ABSTRACT
Plant molecular farming (PMF) is an important growing prospective approach in plant biotechnology; it includes production of recombinant pharmaceutical and industrial proteins in large quantities from engineered plants. Elastin is a major protein component of tissues that require elasticity, it helps keep skin smooth as it stretches to allow normal. Elastin is used as a raw material for the cosmetic industry. In this work, we aimed to use plant as a bioreactor for the expression and production of the full human tropoelastin protein. Agrobacterium- mediated transient expression system into Nicotiana tabacum using syringe agroinfiltration was used to provide fast and convenient way to produce recombinant proteins with greater expression overall the plant leaf. This study aimed to establish an efficient and rapid system for transiently expression and production of human recombinant tropoelastin protein in transgenic N. tabacum plants. Modified elastin (ELN) gene was biosynthesized and cloned into pCambia1390 vector to be used into N. tabacum agroinfilteration. Optimization of codon usage for the human tropoelastin gene, without changing the primary structure of the protein was carried out to ensure high expression in tobacco plants. The obtained data proved that the 5th day post-infiltration is the optimum interval to obtain the maximum production of our recombinant protein. Southern blot analysis was able to detect 2175 bp fragment length representing the ELN orf (open reding frame). On the other hand, ELN -expression within plant's tissue was visualized by RT-PCR during the period 3–10 days post agroinfiltration. At the protein level, western and ELISA confirmed the expression of recombinant tropoelastin protein. Western blot analysis detected the tropoelastin protein as parent band at ∼70 kDa from freshly extracted protein, while two degraded bands of ∼55 and ∼45 kDa, representing a pattern of tropoelastin were appeared with frozen samples. This study showed that biosynthetic ELN gene was successfully expressed into N. tabacum leaves using agroinfiltration technique.
Keywords: agroinfiltration, ELN, ELISA, hTE, Molecular farming, transient expression, tropoelastin, western blot
Introduction
Plants are able to be used as bioreactor by producing full functional, biologically active recombinant proteins. They provide low-cost, large-scale production platform of recombinant industrial and pharmaceutical proteins (Hefferon, 2012). This began with the production of the human serum albumin from tobacco and potato plants (Sijmons et al., 1990), and led to production of several proteins from plants including; antibodies, vaccines, peptides, and enzymes (Ko, 2014; Donini et al., 2005; Aboul-Ata et al., 2014). Moreover, plants utilize eukaryotic endomembrane system which is similar to mammalian cells, this allows proper post-translational modification of proteins including glycosylation and the assembly of multiple-subunit proteins. This ability puts molecular farming technology ahead of those based on prokaryotic systems, such as bacteria (Demain and Vaishnav, 2009).
Agrobacterium-mediated transient expression offers many advantages over other transformation systems. It ensures scalable, fast gene expression without chromosomal integration or position effect (Demain and Vaishnav, 2009). Transient expression via agroinfiltration offers an easy, simple, and effective procedure to obtain high level of protein accumulation few days post-infiltration (Sparkes et al., 2006). Agroinfiltration could be done either by using syringe or vacuum infiltration. The ease in using syringe infiltration made it the method of choice in agroinfilteration (Shamloul et al., 2014). Many plants utilized its efficiency in agroinfiltration, however tobacco plant is well-suited production system for recombinant proteins and most common used as its non-food, non-feed crop with no risk of contamination with neither human nor animal food chain. In addition, it has pulpy thin dermis leaves which are very adapted for agroinfiltration procedures (Rymerson et al., 2002). Tobacco has a high biomass yield and as the expression platform is based on leaves, harvesting occurs prior to flowering minimize the possibility of gene flow into the environment. Most importantly, tobacco is a non-food, non-feed crop, which minimizes regulatory barriers by eliminating the risk of plant-made recombinant proteins entering the food supply (Rymerson et al., 2002; Twyman et al., 2003).
Elastin is the main protein that plays an important role in formation of elastic fibers that presents in skin, lungs and other connective tissues (Rosenbloom et al., 1993). Elastic fibers provide tissues with the required resilience and elasticity. Tropoelastin is 60–72 KDa soluble precursor of elastin that is encoded by ELN gene located on chromosome 7 at position 11.23. It is divided into two domains; hydrophilic domains which is characterized by their high lysine and alanine residues where the cross linking occurs, as well as hydrophobic domains which rich in the non-polar residues glycine, valine and proline (Wise and Weiss, 2009).
During perinatal development, the elastogenic cells produce tropoelastin protein which is moved out to the cell surface by elasitn-binding protein (EBP). the protein molecules then aggregate and are settled onto the microfibrils and get cross-linked by the copper-requiring lysyl oxidase enzymes. Multiple processes of cross-linking result in the mature insoluble elastic fibers (Nivison-Smith and Weiss, 2011). Serious diseases such as cutis laxa (CL) and supravalvular aortic stenosis (SVAS) are associated with deficiency ELN gene or degradation of elastin protein. On the other hand, aging and injuries damage are associated with losing of elastin and consequently losing of cell elasticity (Rnjak et al., 2011; Metcalfe et al., 2000).
In this study, we used agroinfiltration in Nicotiana tabacum plants for transiently express the ELN gene and to evaluate the efficiency of this technique as plant-based expression system for industrial products.
Results
Optimizing the Codon Usage of the ELN Gene for Plant Expression
As the native human ELN gene employs tandem rare codons can reduce the efficiency of translation or even disengage the translational machinery, therefore, the codon usage bias in Arabidopsis thaliana was used by upgrading the codon adoption index (CAI) from 0.70 to 0.88. The GC content and unfavourable peaks have been optimized to prolong the half-life of the mRNA. The Stem-Loop structures, which impact ribosomal binding and stability of mRNA, were broken. In addition, negative cis-acting sites were screened and successfully modified. The modified sequence was submitted to GenBank (KP699575).
Construction of Plant Expression Vectors
The 2175 bp-ELN orf was excised from pUC57-ELN by digesting with both EcoRI and BglII and subcloned into modified pCambia1390-35S binary vector in the corresponding sites and under the control of 35S-promoter (Fig. 1). The transformed colonies were confirmed with restriction digestion. The recombinant plasmid pCambia-ELN was extracted from the selected colony and consequently was transformed into Agrobacterium to carry out agroinfilteration experiments.
Figure 1.
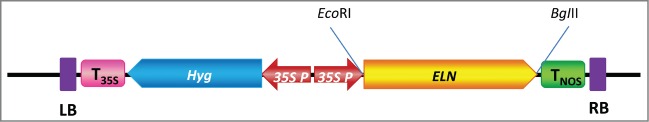
Physical maps of the binary vectors pCambia-ELN used for Agroinfilteration-transformation (ELN: elastin gene, P35S: 35S promoter, TNOS : NOS terminator, T35S : 35S terminator, Hyg: hygromycin resistance gene, RB: right border, LB: left border).
Molecular Analysis
Southern Blot Analysis
Genomic DNA from the agroinfilterated plants were extracted and digested with both EcoRI/BglII and subjected to Southern blot analysis. Results showed that the synthetic ELN orf was successfully transformed into N. tabacum leaves using agroinfiltration. The presence of amplified fragment with the expected size (2175 bp) indicates that the gene was successfully transformed into Nicotiana tabacum leaves via agroinfiltration. Higher molecular weight fragments were visualized due to partial digestion of some DNA of the samples. The digested recombinant vector pUC57-ELN was used as positive control and resulted in the same size band while the un-infiltrated leaves were used as negative control (Fig. 2).
Figure 2.
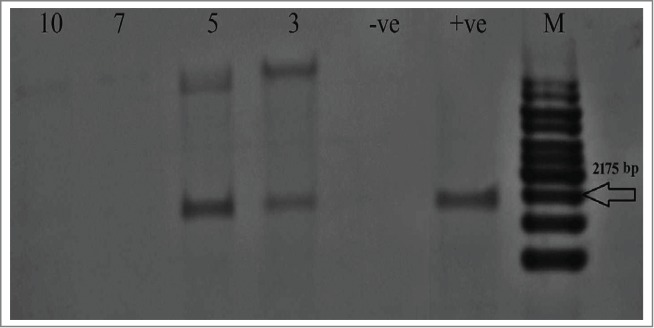
Southern blot results using ELN probe for the total DNA of ELN-infiltrated tobacco leaves after digestion with EcoRI and BglII. Lanes 3, 5, 7 and 10: ELN-infiltrated plants, +ve: digested plasmid pUC57-ELN used as a positive control, -ve: negative control un-infiltrated plant, M: 1Kb ladder DNA marker.
RT-PCR
Transcription of the ELN gene was confirmed using RT-PCR. The extracted RNA samples from infiltrated N. tabacum leaves were subjected to reverse transcription-PCR (RT-PCR) analysis using ELN specific primers to amplify the core region of the gene. The RT-PCR amplified fragment of ∼800 bp indicated that all the infiltrated leaves with pCambi-ELN clearly exhibited the transcription of the ELN gene as shown in Figure 3. While, un-infiltrated leaves showed negative results.
Figure 3.
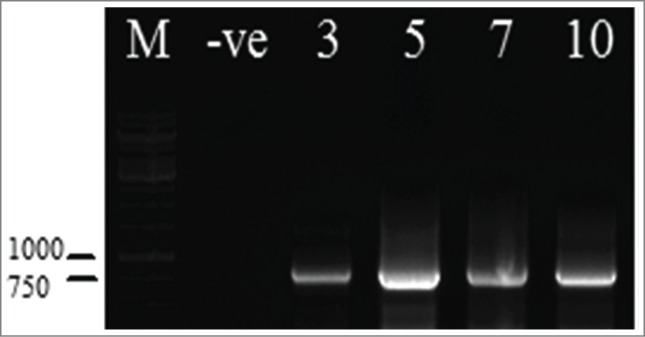
RT-PCR results of ELN-infiltrated Nicotiana leaves using ELN primers. for the RNAs extracted from using EL-F&EL-R primers. RT-PCR from leaves of infiltrated samples No. 3, 5, 7, and 10 days amplified ∼ 800 bp fragment, –ve represents not infiltrated plant used as negative control, M: 1kb ladder DNA molecular marker.
ELISA
Direct ELISA test was performed to test the expression of recombinant tropoelastin protein in total soluble proteins extracted from infiltrated plants. The antigen was used once as a crude lysate. The samples were coated in microtiter 96-well ELISA plate then identified with anti-tropoelastin serum followed by anti-rabbit conjugate.
As shown in Figure 4, ELISA values were expressed as the mean absorbance at wavelength (λ = 630A°). Compared to negative control (C−), the S3, S5, S7 and S10 infiltrated samples showed positive result after 15 min and 30 min of read time. The highest expression level of ELN gene within N. tabacum leaves was obtained at the fifth day post-infiltration followed by descending in expression level at 7th and 10th days post-infiltration.
Figure 4.
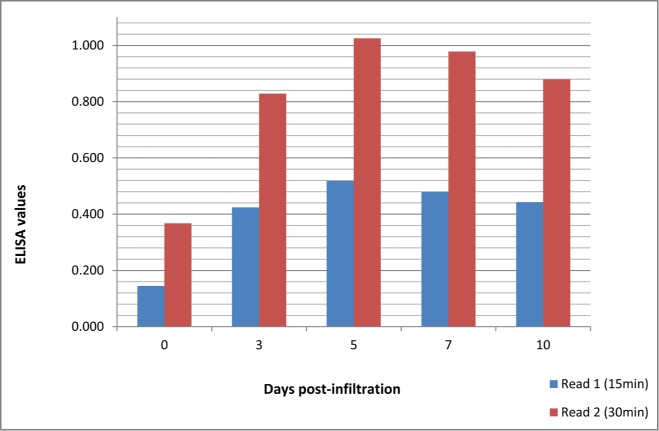
ELISA reading values showing protein expression level over time intervals post inoculation, measurements were taken after 15 and 30 min respectively. “0” represents negative control.
Western Blot Analysis
Western blot was performed to confirm the production of the human recombinant tropoelastin protein within plant's tissue. Total protein was extracted from infiltrated plants and subjected resolved on SDS-PAGE (10%) and then transferred on to nitrocellulose membrane and probed with tropoelastin antibody to detect the expression of the tropoelastin protein. Total protein from collected leaves was either used directly or was storing at -80 until used. Fresh cut leaves gave the expected blotted band of tropoelastin at ∼70 kDA for plant samples number 3 and 5 and their 1/10 dilution, while stored samples showed the two degraded forms of tropoelastin at ∼55 and ∼40 kDA (Fig. 5A and B, respectively). As anticipated it showed no reactivity with un-infiltrate d plants which was used as negative control.
Figure 5.
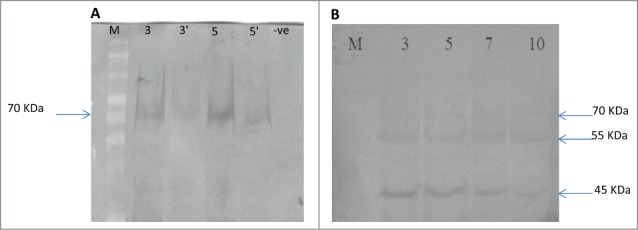
Western blot analysis for ELN-infiltrated into tobacco leaves, (A) from fresh prepared protein for infiltrated plant No. 3 and 5 and their dilutions (3' &5') and B. for stored protein from plants No 3, 5, 7, & 10 showing additional two degraded bands at ∼55 and ∼45 kDa. –ve: un-infilterated plant used as negative control, M: represents Page ruler plus pre-stained protein ladder.
Discussion
During the last decade, significant progresses have been made in the development of novel transient expression platforms to produce industrial and pharmaceutical proteins from plants. These technologies allowed us to produce high amounts of recombinant proteins within few weeks (Schipperus et al., 2009). In case of tropoelastin protein, by the use of transient expression system based on syringe agroinfiltration through pCambia1390-35S binary vector, we was able to adapt the system to produce human tropoelastin (hTE) protein in Nicotiana tabacum plant in a reduced period of time. This protein is similar to that produced by microbial fermentation (Indik et al., 1990).
In general, many studies have been employed to produce elastin like polypeptides (ELPs) based on peptide synthesis whose in vivo biocompatibility is not known yet. This problem was overcame by using recombinant DNA technologies (Schipperus et al., 2009). However many attempts have been carried out to produce elastin sequence-containing proteins (Floss et al., 2010), our research is the first time to express and produce full human tropoelastin protein (hTE) from plant cells.
In this work, Nicotiana tabacum has been selected as a host for expressing of the ELN because of the relative ease of production of transgenic plants among the Solanaceae, particularly (Pen et al., 1993; Komarova, 2010). Nicotiana tabacum plant proved to be the optimal widely used plants whose leaves are ideal for agroinfiltration procedures (Komarova, 2010). The sequence of the Homo sapiens elastin gene was used as a template biosynthesize a modified gene with a good expression into coetaneous plants by upgrading the codon adoption index (CAI) from 0.70 to 0.88.
The Agrobacterium-mediated transient expression system based on agroinfiltration was successfully used in this work and we were able to detect the expression of the synthetic gene in a very short time starting from 48 hr and it can persist for 10 days. To ensure the expression of the ELN gene in the infiltrated plants, compliance and authentic protocols were needed. For nucleic acid based protocols, Southern blot analysis and RT-PCR were used. While, for protein based detection, polyclonal antibodies was used in ELISA and western blot analysis.
Southern blot analysis and RT- PCR screening confirmed the presence and expression of ELN gene in DNA of tobacco leaves as they gave a positive results at 3rd day, 5th, 7th and 10th day post infiltration. This is in consistent with the a previous studies carried out by Ben-Amar et al., (2013) where they used agro-infiltration system for transient gene expression in grapevine (Vitis spp.).
Screening with direct ELISA was used to ensure the expression of the transgene. By using anti-tropoelastin serum, results revealed that maximum expression of tropoelastin protein was obtained 5 days post-infiltration (d.p.i), while the expression peak appeared at the 7th d.p.i with a decrease at the 10th day post-infiltration. Therefore, we are recommending to purify the recombinant protein from infiltrated leaves at 5th day post-infiltration to obtain the maximum of recombinant protein production.
Also, the production of HIV-1 Nef antigen from Nicotiana benthamian leaves was successfully detected via ELISA test (Circelli et al., 2010).
Western blot analysis was also carried out using anti-tropoelastin serum. The obtained results revealed the presence of one parent with fresh prepared protein band and two degraded bands (∼55- and ∼45 kDa) with stored protein (Chen et al., 2005; Pellicoro et al., 2012). The first band at ∼70 kDa represents the recombinant tropoelastin protein while the other two bands at represent the degraded form of the protein. This degradation may happen due to proteolytic activity in the extracted protein. Although tobacco is very convenient for a variety of proof-of-concept experiments, it may not be the ideal host because their vegetative tissue consists of over 90% water and this may result in increasing the activity of the proteolysis. Also, Liu et al. (2014) and Indik et al. (1990) were successfully produced the recombinant tropoelastin using microbial fermentation at ∼70 kDa on SDS-PAGE and immunoblots.
Material and Methods
Plant Material
Nicotiana tabacum seeds were grown in greenhouse. Seedling and germination of N. tabacum plants were carried out under artificial illumination (16h light/ 8h dark cycle) at a constant temperature of 25°C. For all agroinfilteration experiments, 6- to 8-weeks-old N. tabacum plants were used.
Biosynthesis of the ELN Gene
Homo sapiens elastin (NM_000501) was used as a template to biosynthesize the modified ELN gene. The restriction sites for EcoRI and BglII were added to the 5′ and 3′ ends of the gene, respectively. Codon usage was optimized and the gene synthesis was done by Genscript Inc. The 2175 bp-ELN fragment was cloned into pUC57 vector to facilitate gene subcloning into plant expression vector.
Construction of Plant Expression Vectors
The 2175 bp-ELN orf was excised from pUC57-ELN by digesting with both EcoRI and BglII and subcloned into pCambia1390-35S binary vector (modified from pCambia1390 by adding an addition 35S-promoter) in the corresponding sites and under the control of 35S-promoter (Figure 1). The transformed colonies were confirmed by restriction digestion. The recombinant plasmid pCambia-ELN was extracted from the selected colony and consequently was transformed into Agrobacterium to carry out agroinfilteration experiments.
Agroinfiltration of Plant Leaves
The pCambia-ELN construct was transformed into Agrobacterium strain GV3101. (Koncz et al., 1989) using electroporation technique (Sukharev et al., 1992) at 2.5 kV, 25 mF and 400 Ω. The transformed cells were plated on LB agar medium containing 50 mg/ml kanamycin and 50 mg/ml streptomycin.
Agroinfilteration was used for transient expression in N. tabacum with Agrobacterium strains as previously described (Yang et al., 2000). One hundred microlitres of transformed Agrobacterium frozen cells stock was inoculated in 5 ml LB broth supplemented with 50 mg/ml streptomycin and 50 mg/ml kanamycin. The culture was incubated at 28°C at 210 rmp shaking for overnight. Five hundred microlitre (μl) was used to inoculate 50 ml of LB medium. The cultural cells were incubated at 28°C shaking at 210 rpm until the culture had reached an O.D.600 = 0.6. The cells were harvested by centrifugation at 6000 rpm and resuspended in 50 ml MES buffer (10 mM MES; pH 5.5, 10mM MgCl2). This mixture was incubated for 2 hours at room temperature with 100 μM acetosyringone. The leaves 6- to 8-weeks old N. tabacum were pressure injected into the lower epidermis of the leaf through 3 ml disposable syringe. After infiltration, the plants were maintained for 10 days post-infiltration in a controlled growth chamber at 25 °C with a 16-h photoperiod. To avoid variability, leaves and location on the leaf, comparably-sized leaves for each plant of similar age were agro-infiltrated for each experiment.
Southern Blot Analysis
The individual agroinfiltrated leave with the pCambia-ELN was harvested at different time-intervals; 3, 5, 7 and 10 days post-infiltration, in addition to un-infiltrated plant was used as a control plant. DNA of infiltrated leaves was extracted by DNeasy Plant DNA mini kit, QIAGEN and fragmented by endonuclease enzyme; EcoRI. The recombinant ELN-orf released from pUC57-ELN was used as a probe. Labeling and detection were carried out using Biotin Deca Label DNA Labeling Kit, ThermoScientific and Biotin chromogenic Detection kit, ThermoScientific, respectively.
Detection of Chimeric Gene by RT-PCR
Total RNA was extracted from agroinfiltrated leaves using illustra RNAspin mini kit, GE healthcare. Oligonucleotides pair at the core region was designed to detect the presence of ELN gene at the core region; TE-F: 5′-CAGGAGTTTACCCTGGTGGA-3′, and TE-R: 5′-GTATTCCACCCACACCAAC-3′). One step RT-PCR was carried out according to manufacturer instructions using SuperScript®III with Platinum® Taq DNA Polymerase. The reaction was resulted in the expected 805 bp-fragment of the core region of the gene.
Enzyme Linked Immuno Sorbent Assay (ELISA)
Direct ELISA protocol was carried out to confirm the expression of the transgene after agroinfilteration. Total proteins were extracted using ELISA-extraction buffer (2% PVP, 0.02 MNa2SO3). 96-well ELISA plates were coated with 200 μl antigen; total soluble protein and then were incubated at 4°C for overnight. On the next day, plates were washed with washing buffer three times for 5 min each. The remaining protein-binding sites were blocked by adding 200 μl blocking buffer (PBS-Tween 20, 4% low-fat milk) and incubated for 2 hr at room temperature. After washing with PBS + tween 20, the anti-tropoelastin antibody was diluted by 1:1000 in blocking buffer and 200 μl was added each well. The plate was incubated in a humid chamber at 37°C for 3 hr. Then the plate was decanted and washed three times for 5 min each. 200 μl of the substrate buffer (0.2 g (NaN3), 97 ml diethanolamine, 600 ml H2O) was added to the plate and was incubated at room temperature until the color develops. Finally, absorbencies were read at 630 nm wavelength each 15 min in an automated ELISA reader.
Western Blot Analyses
Plant leaves were harvested and then grinded in liquid nitrogen. Total proteins were extracted using SDS-extraction buffer (2% SDS, 0.1% bromopheol blue, 10% glycerol), and the extracts were clarified by centrifugation at 14,000 g for 20 min at 4°C. Supernatants were transferred to fresh tubes, and the protein content was determined (Bradford 1976). Total proteins (40 μg) were separated by SDS-PAGE and then transferred onto polyvinylidene difluoride (PVDF) membranes. Polyvinylidene difluoride membranes were blocked for at least 2 h and then probed with rabbit anti-tropoelastin in a 1:500 dilution. After extensive washing, the membrane was incubated with the appropriate secondary antibody in a 1:5000 dilution and then was conjugated to alkaline phosphatase. BCIP/NBT (Amresco) was used for immunodetection.
Conclusion
A successful expression of the biosynthetic human elastin via agroinfilteration into Nicotiana tabacum was achieved in the present study. However, variation in protein expression was observed with different time post infiltration. Also, fresh extracted protein is recommended for purifying the expressed protein to avoid degradation by the proteolytic enzymes.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- Aboul-Ata A-AE, Vitti A, Nuzzaci M, El-Attar AK, Piazzolla G, Tortorella C, Harandi AM, Olson O, Wright SA, et al. Plant-based vaccines: novel and low-cost possible route for Mediterranean innovative vaccination strategies. Adv Virus Res 2014;89:1-37; PMID:24751193; http://dx.doi.org/ 10.1016/B978-0-12-800172-1.00001-X [DOI] [PubMed] [Google Scholar]
- Ben-Amar A, Cobanov P, Buchholz G, Mliki A, Reustle G. In planta agro-infiltration system for transient gene expression in grapevine (Vitis spp.). Acta Physiol Plant 2013; 35(11):3147-56; http://dx.doi.org/ 10.1007/s11738-013-1348-0 [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72(1-2):248-54; PMID:942051; http://dx.doi.org/ 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Chen Z, Seo JY, Kim YK, Lee SR, Kim KH, Cho KH, Eun HC, Chung JH. Heat modulation of tropoelastin, fibrillin-1, and matrix metalloproteinase-12 in human skin in vivo. J Invest Dermatol 2005; 124(1):70-78; PMID:15654955; http://dx.doi.org/ 10.1111/j.0022-202X.2004.23550.x [DOI] [PubMed] [Google Scholar]
- Circelli P, Donini M, Villani M. Efficient Agrobacterium-based transient expression system for the production of biopharmaceuticals in plants. Bioeng Bugs 2010; 1(3):221-4; PMID:21326930; http://dx.doi.org/ 10.1186/1472-6750-9-96.e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain AL, Vaishnav P. Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv. 2009; 27(3):297-306; PMID:19500547; http://dx.doi.org/ 10.1016/j.biotechadv.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Donini M, Lico C, Baschieri S, Conti S, Magliani W, Polonelli L, Benvenuto E.. Production of an engineered killer peptide in Nicotiana benthamiana by using a potato virus X expression system. Microbiology 2005; 71(10):6360–6367. http://dx.doi.org/ 10.1128/AEM.71.10.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firek S, Draper J, Owen MR, Gandecha A, Cockburn B, Whitelam GC. Secretion of a functional single-chain Fv protein in transgenic tobacco plants and cell suspension cultures. Plant Mol Biol 1993; 23(4):861-70; PMID:8251638; http://www.ncbi.nlm.nih.gov/pubmed/8251638. Accessed February 4, 2015 [DOI] [PubMed] [Google Scholar]
- Floss DM, Schallau K, Rose-John S, Conrad U, Scheller J. Elastin-like polypeptides revolutionize recombinant protein expression and their biomedical application. Trends Biotechnol 2010; 28(1):37-45; PMID:19897265; http://dx.doi.org/ 10.1016/j.tibtech.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Hefferon K. Plant-made vaccines. J Vaccines Vaccin 2012; 03(04):7560; http://dx.doi.org/ 10.4172/2157-7560.1000e108 [DOI] [Google Scholar]
- Indik Z, Abrams WR, Kucich U, Gibson CW, Mecham RP, Rosenbloom J. Production of recombinant human tropoelastin: characterization and demonstration of immunologic and chemotactic activity. Arch Biochem Biophys. 1990; 280(1):80-86; PMID:2191629; http://www.ncbi.nlm.nih.gov/pubmed/2191629 [DOI] [PubMed] [Google Scholar]
- Ko K. Expression of recombinant vaccines and antibodies in plants. Monoclon Antib Immunodiagn Immu 2014; 33(3):192-8; PMID:24937251; http://dx.doi.org/ 10.1089/mab.2014.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova T. Transient expression systems for plant-derived biopharmaceuticals. Expert Rev Vaccines 2010; 4(4):58-74 [DOI] [PubMed] [Google Scholar]
- Koncz C, Martini N, Mayerhofer R, Koncz-Kalman Z, Körber H, Redei GP, Schell J. High-frequency T-DNA-mediated gene tagging in plants. Proc Natl Acad Sci U S A 1989; 86(21):8467-71; PMID:2554318; http://dx.doi.org/ 10.1073/pnas.86.21.8467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wise SG, Rnjak-Kovacina J, Kaplan DL, Bilek MM, Weiss AS, Fei J, Bao S. Biocompatibility of silk-tropoelastin protein polymers. Biomaterials. 2014;35(19):5138-47; PMID:24702962; http://dx.doi.org/ 10.1016/j.biomaterials.2014.03.024 [DOI] [PubMed] [Google Scholar]
- Metcalfe K, Rucka AK, Smoot L, et al. Elastin: mutational spectrum in supravalvular aortic stenosis. Eur J Hum Genet 2000; 8(12):955-63; PMID:11175284; http://dx.doi.org/ 10.1038/sj.ejhg.5200564 [DOI] [PubMed] [Google Scholar]
- Moloney MM. “Molecular Farming” in Plants: Achievements and Prospects. Biotechnol Biotechnol Equip 2014; 9(1):3-9; http://dx.doi.org/ 10.1080/13102818.1995.10818814 [DOI] [Google Scholar]
- Nivison-Smith L, Weiss A. Elastin based constructs. In: Regen Med Tissue Eng Biomater InTech 2011:232-40; http://dx.doi.org/ 10.5772/23660 [DOI] [Google Scholar]
- Pellicoro A, Aucott RL, Ramachandran P, Robson AJ, Fallowfield JA, Snowdon VK, Hartland SN, Vernon M, Duffield JS, Benyon RC, et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology 2012;55(6):1965-75; PMID:22223197; http://dx.doi.org/ 10.1002/hep.25567 [DOI] [PubMed] [Google Scholar]
- Pen J, Verwoerd TC, van Paridon PA, Beudeker RF, van den Elzen PJ M, Geerse K, van der Klis JD, Versteegh HAJ, van Ooyen AJJ, Hoekema A.. Phytase-containing transgenic seeds as a novel feed additive for improved phosphorus utilization. Bio/Technology 1993; 11(5):811-14; http://dx.doi.org/ 10.1038/nbt0793-811 [DOI] [Google Scholar]
- Schipperus R, Teeuwen RLM, Werten MWT, Eggink G, de Wolf FA. Secreted production of an elastin-like polypeptide by Pichia pastoris. Appl Microbiol Biotechnol 2009; 85(2):293-301; PMID:19565236; http://dx.doi.org/ 10.1007/s00253-009-2082-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamloul M, Trusa J, Mett V, Yusibov V. Optimization and utilization of Agrobacterium-mediated transient protein production in Nicotiana. J Vis Exp 2014; (86); PMID:24796351; http://dx.doi.org/ 10.3791/51204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons PC, Dekker BMM, Schrammeijer B, Verwoerd TC, van den Elzen PJM, Hoekema A. Production of correctly processed human serum albumin in transgenic plants. Bio/Technology 1990; 8(3):217-21; http://dx.doi.org/ 10.1038/nbt0390-217. [DOI] [PubMed] [Google Scholar]
- Sparkes I, Runions J, Kearns A, Hawes C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 2006; 1(4):2019-25; PMID:17487191; http://dx.doi.org/ 10.1038/nprot.2006.286 [DOI] [PubMed] [Google Scholar]
- Sukharev SI, Klenchin VA, Serov SM, Chernomordik LV, Chizmadzhev YuA. Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys J 1992; 63(5):1320-7; PMID:1282374; http://dx.doi.org/ 10.1016/S0006-3495(92)81709-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rnjak J, Wise SG, Mithieux SM, Weiss AS. Severe burn injuries and the role of elastin in the design of dermal substitutes. Tissue Eng Part B Rev 2011; 17(2):81-91; PMID:21091393; http://dx.doi.org/ 10.1089/ten.TEB.2010.0452 [DOI] [PubMed] [Google Scholar]
- Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J 1993; 7(13):1208-18; PMID:8405806; http://www.ncbi.nlm.nih.gov/pubmed/8405806 [PubMed] [Google Scholar]
- Rymerson RT, Menassa R, Tobacco JEB. A platform for the production of recombinant proteins. In: Erickson L, Yu W-J, Brandle J, Rymerson R, eds. Molecular farming of plants and animals for human and veterinary medicine. Dordrecht: Taylor & Francis; 2002:1-31; http://dx.doi.org/ 10.1007/978-94-017-2317-6. [DOI] [Google Scholar]
- Twyman RM, Stoger E, Schillberg S, Christou P, Fischer R. Molecular farming in plants: host systems and expression technology. Trends Biotechnol 2003; 21(12):570-8; PMID:14624867; http://dx.doi.org/ 10.1016/j.tibtech.2003.10.002 [DOI] [PubMed] [Google Scholar]
- Wise SG, Weiss AS. Tropoelastin . Int J Biochem Cell Biol 2009; 41(3):494-7; PMID:18468477; http://dx.doi.org/ 10.1016/j.biocel.2008.03.017 [DOI] [PubMed] [Google Scholar]
- Yang Y, Li R, Qi M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 2000; 22(6):543-51; PMID:10886774 [DOI] [PubMed] [Google Scholar]


