Abstract
Glutathione peroxidases (GPXs) are major family of the reactive oxygen species (ROS) scavenging enzymes. Recently, database analysis of the Arabidopsis genome revealed a new open-reading frame, thus increasing the total number of AtGPX gene family to eight (AtGPX1–8). The effect of plant hormones like; i. e. salicylic acid (SA), jasmonic acid (JA), abscisic acid (ABA), indoleacetic acid (IAA), and mannitol on the expression of the genes confirm that the AtGPX genes family is regulated by multiple signaling pathways. The survival rate of AtGPX8 knockout plants (KO8) was significantly decreased under heat stress compared with the wild type. Moreover, the content of malondialdehyde (MDA) and protein oxidation was significantly increased in the KO8 plant cells under heat stress. Results indicating that the deficiency of AtGPX8 accelerates the progression of oxidative stress in KO8 plants. On the other hand, the overexpression of AtGPX8 in E. coli cells enhance the growth of the recombinant enzyme on media supplemented with 0.2 mM cumene hydroperoxide, 0.3 mM H2O2 or 600 mM NaCl.
Keywords: Arabidopsis ATGPX8, heat stress, E. coli, recombinant enzyme, oxidative stress
Introduction
Aerobic organisms require protection from reactive oxygen species (ROS), which are generated continually as byproducts of cellular metabolism.1 Such protection important during exposure to stressors that may promote ROS production. These include oxidative, biotic and abiotic stresses.2-4 Environmental stress is the major limiting factor for plant productivity. Much of the damage to plants imposed by stresses exposure is related to oxidative damage at the cellular level.5 Hence, to increase tolerance against environmental stresses, it is important to enhance ROS scavenging capacity by introducing the corresponding enzymes at suitable levels.5 During more difficult and continued stress conditions an unscavenged accumulation of ROS may occur, which would cause several damages to the cells, including membrane and protein modifications.5 An improved level of lipid and protein peroxidation and activation of antioxidant apparatus indicate the existence of oxidative stress in several plant species.5 To overcome the formation of ROS, plants are equipped with enzymatic and non-enzymatic ROS scavenging systems.6-8 Enzymatic antioxidant systems rely on superoxide dismutase, ascorbate peroxidase, glutathione reductase, thioredoxin peroxidase and glutathione peroxidase (GPX). Non-enzymatic antioxidants, such as ascorbate, glutathione (GSH), a-tocopherol, pigments and phenolic components, prevent cascades of uncontrolled oxidation of cellular compounds.9,10
GPXs are the key enzymes involved in scavenging ROS in animals. Mammalian glutathione peroxidase family is divided into 8 classes based on their primary sequence, substrate specificity, and subcellular localization.11-13 Members in the family could be divided into two groups; the first group (GPX1 to GPX4) has selenium-dependent glutathione peroxidase activity and contains the amino acid selenocysteine (SeC) in their primary sequence, whereas the other group (GPX5 to GPX8) is without selenoenzymes activity because their primary sequence contains cysteine as active site instead of SeC.13,14
In plants, GPX genes are responsive to abiotic stress,15,17 hormone treatments,16 pathogens,18 wounding,19 and immune response.20 Plants GPXs catalyze the reduction of H2O2 as well as different kinds of lipid peroxides by using GSH or thioredoxin as an electron donor.17,21 In Arabidopsis, GPXs comprise of eight isozymes (AtGPX1–8) that defend cells against oxidative damage produced by ROS.17 The assumed subcellular localizations of AtGPX1–7 proteins are the cytosol, chloroplast, mitochondria, and endoplasmic reticulum.16 Recently, it was found that the steady-state transcript and protein levels of AtGPX8 in wild type cells were increased under high-light intensity or with the application of paraquat.17 Interestingly, AtGPX8 is localized in cytosol and nucleus, which plays a significant role in protecting cellular components, particularly nuclear DNA, against oxidative damage.17 Results conclude that AtGPX8 is the first GPX protein in plant cells that is localized in the nucleus and plays an important role in modulating cell survival and protecting it from ROS.
The present research is an attempt to elucidate the expression of all members of the gene family of Arabidopsis GPXs in response to different plant hormones. Moreover, to study the function of AtGPX8 protein under heat stress by studying the effect of the deficiency of AtGPX8 protein on the lipid hydroperoxide content, protein peroxidation and survival rate under normal and heat stress condition, in the wild type and AtGPX8 mutant cells. Furthermore, to study the role of AtGPX8 in the protection of E. coli cells against salt and oxidative stresses by overexpressing the gene in E. coli DH5α strain.
Results and Discussion
Expression of Arabidopsis AtGPX1–8 gene family in response to different plant hormones
The function of plant hormones in the complex biotic and abiotic stress relationship is not sufficiently elucidated. A lot of data relating to higher plants confirm that abscisic acid (ABA) as a plant hormone plays a role to adapt for different kinds of stress.22 According to Jiang and Zhang (2001),23 exogenous application of ABA increases superoxide radical and H2O2 levels. An increase in the activity of the antioxidant enzymes superoxide dismutase, catalse and ascorbate peroxidase was also reported.23 Agrawal et. al.24 reported that the regulation of rice GPX gene is correlated with plant hormones.24 Additionally, jasmonic acid (JA) and its methyl ester as an important molecules of the lipoxygenase signaling pathway mediating the defense response to infection.25 Also, indoleacetic acid (IAA) and ABA are involved in the regulation of plant response to salinity stress and in preventing the unfavorable effect of stress.26 Moreover, salicylic acid (SA) was descript as a protective factor for plants under different abiotic nature,27,28 where as SA increased the resistance of wheat seedlings to salinity27 and water deficit.28
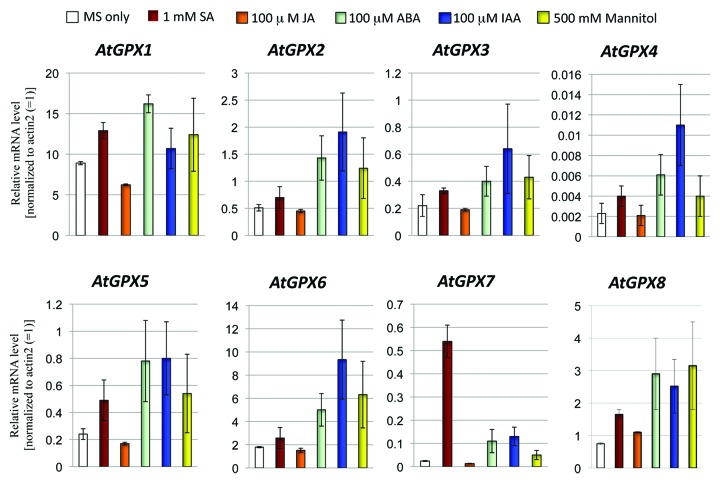
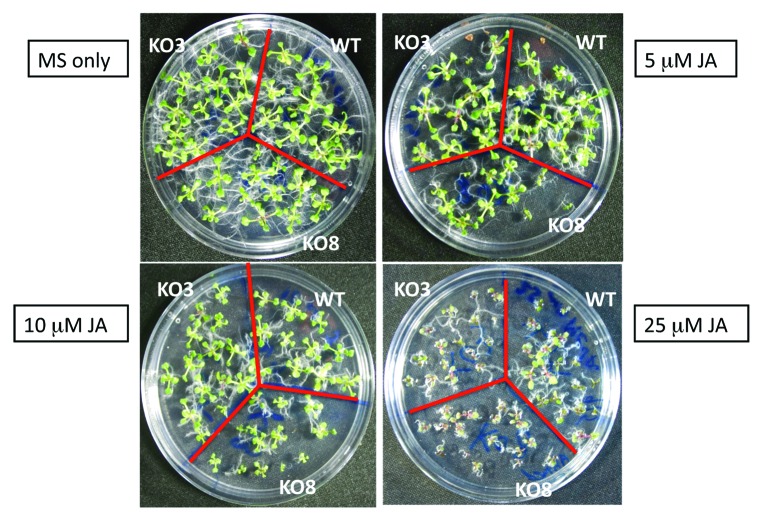
In the present study, the effects of plant hormones on the expression of the AtGPX1–8 genes were investigated. The steady-state transcripts level of AtGPX genes family isolated from 10-d-old seedlings treated with 1 mM SA, 1 mM JA, 0.1 mM ABA, 0.1 mM IAA, and 500 mM mannitol were investigated using quantitative PCR method (Fig. 1). Obviously, the transcriptional levels of AtGPX8 were increased under different treatments used in this study (Fig. 1). An upregulation of AtGPX1 was observed in seedlings treated only with SA and ABA. While, IAA and mannitol treatments resulted in a slight upregulation the expression level of AtGPX1 (Fig. 1). ATGPX2, 3, 4, 5, 6, and AtGPX7 transcripts were increased under the treated of SA, ABA, IAA, and mannitol (Fig. 1). Interestingly, treatment with JA increased the expression level of the AtGPX8 as compared with control seedlings (Fig. 1). In agreement to this result, KO8 mutant plants showed an increase in sensitivity of the growth under high concentration of JA (10 mM and 25 mM) comparing to wild type and KO3 mutant plant cells (Fig. 2). The transcripts level of AtGPX1, AtGPX5, and AtGPX7 were downregulated when treated with 1 mM JA. On the other hand, the transcript levels of AtGPX2, AtGPX3, AtGPX4 and AtGPX6 genes did not showed any changes under the same level of treatment with JA (Fig. 1). In a conclusion, all treatments resulted in enhancing AtGPX8 transcript level. Same results were reported by Agrawal et al. (2002)24 studying rice GPX gene. Milla et al. (2003)16 elucidated that the transcript level of AtGPX6 gene was upregulated under the treatments of SA, JA, ABA, and IAA. On the other hand, this study showed that IAA, ABA and mannitol were the main hormones to enhance transcript levels of AtGPX8. Results designate that AtGPX genes family reacts in different way to plant hormones and indicating a link to disparity regulation of transcripts under stress conditions. These data was in agreement with Dong (1998)29 that reported SA, JA, and ABA, are identified as inducers for the expression of stress defense genes in plants and each hormones using a signal transduction pathway.
Figure 1. The effects of various plant hormones on the steady-state transcript level of AtGPX1–8 gene family. Detailed conditions for experiments in RT-PCR are described in Materials and Methods.
Figure 2. Phenotype of the wild type, AtGPX3 knockout mutant (KO3), and AtGPX8 knockout mutant (KO8) seedlings as affected by different concentration of jasmonic acid (JA).
Deficiency of AtGPX8 protein enhanced sensitivity to oxidative stress under heat stress condition
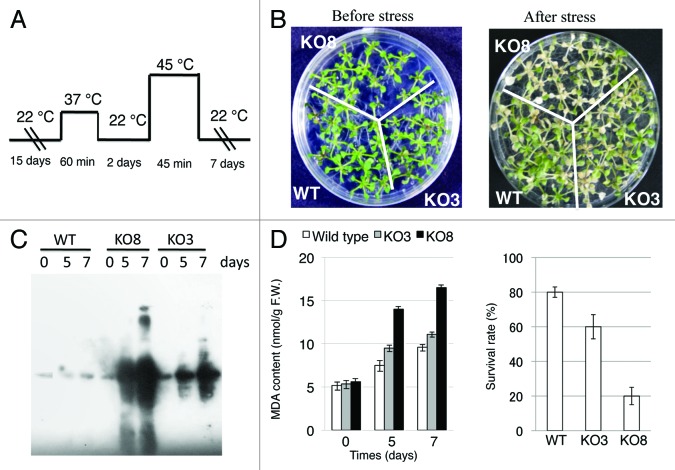
Different studies showed that the use of knockout mutant lines is a useful way to study the physiological roles of the genes in vivo.17,30,31 AtGPX3 and AtGPX8 mutants containing a T-DNA insert in both genes (KO3, SALK_071176 and KO8, SALK_127691) were obtained from the SIGnAL project (signal.salk.edu/tabout.html). T-DNA insertion sites of the homozygous AtGPX3 mutant and AtGPX8 mutant are in the first exon region of the AtGPX3 gene (At2G43350), and in the fifth exon of the AtGPX8 gene (At1G63460).30 Previously, Miao et al. (2006)31 reported that AtGPX3 can play a double function with Arabidopsis plants, initially in the control of H2O2 homeostasis, as well as in the transduction of an H2O2 signal in response to abscisic acid (ABA) and drought stress. Moreover, previous work showed that AtGPX8 play an important role in the suppression of oxidative damage in the nucleus and cytosol of Arabidopsis plants.17 This work was performed in an attempt to examine the contribution of AtGPX3 and AtGPX8 to scavenge ROS generated in Arabidopsis cells under heat stress conditions. The acclimation of the acquired thermotolerance treatment increased survival rates in wild type as well as in the KO3 mutant plants. However, the survival rate of KO8 mutant was 3 and 4-fold lower than that of KO3 mutant and wild type plants, respectively (Fig. 3D). Also, the level of membrane phospholipid peroxidation (MDA product) in wild type, KO3 and KO8 mutant plants was measured. The MDA accumulation was markedly increased in KO8 mutant plants comparing to wild type and KO3 mutant plants (Fig. 3D). These findings indicated that loss of AtGPX8 function in KO8 mutant spoils cell redox homeostasis and that AtGPX8 has an important physiological role in the defense of Arabidopsis cells against peroxidative injury.
Figure 3. The sensitivity of wild type, KO3, and KO8 mutant lines to heat stress. (A) The time scale of the heat stress. (B) Phenotype of the seedlings before and after 7 d from the stress. (C) Immuno-blotting analysis of oxidized proteins during heat stress conditions. (D) Lipid hydroperoxide content and survival rate after 7 d from the stress in the wild type and mutant cells.
Protein carbonylation is an irreparable oxidative process leading to loss-of-function and often to the proteolysis of the modified protein.32 The question about the role of protein oxidation plays is rising as a recent tool to analyzing oxidative stress and damage caused to cells during disease or different abiotic stresses.33 To study whether the accumulation of ROS in leaves of the KO3 and KO8 mutants causes oxidative damage at the cellular level, oxidized proteins in total protein extracts from leaves were analyzed using a protein gel blot assay. The KO8 mutant subject to heat stress accumulated high levels of oxidized proteins (Fig. 3C), compared with that accumulated by the KO3 and wild type plants (Fig. 3C). Study of protein oxidation during stress is mainly a powerful method since it can identify the damage of oxidized proteins in the specific mutants. Also, such study can correlate the enzymatic activities in mutant cells and oxidative injuries at the cellular level. Therefore, high level of oxidized proteins in KO8 mutant than that of the KO3 mutant and wild type cells provides direct evidence for the importance of AtGPX8 protein as oxidative scavenging enzyme for both the nucleus and the cytosol of the Arabidopsis plants (Fig. 3). The idea that each compartment in the cell is protected by its own set of ROS scavenging enzymes should therefore be reconsidered and a new examination of the ROS network should be accepted as an extremely interlinked system that needs the synchronized function of ROS scavenging pathways from different cellular parts to adapt the level of ROS in cells, to avoid cellular damage, and to control ROS signaling.
AtGPX8 is sufficient for increased tolerance to salinity and oxidative stress in E. coli cells
Classical strain engineering approaches are usually both time-consuming and labor intensive. Thus, the coding region of the AtGPX8 gene was amplified by PCR and inserted into the NdeI/BamHI sites of the vector pColdII. The recombinant plasmid with the right reading frame was then prove by DNA sequencing (data not shown). The resulting constructions designated pColdII/GPX8 was used to transform the E. coli strain DH5α in an exponentially growing culture according to the procedure described in the Experimental procedures. The enzymatic properties of AtGPX8 as a recombinant protein in E. coli were previously investigated.17 It was found that the recombinant protein could reduce H2O2, alkyl hydroperoxide and unsaturated fatty acid hydroperoxides using thioredoxin as an electron donor.17 The high degree of amino acids homology of all AtGPXs,17,30 almost 65%, raise the idea to study the cross-reactivity of AtGPX8 antibody with other types of AtGPX1, 2, 5, and -6. Interestingly, previous work showed that AtGPX8 antibody has a unique cross-reactivity only with the recombinant AtGPX8 and there is no reaction occurred with the others AtGPXs (Fig. 4). AtGPX6 mouse antiserum has a wide range of cross-reactivity with AtGPX1, 2, 5 and -6 and a lower reactivity with AtGPX8 (Fig. 4). These data clearly indicated that there are widely shared epitopes in AtGPX1, 2, 5, 6, and 8 proteins which can be recognized by AtGPX6 antibody. However, there is a unique epitope in AtGPX8 protein that was only recognized by AtGPX8 antibody and this epitope is distinct from the widely shared epitopes in other AtGPXs.
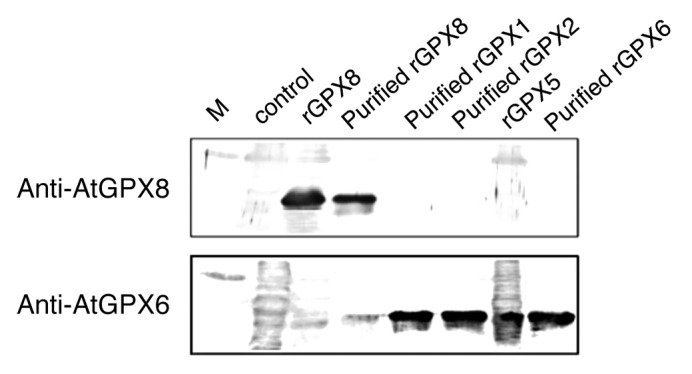
Figure 4. Western blot analysis of recombinant AtGPX1, 2, 3, 5, 6, and 8. Total soluble proteins were subjected to SDS-PAGE on 15% (w/v) gel, following the electrophoresis, proteins were transferred to PVDF membrane and AtGPXs proteins detected by polyclonal antibody raised by AtGPX8 (upper frame) or AtGPX6 (lower frame).
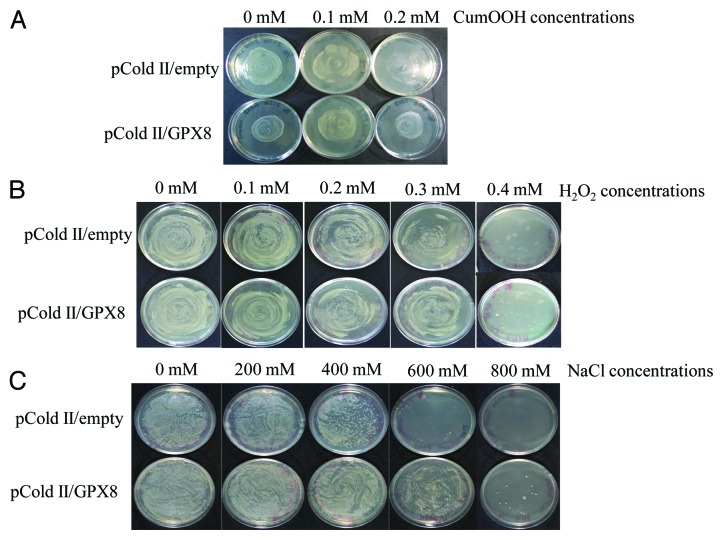
Oxidative damage to microbial hosts often occurs under stressful conditions during bioprocess. So, it has been questioned whether the hydroperoxidase activity of AtGPX8 enzyme plays an important functional role in organisms, and/or whether such activity may be insignificant relative to the regulatory and other functions that GPXs are now known to drive. The pColdII/empty transformed E. coli strain DH5α was sensitive to cumene hydroperoxide (CumOOH), H2O2, and to NaCl. The growth was almost eliminated in the presence of 0.2 mM CumOOH, 0.3 mM H2O2, or 600 mM NaCl (Fig. 5). By contrast, the expression of AtGPX8 by pColdII/GPX8 transformed could favor the growth of the E. coli strain DH5α on the media supplemented with the same concentration of CumOOH, H2O2 and NaCl, showing that the growth was somehow better than those of the pColdII/empty transformed E. coli cells (Fig. 5). Consequently, The survival test of recombinant E. coli cells under high concentration of NaCl, Cumen Hydroperoxide and H2O2 revealed that AtGPX8 might function as an antioxidant to protect cell membranes against hydroperoxide damage (Fig. 5). These results are in agreement with the complementation effect of yeast ScPHGPx3 and radish RsPHGPx in the same case,34,35 suggesting that AtGPX8 might detoxify the lipid peroxides resulting from the autooxidation of exogenous lipid hydroperoxides. These results clearly indicated that AtGPX8 plays an important physiological role to remove ROS from E. coli cells.
Figure 5. Effect of different concentrations of Cumene hydroperoxid (A), H2O2 (B), and NaCl (C) on the growth of E. coli wild type (pcoldII-empty) and recombinant (pColdII-GPX8) cells. Detailed conditions for experiments are described in Materials and Methods.
Materials and Methods
Plant materials and growth condition
Wild type Arabidopsis thaliana (ecotype Columbia), KO3, and KO8 mutant plants were used. Seeds of the T-DNA insertion in the AtGPX3 (SALK_071176) and AtGPX8 (SALK_127691) were kindly obtained from the ABRC (Ohio State University) as pure homozygous lines. The homozygosis of the KO3 and KO8 mutant lines was confirmed using RT-PCR as previously described.30 Seeds were surface sterilized by soaking in 95% ethanol for five min and 50% bleach for 5 min, and rinsed five times each for 2 min with sterilized water. Unless specified treated, seeds were plated on MS medium supplemented with 3% sucrose and the plates were incubated in dark at 4° C for two days to harmonize germination. Then, seedlings were incubated in the growth chamber at 22° C ± 2° C with 16 h light under white fluorescent light (of approximately 75–100 µE m−2 s−1). Heat stress was introduced by exposing two-week-old seedlings to 37° C for one hour and then keeping them for one day under normal conditions. Next, Seedlings were exposed to 45° C for 45 min and then were transferred to normal conditions and were kept for 7 d more. To test the effect of the induction of AtGPX1–8 genes by plant hormones, seedlings were grown under the same conditions described above for 10 d and then were treated with 1 mM SA, 0.1 mM JA, ABA and IAA and finally with 500 mM Mannitol for 12 h. For RNA isolation, tissues were squashed in liquid nitrogen and then were stored at -80° C for RNA extraction.
Analysis of lipid peroxidation
Lipid hydroperoxide content was analyzed by calculating malondialdehyde (MDA) using the 2-thiobarbituric acid (TBA) with a method compatible with the procedure described previously.30 An aliquot of cell extract (500 µl) was treated with 500 µl of 10% (W/V) trichloroacetic acid (TCA) and 1.0 g L−1 ethanolic butylated hydroxytoluene for deproteinization and was then centrifuged at 15,000 × g for 5 min at 4° C. The obtained supernatants were boiled for 30 min and then were reacted with TBA. MDA values were estimated by measuring A515 to A553 and using a molar absorption coefficient of 1.56 × 105.36
Detection of oxidized protein
Oxidized proteins were investigated using a protein gel-blot-assay according to Davletova et al. (2005)37. Briefly, Oxidized proteins were acquired by homogenizing 250 mg of leaves in liquid nitrogen. The crush was suspended in 1 mL of extraction buffer [25 mM Tris-Cl, pH 8, 0.1% Triton X-100, 50 mM DTT, and the protease inhibitors leupeptin (0.5 mg/mL), trypsin inhibitor (0.5 mg/mL), and PMSF (40 mg/mL)]. After centrifugation, the supernatant was stored at -80° C until examination.
Preparation of polyclonal antibodies against AtGPX8
The purified recombinant AtGPX8 protein was used to prepare polyclonal antibody. The purification procedures for the enzyme was performed as previously reported.17,30 The purified enzyme (200 µg) was suspended in 50 mM TRIS-HCl, pH 8.2, and emulsified as 1:1 (v/v) mixture with incomplete Freund’s adjuvant. One ml was injected into white male mice (6 weeks old) and a second injection was performed after additional month. One week after the second injection, blood was gathered from the tail vein of each mouse and the antiserum was separated from the blood. Western blotting tested this antiserum, and the high set titer mouse was given a final injection. One week after the final injection, blood was collected from the orbital vein of each mouse and serum was separated from the blood.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from the rosette leaves of Arabidopsis seedlings (1.0 g FW) using Sepasol reagent (Nacalai tesque) according to the manufacturer's instructions. First strand cDNA was produced using ReverTra Ace (Toyobo, JAPAN) with an oligo(dT)20 primer according to the manufacturer’s procedure. Real-time PCR (RT–PCR) was performed according to Nishizawa et al. (2006)38 using an ABI 7300 Real Time machine (Applied Biosystems). Primer pairs for the quantitative RT–PCR were calculated using Primer ExpressTM software (Applied Biosystems), and the primer sequences were previously described.17 The housekeeping gene of Actin2 was used as internal control in all experiments to normalize the variability in the expression levels.
SDS-PAGE and immunoblotting
Cell extracts were mixed with SDS loading buffer (150 mM TRIS-HCl, pH 6.8, 4% SDS, and 10% 2β-mercaptoethanol). The homogenates were boiled for 5 min and then were centrifuged at 10,000 × g for 5 min at 4° C. The supernatants were analyzed to determine their protein contents and subjected to SDS-PAGE and immuno-blotting. SDS-PAGE was performed in 15% slab gels according to the method of Laemmli (1970).39 The proteins on the gel were stained with Coomassie brilliant blue R-250. The proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA) using a semidry electroblotting system (model 200/2.0, Bio-Rad) at 13 V for 1 h. The membrane was then incubated with polyclonal antibody raised against AtGPX8 protein. The immunocomplexes on the membrane were visualized with horseradish peroxidase-conjugated goat anti-mouse IgG (Bio-Rad).
Assay of oxidative stress tolerance in E. coli cells
E. coli DH5α cells harboring pColdII/GPX8 or pColdII/empty were grown in 50 ml of LB medium containing 50 µg ml−1 of ampicillin and then cells were then transferred and kept for 30 min at 16° C without shaking and the fusion protein expression was induced by the addition of 0.4 mM IPTG. Cells were further incubated for 24 h at 16° C with shaking. Cells were harvested by centrifugation at 3,000 g for 10 min, resuspended in 5 ml of LB medium. Then, the cells were spread on LB medium containing different concentrations of H2O2 (0, 0.1, 0.2, 0.3, and 0.4 mM), of Cumene hydroperoxide (0, 0.1, and 0.2 mM) or of NaCl (0, 200, 400, 600, and 800 mM) and were grown overnight at 37° C.
Glossary
Abbreviations:
- ABA
abscisic acid
- APX
- CumOOH
cumene hydroperoxide
- GPX
glutathione peroxidase
- GSH
reduced glutathione
- IAA
indoleacetic acid
- JA
jasmonic acid
- MDA
malondialdehyde
- ROS
reactive oxygen species
- RT-PCR
reverse transcription-PCR
- SA
salicylic acid
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
I would like to express my thanks and appreciation to Prof Dr Shigeru Shigeoka, Department of Advanced Bioscience, Faculty of Agriculture, Kinki University, 3327–204 Nakamachi, Nara, Japan, for providing the knockout mutant plants, and chemicals. Moreover, most of this work was accomplished in his laboratory. This research was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. P08448) of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, 3rd Ed., Oxford University Press, Oxford 1999. [Google Scholar]
- 2.Prasad TK, Anderson MD, Martin BA, Stewart CR. . Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 1994; 6:65 - 74; PMID: 12244221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H. . A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 1999; 11:1195 - 206; PMID: 10402422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F. . The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 2002; 53:1367 - 76; http://dx.doi.org/ 10.1093/jexbot/53.372.1367; PMID: 11997382 [DOI] [PubMed] [Google Scholar]
- 5.Foyer CH, Noctor G. . Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 2009; 11:861 - 905; http://dx.doi.org/ 10.1089/ars.2008.2177; PMID: 19239350 [DOI] [PubMed] [Google Scholar]
- 6.Foyer CH, Halliwell B. . The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 1976; 133:21 - 5; http://dx.doi.org/ 10.1007/BF00386001 [DOI] [PubMed] [Google Scholar]
- 7.Foyer CH, Lelandais M, Kunert KJ. . Photooxidative stress in plants. Physiol Plant 1994; 92:696 - 717; http://dx.doi.org/ 10.1111/j.1399-3054.1994.tb03042.x [DOI] [Google Scholar]
- 8.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. . Reactive oxygen gene network of plants. Trends Plant Sci 2004; 9:490 - 8; http://dx.doi.org/ 10.1016/j.tplants.2004.08.009; PMID: 15465684 [DOI] [PubMed] [Google Scholar]
- 9.Asada K. Production and action of active oxygen species in photosynthetic tissues. In Causes of photooxidative stress and amelioration of defense systems in plants; Foyer CH and Mullineaux PM, eds (CRC Press) 1994; 77-104. [Google Scholar]
- 10.Noctor G, Foyer CH. . Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 1998; 49:249 - 79; http://dx.doi.org/ 10.1146/annurev.arplant.49.1.249; PMID: 15012235 [DOI] [PubMed] [Google Scholar]
- 11.Drevet JR. . The antioxidant glutathione peroxidase family and spermatozoa: a complex story. Mol Cell Endocrinol 2006; 250:70 - 9; http://dx.doi.org/ 10.1016/j.mce.2005.12.027; PMID: 16427183 [DOI] [PubMed] [Google Scholar]
- 12.Chabory E, Damon C, Lenoir A, Kauselmann G, Kern H, Zevnik B, Garrel C, Saez F, Cadet R, Henry-Berger J, et al. . Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J Clin Invest 2009; 119:2074 - 85; PMID: 19546506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabory E, Damon C, Lenoir A, Henry-Berger J, Vernet P, Cadet R, Saez F, Drevet JR. . Mammalian glutathione peroxidases control acquisition and maintenance of spermatozoa integrity. J Anim Sci 2010; 88:1321 - 31; http://dx.doi.org/ 10.2527/jas.2009-2583; PMID: 20042549 [DOI] [PubMed] [Google Scholar]
- 14.Ghyselinck NB, Dufaure JP. . A mouse cDNA sequence for epididymal androgen-regulated proteins related to glutathione peroxidase. Nucleic Acids Res 1990; 18:7144; http://dx.doi.org/ 10.1093/nar/18.23.7144; PMID: 2263479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaber A, Yoshimura K, Tamoi M, Takeda T, Nakano Y, Shigeoka S. . Induction and functional analysis of two reduced nicotinamide adenine dinucleotide phosphate-dependent glutathione peroxidase-like proteins in Synechocystis PCC 6803 during the progression of oxidative stress. Plant Physiol 2004; 136:2855 - 61; http://dx.doi.org/ 10.1104/pp.104.044842; PMID: 15347790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez Milla MA, Maurer A, Rodriguez Huete A, Gustafson JP. . Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signaling pathways. Plant J 2003; 36:602 - 15; http://dx.doi.org/ 10.1046/j.1365-313X.2003.01901.x; PMID: 14617062 [DOI] [PubMed] [Google Scholar]
- 17.Gaber A, Ogata T, Maruta T, Yoshimura K, Tamoi M, Shigeoka S. . The involvement of Arabidopsis glutathione peroxidase 8 in the suppression of oxidative damage in the nucleus and cytosol. Plant Cell Physiol 2012; 53:1596 - 606; http://dx.doi.org/ 10.1093/pcp/pcs100; PMID: 22773682 [DOI] [PubMed] [Google Scholar]
- 18.Criqui MC, Jamet E, Parmentier Y, Marbach J, Durr A, Fleck J. . Isolation and characterization of a plant cDNA showing homology to animal glutathione peroxidases. Plant Mol Biol 1992; 18:623 - 7; http://dx.doi.org/ 10.1007/BF00040684; PMID: 1536938 [DOI] [PubMed] [Google Scholar]
- 19.Depège N, Drevet J, Boyer N. . Molecular cloning and characterization of tomato cDNAs encoding glutathione peroxidase-like proteins. Eur J Biochem 1998; 253:445 - 51; http://dx.doi.org/ 10.1046/j.1432-1327.1998.2530445.x; PMID: 9654095 [DOI] [PubMed] [Google Scholar]
- 20.Chang CCC, Slesak I, Jordá L, Sotnikov A, Melzer M, Miszalski Z, Mullineaux PM, Parker JE, Karpinska B, Karpinski S. . Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol 2009; 150:670 - 83; http://dx.doi.org/ 10.1104/pp.109.135566; PMID: 19363092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eshdat Y, Holland D, Faltin Z, Ben-Hayyim G. . Plant glutathione peroxidases. Physiol Plant 1997; 100:234 - 40; http://dx.doi.org/ 10.1111/j.1399-3054.1997.tb04779.x [DOI] [Google Scholar]
- 22.Palva ET, Thtiharju S, Tamminen I, Puhakainen T, Laitinen R, Svensson J, Helenius E, Heino P. . Biological mechanisms of low temperature stress response: Cold acclimation and development of freezing tolerance in plants. JIRCAS Working Report 2002; 9 - 15 [Google Scholar]
- 23.Jiang M, Zhang J. . Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 2001; 42:1265 - 73; http://dx.doi.org/ 10.1093/pcp/pce162; PMID: 11726712 [DOI] [PubMed] [Google Scholar]
- 24.Agrawal GK, Rakwal R, Jwa N-S, Agrawal VP. . Effects of signaling molecules, protein phosphatase inhibitors and blast pathogen (Magnaporthe grisea) on the mRNA level of a rice (Oryza sativa L.) phospholipid hydroperoxide glutathione peroxidase (OsPHGPX) gene in seedling leaves. Gene 2002; 283:227 - 36; http://dx.doi.org/ 10.1016/S0378-1119(01)00854-X; PMID: 11867229 [DOI] [PubMed] [Google Scholar]
- 25.Czerpak R, Piotrowska A, Szleska K. . Jasmonic acid affects changes in the growth and some components content in alga Chlorella vulgaris.. Acta Physiol Plant 2006; 28:195 - 203; http://dx.doi.org/ 10.1007/BF02706531 [DOI] [Google Scholar]
- 26.Hamdia MA, Barakat N. . The response of lupine to exogenous application GA3 or ABA under salt stress conditions. J Union Arab Biol Cairo 1999; 99:209 - 20 [Google Scholar]
- 27.Shakirova FM, Bezrukova MV. . Induction of wheat resistance against environmental salinization by salicylic acid. Biol Bull 1997; 24:109 - 12 [Google Scholar]
- 28.Bezrukova MV, Sakhabutdinova R, Fatkhutdinova RA, Kyldiarova I, Shakirova F. . The role of hormonal changes in protective action of salicylic acid on growth of wheat seedlings under water deficit. Agrochemiya (Russ) 2001; 2:51 - 4 [Google Scholar]
- 29.Dong X. . SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol 1998; 1:316 - 23; http://dx.doi.org/ 10.1016/1369-5266(88)80053-0; PMID: 10066607 [DOI] [PubMed] [Google Scholar]
- 30.Gaber A. . Arabidopsis glutathione peroxidase 8 is a key enzyme in response to environmental stresses. Arab J Biotechnol 2011; 14:213 - 24 [Google Scholar]
- 31.Miao Y, Lv D, Wang P, Wang X-C, Chen J, Miao C, Song C-P. . An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 2006; 18:2749 - 66; http://dx.doi.org/ 10.1105/tpc.106.044230; PMID: 16998070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stadtman ER. . Protein oxidation and aging. Science 1992; 257:1220 - 4; http://dx.doi.org/ 10.1126/science.1355616; PMID: 1355616 [DOI] [PubMed] [Google Scholar]
- 33.Rizhsky L, Davletova S, Liang H, Mittler R. . The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis.. J Biol Chem 2004; 279:11736 - 43; http://dx.doi.org/ 10.1074/jbc.M313350200; PMID: 14722088 [DOI] [PubMed] [Google Scholar]
- 34.Avery AM, Avery SV. . Saccharomyces cerevisiae expresses three phospholipid hydroperoxide glutathione peroxidases. J Biol Chem 2001; 276:33730 - 5; http://dx.doi.org/ 10.1074/jbc.M105672200; PMID: 11445588 [DOI] [PubMed] [Google Scholar]
- 35.Yang XD, Li WJ, Liu JY. . Isolation and characterization of a novel PHGPx gene in Raphanus sativus. Biochim Biophys Acta 2005; 1728:199 - 205; http://dx.doi.org/ 10.1016/j.bbaexp.2005.02.003; PMID: 15777688 [DOI] [PubMed] [Google Scholar]
- 36.Gueta-Dahan Y, Yaniv Z, Zilinskas BA, Ben-Hayyim G. . Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in citrus. Planta 1997; 203:460 - 9; http://dx.doi.org/ 10.1007/s004250050215; PMID: 9421931 [DOI] [PubMed] [Google Scholar]
- 37.Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. . Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis.. Plant Cell 2005; 17:268 - 81; http://dx.doi.org/ 10.1105/tpc.104.026971; PMID: 15608336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishizawa A, Yabuta Y, Yoshida E, Maruta T, Yoshimura K, Shigeoka S. . Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J 2006; 48:535 - 47; http://dx.doi.org/ 10.1111/j.1365-313X.2006.02889.x; PMID: 17059409 [DOI] [PubMed] [Google Scholar]
- 39.Laemmli UK. . Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227:680 - 5; http://dx.doi.org/ 10.1038/227680a0; PMID: 5432063 [DOI] [PubMed] [Google Scholar]