Abstract
ABSTRACT. “New Breeding Techniques” (NBTs) are a group of recent innovations in plant breeding using molecular biology tools. It is becoming evident that NBTs can introduce advantageous traits for agriculture that could be commercially available very soon However, there is still a need of clarifying its regulatory status, particularly in regards to worldwide regulations on Genetically Modified Organisms (GMOs).
This article reviews the meaning of the NBTs concept, performs an overall regulatory analysis of these technologies and reports the first regulation in the world that is applied to these technologies, which was issued by the Argentine Government.
KEYWORDS: NBTs, GMO regulation, gene editing, biosafety, gene targeting, genetic modification, agriculture
Abbreviations
- NBTs
New Breeding Techniques
- GMO
genetically modified organisms
- DNA
Deoxyribonucleic acid
- CPB
Cartagena Protocol on Biosafety
- LMO
Living modified organism
- SDN
Site–Directed Nucleases
- ZFNs
Zinc Finger Nucleases
- MNs
Mega Nucleases
- TALENs
TAL Effector Nucleases
- RdDM
RNA-Dependent DNA Methylation
- ODM
Oligonucleotide-Directed Mutation
- RNA
Ribonucleic acid
- RNAi
RNA interference
INTRODUCTION
“New Breeding Techniques” (NBTs) is a term coined recently in reference to emerging technologies for creating genetic diversity in plants using molecular biology techniques. As a category, “NBTs” is neither a science-based nor a strict regulatory term; actually, it does not have a strict definition. Although some lists of technologies have been put together to illustrate the concept (New plant breeding techniques [Internet]), there is no unified universal list.
The term NBTs emerged as a way of referring to an array of technologies where their advocates hope they will not be considered transgenic or “genetically modified” organisms (GMOs) in the usual regulatory sense –and therefore be exempted of the regulation for transgenic products– although it is recognized that regulators still have to consider the issue, and therefore there is a need of debate and clarification.
What Are the NBTs?
As mentioned, the term NBTs was not coined for scientific reasons, there is no definition, and basically it seeks to establish some sort of contrast with “traditional” genetic modification understood as Genetically Modified (GM) crops as those that have been in commercial use for more than 2 decades now (Brookes and Barfoot, 2013).
The main international reference instrument for GMO regulation is the Cartagena Protocol on Biosafety (CPB) (Text of the Cartagena Protocol on Biosafety [Internet]) which is the source for the more accepted definition of GMO (called LMO in CPB):
"Living modified organism” means any living organism that possesses a novel combination of genetic material obtained through the use of modern biotechnology;”
…where modern biotechnology is defined as…
“Modern biotechnology” means the application of:
-
a.
In vitro nucleic acid techniques, including recombinant DNA and direct injection of nucleic acid into cells or organelles, or
-
b.
Fusion of cells beyond the taxonomic family, that overcome natural physiological reproductive or recombination barriers and that are not techniques used in traditional breeding and selection;”
These definitions are used in most regulatory systems in the world for the regulation of LMO, GMO, “biotechnology crops” or “transgenic” organisms.
Until recently, whether a new crop was considered a GMO or not was a straightforward issue for regulators, biotechnology developers and public worldwide. Non GMO are supposed to be obtained by some other breeding techniques, which are collectively and informally named “conventional” breeding.
However, now the so-called NBTs somewhat resemble the production methods of “modern biotechnology” because they employ molecular biology tools, but the genetic modifications that they introduce, in some cases at least, are more alike the new crops obtained by the so-called “conventional” breeding.
During the past decade in certain countries and regions, particularly in Europe (Tiberghien 2009), negative public policies and cumbersome regulation against GMOs lead to any crop biotechnology project having no real chances of becoming a commercial product in those territories. In the remainder of the world, GMO regulation imposes a slow pace of adoption for biotech products developed by multinational companies, and makes it extremely difficult for a public sector or small company product to reach the market.
Therefore, the actual development of crops biotechnology seems severely delayed and biased in comparison with its real potential (Lema, 2012). Such situation rarely motivated to reconsider the regulatory burden by governments, so it led the more disadvantaged developers to seeking shortcuts; one of the earlier attempts was the coining of the “cisgenic” concept in Europe (Schouten et al., 2006). More recently, NBTs are seen by some as promising alternatives for introducing traits using molecular biology tools but potentially without the burden of GMO regulation (Podevin et al., 2012) (not to mention the prejudice against GMOs in some elements of public opinion fostered by misinformation) (Contrary to popular belief (Editorial), 2013; Levidow et al., 2000).
NBTs are described in detail elsewhere (Chair of the UK's Advisory Committee on Releases to the Environment [Internet]). Therefore, we only provide a short summary of some of them for illustrative purposes:
SDN: (Site –Directed Nucleases) (Lusser and Davies 2013): Set of techniques based in the use of nucleases that introduce break in the DNA chain near a defined target sequence. After the action of DNA repair mechanisms and, in some cases, the use of additional nucleic acid molecules, different kind of site-directed modifications may remain. These range from deletions of a few nucleotides to insertion of additional sequences. For example Zinc Finger Nucleases (ZFNs) (Lloyd et al., 2005), Mega Nucleases (MNs) (Honig et al., 2015), TAL Effector Nucleases (TALENs) Christian et al., 2010), and CRISPR-CAS9 (Shan et al., 2013).
Epigenetic Modification: techniques that generate epigenetic changes in the genome, such as RNA-Dependent DNA Methylation (RdDM) (Wu et al., 2010), in a way that the expression of specific genes is changed by heritably means without affecting the primary genomic sequence.
Reverse Breeding (Wijnker et al., 2012): these techniques involve an intermediate generation of GM plants were a transgene is present to suppress meiosis transiently. After its job is done, the transgene is removed by off crossing, and no foreign genetic material is present in the plant variety to be released commercially.
Accelerated Breeding (Comeau et al., 2001): these techniques involve an intermediate generation of GM plants were a transgene is present to shorten the juvenile phase of a plant, hence speeding up the breeding process, for instance in trees. As with reverse breeding, the transgene is expected to be removed later by outcrossing and segregation; in such a case no foreign genetic material needs to be present in the end product.
Grafting on GM Rootstock (Aguero et al., 2005): Cases where there is a GMO rootstock on which a non GMO scion (and its harvest) remains free of transgenic DNA.
Agro-Infiltration (Joh, Vandergheynst,): techniques where transgenes are transiently expressed in a targeted tissue of the plant, usually leaves, which are infiltrated with a liquid suspension of Agrobacterium sp. containing a genetic construct. This includes the original Agroinfiltration technique (Leckie and Stewart, 2010; Agroinfection, Grimsley et al., 1986; Floral Dip, Zhang et al., 2006 and Magnifection. Gleba et al., 2005).
ODM: Oligonucleotide-Directed Mutation is a site-specific gene modification system based on synthetic oligonucleotides having the target genomic sequence with a small mutation. These are introduced into the cells and modify the target sequence after pairing with it and being recognized by the DNA repair mechanism (Schaart and Visser, 2009).
Cisgenesis: A cisgene is an existing natural gene from the crop plant itself or from sexually compatible species with its native regulatory sequences, such as promoter and terminator. Therefore, the gene belongs to the traditional breeders' gene pool and is the already existing result of natural evolution. In theory, the gene of interest could have also been moved from one species to the other using “traditional breeding” with equivalent results (Krens et al., 2015).
Intragenesis: An intragene is a gene comprising of functional elements, such as coding part, promoter and terminator originating from different genes from the crop plant itself or from crossable species. Therefore, all gene elements belong to the traditional breeders‟ gene pool, but in contrast to cisgenesis, an intragene is a manmade genetic construct (Holme et al., 2013).
Regulatory Approaches in the World
So far, there are very few precedents of governmental decisions regarding whether a crop obtained by one or another NBT is under the regulation usually applied specifically for GMOs. A glimpse of current regulatory landscape for products derived from NBTs is provided next, more information can be found elsewhere (International Conference on New Plant Breeding Molecular Technologies. [Internet]; The regulatory status of New Breeding Techniques, June 2015; Wolt et al., 2015):
US
USDA perform a determination of the regulatory status of products derived from NBTs is based in a case by case analysis under the US Plant Protection Act. However, neither EPA nor FDA has specifically articulated a policy approach on NBT products. Some products have been already cleared by USDA as not being under the regulation usually applied to GMOs (Schiemann and Hartung, 2013). However, the US regulatory criterion for biotechnology is based on its very particular legal framework; therefore a determination of regulated/non-regulated status cannot be readily “transplanted” to most other countries which base their regulation on language similar to that in the CPB. The US decisions in this matter have, therefore, mostly a commercial relevance since the country is a major exporter of agricultural products where NBTs-derived ones may be contained in the near future. The US government has announced a major update of the regulatory system, which would include a “horizon-scanning” of the future landscape of biotechnology products (Matz and Hahn, 2015), although the announcement did not mention NBTs specifically, this may entail developing some specific regulatory considerations for them.
Canada
Canada's Biotechnology Regulatory Framework dates from 1993. It is a product-based approach triggered by novelty. The concept for regulatory trigger is “Plants with Novel Traits,” this is plants containing a trait not present in the same species already existing as cultivated populations in Canada. Canada's regulatory system applies to novel plants/foods, irrespective of the breeding technique. Therefore, it is not expected to require any amendment to accommodate case-by-case decisions on products derived from NBTs. However, once more, this is very different to how regulation on crop biotechnology works in virtually the rest of the world, which is triggered by production method. Also in this case, Canadian determination of a NBT-derived product being specifically regulated because it is considered a novel plant/food is not readily transplantable to other regulatory system overseas.
Australia and New Zealand
New Zealand is a party to the CPB, while Australia is not. However, both countries have their particular definitions and regulations to establish what is a regulated GMO.
Current Australian legislation for gene technology came into effect on 2001. Its definitions are applied to determine whether these new plant breeding techniques are covered by the regulatory scheme, on a case by case basis.
Food Standards Australia New Zealand (FSANZ) convened an expert scientific panel in 2012 and 2013 to provide advice on how to regulate different plant breeding techniques (New plant breeding techniques workshops, 2015). The panel grouped NBTs in 3 categories. The first one comprises cisgenesis, intragenesis, SDN-3 and GM rootstock grafting; the expert group concluded that products derived from theses NBTs should be regarded as GM, although a simplified form of safety assessment may be warranted. The second category includes ODM and SDN-1, where products derived from them should not be regarded as GM. Finally, the third category comprises gene technologies at an early stages that are separated from the final plant during the breeding process, such as reverse breeding. For products in this category, the panel concluded that they are not GM but there is a need to confirm the reliability of the breed out process.
In addition to the expert consultations by FSANZ, the Environmental Protection Agency of New Zealand determined that certain products derived from NBTs were not considered GMO under New Zealand regulatory definitions. This was not a product by product determination but rather applicable on the technologies in general. However, this administrative decision was defied in the High Court, which ruled that the EPA did not have authority to decide this since it is a legislative matter in New Zealand (Mokena-Lodge, 2015).
EU
After inquiries from the academic sector as well as the biotechnology industry regarding the regulatory status of plants obtained by NBTs, an ad hoc Working Group was established by the European Commission. It consisted of experts from Competent Authorities from the European Member States and finalized its Report in 2012. Although the Report is not yet published, the European Commission has started a legal analysis of it. The expected outcome of this process, a guideline document, is expected to be circulated to the Member States for comments before the end of 2015.
In addition, the Joint Research Center (JRC) of the EU reviewed the state of the art and published a report in 2011 (Lusser et al., 2011), the European Food Safety Authority (EFSA) has published safety assessment criteria on only some NBTs (EFSA Panel on Genetically Modified Organisms, 2012). These NBTs are cisgenesis, intragenesis and ZFN type 3, which constitute the less debatable NBTs, this is, the ones with higher chances of its derived products to be universally considered GMO (see below in the section referring to the debate in Argentina).
Finally, an European platform consisting of small, medium and large industry representatives, as well as academic and research institutes also conducted a thorough analysis of the legal status of new breeding techniques in 2013 (NBT Platform, 2013 July).
OECD
The Organization for Economic Cooperation and Development has a permanent Working Group on Harmonization of Regulatory Oversight in Biotechnology. This Working Group is addressing NBTs as an emerging issue, and therefore have gathered background information on the technologies and country experiences. A Workshop was held (Working Group on the Harmonisation of Regulatory Oversight in Biotechnology Report of the OECD workshop on environmental risk assessment of products derived from novel plant breeding techniques, 2015 March) and a Questionnaire circulated to gather country by country information. Most have reported that developments are still at research phase at the national level, and authorities are only beginning to consider if and how NBTs-derived products are to be regulated.
From the OECD report and others quoted above, it is clear that important players in the production and trade of products derived of biotechnology crops are, at the best, aware of the issue but have just begun their own analysis of it. This includes India, China, Japan, Korea, The Philippines, Mexico, Peru, Brazil, South Africa, Russia, among others.
In summary, it can be seen that a few countries have functioning mechanisms in place to determine the regulatory status of products derived from NBTs, however these are the less useful as a source of inspiration for most other countries because of gross differences in legal frameworks and definitions. Some other countries are debating internally but have not reached a conclusion. And the remainder, which constitute the majority of countries in the world, still have no policy or criteria regarding products derived from NBT despite its potential importance for agriculture and trade.
Argentinean Regulatory System for Products Derived from NBTs
Argentine regulatory system for GMO is one of the oldest and more recognized ones (Burachik and Traynor, 2002). For instance, recently the Organization for Food and Agriculture of the United Nations (FAO) has recognized the Argentine Biosafety Commission CONABIA as Center of Reference for the Biosafety of GMO (La FAO y Argentina refuerzan la seguridad en biotecnología, 2015).
As one of the leaders among regulatory systems in the world, in Argentina it was also recognized early that products derived from NBTs were appearing in the cutting edge of crop biotechnology. So an early debate among regulators and policymakers was undertaken for more than 3 years, which gave rise to a regulation specifically aimed to clarify the status of any product derived from NBTs under the current GMO regulation.
Of course, as it is usual in these situations, the debate was not linear, it took into consideration many inputs, and some ideas were considered and discarded along the way. For the sake of brevity and clarity, the following is an ordered presentation of the main prevailing criteria that explains the basis of the new Argentine regulation, described later in this article.
Cartagena Protocol Definition
Argentina uses the CPB definitions provided above in its regulatory system. By the time the CPB was framed, Argentina was one of the few countries in the world with a full functioning regulatory system for GMO biosafety assessment. The Argentine experience was taken into account in the formulation of the CPB and the current Argentine regulatory system is fully compatible with it. As a consequence, Argentina and most of its partners for transboundary movements of GMO currently base their regulation on language similar to that in the CPB. Therefore, whatever solution found to the NBTs dilemma could, in principle, be applied in the same way by most other countries.
During our debate, we noted that no difficulties emerged with interpreting the term “organism” or “modern biotechnology” (which in practice means the use of recombinant DNA at some step of the breeding process). Only the term “novel combination of genetic material” was a matter of debate regarding its interpretation.
As a conclusion, “novel combination of genetic material” should be the key to decide if a product derived from NBTs (where NBTs are novel techniques that use recombinant DNA as an aid during the breeding process) is considered or not a GMO.
Flexibility for Future Technologies
As mentioned before, there is no unified reference list of NBTs, nor there should be one since these technologies keep on emerging. For instance, in the seminal lists CRISPR-Cas9 was not included, since that technology was invented later, however at present time this is probably the most promising NBTs. If, hypothetically, an early regulation was based on the lists of the year 2011, it would already be outdated by now, only a few years later. In addition, although in scientific papers a technology name may be perceived as a clear denomination, discussion with policymakers in Argentina revealed that it was not easy to produce “satisfactory” (technically clear, fit to purpose) legal definitions of the various technologies.
As a conclusion, a new regulation on NBTs should not be based on a closed list or description of particular technologies, but instead it should be framed to be flexible and able to be applied to existing or forecoming technologies as much as possible.
Case by Case Analysis
as it has been noted in the preceding paragraph, although certain technology names such as “cisgenesis," “reverse breeding," “site-directed nucleases” may be satisfactory for a loose scientific discussion, when comparing different implementations of an NBTs by different research groups, it can be seen that differences from one case to another makes it difficult to adopt a definition of one of these technologies for regulatory purposes. For similar reasons, it is difficult arriving to a “technology-broad” criterion regarding the regulatory status of end products since these can differ significantly.
As a conclusion, the analysis to establish if a certain NBTs-derived crop is a GMO or not can only be made product by product.
Anticipation at the Development Stage
NBTs may result more convenient than the “older” ways of obtaining GMOs for certain traits. However, in many cases NBTs are being used for introducing traits that have been already obtained or can be easily obtained by “traditional” transgenesis techniques that are widely mastered by many plant molecular biology laboratories. Additionally, in some cases traditional transgenesis strategies could be based on genetic elements that are off patent, while most NBTs will still be proprietary technologies for several years.
Nevertheless, in some cases the developer may favor the use of some NBTs because he/she is aiming to a product that he/she hopes won´t have the heavier burden of GMO regulation (Hou et al., 2014). This happens both in main agricultural biotechnology companies and also in national research institutes with limited resources.
But if the resulting crop ends up being regulated as a GMO, this bet would be a double loss, since in addition to regulation there is the burden of royalties on new technologies and the delay for obtaining and mastering these new technologies and genetic tools. For bigger companies this may mean some negative difference; but for public sector projects and small biotech companies in most cases losing this bet would hamper projects that otherwise could have been successful if based on older “standard” GMO technology.
As a conclusion, a mechanism to determine the regulatory status of NBTs-derived products should be able to provide predictability regarding the regulated / non-regulated status of a particular product at the beginning of the project.
Minding the Regulatory Gap
There is a big elephant in the room of regulation for the introduction of novel plant varieties. Genetic modification through transgenesis may introduce certain risks, and this possibility is the justification for the regulation that is applied worldwide to these products. However, traditional breeding techniques widely used to obtain new varieties, like mutagenesis and crossing with wild relative species, introduce the same kind of risks and, arguably, with a higher degree of ignorance about them, i.e. of risk. Whatever the risk: allergenicity of new proteins, transference of fitness advantages to weeds, impacts on non-target organisms (such as beneficial insects), these risks can be introduced both from GMO and “traditionally bred” varieties, and examples of the latter abound. Therefore, from a purely science-based perspective, if the risks are the same, the controls and regulation should be the same. However, this is not the case in virtually all over the world (excepting perhaps Canada according to what has been described above).
Socially and politically speaking, a population (a country) may freely choose its “appropriate level of protection” for a certain class of products (Atik, 2011) such as new plant varieties. This level cannot be determined purely on scientific grounds, since it involves subjective elements including outweighing more or less controls/safety against accessibility/benefits (Sgrillo, 2015). In addition, from a rational perspective and following the “equivalence principle," the level of protection should be related to the intrinsic characteristics of the product and not from the method of obtaining it, whereas 2 methods can produce products of equivalent characteristics.
Once an “adequate” level of protection is chosen, the same should be applied, for instance, to a GM potato or a potato harboring a gene coming from a wild relative (Song et al., 2003). This is not the case because of historic, commercial and social reasons beyond the scope of this article. This is not a claim that GMOs are over regulated or non GM crops are not under adequate control since, once more, the level of safety is subjectively to be chosen by the population. But the level should be the same for all these products. Neither in Argentina nor the rest of the world it seems that regulation will be “equalized” as it should in the short term.
Therefore, when a product derived from a NBTs is considered to be either in the GMO or non GMO box on the basis of regulatory definition, the technology is also straddling between different levels of safety assessment.
As a conclusion, it may be possible that the GMO regulator establishes that an NBT-derived product is not a GMO but, being proficient in safety assessment, he/she finds a risks hypothesis associated with the novel trait in the product. In such cases, as a practical compromise and a matter of responsibility as public servant, the risk hypothesis must be communicated to the appropriate regulator of non-GMO new plant varieties for further consideration.
Discarded Ideas
during the debate in Argentina, as mentioned, ideas coming from other regions were considered, and some of them discarded. Since these may be still under consideration elsewhere, it may be worthwhile to mention how they were analyzed.
For instance, the “20 bp rule” (see page 8 in ref. EFSA Panel on Genetically Modified Organisms, 2012) for discerning what is a novel combination of genetic material; however very practical as a touchstone, this criterion does not have a sound base. The reasons argued to sustain it (related to detectability and similarity with spontaneous mutations) are arguably true as facts; but in the end they do not relate to regulatory definitions, which are the only acceptable base for establishing the scope of the regulations.
Another example of a discarded idea is RNA interference (RNAi) considered to be among the NBTs (Krens and Kamo 2013). GM crops whose traits rely on RNAi have been around for long both in labs (Waterhouse et al., 1998) and in the fields (Gonsalves, 2002). However, in some regulatory offices there may be little exercise of technology monitoring there have been no applications mentioning RNAi until recently; consequently RNAi may look like a novelty and perhaps this is why it was included in some lists. Nevertheless, RNAi-based crops are modified using quite usual genetic engineering and ordinary genetic constructs that remain in the final product, therefore they are clearly GMOs.
Overall Analysis of Example NBTs
During the process leading to the new piece of regulation for products derived from NBTs, the National Advisory Commission on Agricultural Biotechnology of Argentina reviewed several literature examples of the technologies described above. The following is not a final conclusion, but just an indication of the likeliness of a product derived from these example NBTs to be considered GMO. The final Argentine regulation, as it will be described in the following section, relies on a case by case (product by product) assessment.
Cisgenesis, intragenesis, floral dip, SDN-3 and Synthetic Biology: it was noted that these techniques are usually based in generating a man-made genetic construct introduced in the plant genome. In addition, in most cases the construct may code for a new protein (excepting cisgenesis/intragenesis) or functional element. Thus, the resulting product in most cases would be considered to have incorporated a new combination of genetic material, and therefore would be considered a GMO.
Some experts noted that this conclusion is more arguable in the case of cisgenesis, given the array of different examples in the scientific literature (Eriksson et al., 2014; Eriksson et al., 2014).
It was also pointed out that in the case of Synthetic Biology (Gibson et al., 2010), this conclusion is highly speculative, since there are not real examples of Synthetic Biology crops yet. In addition, the term has been used in very different ways in the scientific literature, from organisms whose whole genome is manmade, to others where the organism has been modified to incorporate a few novel genes.
For grafting between GM and non GM plants, conclusion was that the whole plants to be commercially released are likely to be regulated as GM plants, regardless of the GM part being the rootstock or scion, both for environmental and food safety assessment purposes.
Food safety assessment in cases where the GM part is the scion would be required prima facie because current Argentine regulation demands a food safety assessment process for all GMOs to be authorized for commercialization. Such assessment may be simpler or require as much data as for traditional GM crops, depending on the potential for the modification made in the rootstock to generate a systemic alteration in the composition of the harvested products in the scion.
For SDN-1 and SDN-2, the modification in the plant genome is usually a small deletion of the pre-existing genomic sequence. As a result, it is not expected to find a new combination of genetic material in the plant genome. However, usually the technique involves transgenesis with the SDN gene in some intermediate plant generation. In such cases, the applicant must show evidence of the SDN transgene removal from the final product by outcrossing or otherwise it would be still presumably a GMO.
Similarly, in the case of crops derived from Reverse Breeding, the expected outcome of the technique would not likely be considered a new combination of genetic material. However, as in the earlier case, these techniques usually recur to a transgene that may be removed later, so evidence of such removal is needed.
In regards to RNA-Dependent DNA Methylation (RdDM), and presumably other kinds of Epigenetic Modification, these kinds of modifications are not considered a new combination of genetic material. In some cases there could be a need of removing DNA insertions. However, it was also recognized that these modifications may result unstable and revert after a few generations, therefore they may have little commercial interest in practice.
In the case of ODM, depending on the extent and nature of the modification into the genome plant, in some cases the product derived from these techniques could be considered non-GMO.
Agroinfiltration techniques are mostly used in a research context. It is unlikely that they will be of practical use for commercial purposes in agriculture, except in the case of molecular pharming (Abiri et al., 2015). In any case, genetic modifications are done on mature plants and are not heritable. Actually the GMO to be released into the environment and the appropriate subject of regulation should be the GM microorganism.
How Regulation Works
Resolution no. 173/15 of the Secretariat of Agriculture, Livestock and Fisheries is included in this article as supplementary material. This regulation incorporates the criteria detailed before, and establishes procedures to determine in which cases a crop obtained by breeding techniques involving modern biotechnology does not fall under GMO regulations.
To such end, applicants shall submit each product (NBTs-derived crop) to establish whether the result of the breeding process is a new combination of genetic material or not. A genetic change shall be always regarded as a new combination of genetic material when a stable and joint insertion of one or more genes or DNA sequences that are a part of a defined genetic construct have been introduced permanently into the plant genome.
Also, if appropriate, it must be established if there exists enough scientific evidence to support the absence of the transgenes that may have been used transiently during the crop breeding process.
The procedure includes a 60-day time limit and in the end the applicant receives a reply from the authorities stating if the product described is under the GMO regulations or not.
In case the crop is not required to be regulated as a GMO but its features and/or novelty lead to a significant risk hypothesis, this must be also reported by the regulatory commission and such report is channeled to the appropriate regulator of varieties obtained by “conventional” breeding for consideration.
For projects: Applicants are also allowed to file preliminary inquiries, aiming at anticipating whether a hypothetical expected product would fall under the GMO regulation. This is applicable to projects still in the design stage.
In these cases, the governmental assessment is performed partially on the basis of expectations from the developer, so it will have only a preliminary status. When the new crops are finally obtained, the applicant must still return to the regulator and submit factual determinations about the genetic modification actually generated. Only in case the product possesses those features anticipated in the preliminary inquiry, the earlier assessment regarding its regulatory status would remain.
Please refer to Figure 1, which summarizes how Argentine regulation works.
What About Animals and Microorganisms?
Some variants of the techniques mentioned in this article are nowadays quite standard also for the genetic modification of animals (Yu et al., 2011; Carlson et al., 2013; Tan et al., 2013; Ni et al., 2014; Wang, 2015) and microorganisms. However, the term NBTs is not really used yet beyond plant products. This is because, as mentioned before, the regulatory debate has emerged in relationship with plant products; additionally, regulatory systems for GM plants are in general more developed and sophisticated because more quantity and variety of such products are continuously presented to regulatory systems worldwide for evaluations at different stages.
Nevertheless, it is important to recognize that regulatory frameworks in most cases begin with the same definition of GMO, irrespective of biological kingdom. Therefore, whatever criteria developed for products derived from plant NBTs (also referred to as NPBT) it should be applied to new breeds of animals and microorganism strains developed with the aid of “modern biotechnology” tools.
CONCLUSION
At the time this article was submitted, Argentina is the only country with a regulatory framework where a specific working regulation has been issued for explicitly dealing with products derived from NBTs.
The regulation is the outcome of a 3-year debate which took into account the state of the art in NBTs and parallel discussions overseas. It is a product-by-product consideration of the genetic modifications at the light of the concept of “novel combination of genetic material." It allows developers to anticipate its applicability for a certain product at the design stage, and it takes into consideration the regulatory imbalance between GMOs and “traditional breeding” techniques, as described.
This procedure found to establish if a product derived from NBTs is or not a GMO is fully compliant with the Cartagena Protocol, so it could be also be applied in the same way by most other countries in the world that share the CBP language as the base for their regulation.
It is very important that countries worldwide now work on the harmonization of the regulation of NBTs. Such harmonization is deeply needed to avoid arbitrariness that could lead to regional asymmetries in scientific and technical developments, as well as in the access of farmers and consumers to new products. Of course, this is also needed to prevent conflicts in international trade.
We have learned a lot from the introduction of GMO in agriculture and the formulation of ad-hoc regulations from them. These lessons should not be disregarded in the consideration of the regulatory status and criteria for the risk assessment of products derived from NBTs, which may constitute a bridge to mitigate the tensions derived from the regulatory, commercial and technological imbalance artificially created between GMO and other breeding techniques.
DISCLOSURE
The information and views are those of the authors as individuals and experts in the field, and do not necessarily represent those of the organizations where they work.
Figure 1.
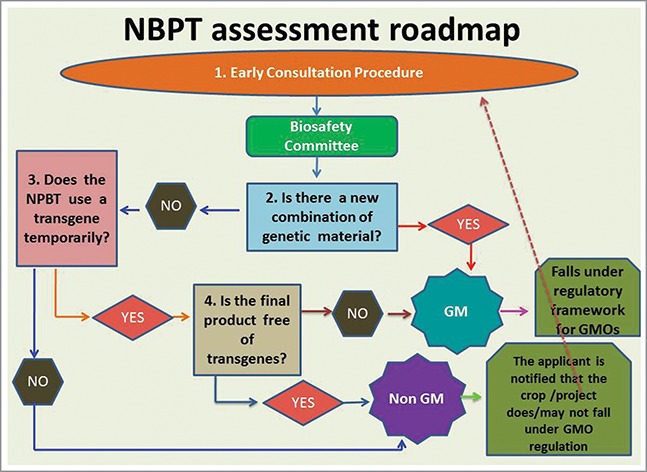
Flow map of NBTs applications for determination of regulated status in Argentina. See text for details.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- Abiri R, Valdiani A, Maziah M, Shaharuddin NA, Sahebi M, Yusof ZY, Atabaki N, Talei D. A critical review of the concept of transgenic plants: Insights into pharmaceutical biotechnology and molecular farming. Transg Plan 2015; 18: 21-42 [PubMed] [Google Scholar]
- Aguero C, Dandekar A, Meredith C. Evaluation of tolerance to Pierce's disease and botrytis in transgenic plants of vitis vinifera L. Expressing the pear pgip gene. VIII international conference on ghrape genetics and breeding. Mol Plant Pathol 2005; 6 (1):43-51; PMID:20565637; http://dx.doi.org/ 10.1111/j.1364-3703.2004.00262.x [DOI] [PubMed] [Google Scholar]
- Atik J. On the efficiency of health measures and the ‘appropriate level of protection.’ Research handbook on environment, health and the WTO, Prevost D, Van Calster G, eds. Los Angeles, CA: Loyola-LA Legal Studies; 2011: 116-38. [Google Scholar]
- Brookes G, Barfoot P. Key environmental impacts of global genetically modified (GM) crop use 1996–2011, GM Crops Food 2013; 4(2):109-19 [DOI] [PubMed] [Google Scholar]
- Burachik M, Traynor PL. Analysis of a national biosafety system: Regulatory policies and procedures in Argentina. The Hague: (NL); 2002. International Service for National Agricultural Research; ISNAR Country Report 63 69 p. [Google Scholar]
- Carlson DF, Tan W, Hackett PB, Fahrenkrug SC. Editing livestock genomes with site-specific nucleases. Reprod Fertil Dev 2013; 26(1):74-82; http://dx.doi.org/ 10.1071/RD13260 [DOI] [PubMed] [Google Scholar]
- Chair of the UK's Advisory Committee on Releases to the Environment Genetically modified organisms: New plant growing methods. London, UK; 2013. Available from: https://www.gov.uk/government/publications/genetically-modified-organisms-new-plant-growing-methods [Google Scholar]
- Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 2010; 186:757-61; PMID:20660643; http://dx.doi.org/ 10.1534/genetics.110.120717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau A, Caetano VR, St-Pierre CA, Haber S. Accelerated breeding: Dream or reality? Wheat Global Environ Dev Plant Breed 2001; 9: 671-9; http://dx.doi.org/ 10.1007/978-94-017-3674-9_90 [DOI] [Google Scholar]
- Contrary to popular belief (Editorial) . Nat Biotechnol 2013; 31:767; PMID:24022131; http://dx.doi.org/ 10.1038/nbt.2700 [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Genetically Modified Organisms Scientific opinion addressing the safety assessment of plants developed using Zinc Finger Nuclease 3 and other Site-Directed Nucleases with similar function. Parma, Italy, EFSA J 2012; 10(10):2943. 31 pp [Google Scholar]
- Eriksson D, Stymne S, Schjoerring JK. The slippery slope of cisgenesis. Nat Biotechnol 2014; 32(8):727-8; PMID:25101741; http://dx.doi.org/ 10.1038/nbt.2980 [DOI] [PubMed] [Google Scholar]
- Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang R-Y, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 2010; 329(5987):52-56; PMID:20488990; http://dx.doi.org/ 10.1126/science.119-0719 [DOI] [PubMed] [Google Scholar]
- Gleba Y, Klimyuk V, Marillonnet S. Magnifection?a new platform for expressing recombinant vaccines in plants. Vaccine 2005; 23(17-18):2042-8; PMID:15755568; http://dx.doi.org/ 10.1016/j.vaccine.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Gonsalves D. Transgenic papaya: A case study on the theoretical and practical application of virus resistance. Plant Biotechnol 2002; 7:115-8 [Google Scholar]
- Grimsley N, Hohn B, Hohn T, Walden R. "Agroinfection," an alternative route for viral infection of plants by using the Ti plasmid. Proc Natl Acad Sci 1986; 83:3282-6; http://dx.doi.org/ 10.1073/pnas.83.10.3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme IB, Wendt T, Holm PB. Intragenesis and cisgenesis as alternatives to transgenic crop development. Plant Biotechnol J 2013; 11:395-407; PMID:23421562; http://dx.doi.org/ 10.1111/pbi.12055 [DOI] [PubMed] [Google Scholar]
- Honig A, Marton I, Rosenthal M, Smith JJ, Nicholson MG, Jantz D, Zuker A, Vainstein A. Transient expression of virally delivered meganuclease in planta generates inherited genomic deletions. Mol Plant 2015; 8:1292-4; PMID:25863166; http://dx.doi.org/ 10.1016/j.molp.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Hou H, Atlihan N, Lu Z-X. New biotechnology enhances the application of cisgenesis in plant breeding. Front Plant Sci 2014; 5:389; PMID:25157261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Conference on New Plant Breeding Molecular Technologies New Delhi: International Life Sciences Institute India n.d. Available from: http://www.ilsi-india.org/international_conference_on_new_plant_breeding_molecular_technologies_technology_development_and_regulation.html [Google Scholar]
- Joh LD, Vandergheynst JS. Agroinfiltration of plant tissues for production of high-value recombinant proteins: an alternative to production in transgenic crops. J Sci Food Agric 2006; 86(13): 2002–2004; http://dx.doi.org/ 10.1002/jsfa.2572 [DOI] [Google Scholar]
- Krens F, Kamo K. Genomic tools and prospects for new breeding techniques in flower bulb crops. Acta Hort 2013; 974:139-47 [Google Scholar]
- Krens FA, Schaart JG, Burgh AMVD, Tinnenbroek-Capel IEM, Groenwold R, Kodde LP, Broggini GAL, Gessler C, Schouten HJ. Cisgenic apple trees; development, characterization, and performance. Front Plant Sci 2015; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La FAO y Argentina refuerzan la seguridad en biotecnología Rome: Organization of the United Nations for Food and Agriculture; n.d. Available from: http://www.fao.org/about/who-we-are/director-gen/faodg-news-archive/detail/es/c/264190/ [Google Scholar]
- Leckie BM, Stewart CN. Agroinfiltration as a technique for rapid assays for evaluating candidate insect resistance transgenes in plants. Plant Cell Rep 2010; 30:325-34; PMID:21140154; http://dx.doi.org/ 10.1007/s00299-010-0961-2 [DOI] [PubMed] [Google Scholar]
- Lema ML. Agrobiotecnología en la Argentina, una nueva etapa. Alimentos Argentinos 2012; 55:5-11 [Google Scholar]
- Levidow L, Carr S, Wield D. Genetically modified crops in the European Union: regulatory conflicts as precautionary opportunities. J Risk Res 2000; 3:189-208 [Google Scholar]
- Lloyd A, Plaisier CL, Carroll D, Drews GN. Targeted mutagenesis using zinc-finger nucleases in Arabidopsis. PNAS 2005;102(6), 232-7; PMID:15623558; http://dx.doi.org/ 10.1073/pnas.0409339102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusser M, Davies HV. Comparative regulatory approaches for groups of new plant breeding techniques. New Biotechnol 2013; 0:437-46; http://dx.doi.org/ 10.1016/j.nbt.2013.02.004 [DOI] [PubMed] [Google Scholar]
- Lusser M, Parisi C, Plan D, Rodrıguez-Cerezo E. New plant breeding techniques, State-of-the-Art and Prospects for Commercial Development. Luxembourg: Joint Research Centre Institute for Prospective Technological Studies; 2011. Report EUR 24760. 220p. [Google Scholar]
- Matz LM, Hahn RA. The White House Announces Plans to Update the U.S. Regulatory Framework for Biotechnology [Internet]. [Washington DC: ]: OFW Law; c2015. [cited 2015 July 31]. Available from: http://www.ofwlaw.com/2015/07/09/the-white-house-announces-plans-to-update-the-u-s-regulatory-framework-for-biotechnology/ [Google Scholar]
- Mokena-Lodge R. Sustainability Council case provides clarity to NZ GM regulations [Internet] [Wellington(NZ)]: McGuinness Institute Blog; c2015 [cited 2015. July 31]. Available from: http://mcguinnessinstituteblog.org/2014/05/22/sustainability-council-case-provides-clarity-to-nz-gm-regulations/ [Google Scholar]
- NBT Platform The regulatory status of plants resulting from new breeding technologies. Brussels (BE: ): NBT Platform Secretariat; 2013 July. 52p [Google Scholar]
- New plant breeding techniques Brussels: European Commission; 2015. Available from: http://ec.europa.eu/food/plant/gmo/new/legislation/plant_breeding/index_en.htm [Google Scholar]
- New plant breeding techniques workshops Kingston, Australia: Food Standards Australia New Zealand; 2014: Available from: http://www.foodstandards.gov.au/consumer/gmfood/Pages/New-plant-breeding-techniques-in-the-spotlight.aspx [Google Scholar]
- Ni W, Qiao J, Hu S, Zhao X, Regouski M, Yang M, Polejaeva IA, Chen C. Efficient gene knockout in goats using CRISPR/Cas9 system. PLoS 2014; 9(9):e106718; http://dx.doi.org/ 10.1371/journal.pone.0106718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podevin N, Devos Y, Davies HV, Nielsen KM. Transgenic or not? No simple answer! EMBO Rep 2012; 13:1057-61; PMID:23154464; http://dx.doi.org/ 10.1038/embor.2012.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The regulatory status of New Breeding Techniques in countries outside the European Union. The Hague, The Netherlands: Schuttelaar & Partners ; June 2015; 65pp [Google Scholar]
- Schaart JG, Visser RGF. Novel plant breeding techniques, consequences of new genetic modification-based plant breeding techniques in comparison to conventional plant breeding. Wageningen(NT): Wageningen University and Research Center; 2009 June. COGEM Report. 60p. [Google Scholar]
- Schiemann J, Hartung F. Safety assessment and regulation of new plant breeding technologies. In Proceedings of the Plant Biotech Denmark Annual meeting; 2013. January 31 – February 1; Copenhagen: 19-20. [Google Scholar]
- Schouten HJ, Krens FA, Jacobsen E. Do cisgenic plants warrant less stringent oversight? Nat Biotechnol 2006; 24:706-753; http://dx.doi.org/ 10.1038/nbt0706-753 [DOI] [PubMed] [Google Scholar]
- Sgrillo R. Considerations on the Appropriate Level of Protection, Acceptable Level of Risk and Phytosanitary Measures [Internet]. [Ilheus, BA, Brazil: ]: [cited 2015. July 31]. Available from: http://www.sgrillo.net/sampling/considerations_on_the_appropriat.htm [Google Scholar]
- Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, Zhang K, Liu J, Xi JJ, Qiu J-L, et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol 2013: 686-8; PMID:23929338; http://dx.doi.org/ 10.1038/nbt.2650 [DOI] [PubMed] [Google Scholar]
- Song J, Bradeen JM, Naess SK, Raasch JA, Wielgus SM, Haberlach GT, Liu J, Kuang H, Austin-Phillips S, Buell CR, et al. Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proc Natl Acad Sci 2003; 16:9128-33; http://dx.doi.org/ 10.1073/pnas.1533501100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Carlson DF, Lancto CA, Garbe JR, Webster DA, Hackett PB, Fahrenkrug SC. Efficient nonmeiotic allele introgression in livestock using custom endonucleases. Proc Natl Acad Sci 2013; 110 (41):16526-31; http://dx.doi.org/ 10.1073/pnas.1310478110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Text of the Cartagena Protocol on Biosafety Montreal: Secretariat of the Convention on Biological Diversity; 2013 Available from: https://bch.cbd.int/protocol/text/ [Google Scholar]
- Tiberghien Y. Competitive Governance and the Quest for Legitimacy in the EU: the Battle over the Regulation of GMOs since the mid‐1990s. J Eur Integration 2009; 31 (3):389-407; http://dx.doi.org/ 10.1080/07036330902782246 [DOI] [Google Scholar]
- Wang Z. Genome engineering in cattle: recent technological advancements. Chromosome Res 2015; 110(41):16526-31 [DOI] [PubMed] [Google Scholar]
- Waterhouse PM, Graham MW, Wang M-B. Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc Natl Acad Sci 1998; 95:13959-64; http://dx.doi.org/ 10.1073/pnas.95.23.13959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnker E, Dun KV, Snoo CBD, Lelivelt CLC, Keurentjes JJB, Naharudin NS, Ravi M, Chan SWL, Jong HD, Dirks R. Reverse breeding in Arabidopsis thaliana generates homozygous parental lines from a heterozygous plant. Nat Genet 2012; 44:467-70; PMID:22406643; http://dx.doi.org/ 10.1038/ng.2203 [DOI] [PubMed] [Google Scholar]
- Wolt JD, Wang K, Yang B. The Regulatory Status of Genome-edited Crops. Plant Biotechnol J 2015: 1-9; PMID:25545722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Working Group on the Harmonisation of Regulatory Oversight in Biotechnology Report of the OECD workshop on environmental risk assessment of products derived from novel plant breeding techniques Paris: (FR): Organisation for Economic Co-operation and Development; 2015 March. ENV/JM/BIO(2015)5. 95p. [Google Scholar]
- Wu L, Zhou H, Zhang Q, Zhang J, Ni F, Liu C, Qi Y. DNA methylation mediated by a MicroRNA pathway. Mol Cell 2010:465-75; PMID:20381393; http://dx.doi.org/ 10.1016/j.molcel.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Yu S, Luo J, Song Z, Ding F, Dai Y, Li N. Highly efficient modification of beta-lactoglobulin (BLG) gene via zinc-finger nucleases in cattle. Cell Res 2011; 21(11):1638-40; PMID:21912434; http://dx.doi.org/ 10.1038/cr.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Henriques R, Lin S-S, Niu Q-W, Chua N-H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 2006; 1:641-6; PMID:17406292; http://dx.doi.org/ 10.1038/nprot.2006.97 [DOI] [PubMed] [Google Scholar]


