Abstract
Tumefactive multiple sclerosis is an inflammatory demyelinating disease of the central nervous system. It has recently been described as a rare subtype of multiple sclerosis (MS) characterised by the appearance of solitary or multiple space-occupying lesions associated with imaging characteristics mimicking neoplasm. Atypical features include plaque size >2 cm with mass effect, oedema, and/or ring enhancement on magnetic resonance (MR) images.
This study is a retrospective review designed to evaluate the prevalence of tumefactive plaques in a selected population of 440 MS patients referred to our MS centre in Southern Italy between 2005 and 2014. We analysed the radiographic features of lesions ranging in size from 0.5 to 2 cm to establish whether smaller plaques with MR characteristics similar to tumefactive plaques present different symptoms, disease evolution and prognosis. We also aimed to ascertain if MR features suggestive of biological aggressiveness could be useful prognostic criteria for a correct diagnosis of the disease and subsequent treatment. Our data suggest that lesions 0.5–2 cm and >2 cm have similar MR features and clinical evolution.
Keywords: Multiple sclerosis, tumefactive demyelinating lesions, magnetic resonance imaging
Introduction
Tumefactive multiple sclerosis (TMS) is an inflammatory demyelinating disease of the central nervous system. It has recently been described as a subtype of multiple sclerosis (MS), characterised by the appearance of solitary or multiple space-occupying lesions associated with imaging characteristics mimicking neoplasm. Atypical features include plaque size >2 cm with and mass effect, perifocal oedema, and/or ring enhancement on magnetic resonance (MR) images.1 The tumefactive condition is an extremely rare MS variant occurring in one per 1000 cases, or in three per million cases per year.2,3
Clinical presentations vary depending on lesion location and size and include headache, cognitive abnormalities, mental confusion, aphasia, apraxia and/or seizures.4,5 Tumefactive demyelinating lesions may occur in patients with or without established MS. They can mimic other lesions of the brain both in clinical presentation and imaging features. Atypical clinical and imaging presentations may mimic brain tumour, cerebral abscess or other inflammatory disorders. Diagnosis may necessitate brain biopsy, although radiological features such as open-ring enhancement may help distinguish tumefactive from neoplastic or other lesions. Choosing when to biopsy a tumefactive lesion to exclude alternative pathology can be challenging.6–9
This study is a retrospective review designed to evaluate the prevalence of tumefactive plaques in a selected population. We analysed the radiographic features of lesions ranging in size from 0.5 to 2 cm to establish whether smaller plaques with MR characteristics similar to tumefactive lesions can also be defined as tumefactive. The study also aimed to ascertain if MR features suggestive of biological aggressiveness could be useful prognostic criteria for a correct diagnosis of the disease and subsequent treatment.10
Materials and methods
Ethical committee approval was obtained for this retrospective study. We reviewed brain MR scans of 440 MS patients who presented to the Multiple Sclerosis Centre of San Salvatore Hospital in L’Aquila, Southern Italy between 2005 and 2014. Out of the 440 patients, 147 were excluded due to partial information. The remaining 293 patients were monitored by 1153 MR scans (Table 1).
Table 1.
Features of multiple sclerosis patients monitored by magnetic resonance (MR) scans.
| Male | 81 |
| Female | 212 |
| Mean age at disease onset | 31 years (12–71) |
| Median disease duration | 12.5 years |
| Mean MR scans in each patient | 3.9 (SD 3.87) |
| Patients with new lesions during monitoring | 216 |
| Patients with n > 10 new lesions during monitoring test | 12 |
| Patients with of n ≤ 10 new lesions during monitoring | 175 |
Standardised contrast-enhanced MR scans were performed on a GE (General Electric Medical Systems, Milwaukee, WI, USA) Signa 1.5T MR device using birdcage head coils, 3 mm slice thickness and no interslice gap. Pre-contrast axial T2-weighted images, axial T1-weighted, axial fluid-attenuated inversion recovery (FLAIR), coronal T2-weighted and post-contrast axial T1-weighted and T1-weighted three-dimensional (3D) fast spoiled gradient echo (FSPGR) volumetric sequences were obtained in all cases.
Each patient underwent spectroscopic analysis to confirm the nature of the plaques in association with clinical diagnosis. Clinical information was obtained by medical record review by our MS centre. We excluded patients with known neoplastic disease, infection, vascular or other non-demyelinating inflammatory diseases of the central nervous system, and neuromyelitis optica.
Each plaque was analysed to acquire the following data: lesion site; margin characteristics (well-defined, diffuse); size range (<2 cm and >2 cm) of the margin-to-margin signal abnormality on T2-weighted scans; presence and degree of mass effect (mild, moderate/severe); presence and degree of oedema (mild, moderate/severe); presence of a hypointense rim on T2-weighted scans, appearing as a smooth, complete and thin border on T2-weighted scans, hypointense to the hyperintense lesion centre and surrounding oedema (Figures 1–3). Enhancement patterns were defined as homogeneous (uniform solid enhancement throughout the lesion), ring-like (circular border around the lesion), or heterogeneous (variable complex enhancement pattern and distribution). We identified more specific patterns in some patients with heterogeneous enhancement, and defined them as diffuse and patchy; ‘fluffy/cotton ball’; nodular and punctate (Figures 4 and 5).
Figure 1.
A 42-year-old woman. Axial T2-weighted image demonstrates tumefactive plaque located in the temporal region. The lesion is >2 cm and with well-defined margins, mild mass effect, mild oedema and a classic T2-weighted hypointense rim.
Figure 2.
A 35-year-old woman. Axial T2-weighted image demonstrates multiple tumefactive plaques. The lesions are >2 cm with well-defined margins, mild mass effect, mild oedema and a classic T2-weighted hypointense rim.
Figure 3.
A 21-year-old man. Axial T1-weighted images demonstrate hypointensity of a tumefactive plaque located in the parietal region.
Figure 4.
A 44-year-old woman. Sagittal T1-weighted image after contrast administration demonstrates a tumefactive plaque located in the temporal region. The lesion is > 2 cm with well-defined margins and ring enhancement.
Figure 5.
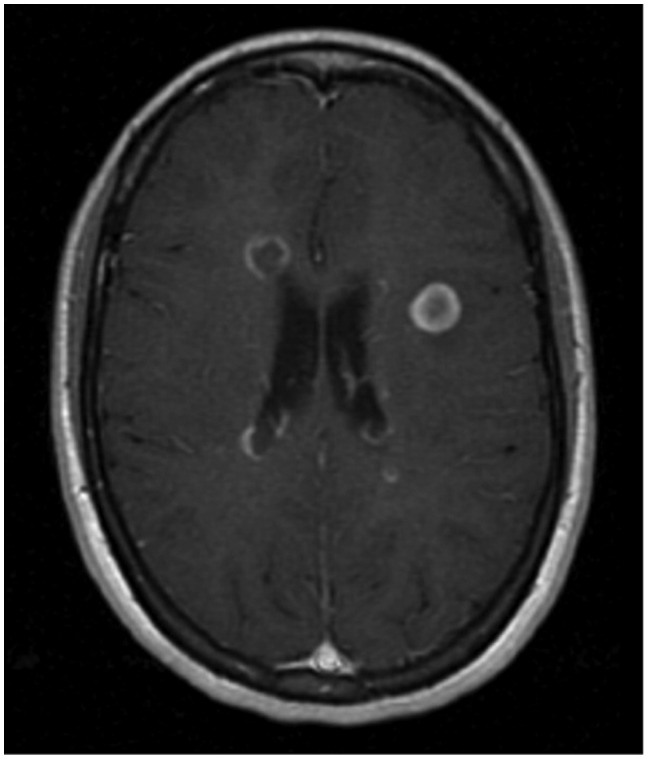
A 20-year-old woman. Axial T1-weighted image after contrast administration demonstrates a tumefactive plaque located in the periventricular region. The lesion is < 2 cm with well-defined margins and concentric enhancement.
A tumefactive demyelinating lesion was defined as a large solitary lesion >2 cm in size associated with mass effect, perilesional oedema and/or ring enhancement (Group A). Smaller plaques (<2 cm in diameter) were also considered, associated with mass effect, perilesional oedema and/or ring enhancement (Group B).
We analysed the number of other lesions appearing hyperintense on T2-weighted scans (<5, 5–10, >10 mm), hypointense on T1-weighted scans (<5, 5–10, >10 mm), and enhancing (<5, 5–10, >10 mm). Follow-up studies were analysed to determine the likelihood that patients with <2 cm plaques could relapse with additional unifocal or multifocal lesions.
Our results were summarised with descriptive statistics, including mean, median, standard deviation (SD), interquartile range (IQR), and frequencies (%). We quantified the degree of heterogeneity using the I-squared (I2) statistic, and its significance was determined based on the accompanying Q p value. Two neuroradiologists evaluated the results based on consensus.
Results
Tumefactive plaque
We identified 24 tumefactive plaques >2 cm (Group A) and seven tumefactive plaques <2 cm (Group B) in 293 MS patients (8.21%) (Table 1). Group A comprised three males (12.5%) and 21 females (87.5%) with a median disease duration of 9.8 years on 31 December 2014. There were no significant differences in sex and age distribution or age at disease onset (Tables 2 and 3). A mean number of 5.8 (SD 4.99) MR scans were reviewed per patient (range 0–21) in Group A and 5.1 (SD 3.87) in Group B (Table 4). Only four patients in Group A showed the lesion at onset, whereas the pseudotumoral plaques appeared during follow-up in the remaining 20 patients.
Table 2.
Group A multiple sclerosis patients.
| Male | 3 |
| Female | 21 |
| Mean age at disease onset | 29 years (15–44) |
| Median disease duration | 9.8 years |
| Mean magnetic resonance scans in each patient | 5.8 (SD 4.99) |
Table 3.
Group B multiple sclerosis patients.
| Male | 1 |
| Female | 6 |
| Mean age at disease onset | 27 years (23–49) |
| Median disease duration | 8.6 years |
| Mean magnetic resonance scans in each patient | 5.1 (SD 3.87) |
Table 4.
Features of Group A and Group B multiple sclerosis patients.
| FEATURES | Group A | Group B |
|---|---|---|
| Number | 24 | 7 |
| Male | 3 (12.5%) | 1 (14.2%) |
| Female | 21 (87.5%) | 6 (85.7%) |
| Age at disease onset | 29 years (15–44) | 27 years (23–49) |
| Median disease duration | 9.8 years | 8.6 years |
| Mean magnetic resonance scans in each patient | 5.8 (SD 4.99) | 5.1 (SD 3.87) |
Lesion location
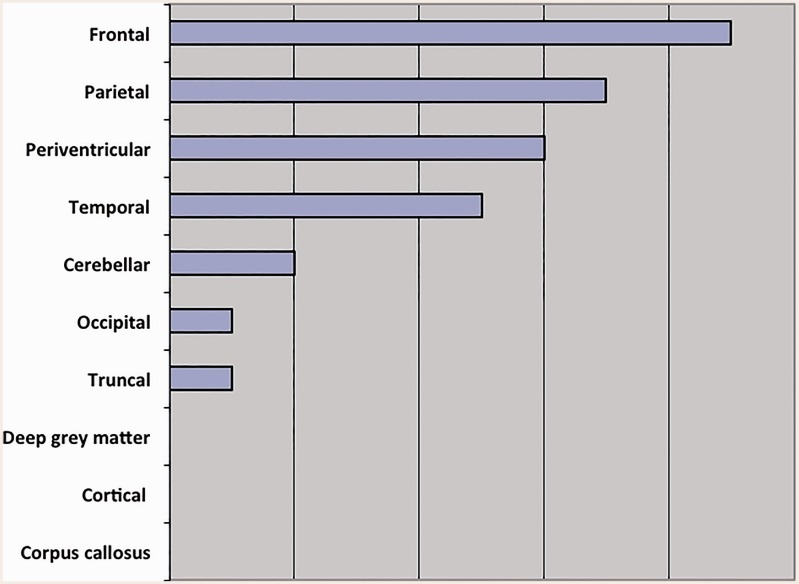
There were no differences in lesion location between the two groups. Group A lesions involved multiple anatomic areas (Figure 6) mainly the frontal, parietal and periventricular regions. Group B lesions involved frontal, parietal and periventricular regions: The frontal region was involved in four cases (57.1%), the parietal in two (28.5%), and the periventricular region in one (14.2%) (Figure 6).
Figure 6.
Location of plaques in Group A multiple sclerosis patients.
Plaque features
In Group A, 23 plaques (95.83%) had well-defined margins and one (4.1%) had diffuse margins. All the lesions were associated with mass effect, which was mild in 19 cases (79.1%) and moderate to severe in five (20.8%). Oedema was most often mild (22 cases, 91.6%) but moderate to severe in two patients (8.3%) (Table 5). Half of the patients (12 cases) showed no contrast enhancement. Ring enhancement was the most common pattern (seven cases, 29.1%). Two patients (8.3%) had fluffy enhancement, whereas only one presented concentric (4.1%), nodular (4.1%) and heterogeneous (4.1%) enhancement. T2-weighted MR sequences depicted a hypointense rim around the lesion in 15 patients (62.5%), whereas no rim was present in the remaining cases. Ten out of the 24 Group A patients (41.6%) were symptomatic when the tumefactive plaques appeared. The estimated mean Expanded Disability Status Scale (EDSS) at the time of clinical symptoms was 2.08 (SD 0.04). Six Group A patients were treated with fingolimod (three patients), interferon beta 1b (two patients), and high-dose interferon beta 1a (one patient).
Table 5.
Mass effect and oedema in multiple sclerosis patients with different lesion sizes.
| Mass effect |
Oedema |
|||
|---|---|---|---|---|
| Mild | Moderate/ Severe | Mild | Moderate/ Severe | |
| Group A | 19 (79.1%) | 5 (20.8%) | 22 (91.6%) | 2 (8.3%) |
| Group B | 7 (100%) | 0 | 7 (100%) | 0 |
In Group B, six plaques (85.7%) showed well-defined margins and one (14.2%) had diffuse margins. All the lesions were associated with mild mass effect. Oedema was always mild. One patient (14.2%) showed no contrast enhancement. Ring enhancement was the most common pattern (six patients, 85.7%). T2-weighted MR sequences showed a hypointense rim around the lesion in all cases.
We also analysed the evolution of black holes. All patients with plaques >2 cm had black holes after 299.5 days (mean value). Six patients (85.7%) with plaques <2 cm had black holes and mean evolution was 437.571 days (Table 6).
Table 6.
Black hole outcome in different lesion sizes.
| Black hole | No black hole | |
|---|---|---|
| Plaque ≥ 2 (24) | 24 (100%) | 0 |
| Plaque < 2 (7 ) | 6 (85.7%) | 1 (14.2%) |
Discussion and conclusions
MS is a chronic inflammatory demyelinating disease of the central nervous system most often diagnosed in young adults although paediatric and older patients have been described.11,12 The disease affects 350,000 individuals in the United States and 2.5 million worldwide with no clear sex predilection.
MS is characterised by relapsing-remitting deficits affecting different functional systems of the central nervous system. Clinical findings include episodes of focal disorders of the optic nerves, spinal cord, and brain, which remit to a varying extent and recur over a period of many years. The neurologic manifestations are protean, being determined by the varied location and extent of the demyelinated foci. The clinical course varies from a benign, largely symptom-free disease to one that is rapidly progressive and disabling. Most patients begin with relapsing and remitting symptoms. At first, recovery from relapses is almost complete, but then neurologic disability gradually accrues. The lesions are therefore multiple in time and space.13–15
TMS is an uncommon manifestation of demyelinating disease and may pose a diagnostic challenge in patients without a pre-existing diagnosis of MS.16–22 TMS may mimic other intracranial processes, such as brain abscesses, malignancies and acute disseminated encephalomyelitis that must be distinguished to avoid inadvertent surgical or toxic chemotherapeutic intervention. Some MR features of tumefactive lesions may help to distinguish TMS from other processes. TMS presents with a large (>2 cm) intracranial lesion with mass effect, perilesional oedema and/or ring enhancement with gadolinium contrast. Open-ring enhancement directed toward the cortical surface has been associated with demyelinating lesions.4,13
Our study identified 24 tumefactive lesions in 293 MS patients (8.21%), a higher proportion than the previous estimate of 0.1%–0.2% of MS patients.23 However, the higher percentage may be explained by the many MR studies carried out in our MS patients and the high quality currently offered by this technique compared to the past. The true incidence of tumefactive lesions is unknown and Poser’s results24,25 are probably an underestimation. However, we are investigating for possible environmental factors, which may be responsible for the high incidence in our region.
Our cohort differs from those of other studies. Although MS usually occurs in female patients, the female predominance in our study (87.5%) is much higher than the proportion reported in other studies investigating TMS (1–2/1000 cases).25 The median age at onset (31 years old) was slightly older than in other studies.24,25 Other distinctive features include a lower EDSS index attack (2.08), higher median disease duration (12.5 years) and tumefactive lesions mainly 0.3–2 cm in size (82.6%). The tumefactive plaques resembled those of other studies, including brain lesions located in frontal and parietal regions (29% and 22.5%, respectively), well-defined margins (74.2%), mild mass effect (93.6%), mild oedema (80.6%) and an enhancing pattern (rim enhancement, 45.2%).2
We also compared the features of patients with and without tumefactive plaques. Our data do not show significant differences in age at disease onset (patients with TMS were slightly younger) or in the female prevalence, which is similar but slightly higher than that seen in classic MS. The main differences are the median disease duration, which is lower in patients with tumefactive lesions (9.8 years vs 12.6 years), and the mean number of MR scans in each patient, which is higher in the group with tumefactive lesions (5.8 vs 3.9).
The definition of ‘tumefactive plaque’ is not consistent in the literature and may refer to various combinations of the following: large size (>2 cm), presence of mass effect or oedema and/or atypical enhancement patterns (ring, heterogeneous, etc.).5 We analysed the morphology of all plaques and noted that plaques <2 cm presented the same mass effect, oedema and ring enhancement as plaques >2 cm. Monitoring the evolution of all 24 tumefactive plaques, we found no significant differences in MR characteristics between plaques <2 cm and >2 cm except for black hole evolution: Plaques <2 cm became black holes in 437.571 days whereas those >2 cm in 299.5 days. This may indicate a slower evolution in <2 cm plaques.
Our study demonstrates that size criteria are not appropriate and that lesions <2 cm and >2 cm have similar MR features and black hole evolution. Further studies on a larger population are required to confirm this theory.
Acknowledgments
The authors thank Angela Martella for language editing.
Professor Splendiani is the principal investigator of this work. All other authors have made equal contributions in drafting/revising the manuscript for content, including medical writing for content, study concept or design and analysis or interpretation of data.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kalanie H, Harandi AA, Bakhshandehpour R, et al. Multiple large tumefactive MS plaques in a young man: A diagnostic enigma and therapeutic challenge. Case Rep Radiol 2012; 2012: 363705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain 2008; 131: 1759–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebers GC. Genetic factors in multiple sclerosis. Neurol Clin 1983; 1: 645. [PubMed] [Google Scholar]
- 4.Confavreux C, Vukusic S. Natural history of multiple sclerosis: Implications for counselling and therapy. Curr Opin Neurol 2002; 15: 257–266. [DOI] [PubMed] [Google Scholar]
- 5.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain 2008; 131: 1759–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altintas A, Petek B, Isik N, et al. Clinical and radiological characteristics of tumefactive demyelinating lesions: Follow-up study. Mult Scler 2012; 18: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 8.Wurm G, Parsaei B, Silye R, et al. Distinct supratentorial lesions mimicking cerebral gliomas. Acta Neurochir (Wien) 2004; 146: 19–26. [DOI] [PubMed] [Google Scholar]
- 9.Hardy TA, Chataway J. Tumefactive demyelination: An approach to diagnosis and management. J Neurol Neurosurg Psychiatry 2013; 84: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 10.Felli V, Di Sibio A, Anselmi M, et al. Progressive multifocal leukoencephalopathy following treatment with rituximab in an HIV-negative patient with non-Hodgkin lymphoma. A case report and literature review. Neuroradiol J 2014; 27: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanton JK, Rovira A, Tintoré M, et al. MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: A multicentre retrospective study. Lancet Neurol 2007; 6: 677–686. [DOI] [PubMed] [Google Scholar]
- 12.Catalucci A, Anselmi M, Splendiani A, et al. Pediatric inflammatory diseases. Part I: Multiple sclerosis. Neuroradiol J 2012; 25: 684–694. [DOI] [PubMed] [Google Scholar]
- 13.Swanton JK, Fernando K, Dalton CM, et al. Modification of MRI criteria for multiple sclerosis in patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry 2006; 77: 830–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triulzi F, Scotti G. Differential diagnosis of multiple sclerosis: Contribution of magnetic resonance techniques. J Neurol Neurosurg Psychiatry 1998; 64(Suppl 1): S6–S14. [PubMed] [Google Scholar]
- 15.Miller DH, Grossman RI, Reingold SC. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain 1998; 121: 3–24. [DOI] [PubMed] [Google Scholar]
- 16.Splendiani A, Catalucci A, Limbucci N, et al. Pediatric inflammatory diseases. Part III: Small vessels vasculitis. Neuroradiol J 2012; 25: 715–724. [DOI] [PubMed] [Google Scholar]
- 17.Splendiani A, Felli V, Di Sibio A, et al. MRI and MR spectroscopy in a young male patient with anti-NMDAR encephalitis and uncommon cerebellar involvement: A case report with review of the literature. Neuroradiol J 2016; 29: 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsecano C, Perri M, Michelini G, et al. Vascular malformation mimicking multiple sclerosis (MS) active plaque: Usefulness of susceptibility weighted imaging (SWI) to perform correct diagnosis. Neuroradiol J 2015; 28: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Splendiani A, Mariani S, Anselmi M, et al. Neuromyelitis optica: Atypical clinical and neuroradiological presentation. Neuroradiol J 2015; 28: 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paonessa A, Limbucci N, Tozzi E, et al. Radiological strategy in acute stroke in children. Eur J Radiol 2010; 74: 77–85. [DOI] [PubMed] [Google Scholar]
- 21.Butteriss DJ, Ismail A, Elison DW, et al. Use of serial proton magnetic resonance spectroscopy to differentiate low grade glioma from tumefactive plaque in a patient with multiple sclerosis. Br J Radiol 2003; 76: 662–665. [DOI] [PubMed] [Google Scholar]
- 22.Metafratzi Z, Argyropoulou MI, Tzoufi M, et al. Conventional MRI and magnetization transfer imaging of tumor-like multiple sclerosis in a child. Neuroradiology 2002; 44: 97–99. [DOI] [PubMed] [Google Scholar]
- 23.Totaro R, Di Carmine C, Splendiani A, et al. Occurrence and long-term outcome of tumefactive demyelinating lesions in multiple sclerosis. Neurol Sci 2016; 37: 1113–1117. [DOI] [PubMed] [Google Scholar]
- 24.Poser S, Lüer W, Bruhn H, et al. Acute demyelinating disease. Classification and non-invasive diagnosis. Acta Neurol Scand 1992; 86: 579–585. [DOI] [PubMed] [Google Scholar]
- 25.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann Neurol 1983; 13: 227–231. [DOI] [PubMed] [Google Scholar]