Abstract
Objectives
To evaluate the correlation between inflammatory measures and whole-body insulin sensitivity in psoriatic arthritis (PsA) patients.
Methods
For the present study, 40 nondiabetic PsA patients were recruited. A standard oral glucose tolerance test (OGTT) was performed. The insulin sensitivity index (ISI), insulinogenic index (IGI) and oral disposition index (ODI) were calculated from dynamic values of glucose and insulin obtained during OGTT.
Results
In our study population, mean ISI was 3.5 ± 2.5, median IGI was 1.2 (0.7–1.8), mean ODI 4.5 ± 4.5. In univariate correlation analysis, ISI correlated inversely with systolic blood pressure (sBP) (R = -0.52, p = 0.001), diastolic blood pressure (dBP) (R = -0.45, p = 0.004) and complement C3 (R = -0.43, p = 0.006) and ODI correlated inversely with sBP (R = -0.38, p = 0.02), dBP (R = -0.35, p = 0.03) and complement C3 (R = -0.37, p = 0.02). No significant correlations were found between analyzed variables and IGI. In a stepwise multiple regression, only complement C3 entered in the regression equation and accounted for approximately 50% of the variance of ISI. Using a receiver operating characteristic (ROC) curve we identified the best cut-off for complement C3 of 1.32 g/L that yielded a sensitivity of 56% and a specificity of 96% for classification of insulin resistant patients.
Conclusions
In conclusion, our data suggest that serum complement C3 could represent a useful marker of whole-body insulin sensitivity in PsA patients.
Introduction
Skin psoriasis (Pso) and psoriatic arthritis (PsA) are inflammatory diseases characterized by an increased risk of major cardiovascular events [1]. Among conventional cardiovascular disease (CVD) risk factors, type 2 diabetes mellitus (T2DM) plays a major role [2]. Glucose homeostasis abnormalities have been considered for a long time a peculiarity of rheumatoid arthritis (RA) [3, 4], but a recent large-scale UK study demonstrated that the risk of incident T2DM in PsA and Pso could be even higher to that conferred by RA [5];moreover a recent work confirmed that abnormal metabolic status is more prevalent in PsA than RA [6].
Metabolic syndrome (MS), a condition characterized by the clustering of different CVD risk factors (including glucose disturbances, visceral adiposity, elevated blood pressure, and abnormal lipid profile), is highly prevalent among patients with Pso [7] and PsA [8]. Although it is now well established that insulin resistance (IR) represents the pathophysiological hallmark of MS [9], the mechanism leading to IR/MS in PsA patients is only partially explained by the abnormal metabolic phenotype [10] and could be accounted for also by the negative impact of TNF-α and other inflammatory cytokines on insulin signaling [11] and altered balance in adipocytokines [12]. The so-called “inflammatory” hypothesis is supported by the evidence that disease-modifying antirheumatic drugs (DMARDs) [13] and biologic agents [14–18] have been demonstrated to improve insulin sensitivity in inflammatory arthritis patients.
The glucose clamp technique by DeFronzo et al. [19] is considered the gold standard for the in-vivo determination of insulin sensitivity. However, this technique is extremely time consuming and requires experienced operators. For these reason, simple surrogate indexes of insulin sensitivity/resistance calculated from fasting state values such as HOmeostasis Model Assessment of Insulin Resistance (HOMA-IR) [20] or from dynamic testing, such as Matsuda Insulin Sensitivity Index (ISI) [21], are widely used in research applications and clinical practice. However, the evaluation of IR as a novel CVD risk factor in the rheumatologic setting remains still complex to perform. The identification of clinician-friendly biomarkers reflecting accurately the metabolic status of the patients could be a simple efficient screening strategy in order to decide which patient should be referred to further evaluation in an appropriate clinical context.
In this view, we previously demonstrated that serum complement C3 is the more accurate inflammatory surrogate of IR in never treated PsA patients [22]. However, our previous study had the limitation of using a surrogate measure of IR, the HOMA-IR, derived from fasting-state only values of glucose and insulin and thus reflecting mainly hepatic IR [23]. The emerging role of skeletal muscle [24] and adipose tissue [25] IR prompted us to perform the present study, in order to evaluate the correlation between inflammation and whole-body insulin sensitivity in PsA patients.
Materials and Methods
Patients
The study protocol was approved by the local Ethics Committee (Comitato Etico Azienda Ospedaliera “Mater Domini”, Catanzaro, Italy). For the present study, 40 nondiabetic PsA patients (12 males and 28 females), were recruited at the Rheumatology Outpatient Clinic, Department of Health Sciences, University of Catanzaro “Magna Graecia”, Catanzaro, Italy and at the Rheumatology Department of Lucania, San Carlo Hospital, Potenza, Italy. All patients satisfied the ClASsification criteria for Psoriatic ARthritis (CASPAR) criteria for PsA [26]. Written informed consent was obtained from all patients involved in the present study according to the Declaration of Helsinki. Exclusion criteria were predefined as a past diagnosis of T2DM, polycystic ovary syndrome, infectious or neoplastic diseases; past or current treatment with insulin-sensitizing agents (i.e. metformin or peroxisome proliferator-activated receptor (PPAR) agonists). According to these criteria, 62 consecutive patients were screened and 22 were excluded.
Anthropometric measurements
Height and weight were measured with patients wearing light clothing and no shoes, to the nearest 0.1 cm and 0.1 kg respectively. Body mass index (BMI) was calculated with the standard formula:
Waist circumference was assessed with a flexible tape at midpoint between the lowest rib margin and the iliac crest. Blood pressure was measured on the left arm with a mercury sphygmomanometer, with the patient supine and after 5 minutes of rest.
Disease activity
The Disease Activity Score including 28 joints (DAS28-CRP) was used, evaluating the number of swollen joints (SJC), number of tender joints (TJC), the patients’ global assessment of health measured on a visual analogic scale (GH-VAS, range 0–100 mm), and high sensitivity C-reactive protein plasma concentration (hsCRP, mg/L). A score of DAS28-CRP between 2.6–3.2 indicates low disease activity, > 3.2- ≤ 5.1 moderate and > 5.1 high disease activity. The presence of dactylitis and enthesitis was recorded as a dichotomic variable. Patients were divided in five subsets according to clinical presentation [27]: “polyarthritis” if ≥ five joints involved; “oligoarthritis” if < five joints involved; “DIP predominant” if more than 50% of total joint count being distal interphalangeal joint (DIP) joints; “arthritis mutilans” if patients presented a destructive form of arthritis; and “spine predominant PsA” if inflammatory spinal pain, reduced spinal movements, and/or radiographic sacroiliitis.
Laboratory evaluation
After overnight fasting, blood samples were obtained for laboratory evaluation. Plasma glucose was measured with automated chemistry analyzer (Cobas 6000/Cobas e411, Roche Diagnostics). Plasma concentration of insulin was determined by chemiluminescence test (Centaur, Siemens HealthCare). Erythrocyte sedimentation rate (ESR) was analyzed by capillary photometry (Test 1, Alifax). High-sensitivity CRP (hsCRP) was measured by immunonephelometry (CardioPhase ® hsCRP, Siemens HealthCare). Serum C3 was measured by nephelometry (Siemens Healthcare Diagnostics, Deerfield, USA).
Oral glucose tolerance test (OGTT) and insulin sensitivity
A standard oral glucose tolerance test (OGTT) was performed in all patients. The test was performed according to the recommendations of World Health Organization (WHO).
Briefly, after overnight fasting, the patient was invited to drink a solution with 75 g of anhydrous glucose dissolved in 200 mL of water over a time of 5 minutes; blood samples were collected before and after 30, 60, 90, and 120 minutes, and plasma glucose and insulin concentrations were measured.
Insulin sensitivity index (ISI) was calculated with the equation proposed by Matsuda et al. [21] which provides a good approximation of measurements of whole-body insulin sensitivity obtained by the glucose clamp technique:
where ISI, insulin sensitivity index; G0, fasting plasma glucose (mg/dL); I0, fasting plasma insulin (mIU/L); Gmean, mean plasma glucose during OGTT (mg/dL); Imean, mean plasma insulin during OGTT (mIU/L).
Although there are no universally accepted cut-off values for the definition of insulin resistant individuals according to ISI, patients were classified as insulin resistant if ISI ≤ 2.5 as suggested by the authors of the original work [21], that corresponded to the lowest tertile of ISI distribution in the study population. This criterion was subsequently adopted by other groups [28].
Insulinogenic index (IGI), a measure of early phase insulin secretion, defined as the ratio of the increment of insulin to that of plasma glucose 30 minutes after a glucose load, was calculated with the formula [29]:
Oral Disposition Index (ODI), a measure of β-cell function integrated with insulin sensitivity, was calculated with the formula [30]:
Statistical Analysis
A sample size of at least 38 patients was calculated to detect a correlation coefficient between insulin sensitivity and disease-related variables of 0.50 (calculated on the basis of a previous study by our group [22] with a type I error rate of 0.05 and a type II error rate of 0.10).
Data are expressed as mean ± standard deviation, median (25th–75th percentile), or number (percentage) as appropriate. Continuous variables that were not normally distributed were ln-transformed before analysis. The Pearson’s product-moment correlation coefficient and stepwise multiple linear regression were used to evaluate correlation between variables. A receiver operating characteristic (ROC) curve was built to evaluate the predictivity of significant variables on the likelihood of being classified as insulin resistant.
A p-value <0.05 was considered statistically significant. All tests were two-tailed. The Statistics Package for Social Sciences (SPSS for Windows, version 17.0, SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
General characteristics of the study population are summarized in Table 1. The mean age of the patients was 50.3 ± 11.5 years while disease duration was 3 (1.25–4.00) years. According to clinical subsets 14/40 patients had polyarthritis, 12/40 oligoarthritis, 3/40 DIP predominant disease, 11/40 spine predominant disease. None of the patients recruited had arthritis mutilans. On average, disease activity was moderate with a mean DAS28-CRP of 4.1 ± 0.9. Fifty percent of patients were obese, with a mean BMI of 30.8 ± 5.9 Kg/m2.
Table 1. General characteristics of the study population.
| PsA (n = 40) | |
|---|---|
| Age, years | 50.3 ± 11.5 |
| Males, n (%) | 12 (30) |
|
PsA subset • Polyarthritis, n (%) • Oligoarthritis, n (%) • DIP predominant, n (%) • Spine predominant, n (%) • Arthritis mutilans, n (%) |
14 (35)12 (30)3 (7.5)11 (27.5)0 (0) |
| Enthesitis, n (%) | 8 (20) |
| Dactylitis, n (%) | 9 (22.5) |
| DAS28-CRP | 4.1 ± 0.9 |
| Disease duration, years | 3 (1.25–4.00) |
| Body Mass Index (BMI), kg/m2 | 30.8 ± 5.9 |
| sBP, mmHg | 128.4 ± 19.3 |
| dBP, mmHg | 80.7 ± 13.1 |
| BMI > 30 kg/m2, n (%) | 20 (50) |
|
Treatment • Methotrexate, n (%) • Sulfasalazine, n (%) • Cyclosporin A, n (%) • Infliximab, n (%) • Adalimumab, n (%) • Etanercept, n (%) • Golimumab, n (%) • Corticosteroids, n (%) |
23(32.5)2 (5)1 (2.5)2 (5)4 (10)3 (7.5)1 (2.5)9 (22.5) |
| ESR, mm/h | 18.2 ± 14.6 |
| hsCRP,mg/L | 5.5 ± 6.0 |
| C4, g/L | 0.26 ± 0.08 |
Legend: PsA, psoriatic arthritis; DIP, distal interphalangeal joint;DAS28-CRP, disease activity score including 28 joints and C-reactive protein; sBP, systolic blood pressure; dBP, diastolic blood pressure; BMI, body mass index; ESR, erythrocyte sedimentation rate; hsCRP, high-sensitivity C-reactive protein
Metabolic characteristics of the study population, including glucose and insulin values during OGTT are summarized in Table 2. According to OGTT results, 24 (52.5%) patients could be classified as normal glucose tolerance (NGT), 8 (20%) as impaired fasting glucose (IFG), 3 (7.5%) as impaired glucose tolerance (IGT), 1 (2.5%) as combined IFG/IGT and 4 (10%) were diagnosed with T2DM.In the whole study population, mean ISI was 3.5 ± 2.5, median IGI was 1.2 (0.7–1.8), mean ODI 4.5 ± 4.5.
Table 2. Metabolic characteristics of the study population.
| PsA (n = 40) | |
|---|---|
| Fasting glucose, mg/dL | 95.1 ± 14.9 |
| • 30 min glucose, mg/dL | 159.4 ± 40.2 |
| • 60 min glucose, mg/dL | 156.2 ± 45.1 |
| • 90 min glucose, mg/dL | 139.1 ± 48.5 |
| • 120 min glucose, mg/dL | 117.0 (98.7–131.3) |
| Fasting insulin, μUI/mL | 11.7 (7.0 18.3) |
| • 30 min insulin, μUI/mL | 102.9 ± 75.1 |
| • 60 min insulin, μUI/mL | 119.0 ± 68.8 |
| • 90 min insulin, μUI/mL | 115.5 ± 81.2 |
| • 120 min insulin, μUI/mL | 69.5 (43.0–126.5) |
| Insulin Sensitivity Index (ISI) ISI ≤ 2.5 | 3.5 ± 2.5 18(45) |
| Insulinogenic Index (IGI) | 1.2 (0.7–1.8) |
| Oral disposition Index (ODI) | 4.5 ± 4.5 |
| Normal Glucose Tolerance (NGT), n (%) | 24 (52.5) |
| Impaired Fasting Glucose (IFG), n (%) | 8 (20) |
| Impaired Glucose Tolerance (IGT), n (%) | 3 (7.5) |
| IFG plus IGT, n (%) | 1 (2.5) |
| Type 2 Diabetes, n (%) | 4 (10) |
Legend: PsA, psoriatic arthritis.
In univariate Pearson’s product-moment correlation analysis, ISI correlated inversely with sBP (R = -0.52, p = 0.001), dBP (R = -0.45, p = 0.004) and complement C3 (R = -0.43, p = 0.006), but not with age, sex, PsA duration, BMI, DAS28-CRP, ESR, C4 or hsCRP; ODI correlated inversely with sBP (R = -0.38, p = 0.02), dBP (R = -0.35, p = 0.03) and complement C3 (R = -0.37, p = 0.02) but not with age, sex, PsA duration, BMI, DAS28-CRP, ESR, C4 or hsCRP. No significant correlations were found between analyzed variables and IGI (Table 3).
Table 3. Univariate correlation analysis between selected variables and measures of insulin sensitivity and β-cell function in the whole study population.
| ISI | IGI | ODI | ||||
|---|---|---|---|---|---|---|
| R | p-value | R | p-value | R | p-value | |
| Age | -0.6 | 0.70 | -0.31 | 0.07 | -0.28 | 0.08 |
| Sex | -0.9 | 0.59 | 0.03 | 0.86 | -0.07 | 0.68 |
| Duration | -0.14 | 0.38 | -0.03 | 0.84 | -0.12 | 0.45 |
| BMI | -0.14 | 0.38 | -0.11 | 0.53 | -0.13 | 0.41 |
| sBP | -0.52 | 0.001 | 0.03 | 0.87 | -0.38 | 0.02 |
| dBP | -0.45 | 0.004 | -0.03 | 0.85 | -0.35 | 0.03 |
| DAS28-CRP | -0.30 | 0.85 | 0.07 | 0.73 | -0.06 | 0.70 |
| ESR | -0.19 | 0.27 | 0.16 | 0.39 | -0.07 | 0.67 |
| C3 | -0.43 | 0.006 | -0.07 | 0.70 | -0.37 | 0.02 |
| C4 | -0.04 | 0.81 | -0.21 | 0.23 | -0.20 | 0.22 |
| hsCRP | -0.17 | 0.29 | 0.17 | 0.33 | -0.21 | 0.18 |
Legend: ISI, insulin sensitivity index; IGI, insulinogenic index; ODI, oral disposition index; BMI, body mass index; sBP, systolic blood pressure; dBP, diastolic blood pressure; DAS28-CRP, disease activity score including 28 joints and C-reactive protein; ESR, erythrocyte sedimentation rate; hsCRP, high-sensitivity C-reactive protein.
Based on univariate correlation analysis above, a stepwise multiple regression model was built using ISI as dependent variable and age, BMI, sBP and complement C3 as predictor variables. At step 1 of the analysis, only complement C3 was entered into the regression equation. Model 1 was statistically significant (F = 5.89, p < 0.02). The standardized β coefficient was -0.50, indicating that approximately 50% of the variance of ISI could be accounted for by C3. A graphical representation of the linear correlation between C3 and ISI is reported in Fig 1.
Fig 1. Linear regression of C3 predicting ISI.
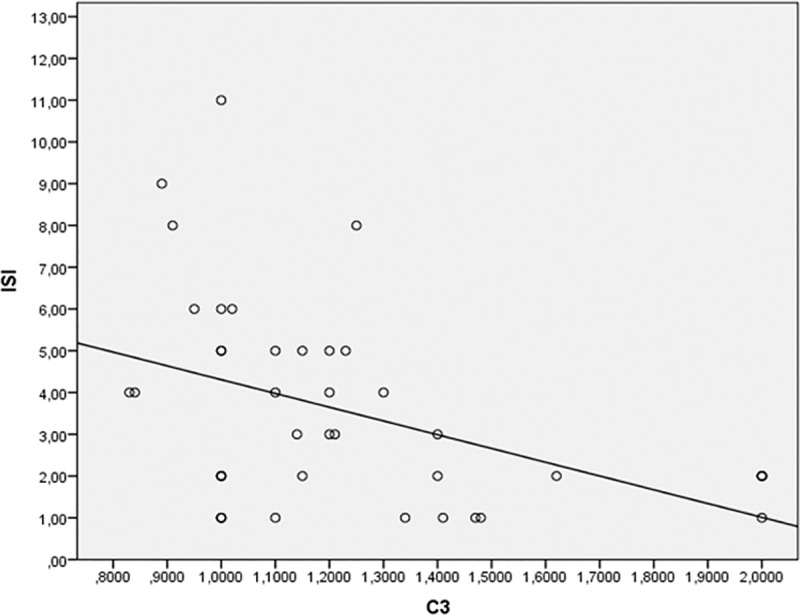
Finally, we constructed a ROC curve to evaluate the predictivity of complement C3 levels on the likelihood of being classified as insulin resistant according to a literature cut-off of ISI ≤ 2.5 (18/40 or 45% patients). The area under the ROC curve was 0.72 (95% CI: 0.55–0.89, p = 0.018). We identified the best cut-off for complement C3 of 1.32 g/L that yielded a sensitivity of 56% and a specificity of 96% for classification of insulin resistant patients.
Discussion
In this study, we demonstrated that complement C3 is the strongest inflammatory predictor of whole-body insulin sensitivity and adjusted β-cell function in PsA patients.
The main strength of our work, compared to other previously published studies, is that insulin sensitivity was evaluated with the surrogate measure ISI [21], obtained from dynamic values of insulin and glucose measured during OGTT. Other measures of insulin sensitivity/resistance, such as HOMA-IR and the Quantitative Insulin Sensitivity Check Index (QUICKI), are calculated from fasting-state values and mirror mainly hepatic insulin sensitivity; in contrast ISI better reflects whole-body insulin sensitivity, including the contribute from adipose tissue and skeletal muscles [23]. For this reason, ISI is able to identify more accurately clamp-defined subjects with IR when compared with other surrogate measures [31] and performs better than HOMA-IR in predicting the future development of T2DM [32].
Not only IR but also β-cell function, defined as the ability of β-cell to produce sufficient amount of insulin to account for peripheral insulin sensitivity, participates in the pathophysiology of T2DM. In our study, we employed the surrogate measure of β-cell function ODI that has been demonstrated to perform better than fasting-state measures and to predict independently the development of T2DM [30].
Although CRP has been historically considered the more reliable marker of cardiometabolic risk, complement C3 is increasingly emerging as a novel predictor of T2DMand IR [33, 34].
In the general population, C3 correlates with cardiovascular diseases and atherosclerosis [35], especially in heavy smokers [36]. In addition, it correlates with measures of β-cell function [37] and insulin sensitivity and predicts the incidence of future T2DM [38]. A similar correlation between complement C3 and IR has been demonstrated in patients with Pso [39], polycystic ovary syndrome [40] and obesity [41].
Serum C3 is produced mainly by the liver, but other sites, in particular visceral adipose tissue (VAT) [42] contribute significantly to circulating C3 levels. The relationship between visceral fat and C3 is mediated mainly by systemic inflammation and IR [39]. Nevertheless, in our population, no significant association between complement C3 and BMI was observed while waist circumference, the more diffuse measure of visceral adiposity, was not measured. This limitation of our study reduces our ability to speculate on the biological contribute of VAT to complement C3 levels in our population. However, the lack of correlation with BMI is partly explainable with the nature itself of this measure, that represents a global estimate of body mass and do not express accurately visceral adiposity, since some people with normal BMI may have abnormal waist circumference and vice versa [43]. In addition, skin cells express complement fractions [44] and C3 has been demonstrated to contribute to skin pathology [45]. Therefore, in the context of Pso and PsA, also skin could contribute to circulating C3 levels.
The increased expression of C3 could be triggered by the systemic inflammatory environment, and several cytokines, such as TNF-α and IL-6, are able to induce C3 expression [46]. Excess of C3 leads to the generation of C3a, that is desarginated by a carboxypeptidase in adipose tissue, generating C3adesArg or acylation-stimulating protein (ASP)[47]. Although previously considered an inactive immune by-product, recent evidence reevaluated its role as a lipogenic hormone. Supporting this hypothesis, circulating levels of ASP are increased in obesity [48] and upon weight loss return to normal values [49]. The binding of ASP to the receptor C5L2 [50] mediates several effects such as increased triacylglycerol synthesis, reduced triglyceride lipolysis, and increased glucose transport [51], thus leading to enhanced fat storage in a vicious circle that leads to incremental IR.
Despite original in methods, our study has some major limitations. First, the relatively low number of patients recruited, albeit appropriately powered to estimate the univariate correlation coefficient, made it impossible to perform more complex multivariate or subgroup analyses to correctly ascertain the influence of other possible confounders. Secondly, the lack of measurements of waist circumference and circulating ASP levels made it impossible to demonstrate the pathophysiological link between circulating C3 levels and insulin sensitivity.
In conclusion, our data suggest that serum C3 > 1.32 g/L could represent a useful marker of insulin sensitivity in PsA patients, easy to use in clinical practice. Larger studies are needed to evaluate the role of C3 in predicting future development of diabetes in PsA and to accurately establish optimal C3 cut-off for the identification of insulin-resistant PsA patients.
Data Availability
All relevant data are within the paper.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74(2):326–32. 10.1136/annrheumdis-2014-205675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA, et al. Risk of myocardial infarction in men and women with type 2 diabetes in the UK: a cohort study using the General Practice Research Database. Diabetologia. 2008;51(9):1639–45. 10.1007/s00125-008-1076-y [DOI] [PubMed] [Google Scholar]
- 3.Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: a systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33(1):115–21. [PubMed] [Google Scholar]
- 4.Ursini F, Russo E, D'Angelo S, Arturi F, Hribal ML, D'Antona L, et al. Prevalence of Undiagnosed Diabetes in Rheumatoid Arthritis: an OGTT Study. Medicine (Baltimore). 2016;95(7):e2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubreuil M, Rho YH, Man A, Zhu Y, Zhang Y, Love TJ, et al. Diabetes incidence in psoriatic arthritis, psoriasis and rheumatoid Epub arthritis: a UK population-based cohort study. Rheumatology (Oxford). 2014;53(2):346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labitigan M, Bahce-Altuntas A, Kremer JM, Reed G, Greenberg JD, Jordan N, et al. Higher rates and clustering of abnormal lipids, obesity, and diabetes mellitus in psoriatic arthritis compared with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66(4):600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bostoen J, Van Praet L, Brochez L, Mielants H, Lambert J. A cross-sectional study on the prevalence of metabolic syndrome in psoriasis compared to psoriatic arthritis. J Eur Acad Dermatol Venereol. 2014;28(4):507–11. [DOI] [PubMed] [Google Scholar]
- 8.Haroon M, Gallagher P, Heffernan E, FitzGerald O. High prevalence of metabolic syndrome and of insulin resistance in psoriatic arthritis is associated with the severity of underlying disease. J Rheumatol. 2014;41(7):1357–65. 10.3899/jrheum.140021 [DOI] [PubMed] [Google Scholar]
- 9.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. [DOI] [PubMed] [Google Scholar]
- 10.Costa L, Caso F, Ramonda R, Del Puente A, Cantarini L, Darda MA, et al. Metabolic syndrome and its relationship with the achievement of minimal disease activity state in psoriatic arthritis patients: an observational study. Immunol Res. 2015;61(1–2):147–53. 10.1007/s12026-014-8595-z [DOI] [PubMed] [Google Scholar]
- 11.Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48(4):751–62. [DOI] [PubMed] [Google Scholar]
- 12.Abella V, Scotece M, Conde J, Lopez V, Lazzaro V, Pino J, et al. Adipokines, metabolic syndrome and rheumatic diseases. J Immunol Res. 2014;2014:343746 10.1155/2014/343746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perdan-Pirkmajer K, Pirkmajer S, Thevis M, Thomas A, Praprotnik S, Hocevar A, et al. Methotrexate reduces HbA1c concentration but does not produce chronic accumulation of ZMP in patients with rheumatoid or psoriatic arthritis. Scand J Rheumatol. 2016:1–9. [DOI] [PubMed] [Google Scholar]
- 14.Ursini F, Naty S, Grembiale RD. Infliximab and insulin resistance. Autoimmun Rev. 2010;9(8):536–9. 10.1016/j.autrev.2009.12.014 [DOI] [PubMed] [Google Scholar]
- 15.Costa L, Caso F, Atteno M, Del Puente A, Darda MA, Caso P, et al. Impact of 24-month treatment with etanercept, adalimumab, or methotrexate on metabolic syndrome components in a cohort of 210 psoriatic arthritis patients. Clin Rheumatol. 2014;33(6):833–9. [DOI] [PubMed] [Google Scholar]
- 16.Ursini F, Russo E, Letizia Hribal M, Mauro D, Savarino F, Bruno C, et al. Abatacept improves whole-body insulin sensitivity in rheumatoid arthritis: an observational study. Medicine (Baltimore). 2015;94(21):e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ursini F, Mauro D, Naty S, Gagliardi D, Grembiale RD. Improvement in insulin resistance after short-term treatment with abatacept: case report and short review. Clin Rheumatol. 2012;31(9):1401–2. 10.1007/s10067-012-2034-0 [DOI] [PubMed] [Google Scholar]
- 18.da Silva BS, Bonfa E, de Moraes JC, Saad CG, Ribeiro AC, Goncalves CR, et al. Effects of anti-TNF therapy on glucose metabolism in patients with ankylosing spondylitis, psoriatic arthritis or juvenile idiopathic arthritis. Biologicals. 2010;38(5):567–9. 10.1016/j.biologicals.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. [DOI] [PubMed] [Google Scholar]
- 22.Ursini F, Grembiale A, Naty S, Grembiale RD. Serum complement C3 correlates with insulin resistance in never treated psoriatic arthritis patients. Clin Rheumatol. 2014;33(12):1759–64. 10.1007/s10067-013-2366-4 [DOI] [PubMed] [Google Scholar]
- 23.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–26. [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32 Suppl 2:S157–63. 10.2337/dc09-S302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalia AZ, Dasari S, Johnson ML, Robinson MM, Konopka AR, Distelmaier K, et al. Predictors of Whole-Body Insulin Sensitivity Across Ages and Adiposity in Adult Humans. J Clin Endocrinol Metab. 2016;101(2):626–34. 10.1210/jc.2015-2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73. [DOI] [PubMed] [Google Scholar]
- 27.Helliwell PS, Porter G, Taylor WJ. Polyarticular psoriatic arthritis is more like oligoarticular psoriatic arthritis, than rheumatoid arthritis. Ann Rheum Dis. 2007;66(1):113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kernan WN, Inzucchi SE, Viscoli CM, Brass LM, Bravata DM, Shulman GI, et al. Pioglitazone improves insulin sensitivity among nondiabetic patients with a recent transient ischemic attack or ischemic stroke. Stroke. 2003;34(6):1431–6. [DOI] [PubMed] [Google Scholar]
- 29.Seltzer HS, Allen EW, Herron AL Jr., Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967;46(3):323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32(2):335–41. 10.2337/dc08-1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorenzo C, Haffner SM, Stancakova A, Kuusisto J, Laakso M. Fasting and OGTT-derived measures of insulin resistance as compared with the euglycemic-hyperinsulinemic clamp in nondiabetic Finnish offspring of type 2 diabetic individuals. J Clin Endocrinol Metab. 2015;100(2):544–50. 10.1210/jc.2014-2299 [DOI] [PubMed] [Google Scholar]
- 32.Lorenzo C, Hazuda HP, Haffner SM. Insulin resistance and excess risk of diabetes in Mexican-Americans: the San Antonio Heart Study. J Clin Endocrinol Metab. 2012;97(3):793–9. 10.1210/jc.2011-2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hertle E, van Greevenbroek MM, Stehouwer CD. Complement C3: an emerging risk factor in cardiometabolic disease. Diabetologia. 2012;55(4):881–4. 10.1007/s00125-012-2462-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlaicu SI, Tatomir A, Boodhoo D, Vesa S, Mircea PA, Rus H. The role of complement system in adipose tissue-related inflammation. Immunol Res. 2016;64(3):653–64. 10.1007/s12026-015-8783-5 [DOI] [PubMed] [Google Scholar]
- 35.Hertle E, van Greevenbroek MM, Arts IC, van der Kallen CJ, Geijselaers SL, Feskens EJ, et al. Distinct associations of complement C3a and its precursor C3 with atherosclerosis and cardiovascular disease. The CODAM study. Thromb Haemost. 2014;111(6):1102–11. 10.1160/TH13-10-0831 [DOI] [PubMed] [Google Scholar]
- 36.van Greevenbroek MM, Jacobs M, van der Kallen CJ, Blaak EE, Jansen EH, Schalkwijk CG, et al. Human plasma complement C3 is independently associated with coronary heart disease, but only in heavy smokers (the CODAM study). Int J Cardiol. 2012;154(2):158–62. 10.1016/j.ijcard.2010.09.017 [DOI] [PubMed] [Google Scholar]
- 37.Fiorentino TV, Hribal ML, Andreozzi F, Perticone M, Sciacqua A, Perticone F, et al. Plasma complement C3 levels are associated with insulin secretion independently of adiposity measures in non-diabetic individuals. Nutr Metab Cardiovasc Dis. 2015;25(5):510–7. 10.1016/j.numecd.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 38.Wlazlo N, van Greevenbroek MM, Ferreira I, Feskens EJ, van der Kallen CJ, Schalkwijk CG, et al. Complement factor 3 is associated with insulin resistance and with incident type 2 diabetes over a 7-year follow-up period: the CODAM Study. Diabetes Care. 2014;37(7):1900–9. 10.2337/dc13-2804 [DOI] [PubMed] [Google Scholar]
- 39.Torres T, Bettencourt N, Mendonca D, Vasconcelos C, Silva BM, Selores M. Complement C3 as a marker of cardiometabolic risk in psoriasis. Arch Dermatol Res. 2014;306(7):653–60. 10.1007/s00403-014-1467-5 [DOI] [PubMed] [Google Scholar]
- 40.Dehdashtihaghighat S, Mehdizadehkashi A, Arbabi A, Pishgahroudsari M, Chaichian S. Assessment of C-reactive Protein and C3 as Inflammatory Markers of Insulin Resistance in Women with Polycystic Ovary Syndrome: A Case-Control Study. J Reprod Infertil. 2013;14(4):197–201. [PMC free article] [PubMed] [Google Scholar]
- 41.Hernandez-Mijares A, Jarabo-Bueno MM, Lopez-Ruiz A, Sola-Izquierdo E, Morillas-Arino C, Martinez-Triguero ML. Levels of C3 in patients with severe, morbid and extreme obesity: its relationship to insulin resistance and different cardiovascular risk factors. Int J Obes (Lond). 2007;31(6):927–32. [DOI] [PubMed] [Google Scholar]
- 42.Naughton MA, Botto M, Carter MJ, Alexander GJ, Goldman JM, Walport MJ. Extrahepatic secreted complement C3 contributes to circulating C3 levels in humans. J Immunol. 1996;156(8):3051–6. [PubMed] [Google Scholar]
- 43.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48 10.1186/1741-7015-9-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dovezenski N, Billetta R, Gigli I. Expression and localization of proteins of the complement system in human skin. J Clin Invest. 1992;90(5):2000–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basset-Seguin N, Porneuf M, Dereure O, Mils V, Tesnieres A, Yancey KB, et al. C3d,g deposits in inflammatory skin diseases: use of psoriatic skin as a model of cutaneous inflammation. J Invest Dermatol. 1993;101(6):827–31. [DOI] [PubMed] [Google Scholar]
- 46.Volanakis JE. Transcriptional regulation of complement genes. Annu Rev Immunol. 1995;13:277–305. [DOI] [PubMed] [Google Scholar]
- 47.Baldo A, Sniderman AD, St-Luce S, Avramoglu RK, Maslowska M, Hoang B, et al. The adipsin-acylation stimulating protein system and regulation of intracellular triglyceride synthesis. J Clin Invest. 1993;92(3):1543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maslowska M, Vu H, Phelis S, Sniderman AD, Rhode BM, Blank D, et al. Plasma acylation stimulating protein, adipsin and lipids in non-obese and obese populations. Eur J Clin Invest. 1999;29(8):679–86. [DOI] [PubMed] [Google Scholar]
- 49.Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88(4):1594–602. [DOI] [PubMed] [Google Scholar]
- 50.Kalant D, MacLaren R, Cui W, Samanta R, Monk PN, Laporte SA, et al. C5L2 is a functional receptor for acylation-stimulating protein. J Biol Chem. 2005;280(25):23936–44. [DOI] [PubMed] [Google Scholar]
- 51.Germinario R, Sniderman AD, Manuel S, Lefebvre SP, Baldo A, Cianflone K. Coordinate regulation of triacylglycerol synthesis and glucose transport by acylation-stimulating protein. Metabolism. 1993;42(5):574–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


