Abstract
Background
Laryngeal squamous cell carcinoma (LSCC) is a head and neck cancer type. In this study, we introduced a novel inflammation- and nutrition-based prognostic system, referred to as COR-BMI (Combination of red blood cell distribution width and body mass index), for LSCC patients.
Methods
A total of 807 LSCC patients (784 male and 23 female, 22–87 y of age) who underwent surgery were enrolled in this retrospective cohort study. The patients were stratified by COR-BMI into three groups: COR-BMI (0) (RDW ≤ 13.1 and BMI ≥ 25); COR-BMI (1) (RDW ≤ 13.1 and BMI < 18.5 or 18.5 ≤ BMI < 25; RDW > 13.1 and 18.5 ≤ BMI < 25 or BMI ≥ 25); or COR-BMI (2) (RDW > 13.1 and BMI < 18.5). Cox regression models were used to investigate the association between COR-BMI and cancer-specific survival (CSS) rate among LSCC patients.
Results
The 5-y, 10-y, and 15-y CSS rates were 71.6%, 60.1%, and 55.4%, respectively. There were significant differences among the COR-BMI groups in age (< 60 versus ≥ 60 y; P = 0.005) and T stage (T1, T2, T3, or T4; P = 0.013). Based on the results, COR-BMI (1 versus 0: HR = 1.76; 95% CI = 0.98–3.15; 2 versus 0: HR = 2.91; 95% CI = 1.53–5.54, P = 0.001) was a significant independent predictor of CSS.
Conclusion
COR-BMI is a novel inflammation- and nutrition-based prognostic system, which could predict long-term survival in LSCC patients who underwent surgery.
Introduction
Laryngeal squamous cell carcinoma (LSCC) is a head and neck cancer type [1]. Approximately 20,875 new cases of LSCC were diagnosed, and 11,488 deaths from LSCC occurred in China in 2011[2]. In spite of recent advances in the field, the National Cancer Data Base reported that the survival rate of LSCC patients has decreased from 57.1% to 51.9% [1]. Therefore, it is important to assess the prognostic factors of patients with LSCC.
There are several clinical prognostic factors in LSCC patients. In clinical practice, the most commonly used predictor of survival in LSCC patients is the tumor-node-metastasis (TNM) classification system; however, its predictive ability is not ideal [3]. Several tumor biomarkers, such as EZH2, MMP11, and P14, may be used individually or combined in LSCC prognosis [4, 5]; however, these biomarkers are rarely used in routine clinical practice due to their high costs, lack of standardization, and limited availability. Therefore, there is a need to identify a valid and reliable clinical prognostic parameter.
Chronic inflammation, which is commonly present in malnourished patients, is associated with poor patient outcomes [6, 7]. The systemic inflammatory response in cancer patients contributes to reduced iron metabolism, diminished response to erythropoietin, and decreased red blood cell survival, which may explain the symptoms and clinical signs in cancer patients: weight loss, anorexia, and cancer-related anemia [8, 9]. Additionally, a poor nutritional status increases the risk of infection due to impaired immune function, delayed wound healing as a result of reduced collagen production, altered blood clotting rates, increased blood vessel wall fragility, and increased risk of postoperative complications [10–13].
Studies have reported that high red blood cell distribution width (RDW) and low body mass index (BMI) values are indicative of malnutrition and chronic inflammation in cancer patients [14, 15]. In this study, we first introduced a novel prognostic system, referred to as COR-BMI (Combination of Red Blood Cell Distribution Width and Body Mass Index), which assesses inflammation and nutritional status in LSCC patients. Inflammation and nutritional status are associated with LSCC prognosis [16, 17]; therefore, we hypothesized that preoperative COR-BMI scores predict LSCC patient outcome.
Materials and Methods
Study Population
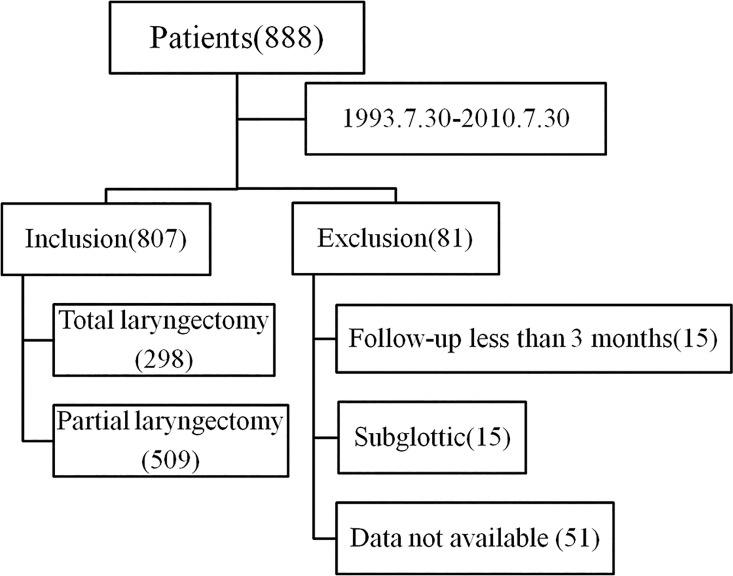
This study was approved by the Institutional Review Board of the Sun Yat-sen University Cancer Center (SYSUCC, Guangdong, China). This retrospective cohort study was conducted in Southern China. We retrospectively analyzed patients who underwent laryngectomy as a 1st curative treatment option for LSCC between July 30th, 1993 and July 30th 2010 at SYSUCC. The exclusion criteria consisted of patients with incomplete preoperative laboratory data, patients with subglottic LSCC, and patients who were lost to follow-up < 3 months post-surgery. A total of 807 LSCC patients were included in the study (Fig 1).
Fig 1. Patient selection process.
Data Collection
Clinical information was extracted from medical records at SYSUCC. Clinicopathological parameters included patient age (y), sex (male/female), smoking (no/yes), alcohol consumption (no/yes), neck dissection (no/yes), tumor subsite (glottic or supraglottic), T stage (T1–T4), N stage (N0–N3), histological differentiation (high/moderate/poor), height (m), and weight (kg). We used the conventional TNM staging system for cancer, which was established by the Union for International Cancer Control and the American Joint Committee on Cancer (AJCC) [18]. Laboratory data, including RDW, were obtained from preoperative examination. Cancer-specific survival (CSS) was defined as the time (in months) from the date of surgery until death due to LSCC. Follow-up evaluations were performed every three months during the first five years and annually thereafter.
Determination of RDW, BMI, and COR-BMI Cut-Off Values
For the RDW, a cutoff of 13.1 was generated according to the receiver operating characteristic (ROC) analysis in the training set for CSS (sensitivity 61.9%, specificity 54.2%, area under the curve [AUC] 0.59, 95% CI 0.55–0.63, P < 0.001) (S1 Fig). RDW values were categorized into two groups: RDW ≤ 13.1 and RDW > 13.1. BMI was categorized into three groups: BMI < 18.5, 18.5 ≤ BMI < 25, and BMI ≥ 25[19]. Patients with RDW ≤ 13.1 and BMI ≥ 25 were defined as COR-BMI (0). Patients with RDW ≤ 13.1 and BMI < 18.5 or 18.5 ≤ BMI < 25, and patients with RDW > 13.1 and 18.5 ≤ BMI < 25 or BMI ≥ 25 were defined as COR-BMI (1). Patients with RDW > 13.1 and BMI < 18.5 were defined as COR-BMI (2).
Statistical Analysis
RDW optimal cut-off values were determined by the ROC curve. Differences among the three COR-BMI groups (0, 1, and 2) were compared by χ2 test or Fisher’s exact test. Clinicopathological parameters that had significant effects (P < 0.05) on CSS based on univariate analyses were subjected to multivariate analyses using the Cox proportional hazards model. Hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) were estimated from Cox regression analysis. Patients’ clinical end points were calculated using the Kaplan-Meier method and compared by the log-rank test. For continuous variables, the data were expressed as mean ± SD. All analyses were carried out using IBM SPSS statistics software, version 20.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered significant.
Results
A total of 807 LSCC patients enrolled in the study. The 5-y, 10-y, and 15-y CSS rates were 71.6%, 60.1%, and 55.4%, respectively. Among the 807 patients, 784 were male (97.1%) and 23 were female (2.9%). The median age was 60 y (22–87 y; Table 1). Approximately 90% of the patients had a history of smoking, and 34.3% of the patients consumed alcohol on a frequently basis. The most common tumor subsite was the glottic larynx (560, 69.4%), followed by the supraglottic larynx (247, 30.6%). The rate of cervical lymph node metastasis was 19.3%. Among all LSCC cases, 24.5% were in T1 stage, 30.6% were in T2 stage, 25.4% were in T3 stage, and 19.5% were in T4 stage. Among the 807 LSCC patients, 39.3%, 43.4%, and 17.3% had high, moderate, and poor differentiation, respectively.
Table 1. Patients’ Clinicopathological Characteristics.
| Variables | COR-BMI 0(N %) | COR-BMI 1(N %) | COR-BMI 2(N %) | P value | |
|---|---|---|---|---|---|
| age | <60 | 30 (50.0) | 340 (50.9) | 25 (31.6) | 0.005 |
| ≥60 | 30 (50.5) | 328 (49.1) | 54 (68.4) | ||
| Gender | Female | 1 (1.7) | 19 (2.8) | 3 (3.8) | 0.756 |
| Male | 59 (98.3) | 649 (97.2) | 76 (96.2) | ||
| Smoking status | No | 6 (10.0) | 72 (10.8) | 9 (11.4) | 0.966 |
| Yes | 54 (90.0) | 596 (89.2) | 70 (88.6) | ||
| Drinking status | No | 43 (71.7) | 429 (64.2) | 58 (73.4) | 0.159 |
| Yes | 17 (28.3) | 239 (35.8) | 21 (26.6) | ||
| Neck dissection | No | 40 (66.7) | 481 (72.0) | 56 (70.9) | 0.675 |
| Yes | 20 (33.3) | 187 (28.0) | 23 (29.1) | ||
| Tumor subsite | Supraglottic | 42 (70.0) | 462 (69.2) | 56 (70.9) | 0.946 |
| Glottic | 18(30.0) | 206 (30.8) | 23 (29.1) | ||
| T | 1 | 16 (26.7) | 171 (25.6) | 11 (13.9) | 0.013 |
| 2 | 23(38.3) | 204 (30.5) | 20 (25.3) | ||
| 3 | 11 (18.3) | 161 (24.1) | 33 (41.8) | ||
| 4 | 10 (16.7) | 132 (19.8) | 15 (19.0) | ||
| N | 0 | 50 (83.3) | 539 (80.7) | 62 (78.5) | 0.855 |
| 1 | 4 (6.7) | 61 (9.1) | 10 (12.7) | ||
| 2 | 6 (10.0) | 63 (9.4) | 7 (8.9) | ||
| 3 | 0 (0.0) | 5 (0.7) | 0 (0.0) | ||
| Histological Differentiation | high | 29 (48.3) | 256 (38.3) | 32 (40.5) | 0.525 |
| moderate | 20 (33.3) | 295 (44.2) | 35 (44.3) | ||
| poor | 11 (18.3) | 117 (17.5) | 12 (15.2) | ||
Abbreviations: COR-BMI = Combination of red blood cell distribution width and body mass index; N = node; T = tumor
Based on COR-BMI scores, LSCC patients were further divided into three groups. Specifically, 60 (7.4%) of the patients had COR-BMI (0), 668 (82.8%) had COR-BMI (1), and 79 (9.8%) had COR-BMI (2). Based on χ2-test results, the only significant differences among the COR-BMI groups were in age (< 60 y versus ≥ 60 y; P = 0.005) and T stage (T1, T2, T3, or and T4; P = 0.013).
Univariate and Multivariate Analyses of CSS
In univariate analysis, age (P = 0.001), alcohol consumption (P = 0.001), neck dissection (P < 0.001), tumor subsite (P < 0.001), T stage (P < 0.001), N stage (P < 0.001), histological differentiation (P < 0.001), and COR-BMI scores were significant predictors of CSS (Table 2). These factors were subsequently subjected to multivariate analyses using the Cox proportional hazards model. Based on the multivariate results, COR-BMI (1 versus 0: HR = 1.76; 95% CI = 0.98–3.15; 2 versus 0: HR = 2.91; 95% CI = 1.53–5.54, P = 0.001) was a significant independent predictor of CSS.
Table 2. Cox Regression Analyses for Cancer-specific Survival in Laryngeal Squamous Cell Carcinoma.
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | P Value | HR(95% CI) | P value | |
| Age | ||||
| <60 | 1 (reference) | 0.001 | 1 (reference) | 0.002 |
| ≥60 | 1.48 (1.18–1.86) | 1.43 (1.13–1.80) | ||
| Gender | ||||
| Female | 1 (reference) | 0.051 | ND | ND |
| Male | 0.38 (0.14–1.01) | |||
| Smoking | ||||
| No | 1 (reference) | 0.139 | ND | ND |
| Yes | 1.35 (0.91–2.02) | |||
| Drinking | ||||
| No | 1 (reference) | 0.001 | 1 (reference) | 0.001 |
| Yes | 1.47 (1.17–1.84) | 1.50 (1.19–1.89) | ||
| Neck dissection | ||||
| No | 1 (reference) | < 0.001 | NS | |
| Yes | 2.01 (1.59–2.55) | |||
| Tumor subsite | ||||
| Supraglottic | 1 (reference) | < 0.001 | NS | |
| Glottic | 1.85 (1.47–2.33) | |||
| T stage | ||||
| T1 | 1 (reference) | < 0.001 | 1 (reference) | <0.001 |
| T2 | 1.37 (0.96–1.94) | 1.22 (0.85–1.73) | ||
| T3 | 2.24 (1.59–3.15) | 1.69 (1.18–2.41) | ||
| T4 | 3.28 (2.31–4.66) | 2.10 (1.43–3.09) | ||
| N stage | ||||
| N0 | 1 (reference) | < 0.001 | 1 (reference) | <0.001 |
| N1 | 2.69 (1.95–3.70) | 1.81 (1.28–2.56) | ||
| N2 | 2.51 (1.78–3.53) | 1.81 (1.25–2.62) | ||
| N3 | 18.68 (7.54–46.30) | 11.71 (4.61–29.79) | ||
| Histological Differentiation | ||||
| high | 1 (reference) | < 0.001 | 1 (reference) | 0.049 |
| moderate | 1.51 (1.17–1.95) | 1.24 (0.95–1.61) | ||
| poor | 2.04 (1.50–2.79) | 1.50 (1.08–2.08) | ||
| COR-BMI | ||||
| 0 | 1 (reference) | < 0.001 | 1 (reference) | 0.001 |
| 1 | 1.92 (1.08–3.43) | 1.76 (0.98–3.15) | ||
| 2 | 3.25 (1.72–6.16) | 2.91 (1.53–5.54) | ||
Abbreviations: COR-BMI = Combination of red blood cell distribution width and body mass index; CI = confidence interval; HR = hazard ratio; N = node; T = tumor; ND = not done; NS = not significant.
CSS Rates by COR-BMI Score
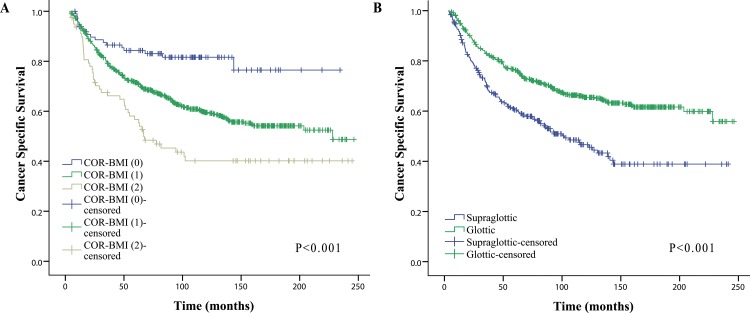
The CSS rates of patients with COR-BMI (2) were significantly lower than those of patients with COR-BMI (1) and COR-BMI (0) (5-y CSS: 56.7% versus 72.5% and 81.0%, respectively; 10-y CSS: 40.2% versus 60.9% and 78.8%, respectively; 15-y CSS: 40.2% vs. 55.4% and 78.8%, respectively log-rank: P < 0.001; Fig 2A).
Fig 2. Kaplan-Meier curves for CSS rates of subgroups.
(A) Kaplan-Meier curves for CSS rates of LSCC patients categorized by COR-BMI score (0/1/2). (B) Kaplan-Meier curves for CSS rates of LSCC patients categorized by tumor subsite. Abbreviations: CSS = cancer-specific survival; LSCC = Laryngeal squamous cell carcinoma; COR-BMI = Combination of red blood cell distribution width and body mass index.
Subgroup Analysis
The 5-y CSS, 10-y CSS and 15-y CSS rates for patients with supraglottic LSCC were 60.4%, 46.6% and 38.9%, respectively; these rates were lower than the 5-year, 10-y CSS and 15-y CSS rates of the glottic LSCC group (76.3%, 65.4% and 61.6%), the differences were statistically significant (P<0.001) (Fig 2B). The baseline characteristics of 807 patients with LSCC based on the stratification with the tumor subsite (see S1 Table). To further investigate prognostic factors in patients with LSCC, we conducted subgroup analysis for CSS in terms of tumor type (supraglottic LSCC group vs glottic LSCC group). Based on the multivariate results, COR-BMI was also a significant independent predictor of CSS for patients with supraglottic LSCC and glottic LSCC (supraglottic LSCC group:1 versus 0: HR = 3.62; 95% CI = 1.47–8.93; 2 versus 0: HR = 6.20; 95% CI = 2.25–17.07, P = 0.002; glottic LSCC group: 1 versus 0: HR = 1.67; 95% CI = 0.94–2.95; 2 versus 0: HR = 2.76; 95% CI = 1.41–5.39,P = 0.008) (see S2 Table).
Neutrophil-to-Lymphocyte Ratio (NLR), Hemoglobin (Hb), T Stage, and COR-BMI
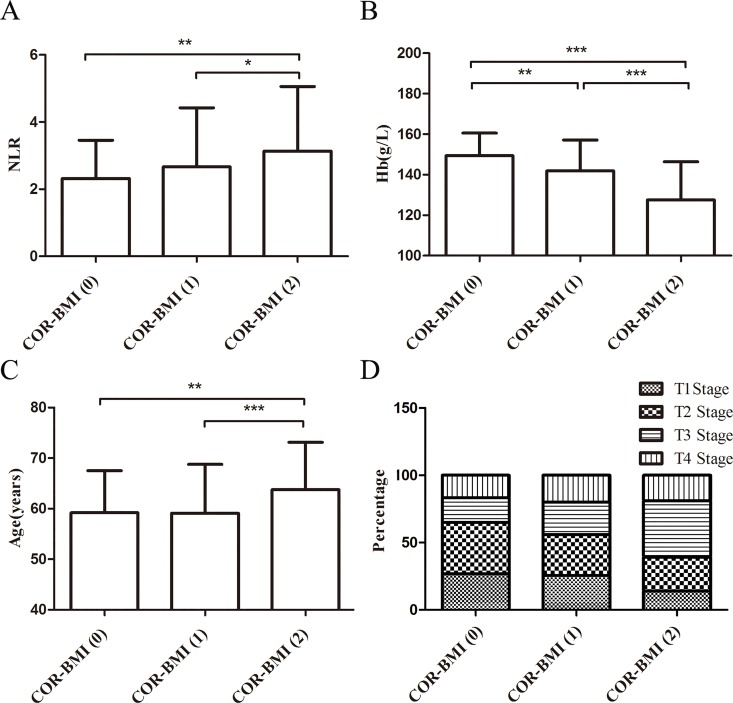
NLR and Hb levels increased with increasing COR-BMI scores (Fig 3A and 3B). As shown in Fig 3C, the average age of patients with COR-BMI (2) was higher than that of patients with COR-BMI (0) or COR-BMI (1). With increasing COR-BMI scores, the number of advanced T stage (T3 and T4) significantly increased (Fig 3D).
Fig 3. The values or distribution of parameters were various in different COR-BMI scores.
(A) -(C) The data were expressed as mean ± SD. (A) NLR in patients with different COR-BMI scores. (B) Hb levels in patients with different COR-BMI scores. (C) Age of patients with different COR-BMI scores. (D) T stage of patients with different COR-BMI scores. (*:0.01<p<0.05;**:0.001<p<0.01;***:p<0.001.) Abbreviations: COR-BMI = Combination of red blood cell distribution width and body mass index; NLR = neutrophil-to-lymphocyte ratio; Hb = hemoglobin; T = tumor stage.
Discussion
This study evaluated the relationship between COR-BMI scores and CSS rates in LSCC patients who underwent surgery. There were three COR-BMI scores: COR-BMI (0), characterized by a high probability of survival; COR-BMI (1), characterized by a medium probability of survival, and COR-BMI (2), characterized by a low probability of survival. To the best of our knowledge, this is the first study that evaluated the association between preoperative COR-BMI scores and CSS rates in LSCC patients.
Chronic inflammation has been identified as a risk factor in several types of cancers [20–23]. Cancer-associated inflammation plays a significant role in the proliferation, angiogenesis, invasion, and migration of cancer cells [24, 25]. Studies have shown that high RDW and low BMI values are indicative of chronic inflammation in cancer patients [14, 15]. Inflammation affects RDW values by impairing iron metabolism, inhibiting the response to erythropoietin, and decreasing red blood cell survival via the production of inflammatory markers [9].Like RDW, low BMI is also a risk factor in cancer patients with inflammation [15]. Accumulating evidence indicates that component of the systemic inflammatory response, for example NLR, has been associated with prognosis of various cancers [26, 27]. NLR is a biomarker of inflammation; high NLR values are correlated with poor clinical outcomes in patients [28]. In this study, NLR values increased with increasing COR-BMI scores, which suggested that there may be a relationship between COR-BMI scores and chronic inflammation. Nutritional status, which is associated with chronic inflammation, is linked to the long-term outcomes of several types of cancers [15, 29]. The metabolic effects of cancer-induced inflammation result in weight loss [30]. Overweight and obese patients have higher nutritional reserves to get through cancer therapy; however, several head and neck cancer patients experience significant weight loss at the time of diagnosis [29, 31, 32]. Different biomarkers of malnutrition have been used to predict outcome in cancer patients, such as RDW and BMI. RDW may be indicative of nutritional deficiencies, especially of iron, vitamin B12, and folic acid, which are common in cancer [33, 34]. In addition, low BMI is a prognostic factor of long-term survival in patients with malignant tumors, including head and neck cancers. Specifically, low BMI is associated with low CSS rates [35]. Recent studies have shown that low BMI is linked with reduced insulin levels, due to low or impaired secretion of insulin-like growth factor I and sex steroids, which suppress cellular proliferation and stimulate apoptosis [36, 37]. Furthermore, it has been reported that LSCC patients with low baseline Hb levels have low CSS rates [38, 39]. Interestingly, in this study, Hb levels decreased with COR-BMI scores. Therefore, COR-BMI is associated with nutritional status.
To evaluate the usefulness of COR-BMI in the prognosis of LSCC, we used ROC curve for survival prediction to verify the optimal cut-off points for RDW. CSS rates among LSCC patients can be divided into two groups based on RDW values: RDW ≤ 13.1 and > 13.1. Increased RDW is associated with poor prognosis in cancer patients [14]. According to WHO, there are three weight categories: underweight (BMI < 18.5), normal weight (18.5 ≤ BMI < 25) and overweight (BMI ≥ 25)[19]. Low BMI values are associated with increased risk of cancer mortality in patients [40]. Based on univariate and multivariate analyses, COR-BMI was a significant independent predictor of CSS in LSCC patients. Moreover, by univariate and multivariate analyses, COR-BMI was also a significant independent predictor of CSS of the patients with supraglottic LSCC and glottic LSCC separately.
Recent studies have shown that age at first diagnosis is the most significant factor that affects prognosis in LSCC patients [41]. The older the patient, the lower the nutritional status. Our results confirmed that the average age of patients with COR-BMI (2) was higher than that of patients with COR-BMI (0) or COR-BMI (1).
Ramroth et al. [41] showed that tumor stage is a significant risk factor in LSCC. Specifically, T3- and T4-stage patients have a two-fold risk of dying compared to T1- and T2-stage patients. With a rapid disease progression, nutritional deficiencies emerge early contributing to a poor disease prognosis[30]. In our study, the proportion of T3 and T4 patients (60.85%) with COR-BMI (2) was higher than that of patients with COR-BMI (0) (35.0%) or COR-BMI (1) (43.9%).
These findings suggest that high COR-BMI scores are predictive of an aggressive LSCC phenotype that contributes to low CSS rates. RDW and BMI are biomarkers of inflammation that are easy to measure and could be performed before surgery.
Our study had some limitations. First, our study had a retrospective design that included 807 patients from a single institution. Therefore, our study findings need to be validated using a larger cohort of patients. Second, several studies have used different RDW cut-off values, which need to be verified [42–45]. Third, COR-BMI, as a novel inflammation- and nutrition-based prognostic system, may be assessed in conjunction with other inflammatory and nutritional biomarkers in LSCC patients, including NLR, C-reactive protein, and Hb. This requires further research.
Conclusion
In conclusion, this is the first study that investigated the prognostic role of COR-BMI in LSCC patients. COR-BMI is an inflammation- and nutrition-based prognostic system of LSCC patients that is easy to measure.
Supporting Information
In this model, sensitivity was 61.9% and specificity was 54.2%. The AUC was 0.59 (95% CI0.55–0.63, P < 0.001).
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank all of the physicians of the Cancer Center, Sun Yat-sen University for allowing us to include their patients. We also thank the anonymous reviewers for their insightful comments and great efforts to help improve our manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hoffman HT, Porter K, Karnell LH, Cooper JS, Weber RS, Langer CJ, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. The Laryngoscope. 2006;116(9 Pt 2 Suppl 111):1–13. Epub 2006/09/02. 10.1097/01.mlg.0000236095.97947.26 . [DOI] [PubMed] [Google Scholar]
- 2.Du L, Li H, Zhu C, Zheng R, Zhang S, Chen W. Incidence and mortality of laryngeal cancer in China, 2011. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2015;27(1):52–8. Epub 2015/02/27. 10.3978/j.issn.1000-9604.2015.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egelmeer AG, Velazquez ER, de Jong JM, Oberije C, Geussens Y, Nuyts S, et al. Development and validation of a nomogram for prediction of survival and local control in laryngeal carcinoma patients treated with radiotherapy alone: a cohort study based on 994 patients. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2011;100(1):108–15. Epub 2011/07/26. 10.1016/j.radonc.2011.06.023 . [DOI] [PubMed] [Google Scholar]
- 4.Yu W, Zhang G, Lu B, Li J, Wu Z, Ma H, et al. miR-340 impedes the progression of laryngeal squamous cell carcinoma by targeting EZH2. Gene. 2015. Epub 2015/12/15. 10.1016/j.gene.2015.11.045 . [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Ding S, Zhong Q, Li G, Zhang Y, Huang XC. Significance of MMP11 and P14(ARF) expressions in clinical outcomes of patients with laryngeal cancer. International journal of clinical and experimental medicine. 2015;8(9):15581–90. Epub 2015/12/03. [PMC free article] [PubMed] [Google Scholar]
- 6.Shibutani M, Maeda K, Nagahara H, Ohtani H, Iseki Y, Ikeya T, et al. The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World journal of surgical oncology. 2015;13:194 Epub 2015/06/05. 10.1186/s12957-015-0609-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439–45. 10.1038/bjc.2012.92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore MM, Chua W, Charles KA, Clarke SJ. Inflammation and cancer: causes and consequences. Clinical pharmacology and therapeutics. 2010;87(4):504–8. Epub 2010/02/12. 10.1038/clpt.2009.254 . [DOI] [PubMed] [Google Scholar]
- 9.de Gonzalo-Calvo D, de Luxan-Delgado B, Rodriguez-Gonzalez S, Garcia-Macia M, Suarez FM, Solano JJ, et al. Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: A translational approach. Cytokine. 2012;58(2):193–8. 10.1016/j.cyto.2012.01.005 . [DOI] [PubMed] [Google Scholar]
- 10.Pacelli F, Bossola M, Rosa F, Tortorelli AP, Papa V, Doglietto GB. Is malnutrition still a risk factor of postoperative complications in gastric cancer surgery? Clin Nutr. 2008;27(3):398–407. 10.1016/j.clnu.2008.03.002 . [DOI] [PubMed] [Google Scholar]
- 11.Sierzega M, Niekowal B, Kulig J, Popiela T. Nutritional status affects the rate of pancreatic fistula after distal pancreatectomy: A multivariate analysis of 132 patients. J Am Coll Surgeons. 2007;205(1):52–9. 10.1016/j.jamcollsurg.2007.02.077 . [DOI] [PubMed] [Google Scholar]
- 12.Schwegler I, von Holzen A, Gutzwiller JP, Schlumpf R, Muhlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Brit J Surg. 2010;97(1):92–7. 10.1002/bjs.6805 . [DOI] [PubMed] [Google Scholar]
- 13.Gao YH, Zhou SF, Jiang WQ, Huang M, Dai XH. Effects of Ganopoly (R) (A Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest. 2003;32(3):201–15. 10.1081/Imm-120022979 . [DOI] [PubMed] [Google Scholar]
- 14.Perisa V, Zibar L, Sincic-Petricevic J, Knezovic A, Perisa I, Barbic J. Red blood cell distribution width as a simple negative prognostic factor in patients with diffuse large B-cell lymphoma: a retrospective study. Croat Med J. 2015;56(4):334–43. 10.3325/cmj.2015.56.334 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen HN, Chen XZ, Zhang WH, Yang K, Chen XL, Zhang B, et al. The Impact of Body Mass Index on the Surgical Outcomes of Patients With Gastric Cancer: A 10-Year, Single-Institution Cohort Study. Medicine. 2015;94(42):e1769 Epub 2015/10/27. 10.1097/MD.0000000000001769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollander D, Kampman E, van Herpen CM. Pretreatment body mass index and head and neck cancer outcome: A review of the literature. Critical reviews in oncology/hematology. 2015;96(2):328–38. Epub 2015/06/29. 10.1016/j.critrevonc.2015.06.002 . [DOI] [PubMed] [Google Scholar]
- 17.Kum RO, Ozcan M, Baklaci D, Kum NY, Yilmaz YF, Gungor V, et al. Elevated neutrophil-to-lymphocyte ratio in squamous cell carcinoma of larynx compared to benign and precancerous laryngeal lesions. Asian Pacific journal of cancer prevention: APJCP. 2014;15(17):7351–5. Epub 2014/09/18. . [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. 10.1245/s10434-010-0985-4 . [DOI] [PubMed] [Google Scholar]
- 19.Kang DH, Guo LF, Guo T, Wang Y, Liu T, Feng XY, et al. Association of body composition with bone mineral density in northern Chinese men by different criteria for obesity. J Endocrinol Invest. 2015;38(3):323–31. 10.1007/s40618-014-0167-5 . [DOI] [PubMed] [Google Scholar]
- 20.Lee SM, Russell A, Hellawell G. Predictive value of pretreatment inflammation-based prognostic scores (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio) for invasive bladder carcinoma. Korean journal of urology. 2015;56(11):749–55. Epub 2015/11/17. 10.4111/kju.2015.56.11.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia WJ, Wu JN, Jia HX, Yang YP, Zhang XL, Chen K, et al. The Peripheral Blood Neutrophil-To-Lymphocyte Ratio Is Superior to the Lymphocyte-To-Monocyte Ratio for Predicting the Long-Term Survival of Triple-Negative Breast Cancer Patients. PloS one. 2015;10(11). ARTN e0143061 10.1371/journal.pone.0143061 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaynar M, Goktas S. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol-Semin Ori. 2015;33(11):497–. 10.1016/j.urolonc.2015.05.017 . [DOI] [PubMed] [Google Scholar]
- 23.Roxburgh CSD, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–63. 10.2217/Fon.09.136 . [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A. Cancer—Inflammation by remote control. Nature. 2005;435(7043):752–3. 10.1038/435752a . [DOI] [PubMed] [Google Scholar]
- 25.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140(6):883–99. 10.1016/j.cell.2010.01.025 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glazer ES, Rashid OM, Pimiento JM, Hodul PJ, Malafa MP. Increased neutrophil-to-lymphocyte ratio after neoadjuvant therapy is associated with worse survival after resection of borderline resectable pancreatic ductal adenocarcinoma. Surgery. 2016. Epub 2016/07/28. 10.1016/j.surg.2016.04.039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong W, Yang S, Yang X, Guo F. Blood preoperative neutrophil-to-lymphocyte ratio is correlated with TNM stage in patients with papillary thyroid cancer. Clinics (Sao Paulo). 2016;71(6):311–4. Epub 2016/07/22. 10.6061/clinics/2016(06)04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer treatment reviews. 2013;39(5):534–40. Epub 2012/09/22. 10.1016/j.ctrv.2012.08.003 . [DOI] [PubMed] [Google Scholar]
- 29.McRackan TR, Watkins JM, Herrin AE, Garrett-Mayer EM, Sharma AK, Day TA, et al. Effect of body mass index on chemoradiation outcomes in head and neck cancer. The Laryngoscope. 2008;118(7):1180–5. Epub 2008/05/14. 10.1097/MLG.0b013e31816fca5c . [DOI] [PubMed] [Google Scholar]
- 30.Laviano A, Koverech A, Mari A. Cachexia: clinical features when inflammation drives malnutrition. The Proceedings of the Nutrition Society. 2015;74(4):348–54. Epub 2015/03/27. 10.1017/S0029665115000117 . [DOI] [PubMed] [Google Scholar]
- 31.Capuano G, Gentile PC, Bianciardi F, Tosti M, Palladino A, Di Palma M. Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Support Care Cancer. 2010;18(4):433–7. 10.1007/s00520-009-0681-8 . [DOI] [PubMed] [Google Scholar]
- 32.den Hollander D, Kampman E, van Herpen CML. Pretreatment body mass index and head and neck cancer outcome: A review of the literature. Crit Rev Oncol Hemat. 2015;96(2):328–38. 10.1016/j.critrevonc.2015.06.002 . [DOI] [PubMed] [Google Scholar]
- 33.Karnad A, Poskitt TR. The automated complete blood cell count. Use of the red blood cell volume distribution width and mean platelet volume in evaluating anemia and thrombocytopenia. Arch Intern Med. 1985;145(7):1270–2. Epub 1985/07/01. . [DOI] [PubMed] [Google Scholar]
- 34.Thompson WG, Meola T, Lipkin M Jr., Freedman ML. Red cell distribution width, mean corpuscular volume, and transferrin saturation in the diagnosis of iron deficiency. Arch Intern Med. 1988;148(10):2128–30. Epub 1988/10/01. . [PubMed] [Google Scholar]
- 35.Langius JAE, Bakker S, Rietveld DHF, Kruizenga HM, Langendijk JA, Weijs PJM, et al. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Brit J Cancer. 2013;109(5):1093–9. 10.1038/bjc.2013.458 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. 10.1038/nrc1408 . [DOI] [PubMed] [Google Scholar]
- 37.Song X, Pukkala E, Dyba T, Tuomilehto J, Moltchanov V, Mannisto S, et al. Body mass index and cancer incidence: the FINRISK study. Eur J Epidemiol. 2014;29(7):477–87. 10.1007/s10654-014-9934-z . [DOI] [PubMed] [Google Scholar]
- 38.Cho EI, Sasaki CT, Haffty BG. Prognostic significance of pretreatment hemoglobin for local control and overall survival in T1-T2N0 larynx cancer treated with external beam radiotherapy. Int J Radiat Oncol. 2004;58(4):1135–40. 10.1016/j.ijrobp.2003.08.002 . [DOI] [PubMed] [Google Scholar]
- 39.Lee WR, Berkey B, Marcial V, Fu KK, Cooper JS, Vikram B, et al. Anemia is associated with decreased survival and increased locoregional failure in patients with locally advanced head and neck carcinoma: a secondary analysis of RTOG 85–27. International journal of radiation oncology, biology, physics. 1998;42(5):1069–75. Epub 1998/12/30. . [DOI] [PubMed] [Google Scholar]
- 40.Adachi T, Hinoi T, Kinugawa Y, Enomoto T, Maruyama S, Hirose H, et al. Lower body mass index predicts worse cancer-specific prognosis in octogenarians with colorectal cancer. Journal of gastroenterology. 2015. Epub 2015/12/15. 10.1007/s00535-015-1147-z . [DOI] [PubMed] [Google Scholar]
- 41.Ramroth H, Schoeps A, Rudolph E, Dyckhoff G, Plinkert P, Lippert B, et al. Factors predicting survival after diagnosis of laryngeal cancer. Oral Oncol. 2011;47(12):1154–8. 10.1016/j.oraloncology.2011.08.003 . [DOI] [PubMed] [Google Scholar]
- 42.Kemal Y, Demirag G, Bas B, Onem S, Teker F, Yucel I. The value of red blood cell distribution width in endometrial cancer. Clinical chemistry and laboratory medicine. 2015;53(5):823–7. Epub 2015/04/11. 10.1515/cclm-2014-0699 . [DOI] [PubMed] [Google Scholar]
- 43.Ay S, Eryilmaz MA, Aksoy N, Okus A, Unlu Y, Sevinc B. Is early detection of colon cancer possible with red blood cell distribution width? Asian Pacific journal of cancer prevention: APJCP. 2015;16(2):753–6. Epub 2015/02/17. . [DOI] [PubMed] [Google Scholar]
- 44.Riedl J, Posch F, Konigsbrugge O, Lotsch F, Reitter EM, Eigenbauer E, et al. Red cell distribution width and other red blood cell parameters in patients with cancer: association with risk of venous thromboembolism and mortality. PloS one. 2014;9(10):e111440 Epub 2014/10/28. 10.1371/journal.pone.0111440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koma Y, Onishi A, Matsuoka H, Oda N, Yokota N, Matsumoto Y, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PloS one. 2013;8(11):e80240 Epub 2013/11/19. 10.1371/journal.pone.0080240 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this model, sensitivity was 61.9% and specificity was 54.2%. The AUC was 0.59 (95% CI0.55–0.63, P < 0.001).
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.