Abstract
As with any technological innovation, time refines the technology, improving upon the original version of the innovative product. The initial GM crops had single traits for either herbicide tolerance or insect resistance. Current varieties have both of these traits stacked together and in many cases other abiotic and biotic traits have also been stacked. This innovation requires investment. While this is relatively straight forward, certain conditions need to exist such that investments can be facilitated. The principle requirement for investment is that regulatory frameworks render consistent and timely decisions. If the certainty of regulatory outcomes weakens, the potential for changes in investment patterns increases.
This article provides a summary background to the leading plant breeding technologies that are either currently being used to develop new crop varieties or are in the pipeline to be applied to plant breeding within the next few years. Challenges for existing regulatory systems are highlighted. Utilizing an option value approach from investment literature, an assessment of uncertainty regarding the regulatory approval for these varying techniques is undertaken. This research highlights which technology development options have the greatest degree of uncertainty and hence, which ones might be expected to see an investment decline.
Keywords: barriers; biotechnology; innovation; investment uncertainty, policy, risk
Introduction
Regulations have been used by societies over time as a means to placing controls and restriction over innovations that triggered uncertainties within those societies. Coffee, train travel, and automobile use all provide examples of this.1 In much of Europe, regulations pertaining to the consumption of coffee banned the use of this product when it first arrived in the 1500s from the Middle East. Five hundred years later, Europe is once again faced with an innovation that governments perceive requires rigorous regulation, with some states going as far as banning the use of agricultural biotechnology. Countries such as Austria, France, and Italy have all banned the use of genetically modified (GM) crops in recent years.
These bans and other delays in the regulation of GM crops and food products in Europe are beginning to have an effect on investment into further research and development (R&D) pertaining to agricultural biotechnology. In January 2012, BASF announced that it was moving its research division from Europe to the Unites States due to the timeliness of regulatory decisions in Europe.2 In part, this decision is based on the fact that it took 13 years to receive approval for a BASF developed GM potato variety in Europe.3 An industry report prepared by Phillips McDougall4 identifies that the average number of months it takes for a GM event to receive regulatory approval in 2011 was 65 months, up from 44 months prior to 2002. If companies such as BASF are making R&D investment decisions based on the lower number of months estimated for regulatory approval, an important question for the ag-biotech industry is: How will investment patterns change if the time for regulatory approval increases even further?
The vast majority of regulatory decisions made to date regarding food/feed use, import, and commercial production, relate to the first generation of GM technologies, that is, single trait varieties. Examples of this would be herbicide tolerance (HT) or insect resistance (IR). These single trait varieties were developed in the late 1980s and early 1990s and commercialized in the mid-1990s. Stacked trait varieties, or second generation GM technologies, began to be approved shortly after this (i.e., varieties with both HT and IR), with their commercial use gaining prominence in 2001.5 Third generation technologies are now in the research pipeline with several of these expected to be ready for regulatory approval within the next few years.6 The application of genetic science to plant breeding continues to advance, with plant breeders using increasingly refined techniques to develop new varieties. Those regulatory agencies that struggled with (and still do in many jurisdictions) managing the regulation of first generation GM varieties will undoubtedly face daunting challenges regarding the regulation of new crop varieties developed by third generation technologies.
The importance of these technologies and investment in them was highlight in the 2012 Gates Foundation’s Annual Letter. Agricultural research is highlighted as one of the crucial areas that deserves greater attention both from increased public and private investment into research on new crop varieties and the techniques used to develop them. The letter observes that “…we can find out precisely which plant contains what gene conferring a specific characteristic. This will make plant breeding happen at a much faster clip.”7 Clearly, there is a global need to improve the investment into new breeding techniques that can provide the developing world with greater options regarding the production of food.
This article provides insights regarding the relationship between the length of time required for a technology development firm to receive a variety approval decision from the respective regulatory authority and the corporate decision to change their R&D investment that either kills the project or causes it to be relocated to a more “regulatory receptive” environment. A survey of the literature on returns to innovation investment allows us to establish a minimum return that can then be compared against the uncertainty of regulatory decision making. The remainder of the paper is structured as follows. Section 2 provides the background to the paper, with Section 3 giving an overview of the three leading innovative plant breeding techniques. Section 4 presents the methodology, while Section 5 provides the data analysis. Section 6 discusses the potential policy impacts that could be expected within various regulatory jurisdictions over the next few years. Section 7 offers some concluding thoughts.
Background
Innovations in agriculture established the criteria for the creation, establishment, and growth of civilizations and cultures.8 Without innovative methods and techniques to feed growing populations, the development and advancement of mankind would not have been possible. Modern day society is faced with a dilemma that is going to require considerable innovation and investment in agriculture: how to feed a global population of 9 billion by the year 2050. The severity of this dilemma is crystalized by the following quote from the Deputy-Director General of the Food and Agriculture Organization (FAO), “Agricultural production needs to increase by 70% worldwide, and by almost 100% in developing countries, in order to meet growing food demand.”9
The FAO10 reports that annual crop yields of 2% are needed to sustain the planet’s existing population. Current yield increases are 1.2% and have slowed considerably (Fig. 1). An earlier report by the FAO11 estimated that the number of people lacking food security in 2006 was 845 million. The report anticipated that there would be a one-third decline by 2015 to just under 600 million. The stark reality is that this figure has grown by over 40% and now stands at approximately 1.2 billion people.12

Figure 1. Growth rates of yields for major cereals, 1960–200010
Brookes et al.13 estimate that without the transgenic crops of canola, corn, and soybeans an additional 2.64 million hectares (6.5 million acres) of land would be needed to meet current production levels. Figure 2 estimates the increase in commodity prices without the use of transgenic crop varieties, using 2007 prices as a baseline. Increasing food security at a global level is dependent on continued investments in genomic breeding technologies and the successful commercialization of the resulting new crop varieties. While rice is not included in this analysis, the price of corn-based food products would rise nearly 6% and with corn-based food products serving as a staple in many developing world diets, further adding to the current food insecurity challenges faced by many developing world consumers.
Figure 2. Increase in world commodity prices with no biotech trait utilization13
Averaging the various commodity price increases across all the commodities listed in Figure 2, reveals that food prices would have increased 4.5% on average had it not been for the production of GM crops. Corn and soybeans are fractionated into a wide variety of processing uses prior to their use an animal feed and due to this widespread food utilization, reductions in corn and soybean production would have impacted all consumers. While the effects of food price increases in this range would have been minimal in industrialized nations, the effects in developing countries would have furthered food insecurity. Innovations in crop genomics contribute to achieving higher yields; therefore it is essential to gain greater insights into how the returns to innovative research affects the firms making those investments.
There have been advances in the estimation of returns to research that use econometrics to examine the effect of R&D investment on agricultural productivity.14-22 These studies imposed an assumed shape and length of adoption lag to calculate the returns. Some of the econometric studies have instead statistically estimated the shape and length of adoption lag and generally have found lower rates of return.23,24 Alston et al.25 also explicitly dealt with the concept of knowledge depreciation, which is not common in the agricultural R&D literature.
An examination of research investments and changes in productivity shows that the time-lag between investment and yield increases is lengthy, possibly reaching 20 years.26 The 1960s through to the end of the 1980s reflect a three decade long period with yield increases of greater than 2% in all but a few instances (Table 1). Developing world yields were well above 2% in all three crops for this period. According to Alston et al.,25 the decline in yield increases that begins to be observed in the 1990s is due to an earlier refocusing of investment strategies.
Table 1. Rates of growth of global average yields for selected crops (% per year)26.
| Crop | Maize | Wheat | Rice | |||
|---|---|---|---|---|---|---|
| Time period | 1961–89 | 1990–06 | 1961–89 | 1990–06 | 1961–89 | 1990–06 |
| Global average | 2.21 | 1.59 | 2.78 | 0.55 | 2.19 | 0.97 |
| Developing world | 2.53 | 1.92 | 3.76 | 1.43 | 2.34 | 1.01 |
| Developed world | 2.50 | 1.67 | 2.41 | -0.13 | 0.77 | 0.73 |
| Western Europe | 3.65 | 1.74 | 3.25 | 0.86 | 0.33 | 0.53 |
| Eastern Europe | 2.62 | 2.45 | 3.29 | -1.27 | -0.61 | 3.63 |
| North America | 2.20 | 1.43 | 1.58 | 0.19 | 1.87 | 1.35 |
Recent predictions regarding investment in primary agriculture is provided by Schmidhuber et al.27 As is illustrated in Figure 3, investment is expected to decline in all regions over the coming decades. If as Alston et al.,26 identify, there is a time lag between investment in R&D and the realization of marketplace benefits, possibly reaching 20 years then the importance of current investment decisions cannot be understated, especially if the impacts of not making these investments may not be fully realized until the 2030s.

Figure 3. Trend in primary agriculture investments27
By its very nature, investment requires a commitment of resources at a point in time with the benefits flowing in future time periods. This dynamic nature of investment precludes the simple comparison of benefits and costs. Specifically the time value of money must be considered in the appraisal of an investment. Fortunately methodologies for valuing investments are well developed and can be used to appraise the investment in biotechnology research. One of the common methods for evaluating investments is to measure the internal rate of return (IRR). The IRR of an investment or project is the discount rate at which the net present value of the future cash revenues and costs is equal to zero.
When economic theory and the literature of returns to research are juxtaposed with the rise of private investment (often for biotechnology-based effort) in the canola sector, Phillips28 estimated gains for research to yield an IRR between 20–95%. This figure may actually be larger for specific biotechnology-based developments because of the reduced cost of the research and the increased array of attributes that can be bred into the seed, which adds new value to consumers.
The uncertainty of investment decisions arises when the net cash flow from the investment is not known. In a thorough analysis, Dixit and Pindyck29 state that uncertainty combined with the irreversibility of an investment creates a value of the option to wait when undertaking large capital investments. The greater the degree of uncertainty, the higher the value of waiting, thus decreasing the investment of firms into projects. This traditional approach of waiting to invest is difficult to apply in a technology field, where there is significant advantage and pay-off to be the first to market.
Typically large capital investments (such as investment into the development of a new crop variety) share similar characteristics: (1) investment decisions and their associated cash outlays occur sequentially over time; (2) there is a maximum rate at which outlays and development can proceed; and (3) the project yields no cash return until it is completed.30A firm investing in R&D has all these factors to consider in their decision-making process when deciding to invest in a project. Agricultural biotechnology firms have an additional factor to consider, the effect of the timing of the cash flow from regulatory approval delays.
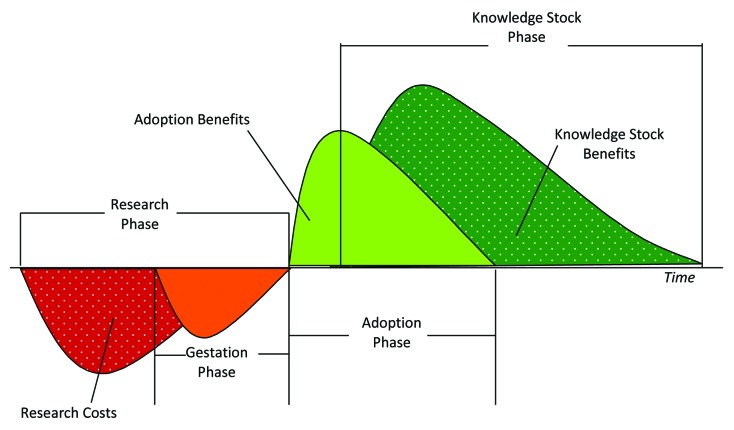
The process of creating new crop varieties can be described in four phases, as shown in Figure 4. During the first phase, or the research phase, research resources are spent to develop a crop variety that has commercially desirable characteristics. This production process depends very much on the stocks of human capital, knowledge, and germplasm as inputs into the creation of a new variety. The attribution of the cost of creating these important stocks is difficult. As a result, the creation of these stocks is often considered to be sunk costs independent of the particular research program. The whole study of research spillovers would be important if these costs were to be attributed. At the end of the research phase a new variety is created.
Figure 4. Four phases of crop development and the path for R&D cost and benefits31
There are many years between the research expenditure to develop a new variety and the variety reaching any end user. Research itself takes a number of years to produce any tangible product. Even after a variety with potential has been created, it must be tested both internally and by external regulatory agencies before it can be licensed for sale. This period is referred to as the “gestation lag of research,” and is defined as the number of years between making the investment and generating new technology or useful knowledge. In practice, the gestation lag is difficult to estimate, because the expenditure to create a new variety is often spread over many years. For instance, a new variety released in year T may have involved research and development expenditure in years T-2 to T-8. To get around this problem of multiple gestation lags most studies have estimated a single gestation lag, which represents the lag between the weighted mean time of expenditure and the commercialization of a licensed variety.
The third relevant period for estimating the returns to research is the adoption phase. During this phase the new variety is adopted and then replaced by other varieties. The typical pattern is low adoption in the first year of introduction, growing to peak adoption in two in more years, then slowly being replaced by other newer varieties. In terms of economic impact the variety has its largest annual impact in the year when the adoption rate reaches its peak.
The final research stage is the depreciation phase or knowledge stock phase. Research often creates a new process or new germplasm. These innovations provide a very important base, onto which subsequent research is built. Thus, innovations in the form of new varieties contribute to the stock of knowledge or germplasm, which continue to play a role long after the particular innovation has been supplanted by newer innovations. For instance, the first semi-dwarf wheat varieties are no longer used but some of the germplasm from these varieties continues to be in many of the varieties grown today. Although durable, the contribution to the stock of germplasm is not permanent and depreciates over time. One of the common reasons cited for depreciation is that pests in the environment eventually adapt themselves to attack a particular germplasm and new germplasm is required.
In neoclassical investment models, it is assumed that the investment being analyzed has an infinite life as does the cash flow associated with it.29 In the area of technology development this is not the case. Old technology supersedes new technology and the old technology frequently becomes obsolete. As mentioned above, germplasm is an excellent example. Gryglewicz et al.32 modify this traditional neoclassical model and evaluate investment uncertainty from the stand point of projects having a finite life. A project with a finite life and with a risk premium in expected rates of return (i.e., being first to market), reduces the value of waiting to invest in the project. Firms are then more likely to invest in projects with higher degrees of uncertainty. This captures the idea of agricultural biotechnology firms where the first to market can have a high level of adoption and capture significant market share. Being first to market with GM canola reaped substantial benefits for the two technology developers. Smyth and Phillips33 have estimated that the developers of the first GM canola varieties in Canada (Bayer and Monsanto) gained an estimated C$100M by commercializing their varieties and identity preserving the GM canola between 1995 and 1997. The GM canola was kept out of the Canadian canola exports while the Canadian canola industry was waiting for the Japanese regulatory system to approve GM canola for import. While the identity preservation system did cost money, the benefits of investing this money to ensure that Japan would approve GM canola for import was a sound investment strategy as opposed to waiting an additional year and then commercializing.
Traditional analysis of investment decisions also fails to account for the timing of the net cash flow from the investment. In all investment decisions, firms look to receive cash flow from the investment as quickly as possible. Delays between the research phase and the adoption phase decreases the returns firms can expect from the investment. With agricultural biotechnology firms, this timing of cash flows is directly related to the uncertainty of time delays in the regulatory approval process. Majd and Pindyck30 conclude that the effects of “time to build” were greatest when uncertainty was greatest, when the opportunity cost of delay is greatest and when the maximum rate of construction is lowest. The authors also list a caveat with this conclusion: this conclusion is reached if the value of the completed project and the opportunity cost of the project are endogenous to the project. For example, this would be the case in the field of agricultural biotechnology where the value of the project and its cash flows are affected by the potential entry of competitors.
A factor further complicating the investment decisions of agricultural biotechnology firms is the effects of regulation and time delays in the approval process. The uncertainty of these time delays affects the investment decisions of the firms. To capture the effects of time on investment decisions, Sarkar34 used a volatility measure of probability at certain time points to find investment triggers. His work concludes that the probability of investing is initially an increasing function of the expected returns, hits a threshold level at a certain time point and then becomes a decreasing function of expected returns after that time point. This conclusion helps to explain why agricultural biotechnology firms are likely to invest in R&D with the ex-ante knowledge that there will be time delays in the regulatory approval process.
Overview of New Breeding Techniques
The following three techniques are the cutting edge of current plant breeding technology. These concise summaries have been synthesized from Lusser et al.6 and are provided to highlight the challenges that regulators will face, when plant varieties that have been developed via these techniques are submitted for regulatory approval.
Targeted mutagenesis techniques
Targeted mutagenesis is a technique that triggers small variations (mutations) in pre-determined specific sites in a plant’s DNA. This is also known as “site-specific mutagenesis.” These techniques consist in exposing plant cells to chemical or physical mutagens in order to obtain random mutations in the plant's DNA and then selecting the best phenotype (the observable traits) for variety development. The value of targeted mutagenesis techniques is the ability to obtain only one mutation at the desired site.35
Several targeted mutagenesis techniques developed in the last decade have been employed in plants, such as oligonucleotide directed mutagenesis (ODM), zinc finger nuclease (ZFN) technique, meganuclease technique, and transcriptional activator like effector-nuclease (TALEN) technique. Oligonucleotide directed mutagenesis employs chemically synthesized oligonucleotides (i.e., short stands of DNA) that are compatible with a targeted DNA sequence. The other targeted mutagenesis techniques in plants involve the use of modified enzymes, called nucleases, which possess the ability of create a break in the plant’s DNA.36 This DNA break activates cellular repair mechanisms and is converted in a mutation. The result is that the mutation caused by the DNA break is directed to the specific desired site.
The technical process to obtain targeted mutations in plants employs molecular biology tools, in which the genes expressing the synthetic nucleases are delivered to the plant cells. For all these techniques, proof of concept has already been demonstrated in plants, in particular model plants like tobacco and Arabidopsis. The most advanced techniques in terms of applications in plants are ZFN and ODM, through which herbicide tolerance (HT) crop plants can be obtained, in particular maize and canola. Plant varieties that have been created through this technique are in the research pipeline and have been submitted for regulatory approval.
From a regulatory point of view, these techniques and their products are not yet clearly classified by existing legislation in countries like the EU or the US,37 since the products are similar to the products of classical mutagenesis but the process involves the use of rDNA, like in the case of GM crop varieties. According to Canadian legislation, only the traits of the final products are considered, independently of the technique employed to obtain them, thus products of targeted mutagenesis do not need to pass an authorization process if the traits (e.g., HT) have been already assessed.
Cisgenesis and intragenesis
Cisgenesis and intragenesis techniques are based on the concept of “all native” transformation, meaning the introduction of genetic material into the plant’s DNA that is derived from the same plant species or from cross-compatible plants. For both techniques a new gene is transferred into the plant cells by means of molecular tools. However, there is no “foreign” DNA present in the final product. If marker genes (from non-compatible organisms) are employed in the process, they must be removed in the final product.
The difference between cisgenic and intragenic plants is the type of genetic construct inserted. In cisgenesis, the gene of interest is transferred to the plant from a plant of the same species, while in intragenesis, the gene of interest can be from the species itself or from cross-compatible species. Proof of concept for cisgenesis and intragenesis has been obtained in several crop plants, in particular potato and apple. The main trait achieved with agronomic interest is fungal resistance in both crops.
Cisgenesis and intragenesis are currently under consideration in several countries (the EU and the US) in the frame of GM legislation. The developers of these techniques have asked for the exclusion of these products from GM legislation, on the grounds that in principle the same products could be obtained by means of classical breeding (crossing and selection), since no foreign DNA sequences are present in the final plant. However, the technical process is completely comparable with transgenesis.
Grafting on GM rootstock
Grafting is an ancient method in which the upper part of a plant (the scion) is inserted onto the lower part (rootstock) of another plant. This allows the breeder to combine the desired characteristics of the scion (high quality fruits) with a rootstock carrying agronomic properties of interest, usually resistance against soil pests, tolerance to specific soil conditions, and good rooting ability.
Rootstocks with favorable characteristics can be obtained by transgenesis. In this case, a non-GM grafted scion would not carry the transgene, neither would the fruits. Several studies are being performed about the possibility of traffic of genetic material between GM rootstock and non-GM scion, and consequent influence on fruit properties. Proof of concept of this technique has been obtained in several plants of agricultural interest, in particular grapevine, apples, and citrus plants. The main traits for which rootstocks are transformed are fungal resistance and improvement of rooting ability.
From a legislative point of view, the transformed rootstock would clearly be GM, but it needs be determined if fruits would also carry the same evaluation. The influence of the rootstock transformation on the properties of the scion is a factor to take into consideration for the final decision.
Methodology
As mentioned above, there are several reasons that uncertainty and risk exist in investment projects. One uncertainty that exists for biotechnology companies is the time variability of the regulatory and approval process and the effect this has on the investment decisions of firms. To illustrate this uncertainty, suppose the current state of the biotechnology industry for the development and regulatory approval of new projects is (X,Y), where X is the cost of the development of the technology and Y is the time in months required for regulatory approval of a new event. Assuming that all technologies have a minimum IRR of 20%, every technology will have different uncertainties associated with the time requirement of the regulatory approval process.
Yearly net cash flow of a new, innovative technology (e.g., a new GM crop variety) after commercialization can be represented by:
where X is the initial investment of the biotechnology firm at time t, and i is the required rate of return for the firm. Y is the time required for the technology to be fully approved through the regulatory process, and will vary depending on the technology being approved and in what country the approval is being sought.
According to Phillips McDougall38 the initial investment cost for the development of a new GM crop variety is US$136 million, requiring an average of 48 months to receive regulatory approval. Using these figures as a base case, the three technologies summarized in the previous section, can be compared using Figure 5 below.
Figure 5. Cash flow and regulatory delay estimates for new technologies
Starting with the base case of (X,Y), Technology 1, Technology 2, and Technology 3 can be analyzed by comparing the loss of net present value of the cash flow that each firm will experience based on the time delays associated with the regulatory approval process.
Firms experience a delay in the return on investment (opportunity cost) for each time period that the technology is held in regulatory assessment. To calculate the value of this delay in earnings to a firm, a simple uniform series payment formula can be used to calculate the present value of that delay.
Where is the present value of the delayed earnings, A is the yearly net cash flow received by the firm, i is the discount rate of the firm and t represents time.
The illustration of Figure 5, and calculation of the net present value of cash flows to the firm that are lost due to delays in the regulatory approval process, will provide initial estimates as to the effects of uncertainty on biotechnology firms. The losses that firms experience from regulatory delays may provide some insight into a biotechnology firm’s decision to move forward with a technology or to abandon the project in the “valley of death”. We pursue two distinct but complementary strategies to examine the investment decision and the consequences of regulatory delays. In the first option, we assume that the innovator will require a set amount of return per year. In the second option, we fix the total amount of return (in real un-discounted terms) and then change the payments required to achieve the set amount of return. This will allow examining this issue from two slightly different perspectives.
This static analysis discussed so far does not consider in detail the potential for risk in the assessment. To introduce risk, we changed the static value of the discount rate in the estimation of a net present value (NPV) to a stochastic probability density function. We then used @Risk to conduct risk simulations, where the program samples from the probability distribution chosen (the input distribution) and then estimates an outcome. In our case the outcome is the NPV resulting from delaying the onset of the stream of benefits up to eight years.39
There are several options for proposing a probability distribution. One of the most popular distributions is the triangular distribution. This distribution is parsimonious as it only requires three parameters for defining the distribution: the minimum, most likely, and maximum values. However, Demont et al.40 proposed that the triangular distribution introduces a heavier emphasis on the probability distribution tails than what the experience has shown in practice.
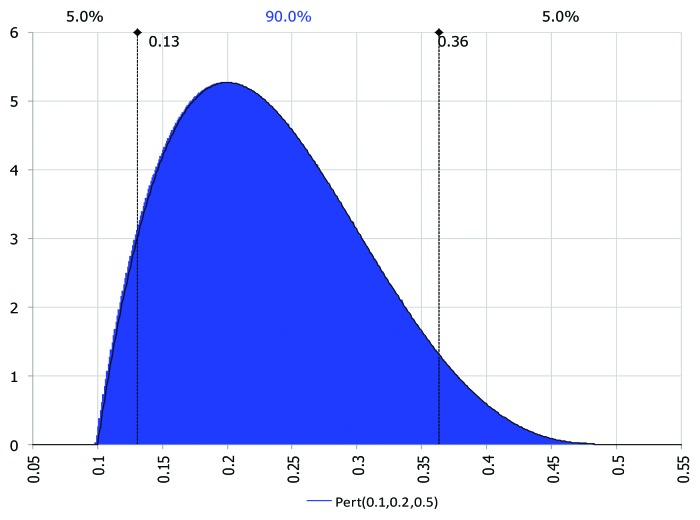
These authors suggest using a PERT (Program Evaluation and Review Technique) probability distribution function—derived from the more general Beta function—as it is also parsimonious and does not put emphasis on the tails of the distribution as the triangular can. In fact, the smooth shape of the PERT places less emphasis in the direction of probability distribution skew. The only disadvantage is that the PERT distribution is bounded on both sides, hence may not be adequate when the desire is to capture tail or extreme events. The PERT distribution is similar to the triangular distribution requiring three parameters for defining the distribution (minimum, most likely and maximum).
In our case, we chose a minimum value of 10% for the discount rate, a most likely value of 20% and a maximum value of 50%. Figure 6 is a representation of the discount rate used and probability distribution used in the analysis. We instructed for @Risk to conduct 10,000 iterations of the sampling and estimation of NPV outcomes, for each one of the years we allowed to onset the stream of benefits up to eight years. @Risk stored the value for each one of the iterations for further analysis.
Figure 6. PERT distribution for discount rate (r)
Analysis
Recuperating a fixed amount of return over time
Based on a survey of seed development firms, the current total cost of discovering, developing and registering a new GM crop variety is US$136 million and the time requirement for regulatory approval is averaging 48 months.38 Applying these conditions with an annual rate of return of 20%, it can be calculated that the biotechnology companies will realize a positive net cash flow of US$27.2 million per year on the initial investment of US$136 million after the commercial sale of the technology.
By estimating this net cash flow, the loss of revenue to the company from hold-ups in the regulatory approval process can also be estimated. The results of those estimations are shown in Figure 7. With an annual rate of return of 20%, biotech firms approach the initial investment of $136 million by year 13 of waiting for regulatory approval. A one year delay in the approval process will cost a biotechnology firm US$22.7 million (assuming a discount rate of 20%), with a seven year delay costing the firm US$98 million. By year 13, the present value of the net cash that firms fail to realize from the sale of the technology due to regulatory delays reaches US$123.3 million.
Figure 7. Present value of net cash loss from delays in regulatory approval process (IRR = 20%)41
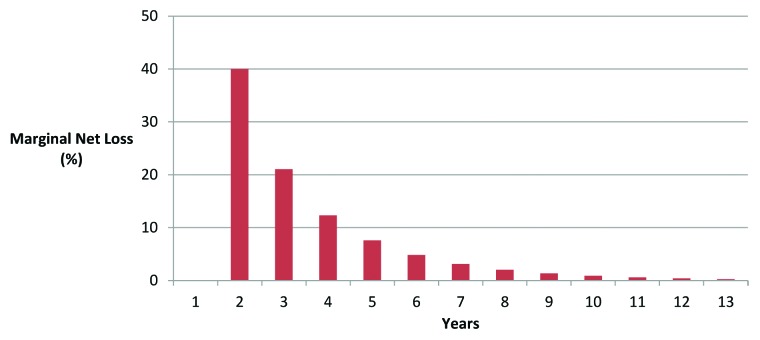
Based on an initial investment of US$136 million, the year over year difference in lost cash flow from delays in the regulatory process can be determined. Figure 8 illustrates that the most significant marginal costs are incurred in the first six years of regulatory delay. By the end of year seven, these marginal losses begin to level off to the point where firms have lost almost all of their initial investment of US$136 million and will not experience positive cash flow on this investment. With an IRR of 20% it would be expected that the end of year six of regulatory delay would be the trigger point for suspending the investment into a R&D project involving new plant varieties.
Figure 8. Marginal loss of net cash flow from delays in regulatory approval process (IRR = 20%)41
Extending this analysis a step further and assuming the annual rate of return of a biotechnology firm is 50%, Figure 9 illustrates that the present value of the net cash loss reaches the initial investment level of US$136 million by year 13. A seven year delay in this scenario will cost the development firm US$128 million. Interestingly, it took EU regulators 13 years to approve BASF’s GM potato. This was subsequently followed by BASF moving all of their crop biotechnology R&D from Europe to the US.
Figure 9. Present value of net cash loss from delays in regulatory approval process (IRR = 50%)41
Continuing the analysis of an annual rate of return of 50%, biotechnology firms experience significant losses in the first two years of regulatory delay and by year five have failed to recoup almost all of their initial investment of US$136 million (Fig. 10). This analysis may provide some insight into firms that abandon projects to the “valley of death” while awaiting regulatory approval. The higher the rate of return of the firm, the less time firms can afford to have varieties awaiting regulatory approval. With current regulatory approvals averaging 48 mo, the upper boundary for this process has likely been reached and any approval process that will be viewed to take longer than this, could cause firms to not proceed with investment into research.
Figure 10. Marginal loss of net cash flow from delays in regulatory approval process (IRR = 50%)41
Recuperating an investment with delays to the onset of benefits
If an investor’s desire is to obtain a 20% rate of return on investment in real terms, that is, the NPV of the investment is US$27.2 million, over a period of recovery of 10 years with an initial investment of US$136 million, then the innovator would need to obtain a stream of payments of US$38.9 million every year for the ten year period of the investment with a discount rate of 20%. A simple analysis to examine the impact of regulatory delays is to shock the stream of benefits by delaying the onset by a number of years.
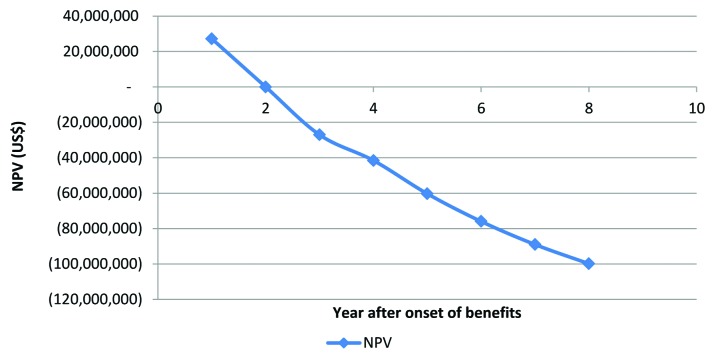
Table 2 and Figure 11 introduce estimations based on this approach. If an innovator invests US$136 million in year 0 and starts receiving the stream of benefits beginning at the end of year 1 during 10 years then the innovator obtains the full NPV. However, if there is a one-year delay, the stream of benefits starts at the end of year 2 and continues for the 10 years and then the NPV becomes zero. The later result is just an artifact of the rate of return being the same as the discount rate. After an onset in starting the stream of benefits in year 3 the NPV becomes negative and thus the innovator will have to examine its investment strategy. This of course is related to the economic decision rule of a project covering its variable costs or not, and when in the project life span is the decision maker making its decision.42
Table 2. Net present value of an investment allowing year delays in onset of benefits.
| Year of income stream onset | NPV (US$) | Percent loss (%) |
|---|---|---|
| 1 | 27,200,000 | n.a. |
| 2 | 0 | n.a. |
| 3 | (27,032,579) | 100% |
| 4 | (41,555,556) | 35% |
| 5 | (60,328,180) | 31% |
| 6 | (75,972,034) | 21% |
| 7 | (89,008,578) | 15% |
| 8 | (99,872,366) | 11% |
Figure 11. NPV and the year of onset of the benefit stream
Figure 12 summarizes the mean, confidence interval and a one standard deviation from the mean for each one of the NPV outcomes from delaying the onset of the stream of benefits over a number of years and allowing for the stochastic distribution of the discount rate using the PERT probability density function. Figure 12 is a summary trend graph displaying how risk changes over time. In the case of Figure 12, the graph is showing the range of NPVs over each one of the eight years we delayed the onset of the NPVs. In such summary trend graphs, the narrower the band compared with the mean, the less uncertainty about the NPV estimates. Conversely, the wider the band around the mean, the greater the possible variance increases and the greater the risk.
Figure 12. Confidence interval and standard deviation for changes in onset of benefit stream
This figure is quite similar to Figure 11 in its overall conclusions. Even small delays on the onset of the stream of benefits make an investment not worth implementing or its value over time may have to be reconsidered pending on the relative cost structure especially that of variable costs to implement a decision. An important conclusion from this graph is that the distribution of the stream of benefits increase over time as the band widens. The change in the wideness of the confidence band changes significantly after year 3. In other words, the longer the delay, the more risk is introduced into the outcome. In this case, outcomes after year 2 are losses.
While some crop varieties developed using these new breeding techniques are currently undergoing regulatory risk assessments, to date, there has been no commercial release of new crop varieties to the market using any of the three technologies identified previously. While applications have been made in the US and EU to have targeted mutagenesis considered a non-GM event, there has been no official decision made on this application. If the decision is made to classify targeted mutagenesis development techniques as non-GM events, the regulatory approval process is much faster, creating less uncertainty for firms using this technology. Commercialized varieties utilizing this technology could then be expected on the market within two to three years.6 The analysis conducted in this article would support reducing the amount of unnecessary delay in the regulatory process.
Similar conditions exist for varieties being developed using cisgenesis and intragenesis technology. While these technologies are currently under consideration as a GM event, arguments are being made to classify varieties developed from these techniques as non-GM events. It can be argued that the same results could be achieved through traditional breeding techniques, but the technology is allowing for the development to occur at a much faster pace. If these technologies are regulated similar to the process used with a GM event, we can only expect that delays introduced by the regulatory system will impact varieties in the same manner. Furthermore, we foresee that some stakeholders may raise the argument that lack of familiarity with cisgenesis and intragenesis introduces more uncertainty in the risk assessment outcome, and thus the need will arise to increase the regulatory oversight and thus the time value of money.
Grafting on GM rootstock are events that create a great deal more uncertainty for firms looking to introduce new varieties utilizing these technologies. Varieties being developed with this technology can only be classified as GM events. Varieties developed with this technology are currently only in phase I and potentially face a great deal of uncertainty with respect to the regulatory process.6 Whether the scion and its products can be maintained as non-GM is still open for discussion and policy decision making. Similar to the case of cisgenesis and intragenesis, if the whole plant that uses grafting on GM rootstock is indeed considered as a GM crop, then the conclusions from this article are likely to apply to this technology too.
In the end, the specific impact of regulatory delays will depend on the technology, the knowledge and familiarity with the crop, trait and transformation and/or production process. The less knowledge and familiarity regulators have about the later, the more likely they are to delay proposing a regulatory outcome.43 This is one limitation of the current analysis. We have not considered the potential gains from delays in terms of the knowledge accumulated during the delay itself. The key policy lesson here is reducing unnecessary delays and to improve the efficiency of the regulatory process itself as much as possible.
Regulatory Implications
Regulatory treatment of these new breeding techniques is unlikely to be problematic for jurisdictions that have science-based regulations as the foundation of their domestic regulatory systems. Indeed, products of these technologies are already undergoing regulatory review in both Canada and the US. Waltz44 identifies that plant varieties developed by oligonucleotide directed mutagenesis are currently under review by the Canadian Food Inspection Agency (CFIA) and that the USDA has reviewed oligonucleotide directed mutagenesis as well as zinc finger nuclease technologies, concluding that the technologies are standard breeding techniques and do not require further oversight. The USDA regulates on a case-by-case basis and companies developing plant varieties with targeted mutagenesis breeding techniques are working with the USDA, which is not to say that at some point greater regulatory oversight may be justified. At present, it is not. Canada has specific regulatory requirement for what they deem to be “plants with novel traits” (PNTs), which triggers additional regulatory oversight. Again the CFIA regulates PNTs on a case-by-case basis and based on previous ruling, it would be expected that the products of these breeding techniques would be treated as PNTs.
Canadian and American regulatory approvals of varieties would be expected to be received within two years, based on previous experiences. Within the European Union, which utilizes a precautionary principle-based regulatory approach, the European Food Safety Authority (EFSA) is governed by Regulation (EC) No 1829/2003.45 This regulation requires that EFSA do its utmost to provide a regulatory decision in six months, but also notes that requests for additional information may push this to 24 mo. Clearly, concern about operating according to this regulation is not a priority for the EU given the 13 years it took to approve BASF’s GM potato variety.
Based on our analysis, regulatory agencies that are able to provide approval decisions on new plant varieties within a two-year time frame should not experience a decline in investment into further development of the techniques behind the development of the new varieties. Technology developers would appear to have accepted that a two-year time frame for regulatory decision is acceptable for the process of new product development. Beyond a two-year time frame, uncertainty increases.
Table 3 provides a list of current traits awaiting regulatory decision within the EU regulatory system. None of the times below, include the time taken by EFSA to reach their opinion. The regulatory guidelines for the EU require that there be a three month maximum of time for the Commission to schedule a vote at the committee level and that there be a two month maximum of time for the Commission to schedule a vote of the Appeal Committee. The top two products have most likely passed the point of no longer having any commercial value as the marginal loss from this regulatory delay will have pushed the two products into the “valley of death”. The important thing to highlight in this table is that while it is taking three, four and even five years, just to have the Commission schedule a vote at the committee level, firms then have to wait an undetermined amount of time for the Commission to schedule the vote of the Appeal Committee. This length of time has not been determined with these products as none have passed this stage of the regulatory approval process. This says nothing of the wait that would be expected to occur for the fourth and final stage, actual variety approval.
Table 3. Timeline for GM products in Europe (as of 5/31/13)46.
| Product | EFSA opinion | Number of days for the Commission to vote at the committee level | Number of days for the Commission to vote in the Appeal Committee | Days after Council/Appeal vote – waiting for approval |
|---|---|---|---|---|
| 1507 maize | 03/03/2005 | Voted after 1462 d | 1549 d and counting | |
| Bt11 maize | 19/05/2005 | Voted after 1385 d | 1549 d and counting | |
| LL rice62 | 30/10/2007 | 2040 d and counting | ||
| NK603 maize | 11/06/2009 | 1450 d and counting | ||
| Mon810 maize | 30/06/2009 | 1431 d and counting | ||
| MS8xRF3 rapeseed | 22/09/2009 | 1347 d and counting | ||
| Gt73 oilseed rape | 15/12/2009 | 1263 d and counting | ||
| MON863 maize | 30/03/2010 | 1158 d and counting | ||
| MON89034x1507x MON88017x59122 maize |
27/09/2010 | 977 d and counting | ||
| MON89034x1507xNK603 maize | 27/09/2010 | 977 d and counting | ||
| MON531 cotton | 16/09/2011 | 623 d and counting | ||
| MON88017 maize | 10/11/2011 | 568 d and counting | ||
| MON1445 cotton | 16/12/2011 | 532 d and counting | ||
| GA21 maize | 16/12/2011 | 532 d and counting | ||
| MON87701xMON89788 soybean | 15/02/2012 | 471 d and counting | ||
| MON531xMON1445 cotton | 28/03/2012 | 429 d and counting |
Depending on a technology development firms rate of return, the window for regulatory approval would appear to be between four and six years. Firms with a rate of return of 20% would have greater flexibility in terms of time for regulatory decisions, with six years being the upper threshold for variety approval, while firms with a higher IRR of 50% will operate on the premise that a four-year time frame for regulatory approval is acceptable but uncertainties increase substantially beyond this period, resulting in a cancelation of that R&D project. Based on BASF’s decision to relocate all of its plant biotechnology R&D capacity from Europe to the US, it could be expected that instead of the “death valley” scenario of other products, that the R&D capacity of firms will relocate to markets that are able to efficiently regulate their products.
Waltz44 indicates that the European Union has not decided how to regulate these new plant breeding techniques. Given that the USDA and CFIA are already evaluating varieties developed using these techniques, it places considerable pressure on the EU regulators to make a decision expeditiously. The uncertainty of EU regulators to make timely regulatory decisions, is having an impact on investment decisions and there are presently no signs indicating that EU regulators and European Commission officials are targeting increases in regulatory efficiency. Given the uncertainty of regulatory decisions of new plant breeding techniques in the EU, the actions of BASF may be simply the first of many.
Conclusions
Trying to measure and quantify uncertainty can be a nebulous exercise. Simply saying that uncertainty will exist past a certain point of reference does not provide much in the way of insight or context to the debate. We have attempted to provide some context to the uncertainty debate as it relates to the regulation of products of new plant breeding techniques. With case-by-case regulation, circumstances will exist that provide exceptions to the norm, but in our analysis, we would expect that regulatory decision that take longer than six years, will justify a level of investment uncertainty that will result in firms suspending further technology investments.
Regulatory agencies that are able to provide timely, consistent and repeatable regulatory decisions should not be unduly concerned about levels of uncertainty. Regulatory agencies that do not rely on science-based regulations and instead rely on the precautionary principle and socio-economic considerations should be concerned about the level of uncertainty that is created as a result of their regulatory approval process.
Europe faces a dire predicament. There is no political commitment to ensuring that regulators or Commission officials comply with the regulations that govern their activities, hence, regulatory decisions that take a decade or longer could become commonplace. The only stakeholder championing a more expeditious regulatory system is the technology development firms and their voice within the European Union is not a strong one. One multinational seed development firm has already indicated that the regulatory delays have created such an environment of uncertainty that it is no longer willing to conduct R&D in Europe. How many more seed development firms are contemplating a similar move? Only time will tell.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
S.J.S. and J.M.’s contribution to this research was supported by VALGEN (Value Addition through Genomics and GE3LS), a project sponsored by the Government of Canada through Genome Canada and Genome Prairie. J.F.-Z.’s contribution made possible by Program for Biosafety Systems (PBS) at the International Food Policy Institute, with support from the Office of Administrator, Bureau for Economic Growth, Agriculture and Trade/Environment and Science Policy, US Agency for International Development, under the terms of Award EEM-A-00–03–00001–00. The opinions expressed herein are those of the authors and do not necessarily reflect the views of Genome Canada or the US Agency for International Development. All responsibility for the content of this article lies solely with the authors.
References
- 1.Smyth SJ, Endres A, Redick T, Kershen D. Innovation and Liability in Biotechnology: Transnational and Comparative Perspectives. Cheltenham, UK: Edward Elgar Publishing Ltd. 2010. [Google Scholar]
- 2.News release: BASF to concentrate plant biotechnology activities on main markets in North and South America [Internet]. Florham Park (NJ): BASF: c2012 [cited 2013 Aug 12]. Available from: http://www.basf.com/group/pressrelease/. 2012.
- 3.News release: European Commission approves Amflora starch potato [Internet]. Florham Park (NJ): BASF: c2012 [cited 2013 Aug 12]. Available from:: http://www.basf.com/group/corporate/en/function/conversions:/publish/content/news-and-media relations/news-releases/downloads/2010/P179-Amflora-e.pdf. 2010.
- 4.Phillips McDougall. The cost and time involved in the discovery, development and authorization of a new plant biotechnology derived trait. A consultancy study for CropLife International. 2011. [Google Scholar]
- 5.Fernandez-Cornejo J. The impacts of adoption of genetically engineered crops on yields, pesticide use and economic returns in the USA [Internet]. Washington [DC]: Presentation to the Farm Foundation Conference on Biotechnology: c2013 [cited 2013 Aug 12]. Available from: http://www.farmfoundation.org/news/articlefiles/372-Fernandez-Cornejo2ndDecadeBiotech.pdf. 2008.
- 6.Lusser M, Parisi C, Plan D, Rodrigues-Cerezo E. New Plant Breeding Techniques: State-of-the-art and Prospects for Commercial Development. Report for the Joint Research Centre, European Commission, Institute for Prospective Technological Studies. Luxembourg: Publications Office of the European Union. 2011. [Google Scholar]
- 7.2012 Annual Letter from Bill Gates [Internet]. Seattle (WA): Bill Gates Foundatin: c2012 [cited 2013 Aug 12]. Available from: http://www.gatesfoundation.org/annual-letter/2012/Pages/home-en.aspx. 2012.
- 8.Diamond J. Guns, Germs, and Steel: The Fate of Human Societies. New York: W.W. Norton & Company. 1997. [Google Scholar]
- 9.Tutwiler A. Abstract for Keynote Address to the GMCC-11 Conference. Vancouver, Canada, October 26-8. 2011.
- 10.How to Feed the World in 2050. Rome, Italy: Food and Agriculture Organization: c2013 [cited 2013 Aug 12]. Available from: http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf. 2010.
- 11.Food and Agriculture Organization. The State of Food Insecurity in the World. Rome: FAO. 2006. [Google Scholar]
- 12.How to feed a hungry world. Nature 2010; 466:531 - 2; http://dx.doi.org/ 10.1038/466531a; PMID: 20671664 [DOI] [PubMed] [Google Scholar]
- 13.Brookes G, Yu T, Tokgoz S, Elobeid A. . The production and price impact of biotech corn, canola, and soybean crops. AgBioForum. 2010; 13:25 - 52 [Google Scholar]
- 14.Thirtle C, Bottomley P. . Is publicly funded agricultural research excessive?. J Agric Econ 1998; 39:99 - 111; http://dx.doi.org/ 10.1111/j.1477-9552.1988.tb00565.x [DOI] [Google Scholar]
- 15.Pardey P, Craig B. . Causal relationships between public sector agricultural research expenditures and output. Am J Agric Econ 1989; 71:9 - 19; http://dx.doi.org/ 10.2307/1241770 [DOI] [Google Scholar]
- 16.Leiby J, Adams G. . The returns to agricultural research in Maine: The case of a small northeastern experiment station. Northeastern Journal of Agricultural and Resource Economics 1991; 20:1 - 14 [Google Scholar]
- 17.Huffman W, Evenson R. Size, specialization, and agricultural growth: How public policy enhances scientific and economic progress in agriculture. Staff General Research Papers 11011. Iowa State University, Department of Economics. 1993. [Google Scholar]
- 18.Huffman W, Evenson R. Contributions of public and private science and technology to U.S. agricultural productivity. Staff General Research Papers 10990. Iowa State University, Department of Economics. 1992. [Google Scholar]
- 19.Huffman W, Evenson R. Supply and demand functions for multiproduct U.S. cash grain farms: Biases caused by research and other policies. Staff General Research Papers 10985. Iowa State University, Department of Economics. 1989. [Google Scholar]
- 20.Chavas J, Cox T. . A nonparametric analysis of the influence of research on agricultural productivity. Am J Agric Econ 1992; 74:583 - 91; http://dx.doi.org/ 10.2307/1242571 [DOI] [Google Scholar]
- 21.Alston J.M and H.O Carter. Valuing California’s agriculture research and extension. Davis: University of California Agriculture Issue Center Publication No. VR-1. March. 1994. [Google Scholar]
- 22.Evenson RE. Two Blades of Grass: Research for U.S. Agriculture. Chapter 11 in J. M. Antle and D. A. Sumner (eds.) The Economics of Agriculture Volume 2, Papers in Honor of D. Gale Johnson. Chicago: University of Chicago Press, 1996; 171-203. [Google Scholar]
- 23.Akgüngör S, Makanda DW, Oehmke JF, Myers RJ, Choe YC. Dynamic analysis of Kenyan wheat research and rate of return. Contributed paper proceedings from the Conference on Global Agricultural Science Policy for the Twenty-First Century. Melbourne, Australia, August 26-28, 1996; 333-366.
- 24.Makki SS, Tweeten LG, Thraeu CS. Returns to agricultural research: Are we assessing right? Contributed paper proceedings from the Conference on Global Agricultural Science Policy for the Twenty-First Century. Melbourne, Australia, August 26-28. 1996;89-114.
- 25.Alston JM, Craig BJ, Pardy PG. Dynamics in the creation and depreciation of knowledge and the returns to research. GPTD Discussion Paper, No. 35 (August). Washington, DC: International Food Policy Research Institute. 1998. [Google Scholar]
- 26.Alston. J. M., J. M. Beddow, and P. G. Pardy. Undated. Agricultural research, productivity, and food commodity prices [Internet]. Giannini Foundation of Agricultural Economics: c2011 [cited 2013 Aug 12]. Available online at: http://giannini.ucop.edu/media/are-update/files/articles/v12n2_5.pdf.
- 27.Schmidhuber J, Bruinsma J, Boedeker G. Capital requirements for agriculture in developing countries to 2050. Paper presented at the FAO Expert Meeting on “How to Feed the World in 2050”, 24-26 June, Rome. 2009.
- 28.Phillips PWB. New Models of Agrifood Innovation and Development. Science and Technology Program, Harvard. 2001. [Google Scholar]
- 29.Dixit AK, Pindyck RS. Investment Under Uncertainty. Princeton University Press. 1994. [Google Scholar]
- 30.Majd S., Pindyck R.S. . Time to build, option value, and investment decisions. . Journal of Financial Economics 1987; 18:7 - 27; http://dx.doi.org/ 10.1016/0304-405X(87)90059-6 [DOI] [Google Scholar]
- 31.Alston JM, Norton GW, Pardey PG. Science Under Scarcity: Principles and Practice for Agricultural Research Evaluation and Priority Setting. Ithaca, NY: Cornell University Press. 1995. (adapted from) [Google Scholar]
- 32.Gryglewicz S, Huisman KJM, Kort PM. . Finite project life and uncertainty effects on investment. J Econ Dyn Control 2008; 32:2191 - 213; http://dx.doi.org/ 10.1016/j.jedc.2007.10.003 [DOI] [Google Scholar]
- 33.Smyth SJ, Phillips PWB. . Competitors co-operating: Establishing a supply chain to manage genetically modified canola. International Food and Agribusiness Management Review 2001; 4:51 - 66; http://dx.doi.org/ 10.1016/S1096-7508(01)00070-2 [DOI] [Google Scholar]
- 34.Sarkar S. . On the investment – uncertainty relationship in a real options model. J Econ Dyn Control 2000; 24:219 - 25; http://dx.doi.org/ 10.1016/S0165-1889(99)00005-6 [DOI] [Google Scholar]
- 35.Podevin N, Davies HV, Hartung F, Nogué F, Casacuberta JM. . Site-directed nucleases: a paradigm shift in predictable, knowledge-based plant breeding. Trends Biotechnol 2013; 31:375 - 83; http://dx.doi.org/ 10.1016/j.tibtech.2013.03.004; PMID: 23601269 [DOI] [PubMed] [Google Scholar]
- 36.Pauwels K, Podevin N, Breyer D, Carroll D, Herman P. . Engineering nucleases for gene targeting: safety and regulatory considerations. N Biotechnol 2014; 31:18 - 27; http://dx.doi.org/ 10.1016/j.nbt.2013.07.001; PMID: 23851284 [DOI] [PubMed] [Google Scholar]
- 37.Cressey D. Transgenics: A new breed. Nature. http://www.nature.com/news/transgenics-a-new-breed-1.12887. 2013. [DOI] [PubMed]
- 38.Phillips McDougall. The cost and time involved in the discovery, development and authorization of a new plant biotechnology derived trait. A consultancy study for CropLife International. 2011. [Google Scholar]
- 39.@Risk uses a quasi-random sampling method named Latin Hypercube. Latin Hypercube sampling is more efficient compared to Monte Carlo methods, relying on the stratification of the input probability distribution and random sampling from each interval or strata of the input distribution. [Google Scholar]
- 40.Demont M, Cerovska M, Daems W, Dillen K, Fogarasi J, Mathijs E, Muska F, Soukup J, Tollens E. . Ex ante impact assessment under imperfect information: Biotechnology in new member states of the EU. J Agric Econ 2008; 59:463 - 86; http://dx.doi.org/ 10.1111/j.1477-9552.2008.00157.x [DOI] [Google Scholar]
- 41.Author’s calculation. [Google Scholar]
- 42.If we examine the situation where the rate of return increases to 50% but keeping the discount rate to 20%, then the NPV continues to be positive until the end of year 3, then it becomes negative starting in year 4. [Google Scholar]
- 43.Bayer JC, Norton GW, Falck-Zepeda JB. . Cost of compliance with biotechnology regulation in the Philippines: Implications for developing countries. AgBioForum 2010; 13:53 - 62 [Google Scholar]
- 44.Waltz E. . Tiptoeing around transgenics. Nat Biotechnol 2012; 30:215 - 7; http://dx.doi.org/ 10.1038/nbt.2143; PMID: 22398610 [DOI] [PubMed] [Google Scholar]
- 45.European Food Safety Authority. FAQ on Genetically Modified Organisms. Online at: http://www.efsa.europa.eu/en/faqs/faqgmo.htm. 2012.
- 46.Updated from Bobo. J. A. Senior Advisor for Biotechnology, United States Department of Agriculture. Personal Communications. 2012. [Google Scholar]