Abstract
Ovarian cancer is a highly aggressive and deadly disease. Currently, the treatment for ovarian cancer entails cytoreductive surgery followed by chemotherapy, mainly cisplatin or carboplatin combined with paclitaxel. Although this regimen is initially effective in a high percentage of cases, unfortunately, after few months of initial treatment, tumor relapse occurs due to platinum-resistance. DOXIL (liposomal preparation of doxorubicin) is a choice of drug for recurrent ovarian cancer. However, its response rate is very low and is accompanied by myocardial toxicity. Resistance to chemotherapy and recurrence of cancer is primarily attributed to the presence of cancer stem cells (CSCs), a small population of cells present in cancer. Effect of DOXIL and withaferin A (WFA), both alone and in combination, was investigated on cell proliferation of ovarian cancer cell line A2780 and tumor growth in SCID mice bearing i.p. ovarian tumors. ALDH1 cells were isolated from A2780 using cell sorter, and effect of DOXIL and WFA both alone and in combination on tumorigenic function of ALDH1 was studied using spheroids formation assays in vitro. Western blots were performed to examine the expression of ALDH1 and Notch 1 genes. In our studies, we showed, for the first time, that DOXIL when combined with withaferin A (WFA) elicits synergistic effect on inhibition of cell proliferation of ovarian cancer cells and inhibits the expression of ALDH1 protein, a marker for ALDH1 positive cancer stem cells (CSCs), and Notch1, a signaling pathway gene required for self-renewal of CSCs. Inhibition of expression of both ALDH1 and Notch1 genes by WFA was found to be dose dependent, whereas DOXIL (200 nM) was found to be ineffective. SCID mice, bearing i.p. ovarian tumors, were treated with a small dose of DOXIL (2 mg/kg) in combination with a sub-optimal dose of WFA (2 mg/kg) which resulted in a highly significant (60% to 70%) reduction in tumor growth, and complete inhibition of metastasis compared to control. In contrast, WFA treatment showed a significant reduction in tumor growth but no change in metastasis compared to control. DOXIL showed non-significant reduction in tumor growth and no change in metastasis compared to control. Isolated ALDH1 positive CSCs treated with the combination of DOXIL and WFA resulted in a significant reduction in spheroids formation (tumorigenic function of CSCs) and expression of ALDH1 protein. WFA when used alone at a concentration of 1.5 μM was found to be highly effective in suppression of ALDH1 expression, whereas DOXIL at a concentration of 200 nM was found to be ineffective. DOXIL in combination with WFA elicits synergistic effects, targets cancer stem cells, and has potential to minimize induction of drug resistance and reoccurrence of cancer. Based on our studies, we conclude that the combination of DOXIL with WFA has the potential to be an effective therapy for ovarian cancer and may ameliorate DOXIL related side effects as well as recurrence of ovarian cancer leading to increase in patients’ survival rate.
Keywords: cancer stem cells, ovarian cancer, DOXIL, withaferin A, combination therapy, ALDH1
INTRODUCTION
Ovarian cancer is the major cause of death in women with gynecological malignancies [1]. Currently, ovarian cancer treatments are based on a surgical cytoreduction followed by cisplatin or platinum/taxane combination chemotherapy [2]. Initially, patients with ovarian cancer respond positively in 70 to 80% of the cases [3], however, within few months of treatment, patients develop platinum-resistance resulting in the recurrence of cancer [4, 5]. Resistance to platinum based chemotherapies have been associated with number of mechanisms; such as increase in glutathione [6], metallothionein levels [7], decrease in drug uptake [8,9], increase in DNA repair mechanisms [10–12], tolerance to the formation of platinum-DNA adducts [13], and changes in status of p53 gene which alters the sensitivity of tumors to cisplatin therapy [14, 15]. DOXIL (liposomal preparation of doxorubicin) is used as a major drug for the treatment of patients with recurrence cancer. However, DOXIL response rate is very low (< 20%) [16], therefore, approximately 70% of patients diagnosed with recurrence ovarian cancer die within 5 years of their diagnosis.
For the last few years, presence of CSCs in tumors has become very clear and these cells have been reported to be the cause of development of drug-resistance and recurrence of cancer. CSCs are a minor sub-population of tumors and possess the capacity for self-renewal and give rise to heterogeneous cancer lineages that comprise the tumor of origin [17]. The presence of CSCs have been reported in ovarian cancer cell lines, ovarian cancer and patients’ ascites [18–22]. CSCs are isolated based on the presence of certain extracellular markers. The most common markers used for ovarian cancer stem cells include CD24, CD34, CD44, CD117, CD133, ALDH1, Oct4, Myd88 and EpCAM. An increase in the number of CSCs in ovarian cancer correlates with a poor prognosis, including shorter overall life and disease free survival [21–29]. In recent studies, Abubaker et al [30] using two ovarian cancer cell lines (epithelial OVCA433 and mesenchymal HEY) demonstrated enrichment for a population of cells with high expression of CSC markers at protein as well as mRNA levels after treatment of cells with cisplatin, paclitaxel and the combination. In our recent study, we showed a significant increase in CD24, CD34, CD44, CD117 and Oct4 positive cells in tumors collected from mice bearing implanted orthotopic ovarian cancer after treatment with cisplatin [31]. These results clearly demonstrate that cisplatin, paclitaxel or carboplatin combination when used as first line chemotherapy for ovarian cancer suppress tumor growth by targeting cancer cells but spare CSCs which undergo enrichment resulting in drug-resistance, ultimately leading to recurrence of cancer. Therefore, developing a chemotherapy that targets both cancer cells and CSCs is mandatory and an appropriate approach to avoid recurrence of ovarian cancer.
In the past few years, many efforts have been devoted to develop drugs that can increase response rate and reduce chemo-resistance and recurrence of cancer including different combinations of DOXIL with other currently used chemo-drugs. Even though some of these combination showed enhanced effects and increase in sensitivity to DOXIL in vitro and in patients with recurrence ovarian cancer [32–34], but no study showing targeting of CSCs by these combinations has been reported. In this context, we explored the combination of DOXIL with withaferin A (WFA) to study its effect on ovarian cancer cell proliferation in vitro and tumor growth in nude mice and its effect on isolated ALDH1 positive cancer stem cells, which has been reported as a major population of CSCs in ovarian cancer [35]. WFA, a bioactive compound isolated from the plant Withania somnifera, is available as an over-the-counter dietary supplement in the U.S. It has been purported to possess anticancer, anti-inflammatory, anti-angiogenic and cardio-protective effects [36–40]. However, its clinical application in combination with DOXIL to treat cancer has not been explored. In our previous studies, we showed that WFA when used alone or in combination with cisplatin to treat mice bearing orthotopic human ovarian tumor reduced tumor growth by 60 to 70% and prevented metastasis to other organs [31], in addition to eliminating CSCs as well as CSCs enhanced by cisplatin. In our present study, we showed that when a low dose of DOXIL is used in combination with suboptimal dose of WFA, it synergistically inhibits proliferation of ovarian cancer cells, and reduces tumor growth and metastasis in SCID mice. In addition, treatment of isolated ALDH1 positive CSCs from A2780 cell line with DOXIL/WFA combination significantly inhibits the spheroid formation (characteristics of cancer stem cells) and ALDH1 expression. Thus, our studies, for the first time, demonstrate that a combination of low dose of DOXIL with suboptimal dose of WFA is highly effective in suppressing tumor growth as well as eliminating putative CSCs. Therefore, this combination has the potential to be an effective therapy for ovarian cancer and may ameliorate DOXIL related side effects as well as recurrence of ovarian cancer, leading to increase in patients’ survival rate.
MATERIAL AND METHODS
Ethical Statement
Animals work reported in the manuscript was performed after approval of the protocol by the University of Louisville Animal Care Use Committee (IACUC).
Cell Culture
Human epithelial ovarian cancer cell line (A2780) was obtained as a gift from Denise Connolly (Fox Chase Cancer Center, Philadelphia, PA). The cell line was originally generated from human ovarian cancer patient prior to treatment [41]. The cisplatin-resistant (A2780/CP70) cell line was obtained as a gift from Dr. Christopher States (University of Louisville, Louisville, KY). This cell line was derived from A2780 cell line after treatment with cisplatin [42]. The third cell line (CaOV3), was purchased from American Type Culture Collection (ATCC). Both A2780 and A2780/CP70 cell lines were cultured in RPMI medium containing 10% fetal bovine serum (FBS), 1% Penicillin/Streptomycin, and 0.05% (v/v) Insulin (Sigma). CaOV3 cell line was cultured in DMEM medium containing 10% FBS and 1% Penicillin/Streptomycin. Withaferin A and DMSO were purchased from Sigma. DOXIL was obtained from Ortho Biotech.
Treatment of cells with DOXIL and WFA
For treatment of ovarian cancer cells with DOXIL and WFA both alone and in combination, cells growing in log phase were harvested using trypsin and plated at 3,000 cells/well into 96 well plates, and allowed to grow for 24 hours before treatment in triplicates with various concentrations of DOXIL, WFA or combination of DOXIL and WFA. Where necessary, DMSO was used as a vehicle control for untreated cells. Cells were incubated for 24 to 72 hr before quantitating by MTT assays as described previously [43]. Color development was assayed by an ELISA reader at 492 nM. DOXIL was diluted in serum free medium whereas WFA was solubilized in DMSO.
Isobologram analysis
A2780 cells were treated in triplicate with 7 different concentrations of DOXIL and WFA both alone and in combination at a constant ratio as described above. The cell proliferation was quantitated using MTT assays and fractions affected were calculated from percent inhibition. Fractions affected were then used in CalcuSyn software to generate dose-dependent curve and isobologram as described previously [43].
SDS-PAGE and Western blot analysis
Cells were plated in 6-well plates and treated with WFA and DOXIL both alone and in combination. After 48 h of treatment, cells were rinsed with PBS and suspended in chilled lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.1% NP-40, 1 mM Na3VO4, and 1 mM NaF] supplemented with complete Protease Inhibitor Cocktail (Sigma). To prepare cell lysate, cells were suspended in lysis buffer, followed by sonication for 15 to 20 sec. Protein concentrations for each sample were determined using Bradford method (Bio-Rad Laboratories) according to supplier’s instructions. Forty μg of protein from each sample was mixed with SDS-PAGE buffer and heat-denatured at 95°C for 5 min and separated on SDS-PAGE. The proteins were transferred to Hybond nitrocellulose membrane (GE HealthCare) and blocked with 5% non-fat milk in TBST buffer [10 mM Tris, 150 mM NaCl and 0.1% (v/v) Tween-20, pH 7.4] for 1 hr at room temperature followed by incubation with primary antibody diluted in non-fat milk at 4°C for overnight. The antibody for ALDH1 (SC-166362), was purchased from Santa Cruz Biotechnology and β-actin (cat # A3854) was obtained from Sigma-Aldrich. Membranes were washed with TBST (three times, 5 min each) and then incubated with appropriate secondary antibody conjugated with horseradish peroxidase (Sigma, diluted 1:2000). Membranes were washed with TBST (three times, 5 min each) and the immuno-reactive bands were visualized by enhanced chemiluminescence (ECL) (GE Healthcare). Membranes were stripped off for 10 min with methanol containing H2O2 and probed with β-actin antibody conjugated with horseradish peroxidase in order to serve as an internal control as described previously [31].
Generation of intraperitoneal ovarian tumor and treatment with DOXIL and WFA
To determine the efficacy of DOXIL in combination with WFA on tumor growth and metastasis in vivo, we generated intraperitoneal (i.p.) tumors in SCID mice followed by treatment with DOXIL and WFA both alone and in combination. i.p. tumors were generated by injecting 1X106 A2780 cells, suspended in serum and antibiotics free RPMI medium/mouse directly into peritoneal cavity of 5 to 6 weeks old SCID mice. After 10 days of post-cell injections, mice were treated with: 1) vehicle control (10% DMSO and 90% glyceryl trioctanoate), 2) DOXIL (2 mg/kg doxorubicin concentration), 3) WFA (2 mg/kg), and 4) DOXIL (2 mg/kg) plus WFA (2 mg/kg). Randomly three animals were included in each group. DOXIL in saline was injected i.p. once a week, whereas WFA was injected in 10% DMSO and 90% glyceryl trioctanoate on every third day. Following three weeks of treatment, animals were sacrificed, visible tumors and other tissues such as ovaries, kidney, liver, adrenal and lungs were collected from each mouse. Tumors were weighed at the time of collection. The tumors and other tissues were divided into two parts; one part was immediately frozen in liquid nitrogen, and the second part was fixed in 10% buffered formalin. The animals’ experiments were approved by the University of Louisville, Institutional Animal Care and USE Committee (IACUC) (protocol # 12063).
Isolation of ALDH1 positive cancer stem cells from A2780 cell line and treatment with DOXIL and WFA
Aldehyde dehydrogenase 1 (ALDH1) is a cancer stem cell marker, and ALDH1 positive cancer stem cells have been reported to play clinical significance role in ovarian cancer [44]. To determine the effect of DOXIL and WFA both alone and in combination on ALDH1 positive cancer stem cells, we isolated ALDH1 positive cancer stem cells from ovarian cancer cell line, A2780. Cells growing in log phase were rinsed with PBS and then dislodged by incubating with non-enzymatic cell dissociation solution (Sigma) in CO2 incubator at 37°C for 60 min. The cells were pelleted by centrifuging at 2,000 rpm for 2 min and suspended in assay buffer (from Aldelfluor kit purchased from Stem Cell Technologies) at 2X106 cells/ml. The cells were incubated in Adelfluor substrate (1 μM/1X106 cells) and incubated for 45 min at 37°C. One sample was treated with 50 mM of diethylaminobenzaldehyde (DEAB, an aldehyde inhibitor), as a negative control. After incubation, cells were centrifuged at 2,000 rpm for 2 min and resuspended in assay buffer. The highly bright fluorescent ALDH1-expressing (ALDH1+) and ALHD1− cells were detected in the green fluorescent channel (520–540 nm) using Beckman Coulter MoFlo XDP and collected in RPMI medium containing 10% FBS.
To determine tumorigenic potential of ALDH1 positive cancer stem cells and examine the effect of DOXIL and WFA, alone and in combination, on tumorigenic potential of ALDH1 positive cancer stem cells, standard spheroid formation assays were performed according to Zhang et al. [45] with some modifications. Initially, isolated ALDH1 positive cancer stem cells or ALDH negative cancer stem cells (1×103) were suspended in RPMI medium supplemented with 5 μg/ml insulin (Sigma), 20 ng/ml human recombinant epidermal growth factor (EGF; Sigma), 10 ng/mL basic fibroblast growth factor (bFGF; Sigma), and 1% FBS. The cells were plated into 6-well ultra-low attachment plates (Corning Costar) and incubated at 37°C/5% CO2 in the incubator. Fresh insulin, bFGF and EGF were added to the medium on every third day. Spheroids that formed within 3 to 4 days after plating were counted and photographed. Spheroids diameters > 50 μm were counted as a single positive colony. Several fields for each well were counted under a phase contrast microscope. For all spheroid formation experiments, a minimum of two wells were used for each condition, and experiments were repeated three times.
Statistical analysis
Statistical comparison of data was carried out by the student’s t test (for single comparison). Probability of p ≤ 0.05 was determined from the two-sided test and was considered significant. The statistical analysis was carried by using SPSS 10.0 software.
RESULTS
DOXIL when combined with WFA elicits synergistic effects on inhibition of ovarian cancer cell proliferation
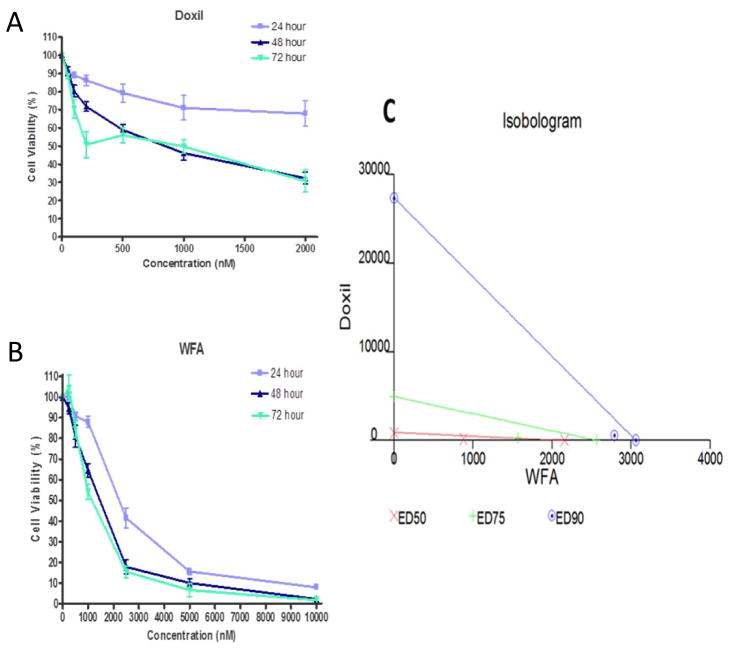
Liposomal preparation of doxorubicin “DOXIL” is preferred over doxorubicin as a second line option for the treatment of recurrent ovarian cancer due to its substantial better toxicity profile. However, it has very low response rate (< 20%) and is still associated with myocardial toxicity [16, 46]. To minimize the side effects as well as to increase its response rate, we explored the possibility of using DOXIL/WFA combination. Treatment of three ovarian tumor cell lines (cisplatin-sensitive, A2780 and CaOV3; and a cisplatin-resistance cell line, A2780/CP70) with various concentrations of DOXIL and WFA both alone and in combination showed a time- and dose-dependent inhibition of cell proliferation. Only results from A2780 cell line are shown (Figure 1). When DOXIL and WFA were used alone, the IC50 values for DOXIL and WFA for A2780 cells (after 48 h of treatment) were found to be approximately 1 μM and 2.5 μM respectively. On co-treatment of cells with DOXIL and WFA, IC50 values for both the agents decreased significantly (Figure 1 A and B). Similar synergistic results were obtained with other two cell lines (CaOV3 and A2780/CP70). Isobologram analysis using both the agents at a constant ratio confirmed the synergistic effect on combination of DOXIL and WFA (Figure 1 C). The combination of WFA with DOXIL requires several-fold lower dose of DOXIL to achieve the same level of cell death compared to DOXIL alone. Therefore, it is expected that the side effects associated with high dose of DOXIL will be eliminated or reduced.
Figure 1.
Effect of WFA and DOXIL both alone and in combination on A2780 cell proliferation. A2780 cells were treated with DOXIL (A) and WFA (B) both alone and in combination for 24 h, 48 h or 72 hr. Cell proliferation was measured using MTT assays (A and B). Isobologram analysis using DOXIL in combination with WFA at constant ratio (C).
DOXIL when combined with WFA suppresses tumor growth and metastasis in nude mice
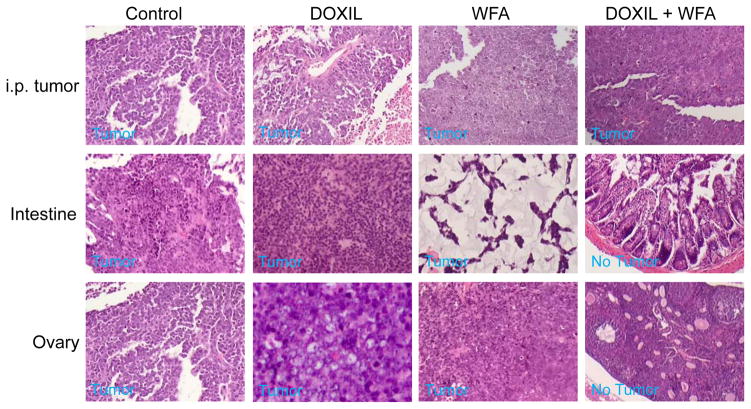
To access the efficacy of WFA/DOXIL combination on tumor growth and metastasis in vivo, we tested the effect of DOXIL and WFA, both alone and in combination, on tumor growth and metastasis in SCID mice bearing i.p. human ovarian tumors, as described in materials and methods. Beginning from 10 days after injection of A2780 cells (106 cells/mouse), mice were treated with vehicle, DOXIL (2 mg/kg), WFA (2 mg/kg) or combination of DOXIL/WFA (2 mg/kg DOXIL + 2 mg/kg WFA). After 3 weeks of treatment, animals were sacrificed. We observed that the control vehicle-treated animals developed highly vascularized and large tumors (Figure 2A) which metastasized to the ovaries (formed bilateral ovarian cancer) (Figure 2B), intestine, omentum and liver (results not shown). Treatment of animals with WFA (2 mg/kg) alone showed a significant reduction in tumor weight but no change in metastasis (Figure 2B and C). Treatment of animals with DOXIL (2 mg/kg) alone showed some reduction in tumor weight but was found to be non-significant compared to control (vehicle-treated) animals (Figure 2C). However, no change in metastasis in these animals was observed. In contrast, animals treated with DOXIL and WFA combination (2 mg/kg each) showed a highly significant reduction (60 to 65%) in tumor weight compared to control animals and interestingly no visible metastasis to any organ was observed (Figure 2A, 2B and 2C). H&E staining of ovarian tissues followed by histo-pathological analysis of the intestine and ovaries collected from the control, as well as treated animals confirmed metastasis to intestine and ovaries in control animals as well as DOXIL or WFA alone treated animals, whereas no cancer cell or metastasis was observed in ovaries collected from animals treated with DOXIL/WFA combination (Figure 3). These results suggest that combination of low dose of DOXIL (2 mg/kg) with suboptimal dose of WFA (2 mg/kg) is highly effective in suppressing tumor growth and metastasis of i.p. ovarian tumor in SCID mice.
Figure 2.
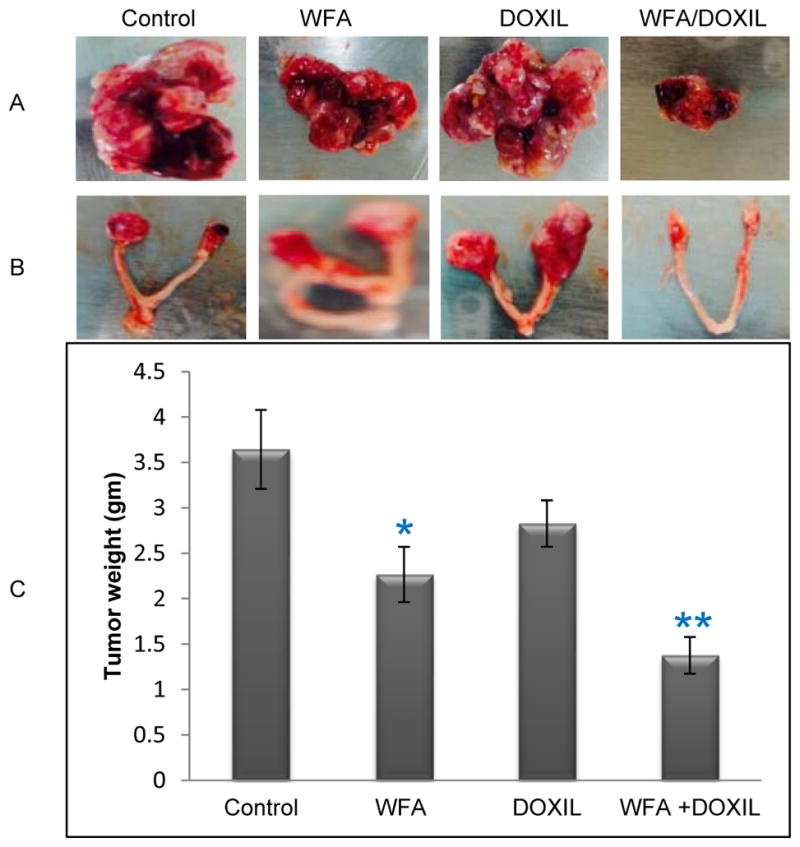
Effect of WFA and DOXIL treatment on tumor growth and metastasis in SCID mice. i.p. tumors were generated in SCID mice by injecting A2780 cells (i.p.) followed by treatment with vehicle (control), DOXIL (2 mg/kg), WFA (2 mg/kg) or DOXIL (2 mg/kg) plus WFA (2 mg/kg). After three weeks of treatment, mice were sacrificed and tumors were weighted and photographed. A = i.p. tumors shown are representative from each group. B = Metastatic ovarian tumors. C = Tumors weights plotted from each group. Results are mean ± SEM (vertical bar). * represents significant at p ≤ 0.05 and ** represents significant at p ≤ 0.001 compared to control.
Figure 3.
Histo-pathological analysis of tumor tissues. Visible tumors, intestine and ovarian tissues from control animals (vehicle treated) and animals treated with DOXIL, WFA or DOXIL plus WFA were collected at the time of sacrificing the animals and processed immediately for immunohistochemical analysis. Tissues sections were stained with H&E staining and analyzed by a trained pathologist for the analysis of metastasis to various organs. In control animals, animals treated with DOXIL or WFA, tumor cells metastasized to intestine and ovaries. No metastasis was observed in intestine or ovaries collected from animals treated with DOXIL and WFA combination.
DOXIL when combined with WFA inhibits tumorigenic potential of ALDH1 positive CSCs
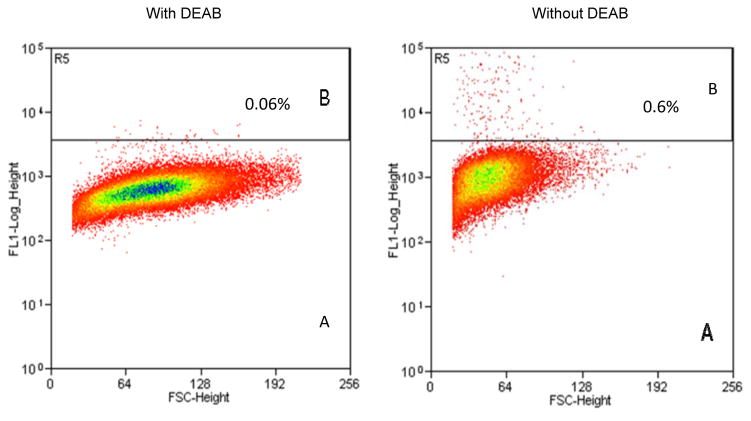
CSCs are capable of forming characteristic compact circular colonies with cobblestone appearance and can survive numerous passages. These spheroid clusters have the potential to be highly tumorigenic and possess the capability to propagate and reconstitute original tumor architecture when injected into permissive hosts [20, 47, 48]. To test that DOXIL and WFA when used in combination inhibits tumorigenic function of CSCs, we isolated ALDH1 positive CSCs from A2780 cell line using Aldelfluor kit as described in materials and methods. Approximately 0.6% to 1% of the cells were found to be AlDH1 positive (Figure 4). Approximately 2000 ALDH1 positive, as well as negative cells were plated on ultra-low attachment plates (corning), respectively. As shown in Figure 5, ALDH1 positive cells, when plated on ultra-low adhering plates, formed large spheroids (colonies) within one week of plating, whereas ALDH1 negative cells did not develop colonies or formed a few and very small size colonies, suggesting tumorigenic characteristic of ALDH1 positive CSCs.
Figure 4.
Analysis of ALDH1 positive cancer stem cells from ovarian cancer cell line A2780. ALDEFLUOR Assay Kit from Stem Cell Technology was used for collection of ALDH1 positive cancer stem cells using Beckman Counter MoFlo XDP. A = ALDH1 negative cells (cells treated with DEAB) and B = ALDH1 positive cells.
Figure 5.
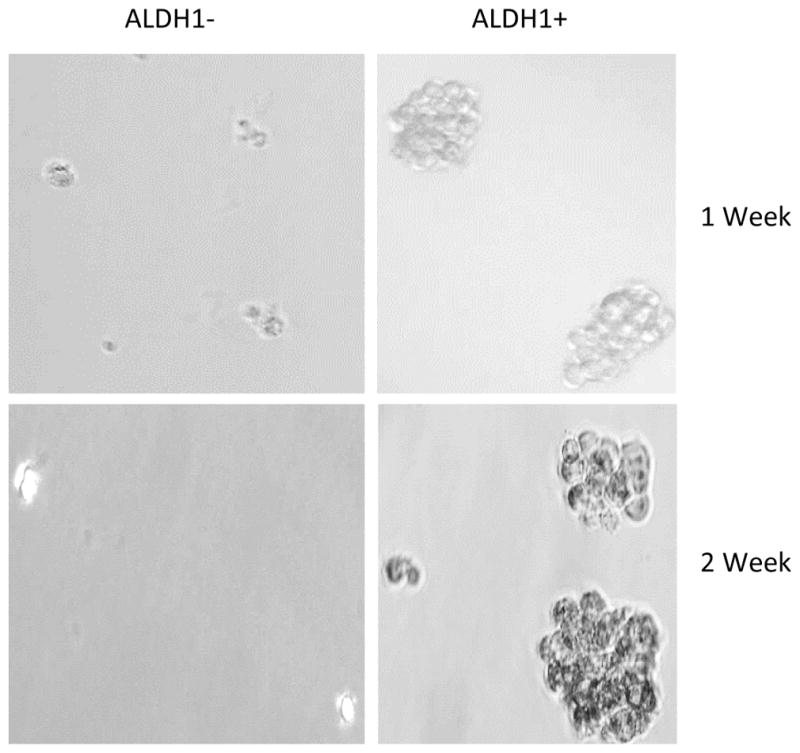
Spheroids formation by ALDH1 positive cells. Isolated ALDH1 negative and positive cancer stem cells were plated into ultra-low attachment plates. The spheroids were examined after one week of plating and photographed. ALDH1 positive cells formed large number and large size spheroids (colonies), whereas ALDH1 negative cells did not form spheroids or formed very small spheroids.
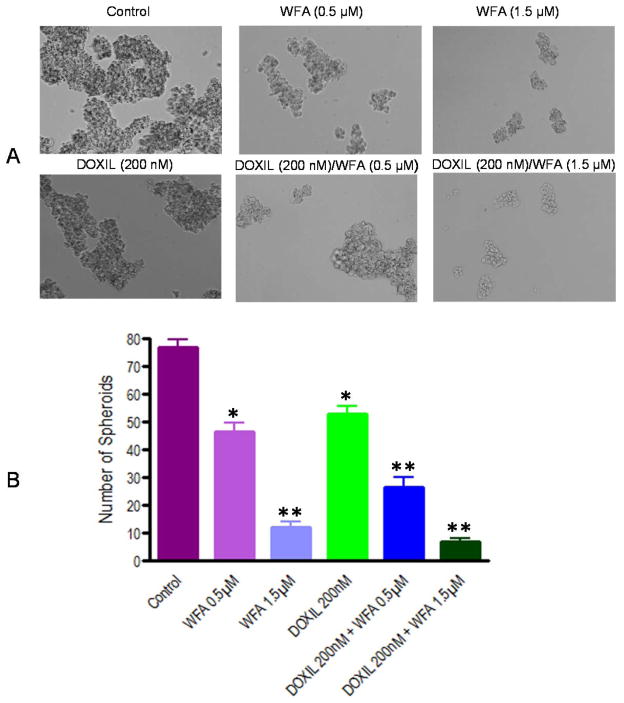
To determine the effect of DOXIL/WFA combination on tumorigenic potential of ALDH1 positive CSCs, spheroids were collected, dispersed mechanically, and plated again on 6 well ultra-low attachment plates. After 24 h of plating, small spheroids formed and were treated with DOXIL and WFA, both alone and in combination. After three days of treatment, spheroids were observed under phase contrast microscope, counted and photographed. As shown in Figure 6 A, a dose dependent deleterious (apoptotic) effect on spheroids formation was observed when treated with WFA alone compared to control. DOXIL also inhibited the spheroid formation. The effects of both WFA (1.5 μM) and DOXIL, when used alone, were found to be significant in reducing the number as well as size of colonies compared to control (Figure 6A and B). However, combination of DOXIL (200 nM) with WFA (1.5 μM) was found to be highly toxic and enhanced the inhibition of colonies formation to a greater extend. Few small disintegrated colonies were observed after treatment with DOXIL and WFA combination especially at higher concentration 1.5 μM of WFA (Figure 6A and B), suggesting that combination of DOXIL with WFA is highly effective in targeting the CSCs and hence may reduce or eliminate the drug-resistance and recurrence of ovarian cancer.
Figure 6.
Effect of DOXIL and WFA on ALDH1 positive cancer stem cells. ALDH1 cells were plated on ultra-low attachment plates. After one week of plating, when large size colonies were formed, colonies were collected, dispersed and plated again on 6-well ultra-low attachment plates. After 24 h of plating when small size spheroids are formed, treated with DOXIL and WFA both alone and in combination. After 3 days of treatment, spheroids formed were counted and photographed. A = spheroids formed after 3 days of treatment. B = Quantitative analysis of spheroids after 3 days of treatment. The colonies were counted in six different fields and were added. The results shown represent mean and standard deviation of three independent experiments. * represents significant at p ≤0.05 and ** represents significant at p ≤0.001 compared to control.
DOXIL when combined with WFA suppresses the expression of ALDH1 protein
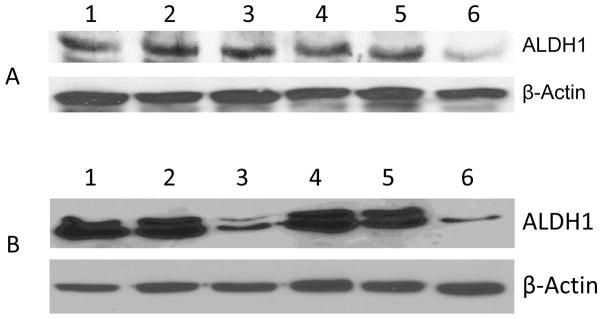
Both DOXIL and WFA were found to inhibit spheroid formation by isolated ALDH1 CSCs. Therefore, to examine the effect of DOXIL and WFA both alone and in combination on the expression of ALDH1 protein, we examined the effect of DOXIL and WFA, both alone and in combination, in A2780 cells and ALDH1 positive isolated CSCs. We treated both A2780 cells and isolated ALDH1 positive cells with DOXIL and WFA, both alone and in combination, as described above. Treatment of A2780 cells with DOXIL (200 nM) and WFA (1.5 μM) combination showed a significant suppression of ALDH1 gene expression compared to control untreated cells. Both DOXIL and WFA showed a non-significant suppression of ALDH1 expression (Figure 7A). In contrast, isolated ALDH1 positive cells when treated with WFA at a concentration of 1.5 μM was highly effective in suppressing the expression of ALDH1 protein, however, DOXIL was found to be ineffective at a concentration of 200 nm. Combining of DOXIL (200 nM) with WFA (1.5 μM) showed enhanced suppression of ALDH1 protein expression (Figure 7B), suggesting that DOXIL, when used alone, is ineffective in targeting CSCs, however, when combined with WFA it enhances the effect of WFA in targeting CSCs.
Figure 7.
Western blot analysis of A2780 cells (A) and isolated ALDH1 positive cancer stem cells (B) after treatment with DOXIL and WFA, both alone and in combination. Cells/spheroids were collected after treatment and lysed using lysis buffer. Forty μg of protein from each sample was used for SDS-PAGE. ALDH1-specfic monoclonal antibody was used for the detection of ALDH1 protein. The data shown is representative of at least two independent experiments. β-Actin-specific monoclonal antibody conjugated to horseradish peroxidase (Sigma) was used as internal control. Lane 1 = control untreated cells, lane 2 = cells treated with WFA (0. 5 μM), lane 3 = cells treated with WFA (1.5 μM), lane 4 = cells treated with DOXIL (200 nM), lane 5 = cells treated with DOXIL (200 nM) plus WFA (0.5 μM), and lane 6 = cells treated with DOXIL (200 nM) plus WFA (1.5 μM).
DOXIL when combined with WFA inhibits Notch1 signaling gene
Self-renewal, drug resistance and differentiation are key characteristics of CSCs. Sonic Hedgehog (Shh), Notch1, Twist, Snail, Slug and Wnt1 signaling transduction pathways play major roles in the self-renewal of CSCs [49–55]. Notch 1 signaling pathway is associated with regulation of cell fate at several distinct developmental stages and has been implicated in cancer initiation and progression [51, 55, 56]. Initially, to show the effect of DOXIL and WFA, both alone and in combination, we treated ovarian cancer cell line, A2780, with different concentrations of DOXIL and WFA, both alone and in combination, for 48 hr. As shown in Figure 8, treatment of A2780 cells with various concentrations of WFA showed a dose dependent suppression of expression of Notch1. DOXIL treatment resulted in an insignificant effect. However, combining DOXIL with WFA showed an enhanced suppressive effect on Notch1 protein expression, suggesting the combination therapy targeted the signaling mechanism involved in self-renewal or CSCs, therefore, resulting in reduction or elimination of drug-resistance and hence recurrence of cancer caused by CSCs.
Figure 8.
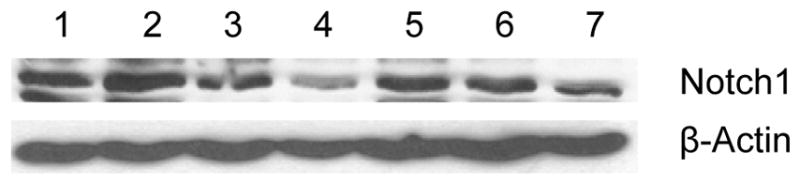
Western blot analysis of A2780 cells after treatment with DOXIL and WFA both alone and in combination. Cells were collected after treatment and lysed using lysis buffer. Forty μg of protein from each sample was used for SDS-PAGE. Notch1-specfic antibody was used for the detection of Notch1 protein. The data shown is representative of at least two independent experiments. β-Actin-specific monoclonal antibody conjugated to horseradish peroxidase was used as internal control. Lane 1 = control untreated cells, lane 2 = cells treated with WFA (0. 5 μM), lane 3 = cells treated with WFA (1.5 μM), lane 4 = cells treated with WFA (3 μM), lane 5 = cells treated with DOXIL (200 nM), lane 6 = cells treated with DOXIL (200 nM) plus WFA (0.5 μM), and lane 7 = cells treated with DOXIL (200 nM) plus WFA (1.5 μM).
DISCUSSION
Ovarian cancer is the leading cause of death from gynecological malignancies [1, 57]. The main reasons for such high mortality rate are due to the lack of symptoms accompanying this tumor, an effective screening strategy, and limited results obtained with medical treatments. The most commonly used first line chemotherapy after cytoreductive surgery is a platinum/taxane combination [2]. Although this strategy initially has high response rate, however, after few treatments, the vast majority of patients develop cisplatin resistance and require further therapy [2–4]. Many strategies have been implanted as second line chemotherapy for the patients that develop cisplatin resistance, and several new drugs have been investigated. Among these, liposomal doxorubicin (DOXIL) is a choice of drug. However, DOXIL alone is not considered an effective drug because it is associated with myocardial toxicity and has a very low response rate.
In recent years, combination of two or more clinically approved drugs for the treatment of cancer has become a common strategy with the hope to improve the clinical outcome. With this respect, to improve the efficacy of DOXIL, DOXIL in combination with various drugs including platinum (carboplatin) [58–61], oxaliplatin [62], gemcitabine [63, 64], paclitaxel [65], topotecan [66], vinorelbine [67], ifosfamide [68], olaparib [69], cyclophosphamide and 5-fluorouracil [33], checkpoint blockers antibodies such as PD-L1, PD-1, and CTLA-4 mAbs [32], and trabectedin [70] have been explored in patients with cisplatin-sensitive or cisplatin-resistance recurrent ovarian cancer. A low to moderate increase in response rate, overall survival (OS), and progression free survival (PFS) has been reported for various combinations [71]. Among these combinations, carboplatin/DOXIL has been reported to be a valid alternate in both first line and recurrent ovarian cancer, compared to actual standard options [71].
Development of cisplatin resistance or chemo-resistance in patients with currently used drugs is a major clinical problem and has been reported due to the presence of cancer stem cells, a small population of cells present in tumors. These cells are chemo-resistant, capable of self-renewal and differentiation and responsible for recurrent cancer [21]. Currently used drugs, including DOXIL or its combination with other commonly used drugs, has not been tested for targeting CSCs. There has been an increasing support for natural compounds when developing new treatments for cancer to enhance the therapeutic effect of an anti-neoplastic agent. This allows a lower dose to be used while achieving the same anti-neoplastic effect and reducing the side effects. WFA is a bioactive, cell permeable compound isolated from the plant Withania somnifera is an anticancer and anti-inflammatory compound and possesses cardio-protective properties [36–40]. In our previous studies, we showed that WFA when combined with doxorubicin elicits synergistic effects on inhibition of cell proliferation of ovarian cancer cells (A2780, A2780/CP70 and CaOV3) and tumor growth in nude mice [43]. In our other studies, we showed for the first time, that WFA alone or in combination with cisplatin (CIS) target CD24, CD34, CD44, CD117 and Oct4 positive CSCs in tumors collected from nude mice treated with WFA or WFA and CIS combination, suggested that WFA target cancer stem cells in addition to cancer cells [31]. DOXIL has a lower toxicity profile compared to doxorubicin, therefore, in our present studies, we tested its efficacy in combination with WFA on ovarian cancer cell proliferation in vitro, tumor growth in SCID mice and targeting of CSCs. As shown in Figure 1, DOXIL when combined with WFA elicits synergistic effect on inhibition of ovarian cancer cell proliferation, tumor growth and metastasis in SCID mice (Figures 2 and 3). These results are consistent with our previous findings for WFA and doxorubicin combination [43]. We examined the effect of DOXIL alone and in combination with WFA on targeting of ALDH1 positive CSCs. AlDH1 positive CSCs have been reported in ovarian tumors as well as in ascites from patients with ovarian cancer. In addition, ALDH1 has been shown to have clinical significance in progression and recurrence of ovarian cancer [44]. DOXIL, when used alone to treat ovarian cancer cell line A2780, was found to be ineffective in downregulation of ALDH1 protein expression. However, combination of DOXIL with WFA showed a significant suppression of ALDH1 protein expression (Figure 7A). Treatment of isolated ALDH1 positive CSCs with DOXIL and WFA both alone showed a significant inhibition of spheroids formation (tumorigenic function of CSCs) and such effects are enhanced significantly on combination of DOXIL with WFA (Figure 7B). Combination of a small dose of DOXIL (200 nM) with suboptimal concentration of WFA (1.5 μM) was found to be highly effective in inhibiting tumorigenic function of ALDH1 (Figure 6 A and B) and its expression (Figure 7), suggesting that DOXIL alone is not significantly effective in inhibiting ovarian cancer cell proliferation or targeting CSCs. However, when combined with WFA, DOXIL is highly efficacious in targeting cancer cells as well as CSCs. Combining DOXIL with WFA also showed a significant suppression of Notch1 gene, a signaling molecule involved in self-renewal of CSCs. Experiments to test the targeting of other CSCs by WFA and DOXIL combination in vivo on tumor growth by isolated CSCs are in process. Based on these results, we conclude that combining a small dose of DOXIL with suboptimal dose of WFA is highly effective in targeting CSCs, which may lead to reduction in development of drug resistance and recurrence of ovarian cancer. Application of a small dose of DOXIL in combination with suboptimal dose of WFA is expected to reduce unwanted side effects caused by high doses of DOXIL used.
Acknowledgments
The authors express their gratitude to Dr. Magdalena Kucia for her help in data analysis, and Dr. Denise Connolly (Fox Chase Cancer Center, Philadelphia) and Dr. Christopher States (University of Louisville) for providing cell lines. This work was supported by a research grant from NCI 124630 and Brown Cancer Center, University of Louisville (SSK).
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTION:
SSK designed and performed most of the experiments and drafted the manuscript. CAW performed sorting of ALDH1 positive and negative cells from A2780 cell line. ZW performed histo-pathological analysis of the tumors. KC performed some experiments, performed analysis of data and editing of the manuscript. MR helped SSK in designing of the experiments. PG generated the i.p. tumors in SCID mice and performed injection of drugs, collection of tumors and other tissues at the time of sacrificing the animals. All authors read the final version of the manuscript and approved the contents.
References
- 1.Siegel R, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2015. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Pfisterer J, Ledermann JA. Management of platinum-sensitive recurrent ovarian cancer. Semin Oncol. 2006;2(Suppl 6):S12–16. doi: 10.1053/j.seminoncol.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo K, Lin YG, Roman LD, Sood AK. Overcoming platinum resistance in ovarian carcinoma. Expert Opin Investig Drugs. 2010;19(11):1339–54. doi: 10.1517/13543784.2010.515585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccart MJ, Bertelsen K, Stuart G, Cassidy J, Mangioni C, Simonsen E, James K, Kaye S, Vergote I, Blom R, Grimshaw R, Atkinson R, Swenerton K, Trope C, Nardi M, Kaern J, Tumolo S, Timmers P, Roy JA, Lhoas F, Lidvall B, Bacon M, Birt A, Andersen J, Zee B, Paul J, Pecorelli S, Baron B, McGuire W. Long- term follow-up confirms a survival advantage of the paclitaxel-cisplatin regimen over the cyclophosphamide-cisplatin combination in advanced ovarian cancer. Int J Gynecol Cancer. 2003;13(Suppl 2):144–148. doi: 10.1111/j.1525-1438.2003.13357.x. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo K, Lin YG, Roman LD, Sood AK. Overcoming platinum resistance in ovarian carcinoma. Expert Opin Investig Drugs. 2010;19(11):1339–1354. doi: 10.1517/13543784.2010.515585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen BA, Brouwer J, Reedijk J. Glutathione induces cellular resistance against cationic dinuclear platinum anticancer drugs. J Inorg Biochem. 2002;89(3–4):197–202. doi: 10.1016/s0162-0134(02)00381-1. [DOI] [PubMed] [Google Scholar]
- 7.Doz F, Roosen N, Rosenblum ML. Metallothionein and anticancer agents: the role of metallothionein in cancer chemotherapy. J Neurooncol. 1993;17(2):123–129. doi: 10.1007/BF01050214. [DOI] [PubMed] [Google Scholar]
- 8.Andrews PA, Velury S, Mann SC, Howell SB. cis-Diamminedichloro platinum(II) accumulation in sensitive and resistant human ovarian carcinoma cells. Cancer Res. 1988;48:68–73. [PubMed] [Google Scholar]
- 9.Kuppen PJ, Schuitemaker H, van’t Veer LJ, de Bruijn EA, van Oosterom AT, Schrier PI. cis-diamminedichloroplatinum(II)-resistant sublines derived from two human ovarian tumor cell lines. Cancer Res. 1988;48(12):3355–3359. [PubMed] [Google Scholar]
- 10.Lai GM, Ozols RF, Smyth JF, Young RF, Hamilton TC. Enhanced DNA repair and resistance to cisplatin in human ovarian cancer. Biochem Pharmacol. 1988;37:4597–4600. doi: 10.1016/0006-2952(88)90325-5. [DOI] [PubMed] [Google Scholar]
- 11.Kelland LR, Mistry P, Abel G, Loh SY, O’Neill CF, Murrer BA, Harrap KR. Mechanism-related circumvention of acquired cis-diamminedichloro platinum (II) resistance using two pairs of human ovarian carcinoma cell lines by ammine/amine platinum IV dicarboxylates. Cancer Res. 1992;52:3857–3864. [PubMed] [Google Scholar]
- 12.Larminat F, Zhen W, Bohr VA. Gene-specific DNA repair of interstrand cross-links induced by chemotherapeutic agents can be preferential. J Biol Chem. 1993;268:2649–2654. [PubMed] [Google Scholar]
- 13.Johnson SW, Perez RP, Godwin AK, Yeung AT, Handel LM, Ozols RF, Hamilton TC. Role of platinum-DNA adduct formation and removal in cisplatin resistance in human ovarian cancer cell lines. Biochem Pharmacol. 1994;47:689–697. doi: 10.1016/0006-2952(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 14.Reles A, Wen WH, Schmider A, Gee C, Runnebaum IB, Kilian U, Jones LA, El-Naggar A, Minguillon C, Schönborn I, Reich O, Kreienberg R, Lichtenegger W, Press MF. Correlation of p53 mutations with resistance to platinum-based chemotherapy and shortened survival in ovarian cancer. Clin Cancer Res. 2001;7(10):2984–2997. [PubMed] [Google Scholar]
- 15.Lavarino C, Pilotti S, Oggionni M, Gatti L, Perego P, Bresciani G, Pierotti MA, Scambia G, Ferrandina G, Fagotti A, Mangioni C, Lucchini V, Vecchione F, Bolis G, Scarfone G, Zunino F. p53 gene status and response to platinum/paclitaxel-based chemotherapy in advanced ovarian carcinoma. J Clin Oncol. 2000;18(23):3936–3945. doi: 10.1200/JCO.2000.18.23.3936. [DOI] [PubMed] [Google Scholar]
- 16.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent Epithelial Ovarian Carcinoma: A Randomized Phase III Study of Pegylated Liposomal Doxorubicin Versus Topotecan. J Clin Oncol. 2001;19(14):3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 17.Tomao F, Papa A, Strudel M, Rossi L, Lo Russo G, Benedetti Panici P, Ciabatta FR, Tomao S. Investigating molecular profiles of ovarian cancer: An Update on Cancer Stem Cells. J Cancer. 2014;5(5):301–310. doi: 10.7150/jca.8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. (2005) Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65(8):3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 19.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I, Mor G. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8(1):158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramdass B, Duggal R, Minev B, Chowdhary A, Koka P. (2013) Functional role of solid tumor stem cells in disease etiology and susceptibility to therapeutic interventions. J Stem Cells. 2013;8(3–4):189–231. [PubMed] [Google Scholar]
- 21.Alvero AB, Fu HH, Holmberg J, Visintin I, Mor L, Marquina CC, Oidtman J, Silasi DA, Mor G. Stem like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells. 2009;7(10):2405–2413. doi: 10.1002/stem.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29(18):2672–2680. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68(11):4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landen CN, Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM, Bast RC, Jr, Coleman RL, Lopez-Berestein G, Sood AK. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9(12):3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo X, Xiong L, Sun T, Peng R, Zou L, Zhu H, Zhang J, Li H, Zhao J. Expression features of SOX9 associate with tumor progression and poor prognosis of hepatocellular carcinoma. Diagn Pathol. 2012;7:44. doi: 10.1186/1746-1596-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surowiak P, Materna V, Maciejczyk A, Kaplenko I, Spaczynski M, Dietel M, Lage H, Zabel M. CD46 expression is indicative of shorter revival-free survival for ovarian cancer patients. Anticancer Res. 2006;26(6C):4943–4948. [PubMed] [Google Scholar]
- 27.Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, Zhang K, Conner M, Landen CN. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2012;18(3):869–881. doi: 10.1158/1078-0432.CCR-11-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103(30):11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vathipadiekal V1, Saxena D, Mok SC, Hauschka PV, Ozbun L, Birrer MJ. Identification of a potential ovarian cancer stem cell gene expression profile from advanced stage papillary serous ovarian cancer. PLoS One. 2012;7(1):e29079. doi: 10.1371/journal.pone.0029079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abubaker K, Latifi A, Luwor R, Nazaretian S, Zhu H, Quinn MA, Thompson EW, Findlay JK, Ahmed N. Short-term single treatment of chemotherapy results in the enrichment of ovarian cancer stem cell like cells leading to an increased tumor burden. Mol Cancer. 2013;12:24. doi: 10.1186/1476-4598-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakar SS, Ratajczak MZ, Powell KS, Moghadamfalahi M, Miller DM, Batra SK, Singh SK. Withaferin a alone and in combination with cisplatin suppresses growth and metastasis of ovarian cancer by targeting putative cancer stem cells. PLoS One. 2014;9(9):e107596. doi: 10.1371/journal.pone.0107596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rios-Doria J, Durham N, Wetzel L, Rothstein R, Chesebrough J, Holoweckyj N, Zhao W, Leow CC, Hollingsworth R. Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia. 2015;17(8):661–670. doi: 10.1016/j.neo.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rau KM, Lin YC, Chen YY, Chen JS, Lee KD, Wang CH, Chang HK. Pegylated liposomal doxorubicin (Lipo-Dox®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: an open-label, multi-center, non-comparative phase II study. BMC Cancer. 2015;15:423. doi: 10.1186/s12885-015-1433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dijkgraaf EM, Santegoets SJ, Reyners AK, Goedemans R, Wouters MC, Kenter GG, van Erkel AR, van Poelgeest MI, Nijman HW, van der Hoeven JJ, Welters MJ, van der Burg SH, Kroep JR. A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti-IL-6R monoclonal antibody, and interferon-α2b in patients with recurrent epithelial ovarian cancer. Ann Oncol. 2015;26(10):2141–2149. doi: 10.1093/annonc/mdv309. [DOI] [PubMed] [Google Scholar]
- 35.Huang R, Li X, Holm R, Trope CG, Nesland JM, Suo Z. The expression of aldehyde dehydrogenase 1 (ALDH1) in ovarian carcinomas and its clinicopathological associations: a retrospective study. BMC Cancer. 2015;5:502. doi: 10.1186/s12885-015-1513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fügner A. Inhibition of immunologically induced inflammation by the plant steroid withaferin A. Arzneimittelforschung. 1973;23(7):932–935. [PubMed] [Google Scholar]
- 37.Rasool M, Varalakshmi P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: An in vivo and in vitro study. Vascul Pharmacol. 2006;44(6):406–410. doi: 10.1016/j.vph.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Gupta SK, Mohanty I, Talwar KK, Dinda A, Joshi S, Bansal P, Saxena A, Arya DS. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: a hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem. 2004;260:39–47. doi: 10.1023/b:mcbi.0000026051.16803.03. [DOI] [PubMed] [Google Scholar]
- 39.Mohan R, Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP, Rougas J, Pribluda VS. (2004) Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 40.Oh JH, Lee TJ, Kim SH, Choi YH, Lee SH, Lee JM, Kim YH, Park JW, Kwon TK. Induction of apoptosis by withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis 2008. 2008;13(12):1494–1504. doi: 10.1007/s10495-008-0273-y. [DOI] [PubMed] [Google Scholar]
- 41.Eva A, Robbins KC, Andersen PR, Srinivasan A, Tronick SR, Reddy EP, Ellmore NW, Galen AT, Lautenberger JA, Papas TS, Westin EH, Wong-Staal F, Gallo RC, Aaronson SA. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- 42.Behrens BC, Hamilton TC, Masuda H, Grotzinger KR, Whang-Peng J, Louie KG, Knutsen T, McKoy WM, Young RC, Ozols RF. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987;47(2):414–418. [PubMed] [Google Scholar]
- 43.Fong MY, Jin S, Rane M, Singh RK, Gupta R, Kakar SS. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PLoS One. 2012;7(7):e42265. doi: 10.1371/journal.pone.0042265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landen CN, Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM, Bast RC, Jr, Coleman RL, Lopez-Berestein G, Sood AK. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9(12):3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68(11):4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabizon AA, Lyass O, Berry GJ, Wildgust M. Cardiac safety of pegylated liposomal doxorubicin (Doxil/Caelyx) demonstrated by endomyocardial biopsy in patients with advanced malignancies. Cancer Invest. 2004;22(5):663–669. doi: 10.1081/cnv-200032899. [DOI] [PubMed] [Google Scholar]
- 47.Latifi A1, Luwor RB, Bilandzic M, Nazaretian S, Stenvers K, Pyman J, Zhu H, Thompson EW, Quinn MA, Findlay JK, Ahmed N. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PLoS One. 2012;7(10):e46858. doi: 10.1371/journal.pone.0046858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng E1, Long B, Sullivan P, McClellan S, Finan MA, Reed E, Shevde L, Rocconi RP. CD44+/CD24− ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metastasis 2012. 2012;29(8):939–948. doi: 10.1007/s10585-012-9482-4. [DOI] [PubMed] [Google Scholar]
- 49.Reya T, Clevers H. Wnt signaling in stem cells and cancer. Nature. 2005;434(7035):843. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 50.Liu S, Dontu ID, Mantle S, Patel NS, Ahn KW, Jackson, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:605–615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 53.Mimeault M, Batra SK. Novel biomarkers and therapeutic targets for optimizing the therapeutic management of melanomas. World J Clin Oncol. 2012;3(3):32–42. doi: 10.5306/wjco.v3.i3.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu KJ, Yang MH. (2011) Epithelial-mesenchymal transition and cancer stemness: the Twist1- Bmi1 connection. Biosci Rep. 2011;31(6):449–55. doi: 10.1042/BSR20100114. [DOI] [PubMed] [Google Scholar]
- 55.Shi W, Harris AL. (2006) Notch signaling in breast cancer and tumor angiogenesis: cross-talk and therapeutic potentials. J Mammary Gland Biol Neoplasia. 2006;1:41–52. doi: 10.1007/s10911-006-9011-7. [DOI] [PubMed] [Google Scholar]
- 56.Gangopadhyay S, Nandy A, Hor P, Mukhopadhyay A. (2013) Breast cancer stem cells: a novel Therapeutic target. Clin Breast Cancer. 2013;13:7–15. doi: 10.1016/j.clbc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90(2):390–396. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 58.Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33(2 Suppl 6):S3–11. doi: 10.1053/j.seminoncol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 59.du Bois A, Pfisterer J, Burchardi N, Loibl S, Huober J, Wimberger P, Burges A, Stähle A, Jackisch C, Kölbl H Arbeitsgemeinschaft Gynäekologische Onkologie Studiengruppe Ovarialkarzinom; Kommission Uterus. Combination therapy with pegylated liposomal doxorubicin and carboplatin in gynecologic malignancies: a prospective phase II study of the Arbeitsgemeinschaft Gynäekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and Kommission Uterus (AGO-K-Ut) Gynecol Oncol. 2007;107(3):518–25. doi: 10.1016/j.ygyno.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Ferrero JM, Weber B, Geay JF, Lepille D, Orfeuvre H, Combe M, Mayer F, Leduc B, Bourgeois H, Paraiso D, Pujade-Lauraine E. Second-line chemotherapy with pegylated liposomal doxorubicin and carboplatin is highly effective in patients with advanced ovarian cancer in late relapse: a GINECO phase II trial. Ann Oncol. 2007;18(2):263–268. doi: 10.1093/annonc/mdl376. [DOI] [PubMed] [Google Scholar]
- 61.Rapoport BL, Vorobiof DA, Slabber C, Alberts AS, Hlophe HS, Mohammed C. Phase II study of pegylated liposomal doxorubicin and carboplatin in patients with platinum-sensitive and partially platinum-sensitive metastatic ovarian cancer. Int J Gynecol Cancer. 2009;19(6):1137–1141. doi: 10.1111/IGC.0b013e3181a8b938. [DOI] [PubMed] [Google Scholar]
- 62.Nicoletto MO, Falci C, Pianalto D, Artioli G, Azzoni P, De Masi G, Ferrazzi E, Perin A, Donach M, Zoli W. Phase II study of pegylated liposomal doxorubicin and oxaliplatin in relapsed advanced ovarian cancer. Gynecol Oncol. 2006;100(2):318–23. doi: 10.1016/j.ygyno.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 63.D’Agostino G, Ferrandina G, Ludovisi M, Testa A, Lorusso D, Gbaguidi N, Breda E, Mancuso S, Scambia G. Phase II study of liposomal doxorubicin and gemcitabine in the salvage treatment of ovarian cancer. Br J Cancer. 2003;89(7):1180–1184. doi: 10.1038/sj.bjc.6601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrandina G1, Paris I, Ludovisi M, D’Agostino G, Testa A, Lorusso D, Zanghi M, Pisconti S, Pezzella G, Adamo V, Breda E, Scambia G. Gemcitabine and liposomal doxorubicin in the salvage treatment of ovarian cancer: updated results and long-term survival. Gynecol Oncol. 2005;98(2):267–73. doi: 10.1016/j.ygyno.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 65.Campos SM, Matulonis UA, Penson RT, Lee H, Berkowitz RS, Duska LR, Fuller AF, Jr, Wilson KS, Puchalski TA, Supko JG, Seiden MV. Phase II study of liposomal doxorubicin and weekly paclitaxel for recurrent Müllerian tumors. Gynecol Oncol. 2003;90(3):610–618. doi: 10.1016/s0090-8258(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 66.Verhaar-Langereis M1, Karakus A, van Eijkeren M, Voest E, Witteveen E. Phase II study of the combination of pegylated liposomal doxorubicin and topotecan in platinum-resistant ovarian cancer. Int J Gynecol Cancer. 2006;16(1):65–70. doi: 10.1111/j.1525-1438.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 67.Katsaros D1, Oletti MV, Rigault de la Longrais IA, Ferrero A, Celano A, Fracchioli S, Donadio M, Passera R, Cattel L, Bumma C. Clinical and pharmacokinetic phase II study of pegylated liposomal doxorubicin and vinorelbine in heavily pretreated recurrent ovarian carcinoma. Ann Oncol. 2005;16(2):300–306. doi: 10.1093/annonc/mdi055. [DOI] [PubMed] [Google Scholar]
- 68.Joly F, Sevin E, Lortholary A, Priou F, Paitel JF, Fabbro M, Henry-Amar M, Hamond K, Bourgeois H. Association of pegylated liposomal doxorubicin and ifosfamide in early recurrent ovarian cancer patients: a multicenter phase II trial. Gynecol Oncol. 2010;116(3):312–316. doi: 10.1016/j.ygyno.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 69.Del Conte G, Sessa C, von Moos R, Viganò L, Digena T, Locatelli A, Gallerani E, Fasolo A, Tessari A, Cathomas R, Gianni L. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer. 2014;111(4):651–659. doi: 10.1038/bjc.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tahir S. Real-life experience using trabectedin plus pegylated liposomal doxorubicin combination to treat patients with relapsed ovarian cancer. EJC Suppl. 2014;12(2):17–20. doi: 10.1016/S1359-6349(15)70006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pisano C, Cecere SC, Di Napoli M, Cavaliere C, Tambaro R, Facchini G, Scaffa C, Losito S, Pizzolorusso A, Pignata S. Clinical trials with pegylated liposomal Doxorubicin in the treatment of ovarian cancer. J Drug Deliv. 2013;2013:898146. doi: 10.1155/2013/898146. [DOI] [PMC free article] [PubMed] [Google Scholar]