Abstract
In older adults with aortic stenosis, we evaluated whether accelerometer-measured physical activity provides distinct clinical information apart from self-reported surveys or performance-based function tests. We employed wrist-mounted accelerometry in 52 subjects with severe aortic stenosis prior to transcatheter aortic valve replacement (TAVR). Daily daytime activity was estimated using the maximum 10 h of daily accelerometer-measured activity (M10) reported in activity counts. Subjects completed baseline surveys (New York Heart Association (NYHA), Short Form 12 (SF12), Kansas City Cardiomyopathy Questionnaire (KCCQ), EuroQol-5D (EQ-5D), Revised Life Orientation Test (LOT-R), Life Space, Detailed Activity Form) and performance-based function tests (Short Physical Performance Battery, 6-min walk test distance, grip strength) to estimate functional status. Simple and multiple linear regression models were used to evaluate the relationship between accelerometer-measured activity and survey data and performance-based function tests. Among all baseline surveys and performance-based function tests, the only statistically significant univariable relationships identified were weak, negative associations between M10 and SF-12 Mental Composite Score (R2=0.1970, P=0.04) and between M10 and grip strength (R2=0.1568, P=0.004). Neither multiple linear regression of overall survey data (R2=0.6159, P=0.23) nor performance-based function tests (R2=0.1743, P=0.10) correlated with M10. Self-reported surveys and performance-based function tests are not meaningfully correlated with daytime accelerometer-measured activity. The results of our study suggest that accelerometer-measured physical activity provides distinct clinical information apart from self-reported surveys or performance-based function tests.
Keywords: Accelerometer, Activity, Aortic stenosis, Valves, Heart failure
Introduction
In older adults with aortic stenosis, one of the primary treatment goals is to improve activity level in those individuals with severe symptoms or to maintain physical activity level among those individuals who are minimally symptomatic. Self-reported patient surveys of symptom status and quality of life have been used to directly or indirectly estimate physical activity in older adults with aortic stenosis [1]. In addition, performance-based function tests, such as the 6-min walk test, have been used to measure functional capacity [2, 3•]. However, both self-reported surveys and objective performance-based function tests are administered at a specific time point and are only indirect measures of true everyday physical activity level in the patient’s environment.
Wearable accelerometer-measured activity, or actigraphy, has emerged as a valuable tool to assess physical activity in research and clinical settings. Wearable accelerometers can objectively quantify and monitor activity counts continuously over an extended period of time, making them an ideal tool for measuring physical activity in older adults with cardiovascular disease [4–6].
Accordingly, in a cohort of older adults with aortic stenosis, we sought to determine whether surveys or performance-based function tests correlated with accelerometer-measured physical activity in order to understand whether accelerometer-measured physical activity provides distinct clinical information apart from self-reported surveys or performance-based function tests.
Methods
Participants
Participants were part of a prospective cohort study, evaluating high-risk patients with severe aortic stenosis (AS) presenting to the outpatient Valve Center at the Columbia University Medical Center/New York-Presbyterian Hospital for transcatheter aortic valve replacement (TAVR) consideration between 2011 and 2012. Subjects were 60 years of age and older and had severe, calcific AS (aortic valve area <0.8 cm2 and mean gradient >40 mmHg or jet velocity >4.0 m/s) and were determined to be TAVR candidates after a careful selection process assessing their overall appropriateness for the procedure. The Columbia University Medical Center Institutional Review Board approved this protocol, and all participants signed informed consent.
Actigraphy
The ActiGraph is a small, wristwatch-like triaxial accelerometer capable of real-time ambulatory monitoring of activity counts at various epochs. Fifty-two subjects were given ActiGraph accelerometer activity monitors worn continuously on the non-dominant wrist prior to their TAVR. Using the ActiLife propriety software, data were collected using a 1-min epoch, generating an activity count for each minute of the day. Accelerometers were worn for an average of 17.6 days (median 15.5, interquartile range 9–25.3). Daily daytime activity was estimated using the maximum 10 h of daily accelerometer-measured activity (M10) [4] reported in activity counts.
Study Measurements
Baseline demographic, clinical, and echocardiographic information were collected for all subjects. Estimates of baseline physical function and quality of life were assessed by surveys. Subjects were classified into one of four New York Heart Association (NYHA) heart failure classes based on a self-reported symptomatology survey [7]. The Kansas City Cardiomyopathy Questionnaire (KCCQ), previously validated as a measure of health status for heart failure [8] and aortic stenosis [9••], was used to calculate an overall KCCQ score (minimum value 0 to maximum value 100, 100=highest functioning). The Short Form 12 (SF12) was used to calculate a physical composite score (SF12-PCS) and mental composite score (SF12-MCS) (minimum value 0 to maximum value 100, 100=highest functioning) [10, 11]. EuroQol-5D (EQ-5D) survey was used to calculate the EQ-5D time trade-off (TTO) utility score (minimum value −0.109 to maximum value 1, 1= highest functioning) validated for the USA [12]. Self-reported lack of energy (anergia) was evaluated with a validated 7-item questionnaire; these results were used to calculate an overall anergia score (minimum value 0 to maximum value 7, 7=least functioning) and patient was classified as anergic or not anergic [13]. The Revised Life Orientation Test (LOT-R), an estimate of positive or negative outlook in life, was used to calculate the LOT score (minimal value 0, maximum value 40, 40=highest functioning) [14]. The Life Space survey was used to calculate the Life Space level score (minimal value 0 to maximum value 40, 40=highest functioning) [15]. A Detailed Activity Form (DAF) (condensed from the original 18 activities of the Minnesota Leisure Time Physical Activity Questionnaire to assess participation in six activities) was used to estimate weekly energy expenditure in kilocalories [16–18]. Independence in activities of daily living was assessed by the Katz ADL survey [19]. The need for assistance with any one of the six ADLs resulted in the subject being considered dependent, and performing all activities independently was required to be considered independent.
Measurement of baseline physical functioning was assessed using several performance-based function tests. Gait speed was assessed according to time in seconds to walk 15 ft [20]. Subjects were instructed to “walk at their comfortable pace” until a few steps past the 15-ft line. Usual assist devices (e.g., walkers, canes) were permitted. If able, each subject completed one 15-ft walk. Gait speed was calculated by dividing 4.57 m (15 ft) by time to walk this distance in seconds and reported in meters/second. Subjects unable to perform the task were assigned a gait speed of 0 m/s. Test of balance included side-by-side, semi-tandem, and tandem stands. For each stand, the interviewer first demonstrated the task, then supported one arm while subjects positioned their feet, asked if they were ready, then released the support and began timing. The timing was stopped when subjects moved their feet or grasped the interviewer for support, or when 10 s had elapsed. Subjects first attempted the side-by-side stand, and if able to stand side-by-side for 10 s, they moved on to the semi-tandem stand, and if able to hold semi-tandem stand for 10 s, they moved on to the tandem stand. To test ability to rise from a chair (chair stand), subjects were seated in a straight-backed chair with their feet on the floor, asked to fold their arms across their chest, and asked to stand from seated position. If subjects were unable to rise without using their arms, they were told to stand using their arms. If successful, subjects were asked to stand up and sit down five times as quickly as possible and were timed from initial sitting position to final standing position after the fifth stand. A Short Physical Performance Battery (SPPB) score (minimum value 0 to maximum value 12, 12=highest functioning) was calculated using each subject’s gait speed, balance testing, and chair stand results [21]. Subjects performed a 6-min walk test (6MWT) in which subjects were asked to walk as far as possible for 6 minutes, without running or jogging [22]. Assist devices (e.g., walkers, canes) were permitted. Dominant hand grip strength was assessed as the average of 3 trials of maximal isometric grip measured in kilograms with a Jamar dynamometer (Sammons Preston, Chicago, Illinois) [23, 24].
Statistical Analysis
The primary outcome variable was daily daytime activity as measured by ActiGraph accelerometer activity, measured as daily M10 activity (M10), and averaged over the total number of days the subject wore their accelerometer. Distribution of M10 measurements was evaluated by histogram analysis. Simple linear regression was used to evaluate the association between M10 and assessments of physical function and quality of life by survey data (SF12-PCS, SF12-MCS, KCCQ overall score, EQ-5D TTO score, LOT-R score, Life Space level score, DAF weekly energy expenditure) and performance-based function tests (SPPB score, 6MWT, grip strength). Multiple linear regressions were used to model M10 with measurements of physical function and quality of life based on survey data (NYHA class, SF12-PCS, SF12-MCS, KCCQ overall score, EQ-5D TTO score, anergia, LOT-R score, Life Space level score, DAF weekly energy expenditure, and Katz ADLs dependence) and with performance-based function tests (SPPB score, 6MWT, grip strength). All analyses were repeated using total 24-h daily counts as the primary outcome. Since results were similar, only analyses for daytime activity (M10) are presented. All analyses were performed with STATA (version SE12, StataCorp LP, College Station, Texas). A P value of <0.05 was considered to be statistically significant.
Results
Fifty-two subjects with severe AS being considered for TAVR and who wore ActiGraph activity monitors prior to TAVR were included in the analysis. The median age was 88 years (interquartile range 85–90) and 63 % were female. All subjects were community dwelling. Subjects had a high prevalence of comorbid illness; 87 % had hyperlipidemia, 94 % had hypertension, and 88 % had coronary artery disease (Table 1).
Table 1.
Baseline characteristics, survey and performance-based assessment summaries, and accelerometer activity
| Variable | |
|---|---|
| Age (years) | 88 [85, 90] |
| Female | 33 (63 %) |
| Caucasian | 50 (96 %) |
| BMI (kg/m2) | 25.2 [22.1, 28.7] |
| Community dwelling | 52 (100 %) |
| Diabetes | 10 (19 %) |
| Hyperlipidemia | 45 (87 %) |
| Hypertension | 49 (94 %) |
| Lung disease | 14 (27 %) |
| Coronary artery disease | 46 (88 %) |
| Arrhythmia | 17 (33 %) |
| Previous percutaneous coronary angioplasty | 4 (8 %) |
| Previous coronary artery bypass | 15 (29 %) |
| Previous aortic valvuloplasty | 1 (2 %) |
| Previous pacemaker | 5 (10 %) |
| Previous stroke | 3 (6 %) |
| NIHSS>0 | 5 (13 %) |
| Peripheral vascular disease | 9 (17 %) |
| Weight loss >10 lbs in past year | 15 (33 %) |
| Aortic valve mean gradient (mmHg) | 42.9 [37.0, 54.9] |
| Aortic valve area (cm2) | 0.70 [0.60, 0.75] |
| Ejection fraction (%) | 63 [54, 67] |
| Albumin (g/dL) | 4.2 [3.8, 4.4] |
| Systolic blood pressure (mmHg) | 138 [119, 150] |
| Diastolic blood pressure (mmHg) | 67 [63, 76] |
| NYHA class 1 | 1 (2 %) |
| NYHA class 2 | 17 (33 %) |
| NYHA class 3 | 34 (65 %) |
| NYHA class 4 | 0 (0 %) |
| SF12-PCS (0–100) | 31.3 [26.8, 38.7] |
| SF12-MCS (0–100) | 55.0 [39.0, 60.1] |
| KCCQ overall score (0–100) | 45.4 [33.7, 68.5] |
| EQ-5D TTO score (−0.109–1) | 0.78 [0.60, 0.84] |
| Anergia, total score (0–7) | 4 [3, 5] |
| Anergia, meets criteria | 32 (62 %) |
| Life Orientation Test score (0–40) | 27 [24, 29] |
| Life Space level score (0–40) | 18.5 [12.0, 23.8] |
| Detailed Activity Form energy expenditure (kcal/week) | 41.9 [0.0, 352.0] |
| Katz ADLs score (0–6) | 6 [5, 6] |
| Katz ADLs <6 (dependent) | 20 (38 %) |
| Short Physical Performance Battery score (0–12) | 7.5 [5.0, 9.0] |
| 6-min walk distance (meters) | 183 [57,237] |
| Grip strength (kg) | 16.3 [11.3, 20.2] |
| M10 accelerometer activity (activity counts) | 966,131 [720,529, 1,267,931] |
The results of the baseline survey assessments and performance-based functional tests are shown in Table 1. Overall, there was a significant symptom burden. Most subjects were NYHA class II or III; 2 % class I, 33 % class II, and 65 % class III, none were class IV. Sixty-two percent of subjects qualified as being anergic based on previously defined criteria [11], and 38 % of subjects were dependent in at least one Katz ADL category. The population demonstrated a low median 6-min walk distance of 183 (interquartile range 57–237) meters and weak median dominant hand grip strength of 16.3 (interquartile range 11.3–20.2) kg. Median daily M10 accelerometer activity was 966,131 (interquartile range 720, 529–1,267,931) activity counts (Table 1, Fig. 1).
Fig. 1.
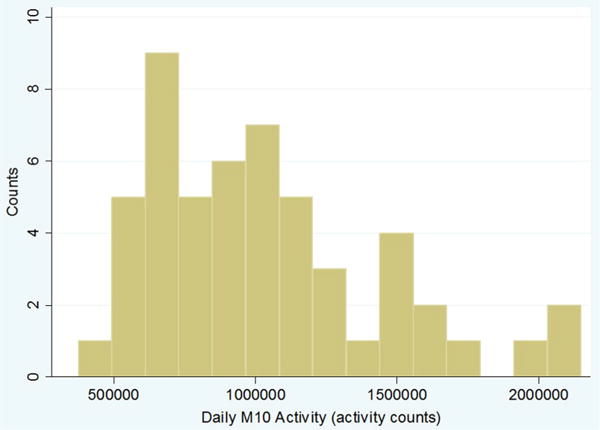
Distribution of daytime activity as estimated by maximum 10 h of daily accelerometer-measured activity counts (M10)
Survey Assessments
There was no association between M10 and SF12-PCS, KCCQ overall score, EQ-5D TTO score, LOT-R score, Life Space level score, or DAF weekly energy expenditure (Table 2). There was a statistically significant but weak, negative association between SF12-MCS and M10 (R2=0.1970, P=0.04) (Fig. 2). Multiple linear regressions of M10 with survey assessments were not statistically significant (R2=0.6159, P=0.23) (Table 2).
Table 2.
Simple and multiple linear regressions of M10 and survey and performance-based assessments
| Variable | R2 | P value | Coefficient |
|---|---|---|---|
| Simple linear regression of M10 and survey and performance-based assessments | |||
| SF12-PCS | 0.0004 | 0.93 | 1245 [−29,282, 31,772] |
| SF12-MCS | 0.1970 | 0.04 | −18055 [−35,058, −1053] |
| KCCQ overall score | 0.0392 | 0.38 | −4214 [−13,939, 5512] |
| EQ-5D TTO score | 0.0016 | 0.86 | −115,084 [−1,462,923, 1,232,755] |
| Life Orientation Test score | 0.0272 | 0.25 | −19989 [−54687,14709] |
| Life Space level score | 0.0088 | 0.52 | 5503 [−11,428, 22,433] |
| Detailed Activity Form energy expenditure | 0.0119 | 0.45 | 92 [−151,334] |
| Short Physical Performance Battery score | 0.0064 | 0.58 | −12,456 [−57,631, 32,718] |
| 6-min walk distance | 0.0071 | 0.62 | 115 [−351,581] |
| Grip strength | 0.1568 | 0.004 | −22,477 [−37,444, −7511] |
| Multiple linear regressions of M10 and survey assessments | |||
| Overall regression | 0.6159 | 0.23 | |
| NYHA class | 1.00 | −1303 [−715,024, 712,418] | |
| SF12-PCS | 0.71 | −11,652 [−78,482, 55,178] | |
| SF12-MCS | 0.15 | −25,702 [−62,432, 11,028] | |
| KCCQ overall score | 0.94 | 854 [−24,409, 26,118] | |
| EQ-5D TTO score | 0.78 | −288,278 [−2,556,553, 1,979,998] | |
| Anergia, meets criteria | 0.98 | 7004 [−553,418, 567,425] | |
| Life Orientation Test score | 0.56 | −25280 [−118,711, 68,151] | |
| Life Space level score | 0.16 | 25,426 [−12,060, 62,912] | |
| Detailed Activity Form energy expenditure | 0.28 | 323 [−313,959] | |
| Katz ADLs <6 (dependent) | 0.79 | 97,263 [−691573, 886,099] | |
| Multiple linear regressions of M10 and performance-based assessments | |||
| Overall regression | 0.1743 | 0.10 | |
| Short Physical Performance Battery score | 0.92 | −2607 [−57,824, 52,610] | |
| 6-min walk distance | 0.45 | 179 [−300,657] | |
| Grip strength | 0.02 | −23,022 [−41,988, −4055] | |
Fig. 2.
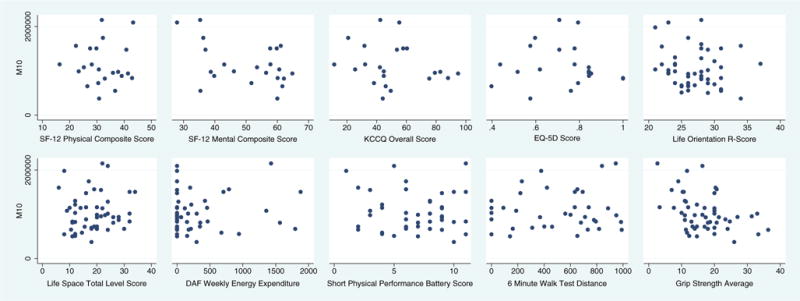
Scatterplots of maximum 10 h of daily accelerometer-measured activity counts (M10) vs. survey and performance-based assessments
Performance-Based Function Assessments
M10 was not associated with SPPB score or 6MWT. There was a statistically significant but weak, negative association between grip strength and M10 (R2=0.1568, P=0.004) (Table 2, Fig. 2). Multiple linear regressions of M10 with performance-based function tests were not significant (2=0.1743, P=0.10) (Table 2).
Discussion
In a cohort of well-characterized older adults with severe symptomatic aortic stenosis, we evaluated the relationship between symptom and functional status as assessed by surveys and performance-based functional test and objectively measured activity using continuous wrist-worn accelerometry. We found that in older adults with severe AS, self-reported surveys and performance-based function tests are not meaningfully correlated with daytime activity as estimated by maximum 10 h of daily accelerometer-measured activity. The results of our study suggest that accelerometer-measured physical activity provides distinct clinical information apart from self-reported surveys or performance-based function tests. This underscores the importance of using objective measures of physical activity to accurately quantify physical function in older adults with severe aortic stenosis.
Self-reported surveys have been traditionally used to estimate quality of life and functional capacity in adults with heart failure and to predict morbidity and mortality. However, because self-reported surveys are subjective and indirect in nature, they fall short in estimating true daily physical activity. Surveys are prone to recall bias; subjects may exaggerate responses one way or another based on a multitude of factors, including their state of mind when the survey was taken and who the intended recipients of the survey results are, in most cases clinicians or research investigators. Moreover, surveys, which ask patients to recall previous events over an extended period of time, are administered at one point in time, making results prone to error and inaccuracy. As with self-reported surveys, performance-based function tests have been used to estimate functional capacity in adults with heart failure and predict morbidity and mortality but are also imperfect surrogates for daily physical activity. Although objective in nature, performance-based tests such as the 6MWT, grip strength, and Short Physical Performance Battery tests are measured at only one point in time. Therefore, although they estimate maximal and submaximal functional capacity, they may not correlate well with true physical activity over an extended period of time. Andrews et al. found that both cross-sectional and longitudinal KCCQ survey scores and 6MWT distances were statistically correlated, suggesting survey and performance-based functional tests may provide complimentary information [25]. However, both survey and performance-based assessments have their limitations; accelerometer-measured physical activity is both objective and a continuous measurement of a subject’s activity in their home environment, thus offering unique clinical information apart from subjective self-reported surveys and point-in-time performance-based tests.
The concept of frailty in aging adults has been associated with greater morbidity and mortality. Frailty as a distinct physiologic phenotype was first validated by Fried who offered a standardized definition for frailty which predicted falls, disability, hospitalizations, and death in the elderly [24]. Her standardized definition included both subjective and objective measures of functional status as follows: weight loss, grip strength, self-reported exhaustion and physical activity, and 15-ft walk time. Further studies have modified Fried’s original definition of frailty and adapted its use to predict outcomes in older adults with coronary artery disease [26, 27], recovery after general [28] and cardiac surgery [29], and survival after transcatheter aortic valve replacement (TAVR) [30•]. However, no study has used objective measurements of daily activity in their standardized definition of frailty. Accelerometer-measured activity may therefore serve as a valuable and distinct adjunct to future frailty studies. Moreover, accelerometer-measured activity may be used to evaluate and monitor therapeutic benefit following surgical and medical interventions. Several studies have begun to use accelerometer-measured activity to assess daytime and sleep activity following orthopedic [31, 32] and cardiac surgeries [33, 34]. In our cohort of subjects who all underwent TAVR, further studies will evaluate whether pre-TAVR accelerometer-measured physical activity predict post-operative outcomes. We will also be able to monitor improvements in functional capacity longitudinally by comparing changes in accelerometer-measured physical activity after TAVR.
It must be noted that we did find a statistically significant, albeit weak, negative association between the SF12 Mental Composite Score (SF12-MCS) and M10. The SF12-MCS estimates subjects’ self-reported emotional stress and anxiety. We speculate that a potential explanation for this finding is subjects who reported a greater level of anxiety also had a greater amount of responsibility in their day-to-day lives and thus were more physically active. On the other hand, less active subjects who were sedentary and exerted themselves less reported a lesser degree of anxiety. Another unexpected finding was the statistically significant, although weak, negative association between grip strength and M10. Poor grip strength, a surrogate marker for overall decreased muscular strength, is a marker of frailty and has been previously shown to predict mortality and morbidity in older adults [24, 35]. However, in our cohort of subjects with severe AS and poor cardiovascular reserve, subjects who were more sedentary may have compensated for lack of mobility with greater use of upper extremities to perform day-to-day tasks and thus preserved relative grip strength. Moreover, greater grip strength does not necessarily imply greater lower extremity strength and therefore, mobility and activity. These unexpected inverse relationships between anxiety and activity and between grip strength and activity must be confirmed in larger cohorts.
There are several methodological issues to be addressed. First, all subjects included in this study were carefully evaluated and deemed appropriate candidates for TAVR by meeting strict inclusion criteria, resulting in a cohort with a high burden of comorbid illness. Therefore, the generalizability of these findings to lower risk populations and to subjects without severe AS is unknown. Moreover, our study includes a relatively small sample size and not every subject completed all surveys or performance-based tests due to poor follow-up. Another limitation was the variability in the amount of time subjects wore their accelerometers. As a result, daily accelerometer-measured activity for subjects who wore accelerometers for fewer days may not accurately reflect their average activity over a longer period of time. The cross-sectional nature of our study also limits inferring causality. Because of these study limitations, larger longitudinal studies, which include healthier subjects and maintain better follow-up, are needed to better characterize the relationship between accelerometer-measured activity and self-reported or performance-based function.
Conclusion
Self-reported surveys and performance-based function tests are not meaningfully correlated with daytime activity as estimated by maximum 10 h of daily accelerometer-measured activity. The results of our study suggest that accelerometer-measured physical activity provides distinct clinical information apart from self-reported surveys or performance-based function tests, thus underscoring the importance of using objective real-world measures to understand physical activity in older adults with cardiovascular disease.
Acknowledgments
Dr. Green is supported by Career Development Grant Awards K23 HL12114 from the National Heart, Lung, and Blood Institute. Dr. Maurer is supported by a Mid-Career Mentoring Award from the NIA K24AG036778. Ms. Lazarte is supported by a grant from the NIA.
Footnotes
Conflict of Interest Yufei Tang declares that he has no conflict of interest.
Philip Green declares that he has no conflict of interest.
Mathew Maurer declares that he has no conflict of interest.
Rosa Lazarte declares that she has no conflict of interest.
Jonathan Rubin Kuzniecky declares that he has no conflict of interest.
Ming Yang Hung declares that he has no conflict of interest.
Melissa Garcia declares that she has no conflict of interest.
Susheel Kodali is the national P.I. for the Sapien 3 trial utilizing the Edwards Sapien 3 valve. This trial was funded by Edwards Lifesciences, and Columbia University received funding for the research. Dr. Kodali has also received compensation from Edwards Lifesciences for service as a consultant, but has not received any financial compensation in the past 12 months as of the submission of this article, and has also served as an unpaid consultant for Medtronic.
Tamara Harris declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Yufei Tang, Email: ygt1@columbia.edu.
Philip Green, Email: pg2336@columbia.edu.
Mathew Maurer, Email: msm10@columbia.edu.
Rosa Lazarte, Email: rl2345@columbia.edu.
Jonathan Rubin Kuzniecky, Email: jr3466@columbia.edu.
Ming Yang Hung, Email: mh1515@georgetown.edu.
Melissa Garcia, Email: garciamel@mail.nih.gov.
Susheel Kodali, Email: skodali@columbia.edu.
Tamara Harris, Email: harrista@nia.nih.gov.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Reynolds MR, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124(18):1964–72. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 2.de Arenaza DP, et al. Preoperative 6-minute walk test adds prognostic information to Euroscore in patients undergoing aortic valve replacement. Heart. 2010;96(2):113–7. doi: 10.1136/hrt.2008.161174. [DOI] [PubMed] [Google Scholar]
- 3•.Green P, et al. Relation between six-minute walk test performance and outcomes after transcatheter aortic valve implantation (from the PARTNER trial) Am J Cardiol. 2013;112(5):700–6. doi: 10.1016/j.amjcard.2013.04.046. This study showed an association between shorter 6MWT distance and poorer long-term survival. Moreover, patients with poor baseline functional status exhibited the greatest improvement in 6MWT distance. These results suggest that more objective measurements of functional status, such as accelerometer-measured activity, have the potential to predict mortality after TAVR and predict which patients benefit most from TAVR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howell J, et al. Maximum daily 6 minutes of activity: an index of functional capacity derived from actigraphy and its application to older adults with heart failure. J Am Geriatr Soc. 2010;58(5):931–6. doi: 10.1111/j.1532-5415.2010.02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jehn M, et al. Accelerometer-based quantification of 6-minute walk test performance in patients with chronic heart failure: applicability in telemedicine. J Card Fail. 2009;15(4):334–40. doi: 10.1016/j.cardfail.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Maurer MS, et al. The prevalence and impact of anergia (lack of energy) in subjects with heart failure and its associations with actigraphy. J Card Fail. 2009;15(2):145–51. doi: 10.1016/j.cardfail.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The criteria committee of the New York Heart Association: nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th. Boston: Little, Brown & Co; 1994. [Google Scholar]
- 8.Green CP, et al. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 9••.Arnold SV, et al. How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes. 2013;6(5):591–7. doi: 10.1161/CIRCOUTCOMES.113.000354. This study established a standard method of assessing poor outcomes following TAVR utilizing the KCCQ survey and mortality rate. Our study alludes to using accelerometer-measured activity before and after TAVR as an objective measurement of patient recovery following TAVR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Muller-Nordhorn J, Roll S, Willich SN. Comparison of the short form (SF)-12 health status instrument with the SF-36 in patients with coronary heart disease. Heart. 2004;90(5):523–7. doi: 10.1136/hrt.2003.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kind P. Quality of life and PharmacoEconomics in clinical trials. 2nd. Lippincott-Raven; 1996. The EuroQol instrument: an index of health related quality of life. [Google Scholar]
- 13.Cheng H, Gurland BJ, Maurer MS. Self-reported lack of energy (anergia) among elders in a multiethnic community. J Gerontol A Biol Sci Med Sci. 2008;63(7):707–14. doi: 10.1093/gerona/63.7.707. [DOI] [PubMed] [Google Scholar]
- 14.Burke KL, et al. An investigation of concurrent validity between two optimism/pessimism questionnaires: the life orientation test-revised and the optimism/pessimism scale. Curr Psychol. 2000;19(2):129–36. [Google Scholar]
- 15.Peel C, et al. Assessing mobility in older adults: the UAB study of aging life-space assessment. Phys Ther. 2005;85(10):1008–119. [PubMed] [Google Scholar]
- 16.Nicklett EJ, et al. Fruit and vegetable intake, physical activity, and mortality in older community-dwelling women. J Am Geriatr Soc. 2012;60(5):862–8. doi: 10.1111/j.1532-5415.2012.03924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ainsworth BE, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 18.Taylor HL, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 19.Katz S, et al. Studies of illness in the aged. The index of Adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 20.Green P, et al. Gait speed and dependence in activities of daily living in older adults with severe aortic stenosis. Clin Cardiol. 2012;35(5):307–14. doi: 10.1002/clc.21974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guralnik JM, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 22.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 23.Roberts HC, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–9. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 25.Andrew Kao FM, et al. The correlation between the Kansas City cardiomyopathy questionnaire and the six minute walk test in ambulatory heart failure patients. J Card Fail. 2007;13(6):181–2. [Google Scholar]
- 26.Purser JL, et al. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54(11):1674–81. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 27.Ekerstad N, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124(22):2397–404. doi: 10.1161/CIRCULATIONAHA.111.025452. [DOI] [PubMed] [Google Scholar]
- 28.Makary MA, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Sepehri A, et al. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg. 2014;148(6):3110–7. doi: 10.1016/j.jtcvs.2014.07.087. [DOI] [PubMed] [Google Scholar]
- 30•.Green P, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5(9):974–81. doi: 10.1016/j.jcin.2012.06.011. This study showed that frailty status was associated with increased 1-year mortality after TAVR. Our study suggests that accelerometer-measured activity may be used as an adjunct to frailty status in identifying patients at risk for poor outcomes following TAVR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harding P, et al. Do activity levels increase after total hip and knee arthroplasty? Clin Orthop Relat Res. 2014;472(5):1502–11. doi: 10.1007/s11999-013-3427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutzner C, Kirschner S, Lutzner J. Patient activity after TKA depends on patient-specific parameters. Clin Orthop Relat Res. 2014;472(12):3933–40. doi: 10.1007/s11999-014-3813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redeker NS, Ruggiero JS, Hedges C. Sleep is related to physical function and emotional well-being after cardiac surgery. Nurs Res. 2004;53(3):154–62. doi: 10.1097/00006199-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Redeker NS, Wykpisz E. Effects of age on activity patterns after coronary artery bypass surgery. Heart Lung. 1999;28(1):5–14. doi: 10.1016/s0147-9563(99)70038-5. [DOI] [PubMed] [Google Scholar]
- 35.Ling CH, et al. Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ. 2010;182(5):429–35. doi: 10.1503/cmaj.091278. [DOI] [PMC free article] [PubMed] [Google Scholar]


