One 2013; 8: e79008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inano R, Oishi N, Kunieda T, et al. Voxel-based clustered imaging by multiparameter diffusion tensor images for glioma grading. Neuroimage Clin 2014; 5: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smitha KA, Gupta AK, Jayasree RS. Total magnitude of diffusion tensor imaging as an effective tool for the differentiation of glioma. Eur J Radiol 2013; 82: 857–861. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Steward CE, Desmond PM. Diffusion tensor imaging in glioblastoma multiforme and brain metastases: The role of p, q, L, and fractional anisotropy. AJNR Am J Neuroradiol 2009; 30: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razek AA. Diffusion-weighted magnetic resonance imaging of head and neck. J Comput Assist Tomogr 2010; 34: 808–815. [DOI] [PubMed] [Google Scholar]
- 15.Razek AA. Diffusion magnetic resonance imaging of chest tumors. Cancer Imaging 2012; 12: 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamasaki F, Kurisu K, Satoh K, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 2005; 235: 985–991. [DOI] [PubMed] [Google Scholar]
- 17.Liang R, Wang X, Li M, et al. Potential role of fractional anisotropy derived from diffusion tensor imaging in differentiating high-grade gliomas from low-grade gliomas: A meta-analysis. Int J Clin Exp Med 2014; 7: 3647–3653. [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar M, Gupta RK, Nath K, et al. Can we differentiate true white matter fibers from pseudofibers inside a brain abscess cavity using geometrical diffusion tensor imaging metrics? NMR Biomed 2008; 21: 581–588. [DOI] [PubMed] [Google Scholar]
- 19.Beppu T, Inoue T, Shibata Y, et al. Measurement of fractional anisotropy using diffusion tensor MRI in supratentorial astrocytic tumors. J Neuro Oncol 2003; 63: 109–116. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita M, Hashimoto N, Goto T, et al. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage 2008; 43: 29–35. [DOI] [PubMed] [Google Scholar]
- 21.Toh CH, Castillo M, Wong AM, et al. Primary cerebral lymphoma and glioblastoma multiforme: Differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol 2008; 29: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westin CF, Maier SE, Mamata H, et al. Processing and visualization for diffusion tensor MRI. Med Image Anal 2002; 6: 93–108. [DOI] [PubMed] [Google Scholar]
- 23.Alexander AL, Hasan K, Kindlmann G, et al. A geometric analysis of diffusion tensor measurements of the human brain. Mag Res Med 2000; 44: 283–291. [DOI] [PubMed] [Google Scholar]
- 24.Inano R, Oishi N, Kunieda T, et al. Voxel-based clustered imaging by multiparameter diffusion tensor images for glioma grading. Neuroimage Clin 2014; 5: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fudaba H, Shimomura T, Abe T, et al. Comparison of multiple parameters obtained on 3T pulsed arterial spin-labeling, diffusion tensor imaging, and MRS and the Ki-67 labeling index in evaluating glioma grading. AJNR Am J Neuroradiol 2014; 35: 2091–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piyapittayanan S, Chawalparit O, Tritakarn SO, et al. Value of diffusion tensor imaging in differentiating high-grade from low-grade gliomas. J Med Assoc Thai 2013; 96: 716–721. [PubMed] [Google Scholar]
- 27.Wang S, Kim S, Chawla S, et al. Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 2011; 32: 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou QG, Xu HB, Liu F, et al. In the assessment of supratentorial glioma grade: The combined role of multivoxel proton MR spectroscopy and diffusion tensor imaging. Clin Radiol 2011; 66: 953–960. [DOI] [PubMed] [Google Scholar]
- 29.Ferda J, Kastner J, Mukensnabl P, et al. Diffusion tensor magnetic resonance imaging of glial brain tumors. Eur J Radiol 2010; 74: 428–436. [DOI] [PubMed] [Google Scholar]
- 30.Lee HY, Na DG, Song IC, et al. Diffusion-tensor imaging for glioma grading at 3-T magnetic resonance imaging: Analysis of fractional anisotropy and mean diffusivity. J Comput Assist Tomogr 2008; 32: 298–303. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Bastin ME, Laidlaw DH, et al. Visualization and analysis of white matter structural asymmetry in diffusion tensor MRI data. Magn Reson Med 2004; 51: 140–147. [DOI] [PubMed] [Google Scholar]
- 32.Ma L, Song ZJ. Differentiation between low-grade and high-grade glioma using combined diffusion tensor imaging metrics. Clin Neurol Neurosurg 2013; 115: 2489–2495. [DOI] [PubMed] [Google Scholar]
- 33.Bieza A, Krumina G. Magnetic resonance study on fractional anisotropy and neuronal metabolite ratios in peri-tumoral area of cerebral gliomas. Medicina (Kaunas) 2012; 48: 497–506. [PubMed] [Google Scholar]
- 34.Razek AA, Elkhamary S, Mousa A. Differentiation between benign and malignant orbital tumors at 3-T diffusion MR-imaging. Neuroradiology 2011; 53: 517–522. [DOI] [PubMed] [Google Scholar]
- 35.Bai Y, Lin Y, Tian J, et al. Grading of gliomas by using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR imaging. Radiology 2016; 278: 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel Razek AA, Khairy M, Nada N. Diffusion-weighted MR imaging in thymic epithelial tumors: Correlation with World Health Organization classification and clinical staging. Radiology 2014; 273: 268–275. [DOI] [PubMed] [Google Scholar]
- 37.Abdel Razek AA, Gaballa G. Role of perfusion magnetic resonance imaging in cervical lymphadenopathy. J Comput Assist Tomogr 2011; 35: 21–25. [DOI] [PubMed] [Google Scholar]
- 38.Razek AA, Elsorogy LG, Soliman NY, et al. Dynamic susceptibility contrast perfusion MR imaging in distinguishing malignant from benign head and neck tumors: A pilot study. Eur J Radiol 2011; 77: 73–79. [DOI] [PubMed] [Google Scholar]
- 39.Abdel Razek AA, Gaballa G, Ashamalla G, et al. Dynamic susceptibility contrast perfusion-weighted MR imaging and diffusion-weighted MR imaging in differentiating recurrent head and neck cancer from post-radiation changes. J Comput Assist Tomogr 2015; 39: 849–854. [DOI] [PubMed] [Google Scholar]
- 40.Abdel Razek AA, Poptani H. MR spectroscopy of head and neck cancer. Eur J Radiol 2013; 82: 982–989. [DOI] [PubMed] [Google Scholar]
- 41.Razek AA, Nada N. Correlation of choline/creatine and apparent diffusion coefficient values with the prognostic parameters of head and neck squamous cell carcinoma. NMR Biomed 2016; 29: 483–489. [DOI] [PubMed] [Google Scholar]