Abstract
Background
Individuals at high risk of cardiovascular disease (CVD) are undertreated.
Aim
To evaluate the effectiveness of a programme of targeted, nurse-led case finding for CVD prevention in primary care.
Design and setting
Targeted case finding for CVD prevention was implemented in urban West Midlands general practices between February 2009 and August 2012, and evaluated as a stepped wedge cluster randomised trial.
Method
Untreated patients aged 35–74 years and at ≥20% 10-year CVD risk were identified, invited for assessment by a project nurse, and referred to their GP for treatment initiation. The primary outcome was the proportion of high-risk patients prescribed antihypertensives or statins after exposure to the intervention compared with an equivalent period of time prior to exposure. Secondary outcomes included assessment of CVD risk factors.
Results
In 26 sequentially randomised general practices the exposed group consisted of 2926 untreated high-risk patients identified at the start of the intervention, with 2969 patients identified at the start of the unexposed period. The trial was well balanced in terms of age, sex, and cardiovascular risk factors. In the exposed period 19.7% of patients were prescribed antihypertensives or statins, and 10.8% of patients in the unexposed period. After adjustment for clustering and temporal effects the risk difference was 15.5% (95% CI = 3.9 to 27.1, P = 0.009). Assessment of lipid levels increased significantly, at 26.4% (99% CI = 5.3 to 47.5, P = 0.001)
Conclusion
Targeted case finding programmes can increase the number of high-risk patients started on antihypertensive and statin treatment.
Keywords: cardiovascular diseases, case-finding, CVD risk, nurse-led, prevention, primary care, randomised controlled trials
INTRODUCTION
Statins and antihypertensive drug treatments are effective, and are recommended for primary prevention of cardiovascular disease (CVD) in high-risk patients, but many remain untreated.1–12
Identification and treatment of high-risk patients in primary care can be opportunistic or by systematic invitation and CVD risk factor assessment. Systematic health checks are policy in the UK and have been proposed across a number of countries.13,14
Systematic health checks have not been shown to be effective and this may be due to a failure to identify eligible patients, initiate treatment, or continue with treatment.15,16 If they are to be effective, health checks must identify and treat high-risk patients. However, attendees are typically healthy and non-smokers.17,18 In the UK, implementation has focused on risk factor measurement rather than treatment and, although not all are offered health checks, those most likely to benefit are not systematically prioritised.19–21
Using pre-estimated CVD risk is the most efficient method of identifying high-risk patients.22 In a pilot study, an externally provided nurse identified high-risk patients from patient records using this method, invited them for assessment within their own general practice, and referred appropriate patients to their GP to prescribe treatment.23
The aim was to evaluate a programme of targeted case finding for prevention of CVD compared with usual care (opportunistic case finding) in UK primary care. A dedicated nurse, resourced and managed separately from the primary care team, was responsible for inviting and assessing patients while avoiding competing responsibilities affecting programme delivery. The programme was integrated with existing primary care, making use of medical records to identify high-risk patients, primary care facilities to assess patients, and GPs to initiate treatment. The primary objective was to determine the effect of the case-finding programme on the number of high-risk individuals started on treatment. Secondary objectives included effects on cardiovascular risk factors assessment and referrals to lifestyle advice services.
It was not possible to implement a parallel design across all general practices simultaneously. Therefore, it was implemented sequentially, as a stepped wedge cluster randomised controlled trial.24
METHOD
Between February 2009 and August 2012 targeted case finding for CVD prevention was implemented and evaluated separately in two urban areas of the West Midlands, UK. General practices in one area underwent major structural changes during the period. Therefore, the this study reports findings from 26 general practices in Sandwell, a deprived, multiethnic, metropolitan area.
How this fits in
Health checks to prevent cardiovascular disease are offered to all middle-aged and older adults in many countries including the UK, but they have not been shown to be effective. Health checks are only likely to be effective if additional high-risk patients are identified and start treatment. It is possible to pre-select high-risk patients for health checks using information in electronic patient records. This robust study design demonstrates that targeted, nurse-led case finding aimed at preselected high-risk patients increases the number of high-risk patients started on treatment.
The targeted case finding was in three steps, each undertaken by a project nurse external to the practice. These were: the use of software to search and identify untreated high-risk patients from electronic patient records; invitation to identified high-risk patients for assessment; and referral of appropriate eligible patients for lifestyle advice and to their GP for treatment initiation. Once all identified high-risk patients had been invited in one practice, the nurse moved on to the next practice.
Participants
Sandwell general practices were eligible for inclusion if they had not already implemented targeted case finding, had sufficient consultation rooms, and had MSDi Clinical Manager software. Patients were eligible for inclusion in this study if they:
were registered in one of the 26 practices for at least 90 days;
were aged between 35 and 74 years;
were not currently identified as having coronary heart disease, stroke, or diabetes;
were not currently receiving antihypertensive or statin treatment (no prescription within 90 days); and
had an estimated 10-year CVD risk of ≥20%.
The modified Framingham risk equation recommended in 2008 National Institute for Health and Care Excellence guidelines5,25 was used to estimate 10-year CVD risk. A previously described method addressed missing risk factor data.26 Patients with missing smoking status were assumed to be non-smokers. Missing blood pressure or cholesterol values were replaced with the average for an untreated person of that age, sex, and smoking and diabetic status.
Intervention
During the intervention period, the software generated a list of patients meeting the inclusion criteria. Letters were sent to these patients indicating they may be at increased risk of developing heart disease or stroke, offering assessment, and mentioning that they may be offered lifestyle advice and medication. The letter included the time and date of an appointment for their CVD assessment, which the patient could reschedule. Two further attempts, either by letter or telephone, were made to contact non-responders. The GP could exclude patients who had died, moved away, or were terminally ill.
Eligible patients were invited in descending order of estimated ≥20% 10-year CVD risk, until all had been invited. On attending, patients underwent a cardiovascular risk factor assessment, including smoking status, blood pressure measurement, and fasting glucose and lipid levels, and their 10-year CVD risk was recalculated with up-to-date measurements. The project nurse gave advice and also referred appropriate patients to smoking cessation services, and for advice on physical activity or diet. If they were eligible for drug treatment under guidelines, the nurse discussed treatment and informed the GP.5
During the control period general practices followed usual care, measuring CVD risk factors and identifying patients for treatment opportunistically.
Trial design
This was a stepped wedge cluster randomised controlled trial of 26 general practices (clusters). The methods are described in detail in a separate publication.27
From February 2009 to August 2012 clusters were sequentially randomised to be exposed to the intervention (targeted case finding). The step lengths were approximately 3 months, but exact duration was determined by the time taken for the project nurse to invite and assess all untreated high-risk patients in the practice. This took longer in larger practices. It was expected that implementation in all practices would take 18 months.
For the evaluation, all eligible patients were identified at two time points. Eligible patients in the exposed period were identified on the date on which the intervention began in the general practice. They were followed up for the duration that the practice was exposed to the intervention, allowing sufficient time for all eligible patients in the exposed period to be invited for assessment and prescribed treatment.
Eligible patients in the unexposed period were identified on a fixed date before the practice was exposed to the intervention. For each practice, the date was selected to allow for an equal length of follow-up in the unexposed period as in the exposed period. The unexposed period immediately preceded the exposed period (Figure 1).
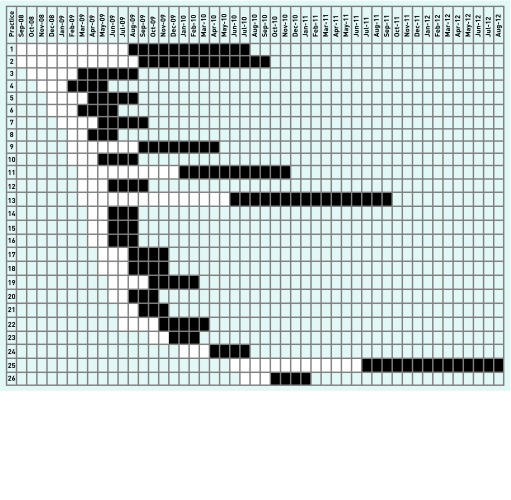
Figure 1.
Schematic diagram of exposed and unexposed periods in participating general practices.
White = unexposed period. Black = exposed period. Periods are rounded to the nearest month.
Outcomes
The primary outcome was the proportion of eligible patients (untreated high-risk) started on either an antihypertensive drug or a statin during the period of the intervention or the equivalent control period. Drugs were defined by their British National Formulary chapter codes.28 Secondary outcomes included the number of patients having blood pressure and lipid levels assessed, the number of patients referred for lifestyle advice (smoking cessation, physical activity, and dietary advice), changes in cardiovascular risk factors (blood pressure and lipid levels), and cardiovascular events (myocardial infarction [MI] and new diagnoses of angina, stroke, transient ischaemic attack [TIA], or peripheral vascular disease). All outcomes were at the level of the individual participant.
Anonymised data on outcomes and covariates were obtained from routinely recorded data in electronic primary care records.
Sample size
This was a pragmatic evaluation and the study sample size was limited to the 26 practices that agreed to participate. It was estimated there would be 6240 untreated high-risk patients in these practices, giving an average of 240 per practice, with a between-practice coefficient of variation in size of 0.74.
The detectable difference depends on the level of intracluster correlation coefficient (ICC), the variation in sizes of practices, and the proportion of patients treated in the control arm: 13% per year in the pilot study.23 The stepped wedge design should be less sensitive to increases in ICC than conventional cluster trials.4
At the time of designing this study no methodology existed that allowed for the varying cluster sizes, the mixture of cohort and cross-sectional design, or the variation in the design from the conventional stepped wedge study. Therefore conventional cluster randomised controlled trial (RCT) power calculation methods were used, as the variation in the design prohibited the use of the Hussey and Hughes approach.29 The study had a fixed sample size by design that could not be modified, so the power calculations did not inform any sample size targets.
Using several different ICC estimates it was determined that the study had 80% power to detect an increase in the percentage of patients treated to 19% (at 5% significance and a coefficient of variation of 0.74).
Randomisation
The unit of randomisation was the general practice. In all, 11 of the 26 practices were a higher priority for the case-finding intervention and therefore higher-priority practices received the intervention first in random order, followed by lower-priority practices in random order. Each practice was allocated a unique random number by the principal investigator, and within each priority group these were ranked in descending order. The implementation sequence was high-priority group, then low-priority group, following the order determined by the randomisation ranking.
Allocation was concealed from individual participants because there was no individual recruitment and therefore no individual consent. A representative of the general practice (usually the lead GP) consented to participate in the study but the practice was not informed of the timing of the intervention until approximately 1 week before it was to be delivered. The exact date of the intervention was determined by completion of the intervention in the previous practice. Due to the nature of the intervention it was not possible to blind general practices or patients to the intervention.
Statistical methods
The characteristics of the exposed and unexposed population included in the study are summarised using frequencies and percentages, means and standard deviations, or medians and interquartile ranges (IQR) as appropriate.
The null hypothesis — that there was no difference in the proportion of eligible patients started on treatment before and after exposure to the intervention — was tested using a mixed-effects regression model with a binary outcome, prescription of the appropriate medication. Important independent variables to consider are the clustering effect (general practice), calendar time (because the intervention is sequentially rolled out), dependence due to the same person contributing exposure time before and after the intervention (using a random effect), and an indicator of intervention exposure for each practice at each time point. The authors called this the time-adjusted model. Crucially, because this is a stepped wedge study it is essential to adjust for the effect of time and cluster.
The authors had anticipated fitting logistic random effects regression models (using generalised estimating equation [GEE] methods) and reporting corresponding odds ratios (OR). However, because these models did not converge, the authors fitted linear mixed models with dichotomous dependent variables and reported risk differences (RD) as opposed to ORs. This method has been used by others in the analysis of stepped wedge studies, and more generally has been found to give a good approximation to exact methods for large degrees of freedom.30,31
Null hypotheses and analyses for secondary outcomes took a similar form to that for the primary outcome. The primary outcome was considered significant at the 5% level (and so 95% confidence intervals [CIs] were reported) and the secondary at the 1% level (and so 99% CIs were reported). All analysis was by intention to treat.
RESULTS
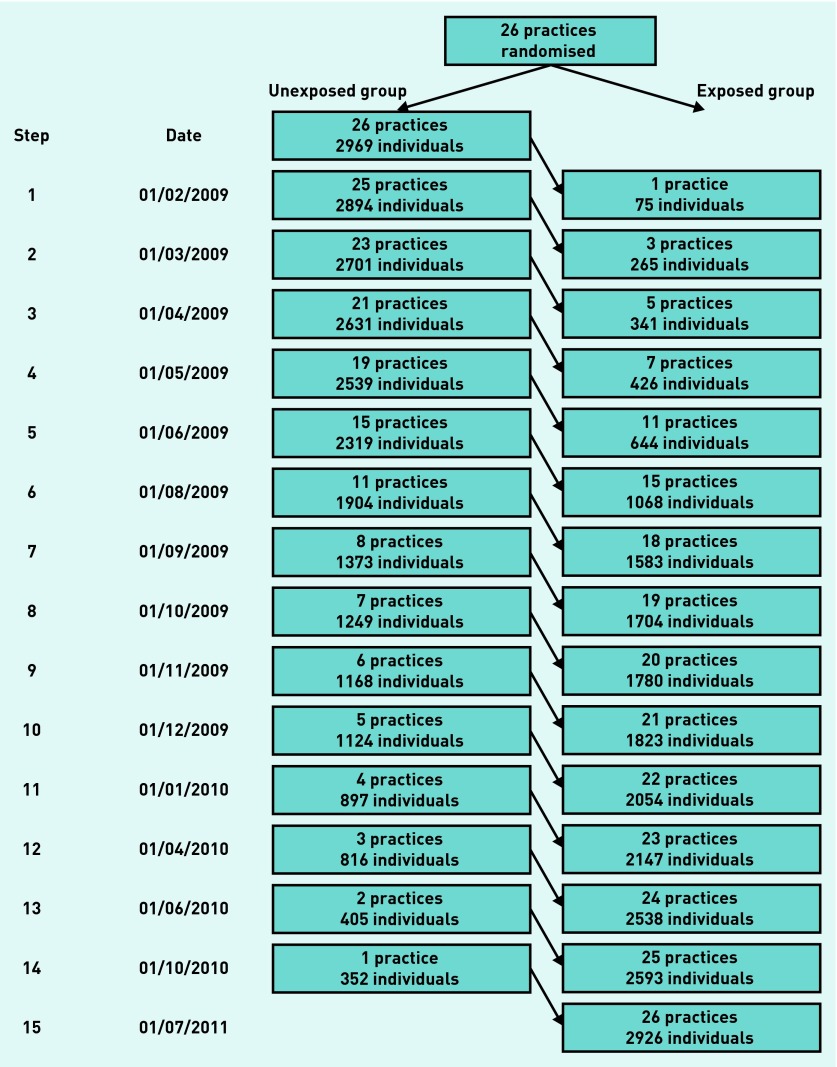
In all, 26 practices participated in the study, with a total list size of 126 535 and a median of 3114 (IQR = 2458 to 5236).32 The intervention began in the first practice on 1 February 2009 and ended in the last practice on 10 August 2012 (Figure 2). The median duration of the intervention was 111 days (IQR = 91–170 days). All practices participated for the duration of the study (Figure 2).
Figure 2.
Flow diagram of stepped wedge randomised controlled trial.
At the start of the unexposed period 2969 untreated high-risk patients were identified (2104 person years), and at the start of the exposed period 2926 untreated high-risk patients (2069 person years). In both unexposed and exposed groups average age was 63.9 years, 90% were male, cardiovascular risk factors were similar, and median Framingham 10-year CVD risks were 26% (IQR = 23% to 30%) (Table 1).
Table 1.
Baseline characteristics of exposed and unexposed patients
| Practice variables | Unexposed arm | Exposed arm |
|
| ||
| Number of registered patients,a n (%) | ||
| 35–54 years | 24 176 (51.3) | 24 809 (51.8) |
| 55–74 years | 22 911 (48.7) | 23 127 (48.2) |
|
| ||
| Eligible patient variables | Unexposed arm | Exposed arm |
|
| ||
| Number | 2969 | 2926 |
|
| ||
| Person years of follow-up (total) | 2104 | 2069 |
|
| ||
| Person days of follow-up (average per person), median (IQR) | 228 (112 to 368) | 229 (113 to 369) |
|
| ||
| Age, years | 63.9 (6.6) | 63.9 (6.6) |
|
| ||
| Male, n (%) | 2676 (90.1) | 2620 (89.5) |
|
| ||
| Ethnicity, n (%) | ||
| White British | 378 (12.7) | 488 (16.7) |
| South Asian | 202 (6.8) | 232 (7.9) |
| African–Caribbean | 6 (0.2) | 12 (0.4) |
| Mixed | 4 (0.1) | 5 (0.2) |
| Other | 3 (0.1) | 4 (0.1) |
| Not known | 2376 (80.0) | 2185 (74.7) |
|
| ||
| Systolic blood pressure, mmHg (SD)b | 145.2 (16.8) | 145.0 (16.3) |
| Diastolic blood pressure, mmHg (SD)b | 83.7 (9.8) | 83.5 (10.1) |
|
| ||
| Lipids | ||
| Total cholesterol, mmol/L (SD)c | 5.6 (0.99) | 5.7 (1.00) |
| HDL cholesterol, mmol/L (SD)c | 1.26 (0.32) | 1.26 (0.32) |
|
| ||
| Medication use, n (%) | ||
| Over-the-counter aspirin | 235 (7.9) | 263 (9.0) |
|
| ||
| Current smoker, n (%) | 1490 (50.2) | 1484 (50.7) |
|
| ||
| Framingham 10-year CVD risk, % median (IQR)b | 26 (23 to 30) | 26 (23 to 30) |
|
| ||
| Family history of premature CHD, n (%) | 367 (12.4) | 363 (12.4) |
After 90-day prior registration requirement applied, and before exclusions for prior disease.
Blood pressure (unexposed n = 1747, exposed n = 1738).
Total cholesterol (unexposed n = 848, exposed n = 862), HDL cholesterol (unexposed n = 641, exposed n = 707). CHD = coronary heart disease. CVD = cardiovascular disease. HDL = high-density lipoprotein. IQR = interquartile range.
A total of 321 (10.8%) unexposed patients were started on either antihypertensives or statins, and 577 (19.7%) exposed patients. The time-adjusted mean difference in proportion of patients initiating either treatment was 15.5% (95% CI = 3.9 to 27.1). In all, 222 (7.5%) unexposed patients and 333 (11.4%) exposed patients were started on antihypertensives. The time adjusted mean difference was 7.7% (95% CI = −0.1 to 15.5). In addition, 188 (6.3%) unexposed patients and 405 (13.8%) exposed patients were started on statins. The time-adjusted mean difference for initiating statins was 9.5% (95% CI = 3.0 to 16.0) (Table 2). The ICC in the time-adjusted analysis for initiation of either treatment was 0.014 (95% CI = 0.005 to 0.038).
Table 2.
Summary of outcomes by exposure to the intervention, with adjusted and unadjusted intervention effects
| Unexposed arm | Exposed arm | Percentage difference (95% CI for primary outcomes and 99% CI for secondary outcomes) | ||
|---|---|---|---|---|
|
| ||||
| Unadjusteda | Time adjustedb | |||
| Persons (person years) | 2969 (1939.33) | 2926 (1795.48) | ||
|
| ||||
| Treatment initiation, n(%) | ||||
| Antihypertensives or statins | 321 (10.8) | 577 (19.7) | 9.7 (7.9 to 11.4) P<0.001 | 15.5 (3.9 to 27.1) P= 0.009 |
| Antihypertensives | 222 (7.5) | 333 (11.4) | 4.7 (3.4 to 6.1) P<0.001 | 7.7 (−0.1 to 15.5) P= 0.054 |
| Statins | 188 (6.3) | 405 (13.8) | 7.7 (6.2 to 9.2) P<0.001 | 9.5 (3.0 to 16.0) P= 0.004 |
| Antiplatelet agents or over-the-counter aspirin | 235 (7.9) | 263 (9.0) | 2.5 (1.8 to 3.2) P<0.001 | 2.5 (−1.6 to 6.7) P= 0.230 |
|
| ||||
| Measurement of CVD risk factors, n(%) | ||||
| Patients with BP assessed | 825 (27.8) | 1285 (43.9) | 17.2 (14.3 to 20.0) P<0.001 | 28.8 (−3.5 to 59.1) P= 0.022 |
| Patients with lipids assessed | 437 (14.7) | 1046 (35.7) | 21.1 (18.4 to 23.9) P<0.001 | 26.4 (5.3 to 47.5) P= 0.001 |
|
| ||||
| Cardiovascular events, n(%) | ||||
| CVD events | 27 (0.91) | 17 (0.58) | 0.1 (−0.3 to 0.6) P= 0.486 | 0.3 (−1.6 to 2.2) P= 0.685 |
|
| ||||
| Referrals, n(%) | ||||
| Referral to smoking cessation | 12 (0.40) | 30 (1.03) | 0.6 (0.1 to 1.2) P= 0.002 | 3.3 (0.8 to 5.9) P= 0.001 |
| Referral to diet or physical activity advice | 4 (0.13) | 5 (0.17) | 0.0 (−0.2 to 0.3) P= 0.730 | −0.6 (−1.5 to 0.2) P= 0.055 |
The unadjusted model is adjusted for clustering.
The time-adjusted model is adjusted for the effect of time, clustering, and practice priority.
BP = blood pressure. CVD = cardiovascular disease.
Blood pressure was assessed in 825 (27.8%) of unexposed and 1285 (43.9%) of exposed patients. Lipid levels (total cholesterol and high density lipoprotein (HDL) cholesterol) were assessed in 437 (14.7%) and 1046 (35.7%), respectively. After adjusting for time and clustering, the difference in proportion with blood pressure measured was not statistically significant at the 1% level (P = 0.022), but the difference in proportion with lipid levels measured was statistically significant (P = 0.001). Referrals to smoking cessation services were higher among exposed patients — 12 (0.40%) to 30 (1.03%) — and these differences were statistically significant after adjustment. There were no statistically significant differences in frequency of referrals for dietary advice or cardiovascular events. There were few blood pressure and cholesterol measurements after exposure. Most of these were taken at assessment, and therefore before any treatment had been initiated. Thus, they could not reflect any changes due to the intervention and were not reported (Table 2).
Most of the differences in prescribing could be linked to risk factor assessment: 85.6% (769/898) of those started on treatment had both blood pressure and lipid levels recorded either at baseline, or during the unexposed or exposed periods. Most of those assessed (68.2% unexposed, 67.7% exposed) were confirmed to be at high risk. Of those who had blood pressure and lipid levels recorded, 33.3% were started on treatment during the unexposed period and 38.7% during the exposed period. High-risk patients were less frequently started on treatment during unexposed (28.6%) than exposed (38.9%) periods. Low-risk patients were more frequently started on treatment during unexposed (43.5%) than exposed (38.5%) periods (Table 3).
Table 3.
Proportion of patients with risk factors recorded, classified high and low risk, and treated in unexposed and exposed groups
| Unexposed period (n= 2969) | Risk factors recordeda (% of total in group) | 10-year CVD risk group (% of 10-year CVD risk group) | Treated (% of 10-year CVD risk group) | |||
|
|
|
|
||||
| Recorded | 774 (26.1) | CVD risk <20% | 246 (31.8) | 107 (43.5) | 258 (33.3) | |
| CVD risk ≥20% | 528 (68.2) | 151 (28.6) | ||||
| Not recorded | 2195 (73.9) | Not known | 2195 (100) | 63 (2.9) | 63 (2.9) | |
|
| ||||||
| Exposed period (n= 2926) | Risk factors recorded (% of total in group) | 10-year CVD risk (% of known risk) | Treated (% of 10-year CVD risk group) | |||
|
|
|
|
||||
| Recorded | 1319 (45.1) | CVD risk <20% | 426 (32.3) | 164 (38.5) | 511 (38.7) | |
| CVD risk ≥20% | 893 (67.7) | 347 (38.9) | ||||
| Not recorded | 1607 (54.9) | Not known | 1607 (100) | 66 (4.1) | 66 (4.1) | |
A record of blood pressure and lipid levels after baseline risk estimation. This is taken to mean that the patient was assessed. CVD = cardiovascular disease.
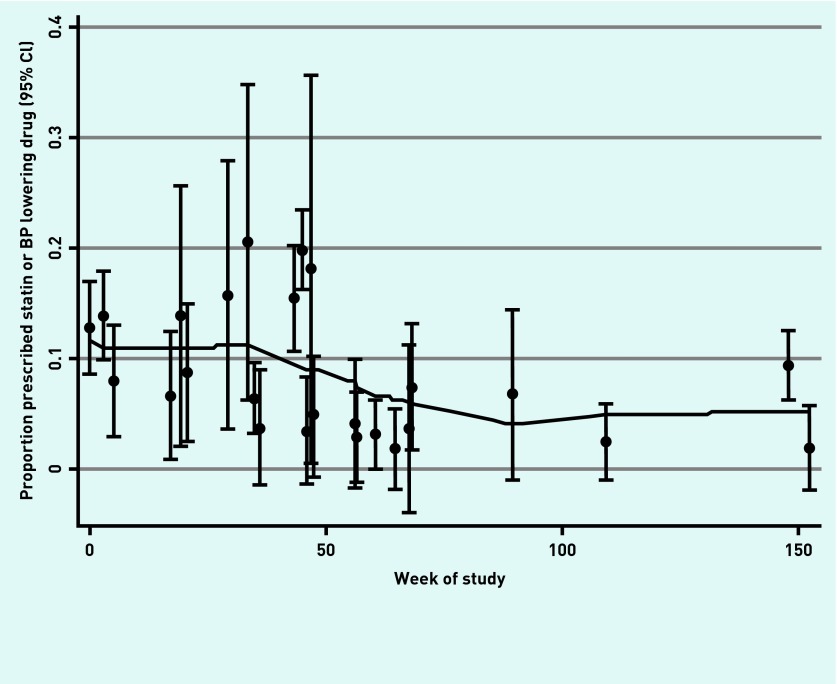
The differences in initiation of treatment between the unexposed and exposed periods increased after adjustment for time. This is because there was a declining rate of initiation of treatment over time during the unexposed period (Appendix 1).
DISCUSSION
Summary
Targeted case finding by a dedicated nurse increases the proportion of untreated high-risk patients started on either antihypertensive treatment or statins. The increased numbers of patients started on treatment can probably be attributed to higher rates of risk factor assessment during the time when the programme was implemented. It is unclear what caused the declining rate of treatment initiation in the control group. It may be because those patients easiest to start on treatment — because they attended more frequently or clinicians more readily considered cardiovascular risk — were initiated on treatment earlier, leaving an increasing proportion of harder-to-reach patients. The programme has a number of key elements; externally provided dedicated staff, access to primary care records for patient identification, targeting patients most likely to need treatment, and access to primary care facilities for assessment.
This evaluation shows that nurse-led, targeted case finding for CVD prevention in primary care should be the preferred model for provision of CVD case finding in primary care.
Strengths and limitations
A limitation of this study is that outcomes were assessed from patient records rather than directly measured. These record that prescriptions were issued but do not indicate that patients took the prescribed medication, nor are there data on persistence with medications. However, these limitations affect patients initiated on treatment in both the exposed and unexposed periods. Estimating CVD risk from previously recorded risk factors means some high-risk patients were missed and some low-risk patients invited. Low-risk invited patients were less often treated, reducing the apparent effectiveness of the intervention. But, as CVD risk was less frequently confirmed in the unexposed period, to confine analysis to assessed patients would introduce bias. This is because this would be comparing the proportions of a small number of patients confirmed to be at high risk and treated in the control group with a large number of patients confirmed to be at high risk and treated in the intervention group. As with any stepped wedge cluster randomised controlled trial these findings could be affected by time-varying confounders such as a change in frequency of cardiovascular risk factor assessment during the course of the study. However, as previously observed the rates of cardiovascular risk factor assessment appeared to decline over time.
Working with local stakeholders a form of roll-out was designed that was both random and practical to implement, allowing for a robust evaluation. Although a novel study design, the stepped wedge provides a higher level of evidence than a simple or controlled before and after study. The adjustment for temporal trends (that is, what is happening in the absence of any intervention) increased the apparent effectiveness of the intervention. This may be because the many high-risk patients identified in the unexposed and exposed periods were the same patients. Those in whom it was easier to start treatment were started during the unexposed period.
Comparison with existing literature
This study’s results are similar to the 15% increase in statin prescribing observed in a previous evaluation of externally provided nurse-led prevention targeted at high-risk patients in primary care.33 However, the target population included patients with diabetes. By contrast, untargeted CVD prevention does not reduce CVD events.15,16 Recent evaluations of general practice-led, untargeted case finding used simple before and after designs, which are prone to confounding.34,35
Implications for research and practice
Although this study was funded by a primary care trust (PCT), towards the end of the programme a national health checks programme was announced, PCTs were abolished, and responsibility for health checks was passed to the local government. Locally, the essence of targeted case finding was maintained by continuing to invite and offer nurse assessment to patients at ≥20% 10-year cardiovascular risk, while offering assessment by a health trainer to those at lower cardiovascular risk.
The provision of a dedicated staff member whose sole responsibility was invitation and assessment was critical to the success of this intervention. Although any UK general practice could identify, invite, and assess high-risk patients, practices neither identified nor invited high-risk patients during the unexposed periods. During the pilot study, control practices provided with a list of high-risk patients did not invite any for assessment.23
The findings are applicable to settings where external dedicated staff could use electronic primary care records to target patients at highest risk of CVD. A recent reduction in the recommended 10-year risk threshold for initiation of statins to 10% would increase the proportion of patients eligible for preventive treatments.36
There is an absence of robust evidence to support untargeted case finding in primary care, or for paying general practices to use existing staff for case finding. Advocates of these approaches should provide evidence for their effectiveness.
Acknowledgments
The authors wish to thank Sandwell Primary Care Trust for supporting this project, Sandwell general practices who participated, the project nurses who implemented the programme, and the patients who took part.
Appendix 1.
Underlying secular trend in rate of prescription of antihypertensives or statins (in absence of the intervention). BP = blood pressure. LOWESS = locally weighted scatterplot smoothing. Solid line is a smooth LOWESS fit to each of the point estimates.
Funding
The targeted case-finding programme was funded by Sandwell Primary Care Trust and covered the costs of nurses’ salaries, and in some cases postal costs. No specific payments were made to general practices to encourage them to take part. Tom Marshall, Karla Hemming, Kate Jolly, and Paramjit Gill were partly or wholly funded by the National Institute for Health Research (NIHR) through the Collaboration for Leadership in Applied Health Research and Care for Birmingham and Black Country (CLAHRC-BBC) programme. Tom Marshall and Kate Jolly are partly funded by CLAHRC West Midlands. The views expressed in this publication are not necessarily those of the NIHR, the Department of Health, NHS Partner Trusts, University of Birmingham, or the CLAHRC-BBC management group.
Ethical approval
Not applicable.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McConnachie A, Walker A, Robertson M, et al. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur Heart J. 2014;35(5):290–298. doi: 10.1093/eurheartj/eht232. Epub 2013 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev. 2009;4:CD000028. doi: 10.1002/14651858.CD000028.pub2. [DOI] [PubMed] [Google Scholar]
- 4.National Clinical Guideline Centre . Hypertension: the clinical management of primary hypertension in adults: update of clinical guidelines 18 and 34 CG127. London: Royal College of Physicians; 2011. Appendix I, Cost-effectiveness analysis — pharmacological treatment (updated 2011) [PubMed] [Google Scholar]
- 5.Cooper A, O’Flynn N. Risk assessment and lipid modification for primary and secondary prevention of cardiovascular disease: summary of NICE guidance. BMJ. 2008;336:1246–1248. doi: 10.1136/bmj.39554.624086.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence . Hypertension: clinical management of primary hypertension in adults CG127. London: NICE; 2011. https://www.nice.org.uk/guidance/Cg127 (accessed 18 Jul 2016). [Google Scholar]
- 7.Wu J, Yao GL, Zhu S, et al. Patient factors influencing the prescribing of lipid lowering drugs for primary prevention of cardiovascular disease in UK general practice: a national retrospective cohort study. PLoS One. 2013;8(7):e67611. doi: 10.1371/journal.pone.0067611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Staa TP, Smeeth L, Ng ES, et al. The efficiency of cardiovascular risk assessment: do the right patients get statin treatment? Heart. 2013;99(21):1597–1602. doi: 10.1136/heartjnl-2013-303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heeley EL, Peiris DP, Patel AA, et al. Cardiovascular risk perception and evidence — practice gaps in Australian general practice (the AusHEART study) Med J Aust. 2010;192(5):254–259. doi: 10.5694/j.1326-5377.2010.tb03502.x. [DOI] [PubMed] [Google Scholar]
- 10.Ma J, Sehgal NL, Ayanian JZ, Stafford RS. National trends in statin use by coronary heart disease risk category. PLoS Med. 2005;2(5):e123. doi: 10.1371/journal.pmed.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Wyk JT, Picelli G, Dieleman JP, et al. Management of hypertension and hypercholesterolaemia in primary care in The Netherlands. Curr Med Res Opin. 2005;21(6):839–848. doi: 10.1185/030079905X46368. [DOI] [PubMed] [Google Scholar]
- 12.Pereira M, Lunet N, Azevedo A, Barros H. Differences in prevalence, awareness, treatment and control of hypertension between developing and developed countries. J Hypertens. 2009;27(5):963–975. doi: 10.1097/hjh.0b013e3283282f65. [DOI] [PubMed] [Google Scholar]
- 13.Department of Health . Putting prevention first: vascular checks: risk assessment and management. London: Department of Health; 2008. [Google Scholar]
- 14.Schuetz CA, Alperin P, Guda S, et al. A standardized vascular disease health check in Europe: a cost-effectiveness analysis. PLoS One. 2013;8(7):e66454. doi: 10.1371/journal.pone.0066454. Print 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jørgensen T, Jacobsen RK, Toft U, et al. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ. 2014;348:g3617. doi: 10.1136/bmj.g3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krogsbøll LT, Jørgensen KJ, Grønhøj Larsen C, Gøtzsche PC. General health checks in adults for reducing morbidity and mortality from disease. Cochrane Database Syst Rev. 2012;10:CD009009. doi: 10.1002/14651858.CD009009.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Hoebel J, Starker A, Jordan S, et al. Determinants of health check attendance in adults: findings from the cross-sectional German Health Update (GEDA) study. BMC Public Health. 2014;14:913. doi: 10.1186/1471-2458-14-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dryden R, Williams B, McCowan C, Themessl-Huber M. What do we know about who does and does not attend general health checks? Findings from a narrative scoping review. BMC Public Health. 2012;12:723. doi: 10.1186/1471-2458-12-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chipchase L, Waterall J, Hill P. Understanding how the NHS Health Check works in practice. Practice Nursing. 2013;24(1):24–29. [Google Scholar]
- 20.Nicholas JM, Burgess C, Dodhia H, et al. Variations in the organization and delivery of the ‘NHS health check’ in primary care. J Public Health. 2013;35(1):85–91. doi: 10.1093/pubmed/fds062. [DOI] [PubMed] [Google Scholar]
- 21.Artac M, Dalton ARH, Babu H, et al. Primary care and population factors associated with NHS Health Check coverage: a national cross-sectional study. J Public Health. 2013;35(3):431–439. doi: 10.1093/pubmed/fdt069. [DOI] [PubMed] [Google Scholar]
- 22.Marshall T, Rouse A. Resource implications and health benefits of primary prevention strategies for cardiovascular disease in people aged 30 to 74: mathematical modelling study. BMJ. 2002;325(7357):197–199. doi: 10.1136/bmj.325.7357.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall T, Westerby P, Chen J, et al. The Sandwell Project: a controlled evaluation of a programme of targeted screening for prevention of cardiovascular disease in primary care. BMC Public Health. 2008;8:73. doi: 10.1186/1471-2458-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemming K, Haines TP, Chilton PJ, et al. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015;350:h391. doi: 10.1136/bmj.h391. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121(1 Pt 2):293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 26.Marshall T. Identification of patients for clinical risk assessment by prediction of cardiovascular risk using default risk factor values. BMC Public Health. 2008;8:25. doi: 10.1186/1471-2458-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall T, Caley M, Hemming K, et al. Mixed methods evaluation of targeted case finding for cardiovascular disease prevention using a stepped wedged cluster RCT. BMC Public Health. 2012;12:908. doi: 10.1186/1471-2458-12-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.BMJ Group, RCPCH Publications Ltd Royal Pharmaceutical Society of Great Britain. British National Formulary. http://bnf.org/bnf/index.htm (accessed 18 Jul 2016). [Google Scholar]
- 29.Hemming K, Girling AJ, Sitch AJ, et al. Sample size calculations for cluster randomised controlled trials with a fixed number of clusters. BMC Med Res Methodol. 2011;11:102. doi: 10.1186/1471-2288-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannan P, Murray D. Gauss or Bernoulli? A Monte Carlo comparison of the performance of the linear mixed-model and the logistic mixed-model analyses in simulated community trials with a dichotomous outcome variable at the individual level. Eval Rev. 1996;20(3):338–352. doi: 10.1177/0193841X9602000306. [DOI] [PubMed] [Google Scholar]
- 31.Leontjevas R, Gerritsen DL, Smalbrugge M, et al. A structural multidisciplinary approach to depression management in nursing-home residents: a multicentre, stepped-wedge cluster-randomised trial. Lancet. 2013;381(9885):2255–2264. doi: 10.1016/S0140-6736(13)60590-5. [DOI] [PubMed] [Google Scholar]
- 32.Health and Social Care Information Centre Quality and Outcomes Framework — 2009–10 practice level. 2010. http://www.hscic.gov.uk/catalogue/PUB05634 (accessed 18 Jul 2016).
- 33.Wood DA, Kotseva K, Connolly S, et al. EUROACTION Study Group. Nurse-coordinated multidisciplinary, family-based cardiovascular disease prevention programme (EUROACTION) for patients with coronary heart disease and asymptomatic individuals at high risk of cardiovascular disease: a paired, cluster-randomised controlled trial. Lancet. 2008;371(9629):1999–2012. doi: 10.1016/S0140-6736(08)60868-5. [DOI] [PubMed] [Google Scholar]
- 34.Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii–x. 1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 35.Artac M, Dalton AR, Majeed A, et al. Uptake of the NHS Health Check programme in an urban setting. Fam Pract. 2013;30(4):426–435. doi: 10.1093/fampra/cmt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Institute for Health and Care Excellence . Cardiovascular disease: risk assessment and reduction, including lipid modification CG181. London: NICE; 2014. https://www.nice.org.uk/guidance/cg181 (accessed 18 Jul 2016). [Google Scholar]