With frog populations in decline, it is important to understand the impact of changing environmental stressors, such as increased solar UV-B radiation, on frog health. This study investigated the impact of elevated UV-B radiation on tadpole immune function, and the carry-over effects of early UV-B exposure on immune function, post-metamorphosis.
Keywords: Amphibian declines, disease, immunocompetence, leucocyte, phytohaemagglutinin, ultraviolet radiation
Abstract
Amphibians have declined dramatically worldwide. Many of these declines are occurring in areas where no obvious anthropogenic stressors are present. It is proposed that in these areas, environmental factors such as elevated solar ultraviolet-B (UV-B) radiation could be responsible. Ultraviolet-B levels have increased in many parts of the world as a consequence of the anthropogenic destruction of the ozone layer. Amphibian tadpoles are particularly sensitive to the damaging effects of UV-B radiation, with exposure disrupting growth and fitness in many species. Given that UV-B can disrupt immune function in other animals, we tested the hypothesis that early UV-B exposure suppresses the immune responses of amphibian tadpoles and subsequent juvenile frogs. We exposed Limnodynastes peronii tadpoles to sublethal levels of UV-B radiation for 6 weeks after hatching, then examined indices of immune function in both the tadpoles and the subsequent metamorphs. There was no significant effect of UV-B on tadpole leucocyte counts or on their response to an acute antigen (phytohaemagglutinin) challenge. However, early UV-B exposure resulted in a significant reduction in both metamorph leucocyte abundance and their response to an acute phytohaemagglutinin challenge. These data demonstrate that early UV-B exposure can have carry-over effects on later life-history traits even if the applied stressor has no immediately discernible effect. These findings have important implications for our understanding of the effects of UV-B exposure on amphibian health and susceptibility to diseases such as chytridiomycosis.
Introduction
Amphibians have been experiencing dramatic declines worldwide, with populations from 42% of species known to be decreasing (Alford and Richards, 1999; Stuart et al., 2004; IUCN, 2016). Most of these declines have been attributed to anthropogenic factors; however, some declines are more enigmatic, as they occur in habitats that are free from any obvious anthropogenic pressures (Stuart et al., 2004). In these areas, it has been hypothesized that altered environmental factors, such as increased ultraviolet-B (UV-B) radiation, could be responsible (Collins and Storfer, 2003).
The destruction of the ozone layer in the upper atmosphere has led to an increase in ultraviolet (UV) radiation reaching the Earth's surface (Kerr and McElroy, 1993; Herman et al., 1996; McKenzie et al., 1999; Stuart et al., 2004). Exposure to solar radiation in the UV-B range (290–320 nm) can cause a wide range of sublethal effects on eggs and tadpoles, including reduced growth rates (Ankley et al., 2002), increased occurrence of developmental mortalities (Pahkala et al., 2002), decreased locomotor performance (Blaustein et al., 1994) and altered behaviours (Kats et al., 2000; Han et al., 2007). Exposure to UV-B radiation also synergistically enhances the negative effects of other stressors (Kiesecker and Blaustein, 1995; Kats et al., 2000; Pahkala et al., 2002; Hatch and Blaustein, 2003; van Uitregt et al., 2007; Alton et al., 2010; Mitchell et al., 2012). Sensitivity to UV-B radiation varies between species (Blaustein et al., 1994) and between populations, with populations at higher elevations considered to be at greater risk of UV-B-associated damage than populations at lower elevations, because they receive higher levels of solar UV-B radiation (Belden and Blaustein, 2002).
The exact mechanisms underlying the effect of UV-B radiation on amphibians are unknown, although it is hypothesized that the damage to DNA caused by UV-B exposure may be responsible. Ultraviolet-B exposure results in the synthesis of pyrimidine dimers between adjacent DNA nucleotides, which impedes the transcription of the affected gene, leading to cellular mutation or death (Jagger, 1985; Hearst, 1995; Friedberg et al., 2006). Amphibians, like other vertebrates, have evolved DNA repair mechanisms to reverse the damage caused by UV-B radiation through the activation of the photo-repair enzymes, the photolyases (Hearst, 1995). However, this repair is considered energetically costly (Sancar and Tang, 1993) and, therefore, it has been proposed that a trade-off may exist between costly repair and ‘regular’ functions, such as growth and performance (Jagger, 1985; Sancar and Tang, 1993; Hearst, 1995; Friedberg et al., 2006; Alton et al., 2012).
Ultraviolet-B exposure suppresses immune function in many vertebrate species, including fish (Jokinen et al., 2008), mice (Kripke, 1984), rats (Goettsch et al., 1994) and humans (Poon et al., 2005). The mechanisms for this immunosuppressive effect vary from local damage or killing of important antigen-presenting cells in the skin (Stingl et al., 1983) to stimulation of keratinocytes to release cytokines that induce systemic immune suppression (Rivas and Ullrich, 1992) or, indirectly, through an increase in concentrations of corticosteroids (cortisol or corticosterone), important stress hormones that also have an immunosuppressive function (Jokinen et al., 2000). Given that the immune function of fish and mammals can be impaired through exposure to elevated UV-B, negative effects of exposure on the amphibian immune system are likely.
In recent years, the emergence of novel diseases and pathogens has had a devastating effect on amphibian populations worldwide (Carey et al., 1999). One of the most influential of amphibian pathogens is the fungus Batrachochytrium dendrobatidis (hereafter referred to as Bd), which causes the disease chytridiomycosis (Berger et al., 1998; Pessier et al., 1999). Batrachochytrium dendrobatidis has been documented in >500 amphibian species worldwide and is implicated in the decline or extinction of several hundred of these (van Rooij et al., 2015). Indeed, Bd is now thought to be responsible for the greatest loss of vertebrate biodiversity ever attributed to disease (Skerratt et al., 2007). Although recent data suggest that several Batrachochytrium lineages appear to have coexisted with amphibian populations for some time (James et al., 2015), it is still unclear whether the global devastation caused by Bd is solely the result of exposure to a novel lineage or whether exposure to environmental stressors, such as exposure to increases in UV-B radiation, may have increased the susceptibility of amphibians to this pathogen. Levels of UV-B radiation have increased substantially over recent decades in many regions of the world (Herman, 2010). Ultraviolet-B suppresses immune function and impairs resistance to pathogens in fish and mammals (Salo et al., 2000; Jokinen et al., 2008), but its effects on amphibian immune function remain largely untested.
Although the direct effects of elevated UV-B exposure on eggs and tadpoles are well established, the potential for these effects to persist into other life-history stages, known as carry-over effects, remains unexplored. Environmental stress during embryonic and larval stages in amphibians can impact on locomotion (Boes and Benard, 2013), morphology (Relyea, 2001; Tejedo et al., 2010) and survival in later life-history stages (Rasanen et al., 2002). Exposure of embryos to UV-B has been shown to elicit carry-over effects in the form of increased occurrence of developmental abnormalities and slower development in the subsequent Rana temporaria tadpoles, despite having no apparent effect on the embryonic stage (Pahkala et al., 2001). Therefore, it is possible that the effect of exposure to elevated UV-B in early life-history stages on immune function in tadpoles could persist and impact upon fitness in later life.
To investigate the impact of early (larval) UV-B exposure on tadpole and subsequent metamorph immune function, we exposed tadpoles of the brown striped marsh frog, Limnodynastes peronii, to sublethal ecologically relevant levels of UV-B radiation for 6 weeks and measured several indices of physiological performance and immune function in the tadpoles and resulting metamorphs. The tadpoles of L. peronii are sensitive to the ecologically relevant levels of UV-B used in the present study (van Uitregt et al., 2007; Alton et al., 2011; Bernal et al., 2011). Limnodynastes peronii produces foamy egg masses, which are laid on the water surface during spring and summer in both open and shaded positions (Anstis, 2002). Larvae then hatch from the foam nest after a few days and remain free swimming in the water until metamorphosis. Given that early life-history exposure to other environmental stressors can have a lasting effect on post-metamorphic traits, we hypothesized that the sublethal exposure of early tadpoles to UV-B would disrupt immune function in both the tadpoles and the subsequent metamorphs.
Materials and methods
Ethics statement
This research was approved by the University of Queensland Animal Welfare Unit (permit number SBS/085/13/URG) and the Queensland Department of Environment and Heritage (WISP07785810).
Animal collection and maintenance
Four recently laid L. peronii egg foam masses were collected from The University of Queensland campus, Brisbane, Australia (27°29′′50.54″S, 153° 1′4.12″E). Seven days after hatching, 210 tadpoles were distributed evenly among 21 2 litre containers, each containing 1 litre of aged tap water. Twelve containers were randomly assigned to a UV-B treatment (n = 120 individual tadpoles) and nine to the control treatment (n = 90). The larger sample size of tadpoles assigned to the UV-B treatment was to account for any mortality expected to be experienced in the UV-B treatment group. Tadpoles were kept at 24 ± 2°C and were fed frozen spinach twice a week. Ambient, (non-UV) fluorescent lighting in the room maintained the photoperiod at 12 h light–12 h dark.
Ultraviolet-B treatments
Ultraviolet radiation was emitted from four fluorescent sources [(Reptile One T8 36 W) two UV 5.0 and two UV 10.0]. Control group containers were protected from UV-B by placement of metallized window film (Clear grey; Handi Home Supplies, Thomastown, Victoria, Australia), which was suspended over the control containers. The lights were set to a regimen in which the UV lights would emit a baseline level of UV-B from 10.00 to 11.00 h and again from 13.00 to 14.00 h daily, with a peak level of UV-B delivered during the daily midpoint (10.00–13.00 h; Table 1). After 20 days (beginning during week 3 of the exposure period and continuing until the completion of the exposure period in week 6), levels of UV-B were increased by replacement with stronger UV-B lights [two UV-B 8.0 (Repti-Glo Exo-Terra 40 W) and two UV 10.0 (Reptile One T8 36 W)] that emitted UV-B radiation simultaneously for 4 h daily (10.00–14.00 h; Table 2). This increase in UV-B radiation of was applied to account for the increased water depth of the containers as the tadpoles progressed through development.
Table 1:
Calculated UV spectra measurements, including absolute irradiance levels of UV-A and UV-B and absolute cumulative daily does rates of baseline and peak levels of UV-B radiation during the initial exposure period (experienced during weeks 0–3 of the exposure period)
| Fluctuating daily cycle | Baseline irradiance (µW cm−2), 4 h day−1 | Peak irradiance (µW cm−2), 2 h day−1 | Cumulative daily dose (kJ m−2) | |||
|---|---|---|---|---|---|---|
| UV-B | UV-A | UV-B | UV-A | UV-B | UV-A | |
| UV-B treatment | 7.59 ± 1.59 | 83.85 ± 16.04 | 12.90 ± 3.18 | 63.89 ± 10.87 | 1.904 ± 0.394 | 11.468 ± 2.062 |
| Control treatment | 1.42 ± 0.95 | 3.05 ± 0.70 | −2.08 ± 1.93 | 1.62 ± 0.99 | 0.076 ± 0.199 | 0.521 ± 0.167 |
Values are means ± SD. Abbreviation: UV, ultraviolet.
Table 2:
Calculated UV spectra measurements, including absolute irradiance levels of UV-A and UV-B and absolute cumulative daily does rates of baseline levels of UV-B radiation during the final exposure period (experienced during weeks 3–6 of the exposure period)
| Daily cycle | Absolute irradiance (µW cm−2), 4 h day−1 | Cumulative daily dose (kJ m−2) | ||
|---|---|---|---|---|
| UV-B | UV-A | UV-B | UV-A | |
| UV-B treatment | 18.41 ± 3.55 | 132.48 ± 21.67 | 2.43 ± 0.65 | 19.06 ± 3.25 |
| Control treatment | 1.65 ± 0.64 | 9.80 ± 1.90 | 0.24 ± 0.10 | 1.40 ± 0.29 |
Values are means ± SD. Abbreviation: UV, ultraviolet.
All UV-B treatment levels were substantially lower than the ambient levels of 500 µW cm−2 measured during summer in the middle of the day in Brisbane recorded by van Uitregt et al. (2007). Given that UV-B penetration into water is attenuated by suspended particulate matter and dissolved carbon, it is likely that tadpoles would experience only a small fraction of the total solar irradiance reported by van Uitregt et al. (2007). Ultraviolet-B radiation was measured using a cosine corrector (CC-3-UV-S; Ocean Optics, Dunedin, FL, USA) and fibre-optic cable (400 µm Premium Fiber; Ocean Optics) attached to a spectrometer (USB2000+ Miniature Fiber Optic Spectrometer; Ocean Optics). Measurements were taken from the midpoint position of each container at the level of the water surface. The irradiance of UV-B and ultraviolet-A (UV-A) at each container midpoint was calculated by integrating the spectral irradiance data between 300 and 320 nm and between 320 and 400 nm, respectively (Alton et al., 2011).
After the 40 day exposure period, the UV lights were switched off, and a subset of tadpoles was tested immediately for immune function [leucocyte count and response to phytohaemagglutinin (PHA)]. The remaining tadpoles were maintained under 12 h–12 h rooftop fluorescent lighting until metamorphosis (determined as the point at which the tail was fully resorbed). Metamorphs were measured for immunocompetence (leucocyte counts and response to PHA) 14 days after each metamorph had achieved metamorphosis. By measuring each metamorph 14 days after metamorphosis the age at which all the metamorphs underwent the analysis of immune function was kept constant. This was done to avoid testing in the immunosuppressed stage of development that follows metamorphosis prior to the development of the adult-type immune system (Rollins-Smith, 1998).
Growth rate measurements
Growth rates were measured periodically during larval development (0, 2 and 4 weeks after UV-B exposure had begun) and upon completion of metamorphosis (i.e. tail reabsorption stage completed). Photographs were taken fortnightly to determine the growth rate at three different time points during the exposure period. Four tadpoles from each container were photographed with a digital camera (EX-ZR200 Exilim; Casio, Tokyo, Japan) for size measurements, such that 48 tadpoles from the UV-B treatment group and 36 tadpoles from control group were analysed at each time point. Total length measurements, the length from the top of the head to the end of the tail, of each tadpole were measured from the images using the analysis program ImageJ (National Institutes of Health, Bethesda, MD, USA). The size at metamorphosis was determined by analysing the mass and snout-vent length of the animals measured once metamorphosishad been completed (defined as larval tail resorbed to < 2 mm in length).
Metabolic rate of tadpoles
To assess the metabolic cost of UV-B exposure for tadpoles, the standard metabolic rate was determined at two different time points during UV-B exposure; firstly, during the initial UV-B exposure period (experienced from the beginning of the exposure period to week 3; Gosner developmental stage 21–25 (Gosner, 1960); absolute irradiance of UV-B during peak = 7.59 ± 1.59 µW cm−2, mean ± SEM) and secondly, during the final UV-B exposure period (experienced from week 3 until the completion of exposure in week 10; Gosner developmental stage 25–38 (Gosner, 1960); absolute irradiance of UV-B during peak = 18.41 ± 3.55 µW cm−2, mean ± SEM). Measurements were taken from three tadpoles per container from UV-B and control groups (initial exposure period, n = 36 and n = 27, respectively; and final exposure period, n = 25 and n = 27, respectively). Metabolic rate was measured using closed-system respirometry, by placing each tadpole in a 25 ml syringe equipped with an integrated oxygen-sensitive fluorescent Sensor Spot (PreSens, Regensburg, Germany) filled with air-saturated aged water. The oxygen concentration was measured every 30 min for an hour using a Fibox3 reader (PreSens).
The oxygen consumption rate (; in millilitres of O2 per hour) of the tadpoles was corrected for water temperature and for any background respiration, using the following formula:
where ΔO2 is the change in oxygen concentration after accounting for background respiration (in millilitres of O2 per litre), V is the volume of the syringe (in millilitres) and t is time (in minutes). Body mass was incorporated into statistical analysis as a covariate.
Phytohaemagglutinin challenge
A subset of tadpoles from the UV-B treatment group (n = 10) and the control group (n = 7) were lightly anaesthetized in Aqualife TMS (Syndel Laboratories, Nanaimo, BC, Canada; 0.125 g l−1), and the dorsal plane of each tadpole was photographed. Tadpoles’ tails were then injected with 3 µl of PHA (12 mg ml−1; Sigma-Aldrich, St Louis, MO, USA) dissolved in sterile amphibian Ringer solution (6.6 g NaCl, 0.15 g KCl, 0.15 g CaCl2 and 0.2 g NaHCO3 in 1 litre of ultrapure water, sterilized with a 0.22 µm filter) using a glass Hamilton microlitre syringe (Hamilton, Reno, NV, USA). Injections were made into the right side of the tail, 5 mm from the end of the body towards the posterior tip of the tail. Tadpoles were then placed into individual 200 ml cups with aged water to recover. The tadpoles were re-anaesthetized at 24 and 48 h post-injection, and the injection sites were re-photographed. The degree of swelling in response to PHA was determined by subtracting the initial tail width from the tail width at 24 h or maximum 48 h post-injection from measurements made from images using the analysis program ImageJ. Likewise, a subset of metamorphs from the high- (n = 12) and low-UV-B groups (n = 8) were anaesthetized (Tricaine-S, 0.125 g l−1) and administered 5 µl of PHA (12 mg ml−1) using a BD Ultra-Fine II 0.3 ml 31 gauge insulin needle (North Ryde, NSW, Australia) subcutaneously into the skin surrounding the right triceps femoris. The thickness of the area where the injections were made was measured prior to the injections using fine-gauged digital callipers and again at 24 and 48 h post injection. Animals in which the swelling response was negative were excluded from further analysis. Final sample sizes for tadpole responses were as follows: UV-B treatment group, n = 8; and control group n = 6. Sample sizes for metamorph responses were as follows: UV-B treatment group, n = 6; and control group, n = 4.
Leucocyte counts
Tadpoles (n = 19 and n = 15 for the UV-B treatment and control groups, respectively) and metamorphs (n = 11 and n = 8 for the UV-B treatment and control groups, respectively) were euthanized in buffered Tricaine-S (0.25 mg ml−1), and blood samples were obtained directly from the heart into heparinized capillary tubes. Blood smears were made, and leucocytes were stained with Quick Dipstain (POCD Healthcare, Artarmon, NSW, Australia). Blood smears were analysed at ×200 magnification (Olympus BH-2 microscope, Japan), and the percentage of leucocytes was determined for each animal from five different fields of view.
Statistical analysis
All data were analysed using the statistical program R (R Core Team, 2013). Four tadpoles per tank within each treatment group were randomly selected and body size indices measured at three separate time points during the exposure period (at weeks 0, 2 and 4 after UV-B exposure began). The effects of UV-B on tadpole growth and developmental rates and tadpole immune function were analysed using a mixed-model approach, with ‘UV treatment’ as the fixed effect and ‘tank’ as a random factor. Tadpole metabolic rates were measured at two different time points during the experiment, once at 2 weeks after exposure began and again at 5 weeks. The data obtained from the first measurement at 2 weeks were analysed using a mixed-model approach, with ‘tank’ as a random factor and ‘body mass’ as a covariate. The data obtained from the second measurement at 5 weeks were analysed using a weight least-squares regression, using ‘tank’ as a random factor and ‘body mass’ as a covariate. The time taken to reach metamorphosis, mass at metamorphosis and length at metamorphosis were also analysed using mixed-effects models, with ‘UV treatment’ as the fixed factor and ‘tank’ as a random factor. The effect of UV-B treatment on the swelling response of metamorphs (one individual from each tadpole ‘tank’) following PHA injection was analysed using a robust regression method, and the effect of tadpole UV-B exposure on metamorph white blood cell counts was examined using an anlysis of variance (one metamorph from each tadpole ‘tank’).
Results
Growth rate and development
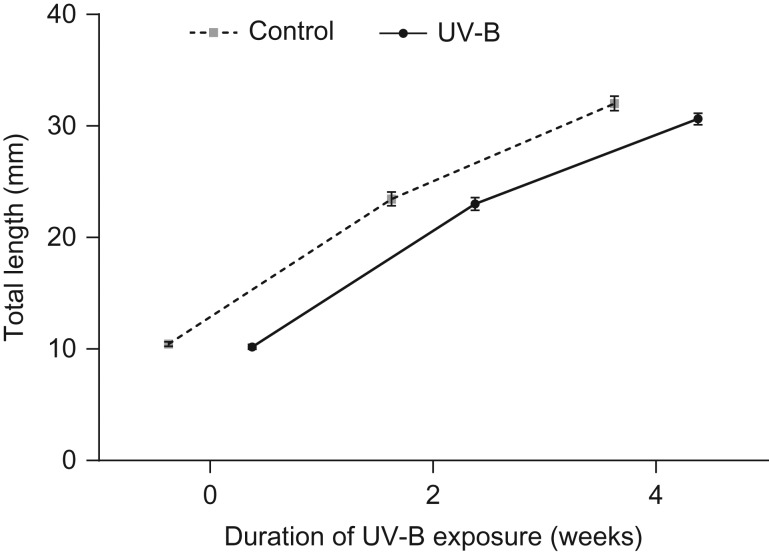
There was no significant effect of larval UV-B exposure on the growth rate of tadpoles (F1,19 = 2.68, P = 0.12; Fig. 1). There was no effect of treatment on the developmental rate of tadpoles at week 4 of exposure (F1,19 = 0.62, P = 0.44), or their mass at metamorphosis (F1,96 = 0.44, P = 0.51; Table 3). Time taken to reach metamorphosis and length at metamorphosis were unaffected by UV-B exposure (F1,19.7 = 0.02, P = 0.89 and F1,96 = 2.98, P = 0.09, respectively; Table 3).
Figure 1:
The mean length (in millimetres) of Limnodynastes peronii tadpoles in the ultraviolet-B (UV-B) treatment (n = 48) or control treatment (n = 36) measured at three different time points during development. There was no significant effect of larval UV-B exposure on growth rates (F1,19= 2.68, P = 0.12). Data are presented as mean values ± SEM.
Table 3:
The effect of 6 weeks of UV-B exposure during the tadpole stage on the time to metamorphosis and size at metamorphosis in Limnodynastes peronii
| Metamorph traits | Control | UV-B |
|---|---|---|
| Time to metamorphosis (days) | 77.79 ± 1.87 | 78.13 ± 1.58 |
| Mass at metamorphosis (g) | 0.3 ± 0.01 | 0.31 ± 0.01 |
| Snout-to-vent length (mm) | 15.7 ± 0.2 | 16.1 ± 0.2 |
Values are presented as means ± SEM. Abbreviation: UV, ultraviolet.
Metabolic rate of tadpoles
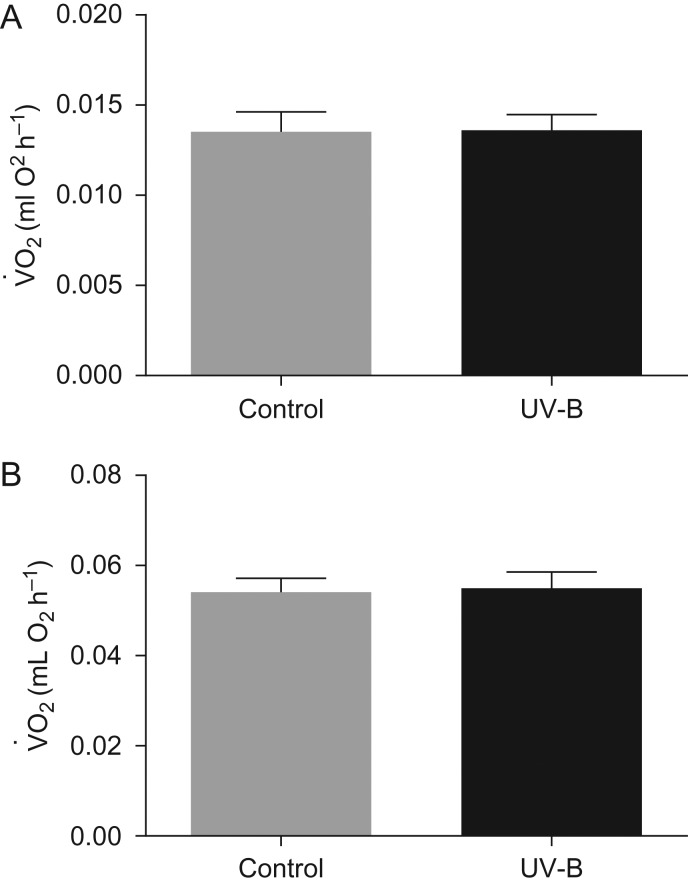
Standard metabolic rates (; in millilitres of O2 per hour) of tadpoles were significantly influenced by body mass at both 2 and 5 weeks of UV-B exposure (F1,33 = 48.2, P ≤ 0.001 and F1,58.9 = 42.27, P ≤ 0.001, respectively) but were unaffected by UV-B levels at both points (F1,16 = 0.21, P = 0.65 and F1,19.1 = 0.13, P = 0.72, respectively; Fig. 2).
Figure 2:
The standard metabolic rate (; in millilitres of O2 per hour) of L. peronii tadpoles exposed to control and UV-B treatments measured during the initial stage of exposure (A; experienced from week 0 to 3 of the exposure period; absolute irradiance of UV-B during peak = 12.9 ± 3.18 µW cm−2; control, n = 28 and UV-B, n = 35) and during the final stage of exposure (B; experienced from week 3 to 6 of the exposure period; absolute irradiance of UV-B during peak = 18.41 ± 3.55 µW cm−2; control, n = 27 and UV-B, n = 25). There was no significant effect of larval UV-B exposure on standard metabolic rate rate at either the initial or the final stage of the exposure period. Data are presented as mean values + SEM.
Immune function of tadpoles
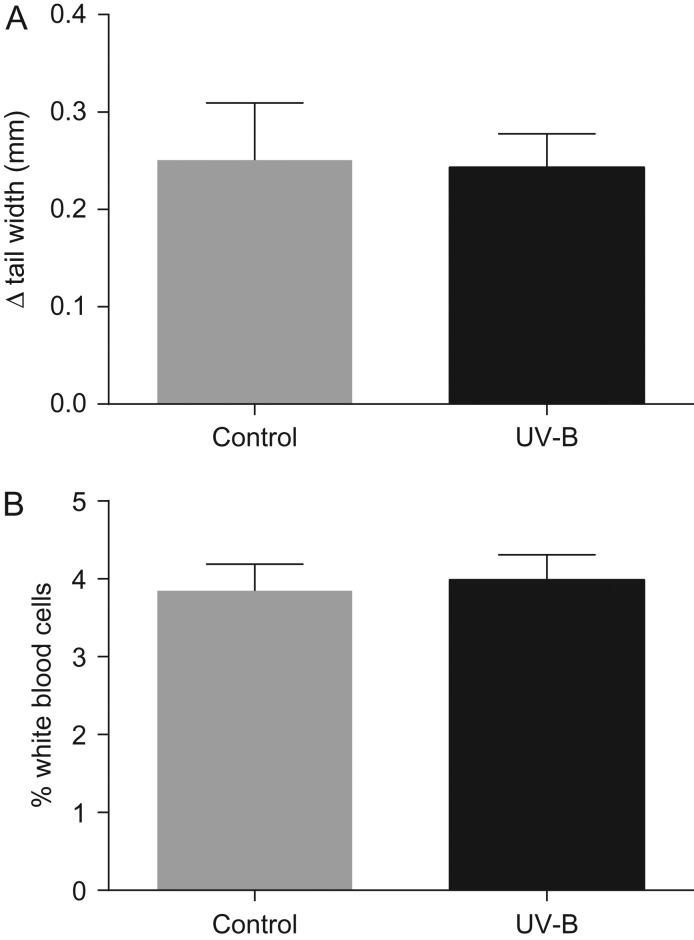
There was no significant effect of UV-B treatment on indices of tadpole immune function. Exposure to UV-B radiation did not affect the tissue swelling response to PHA injection (F1,11 = 0.2, P = 0.67; Fig. 3A) nor the proportion of leucocytes in the blood (F1,18.2 = 0.009, P = 0.92; Fig. 3B).
Figure 3:
(A) The mean maximal tail swelling response (in millimetres) to phytohaemagglutinin administration in L. peronii tadpoles in the control treatment (n = 6) and UV-B treatment (n = 8). (B) The mean percentage of leucocytes per 200 blood cells in tadpoles exposed to the control treatment (n = 15) and UV-B treatment (n = 19). There was no significant effect of UV-B exposure on either parameter. Data are presented as mean values + SEM.
Immune function of metamorphs
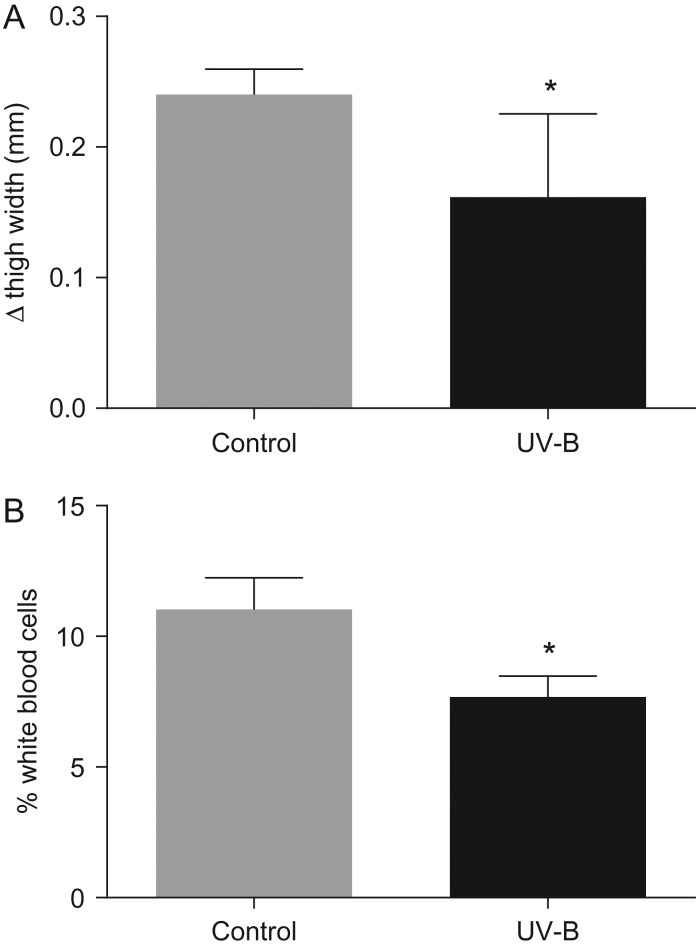
Early exposure to UV-B had a significant effect on the immune function of metamorphs. Metamorphs exposed to UV-B as tadpoles exhibited a 30% weaker response to PHA injection relative to the metamorphs not exposed to UV-B as tadpoles (F1,8 = 0.93, P = 0.002; Fig. 4A). The leucocyte count of metamorphs exposed to high UV-B as tadpoles was 4.1% lower in comparison to the metamorphs not exposed to UV-B as tadpoles (F1,17 = 5.6, P = 0.03; Fig. 4B).
Figure 4:
(A) The mean maximal thigh swelling response (in millimetres) to phytohaemagglutinin injection of L. peronii metamorphs in the control treatment (n = 4) and UV-B treatment (n = 6) during development as tadpoles. (B) The mean percentage of leucocytes of L. peronii metamorphs in the UV-B treatment (n = 11) and control treatment (n = 8) during development as tadpoles. The maximal response to phytohaemagglutinin was greatest in the metamorphs from the control treatment group (F1,8 = 0.93, P = 0.002). The proportion of leucocytes was also greatest in the control treatment group (F1,17 = 5.6, P = 0.03). *Significant difference (P < 0.05) between the UV-B and control treatment groups.
Discussion
We showed that UV-B exposure in early amphibian life stages had no immediate effect on the immune function of tadpoles, but elicited a carry-over effect on metamorphs, demonstrated by a decreased abundance of leucocytes and a reduced response to foreign antigen challenge. Despite the relatively low levels of UV-B received by tadpoles during the study, an effect on subsequent metamorph immune parameters was nonetheless apparent. Similar effects of UV-B exposure on fish and mammalian immune function have previously been established (Jeevan and Kripke, 1990; Jokinen et al., 2008), but this is the first time that an effect from prior UV-B exposure on immune function has been identified in amphibians.
This carry-over effect in metamorphs indicates that although UV-B exposure did not appear to have a direct effect on larval immune function, the development of the adult-type immune system was somehow disrupted by exposure to UV during the larval stages. The mechanism by which this disruption occurred remains unclear; however, it is possible that the cost of repair of UV-B-induced damage, although not significant enough to impair tadpoles, could have compromised the development of the immune system of individuals later in life. For example, tadpole growth or development rates that are faced with exposure to UV-B have an increased risk of predation, therefore impacting subsequent fitness (Alton et al., 2010). A similar trade-off may occur in immune function as a response to UV-B, in which tadpoles are able to manage the immediate damage caused by UV-B, but the development of resulting metamorphs is compromised. Mechanisms to repair UV-associated damage are inherent within cells (photolyase and nucleotide excision repair pathways), although activation of these processes incurs an energetic cost (Sancar and Tang, 1993). It is possible that the carry-over effect of early UV-B exposure is a product of a trade-off for resource allocation between immune function development and UV-B-induced repair mechanisms, leading to decreased immunocompetence in metamorphs. The effect of early larval UV-B exposure on subsequent immune function in metamorphs could also be due to direct damage to genes and/or the biological machinery responsible for the development of adult-type immune function. Whether the effects of UV-B on immune function exist as a result of direct damage or indirectly, as a result of an energy allocation trade-off, remains undetermined.
Whether the impact of early UV-B exposure on metamorph immune function remains an irreversible consequence of damage acquired during the larval period or reflects a more temporary response is unknown. During metamorphosis, amphibians experience a natural phase of immunosuppression, during which the immune system is reorganized from the ‘larval-type’ into the ‘adult-type’ immune system (Flajnik et al., 1987). In the period leading up to metamorphosis, the number of circulating leucocytes in the blood declines substantially until development of the ‘adult-type’ immune system commences, in which leucocyte density is again restored (Du Pasquier and Weiss, 1973). Metamorphs exposed to UV-B as tadpoles had lower leucocyte densities than those that received no UV-B as tadpoles, a potential indicator of an underdeveloped or immature immune system. A decrease in leucocytes in metamorphs suggests that exposure to UV-B may have disrupted white blood cell proliferation during development of the ‘adult-type’ immune system, resulting in fewer cells at the completion of metamorphosis. It is possible that larval exposure to UV-B might have delayed development of the adult-type immune system and that, given time, full immunocompetence could be restored. Alternatively, exposure to UV-B during development might have resulted in permanent damage to immune function, so that the immune function remains in an underdeveloped state. Analysis of immunocompetence at additional time points following metamorphosis is required to determine whether immune function is delayed or permanently disrupted following larval UV-B exposure.
The results of this study have important implications for our understanding of disease risks in amphibians. Our data show that early larval UV-B exposure can influence the subsequent immune capacity of resulting juvenile frogs, which has the potential to increase their risk of susceptibility to pathogens such as Bd. Moreover, amphibian species differ in their susceptibility to pathogens such as Bd (e.g. Briggs et al., 2010) and also in their capacity to repair UV-B-induced DNA damage (van de Mortel et al., 1998). However, whether UV-B exposure increases the susceptibility of amphibian larvae to Bd infection remains unclear. Several studies have shown contrasting results; simultaneous UV-B and Bd exposure either has no effect on infection risk in amphibian larvae (Garcia et al., 2006; Searle et al., 2010) or the two factors interact antagonistically to lower infection risk (Ortiz-Santaliestra et al., 2011). However, these studies do not take into consideration the potential for early UV-B exposure to have a latent influence on Bd susceptibility in subsequent developmental stages. The results of the present study suggest that the impacts of UV-B may be long lasting, and further work in this area is needed to elucidate the mechanisms through which UV-B may influence disease susceptibility through immune system dysfunction.
The risk to effective amphibian conservation lies in the inability of immunocompromised individuals to respond adequately to pathogens, which may elevate their risk of disease (Berger et al., 1998; Longcore et al., 1999; Voyles et al., 2007, 2009; Garner et al., 2009). Therefore, decreased immune function in metamorphs, as a carry-over effect of UV-B exposure, has the potential to increase susceptibility to Bd and chytridiomycosis, given that amphibians are most vulnerable to this pathogen during the post-metamorphosis life stage (Berger et al., 1998; Pessier et al., 1999). Other studies have shown that physiological stressors applied in the larval period of amphibians can influence immunological fitness in the resulting metamorphs (Gervasi and Foufopoulos, 2008). Like UV-B exposure, the impacts of early life-history stresses may not necessarily manifest immediately, but may subsequently influence immune function and disease susceptibility following metamorphosis. Predictions of the effects of early larval exposure to environmental stressors such as UV-B on disease ecology should not be restricted to Bd; immunosuppression and/or delayed immune system development in post-metamorphic amphibians could be detrimental in the presence of any other disease-causing pathogens.
In light of recent dramatic amphibian declines, the results of the present study provide important insight for our understanding of UV-B radiation as an influential stressor of amphibians. This study demonstrates that exposure to environmental stressors, such as UV-B radiation, during early development could impose significant fitness consequences for individuals later in life as a result of the impact of UV-B on immune function.
Acknowledgements
We would like to thank Dr Simon Bloomberg for providing statistical support.
Funding
This work was supported by an Australian Research Council Discovery grant to C.E.F. and F.S. (DP140102773).
References
- Alford RA, Richards SJ (1999) Global amphibian declines: a problem in applied ecology. Annu Rev Ecol Syst 30: 133–165. [Google Scholar]
- Alton LA, Wilson RS, Franklin CE (2010) Risk of predation enhances the lethal effects of UV-B in amphibians. Glob Change Biol 16: 538–545. [Google Scholar]
- Alton LA, Wilson RS, Franklin CE (2011) A small increase in UV-B increases the susceptibility of tadpoles to predation. Proc Biol Sci 278: 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton LA, White CR, Wilson RS, Franklin CE (2012) The energetic cost of exposure to UV radiation for tadpoles is greater when they live with predators. Funct Ecol 26: 94–103. [Google Scholar]
- Ankley GT, Diamond SA, Tietge JE, Holcombe GW, Jensen KM, DeFoe DL, Peterson R (2002) Assessment of the risk of solar ultraviolet radiation to amphibians. I. Dose-dependent induction of hindlimb malformations in the Northern leopard frog (Rana pipiens). Environ Sci Technol 36: 2853–2858. [DOI] [PubMed] [Google Scholar]
- Anstis M. (2002) Tadpoles of South-Eastern Australia: a Guide with Keys. New Holland in association with the World Wide Fund for Nature, Frenchs Forest, NSW.
- Belden LK, Blaustein AR (2002) Population differences in sensitivity to UV-B radiation for larval long-toed salamanders. Ecology 83: 1586–1590. [Google Scholar]
- Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR et al. (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95: 9031–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal MH, Alton LA, Cramp RL, Franklin CE (2011) Does simultaneous UV-B exposure enhance the lethal and sub-lethal effects of aquatic hypoxia on developing anuran embryos and larvae. J Comp Physiol B 181: 973–980. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Hoffman PD, Hokit DG, Kiesecker JM, Walls SC, Hays JB (1994) UV repair and resistance to solar UV-B in amphibian eggs: a link to population declines. Proc Natl Acad Sci USA 91: 1791–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes MW, Benard MF (2013) Carry-over effects in nature: effects of canopy cover and individual pond on size, shape, and locomotor performance of metamorphosing wood frogs. Copeia 2013: 717–722. [Google Scholar]
- Briggs CJ, Knapp RA, Vredenburg VT (2010) Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci USA 107: 9695–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey C, Cohen N, Rollins-Smith L (1999) Amphibian declines: an immunological perspective. Dev Comp Immunol 23: 459–472. doi:10.1016/S0145-305x(99)00028-2. [DOI] [PubMed] [Google Scholar]
- Collins JP, Storfer A (2003) Global amphibian declines: sorting the hypotheses. Divers Distrib 9: 89–98. [Google Scholar]
- Du Pasquier L, Weiss N (1973) The thymus during the ontogeny of the toad Xenopus laevis: growth, membrane-bound immunoglobulins and mixed lymphocyte reaction. Eur J Immunol 3: 773–777. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Hsu E, Kaufman JF, Dupasquier L (1987) Changes in the immune system during metamorphosis of Xenopus. Immunol Today 8: 58–64. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T (2006) DNA Repair and Mutagenesis. ASM Press, Washington, DC. [Google Scholar]
- Garcia TS, Romansic JM, Blaustein AR (2006) Survival of three species of anuran metamorphs exposed to UV-B radiation and the pathogenic fungus Batrachochytrium dendrobatidis. Dis Aquat Organ 72: 163–169. [DOI] [PubMed] [Google Scholar]
- Garner TWJ, Walker S, Bosch J, Leech S, Rowcliffe JM, Cunningham AA, Fisher MC (2009) Life history tradeoffs influence mortality associated with the amphibian pathogen Batrachochytrium dendrobatidis. Oikos 118: 783–791. [Google Scholar]
- Gervasi SS, Foufopoulos J (2008) Costs of plasticity: responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct Ecol 22: 100–108. [Google Scholar]
- Goettsch W, Garssen J, De Gruijl FR, Van Loveren H (1994) Effects of UV-B on the resistance against infectious diseases. Toxicol Lett 72: 359–363. [DOI] [PubMed] [Google Scholar]
- Gosner KL. (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183–190. doi:10.2307/3890061. [Google Scholar]
- Han BA, Kats LB, Pommerening RC, Ferrer RP, Murry-Ewers M, Blaustein AR (2007) Behavioral avoidance of ultraviolet-B radiation by two species of neotropical poison-dart frogs. Biotropica 39: 433–435. [Google Scholar]
- Hatch AC, Blaustein AR (2003) Combined effects of UV-B radiation and nitrate fertilizer on larval amphibians. Ecol Appl 13: 1083–1093. [Google Scholar]
- Hearst JE. (1995) The structure of photolyase: using photon energy for DNA repair. Science 268: 1858–1859. [DOI] [PubMed] [Google Scholar]
- Herman JR, Bhartia PK, Ziemke J, Ahmad Z, Larko D (1996) UV-B increases (1979–1992) from decreases in total ozone. Geophys Res Lett 23: 2117–2120. [Google Scholar]
- Herman JR. (2010) Global increase in UV irradiance during the past 30 years (1979–2008) estimated from satellite data. J Geophys Res-Atmos 115: 2156–2202. doi:10.1029/2009JD012219. [Google Scholar]
- IUCN (2016) The IUCN Red List of Threatened Species. Version 2016-2. http://www.iucnredlist.org. Downloaded on 04 September 2016.
- Jagger J. (1985) Solar-UV Actions on Living Cells. Praeger, New York. [Google Scholar]
- James TY, Toledo LF, Rodder D, Leite DD, Belasen AM, Betancourt-Roman CM, Jenkinson TS, Soto-Azat C, Lambertini C, Longo AV et al. (2015) Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: lessons from the first 15 years of amphibian chytridiomycosis research. Ecol Evol 5: 4079–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevan A, Kripke ML (1990) Alteration of the immune response to Mycobacterium bovis BCG in mice exposed chronically to low doses of UV radiation. Cell Immunol 130: 32–41. [DOI] [PubMed] [Google Scholar]
- Jokinen EI, Salo HM, Markkula SE, Aaltonen TM, Immonen AK (2000) Effects of ultraviolet light on immune parameters of the roach. Toxicol Lett 112–113: 303–310. [DOI] [PubMed] [Google Scholar]
- Jokinen IE, Markkula ES, Salo HM, Kuhn P, Nikoskelainen S, Arts MT, Browman HI (2008) Exposure to increased ambient ultraviolet B radiation has negative effects on growth, condition and immune function of juvenile Atlantic salmon (Salmo salar). Photochem Photobiol 84: 1265–1271. [DOI] [PubMed] [Google Scholar]
- Kats LB, Kiesecker JM, Chivers DP, Blaustein AR (2000) Effects of UV-B radiation on anti-predator behavior in three species of amphibians. Ethology 106: 921–931. [Google Scholar]
- Kerr JB, McElroy CT (1993) Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science 262: 1032–1034. [DOI] [PubMed] [Google Scholar]
- Kiesecker JM, Blaustein AR (1995) Synergism between UV-B radiation and a pathogen magnifies amphibian embryo mortality in nature. Proc Natl Acad Sci USA 92: 11049–11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke ML. (1984) Immunological unresponsiveness induced by ultraviolet radiation. Immunol Rev 80: 87–102. [DOI] [PubMed] [Google Scholar]
- Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91: 219–227. [Google Scholar]
- McKenzie R, Conner B, Bodeker G (1999) Increased summertime UV radiation in New Zealand in response to ozone loss. Science 285: 1709–1711. [DOI] [PubMed] [Google Scholar]
- Mitchell T, Alton LA, White CR, Franklin CE (2012) Relations between conspecific density and effects of ultraviolet-B radiation on tadpole size in the striped marsh frog. Conserv Biol 26: 1112–1120. [DOI] [PubMed] [Google Scholar]
- Ortiz-Santaliestra ME, Fisher MC, Fernandez-Beaskoetxea S, Fernandez-Beneitez MJ, Bosch J (2011) Ambient ultraviolet B radiation and prevalence of infection by Batrachochytrium dendrobatidis in two amphibian species. Conserv Biol 25 doi:10.1111/j.1523-1739.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- Pahkala M, Laurila A, Merila J (2001) Carry-over effects of ultraviolet-B radiation on larval fitness in Rana temporaria. Proc Biol Sci 268: 1699–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahkala M, Rasanen K, Laurila A, Johanson U, Bjorn LO, Merila J (2002) Lethal and sublethal effects of UV-B/pH synergism on common frog embryos. Conserv Biol 16: 1063–1073. [Google Scholar]
- Pessier AP, Nichols DK, Longcore JE, Fuller MS (1999) Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White's tree frogs (Litoria caerulea). J Vet Diagn Investig 11: 194–199. [DOI] [PubMed] [Google Scholar]
- Poon TSC, Barnetson RSC, Halliday GM (2005) Sunlight-induced immunosuppression in humans is initially because of UVB, then UVA, followed by interactive effects. J Invest Dermatol 125: 840–846. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- Rasanen K, Laurila A, Merila J (2002) Carry-over effects of embryonic acid conditions on development and growth of Rana temporaria tadpoles. Freshw Biol 47: 19–30. [Google Scholar]
- Relyea RA. (2001) The lasting effects of adaptive plasticity: predator-induced tadpoles become long-legged frogs. Ecology 82: 1947–1955. [Google Scholar]
- Rivas JM, Ullrich SE (1992) Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol 149: 3865–3871. [PubMed] [Google Scholar]
- Rollins-Smith LA. (1998) Metamorphosis and the amphibian immune system. Immunol Rev 166: 221–230. [DOI] [PubMed] [Google Scholar]
- Salo HM, Jokinen EI, Markkula SE, Aaltonen TM, Penttilä HT (2000) Comparative effects of UVA and UVB irradiation on the immune system of fish. J Photochem Photobiol B 56: 154–162. [DOI] [PubMed] [Google Scholar]
- Sancar A, Tang MS (1993) Nucleotide excision repair. Photochem Photobiol 57: 905–921. [DOI] [PubMed] [Google Scholar]
- Searle CL, Belden LK, Bancroft BA, Han BA, Biga LM, Blaustein AR (2010) Experimental examination of the effects of ultraviolet-B radiation in combination with other stressors on frog larvae. Oecologia 162: 237–245. [DOI] [PubMed] [Google Scholar]
- Skerratt L, Berger L, Speare R, Cashins S, McDonald K, Phillott A, Hines H, Kenyon N (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4: 125–134. [Google Scholar]
- Stingl LA, Sauder DN, Iijima M, Wolff K, Pehamberger H, Stingl G (1983) Mechanism of UV-B-induced impairment of the antigen-presenting capacity of murine epidermal cells. J Immunol 130: 1586–1591. [PubMed] [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306: 1783–1786. [DOI] [PubMed] [Google Scholar]
- Tejedo M, Marangoni F, Pertoldi C, Richter-Boix A, Laurila A, Orizaola G, Nicieza AG, Alvarez D, Gomez-Mestre I (2010) Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Clim Res 43: 31–46. [Google Scholar]
- van de Mortel T, Buttemer W, Hoffman P, Hays J, Blaustein A (1998) A comparison of photolyase activity in three Australian tree frogs. Oecologia 115: 366–369. [DOI] [PubMed] [Google Scholar]
- van Rooij P, Martel A, Haesebrouck F, Pasmans F (2015) Amphibian chytridiomycosis: a review with focus on fungus-host interactions. Vet Res 46 Artn 137; doi:10.1186/S13567-015-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uitregt VO, Wilson RS, Franklin CE (2007) Cooler temperatures increase sensitivity to ultraviolet B radiation in embryos and larvae of the frog Limnodynastes peronii. Glob Change Biol 13: 1114–1121. [Google Scholar]
- Voyles J, Berger L, Young S, Speare R, Webb R, Warner J, Rudd D, Campbell R, Skerratt LF (2007) Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis Aquat Organ 77: 113–118. [DOI] [PubMed] [Google Scholar]
- Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Cook D, Webb R, Alford RA, Skerratt LF et al. (2009) Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326: 582–585. [DOI] [PubMed] [Google Scholar]