Abstract
Diabetic ketoacidosis (DKA) is a state of severe insulin deficiency and a serious complication in children with diabetes mellitus type 1. In a small number of children, DKA is complicated by injury of the central nervous system. These children have a significant mortality and high long-term neurological morbidity. Cerebral edema is the most common neuroimaging finding in children with DKA and may cause brain herniation. Ischemic or hemorrhagic stroke during the acute DKA episode is less common and accounts for approximately 10% of intracerebral complications of DKA. Here we present the neuroimaging findings of two children with DKA and brain injury. Familiarity with the spectrum of neuroimaging findings seen in pediatric DKA is important to allow early detection as well as initiation of therapy and, hence, prevent complications of the central nervous system.
Keywords: Diabetic ketoacidosis, children, brain, MRI, stroke
Introduction
Diabetes mellitus type 1 (DM1) is one of the most common chronic pediatric illnesses. Diabetic ketoacidosis (DKA) is serious complication in children with DM1 and has an increasing incidence.1 DKA is a state of severe insulin deficiency, either absolute or relative, resulting in hyperglycemia, ketonemia, acidemia, and systemic inflammation. Treatment involves fluid resuscitation with insulin and electrolyte replacement.2 When DKA is recognized and immediately treated, the prognosis is excellent. In about 1% of pediatric patients DKA is complicated by involvement/injury of the central nervous system (CNS).3 These children have a significant mortality and high long-term neurological morbidity. Cerebral edema is the most common neuroimaging finding in children with DKA and may cause brain herniation with possible subsequent cerebral infarctions.4 Primary ischemic or hemorrhagic stroke during the acute DKA episode is less common and accounts for approximately 10% of intracerebral complications of DKA.5
We report on two adolescent females with DKA and acute CNS involvement. We will focus on the neuroimaging findings and review the available literature.
Case report
Patient 1
The first patient is a girl who was first diagnosed with DM1 at the age of 11 years. A medicamentous therapy and diet were started, but her blood sugar levels were poorly controlled and she had two episodes of DKA causing short admissions to our tertiary pediatric hospital. A few months later she presented at the emergency department (ED) with a new episode of acute onset of abdominal pain, headaches, emesis, and an altered mental status. The diagnosis of a new DKA episode was made. The mental status deteriorated rapidly with a drop of the Glasgow Coma Scale (GCS) from 11 to 3 and the child was intubated and transferred to the pediatric intensive care unit (PICU). An acute head computed tomography (CT) study showed a diffuse hypodensity of the cerebral white matter with effacement of the sulci, third ventricle, and basal cisterns and crowding of the foramen magnum suggestive of severe, diffuse brain edema with early tonsillar herniation (Figure 1). An intracranial pressure monitoring device was placed and conservative management of acute intracranial hypertension was started. The GCS increased up to 8–9. A brain magnetic resonance imaging (MRI) performed one week after admission showed focal areas of hyperintense signal on T2-weighted and fluid-attenuation inversion recovery (FLAIR) images with matching bright signal on trace of diffusion maps and low apparent diffusion coefficient (ADC) values within the left posterior lateral aspect of the pons, left posterior limb of the internal capsule, bilateral anterior medial thalami, and body of the corpus callosum (Figure 2). In addition, focal areas of hyperintense signal on T2-weighted and FLAIR images with matching high ADC values were seen within the left inferior olivary nucleus, left paracentral posterior pons, left side of the posterior lateral thalamus, right posterior limb of internal capsule, right genu of the internal capsule, and bilateral medial thalamus (Figure 2). These findings are compatible with multiple areas of acute and late subacute to early chronic ischemic injuries and were interpreted as most likely secondary to the increased intracranial hypertension. After two weeks in the PICU, she was extubated and transferred to the clinical ward, where she was partially responsive to voice or painful stimuli and showed an extensor posturing in her legs and arms. On the floor, rehabilitation therapy was started and she continued to improve neurologically. Six months after admission, she was able to speak, but was dysarthric, was wheelchair dependent, and had a bilateral dysmetria, left facial nerve palsy, spasticity of both arms and legs, and dysphagia requiring a gastrostomy tube feeding.
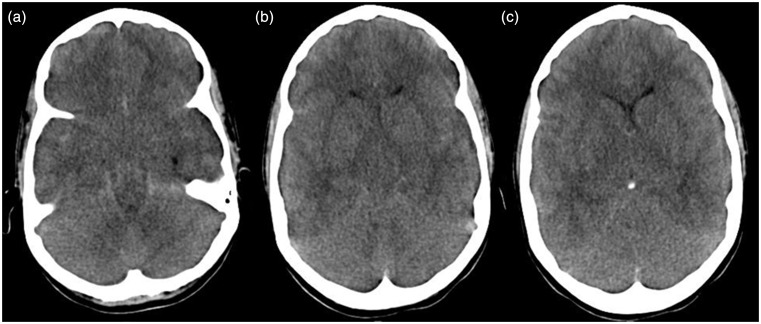
Figure 1.
((a)–(c)) Acute axial CT images of patient 1 show diffuse cerebral edema with hypodense brain parenchyma and effacement of sulci and basal cisterns. CT: computed tomography.
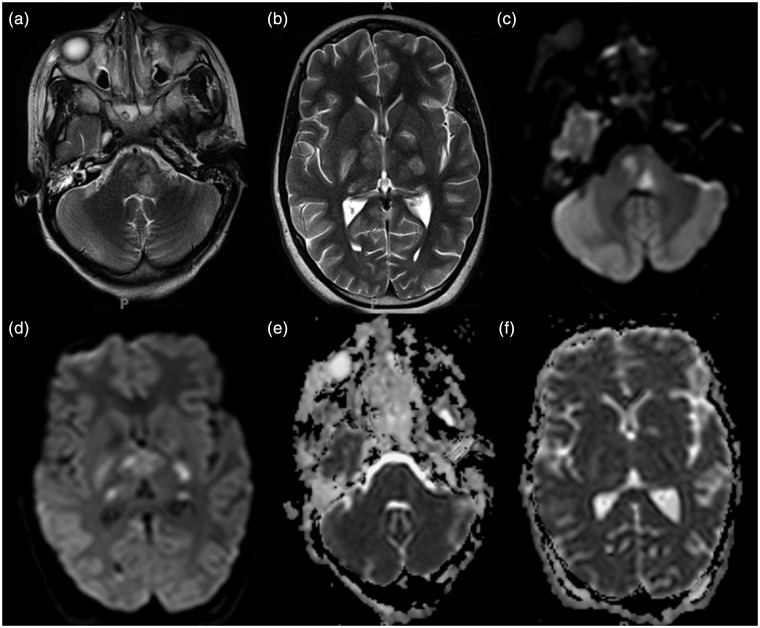
Figure 2.
((a), (b)) Follow-up axial T2-weighted MR images, ((c), (d)) trace of diffusion images and ((e), (f)) ADC maps of patient 1 performed one week after the CT (Figure 1) show T2-hyperintense signal with matching bright signal on trace of diffusion and low ADC values within the left posterior lateral aspect of the pons, left posterior limb of the internal capsule, bilateral anterior medial thalami, and genu of the corpus callosum. In addition, focal areas of T2-hyperintense signal with matching high ADC values are seen within the left inferior olivary nucleus, left paracentral posterior pons, left side of the posterior lateral thalamus, right posterior limb of internal capsule, right genu of the internal capsule, and bilateral medial thalamus. MR: magnetic resonance; ADC: apparent diffusion coefficient; CT: computed tomography.
Patient 2
The second patient is a 5.8-year-old girl who was first diagnosed with DM1 at the age of 3.5 years. A medicamentous therapy and diet was started, but her blood sugar levels were poorly controlled (high level of glycated hemoglobin). She presented at the ED of our tertiary pediatric hospital because of acute onset of vomiting, diarrhea, and altered mental status. DKA was diagnosed. The clinical exam showed absent pupillary reaction bilaterally, no spontaneous movement of the extremities, and no reaction to stimuli. She was intubated and transferred to the PICU. A head CT showed a diffuse effacement of the sulci and basal and suprasellar cisterns suggestive of global cerebral edema with impending transtentorial herniation (Figure 3). In addition, in the left posterior cerebral artery (PCA) territory a hypodensity was seen concerning for an acute/subacute ischemic stroke (Figure 3). Conservative management for the cerebral edema was initiated. A brain MRI on the subsequent day revealed bright signal on trace of diffusion maps with matching reduced ADC values in the left PCA distribution confirming the ischemic stroke (Figure 4). In addition, small areas of restricted diffusion were seen within the medial thalami bilaterally, midbrain, bilateral amygdala, posterior inferior aspect of bilateral frontal lobes, and right medial temporal lobes (Figure 4). The distribution of the predominant midline ischemic lesions is most likely secondary to transtentorial herniation. Susceptibility-weighted imaging (SWI) showed multiple small areas of susceptibility artifact in the left PCA infarct representing partial hemorrhagic transformation. The neurological status of the child progressively improved and she was extubated and transferred to the clinical ward after about two weeks in the PICU. On the floor, rehabilitation therapy was started and she continued to improve neurologically. Five months after admission she was alert and cooperative, had fluent speech as well as a normal sensory and motor exam of all extremities. Her neurological exam showed right anisocoria and homonymous hemianopsia. In addition, she had a reduced short-term memory. A follow-up MRI at this point showed chronic encephalomalacic changes in the left PCA territory, medial thalami bilaterally, midbrain, bilateral amygdala, posterior inferior aspect of bilateral frontal lobes, and right medial temporal lobes (not shown). At the last follow-up two years after admission, her neurological status was stable.
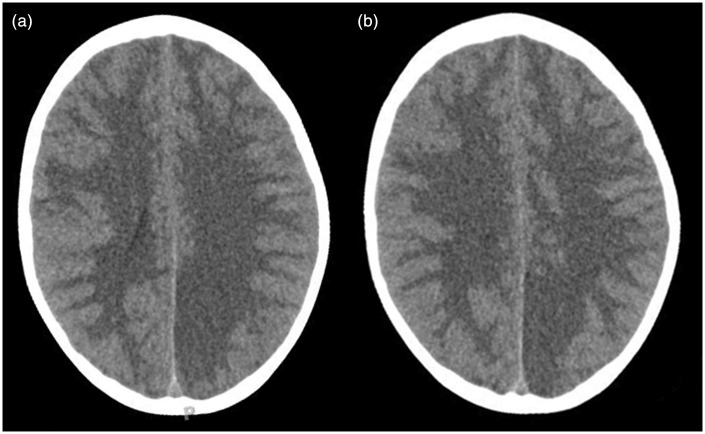
Figure 3.
((a), (b)) Acute axial CT images of patient 2 show diffuse effacement of the sulci and basal and suprasellar cisterns suggestive of global cerebral edema. In addition, a cortical/subcortical hypodensity is noted within the vascular territory of the left posterior cerebral artery concerning for an acute/subacute ischemic stroke. CT: computed tomography.
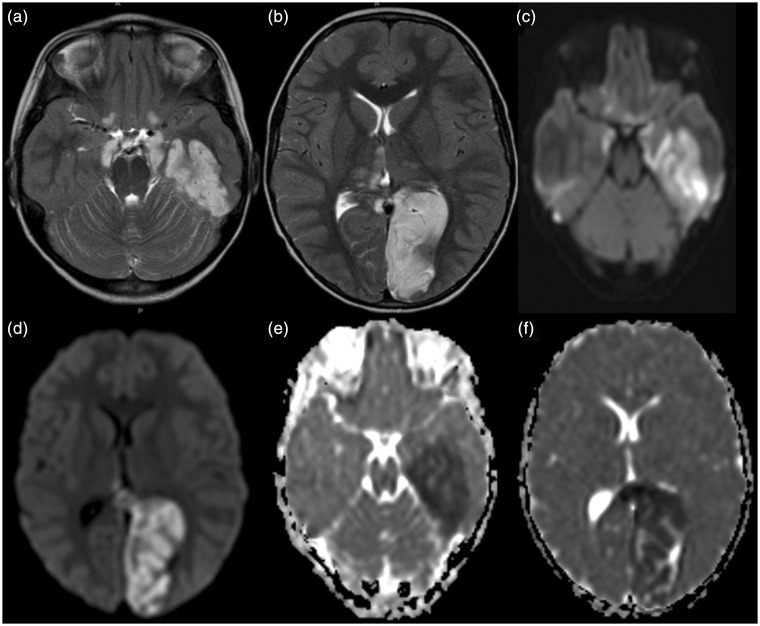
Figure 4.
((a), (b)) Axial T2-weighted MR images, ((c), (d)) trace of diffusion and ((e), (f)) ADC maps of patient 2 performed the subsequent day compared to the CT (Figure 3) reveal T2-hyperintense signal with matching bright signal on trace of diffusion images and reduced ADC values within the vascular territory of the left posterior cerebral artery confirming the acute/subacute arterial ischemic stroke. In addition, T2-hyperintense foci with restricted diffusion are seen within the medial thalami bilaterally, midbrain, bilateral amygdala, posterior inferior aspect of bilateral frontal lobes, and left medial temporal lobe. The last ones are most likely secondary to transtentorial herniation. MR: magnetic resonance; ADC: apparent diffusion coefficient; CT: computed tomography.
Discussion
DKA is a rather common presentation or complication of pediatric DM1 patients and occurs in about 36% of diabetic children younger than 5 years of age and about 16% of teenagers older than 14 years.6 In the majority of pediatric patients, DKA has an excellent prognosis. In about 1% of the children DKA results in acute morphologic and functional brain changes that are associated with poor long-term neurocognitive outcome. Neuroimaging plays a key role in the early diagnosis of diabetic children with DKA and acute CNS involvement. Accordingly, it important that neuroradiologists are aware of the possible neuroimaging findings associated with DKA.
Cerebral edema is the most common neuroimaging finding associated with pediatric DKA and occurs in about 0.5%–1% of pediatric DKA.7–9 Cerebral edema in DKA is associated with increased morbidity and mortality in up to 50% of affected children and occurs almost exclusively in pediatric patients.7,8 Children with more severe acidosis, hypocapnia, and dehydration have a higher risk of developing cerebral edema.7,8,10 The exact pathomechanism of cerebral edema in DKA, however, remains unclear and is most likely multifactorial. Possible pathomechanisms include (1) osmotic gradients drawing fluid into hypertonic astrocytes during intravenous therapy,11 (2) intracellular accumulation of sodium and water as a correction of intracellular acidosis due to the accumulation of lactate, free fatty acids, and ketone bodies,12 and (3) vasodilatation and reperfusion following cerebral vasoconstriction due to hypoocapnia.4
Children with DKA and cerebral edema (including our two patients) usually present with symptoms/signs of cerebral dysfunction and/or increased intracranial pressure such as headache, altered mental status, abnormal pupillary reflexes, posturing, hypertension with bradycardia, or apnea. This acute presentation should prompt clinicians to request acute neuroimaging in children with DKA. However, in up to 40% of children with DKA and acute neurological presentation, the first neuroimaging study (especially head CT) may show no obvious acute abnormalities suggesting that (1) brain MRI instead of CT should be performed in children with DKA and acute neurological symptoms and (2) the neurological presentation may be due to other causes and cerebral edema may be of secondary nature.13 On neuroimaging (CT and MRI), cerebral edema in children with DKA is characterized by effacement of the sulci and basilar cisternal spaces (especially the suprasellar, quadrigeminal plate and ambient cisterns), compression and decreased size of the cerebral ventricles, and reduction of the gray-white matter differentiation.4 Diffusion-weighted and diffusion-tensor imaging may further characterize cerebral edema and differentiate between vasogenic and cytotoxic edema. Increased ADC values representing vasogenic edema have been shown in the basal ganglia, thalamus, frontal white matter, and periaqueductal gray matter of children with DKA and cerebral edema.14 Increase in ADC values correlates with the degree of dehydration and hyperventilation at presentation, but not with factors related to initial osmolality or osmotic changes during treatment.14 These findings suggest that cerebral hypoperfusion may be a key role in the pathomechanism of cerebral edema in DKA. The role of cerebral hypoperfusion is supported by perfusion-weighted imaging studies showing shorter mean transit times and higher peak tracer concentrations, again suggesting vasogenic edema.15–17
Severe cerebral edema may cause complications with high morbidity and mortality such as focal stroke (as in patient 1) and subfalcine or transtentorial herniation (as in patient 2). Focal infarction secondary to cerebral edema may occur in up to 20% of children with DKA and typically involves the mesial basal ganglia, thalamus, periaqueductal gray matter, and dorsal pontine nuclei.13,18 Brain herniation is an uncommon complication of cerebral edema in DKA that typically occurs 6–13 hours after onset of symptoms and is clinically characterized by sudden deterioration of consciousness, absent brainstem responses, and eventual respiratory arrest.19,20 Compression of the major vessels of the circle of Willis due to downward herniation may play an additional significant factor in the occurrence of focal ischemic strokes.
Other possible neuroimaging findings in pediatric DKA include ischemic and hemorrhagic stroke, sinovenous thrombosis, and extrapontine myelinolysis. Ischemic stroke in DKA may occur independently from cerebral edema and be due to increased blood viscosity and thrombosis due to hyperosmolality, systemic inflammatory reaction, vascular endothelial injury, acidemia-induced red blood cell rigidity, and hyperglycemia-induced vasoconstriction. Associated coagulopathies or vasculopathies such as factor V Leiden mutation or Adams–Oliver syndrome may further increase the risk for a primary ischemic stroke in children with DKA.21,22 Hemorrhagic stroke is less common in acute DKA and may be secondary to sinovenous thrombosis.23,24 In addition, hyperglycemia and acidosis could enhance endothelial damage with subsequent extravasation of red blood cells through the leaky vessels and increase the risk of hemorrhagic transformation of ischemic strokes.25,26 Finally, extrapontine myelinolysis is a very rare complication of DKA in children.27,28 Extrapontine myelinolysis is characterized by T2-/FLAIR-hyperintense signal and restricted diffusion in the claustrum and putamen with inconsistent involvement of the hippocampi and subcortical white matter and may be associated with pontine myelinolysis. The exact pathomechanism of extrapontine myelinolysis in pediatric DKA is unknown, but is most likely related to electrolyte imbalance in the context of DKA and its rapid correction.
In conclusion, our patients show the various CNS injuries that can be seen on neuroimaging in pediatric DKA. It is important that pediatric neuroradiologists are familiar with the spectrum of neuroimaging findings of pediatric DKA for early detection as well as guidance of acute therapy and, hence, avoid devastating long term complications.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Olivieri L, Chasm R. Diabetic ketoacidosis in the pediatric emergency department. Emerg Med Clin North Am 2013; 31: 755–773. [DOI] [PubMed] [Google Scholar]
- 2.Wolfsdorf JI. The International Society of Pediatric and Adolescent Diabetes guidelines for management of diabetic ketoacidosis: Do the guidelines need to be modified? Pediatr Diabetes 2014; 15: 277–286. [DOI] [PubMed] [Google Scholar]
- 3.Ho J, Pacaud D, Hill MD, et al. Diabetic ketoacidosis and pediatric stroke. CMAJ 2005; 172: 327–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wootton-Gorges SL, Glaser NS. Imaging of the brain in children with type I diabetes mellitus. Pediatr Radiol 2007; 37: 863–869. [DOI] [PubMed] [Google Scholar]
- 5.Roe TF, Crawford TO, Huff KR, et al. Brain infarction in children with diabetic ketoacidosis. J Diabetes Complications 1996; 10: 100–108. [DOI] [PubMed] [Google Scholar]
- 6.Wolfsdorf J, Glaser N, Sperling MA, et al. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care 2006; 29: 1150–1159. [DOI] [PubMed] [Google Scholar]
- 7.Edge JA, Hawkins MM, Winter DL, et al. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child 2001; 85: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunger DB, Sperling MA, Acerini CL, et al. ESPE/LWPES consensus statement on diabetic ketoacidosis in children and adolescents. Arch Dis Child 2004; 89: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin DL. Cerebral edema in diabetic ketoacidosis. Pediatr Crit Care Med 2008; 9: 320–329. [DOI] [PubMed] [Google Scholar]
- 10.Glaser N, Barnett P, McCaslin I, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med 2001; 344: 264–269. [DOI] [PubMed] [Google Scholar]
- 11.Bohn D, Daneman D. Diabetic ketoacidosis and cerebral edema. Curr Opin Pediatr 2002; 14: 287–291. [DOI] [PubMed] [Google Scholar]
- 12.Glaser N. Cerebral edema in children with diabetic ketoacidosis. Curr Diab Rep 2001; 1: 41–46. [DOI] [PubMed] [Google Scholar]
- 13.Muir AB, Quisling RG, Yang MC, et al. Cerebral edema in childhood diabetic ketoacidosis: Natural history, radiographic findings, and early identification. Diabetes Care 2004; 27: 1541–1546. [DOI] [PubMed] [Google Scholar]
- 14.Glaser NS, Marcin JP, Wootton-Gorges SL, et al. Correlation of clinical and biochemical findings with diabetic ketoacidosis-related cerebral edema in children using magnetic resonance diffusion-weighted imaging. J Pediatr 2008; 153: 541–546. [DOI] [PubMed] [Google Scholar]
- 15.Glaser NS, Wootton-Gorges SL, Marcin JP, et al. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr 2004; 145: 164–171. [DOI] [PubMed] [Google Scholar]
- 16.Glaser N, Ngo C, Anderson S, et al. Effects of hyperglycemia and effects of ketosis on cerebral perfusion, cerebral water distribution, and cerebral metabolism. Diabetes 2012; 61: 1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vavilala MS, Marro KI, Richards TL, et al. Change in mean transit time, apparent diffusion coefficient, and cerebral blood volume during pediatric diabetic ketoacidosis treatment. Pediatr Crit Care Med 2011; 12: e344–e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers B, Sills I, Cohen M, et al. Diabetic ketoacidosis. Neurologic collapse during treatment followed by severe developmental morbidity. Clin Pediatr (Phila) 1990; 29: 451–456. [DOI] [PubMed] [Google Scholar]
- 19.Eskandar EN, Weller SJ, Frim DM. Hydrocephalus requiring urgent external ventricular drainage in a patient with diabetic ketoacidosis and cerebral edema: Case report. Neurosurgery 1997; 40: 836–838. discussion 838–839. [DOI] [PubMed] [Google Scholar]
- 20.Shrier DA, Shibata DK, Wang HZ, et al. Central brain herniation secondary to juvenile diabetic ketoacidosis. AJNR Am J Neuroradiol 1999; 20: 1885–1888. [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbloom AL. Fatal cerebral infarctions in diabetic ketoacidosis in a child with previously unknown heterozygosity for factor V Leiden deficiency. J Pediatr 2004; 145: 561–562. [DOI] [PubMed] [Google Scholar]
- 22.Nwosu BU, Adhami S, Rogol AD. Stroke in a child with Adams–Oliver syndrome and mixed diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. J Pediatr Endocrinol Metab 2012; 25: 357–361. [DOI] [PubMed] [Google Scholar]
- 23.Sasiadek MJ, Sosnowska-Pacuszko D, Zielinska M, et al. Cerebral venous thrombosis as a first presentation of diabetes. Pediatr Neurol 2006; 35: 135–138. [DOI] [PubMed] [Google Scholar]
- 24.Zerah M, Patterson R, Hansen I, et al. Resolution of severe sinus vein thrombosis with super selective thrombolysis in a pre-adolescent with diabetic ketoacidosis and a prothrombin gene mutation. J Pediatr Endocrinol Metab 2007; 20: 725–731. [DOI] [PubMed] [Google Scholar]
- 25.Lin JJ, Lin KL, Wang HS, et al. Occult infarct with acute hemorrhagic stroke in juvenile diabetic ketoacidosis. Brain Dev 2008; 30: 91–93. [DOI] [PubMed] [Google Scholar]
- 26.Shoar Z, Dunne C, Yorns W, et al. Diabetic ketoacidosis with cerebral hemorrhage and alpha coma in an adolescent female. J Pediatr Endocrinol Metab 2013; 26: 561–564. [DOI] [PubMed] [Google Scholar]
- 27.Bonkowsky JL, Filloux FM. Extrapontine myelinolysis in a pediatric case of diabetic ketoacidosis and cerebral edema. J Child Neurol 2003; 18: 144–147. [DOI] [PubMed] [Google Scholar]
- 28.Sivaswamy L, Karia S. Extrapontine myelinolysis in a 4 year old with diabetic ketoacidosis. Eur J Paediatr Neurol 2007; 11: 389–393. [DOI] [PubMed] [Google Scholar]