Abstract
Phosphatidylinositol-specific phospholipase C2 (PLC2) is a signaling enzyme with hydrolytic activity against membrane-bound phosphoinositides. It catalyzes the cleavage of phosphatidylinositol(4,5)bisphosphate (PtdIns(4,5)P2) into two initial second messengers, myo-inositol-1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG). The former, as well as its fully phosphorylated derivative, myo-inositol-1,2,3,4,5,6-hexakisphosphate (InsP6), play a major role in calcium signaling events within the cell, while DAG may be used in the regeneration of phospholipids or as a precursor for phosphatidic acid (PA) biosynthesis, an important signaling molecule involved in both biotic and abiotic types of stress tolerance. Overexpression of the gene for Brassica napus phospholipase C2 (BnPLC2) in Brassica napus has been shown to enhance drought tolerance, modulate multiple genes involved in different processes and favorably affect hormonal levels in different tissues. We, therefore, undertook the current study with a view to examining, at the metabolome level, its effect on both abiotic (low temperature) and biotic (stem white rot disease) types of stress in canola. Thus, while transgenic plants exhibited a significant rise in maltose levels and a concomitant elevation in some unsaturated free fatty acids (FFAs), glycerol, and glycerol 3-phosphate under subzero temperatures, they accumulated high levels of raffinose, stachyose and other sugars as well as some flavonoids under acclimatization conditions. Collectively, overexpression of BnPLC2 appears to have triggered different metabolite patterns consistent with its abiotic and, to a limited extent, biotic stress tolerance phenotypes.
Keywords: abiotic stress, biotic stress, canola, cold, metabolomics, phospholipase C, sclerotinia, signal transduction
Introduction
Low temperature stress in the Canadian Prairies is one of several types of stress conditions that threaten Western agriculture and determine success or failure of several crops that are grown in the region, e.g., canola and cereals. Frost conditions and subzero temperatures can induce cellular damage through several mechanisms, all of which are caused by what we refer to as “disruptive water status” (DWS). The latter is a consequence of impaired water utilization through freezing, excessive transpiration, severe dehydration, and osmotic imbalance. DWS occurs when the availability of water, necessary for vital cellular activities and metabolic processes, becomes halted or severely limited. Plant cells usually adapt to mild conditions of DWS through acclimatization or by accumulating osmolytes, e.g., simple sugars, which provide proper water balance across membranes and maintain turgor pressure at optimal levels for membrane integrity.
Similarly, yield losses due to different pathogens can reach detrimental proportions if not properly controlled. For example, white stem rot, caused by the fungus Sclerotinia sclerotiorum, is listed as a major disease of canola by the Canola Council of Canada,1 causing yield losses approximately equivalent to 0.5 × percentage infection; yield losses can be nearly 50% when the pathogen is growing under ideal conditions.1
Although many plants have evolved to adapt to different stresses through various mechanisms, unpredictable weather patterns continue to pose serious challenges for agricultural crop species. Therefore, it becomes crucial that crop failures due to biotic and abiotic stresses be minimized and crop yields be maximized. Thus, an important objective of plant biotechnology is to produce crops that can tolerate sudden changes in weather patterns and have improved tolerance to pathogens. Since both types of stress (biotic and abiotic) require the deployment of adaptive molecular mechanisms involving complex multigenic signals, the engineering of such traits into crop plants must rely on the use of factors that are capable of setting in motion such complex molecular networks.
Previously, we have shown that transgenic canola plants overexpressing the gene for BnPLC2 developed faster and gave higher seed yield and oil % than non-transgenic controls (NTCs).2 Overexpression of BnPLC2 also had a significant effect on differential gene expression and hormone synthesis and distribution.2 Some of the genes identified are known to be associated with both biotic and abiotic stress tolerance, and we showed these plants to be considerably more tolerant of drought than their corresponding NTCs. Of particular interest to the current study are genes such as lipoxygenase 2 (LOX2), a principal enzyme in jasmonic acid (JA) biosynthesis, and several JA-responsive genes and proteins, including strictosidine synthase and other disease-associated defense genes (e.g., glucanases and chitinases), whose change in expression implies an effect of BnPLC2 enhancement toward priming stress tolerance,2 presumably synergistically with innate defense mechanisms. JA and its derivatives are ubiquitous lipid-derived signal compounds that are produced in response to both biotic and abiotic stresses. They act as systemic signals and can interact with other hormones and modulate production of transcription factors (TFs), protein kinases3 as well as some phospholipases such as PLC.4 The involvement of phospholipases in plant immunity to different stresses is due in part to early activation of signaling events.5
Phospholipases have been shown to play important roles in resistance to various biotic and abiotic stress conditions. For example, phospholipase A (PLA) produces FFA second messengers and precursors of JA and derivatives, while phospholipase D (PLD) hydrolyzes structural phospholipids such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE) to produce phosphatidic acid (PA) which is associated with various stress responses. Further, PLC is a key enzyme in phospholipid signal transduction processes, located in plasma membranes where it catalyzes the hydrolysis of the membrane-bound phospholipid, phosphatidylinositol(4,5)bisphosphate (PtdIns(4,5)P2), to produce two second messengers, myo-inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) and sn-1,2-diacylglycerol (DAG). Ins(1,4,5)P3, being water soluble, migrates to the cytosol where it regulates Ca2+ release from internal stores, directly or after being converted to Ins(1,2,3,4,5,6)P6 (InsP6) by the action of inositol polyphosphate kinases.6 Although DAG is known to play a role in activating protein kinase C (PKC) in animal systems, in plants, which lack PKC, it is rapidly converted into phosphatidic acid (PA) by the action of DAG kinase (DAGK). PA is known to perform a wide range of second messenger functions in plant cells, and its level is reported to increase in response to different types of stresses, including low temperature, osmotic, oxidative, wounding and pathogen infection.7,8
PA can be generated by two phospholipid signaling systems, PLD and PLC/DAGK. Simultaneous activation of the two pathway systems was shown to be among the early events following exposure to low temperature stress.9 Although the product from both pathways is PA, the molecular species of PA produced by each pathway can be different due to the variant types of the sn-1 and sn-2 acyl substituents in each case.8,10 The PLC/DAGK route has been shown to be the predominant source of PA production under low temperature stress conditions in Arabidopsis cell suspension cultures, seedlings, and leaf discs.9,11 This is of relevance to the present work in view of the fact that in Brassica napus, expression of PLC2 was found to increase in response to treatment with different stresses such as drought, NaCl, and cold.12 Important roles of PLC in plant cell responses to hyperosmotic stress and drought in different plant systems are well documented.13,14
The PLC/DAGK-derived PA also seems to be the prominent species involved in biotic stress tolerance. Thus, tomato cell suspensions responded to a number of pathogenic elicitors by increasing their PA contents within minutes of elicitation, which arose mostly via DAG phosphorylation and was correlated with decreased levels of PtdIns(4,5)P2.15,16 Furthermore, expression of the rice gene, OsPLC1, was shown to be induced by diverse inducers of plant defense pathways as well as during the incompatible interaction between a resistant genotype of rice and the fungal pathogen, Magnaporthe grisea, suggesting that OsPLC1 might be involved in the activation of the signaling pathway leading to disease resistance response in rice.17
Thus, its potential for biotic stress tolerance, together with the existing evidence on its involvement in abiotic stress tolerance, prompted us to investigate the effect of BnPLC2 enhancement on low temperature stress response in transgenic B. napus plants, while examining prominent changes to the metabolome configuration in those lines as it relates to cold tolerance. Metabolomes are modulated by factors that influence the expression of multiple genes and pathways, such as PLC. As well, we report an analysis of response of a transgenic line, with enhanced BnPLC2, to a common fungal pathogen, Sclerotinia sclerotiorum, the main cause of the white stem rot disease in canola which results in substantial yield losses.1
Results
Cold stress
To evaluate the performance of transgenic plants overexpressing BnPLC2 under conditions of subzero temperatures, the Phytotron temperature was lowered gradually to -5 °C at the rate of 2 °C/h. Plants were maintained at this temperature for 12 h, at which point the temperature was gradually increased at the same rate back to the normal growth cycle conditions. To evaluate their subzero stress tolerance after acclimatization, transgenic plants were first acclimatized by incubation at +4 °C for 7 d, followed by a gradual temperature descent (2 °C/h) to -5 °C. Plants were kept at -5 °C for 24 h, after which time temperature was gradually raised at the same rate back to the normal growth conditions. Recovery of plants was evaluated by the appearance of leaves and their relative turgidity with reference to NTC plants.
After subzero treatments at -5 °C without acclimatization, recovered transgenic plants showed less leaf wilt than NTCs. They also had stronger stem turgidity. Transgenic plants subjected to prior acclimatization showed healthier, turgid leaves and faster recovery after subzero treatment than NTCs (Fig. 1).
Figure 1. Plant performance after subzero temperature treatments. (A) NTC and transgenic BnPLC2 line (S18) were maintained at -5 °C for 12 h. Pictures were taken 2 d after recovery. (B) NTC line and transgenic S18 line were first acclimatized at +4 °C for 7 d and then treated at -5 °C for 24 h. Pictures were taken 7 d after recovery.
Metabolomic studies of transgenic plants overexpressing BnPLC2 under control, subzero stress, and acclimatization conditions
We performed metabolome analyses in transgenic lines overexpressing BnPLC2 under normal growth conditions, subzero temperature conditions (-5 °C) and acclimatization at +4 °C for 7 d. A total of 162 biochemicals were analyzed as a data set in these studies (Table S1). Our overall observation was that the metabolome of transgenic lines under normal growth conditions was similar to that in NTC plants. However, we found consistent metabolite changes in lines overexpressing BnPLC2 in response to subzero and acclimatization treatments.
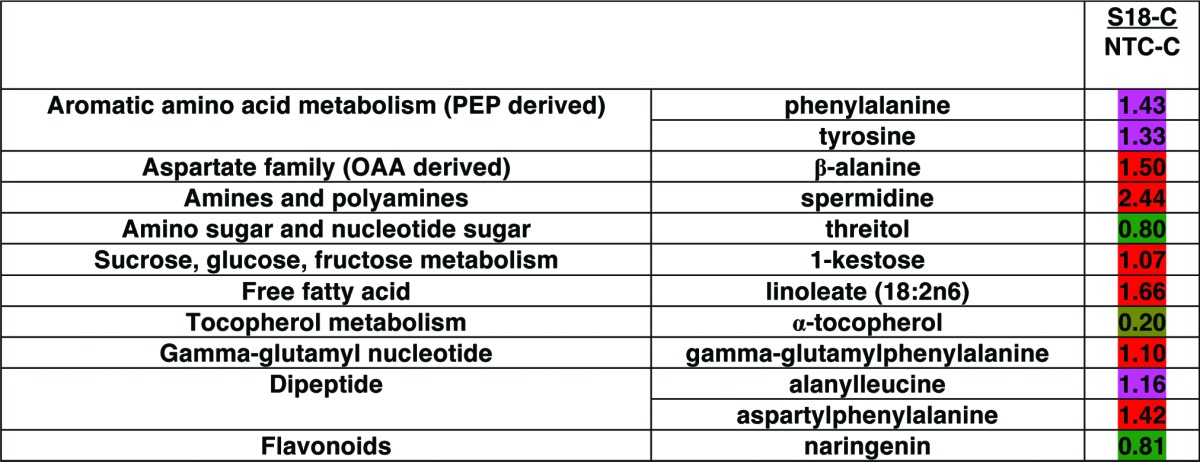
Pairwise comparison analysis contrasting a transgenic line under normal conditions to subzero temperature and acclimatization and non-transgenic control (NTC) under respective conditions, was performed. After performing statistical comparison by Welch’s two-samples t-Test, biochemicals that were significantly different (P < 0.05) or approaching significance level (P < 0.1) in transgenic plants compared with NTCs under the same conditions were selected. When contrasting S18 grown under normal conditions to NTC grown under the same conditions (S18-C/NTC-C), changes in 12 metabolites were found significant (9 increased and 3 decreased). Likewise, when contrasting S18 grown under acclimatization conditions to NTC under acclimatization conditions (S18-A/NTC-A), changes in 23 metabolites (17 increased and 6 decreased) were found significant. Changes in 14 metabolites (7 increased and 7 decreased) were found significant between S18 after subzero stress and NTC after subzero stress (S18-S/NTC-S). Metabolites that were significantly increased under non-stressed conditions in transgenic compared with NTC lines were: the amino acids, phenylalanine, tyrosine and β-alanine in addition to the peptide, gamma-glutamylphenylalanine, the dipeptides alanylleucine and aspartylphenylalanine, as well as the polyamine, spermidine and the FFA, linoleate (18:2n6). Also under non-stressed conditions, significant decrease was found in threitol, α-tocopherol, and the flavonoid naringenin (Table 1).
Table 1. Pairwise comparison of significantly different metabolites in line S18 compared with NTC under normal growth conditions. The number in each cell indicates fold change between S18 and NTC. Welch’s two-sample t-Test was used to estimate significance (red, increased in S18 compared with NTC at P < 0.05, pink, increased in S18 compared with NTC at P < 0.1; green, decreased in S18 compared with NTC at P < 0.05, light green, decreased in S18 compared with NTC at P < 0.1).
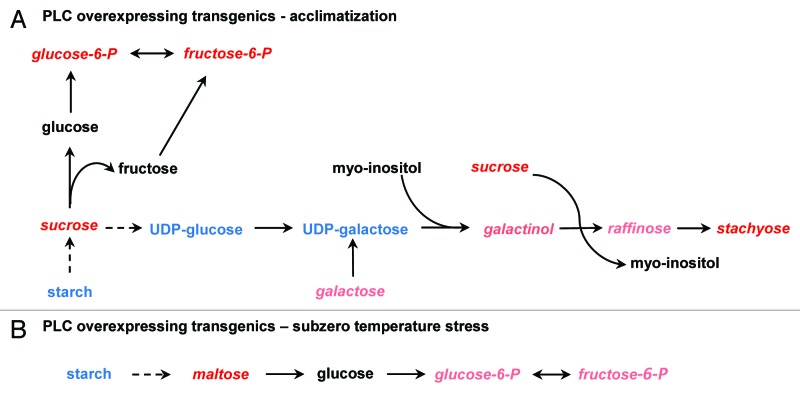
Metabolite analyses revealed that transgenic lines mounted a robust carbohydrate metabolic response to acclimatization and subzero treatments (Table 2). The carbohydrate metabolic response was different for each type of treatment (acclimatization vs. subzero treatment). Thus, BnPLC2 transgenic plants showed a 4.98 times increase in sucrose under acclimatization conditions but responded to subzero stress with a 4.94 times increase in maltose compared with NTCs (Table 2). The maltose increase was also found in other biological replicates of this experiment (data not shown). This notable increase in the level of maltose is indicative of starch mobilization, where transgenic leaves may be exhibiting sink tissue properties, drawing on stored starch in the acute response to subzero temperatures, while in the longer term acclimatization, the carbon appeared in the form of sucrose (Fig. 2). NTC leaves did not accumulate maltose under stress.
Table 2. Pairwise comparison data of changes in carbohydrates metabolism in leaves of transgenic line S18, grown under normal control conditions (C) and subjected to acclimatization (+4 °C for 7 d) (A) and subzero stress treatments (-5 °C for 12h) (S). The number in each cell indicates fold change between S18 and NTC lines under equal treatment conditions. Statistical comparisons were estimated according to Welch’s two-sample t-Test; red, P < 0.05; pink, P < 0.1 (red, increased in S18 compared with NTC at P < 0.05; pink, increased in S18 compared with NTC at P < 0.1).
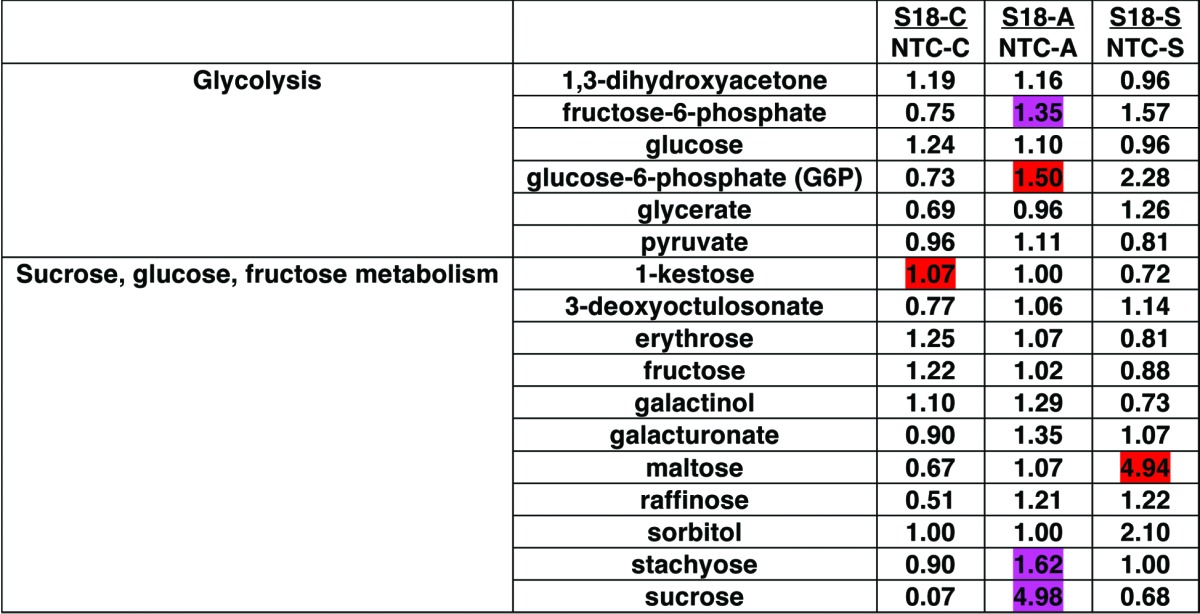
Figure 2. Changes in carbohydrates metabolism in transgenic line (S18) overexpressing BnPLC2 gene under the conditions of acclimatization (A) and subzero temperature stress (B). Metabolites in red italics indicate higher level at this treatment conditions compared with control. Pink italics indicate biochemicals stably increased in other biological replicates of this experiment. Blue font indicates biochemicals not detected in this study and black font, unchanged metabolites.
The increase in sucrose under acclimatization conditions in transgenic lines was accompanied by increased levels of stachyose, glucose-6-phosphate, and fructose-6-phosphate (Table 2). Levels of raffinose and galactinol appeared to increase in this experiment without reaching statistical significance. However, in other biological replicates of this experiment these were found significantly higher (data not shown). This is consistent with the accumulation pattern of the raffinose family of oligosaccharides (RFOs) in plant tissues during acclimatization. RFOs are known for their osmoprotection and cryopreservation properties as well as being protective agents against oxidative damage.18
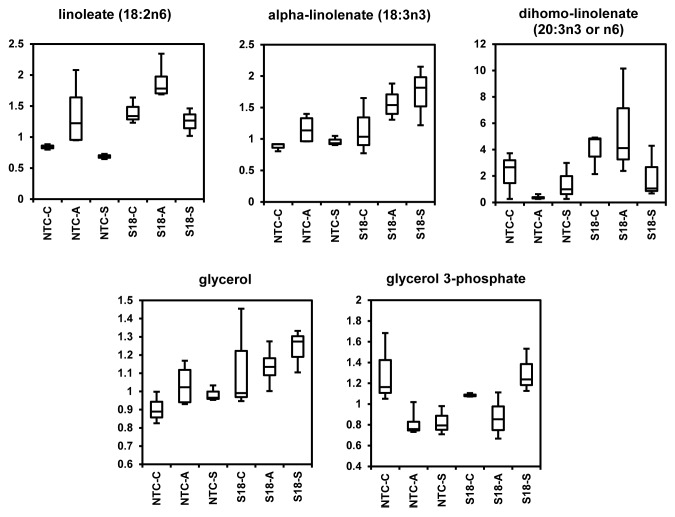
FFA metabolism was consistently affected in transgenic lines overexpressing Bn-PLC2 (Table 3; Fig. 3). Thus, elevated levels of linoleate (18:2n6) were found in transgenic lines growing under regular growth conditions. It was also significantly increased following subzero temperature treatment, along with α-linolenate (18:3n3). On the other hand, the acclimatization treatment led to a significant increase in the levels of α-linolenate (18:3n3), 2-hydroxypalmitate, and dihomo-linolenate (20:3).
Table 3. Pairwise comparison of changes in metabolites between S18 transgenic line overexpressing BnPLC2 gene and non-transgenic control (NTC) line grown under normal growth conditions (C) and subjected to acclimatization (A) and subzero temperature stress treatment (S). The number in each cell indicates the fold change between S18 and NTC under equal treatment conditions. Welch’s two-samples t-Test was used to estimate significance. Red, increased in transgenic line compared with control, P < 0.05; pink, increased in transgenic line compared with control, P < 0.1; green, decreased in transgenic line compared with non-transgenic control line P < 0.05; light green, decreased in transgenic line compared with non-transgenic control, P < 0.1.
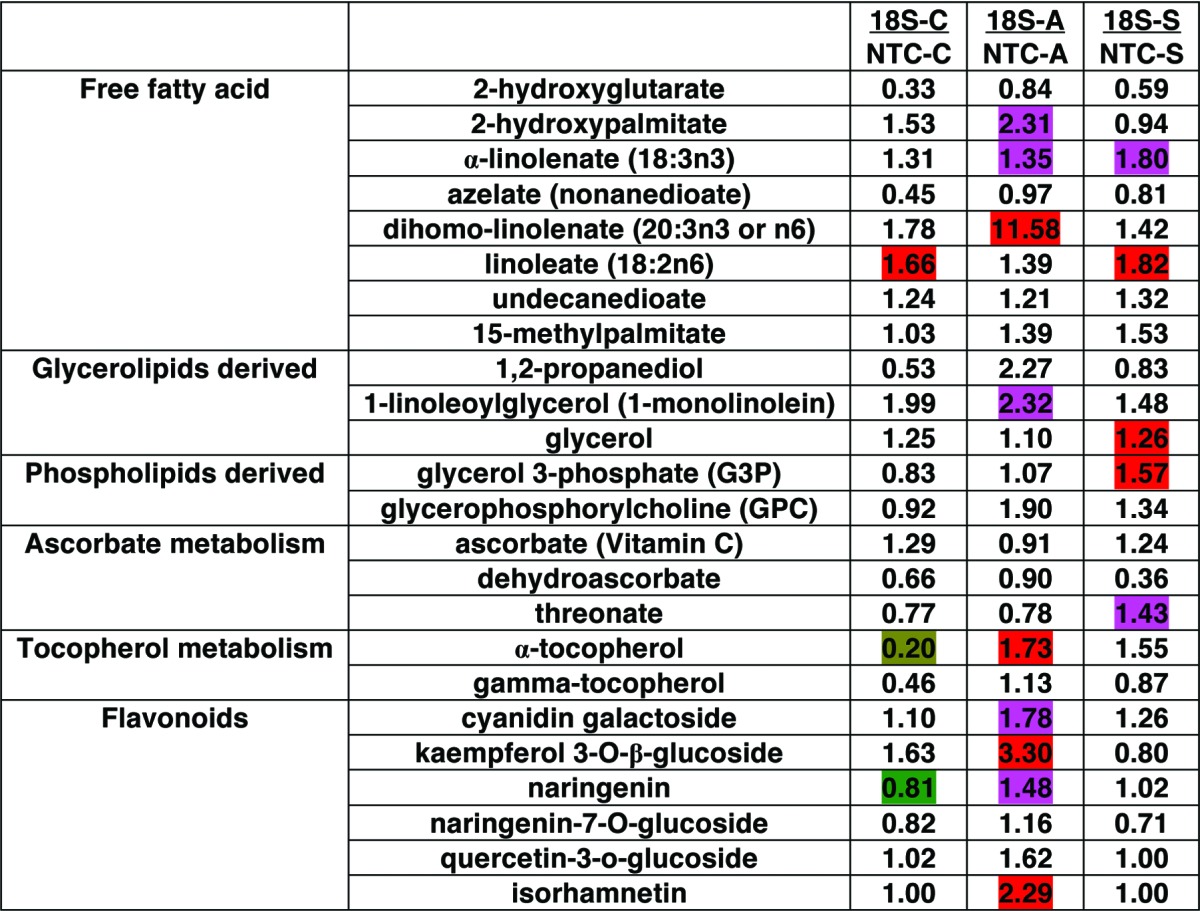
Figure 3. Box Plots representation of the amounts of linoleate (18:2n6), α-linolenate (18:3n3), dihomo-linolenate (20:3n3 or n6), glycerol and glycerol 3-phosphate in line S18 overexpressing BnPLC2 gene relative to NTC, grown under normal control conditions (C) and subjected to acclimatization (A) at +4 °C for 7 d and subzero stress (S) at -5 °C for 12h. The box represents the middle 50% of the distribution and upper and lower whiskers represent the entire spread of data. Horizontal line in the box body represents the median value. The y axis is a relative level (median scaled value). The P values for all comparisons are referenced in Table S2.
Unesterified fatty acids are known to perform different functions in cell metabolism. They serve as energy sources and can interact with a wide range of enzyme systems in specific and non-specific ways. They influence the activities of protein kinases, phospholipases, G-proteins, adenylate and guanylate cyclases, and many other metabolic processes at specific intracellular locations.19 In addition, FFAs can act as second messengers that mediate cell responses to extracellular signals.20 In animals, polyunsaturated FFAs regulate the expression of genes involved in lipid and energy metabolism.21 They can be produced rapidly by a variety of lipolytic enzymes, e.g., lipoprotein lipase, phospholipases A, and hormone-sensitive lipases.
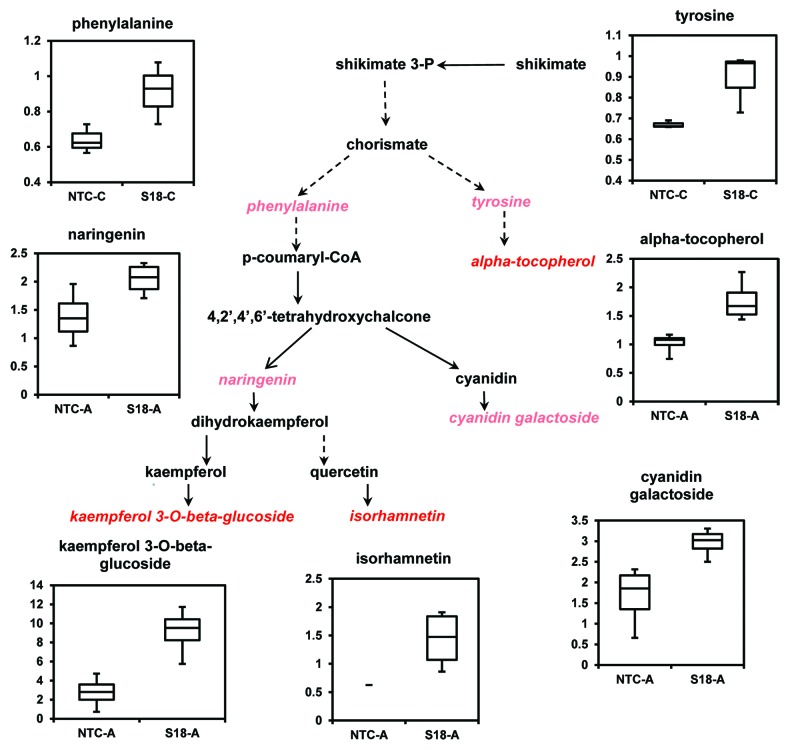
In response to acclimatization we also observed increases in compounds with antioxidant properties in the transgenic lines compared with NTCs. Thus, α-tocopherol and a group of flavonoids, such as cyanidin galactoside, kaempferol 3-O-β-glucoside, naringenin, and isorhamnetin, were increased as a result of acclimatization (Table 3; Fig. 4). Transcriptome profiling studies suggest that enhanced freezing tolerance is associated with the induction of flavonoid metabolism.22
Figure 4. Elevation of flavonoids and α-tocopherol biosynthesis in transgenic line S18, overexpressing BnPLC2 gene after acclimatization. The metabolites in pink and red italics indicate higher level in transgenic line under acclimatization conditions (A), except for aromatic amino acids, phenylalanine and tyrosine, which are elevated under normal growth conditions (C). Red, significantly elevated (P < 0.05); pink, approaches significance (P < 0.1) by Welch’s two-sample t-Test. The relative levels of the significantly altered metabolites were displayed using Box Plots. The P values for all comparisons are referenced in Table S2.
Inoculation of plant stems with the fungal pathogen
Transgenic line S1-31, overexpressing BnPLC22, was used to assess its susceptibility to fungal pathogens. Plants were inoculated with three strains of Sclerotinia and disease progress was noted, relative to the corresponding NTC line. The strains tested varied in virulence, with 243 being the least, 321 the most, and TAN being intermediately virulent. In the challenge with the 321 strain, the experiment was terminated at 14 d after inoculation (DAI) as the damage to all plants in the experiment was severe.
As a measure of quantitative disease resistance, the area under the disease progress curve (AUDPC), which includes lengths of stem lesions, was found to be significantly lower in the S1-31 line than in the NTCs for all three strains of Sclerotinia tested (Fig. 5). However, the percentage of soft and collapsed lesions (% [S+C]) data suggests less softening and collapsing of the NTC stems in all cases (data not shown).
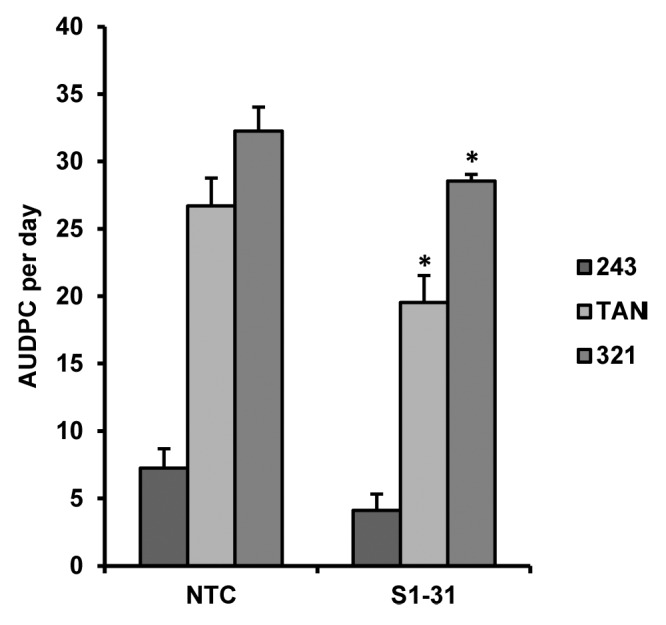
Figure 5. AUDPC per day values for transgenic line S1-31, overexpressing BnPLC2 gene relative to Westar NTC line, challenged with three strains of S. sclerotiorum (243, TAN, and 321). Evaluation with strain 321 was stopped at 14 DAI, while evaluation with strains TAN and 243 were concluded at the usual 21DAI.*Values significantly different from NTC line at P < 0.05 as determined by Student’s t test.
The general trend of this series of experiments shows the transgenic line to outperform NTCs in the rate of disease spreading, pointing to a relatively stronger drive for disease management, conferred by the enhanced BnPLC2 expression. However, although the lesions were smaller in the transgenic line as reflected in the AUDPC values, which may indicate a stronger localized defense response to the infection, the impact of the infection seemed greater as reflected in the %(S+C) data.
Discussion
We performed metabolomics studies in leaves of plants overexpressing BnPLC2 grown under normal growth conditions and subjected to subzero and acclimatization temperature treatments in order to assess the effects of BnPLC2 enhancement on metabolites pertaining to the applied stress. Under normal growth conditions, transgenic plants show fairly similar metabolomes to those found in the corresponding NTCs. However, under low temperature stress conditions (acclimatization and subzero temperature), the metabolome of transgenic plants showed some significant changes compared with NTCs. These appeared to be dependent on the type of treatment, indicating that the nature of metabolites in the BnPLC2 transgenic plants is influenced by growth condition-specific physiological programs. Thus, unlike the NTCs, under subzero temperatures, transgenic plants responded by showing increased levels of maltose (Table 2; Fig. 2). While the reason for the elevated levels of this disaccharide is not immediately obvious, it is possible that transgenic plants are capable of accumulating more transient starch than NTCs and that starch degrading enzymes are sufficiently active in these plants. Regardless of the mechanism involved in its production, maltose may function both as an osmoprotectant and as a source of glucose. Since the latter is a substrate for glycolysis, it can provide for an additional energy source under conditions of impaired photosynthesis and respiration in subzero temperatures.
Central carbohydrate metabolism is one of the most important resources for cellular protection against freezing. Its importance in freezing tolerance was demonstrated by the identification of 15 metabolites significantly correlated with acclimatized freezing tolerance in different Arabidopsis accessions, including xylose, glucose, fructose, galactose, sucrose, and raffinose.22 Also, in Arabidopsis, under acclimatization conditions (+4 °C) for four days, accumulation of cold responsive sugars and sugar phosphates was observed.23
After acclimatization, transgenic plants overexpressing BnPLC2 showed significantly elevated levels of carbohydrates associated with cellular protection, such as RFOs and smaller sugars (e.g., glucose-6-P, fructose-6-P, sucrose). Accumulation of the RFOs in transgenic lines upon acclimatization may be due to activation by the transgene of cold responsive TFs and increased availability of myo-inositol-releasing substrates such as myo-inositol phosphates, in addition to the fact that overexpression of BnPLC2 has been shown to upregulate the expression of myo-inositol 1-phosphate synthase2 for de novo synthesis of myo-inositol. The first committed step in RFO biosynthesis is the formation of galactinol from myo-inositol and UDP-galactose by the enzyme, galactinol synthase (GolS). Under normal and subzero stress conditions, galactinol levels in B. napus leaves are very low. These levels are significantly increased following acclimatization (data not shown), suggesting induction of the GolS expression as a result of exposure to low, non-freezing temperatures.38 This gene is known to be regulated by CBF, the transcription activator of the cold-regulated (COR) genes.24
The significant elevation of hexose phosphates in transgenic leaves after acclimatization suggests their possible participation as osmoprotective and cryoprotective osmolytes. In addition, hexoses can be used as added substrates for glycolysis under conditions of reduced transpiration and photosynthesis at low temperatures as well.
Following a similar trend, the levels of unsaturated FFA contents in lines overexpressing BnPLC2 were also increased compared with NTCs in all physiological states (Table 3; Fig. 3). Thus, in transgenic plants growing under normal conditions, elevated levels of linoleate (18:2n6) were observed. After subzero temperature treatment, the rise in linoleate (18:2n6) levels was accompanied by an increase in α-linolenate (18:3n3). Levels of the latter fatty acid, as well as those of dihomo-linolenate (20:3n3 or n6) were also increased upon acclimatization.
FFAs are reported to have second messenger functions in addition to controlling a wide variety of targets in the cells of all living organisms; hence, their levels have to be tightly controlled.19,20 Accordingly, various metabolic destinations of FFAs are determined by the cell's physiological state. For example, under normal as well as acclimatization conditions FFAs can undergo further desaturation and/or elongation and become incorporated into phospholipids pools. In this way, further desaturation and elongation of linoleate (18:2n6) will lead to the production of 18:3 FA, which is relatively elevated after acclimatization. Generally, under normal growth conditions this compound is a precursor of JA, which may explain the increase in the expression of the LOX2 enzyme in BnPLC2 overexpressing transgenic lines.2
FFAs can also be degraded quickly by β-oxidation pathways to generate energy for the cell. Also produced through β-oxidation is acetyl-CoA which may enter the glyoxylate cycle and, through gluconeogenesis, generates additional glucose molecules. It is possible that this pathway could be operational in transgenic plants under normal growth conditions, which may explain their seemingly increased level of transient starch. Glucose produced in this manner may also be utilized through glycolysis to supplement the ATP pool in transgenic plants as part of coping with stress under unfavorable growth conditions, such as subzero temperatures. Alternatively, it may be utilized in other biosynthetic needs under suitable conditions such as acclimatization.
It was shown in many plant systems that subzero temperatures activated phospholipid hydrolyzing enzymes such as phospholipase D (PLD) and phospholipase A, which leads to phospholipid degradation and possibly reutilization of available substrates. The significant accumulation of glycerol and glycerol-3-P in the transgenics compared with NTCs after subzero temperature treatment indicates that this process is more active as a result of increased BnPLC2 presence (Fig. 3). Increased glycerol can perform a number of protective functions such as osmoprotection and cryopreservation or, through dihydroxyacetone and glycolysis, may serve as additional energy source.
The increase in transgenic plants in α-linolenate (18:3(n-3)) and the 11-fold increase in dihomo-linolenate (20:3n3 or 6) after acclimatization is noteworthy. This may be a consequence of maintaining active biosynthesis of plasma membrane phospholipids during acclimatization, leading to increased activity of phospholipid metabolic enzymes that are responsible for desaturation and elongation of fatty acids. Thus, the significant accumulation of 20:3 FA in transgenic lines during acclimatization could be a result of increased activity of desaturases and elongases that utilize the 18:2 FA, which is present in significantly elevated levels in transgenic plants under normal growth conditions.
Previous evidence of the upregulation of the shikimate kinase-like gene in transgenic plants overexpressing BnPLC22 supports the possibility of an induced shikimate pathway, which ultimately leads to increased production of phenylalanine and tyrosine through chorismate. This is further supported by the current metabolite analysis results, which show that under normal growth conditions transgenic BnPLC2 plants accumulated higher levels of these aromatic amino acids. This is of significance since aromatic amino acids are important precursors in the biosynthesis of protection and repair compounds.25 For example, phenylalanine is a precursor in flavonoid biosynthesis and tyrosine is a precursor in tocopherol biosynthesis. Indeed, our metabolomics analysis revealed that acclimatization has induced significant accumulation of flavonoids and α-tocopherol in the transgenics overexpressing BnPLC2 as compared with the NTCs. Accordingly, higher levels of cyanidin galactoside, kaempferol 3-O-β-glucoside, naringenin and isorhamnetin were obtained in transgenic plants after acclimatization (Fig. 4).
Flavonoids and tocopherols have been shown to be important antioxidants and scavengers of lipid radicals and reactive oxygen species in plants.26,27 The reactive oxygen species scavenging properties of flavonoids are most prominent in structures with dihydroxy B-ring substituted glycosides. The biosynthesis of flavonoids is induced by stresses of different nature, ranging from nitrogen/phosphate starvation to cold, salinity and drought.28 Therefore, it is tempting to speculate that BnPLC2 transgenics may be predisposed to cold stress tolerance by accumulating the corresponding aromatic amino acid precursors under normal conditions. In order to trigger their utilization in the biosynthesis of flavonoids and α-tocopherol under acclimatization conditions, additional factors may have to be activated due to the decrease in temperature. In this context, while we observe a significant decrease in α-tocopherol and naringenin under normal growth conditions (Table 1), upon acclimatization their biosynthesis is activated as compared with NTCs (Fig. 4).
Pathogen-related (PR) gene expression and the synthesis of defense compounds associated with local and systemic acquired resistance (LAR and SAR) in plants require salicylic acid (SA).29 In Arabidopsis, SA is synthesized by two different mechanisms: first, from chorismate by the action of isochorismate synthase (ICS); and second, from phenylalanine and conversion by phenylalanine ammonia lyase (PAL) and benzoic acid 2-hydrolase (BA2H).30,31 It has been suggested that SA synthesized through each pathway has a different role in plant defense mechanisms against pathogens.30 If so, ICS would have an important role in LAR and SAR defense responses and specifically is required for PR1 gene expression.
Overexpression of BnPLC2 in B. napus was shown to induce transcription of a number of defense-related factors such as glucanases, chitinases, and lipoxygenase 2 (LOX2), an important enzyme in JA biosynthesis, as well as several jasmonate-responsive genes and proteins, including strictosidine synthase and other disease-related defense enzymes.2 This, in addition to our findings in the current study that BnPLC2 enhancement induced, at the metabolomic level under normal growth conditions, the accumulation of higher levels of linoleate (18:2n6) and α-linolenate (18:3n3), and the fact that the latter is the precursor in the biosynthesis of JA, as well as the prominent standing of PLC/DAGK-derived PA in biotic stress tolerance,15,16 prompted us to investigate the extent of disease resistance of transgenic plants against fungal pathogens. Sclerotinia was the pathogen of choice due to its economic importance in the canola industry.
When challenged with the pathogen, transgenic plants appeared to accumulate less infection over a given period of time than do the NTC group (Fig. 5). This suggests a relatively stronger effort for disease resistance by the transgenic plants with enhanced BnPLC2 production. However, although the lesions were smaller in the transgenic line as reflected in the AUDPC values, which may indicate a stronger localized defense response that limits the spread of the invasive fungus, the impact of the infection seemed greater as reflected in the %(S+C) data (not shown). Thus, it would appear that while overexpression of BnPLC2 does not confer full resistance, it does confer a certain degree of tolerance, and a slower rate of infection spread, suggesting that there may be a pre-existent disease resistance, albeit weak, in these plants.
While the reasons for the partial disease resistance in the transgenic line are not clear at this time, there may be opposing factors behind the fine balance that separates resistance from susceptibility, which may be imposed by the very same mechanism that seemed likely to confer resistance, i.e., PLC enhancement. In this context, it should be noted that PLC-mediated calcium regulation is important for fungal pathogenesis.32 Thus, the solution to the seeming wobble between resistance and susceptibility in the PLC-Sclerotinia scenario may simply rest within the achievement of a “median balance” between calcium regulation and the enhancement of defense components. Therefore, future studies could focus on elucidating this possibility and work toward establishing such balance. It remains important, however, to evaluate the biotic stress-tolerance attributes of the BnPLC2 transgenic line against other pathogens.
Conclusions
During the preparation of this manuscript, a comprehensive review was published,37 describing different features of plant PI-PLCs including expression patterns of different isoforms in conjunction with biotic and abiotic challenges in different plant species. Of the various isoforms known, the PLC2 isoform, used in this study, although constitutively expressed in Arabidopsis, is not significantly induced by low temperature or biotic stress conditions. Interestingly, while the same isoform is shown to be upregulated by cold and downregulated by biotic stress in wheat, its expression follows an opposite trend in rice.37 What is more, in B. napus, unlike Arabidopsis, this isoform is significantly induced by cold,12 implying the possibility of species-specific expression regulation.
Thus, by expression enhancement of this isoform in B. napus, we were able to show its influence on trait modification both at the gene expression level2 and, for the first time in a transgenic model, at the metabolome level under normal and low temperature stress conditions. Moreover, we were able to reveal its possible potential in biotic stress resistance as well as its effectiveness, through metabolome reconfiguration, in priming protection mechanisms against different applied stresses.
Materials and Methods
Plant material
Two transgenic lines were used in this study, S18 and S1-31. The first was genetically transformed into DH12075 background and the second into Westar background. Plants were genetically transformed with an ORF gene for phosphatidylinositol-specific phospholipase C2 (BnPLC2)12 (Gene bank accession number AF108123) in sense orientation under the control of CaMV-35S promoter.2
Whole plant freezing test and acclimatization treatments
Cold treatments were performed in the Phytotron facilities of the Plant Biotechnology Institute, National Research Council of Canada. Plants were grown in 6 inch pots on Sunshine #3 soil at a 16 h photoperiod (22 °C day/16 °C night), watered and fertilized regularly with 20-20-20 NPK fertilizer. At the age of 5 wk, 5–6 true leaves, plants were divided into three groups. The first group served as a control and was maintained under normal growth conditions for the duration of the experiment. The second group was subjected to sub-zero temperature treatment. These plants were transferred into a growth cabinet in which night temperature was gradually decreased from +16 °C to -5 °C at a rate of 2 °C/h and then maintained at this temperature for 12 h. At the end of the treatment, temperature was increased at a rate of 2 °C/h up to +16 °C and then returned to normal conditions. The third group was subjected to cold acclimatization by transferring plants into a growth chamber in which night temperature was gradually decreased from +16 °C to +4 °C at a rate of 2 °C/h and then maintained at this temperature for 7 d (+4 °C day and +4 °C night). At the end of acclimatization, temperature was gradually decreased to -5 °C and plants were maintained at this low temperature for 24 h. The experiment was repeated 3 times in a randomized design with 6–10 plants/treatment. For metabolomics studies leaf samples from plants subjected to sub-zero temperature stress were collected after stress treatment when temperature was gradually increased and reached +1 to +4 °C, acclimatized samples were collected at the end of treatment, at +4 °C. Samples in three to four replicates were collected for metabolomic studies from individual plants under each treatment.
Metabolomic profiling platform and sample preparation
Metabolomics profiling was done at Metabolon, Inc (www.metabolon.com). The global unbiased metabolic profiling platform was based on a combination of three independent platforms: UHLC/MS/MS2 optimized for basic species, UHLC/MS/MS2 optimized for acidic species, and GC/MS. This platform was described in detail in previous publications.33,34
The sample preparation process was performed using the automated MicroLab STAR® system from Hamilton Company. Extraction was conducted using series of organic (methanol) and aqueous extractions to remove the protein fraction while allowing maximum recovery of small molecules. Recovery standards were added prior to the first step in the extraction process for QC purposes. The resulting extract was divided into two fractions; one for analysis by LC and one for analysis by GC. Each sample was then frozen and dried under vacuum. Samples were then prepared for the appropriate instrument, either LC/MS or GC/MS.
Liquid chromatography/mass spectrometry (LC/MS, LC/MS2)
The LC/MS portion of the platform was based on a Waters ACQUITY UPLC and a Thermo-Finnigan LTQ mass spectrometer, which consisted of an electrospray ionization (ESI) source and linear ion-trap (LIT) mass analyzer. The sample extract was split into two aliquots, dried, then reconstituted in acidic or basic LC-compatible solvents, each of which contained 11 or more injection standards at fixed concentrations. One aliquot was analyzed using acidic positive ion optimized conditions and the other using basic negative ion optimized conditions in two independent injections using separate dedicated columns. Extracts reconstituted in acidic conditions were gradient eluted using water and methanol, both containing 0.1% formic acid, while the basic extracts, which also used water and/or methanol, contained 6.5 mM ammonium bicarbonate. The MS analysis alternated between MS and data-dependent MS2 scans using dynamic exclusion.
Gas chromatography/mass spectrometry (GC/MS)
Samples for GC/MS analysis were re-dried under vacuum desiccation for a minimum of 24 h prior to being derivatized under dried nitrogen using bistrimethyl-silyl-triflouroacetamide (BSTFA). The GC column was 5% phenyl-methyl polysiloxane and the temperature ramp was from 40° to 300 °C in a 16 min period. Samples were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization.
Chromatographic separation, followed by full-scan mass spectra, was performed to record retention time, molecular weight (expressed as mass-to-charge ratio or m/z), and MS/MS2 of all detectable ions present in the samples. Metabolites were identified by automated comparison of the ion features in the experimental samples to a reference library of chemical standard entries that included retention time, molecular weight (m/z), preferred adducts, and in-source fragments, as well as their associated MS/MS2 spectra. This library allowed the rapid identification of metabolites in the experimental samples with high confidence. Comparison of experimental samples to process blanks (water only) and solvent blanks allowed the removal of artifactual peaks.
A list of metabolites detected in this study with their Metabolon compound ID, MS platform of detection, retention index (RI), detected quantum mass and CAS number are presented in Supplemental data set 1 of the Supplemental Table.
Data imputation and statistical analysis
Data were corrected for minor variations resulting from instrument day to day tuning differences,33 the missing values for a given metabolite were assigned the observed minimum detection value, based on the assumption that the missing values were below the limits of detection. The integrated peak areas of the quantitation ion for each biochemical were rescaled by dividing each sample value by the median value for the specific biochemical in each run day block, and then the null values were imputed with the minimum observed values across all samples for that compound. Statistical analysis of the data was performed using JMP (SAS, http://www.jmp.com) and R (http://cran.r-project.org/). To visualize the entire data set, a heat map was generated to show fold change for each compound identified from GC-MS and LC-MS analyses of the tissue samples (see Table S2). Fold change was calculated as the means ratios of each treatment compared with non-transgenic control line for each treatment. Welch’s two-sample t-Tests were then used to determine whether each metabolite was significantly increased or decreased in abundance.
Inoculation of plant stems with the fungal pathogen
Westar was used as the NTC line relative to the sense BnPLC2 transformant S1-31.2 Seeds were sown in Sunshine Mix 3 (SunGro Horticulture Canada Ltd) amended with 7 g Nutricote Controlled Release Fertilizer 14-14-14 (Chisso-Asahi Fertilizer Co Ltd). The plants were maintained in a growth chamber under 200–300 μmol/m2/s light with a 16 h photoperiod (Philips, Silhouette High Output, TL5 fluorescent, F39T5/841 HO (4000 K, Cool white), at 20 °C in the light and 16 °C in the dark. Two seeds per pot were sown and thinned to one plant per pot after the presence of the BnPLC2 gene was confirmed in the plants by PCR. Plants were placed in a randomized design with two biological replicates and two treatments for each cultivar. Each replicate consisted of six plants. Replicates were spaced at a 10 d interval.
Three strains of S. sclerotiorum of increasing virulence were tested. Strain 321, the most virulent, is representative of a clone common in canola fields in western Canada,35 while Tan and 243 were somewhat less virulent. They were grown as plate cultures on minimal salts-glucose agar.36 At the full flowering stage, plants were inoculated with 4 mm plugs cut from the fungal growth front and affixed to the stem with ParafilmTM at two sites. Each main stem was inoculated at two internodes separated by an uninoculated internode. In susceptible plant lines, lesions may appear as early as 24 h after inoculation. Lesion length and depth penetration were recorded at 7, 14, and 21 d after inoculation. The length of the brown, necrotic tissue, sometimes surrounded by a dark margin, was measured. From these data, the area under the disease progress curve (AUDPC) was calculated. The depth of fungal penetration was described as firm (f), soft (s), or collapsed (c). For each line, the % (S+C) was calculated using all lesions at the three rating dates.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgements
We thank Metabolon Inc, for helping with metabolomics analyses and statistical evaluations, and Dr Lone Buchwaldt for providing the fungal strains and for valuable discussions. The abiotic stress part of this work was supported by a grant to K.N. from the Government of Saskatchewan's Agricultural Development Funds (ADF) Program. This is NRCC publication No. 55990.
References
- 1.Canola encyclopedia, diseases, sclerotinia [Internet]. Winnipeg, Canada; Canola Council of Canada: c2014 [cited 25 Jan 2014]. Available from: http://www.canolacouncil.org/canola-encyclopedia/diseases/sclerotinia/.
- 2.Georges F, Das S, Ray H, Bock C, Nokhrina K, Kolla VA, Keller W. . Over-expression of Brassica napus phosphatidylinositol-phospholipase C2 in canola induces significant changes in gene expression and phytohormone distribution patterns, enhances drought tolerance and promotes early flowering and maturation. Plant Cell Environ 2009; 32:1664 - 81; http://dx.doi.org/ 10.1111/j.1365-3040.2009.02027.x; PMID: 19671099 [DOI] [PubMed] [Google Scholar]
- 3.Wasternack C. . Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 2007; 100:681 - 97; http://dx.doi.org/ 10.1093/aob/mcm079; PMID: 17513307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Profotová B, Burketová L, Novotná Z, Martinec J, Valentová O. . Involvement of phospholipases C and D in early response to SAR and ISR inducers in Brassica napus plants. Plant Physiol Biochem 2006; 44:143 - 51; PMID: 16644231 [DOI] [PubMed] [Google Scholar]
- 5.Canonne J, Froidure-Nicolas S, Rivas S. . Phospholipases in action during plant defense signaling. Plant Signal Behav 2011; 6:13 - 8; http://dx.doi.org/ 10.4161/psb.6.1.14037; PMID: 21248491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munnik T, Testerink C. . Plant phospholipid signaling: “in a nutshell”. J Lipid Res 2009; 50:Suppl S260 - 5; http://dx.doi.org/ 10.1194/jlr.R800098-JLR200; PMID: 19098305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munnik T. . Phosphatidic acid: an emerging plant lipid second messenger. Trends Plant Sci 2001; 6:227 - 33; http://dx.doi.org/ 10.1016/S1360-1385(01)01918-5; PMID: 11335176 [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Devaiah SP, Zhang W, Welti R. . Signaling functions of phosphatidic acid. Prog Lipid Res 2006; 45:250 - 78; http://dx.doi.org/ 10.1016/j.plipres.2006.01.005; PMID: 16574237 [DOI] [PubMed] [Google Scholar]
- 9.Ruelland E, Cantrel C, Gawer M, Kader JC, Zachowski A. . Activation of phospholipases C and D is an early response to a cold exposure in Arabidopsis suspension cells. Plant Physiol 2002; 130:999 - 1007; http://dx.doi.org/ 10.1104/pp.006080; PMID: 12376663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergnolle C, Vaultier MN, Taconnat L, Renou JP, Kader JC, Zachowski A, Ruelland E. . The cold-induced early activation of phospholipase C and D pathways determines the response of two distinct clusters of genes in Arabidopsis cell suspensions. Plant Physiol 2005; 139:1217 - 33; http://dx.doi.org/ 10.1104/pp.105.068171; PMID: 16258011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arisz SA, van Wijk R, Roels W, Zhu JK, Haring MA, Munnik T. . Rapid phosphatidic acid accumulation in response to low temperature stress in Arabidopsis is generated through diacylglycerol kinase. Front Plant Sci 2013; 4:1 - 15; http://dx.doi.org/ 10.3389/fpls.2013.00001; PMID: 23346092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das S, Hussain A, Bock C, Keller WA, Georges F. . Cloning of Brassica napus phospholipase C2 (BnPLC2), phosphatidylinositol 3-kinase (BnVPS34) and phosphatidylinositol synthase1 (BnPtdIns S1)--comparative analysis of the effect of abiotic stresses on the expression of phosphatidylinositol signal transduction-related genes in B. napus.. Planta 2005; 220:777 - 84; http://dx.doi.org/ 10.1007/s00425-004-1389-0; PMID: 15480754 [DOI] [PubMed] [Google Scholar]
- 13.DeWald DB, Torabinejad J, Jones CA, Shope JC, Cangelosi AR, Thompson JE, Prestwich GD, Hama H. . Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed arabidopsis. Plant Physiol 2001; 126:759 - 69; http://dx.doi.org/ 10.1104/pp.126.2.759; PMID: 11402204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munnik T, Vermeer JEM. . Osmotic stress-induced phosphoinositide and inositol phosphate signalling in plants. Plant Cell Environ 2010; 33:655 - 69; http://dx.doi.org/ 10.1111/j.1365-3040.2009.02097.x; PMID: 20429089 [DOI] [PubMed] [Google Scholar]
- 15.van der Luit AH, Piatti T, van Doorn A, Musgrave A, Felix G, Boller T, Munnik T. . Elicitation of suspension-cultured tomato cells triggers the formation of phosphatidic acid and diacylglycerol pyrophosphate. Plant Physiol 2000; 123:1507 - 16; http://dx.doi.org/ 10.1104/pp.123.4.1507; PMID: 10938366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong CF, Laxalt AM, Bargmann BO, de Wit PJ, Joosten MH, Munnik T. . Phosphatidic acid accumulation is an early response in the Cf-4/Avr4 interaction. Plant J 2004; 39:1 - 12; http://dx.doi.org/ 10.1111/j.1365-313X.2004.02110.x; PMID: 15200638 [DOI] [PubMed] [Google Scholar]
- 17.Song F, Goodman RM. . Molecular cloning and characterization of a rice phosphoinositide-specific phospholipase C gene, OsPI-PLC1, that is activated in systemic acquired resistance. Physiol Mol Plant Pathol 2002; 61:31 - 40 [Google Scholar]
- 18.Nishizawa A, Yabuta Y, Shigeoka S. . Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol 2008; 147:1251 - 63; http://dx.doi.org/ 10.1104/pp.108.122465; PMID: 18502973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calder PC, Burdge GC. Bioactive Lipids. Bridgwater: Oily Press; c2004. Fatty Acids; p. 1-36. [Google Scholar]
- 20.Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock D, Landrock KK, et al. . Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids 2008; 43:1 - 17; http://dx.doi.org/ 10.1007/s11745-007-3111-z; PMID: 17882463 [DOI] [PubMed] [Google Scholar]
- 21.Nakamura MT, Cheon Y, Li Y, Nara TY. . Mechanisms of regulation of gene expression by fatty acids. Lipids 2004; 39:1077 - 83; http://dx.doi.org/ 10.1007/s11745-004-1333-0; PMID: 15726822 [DOI] [PubMed] [Google Scholar]
- 22.Hannah MA, Wiese D, Freund S, Fiehn O, Heyer AG, Hincha DK. . Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol 2006; 142:98 - 112; http://dx.doi.org/ 10.1104/pp.106.081141; PMID: 16844837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. . Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol 2004; 136:4159 - 68; http://dx.doi.org/ 10.1104/pp.104.052142; PMID: 15557093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook D, Fowler S, Fiehn O, Thomashow MF. . A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc Natl Acad Sci U S A 2004; 101:15243 - 8; http://dx.doi.org/ 10.1073/pnas.0406069101; PMID: 15383661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzin V, Galili G. . New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol Plant 2010; 3:956 - 72; http://dx.doi.org/ 10.1093/mp/ssq048; PMID: 20817774 [DOI] [PubMed] [Google Scholar]
- 26.Agati G, Azzarello E, Pollastri S, Tattini M. . Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 2012; 196:67 - 76; http://dx.doi.org/ 10.1016/j.plantsci.2012.07.014; PMID: 23017900 [DOI] [PubMed] [Google Scholar]
- 27.Holländer-Czytko H, Grabowski J, Sandorf I, Weckermann K, Weiler EW. . Tocopherol content and activities of tyrosine aminotransferase and cystine lyase in Arabidopsis under stress conditions. J Plant Physiol 2005; 162:767 - 70; http://dx.doi.org/ 10.1016/j.jplph.2005.04.019; PMID: 16008101 [DOI] [PubMed] [Google Scholar]
- 28.Fernando M, Brunetti C, Fini A, Tattini M. Abiotic stress responses in plants: metabolism, productivity and sustainability. New York: Springer; c2012. Chapter 9, Flavonoids as antioxidants in plants under abiotic stresses; p. 159-179. [Google Scholar]
- 29.Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner H-Y, Hunt MD. . Systemic acquired resistance. Plant Cell 1996; 8:1809 - 19; http://dx.doi.org/ 10.1105/tpc.8.10.1809; PMID: 12239363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. . Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001; 414:562 - 5; http://dx.doi.org/ 10.1038/35107108; PMID: 11734859 [DOI] [PubMed] [Google Scholar]
- 31.Vermerris W, Nicholson R. Phenolic compound biochemistry. The Netherlands: Springer; c2008. [Google Scholar]
- 32.Rho H-S, Jeon J, Lee Y-H. . Phospholipase C-mediated calcium signalling is required for fungal development and pathogenicity in Magnaporthe oryzae.. Mol Plant Pathol 2009; 10:337 - 46; http://dx.doi.org/ 10.1111/j.1364-3703.2009.00536.x; PMID: 19400837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. . Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009; 81:6656 - 67; http://dx.doi.org/ 10.1021/ac901536h; PMID: 19624122 [DOI] [PubMed] [Google Scholar]
- 34.Oliver MJ, Guo L, Alexander DC, Ryals JA, Wone BWM, Cushman JC. . A sister group contrast using untargeted global metabolomic analysis delineates the biochemical regulation underlying desiccation tolerance in Sporobolus stapfianus. Plant Cell 2011; 23:1231 - 48; http://dx.doi.org/ 10.1105/tpc.110.082800; PMID: 21467579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohli Y, Kohn LM. . Random association among alleles in clonal populations of Sclerotinia sclerotiorum.. Fungal Genet Biol 1998; 23:139 - 49; http://dx.doi.org/ 10.1006/fgbi.1997.1026; PMID: 9578627 [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Buchwaldt L, Rimmer SR, Sharpe A, McGregor L, Bekkaoui D, Hegedus D. . Patterns of differential gene expression in Brassica napus cultivars infected with Sclerotinia sclerotiorum. Mol Plant Pathol 2009; 10:635 - 49; http://dx.doi.org/ 10.1111/j.1364-3703.2009.00558.x; PMID: 19694954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pokotylo I, Kolesnikov Y, Kravets V, Zachowski A, Ruelland E. . Plant phosphoinositide-dependent phospholipases C: variations around a canonical theme. Biochimie 2014; 96:144 - 57; http://dx.doi.org/ 10.1016/j.biochi.2013.07.004; PMID: 23856562 [DOI] [PubMed] [Google Scholar]
- 38.Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K. . Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 2002; 29:417 - 26; http://dx.doi.org/ 10.1046/j.0960-7412.2001.01227.x; PMID: 11846875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.