Abstract
Background
The mechanisms through which physical activity supports healthy brain function remain to be elucidated. One hypothesis suggests that increased brain-derived neurotrophic factor (BDNF) mediates some cognitive and mood benefits. This meta-analysis sought to determine the effect of exercise training on resting concentrations of BDNF in peripheral blood.
Methods
MEDLINE, Embase, PsycINFO, SPORTDiscus, Rehabilitation & Sports Medicine Source, and CINAHL databases were searched for original, peer-reviewed reports of peripheral blood BDNF concentrations before and after exercise interventions ≥ 2 weeks. Risk of bias was assessed using standardized criteria. Standardized mean differences (SMDs) were generated from random effects models. Risk of publication bias was assessed using funnel plots and Egger’s test. Potential sources of heterogeneity were explored in subgroup analyses.
Results
In 29 studies that met inclusion criteria, resting concentrations of peripheral blood BDNF were higher after intervention (SMD = 0.39, 95% CI: 0.17–0.60, p < 0.001). Subgroup analyses suggested a significant effect in aerobic (SMD = 0.66, 95% CI: 0.33–0.99, p < 0.001) but not resistance training (SMD = 0.07, 95% CI: -0.15–0.30, p = 0.52) interventions. No significant difference in effect was observed between males and females, nor in serum vs plasma.
Conclusion
Aerobic but not resistance training interventions increased resting BDNF concentrations in peripheral blood.
Introduction
Evidence suggests that physical activity confers cognitive benefits and can alleviate symptoms of psychiatric disorders in many individuals [1–11]. However, the mechanisms through which physical activity confers these benefits have not been fully elucidated. It is likely that a combination of physiological changes induced by physical activity results in these beneficial effects [12–14]. One current hypothesis is that physical activity stimulates the production of brain-derived neurotrophic factor (BDNF), a protein of the neurotrophin family involved in the growth, differentiation, and survival of neurons [12, 15–18]. An increase in BDNF resulting from physical activity is thought to increase adult neurogenesis and synaptogenesis, and prevent neuronal loss, possibly contributing to cognitive benefits and reduced psychiatric symptoms [19–25]. Importantly, studies suggest that peripheral BDNF concentrations may reflect CNS health to some extent, with concentrations typically being lower in patients with psychiatric disorders [26–32] and metabolic disorders [33].
Human and animal studies have examined the impact of physical activity on BDNF concentrations in different body compartments such as blood, muscle, and the brain [34–47]. Animal studies have reported an increase in BDNF after physical activity in various regions of the brain including the hippocampus, prefrontal cortex, motor cortex, lateral septum, cerebellum, striatum, and amygdala [42–56]. Peripheral blood studies in humans have been inconsistent, with some studies reporting increases in BDNF after physical activity and others reporting no significant change or even decreases in BDNF concentrations [20, 40, 57–66]. Possible reasons for this inconsistency include population heterogeneity, differences in type, intensity, and duration of the exercise intervention, and BDNF measurement from different blood components such as serum or plasma [67].
Previous reviews on the effect of exercise on BDNF have highlighted the impact of those study differences [68–71]. A systematic review by Knaepen et al. found a transient increase in peripheral BDNF after acute aerobic exercise but not after resistance training [69]. Furthermore, they concluded that chronic exercise training was unlikely to increase resting concentrations of BDNF but that more research was needed in this area. A review by Zoladz et al. on the same topic found similar results regarding acute exercise but reported mixed results regarding the effect of chronic exercise training on resting peripheral BDNF concentrations [70]. More recently, a systematic review by Huang et al. concluded that both acute and chronic aerobic exercise result in an increase in peripheral BDNF [68]. Consistent with previous reviews, no effect of resistance training on peripheral BDNF concentrations was found. A meta-analysis on the effect of exercise on peripheral BDNF concentrations in humans was published in 2015 by Szuhany et al. [72]. In their analysis, they found a moderate effect size for increases in BDNF following acute exercise and a small but significant increase in resting BDNF concentrations after exercise training. The effect of acute exercise on peripheral blood BDNF concentrations appears to be consistent; however, the effect of chronic exercise training on resting BDNF concentrations is less clear.
Since the last review, more than 20 clinical studies investigating the effect of exercise on peripheral BDNF concentrations have been published, underscoring the importance of this topic. Thus, there is opportunity to re-evaluate the evidence to date and to further investigate moderators of this effect, such as exercise intensity and potential gender differences. This meta-analysis of human studies sought to quantify the magnitude and consistency of the effect of exercise training on resting concentrations of BDNF in peripheral blood. Potential differences in this effect across gender, age, and blood component (i.e. serum vs plasma) were also examined. Furthermore, the impacts of exercise modality, intensity, and duration on this effect were examined as possible sources of heterogeneity.
Methods
Data Sources
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [73] were followed for this meta-anlysis (S1 Text). English-language literature was searched using MEDLINE, Embase, PsycINFO, SPORTDiscus, Rehabilitation & Sports Medicine Source, and Cumulated Index to Nursing and Allied Health Literature (CINAHL) databases. One non-English language study was included (Koichiro et al. 2015), as a translator was available to extract information from that study. Databases were searched up to February 2016 for original reports of BDNF changes after exercise. A sample search strategy (MEDLINE) is presented in S2 Text. Reference lists of retrieved studies were searched for additional reports.
Study Selection
Inclusion criteria were as follows: 1) measured serum, plasma, or whole blood BDNF concentration; 2) BDNF measured before and after an exercise intervention; 3) intervention ≥ 2 weeks; 4) exercise intensity ≥ 50% of peak oxygen uptake (VO2Peak), or if exercise intensity was not reported, exercise described as running, cycling, or resistance training. Exclusion criteria were as follows: 1) study included a diseased population (e.g. diabetes, Parkinson’s disease, multiple sclerosis, etc.) or a psychiatric population (e.g. depression, schizophrenia, etc.); 2) study population consisted of children below the age of 18; or 3) study had significant co-interventions likely to impact the effect of exercise on BDNF concentrations (e.g. military training [74], restricted sleep, etc.) as these groups have altered BDNF concentrations which may modify the effect of exercise training on BDNF concentrations [33, 75–79]. Some studies that met these initial eligibility criteria were not included in this meta-analysis as the data were not extractable (e.g. standard deviation not reported) or the exercise intensity description implied that the intervention intensity was < 50% VO2Peak (e.g. yoga or easy walking).
Data Extraction
Two independent raters examined each article for eligibility. Disagreements regarding inclusion were settled by consensus with a third rater. Data on pre- and post-intervention mean BDNF concentrations and standard deviations [picograms/millilitre], population characteristics, exercise intervention characteristics, risk of bias items, and other study details were extracted into a pre-formatted spreadsheet by two raters. Missing data were requested from the corresponding authors. Exercise intensity prescriptions for percentage of maximum heart rate were converted to percentage of maximum VO2Peak as described by the National Council on Strength & Fitness [80]. In studies with multiple exercise intervention groups, groups were combined for the overall analysis. Studies were additionally categorized by whether the exercise intervention was aerobic or resistance training. In studies with an exercise intervention consisting of both aerobic and resistance training, interventions in which >50% of the time was spent performing aerobic exercise were considered aerobic and vice-versa.
Statistical Analyses
Standardized mean differences (SMD) and 95% confidence intervals (CI) were calculated using random-effects models [81]. SMDs were chosen because of variability in absolute BDNF concentrations between assays used by different laboratories and between measures of BDNF in different components of blood [82]. Random-effects models are preferred if significant heterogeneity is expected, as they account for variable underlying effects in estimates of uncertainty, including both within- and between- study variance. Heterogeneity across studies was summarized by Q statistics calculated in Chi-square analysis and I2 indices were calculated to investigate inconsistencies among results of the included studies [83]. Heterogeneity was further explored via subgroup analysis. In one study that measured BDNF in both serum and plasma [41], serum measurements were used in all analyses except subgroup analysis of serum versus plasma (in which both measurements were used), as serum BDNF measurements were more common across studies than plasma measurements. Inverse variance-weighted meta-regression analyses were used to investigate associations between SMD and population characteristics and intervention characteristics. Risk of publication bias was assessed visually using funnel plots and quantitatively with Egger’s test [84]. Study quality was assessed using criteria adapted from the Cochrane Collaboration’s Risk of Bias tool as done previously [85]. Analyses were conducted using Review Manager Version 5.3 (Cochrane Collaboration, Oxford, UK) and Stata (Release 14.1; StataCorp, College Station, TX).
Results
Population Characteristics
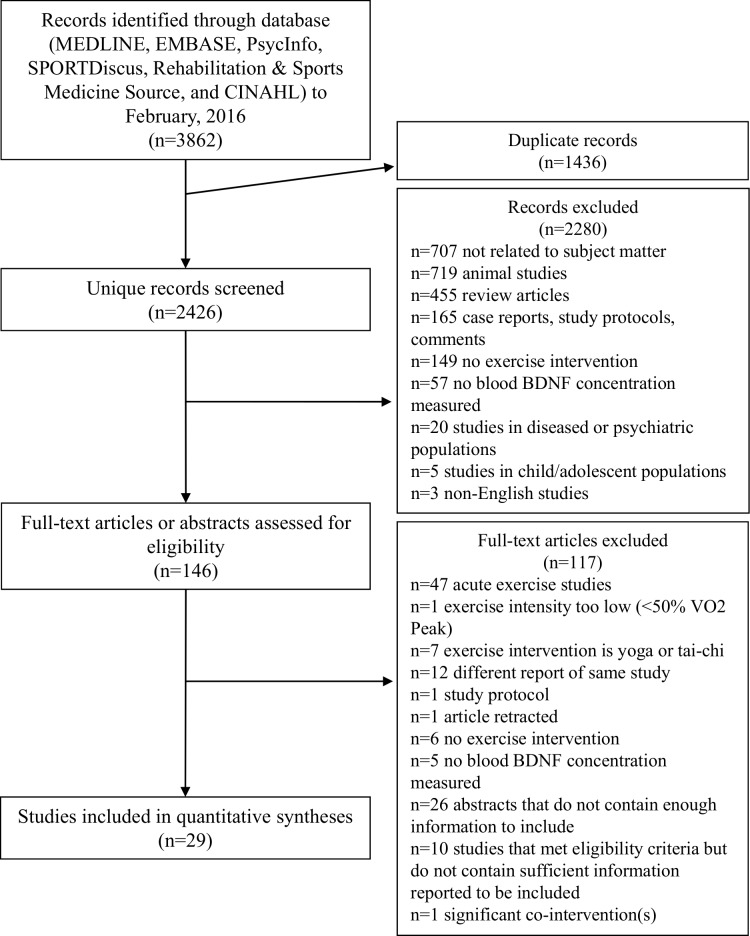
Twenty-nine studies met inclusion criteria and presented sufficient data to be included in this meta-analysis (Fig 1). Disagreement on whether a study met inclusion criteria arose for one study, and consensus was reached. Reasons for exclusion are shown in Fig 1. 910 participants (61.3% male, mean age 42.2 ± 22.4, mean BMI 25.8 ± 2.3) were included. Included studies ranged in size from 7 to 304 participants (Table 1) completing an exercise intervention. Mean exercise prescriptions were 50.9 ± 16.3 (20–90) minutes for 3.1 ± 1.0 (2–7) sessions per week for 12.4 ± 8.7 (5–52) weeks (Table 2).
Fig 1. Search and selection of articles.
Table 1. Baseline study population characteristics.
| Author, year | n | Gender (% male) | Mean age (years) | Mean BMI (kg/m2) | Mean VO2 Peak (mL∙kg-1∙min-1) |
|---|---|---|---|---|---|
| Araya et al [41], 2013 | 15 | 40 | 38.3 | 30.6 | ? |
| Babaei et al [86], 2013 | 22 | 100 | 55.7 | 27.6 | ? |
| Bos et al [87], 2013 | 24 | 37.5 | 32.1 | 24 | 38.3 |
| Cho et al [88], 2014 | 23 | 0 | 42.7 | 23.4 | 29.4 |
| Coelho et al [89], 2012 | 20 | 0 | 71.5 | 29.7 | ? |
| Damirchi et al [58], 2014 | 11 | 100 | 54.1 | 29.8 | 28.4 |
| Erickson et al [20], 2011 | 60 | 27 | 67.6 | ? | 21.4 |
| Ferris et al [90], 2006 | 18 | 16.7 | 20 | ? | ? |
| Forti et al [60], 2014 | 19 | 55 | 65.7 | 24.7 | ? |
| Forti et al [91], 2015 | 49 | 49 | 68 | 27.1 | ? |
| Fragala et al [92], 2014 | 13 | ? | ? | ? | ? |
| Gapin et al [93], 2013 | 20 | 40 | 51.4 | 30.2 | ? |
| Goekint et al [61], 2010a | 15 | 80 | 20.1 | 23.9 | ? |
| Goekint et al [94], 2010b | 9 | ? | 21.2 | 23.2 | ? |
| Kim H et al [95], 2015 | 66 | 0 | 81.1 | ? | ? |
| Kim Y [96], 2015 | 7 | 100 | 20.6 | 21.8 | ? |
| Koichiro et al [97], 2015 | 12 | 100 | 35 | 24.6 | 24.5 |
| Lemos et al [98], 2016 | 304 | 100 | 24.3 | 24.8 | ? |
| Levinger et al [99], 2008 | 23 | 48 | 50.7 | 28.5 | ? |
| Mueller et al [100], 2015 | 16 | 43.8 | 27.2 | 33.6 | ? |
| Murawska-Cialowicz et al [101], 2015 | 12 | 58.3 | 25.6 | 24.2 | 38.3 |
| Prestes et al [102], 2015 | 39 | 0 | 67.4 | 27.9 | ? |
| Ruiz et al [103], 2015 | 20 | 20 | 92.3 | 25.5 | ? |
| Schiffer et al [104], 2009 | 18 | ? | 22.4 | ? | ? |
| Seifert et al [105], 2010 | 7 | 100 | 29 | 27.3 | ? |
| Wagner et al [106], 2015 | 17 | 100 | 25 | 23.8 | 45.9 |
| Williams & Ferris [107], 2012 | 18 | 16.7 | 20 | 21.9 | 33.8 |
| Yarrow et al [108], 2010 | 20 | 100 | 21.9 | 25.9 | ? |
| Zoladz et al [109], 2008 | 13 | 100 | 22.7 | 23.7 | 45.3 |
? indicates uncertain
Table 2. Exercise intervention characteristics of included studies.
| Author, year | Duration (weeks) | Frequency (sessions per week) | Intervention | Intensity | Session time (mins) | Modality |
|---|---|---|---|---|---|---|
| Araya et al [41], 2013 | 10 | 3 | Exercise | ≥ 65% VO2 Peak | 60 | running or cycling |
| Babaei et al [86], 2013 | 6 | 3 | Exercise | 50–60% VO2 Peak | 60 | walking and running |
| Bos et al [87], 2013 | 12 | 3 | Exercise | ? | 29.5 | walking and running |
| Cho et al [88], 2014 | 24 | 4 | Exercise | Aerobic training group: 50–80% VO2 Peak; Combined exercise group:? | 60 | Resistance training, ab exercises, running, jogging |
| Coelho et al [89], 2012 | 10 | 3 | Exercise | 50–75% of 1RM, 8 reps for each exercise | 60 | resistance training |
| Damirchi et al [58], 2014 | 6 | 3 | Exercise | 50–60% VO2 Peak | 62.5 | walking and running |
| Erickson et al [20], 2011 | 52 | 3 | Exercise | Weeks 1–7: 50–60% HR Max Weeks 8–52: 60–75% HR Max | 40 | walking |
| Ferris et al [90], 2006 | 12 | 3 | Exercise | 65–75% VO2 Peak | 30 | running |
| Forti et al [60], 2014 | 12 | 3 | Exercise | 50–80% of 1RM, 10 reps x 3 sets for each exercise | 60 | resistance training |
| Forti et al [91], 2015 | 12 | 3 | Exercise | 6 on the OMNI scale of perceived exertion | 37.5 | resistance training |
| Fragala et al [92], 2014 | 6 | 2 | Exercise | 5 or 6 on the OMNI scale of perceived exertion | ? | resistance training |
| Gapin et al [93], 2013 | 13 | ? | Exercise, Dietary Restrictions | ? | ? | ? |
| Goekint et al [61], 2010a | 10 | 3 | Exercise | 50–80% of 1RM, 10 reps x 3 sets for each exercise | 45 | resistance training |
| Goekint et al [94], 2010b | 8 | 3 | Exercise | ? | 30 | walking, running, or cycling |
| Kim H et al [95], 2015 | 13 | 2 | Exercise, One group given milk fat globule membrane supplements | 12–14 RPE on the Borg Scale | 60 | resistance training |
| Kim Y [96], 2015 | 8 | 5 | Exercise | 11–15 RPE on the Borg Scale | 80 | running and taekwondo |
| Koichiro et al [97], 2015 | 16 | 2 | Exercise | High intensity interval training; >90% VO2 peak separated by periods of 60 sec active rest | 20 | cycling or arm and leg ergometer |
| Lemos et al [98], 2016 | 17 | 3 | Exercise | 80% VO2 Peak | 90 | jogging and running, body weight exercises |
| Levinger et al [99], 2008 | 10 | 3 | Exercise | Week 1: 40–50% of 1RM, 15–20 reps x 2 sets for each exercise; Week 2–10: 50–85% of 1RM, 8–20 reps x 3 sets for each exercise | 55 | resistance training |
| Mueller et al [100], 2015 | 13 | 2 | Exercise | 70–80% HR Max | 60 | resistance training |
| Murawska-Cialowicz et al [101], 2015 | 13 | 2 | Exercise | 85–95% HR Max | 60 | crossfit |
| Prestes et al [102], 2015 | 16 | 2 | Exercise | ? | 45 | resistance training |
| Ruiz et al [103], 2015 | 8 | 3 | Exercise | Aerobic training group: 10–12 RPE on Borg Scale; Resistance training group: 30–70% of 1RM, 8–10 reps x 2–3 sets for each exercise | 42.5 | cycling and resistance training |
| Schiffer et al [104], 2009 | 12 | 3 | Exercise | Aerobic training group: 50–60% HR Max; Resistance training group: 70–80% of 1RM, 8–10 reps x 3 sets for each exercise | 45 | resistance training and running |
| Seifert et al [105], 2010 | 12 | 7 | Exercise, Dietary Restrictions | 70% HR Max or 65% VO2 Peak | 60 | cycling, running, swimming, or rowing |
| Wagner et al [106], 2015 | 6 | 3 | Exercise | 77% VO2 Peak | 60 | cycling |
| Williams & Ferris [107], 2012 | 12 | 3 | Exercise | 65–70% HR Max | 30 | jogging |
| Yarrow et al [108], 2010 | 5 | 3 | Exercise | 70% of 1RM, 6 reps for each exercise | ? | resistance training |
| Zoladz et al [109], 2008 | 5 | 4 | Exercise | 90% of VO2 at Lactate Threshold | 42.5 | cycling |
? indicates uncertain
Comparison of Pre- and Post-Intervention BDNF Concentration
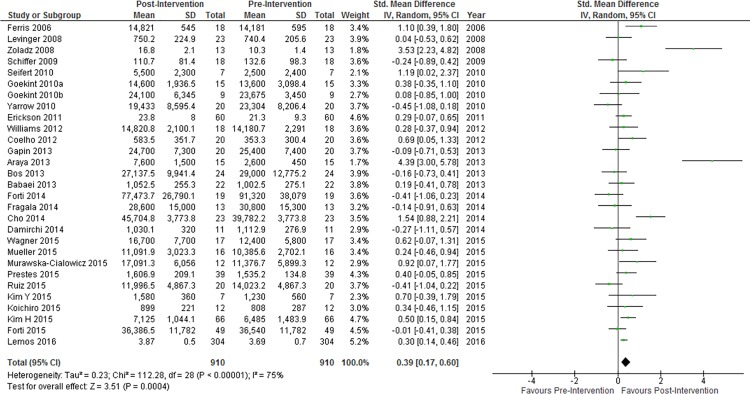
Resting concentrations of peripheral blood BDNF were significantly higher after an exercise training intervention (Fig 2). Neither a Funnel plot (S1 Fig) nor Egger’s test (p = 0.18) revealed significant risk of small-study effects. A Q2 value of 112.28 and I2 index of 75% signify considerable heterogeneity and inconsistency, respectively, among included studies. Qualitatively, nine of twenty-nine studies (31.0%) reported a significant increase in resting peripheral BDNF concentrations, no studies reported a significant decrease in resting peripheral BDNF concentrations, and twenty studies (69.0%) reported no significant change. Twenty-eight of the twenty-nine included studies were deemed to have high methodological quality (S1 Table).
Fig 2. Resting peripheral BDNF concentration change pre- to post-intervention.
Diamond indicates SMD and 95% CI. Significance of overall effect: Z = 5.00, P < 0.00
Investigations of Heterogeneity
Aerobic Exercise vs Resistance Training
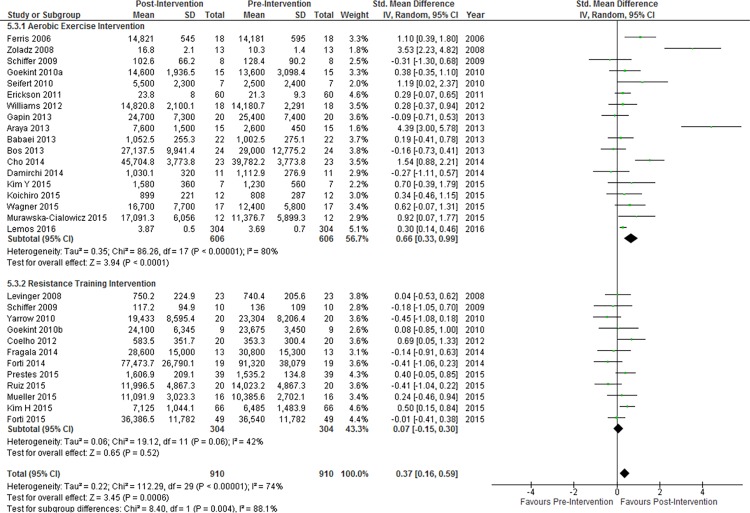
In eighteen studies in which the exercise intervention consisted of entirely or mostly (>50% of the time) of aerobic exercise, a greater increase in resting peripheral BDNF concentration was found (SMD = 0.66, 95% CI: 0.33–0.99, p < 0.001) than that found when all studies were combined. Substantial heterogeneity and inconsistency were present in this subgroup (Q2 = 86.26, I2 = 80%). In contrast, in twelve studies in which the exercise intervention consisted entirely or mostly of resistance training, there was no change in resting peripheral BDNF concentration after exercise (SMD = 0.07, 95% CI: -0.15–0.30, p = 0.52, Q2 = 19.12, I2 = 42%). The difference in effect size between these two subgroups was significant (Fig 3). Of note, one study [104] included both an aerobic exercise group and a resistance training group and these separate groups were included in their respective subgroups.
Fig 3. Subgroup analysis of aerobic exercise interventions vs resistance training interventions.
Diamonds indicate SMD and 95% CI. Test for subgroup differences: P = 0.004.
Other Exercise Intervention Characteristics
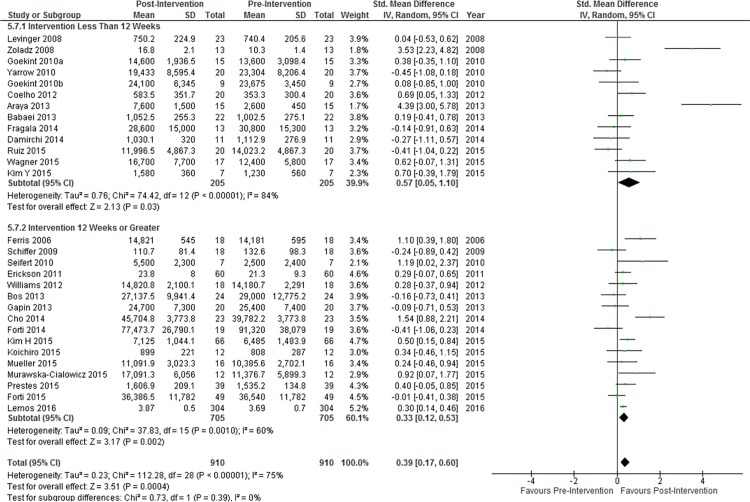
In meta-regression analyses, duration of exercise intervention (β = 0.038, p = 0.97, df = 28), exercise session time (β = 0.520, p = 0.61, df = 25), and number of exercise sessions per week (β = 1.471, p = 0.15, df = 27) were not significantly associated with changes in resting BDNF concentration (S2 Fig). Among studies in which exercise intensity was reported in percentage of VO2Peak or percentage of maximum heart rate (n = 15), there was no association between exercise intensity and changes in resting BDNF concentration (β = 1.478, p = 0.16, df = 14). The median and modal exercise intervention duration was 12 weeks. Effect sizes in studies with an intervention duration of less than 12 weeks (n = 13) were not significantly different from effect sizes in studies with an intervention duration of 12 weeks or greater (Fig 4). Significant heterogeneity and inconsistency were observed in both subgroups (Fig 4).
Fig 4. Subgroup analysis of studies with an intervention duration less than 12 weeks vs studies with an intervention duration of 12 weeks or greater.
Diamonds indicate SMD and 95% CI. Test for subgroup differences: P = 0.39.
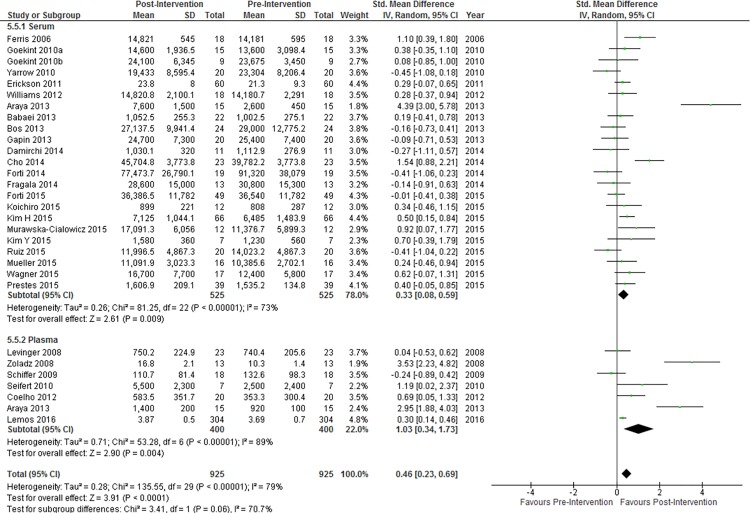
Serum vs Plasma
BDNF was measured more commonly in serum than in plasma (Fig 5). Fig 5 shows subgroup differences in change in BDNF concentration after exercise between serum and plasma. The difference between these two subgroups was not significant.
Fig 5. Subgroup analysis of BDNF concentration changes in serum vs plasma.
Diamonds indicate SMD and 95% CI. Test for subgroup differences: P = 0.06.
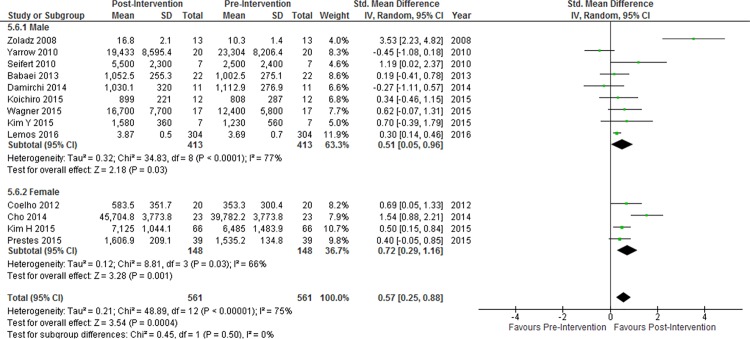
Gender
Nine studies included only men in their study population and four studies consisted entirely of females. Subgroup analysis showed no significant difference between effect sizes in studies that included only males and studies that included only females (Fig 6). Among studies in which the number of male and female participants was reported, no association was observed between percentage of male study participants and changes in resting BDNF concentration (β = -0.070, p = 0.95, df = 25).
Fig 6. Subgroup analysis of difference in effect sizes between males and females.
Diamonds indicate SMD and 95% CI. Test for subgroup differences: P = 0.50.
Age and Body Mass Index (BMI)
The mean age of the study population was not associated with change in resting BDNF concentration (β = -1.182, p = 0.25, df = 27). Among studies reporting mean baseline BMI of participants, BMI was not associated with changes in resting BDNF concentration (β = 0.046, p = 0.96, df = 23).
Discussion
Collectively, the evidence suggests an increase in resting peripheral BDNF concentrations after aerobic exercise training interventions but not after resistance training interventions. The increase in resting peripheral BDNF concentrations after an exercise intervention is heterogeneous and was found to have a small effect size [110]. This is important, as an increase in BDNF is proposed to be a mechanism through which physical activity enhances cognition and alleviates psychiatric symptoms [24, 25, 111–113]. This finding strengthens the evidence that aerobic exercise and resistance training constitute different physiological stimuli with respect to neurotrophin concentrations. For brain health, this finding suggests a basis for possible differential benefits from different exercise modalities. Furthermore, since BDNF is significantly lower in individuals with psychiatric disorders [26, 28–31], an increase in BDNF via exercise may confer clinical benefit by ameliorating this abnormality.
These results are similar to those reported in a previous meta-analysis on the effect of exercise on resting peripheral BDNF concentrations [72]. This current meta-analysis differs from that one due to the inclusion of 21 additional reports that were included due to a different search strategy and a later search period (February 2016). The larger number of included studies allowed for a more robust exploration of heterogeneity across studies and a stronger evaluation of current evidence.
As suggested by high Q2 and I2 values, heterogeneity across studies, as opposed to random sampling error, contributed considerably to variability in effect estimates. Inconsistency in effect estimates may be due to differences in study populations, exercise intervention characteristics, measurement techniques, and study quality. Our explorations of heterogeneity suggested that inconsistency was not significantly related to gender or age. This is important as exercise is recommended in elderly to promote brain health [114–116]. Similarly, exercise intervention duration, intensity and session time were not associated with degree of change in resting peripheral BDNF concentrations. As such, the current evidence could not determine an ideal exercise prescription for increasing resting peripheral BDNF concentrations.
No significant differences were found between studies measuring BDNF in serum versus plasma, although this subgroup analysis was limited by the relatively small number of studies measuring BDNF in plasma. As BDNF is stored in platelets, which are activated and release their contents in serum, serum concentrations of BDNF are substantially higher than plasma BDNF concentrations [67, 117]. Plasma BDNF concentration represents freely-floating, not stored, BDNF and thus may have a different physiological role from serum BDNF [118].
It is likely that there is a combination of different mechanisms through which physical activity confers benefits to neural health [12–14]. These may include increases in cerebral blood flow, changes in neuroendocrine responses, changes in endocannabinoid and neurotransmitter release, and structural changes in the central nervous system (CNS) [12, 113]. Increased BDNF is one proposed mechanism by physical activity confers these benefits [119]. Increased resting peripheral BDNF concentrations may indicate an increase in central BDNF production [120–123]. Increased BDNF production in the CNS may result in enhanced synaptogenesis and neuronal survival, resulting in structural changes and enhanced cognition [20, 22, 124–127]. While the findings support the hypothesis that BDNF concentration is increased in the blood after an exercise training intervention, it cannot be inferred that BDNF concentration is increased in the brain. Although one human study suggested an association between peripheral and central BDNF concentrations [121], the evidence in humans is limited. Since the human BBB is structurally and functionally different from those in animal models, we cannot infer from those models that BDNF can cross the human BBB [128, 129]. The roles of BDNF in the periphery are not well characterized and may involve regulation of energy homeostasis, modification of insulin activity, and modification of neuronal function in certain neuronal subpopulations in the peripheral nervous system [130–132].
Interestingly, a single nucleotide polymorphism (SNP) in the gene encoding BDNF, resulting in an amino acid substitution from valine to methionine, may impact the effect of exercise on BDNF concentrations [37, 133]. This SNP is present in approximately 30% of the global population and is associated with altered secretion of BDNF as well as a possible increase in serum BDNF concentration relative to those without the SNP [134–136]. The presence of this polymorphism may alter the relationship between fitness and brain outcomes such as cognition, mood, and response to mood treatments [133, 137, 138]. Few studies have assessed the potential impact of this SNP on the effect of exercise training on peripheral BDNF and brain outcomes. Findings from these reports suggest an increase in BDNF and improvements in memory after exercise only in those without the SNP [37, 133, 137]. However, those findings are limited by the small number of studies. Future work assessing the potential impact of this polymorphism on this effect and on the relationship between exercise and brain outcomes is required to determine whether exercise interacts equally with BDNF and brain outcomes in those with and without the polymorphism.
The large number of studies assessing the impact of exercise on resting concentrations of peripheral blood BDNF indicates the importance of this topic and allowed for a robust exploration of this effect. Nevertheless, this meta-analysis was limited by the heterogeneity in study populations and exercise interventions prescribed across different studies. A variety of populations and exercise interventions were included in this report, which we attempted to address via subgroup and meta-regression analyses. In addition, variable adherence to the intervention may have detracted from the quality of evidence.
Overall, this meta-analysis provides evidence for an increase in resting concentrations of peripheral blood BDNF after an exercise intervention. This effect is heterogeneous and may not be present in all individuals, suggesting the importance of further research to elucidate predictors of response. Interestingly, an increase in BDNF concentration may occur after aerobic exercise interventions but not resistance training interventions. Future studies in humans to determine whether peripheral concentrations of BDNF reflect central BDNF concentrations and whether BDNF can cross the human BBB are needed to assess the importance of these findings. In addition, further work to determine if changes in blood BDNF concentrations mediate clinical benefits, such as those on mood, cognition and other psychiatric disorders, would help to determine the usefulness of peripheral BDNF as a putative biomarker.
Supporting Information
(DOCX)
(DOCX)
+ indicates yes;—indicates no;? indicates uncertain; NA indicates not applicable.
(XLSX)
(DOC)
(DOCX)
Acknowledgments
The authors thank Maureen Pakosh, BA, MISt, for information resources support, Alex Adibfar, BSc, for editing, Daniel Wang for translating, and Drs. Ivan Bautmans, Mark Powers, Jaroslaw Marusiak, Mârcia Maria Oliveira Lima, and Christian Roth for providing data.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Operating funds from the Canadian Institutes of Health Research (MOP 114913) and Ontario Mental Health Foundation (http://www.omhf.on.ca/guidelines/researchgrants/) supported this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Basso JC, Shang A, Elman M, Karmouta R, Suzuki WA. Acute Exercise Improves Prefrontal Cortex but not Hippocampal Function in Healthy Adults. J Int Neuropsychol Soc. 2015;21(10):791–801. 10.1017/S135561771500106X . [DOI] [PubMed] [Google Scholar]
- 2.Best JR, Chiu BK, Liang Hsu C, Nagamatsu LS, Liu-Ambrose T. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J Int Neuropsychol Soc. 2015;21(10):745–56. 10.1017/S1355617715000673 . [DOI] [PubMed] [Google Scholar]
- 3.Bossers WJ, van der Woude LH, Boersma F, Hortobagyi T, Scherder EJ, van Heuvelen MJ. A 9-Week Aerobic and Strength Training Program Improves Cognitive and Motor Function in Patients with Dementia: A Randomized, Controlled Trial. Am J Geriatr Psychiatry. 2015. 10.1016/j.jagp.2014.12.191 . [DOI] [PubMed] [Google Scholar]
- 4.Hagovska M, Takac P, Dzvonik O. Effect of a combining cognitive and balanced training on the cognitive postural and functional status of seniors with a mild cognitive deficit in a randomized, controlled trial. Eur J Phys Rehabil Med. 2015. . [PubMed] [Google Scholar]
- 5.Dauwan M, Begemann MJ, Heringa SM, Sommer IE. Exercise Improves Clinical Symptoms, Quality of Life, Global Functioning, and Depression in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr Bull. 2015. 10.1093/schbul/sbv164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza Moura AM, Lamego MK, Paes F, Rocha NB, Simoes-Silva V, Rocha SA, et al. Effects of aerobic exercise on anxiety disorders: a systematic review. CNS & neurological disorders drug targets. 2015. . [DOI] [PubMed] [Google Scholar]
- 7.Kerling A, Tegtbur U, Gutzlaff E, Kuck M, Borchert L, Ates Z, et al. Effects of adjunctive exercise on physiological and psychological parameters in depression: a randomized pilot trial. J Affect Disord. 2015;177:1–6. 10.1016/j.jad.2015.01.006 . [DOI] [PubMed] [Google Scholar]
- 8.Knapen J, Vancampfort D, Morien Y, Marchal Y. Exercise therapy improves both mental and physical health in patients with major depression. Disabil Rehabil. 2015;37(16):1490–5. 10.3109/09638288.2014.972579 . [DOI] [PubMed] [Google Scholar]
- 9.Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25 Suppl 3:1–72. 10.1111/sms.12581 . [DOI] [PubMed] [Google Scholar]
- 10.Rethorst CD, Wipfli BM, Landers DM. The Antidepressive Effects of Exercise: A Meta-Analysis of Randomized Trials. Sports Med. 2009;39(6):491–511. 10.2165/00007256-200939060-00004 [DOI] [PubMed] [Google Scholar]
- 11.Wipfli BM, Rethorst CD, Landers DM. The anxiolytic effects of exercise: a meta-analysis of randomized trials and dose-response analysis. J Sport Exerc Psychol. 2008;30(4):392–410. . [DOI] [PubMed] [Google Scholar]
- 12.Gligoroska JP, Manchevska S. The effect of physical activity on cognition—physiological mechanisms. Mater. 2012;24(3):198–202. 10.5455/msm.2012.24.198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol (1985). 2006;101(4):1237–42. 10.1152/japplphysiol.00500.2006 . [DOI] [PubMed] [Google Scholar]
- 14.Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741–60. . [DOI] [PubMed] [Google Scholar]
- 15.Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374(6521):450–3. 10.1038/374450a0 . [DOI] [PubMed] [Google Scholar]
- 16.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(41):12764–7. 10.1523/JNEUROSCI.3566-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. 10.1146/annurev.neuro.24.1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25(2):237–58. . [DOI] [PubMed] [Google Scholar]
- 19.Erickson K, Weinstein AM, Verstynen TD, Voss MW, Prakash RS, Woods J, et al. The influence of an aerobic exercise intervention on brain volume in late adulthood. Alzheimer's and Dementia. 2012;1):P81 10.1016/j.jalz.2012.05.197. . [DOI] [Google Scholar]
- 20.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22. 10.1073/pnas.1015950108. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–56. 10.1016/j.expneurol.2004.11.016 . [DOI] [PubMed] [Google Scholar]
- 22.Tolwani RJ, Buckmaster PS, Varma S, Cosgaya JM, Wu Y, Suri C, et al. BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience. 2002;114(3):795–805. . [DOI] [PubMed] [Google Scholar]
- 23.Arancibia S, Silhol M, Mouliere F, Meffre J, Hollinger I, Maurice T, et al. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 2008;31(3):316–26. 10.1016/j.nbd.2008.05.012 . [DOI] [PubMed] [Google Scholar]
- 24.M Q.. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci Bull. 2008;24(4):265–70. 10.1007/s12264-008-0402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: learning from animal models. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2007;3(2 Suppl):S30–7. 10.1016/j.jalz.2007.01.013 . [DOI] [PubMed] [Google Scholar]
- 26.Fernandes BS, Molendijk ML, Kohler CA, Soares JC, Leite CM, Machado-Vieira R, et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med. 2015;13:289 10.1186/s12916-015-0529-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord. 2015;174:432–40. 10.1016/j.jad.2014.11.044 . [DOI] [PubMed] [Google Scholar]
- 28.Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol Psychiatry. 2014;19(7):791–800. 10.1038/mp.2013.105 . [DOI] [PubMed] [Google Scholar]
- 29.Fernandes BS, Steiner J, Berk M, Molendijk ML, Gonzalez-Pinto A, Turck CW, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiatry. 2015;20(9):1108–19. 10.1038/mp.2014.117 . [DOI] [PubMed] [Google Scholar]
- 30.Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16(9):960–72. 10.1038/mp.2010.88 . [DOI] [PubMed] [Google Scholar]
- 31.Suliman S, Hemmings SM, Seedat S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front Integr Neurosci. 2013;7:55 10.3389/fnint.2013.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libman-Sokolowska M, Drozdowicz E, Nasierowski T. BDNF as a biomarker in the course and treatment of schizophrenia. Psychiatr Pol. 2015;49(6):1149–58. 10.12740/PP/37705 . [DOI] [PubMed] [Google Scholar]
- 33.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50(2):431–8. 10.1007/s00125-006-0537-4 . [DOI] [PubMed] [Google Scholar]
- 34.Wens I, Keytsmen C, Dalgas U, Eijinde BO. The impact of 24 weeks combined exercise on BDNF expression in relapsing-remitting multiple sclerosis patients. Annual Rehabilitation in Multiple Sclerosis Conference. 2015;21(4):533–4. [Google Scholar]
- 35.Tonoli C, Heyman E, Roelands B, Buyse L, Piacentini F, Berthoin S, et al. BDNF, IGF-I, Glucose and Insulin during Continuous and Interval Exercise in Type 1 Diabetes. Int J Sports Med. 2015;36(12):955–9. 10.1055/s-0035-1548886 . [DOI] [PubMed] [Google Scholar]
- 36.Schuch FB, da Silveira LE, de Zeni TC, da Silva DP, Wollenhaupt-Aguiar B, Ferrari P, et al. Effects of a single bout of maximal aerobic exercise on BDNF in bipolar disorder: A gender-based response. Psychiatry Res. 2015;229(1–2):57–62. 10.1016/j.psychres.2015.07.072 . [DOI] [PubMed] [Google Scholar]
- 37.Nascimento CM, Pereira JR, Pires de Andrade L, Garuffi M, Ayan C, Kerr DS, et al. Physical exercise improves peripheral BDNF levels and cognitive functions in mild cognitive impairment elderly with different bdnf Val66Met genotypes. J Alzheimers Dis. 2015;43(1):81–91. 10.3233/JAD-140576 . [DOI] [PubMed] [Google Scholar]
- 38.Zoladz JA, Majerczak J, Zeligowska E, Mencel J, Jaskolski A, Jaskolska A, et al. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in parkinson's disease patients. Journal of Physiology and Pharmacology. 2014;65(3):441–8. . [PubMed] [Google Scholar]
- 39.Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, et al. BDNF mediates improvements in executive function following a 1-year exercise intervention. Frontiers in human neuroscience. 2014;8:985 10.3389/fnhum.2014.00985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmolesky MT, Webb DL, Hansen RA. The effects of aerobic exercise intensity and duration on levels of brain-derived neurotrophic factor in healthy men. J Sports Sci Med. 2013;12(3):502–11. [PMC free article] [PubMed] [Google Scholar]
- 41.Araya AV, Orellana X, Godoy D, Soto L, Fiedler J. Effect of exercise on circulating levels of brain-derived neurotrophic factor (BDNF) in overweight and obese subjects. Horm Metab Res. 2013;45(7):541–4. 10.1055/s-0032-1333237. 10.1055/s-0032-1333237 [DOI] [PubMed] [Google Scholar]
- 42.Berchtold NC, Adlard PA, Kesslak JP, Pike CJ, Cotman CW. Hippocampal BDNF protein is increased by exercise and estrogen. Society for Neuroscience Abstracts. 2001;27(1):280-. . [Google Scholar]
- 43.Koo JH, Kwon IS, Kang EB, Lee CK, Lee NH, Kwon MG, et al. Neuroprotective effects of treadmill exercise on BDNF and PI3-K/Akt signaling pathway in the cortex of transgenic mice model of Alzheimer's disease. Journal of exercise nutrition & biochemistry. 2013;17(4):151–60. 10.5717/jenb.2013.17.4.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geng YM, Yu F, Wang Z. Effect of Exercise on BDNF, HSP70, and Oxidative Stress Level in Prefrontal Cortex of Depression Rat. Journal of Xi'an Institute of Physical Education. 2013;30(4):448–52,58. ; 18671602. [Google Scholar]
- 45.Groves-Chapman JL, Murray PS, Stevens KL, Monroe DC, Koch LG, Britton SL, et al. Changes in mRNA levels for brain-derived neurotrophic factor after wheel running in rats selectively bred for high- and low-aerobic capacity. Brain Res. 2011;1425:90–7. 10.1016/j.brainres.2011.09.059. ; 16058287. 10.1016/j.brainres.2011.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustafsson S, Liang W, Hilke S. Effects of voluntary running in the female mice lateral septum on BDNF and corticotropin-releasing factor receptor 2. Int J Pept. 2011;2011:932361 10.1155/2011/932361. 10.1155/2011/932361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028(1):92–104. 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Fang Z, Lee C, Seo M, Cho H, Lee J, Lee B, et al. Effect of treadmill exercise on the BDNF-mediated pathway in the hippocampus of stressed rats. Neuroscience Research. 2013;76(1–2):187–94. 10.1016/j.neures.2013.04.005. ; 19260602. [DOI] [PubMed] [Google Scholar]
- 49.Liu YF, Chen HI, Wu CL, Kuo YM, Yu L, Huang AM, et al. Differential effects of treadmill running and wheel running on spatial or aversive learning and memory: Roles of amygdalar brain-derived neurotrophic factor and synaptotagmin I. J Physiol (Lond). 2009;587(13):3221–31. 10.1113/jphysiol.2009.173088. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu J, Xu Y, Hu W, Gao Y, Ni X, Sheng H, et al. Exercise ameliorates depression-like behavior and increases hippocampal BDNF level in ovariectomized rats. Neurosci Lett. 2014;573:13–8. 10.1016/j.neulet.2014.04.053. 10.1016/j.neulet.2014.04.053 [DOI] [PubMed] [Google Scholar]
- 51.Marais L, Stein DJ, Daniels WMU. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metabolic Brain Disease. 2009;24(4):587–97. 10.1007/s11011-009-9157-2. ; 11893486. 10.1007/s11011-009-9157-2 [DOI] [PubMed] [Google Scholar]
- 52.Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Developmental Neurobiology. 2012;72(6):943–52. 10.1002/dneu.22009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mojtahedi S, Kordi MR, Hosseini SE, Omran SF, Soleimani M. Effect of treadmill running on the expression of genes that are involved in neuronal differentiation in the hippocampus of adult male rats. Cell Biology International. 2013;37(4):276–83. 10.1002/cbin.10022 . [DOI] [PubMed] [Google Scholar]
- 54.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Physical activity up-regulates BDNF and NGF mRNA in the rat brain. Society for Neuroscience Abstracts. 1995;21(1–3):296-. . [Google Scholar]
- 55.Pedroso J, Chadi G. Effects of Spontaneous Running in mRNA BDNF Expression in Rat Hippocampus and in Anxiety and Memory Responses Through an Elevated T-Maze. Motor Control. 2007;11:1 ; 7553429.17392564 [Google Scholar]
- 56.Neeper SA, GomezPinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(1–2):49–56. 10.1016/s0006-8993(96)00273-9 . [DOI] [PubMed] [Google Scholar]
- 57.Currie J, Ramsbottom R, Gilder M. Serum and plasma concentrations of brain derived neurotrophic factor in response to maximal exercise. J Sports Sci Med. 2009;8 ; 13551504. [Google Scholar]
- 58.Damirchi A, Tehrani BS, Alamdari KA, Babaei P. Influence of aerobic training and detraining on serum BDNF, insulin resistance, and metabolic risk factors in middle-aged men diagnosed with metabolic syndrome. Clin J Sport Med. 2014;24(6):513–8. 10.1097/JSM.0000000000000082 . [DOI] [PubMed] [Google Scholar]
- 59.Ferris LT, Williams JS, Shen C-L. Serum Neurotrophin Levels Following Acute Exercise In Humans. Medicine and Science in Sports and Exercise. 2005;37:S108–S. 10.1097/00005768-200505001-00565 . [DOI] [PubMed] [Google Scholar]
- 60.Forti LN, Njemini R, Beyer I, Eelbode E, Meeusen R, Mets T, et al. Strength training reduces circulating interleukin-6 but not brain-derived neurotrophic factor in community-dwelling elderly individuals. Age (Dordr). 2014;36(5):9704 10.1007/s11357-014-9704-6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goekint M, De Pauw K, Roelands B, Njemini R, Bautmans I, Mets T, et al. Strength training does not influence serum brain-derived neurotrophic factor. Eur J Appl Physiol. 2010;110(2):285–93. 10.1007/s00421-010-1461-3. 10.1007/s00421-010-1461-3 [DOI] [PubMed] [Google Scholar]
- 62.Gomes WF, Lacerda AC, Mendonca VA, Arrieiro AN, Fonseca SF, Amorim MR, et al. Effect of exercise on the plasma BDNF levels in elderly women with knee osteoarthritis. Rheumatol Int. 2014;34(6):841–6. 10.1007/s00296-013-2786-0. 10.1007/s00296-013-2786-0 [DOI] [PubMed] [Google Scholar]
- 63.Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104(5):934–41. 10.1016/j.physbeh.2011.06.005. 10.1016/j.physbeh.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 64.Toups MS, Greer TL, Kurian BT, Grannemann BD, Carmody TJ, Huebinger R, et al. Effects of serum Brain Derived Neurotrophic Factor on exercise augmentation treatment of depression. J Psychiatr Res. 2011;45(10):1301–6. 10.1016/j.jpsychires.2011.05.002. 10.1016/j.jpsychires.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer's disease. J Alzheimers Dis. 2010;22(2):569–79. 10.3233/JAD-2010-100768. 10.3233/JAD-2010-100768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Archives of Neurology. 2010;67(1):71–9. 10.1001/archneurol.2009.307. 10.1001/archneurol.2009.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pareja-Galeano H, Alis R, Sanchis-Gomar F, Cabo H, Cortell-Ballester J, Gomez-Cabrera MC, et al. Methodological considerations to determine the effect of exercise on brain-derived neurotrophic factor levels. Clin Biochem. 2015;48(3):162–6. 10.1016/j.clinbiochem.2014.11.013 . [DOI] [PubMed] [Google Scholar]
- 68.Huang T, Larsen KT, Ried-Larsen M, Moller NC, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: a review. Scand J Med Sci Sports. 2014;24(1):1–10. 10.1111/sms.12069. 10.1111/sms.12069 [DOI] [PubMed] [Google Scholar]
- 69.Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity—Exercise-Induced Response of Peripheral Brain-Derived Neurotrophic Factor: A Systematic Review of Experimental Studies in Human Subjects. Sports Med. 2010;40(9):765–801. 10.2165/11534530-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 70.Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61(5):533–41. . [PubMed] [Google Scholar]
- 71.Coelho FGDM, Gobbi S, Andreatto CAA, Corazza DI, Pedroso RV, Santos-Galduroz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): A systematic review of experimental studies in the elderly. Archives of Gerontology and Geriatrics. 2013;56(1):10–5. 10.1016/j.archger.2012.06.003. 10.1016/j.archger.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 72.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64. 10.1016/j.jpsychires.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henning PC, Scofield DE, Spiering BA, Staab JS, Matheny RW Jr, Smith MA, et al. Recovery of endocrine and inflammatory mediators following an extended energy deficit. Journal of Clinical Endocrinology and Metabolism. 2014;99(3):956–64. 10.1210/jc.2013-3046. 10.1210/jc.2013-3046 [DOI] [PubMed] [Google Scholar]
- 75.Iughetti L, Casarosa E, Predieri B, Patianna V, Luisi S. Plasma brain-derived neurotrophic factor concentrations in children and adolescents. Neuropeptides. 2011;45(3):205–11. 10.1016/j.npep.2011.02.002 . [DOI] [PubMed] [Google Scholar]
- 76.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–8. 10.1016/s0165-1781(02)00005-7 . [DOI] [PubMed] [Google Scholar]
- 77.Palomino A, Vallejo-Illarramendi A, Gonzalez-Pinto A, Aldama A, Gonzalez-Gomez C, Mosquera F, et al. Decreased levels of plasma BDNF in first-episode schizophrenia and bipolar disorder patients. Schizophr Res. 2006;86(1–3):321–2. 10.1016/j.schres.2006.05.028 . [DOI] [PubMed] [Google Scholar]
- 78.Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Brain Res Mol Brain Res. 1997;49(1–2):71–81. . [DOI] [PubMed] [Google Scholar]
- 79.Frota ER, Rodrigues DH, Donadi EA, Brum DG, Maciel DR, Teixeira AL. Increased plasma levels of brain derived neurotrophic factor (BDNF) after multiple sclerosis relapse. Neurosci Lett. 2009;460(2):130–2. 10.1016/j.neulet.2009.05.057 . [DOI] [PubMed] [Google Scholar]
- 80.Fitness NCoS. Relationship between Percent HR Max and Percent VO2 Max.
- 81.Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JAC. metan: fixed- and random-effects meta-analysis. The Stata Journal. 2008;8(1):3–28. [Google Scholar]
- 82.Noble JE, Wang L, Cerasoli E, Knight AE, Porter RA, Gray E, et al. An international comparability study to determine the sources of uncertainty associated with a non-competitive sandwich fluorescent ELISA. Clin Chem Lab Med. 2008;46(7):1033–45. 10.1515/CCLM.2008.182 . [DOI] [PubMed] [Google Scholar]
- 83.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. 10.1002/sim.1186 . [DOI] [PubMed] [Google Scholar]
- 84.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swardfager W, Herrmann N, Cornish S, Mazereeuw G, Marzolini S, Sham L, et al. Exercise intervention and inflammatory markers in coronary artery disease: a meta-analysis. Am Heart J. 2012;163(4):666–76 e1-3. 10.1016/j.ahj.2011.12.017 . [DOI] [PubMed] [Google Scholar]
- 86.Babaei P, Alamdari KA, Tehrani BS, Damirchi A. Effect of six weeks of endurance exercise and following detraining on serum brain derived neurotrophic factor and memory performance in middle aged males with metabolic syndrome. Journal of Sports Medicine and Physical Fitness. 2013;53(4):437–43. . [PubMed] [Google Scholar]
- 87.Bos I, De Boever P, Vanparijs J, Pattyn N, Panis LI, Meeusen R. Subclinical effects of aerobic training in urban environment. Med Sci Sports Exerc. 2013;45(3):439–47. 10.1249/MSS.0b013e31827767fc. 10.1249/MSS.0b013e31827767fc [DOI] [PubMed] [Google Scholar]
- 88.Cho HC, Kim JK, Lee NJ, Kim SY, Yoon NK. Effects of combined exercise on cardiovascular risk factors and serum BDNF level in mid-aged women. Journal of exercise nutrition & biochemistry. 2014;18(1):61–7. 10.5717/jenb.2014.18.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coelho FM, Pereira DS, Lustosa LP, Silva JP, Dias JM, Dias RC, et al. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch Gerontol Geriatr. 2012;54(3):415–20. 10.1016/j.archger.2011.05.014. 10.1016/j.archger.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 90.Ferris LT, Williams JS, Akerhielm TD, Hale KB, Parsons SM, Jordan SL, et al. Serum Brain-Derived Neurotrophic Factor Responses to Chronic Exercise Training. Medicine and Science in Sports and Exercise. 2006;38(5):S522–S3. . [Google Scholar]
- 91.Forti LN, Van Roie E, Njemini R, Coudyzer W, Beyer I, Delecluse C, et al. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp Gerontol. 2015;70:144–9. 10.1016/j.exger.2015.08.004 . [DOI] [PubMed] [Google Scholar]
- 92.Fragala MS, Beyer KS, Jajtner AR, Townsend JR, Pruna GJ, Boone CH, et al. Resistance exercise may improve spatial awareness and visual reaction in older adults. J Strength Cond Res. 2014;28(8):2079–87. 10.1519/JSC.0000000000000520. 10.1519/JSC.0000000000000520 [DOI] [PubMed] [Google Scholar]
- 93.Gapin JI, Wooten JS, Bohall SC, Stout JJ, Smith B, Kirk EP. Associations Between Cognition, Weight Loss, and BDNF Following Exercise Training in Overweight and Obese Adults. Medicine and Science in Sports and Exercise. 2013;45(5):559-. . [Google Scholar]
- 94.Goekint M, Roelands B, De Pauw K, Knaepen K, Bos I, Meeusen R. Does a period of detraining cause a decrease in serum brain-derived neurotrophic factor? Neurosci Lett. 2010;486(3):146–9. 10.1016/j.neulet.2010.09.032. 10.1016/j.neulet.2010.09.032 [DOI] [PubMed] [Google Scholar]
- 95.Kim H, Suzuki T, Kim M, Kojima N, Ota N, Shimotoyodome A, et al. Effects of Exercise and Milk Fat Globule Membrane (MFGM) Supplementation on Body Composition, Physical Function, and Hematological Parameters in Community-Dwelling Frail Japanese Women: A Randomized Double Blind, Placebo-Controlled, Follow-Up Trial. PLoS ONE. 2015;10(2):e0116256 10.1371/journal.pone.0116256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim Y. The effect of regular Taekwondo exercise on Brain-derived neurotrophic factor and Stroop test in undergraduate student. Journal of exercise nutrition & biochemistry. 2015;19(2):73–9. 10.5717/jenb.2015.15060904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koichiro A, Yusuke O, Shogo T, Shiori H, Fuminori K, Hiroyuki I, et al. Association of serum BDNF concentration with high-intensity interval training. Japanese Journal of Physical Fitness & Sports Medicine. 2015;64(2):227–32. [Google Scholar]
- 98.Lemos JR Jr., Alves CR, de Souza SB, Marsiglia JD, Silva MS, Pereira AC, et al. Peripheral vascular reactivity and serum BDNF responses to aerobic training are impaired by the BDNF Val66Met polymorphism. Physiol Genomics. 2016;48(2):116–23. 10.1152/physiolgenomics.00086.2015 . [DOI] [PubMed] [Google Scholar]
- 99.Levinger I, Goodman C, Matthews V, Hare DL, Jerums G, Garnham A, et al. BDNF, metabolic risk factors, and resistance training in middle-aged individuals. Med Sci Sports Exerc. 2008;40(3):535–41. 10.1249/MSS.0b013e31815dd057. 10.1249/MSS.0b013e31815dd057 [DOI] [PubMed] [Google Scholar]
- 100.Mueller K, Moller HE, Horstmann A, Busse F, Lepsien J, Bluher M, et al. Physical exercise in overweight to obese individuals induces metabolic- and neurotrophic-related structural brain plasticity. Frontiers in human neuroscience. 2015;9:372 10.3389/fnhum.2015.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murawska-Cialowicz E, Wojna J, Zuwala-Jagiello J. Crossfit training changes brain-derived neurotrophic factor and irisin levels at rest, after wingate and progressive tests, and improves aerobic capacity and body composition of young physically active men and women. J Physiol Pharmacol. 2015;66(6):811–21. . [PubMed] [Google Scholar]
- 102.Prestes J, da Cunha Nascimento D, Tibana RA, Teixeira TG, Vieira DC, Tajra V, et al. Understanding the individual responsiveness to resistance training periodization. Age (Dordr). 2015;37(3):9793 10.1007/s11357-015-9793-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruiz JR, Gil-Bea F, Bustamante-Ara N, Rodriguez-Romo G, Fiuza-Luces C, Serra-Rexach JA, et al. Resistance training does not have an effect on cognition or related serum biomarkers in nonagenarians: a randomized controlled trial. Int J Sports Med. 2015;36(1):54–60. 10.1055/s-0034-1375693 . [DOI] [PubMed] [Google Scholar]
- 104.Schiffer T, Schulte S, Hollmann W, Bloch W, Struder HK. Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Horm Metab Res. 2009;41(3):250–4. 10.1055/s-0028-1093322. 10.1055/s-0028-1093322 [DOI] [PubMed] [Google Scholar]
- 105.Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R372–7. 10.1152/ajpregu.00525.2009. 10.1152/ajpregu.00525.2009 [DOI] [PubMed] [Google Scholar]
- 106.Wagner G, Herbsleb M, Cruz Fde L, Schumann A, Brunner F, Schachtzabel C, et al. Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35(10):1570–8. 10.1038/jcbfm.2015.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams JS, Ferris LT. Effects of Endurance Exercise Training on Brain-Derived Neurotrophic Factor. Journal of Exercise Physiology. 2012;15(4):11–7. ; 17706373. [Google Scholar]
- 108.Yarrow JF, White LJ, McCoy SC, Borst SE. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci Lett. 2010;479(2):161–5. 10.1016/j.neulet.2010.05.058. 10.1016/j.neulet.2010.05.058 [DOI] [PubMed] [Google Scholar]
- 109.Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol. 2008;59 Suppl 7:119–32. . [PubMed] [Google Scholar]
- 110.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 111.Marais L, Stein DJ, Daniels WM. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab Brain Dis. 2009;24(4):587–97. 10.1007/s11011-009-9157-2 . [DOI] [PubMed] [Google Scholar]
- 112.Laske C, Banschbach S, Stransky E, Bosch S, Straten G, Machann J, et al. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int J Neuropsychopharmcol. 2010;13(5):595–602. 10.1017/S1461145709991234. ; 12928638. [DOI] [PubMed] [Google Scholar]
- 113.Heijnen S, Hommel B, Kibele A, Colzato LS. Neuromodulation of Aerobic Exercise-A Review. Front Psychol. 2015;6:1890 10.3389/fpsyg.2015.01890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Catalan-Matamoros D, Gomez-Conesa A, Stubbs B, Vancampfort D. Exercise improves depressive symptoms in older adults: An umbrella review of systematic reviews and meta-analyses. Psychiatry Res. 2016;244:202–9. 10.1016/j.psychres.2016.07.028 . [DOI] [PubMed] [Google Scholar]
- 115.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. . [DOI] [PubMed] [Google Scholar]
- 116.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–72. 10.1016/j.tins.2007.06.011 . [DOI] [PubMed] [Google Scholar]
- 117.Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87(4):728–34. . [PubMed] [Google Scholar]
- 118.Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26(1):115–23. 10.1016/j.neurobiolaging.2004.03.002 . [DOI] [PubMed] [Google Scholar]
- 119.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28(11):2278–87. 10.1111/j.1460-9568.2008.06524.x. ; 8821297. 10.1111/j.1460-9568.2008.06524.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14(3):347–53. 10.1017/S1461145710000738 . [DOI] [PubMed] [Google Scholar]
- 121.Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, et al. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13(4):535–9. 10.1017/S1461145709991015 . [DOI] [PubMed] [Google Scholar]
- 122.Angelucci F, Gelfo F, De Bartolo P, Caltagirone C, Petrosini L. BDNF concentrations are decreased in serum and parietal cortex in immunotoxin 192 IgG-Saporin rat model of cholinergic degeneration. Neurochem Int. 2011;59(1):1–4. 10.1016/j.neuint.2011.04.010 . [DOI] [PubMed] [Google Scholar]
- 123.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328(3):261–4. . [DOI] [PubMed] [Google Scholar]
- 124.Swardfager W, Herrmann N, Marzolini S, Saleem M, Shammi P, Oh P, et al. Brain derived neurotrophic factor, cardiopulmonary fitness and cognition in patients with coronary artery disease. Brain, Behavior, and Immunity. 2011;25(6):1264–71. 10.1016/j.bbi.2011.04.017. 10.1016/j.bbi.2011.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017–22. 10.1073/pnas.1015950108. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences, USA. 2011;108(7):3017–22. ; 14388032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22. 10.1073/pnas.1015950108. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Syvanen S, Lindhe O, Palner M, Kornum BR, Rahman O, Langstrom B, et al. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab Dispos. 2009;37(3):635–43. 10.1124/dmd.108.024745 . [DOI] [PubMed] [Google Scholar]
- 129.Lacombe O, Videau O, Chevillon D, Guyot AC, Contreras C, Blondel S, et al. In vitro primary human and animal cell-based blood-brain barrier models as a screening tool in drug discovery. Mol Pharm. 2011;8(3):651–63. 10.1021/mp1004614 . [DOI] [PubMed] [Google Scholar]
- 130.Vanevski F, Xu B. Molecular and neural bases underlying roles of BDNF in the control of body weight. Front Neurosci. 2013;7:37 10.3389/fnins.2013.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ono M, Ichihara J, Nonomura T, Itakura Y, Taiji M, Nakayama C, et al. Brain-derived neurotrophic factor reduces blood glucose level in obese diabetic mice but not in normal mice. Biochem Biophys Res Commun. 1997;238(2):633–7. 10.1006/bbrc.1997.7220 . [DOI] [PubMed] [Google Scholar]
- 132.Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, et al. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. Am J Pathol. 1999;155(4):1183–93. 10.1016/S0002-9440(10)65221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lemos J Jr., De Souza S, Ribeiro R Jr., Dias R, Silva M, Alves C, et al. Aerobic exercise increases parasympathetic activity on BDNF genetic variant Val66Met carriers. FASEB Journal. 2015. [Google Scholar]
- 134.Arija V, Ferrer-Barcala M, Aranda N, Canals J. BDNF Val66Met polymorphism, energy intake and BMI: a follow-up study in schoolchildren at risk of eating disorders. BMC Public Health. 2010;10:363 10.1186/1471-2458-10-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lang UE, Hellweg R, Sander T, Gallinat J. The Met allele of the BDNF Val66Met polymorphism is associated with increased BDNF serum concentrations. Mol Psychiatry. 2009;14(2):120–2. 10.1038/mp.2008.80 . [DOI] [PubMed] [Google Scholar]
- 136.Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry. 2010;15(8):810–5. 10.1038/mp.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Canivet A, Albinet CT, Andre N, Pylouster J, Rodriguez-Ballesteros M, Kitzis A, et al. Effects of BDNF polymorphism and physical activity on episodic memory in the elderly: a cross sectional study. Eur Rev Aging Phys Act. 2015;12:15 10.1186/s11556-015-0159-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kang HJ, Bae KY, Kim SW, Shin IS, Hong YJ, Ahn Y, et al. BDNF val66met polymorphism and depressive disorders in patients with acute coronary syndrome. J Affect Disord. 2016;194:1–8. 10.1016/j.jad.2016.01.033 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
+ indicates yes;—indicates no;? indicates uncertain; NA indicates not applicable.
(XLSX)
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.