The uncontrolled rapid growth of diabetes represents a global burden. Subjects with diabetes are at increased risk of cardiovascular disease, including a higher case fatality rate in myocardial infarction or stroke. However, the underlying pathogenesis and distinct mechanisms of diabetic cardiovascular complications are not clearly understood, thus limiting the development of novel preventive and treatment strategies. Cardiac cell communication via extracellular vesicles in healthy and pathological conditions is an emerging area of research. Exosomes are endogenous nanovesicles (30–100 nm circa), which are actively secreted out of cells of different types and are present in all studied biological fluids. Exosomes carry a composite cargo of molecules, including microRNAs, proteins, and lipids. Interestingly, it is emerging that the quality and quantity of molecules in such cargo vary with cell types and the environmental conditions. Moreover, the exosome cargo is at least in part transferrable to other cell types, with variable capacities for receiving such exosomes. Recipient cells respond to exosome uptake with expressional and functional changes.
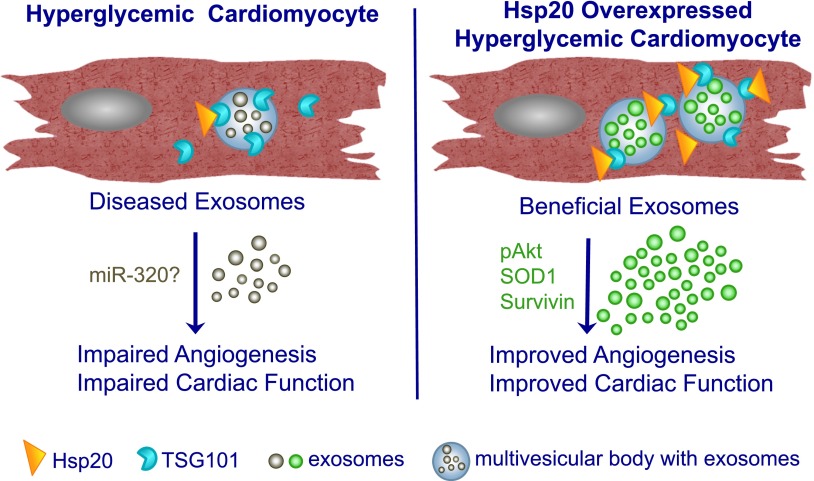
The study by Wang et al. (1) advances our understanding of the role of cardiomyocyte exosomes and hints at the complexity of exosomal cargo under diabetic conditions. One of the novel findings from the study demonstrates that heat shock protein (Hsp) 20, a chaperone protein from the HSP family that plays an important role in cellular intrinsic defense mechanisms, may enhance production of exosomes in cardiomyocytes via directly interacting with Tsg101, an upstream endosomal membrane transport protein involved in the exosome biogenesis pathway. Further, the authors used GW4869, an inhibitor of neutral sphingomyelinase/ceramide, to block the release of exosomes from cardiac cells in vivo, which ameliorated cardiac function in wild-type mice with streptozotocin-mediated induction of type 1 diabetes. This observation suggests that pathogenic exosomes carrying and diffusing detrimental signals contribute to the development of diabetic cardiomyopathy. However, as Wang et al. (1) noted, we need to be cautious about this observation, as GW4869 is not a specific inhibitor of exosome secretion and may affect other nonexosomal, nonvesicular secretions from cells in vivo and in vitro (2,3). Moreover, GW4869 may not even inhibit proteins associated with exosome secretion, or it may inhibit the secretion of a subset of vesicles but not all types of vesicles/exosomes released from certain cells (2,3). Prior investigations from the same team already provided evidence that cardiomyocyte-derived exosomes influence the behavior of endothelial cells (ECs) in a direction that varies upon the condition to what the producing cells are exposed (4). In particular, the proangiogenic response to cardiomyocyte-derived exosomes observed under physiological conditions was completely reverted when the cells were prepared from rats with type 2 diabetes. The group identified increased miR-320 levels in diabetic exosomes as the culprit of this dysfunction (4). In this new study, Wang et al. (1) provide complementary evidence that the exosomes released from diabetic cardiomyocytes carry detrimental components that are able to initiate a cascade of events amplifying and diffusing the disease signals. Neighboring cells such as ECs receive this disease signal, resulting in impaired angiogenic function. Importantly, the authors found that Hsp20 could convert this disease signal into a beneficial one via altering cardiomyocyte exosome secretion, thus restoring cardiac function under hyperglycemic conditions. In the current study, Hsp20, which interestingly is directly inhibited by miR-320 at the posttranscriptional level, was found to be decreased in diabetic cardiomyocytes. By contrast, diabetes did not affect any of the other HSP family of proteins screened by the authors. Moreover, Hsp20 was responsive to both acute and chronic hyperglycemia in mouse hearts, thus suggesting that decreased Hsp20 contributes to the different developmental stages of diabetic cardiomyopathy. To decipher the underlying mechanisms and cellular events, the authors have used a series of in vivo experiments including inducing streptozotocin-induced type 1 diabetes in transgenic mice with cardiac-specific Hsp20 overexpression. The authors have shown that myocardial overexpression of Hsp20 induces both qualitative and quantitative alterations to the composition and number of the exosomes secreted by cardiomyocytes, transforming the injurious exosomes into beneficial exosomes. The altered exosomes, the authors claim, now contain cellular protective proteins, such as phosphorylated Akt, SOD1, and survivin, that could be delivered to neighboring cardiac cells, thus promoting myocardial angiogenesis and alleviating oxidative stress, fibrosis, and apoptosis in the diabetic heart. Moreover, the “corrected” exosomes were shown to pass a presumably functionally active Hsp20 to the recipient cardiac cells in vivo. This process seems to have long-lasting (∼6 weeks) functional effects that are retained by the myocardium after the injection of the last dose of the therapeutic exosomes (1).
The precise biodistribution and half-life of individual exosomes delivered from an external source is still an unexplored question yet to be investigated; the study is rendered difficult particularly by the miniature nanometer size of the exosomes and the unavailability of suitable exosome markers, methods, and technology for their efficient detection in vivo. However, it is plausible that by the time of the last functional evaluation of cardiac function by Wang et al. (1) the injected exosomes had already been taken up and metabolized by the cardiac cells and/or eliminated from the heart and the circulation. Hence, this important observation contributes to the hypothesis of a long-lasting effect of exosomes. From here, we extrapolate to suggest the presence of an exosome “epigenetic” memory into the recipient cells that is mediated by microRNAs and other epigenetic modulatory factors embedded in the exosome cargo. The duration of such memory still needs to be proved and elucidated. In our opinion, the possibility that exosomes can transmit durable information between cardiovascular cells in a diabetes milieu has wider implications within the field of diabetes cardiovascular complications. In fact, although a number of genes involved in susceptibility to type 2 diabetes and its complications have been already identified by complementary approaches (such as genome-wide association studies, linkage studies, candidate gene association studies, and meta-analyses), the clinical risk of vascular complications in type 2 diabetes is only partially attributable to genetic predisposition and epigenetic mechanisms are suspected to play a role in the diabetes metabolic memory (5). This, together with the well-investigated lifestyle-dependent origin of type 2 diabetes and the emerging notion that diet-derived exosomes, such as the ones found in cow milk, are taken up by the human digestive tract cells, ECs, and inflammatory cells and deliver functional molecular material, opens completely new avenues for the understanding of the mechanisms behind the diabetes epidemics in our westernized world (6,7).
The functional relevance of circulating exosomes, including after the systemic delivery of exosomes prepared from culture cells, on the cardiovascular system is still debated within the cardiovascular research community. The study by Wang et al. (1) provides additional evidence in favor of a cardiovascular regulatory role for circulating exosomes derived from autologous, allogeneic, and xenogeneic sources. In particular, stem cell–derived exosomes proved able to induce cardioprotective and proangiogenic effects (8), which appear advantageous for preventing the evolution of the ischemic complications of diabetes.
Importantly, the exosome secretion by cardiac myocytes appears to be a regulated process that encompasses local and systemic consequences. As observed in the article by Wang et al. (1), exposure to high glucose affects the quantity and quality of cardiomyocyte exosomes, thus impacting the myocardial microvascular cells. Cardiomyocyte-derived exosomes are also reported to be able of systemic effects. For example, Pironti et al. (9) showed that cellular stretch in vitro and pressure overload in an in vivo mouse model induce the cardiomyocytes to release exosomes enriched with angiotensin II type 1 receptor (AT1R). Such particles appear able to transfer an active AT1R at distant sites (mesenteric artery, skeletal muscles), modulating peripheral vascular resistance and blood pressure, when injected in the circulation of AT1 knockout mice. It is possible that this and other exosome-based regulatory mechanisms are altered in diabetes and contribute to its several cardiovascular complications. It also suggests that therapeutic targeting of dysfunctional exosomes could represent new hope for the prevention and treatment of diabetic cardiovascular complications.
The microcommunication mechanisms involving exosomes are often multidimensional, with exosomes carrying signature signaling molecules from the cell of their origin to different cells and tissues at the vicinity or at a distance. The observation that detrimental exosomes released from cardiomyocytes in diabetic hearts can be switched to beneficial exosomes after Hsp20 overexpression is intriguing and warrants further investigation. It would be interesting to deeply characterize the molecular changes induced in Hsp20 transgenic cardiomyocytes under hyperglycemia and define what mediates such switch from releasing injurious exosomes to beneficial exosomes. A side-by-side molecular profiling in the cardiomyocytes and their released exosomes could provide a thorough insight on this mechanism and identify new therapeutic targets for validation.
A weakness of the studies by Wang et al. (1) and Pironti et al. (9) is that they could not expand from the narrow use of genetic models to demonstrate the functional and therapeutic relevance of their findings. Studies applying clinical-relevant models that are alternative to cardiomyocyte-selective genetic engineering are now needed. Moreover, exosomes from other important cardiac cells, such as ECs, fibroblasts, and whole tissue–derived exosomes, should be investigated to broaden our understanding of the in vivo function of exosomes under disease conditions. The expressional and functional phenotyping of exosomes from the different cell types contributing to the cardiovascular complications of diabetes could teach us alternative therapeutic approaches based on the use of bioinspired “artificial” exosomes that are able to correct the molecular defects and their propagation.
In summary, the study by Wang et al. (1) has provided novel insight that cardiac-specific overexpression of Hsp20 remarkably attenuated diabetes-induced cardiac dysfunction and adverse remodeling via modulating the cardiomyocyte exosome secretion (1) (Fig. 1). The results add to the current understanding of the exosome-mediated microcommunication mechanisms in the setting of diabetic cardiomyopathy. Although this is a valuable proof-of-concept study using a mouse model, it remains to be determined whether pathophysiological remodeling under diabetic cardiomyopathy in human hearts is regulated by similar mechanisms and whether Hsp20-mediated reprogramming of the myocardial exosomes can provide a novel platform for therapeutic strategies.
Figure 1.
Schematic representation of Hsp20-induced cardiac function. Hsp20 induces qualitative and quantitative changes in cardiomyocyte-derived exosomes, converting them to beneficial exosomes, which improves angiogenesis and cardiac function in the Hsp20-overexpressed heart. p, phosphorylated.
Article Information
Funding. This work is supported by the National Institutes of Health National Heart, Lung, and Blood Institute (R01 HL124187-01 to S.S.), the American Heart Association–The Davee Foundation (12SDG12160052 to S.S.), and a British Heart Foundation research program grant to C.E.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 3111.
References
- 1.Wang X, Gu H, Huang W, et al. Hsp20-mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes 2016;65:3111–3128 [DOI] [PMC free article] [PubMed]
- 2.Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008;319:1244–1247 [DOI] [PubMed] [Google Scholar]
- 3.Guo BB, Bellingham SA, Hill AF. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem 2015;290:3455–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Huang W, Liu G, et al. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol 2014;74:139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prattichizzo F, Giuliani A, De Nigris V, et al. Extracellular microRNAs and endothelial hyperglycemic memory: a therapeutic opportunity? Diabetes Obes Metab 2016;18:855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melnik BC. Milk: an epigenetic amplifier of FTO-mediated transcription? Implications for Western diseases. J Transl Med 2015;13:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusuma RJ, Manca S, Friemel T, Sukreet S, Nguyen C, Zempleni J. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. Am J Physiol Cell Physiol 2016;310:C800–C807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emanueli C, Shearn AI, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol 2015;71:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pironti G, Strachan RT, Abraham D, et al. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 2015;131:2120–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]