Abstract
Purpose
This study was designed to investigate the biomechanical properties of nonirradiated (NI) and irradiated (IR) peroneus tendons to determine if they would be suitable allografts, in regards to biomechanical properties, for anterior cruciate ligament reconstruction after a dose of 1.5–2.5 Mrad.
Methods
Seven pairs of peroneus longus (PL) and ten pairs of peroneus brevis (PB) tendons were procured from human cadavers. The diameter of each allograft was measured. The left side of each allograft was IR at 1.5–2.5 Mrad, whereas the right side was kept aseptic and NI. The allografts were thawed, kept wet with saline, and attached in a single-strand fashion to custom freeze grips using liquid nitrogen. A preload of 10 N was then applied and, after it had reached steady state, the allografts were pulled at 4 cm/sec. The parameters recorded were the displacement and force.
Results
The elongation at the peak load was 10.3±2.3 mm for the PB NI side and 13.5±3.3 mm for the PB IR side. The elongation at the peak load was 17.4±5.3 mm for the PL NI side and 16.3±2.0 mm for the PL IR side. For PL, the ultimate load was 2,091.6±148.7 N for NI and 2,122.8±380.0 N for IR. The ultimate load for the PB tendons was 1,485.7±209.3 N for NI and 1,318.4±296.9 N for the IR group. The ultimate stress calculations for PL were 90.3±11.3 MPa for NI and 94.8±21.0 MPa for IR. For the PB, the ultimate stress was 82.4±19.0 MPa for NI and 72.5±16.6 MPa for the IR group. The structural stiffness was 216.1±59.0 N/mm for the NI PL and 195.7±51.4 N/mm for the IR side. None of these measures were significantly different between the NI and IR groups. The structural stiffness was 232.1±45.7 N/mm for the NI PB and 161.9±74.0 N/mm for the IR side, and this was the only statistically significant difference found in this study (P=0.034).
Conclusion
Our statistical comparisons found no significant differences in terms of elongation, ultimate load, or ultimate stress between IR and NI PB and PL tendons. Only the PB structural stiffness was affected by irradiation. Thus, sterilizing allografts at 1.5–2.5 Mrad of gamma irradiation does not cause major alterations in the tendons’ biomechanical properties while still providing a suitable amount of sterilization for anterior cruciate ligament reconstruction.
Keywords: ACL reconstruction, allograft, sterilization, tissue banking
Introduction
In the United States alone, over 900,000 allografts are transplanted per year.1 The American Association of Tissue Banks estimated the use of 1,100,000 musculoskeletal allografts in 2009.1 Anterior cruciate ligament (ACL) reconstruction is commonly studied in the United States. An estimated 200,000 ACL ruptures occur annually, and use of musculoskeletal allografts has increased over the past years.1–4 Patellar bone-tendon-bone (PBTB) allografts are commonly used in ACL reconstruction,3,5,6 while iliotibial bands are gaining popularity.7–9 Other allografts such as the peroneus longus (PL) and peroneus brevis (PB) tendons have shown strength properties that are similar to the ACL.7,10,11
Allograft implantation brings a higher risk for harmful pathogenic transmission compared to an autograft. Traditional sterilization methods for implanting medical devices include dry heat, moist heat, ethylene oxide, electron beam exposure, and gamma irradiation to prevent possible bacterial and disease transmission.3,12–14 Tissue banks usually favor the use of gamma irradiation for allografts.15 Several studies have shown that the effects on biomechanical properties among allografts are dose dependent.1,12,16–18 For example, a study demonstrated that 15 PBTB allografts that received a gamma irradiation dose of 3.0 and 4.0 Mrad showed a dramatic reduction in stiffness, maximum force, maximum elongation, strain energy, and maximum stress, while 15 PBTB allografts that received a gamma irradiation dose of 2.0 Mrad only showed a significant difference in maximum force, strain energy, and maximum stress.16 Along with the previous study, another study has shown that a dose of 2 Mrad can affect the strength of allografts as well.19 The dosage that the allografts receive typically depends on the protocol of the tissue bank. The American Association of Tissue Banks conducted a survey and found that 29 out of 36 tissue banks use a standard gamma irradiation dose ranging from 1.0 to 3.5 Mrad, with the majority of the tissue banks utilizing a gamma irradiation dose ranging from 1.0 to 1.8 Mrad.15,20
Purpose
Throughout our literature research, we found no studies that investigated ultimate load, ultimate stress, elongation, and strain differences among NI and IR PL and PB tendons. This study was designed to investigate the biomechanical properties of nonirradiated (NI) and irradiated (IR) PL and PB. We hypothesized that there would be no statistically significant difference in terms of ultimate load, ultimate stress, elongation, ultimate strain, and structural stiffness between the NI and IR tendons (PL and PB).
Materials and methods
Preparation of the allografts
Seven pairs of PL tendons and ten pairs of PB tendons were procured from male and female cadavers at the UMTB (Vivex Biomedical Inc.) following the current AATB standards and FDA regulations. Institutional review board approval was not required, as in the context of tissue banking, the gift of tissue and consent is for both transplantation and research. Ages of cadavers ranged from 13 to 57 years, with a mean age of 41.6 years. Tendons were cleaned of remaining connective tissues and rinsed in saline solution prior to packaging and freezing at −80°C. The left side of each allograft was gamma IR at 1.5–2.5 Mrad on dry ice, while the right side was kept aseptic and NI. Gamma IR was performed by Sterigenics, Charlotte, NC, USA.
Biomechanical testing
Allografts were thawed for 2–3 hours at room temperature (23°C) before testing. The diameter of each allograft was measured in a double loop configuration using a tendon sizer after being sprayed with saline. Allografts were then mounted in custom freeze grips, with an active length of 4 cm. Both grips were then frozen in liquid nitrogen for 20 seconds before being placed in the MTS model 858 MiniBionix II (Eden Prairie, MN, USA). The expected length of a reconstructed allograft between the tibial and femoral anchors was approximately 4 cm. After mounting each specimen in the soft tissue grips in a single-strand fashion, a preload of 10 N was applied, and when it had reached steady state, the allografts were finally pulled in displacement control at a constant displacement of 4 cm/sec to create a strain rate of 100%/sec. Failure mode was recorded, and only specimens showing intraligamentous rupture were included in the analysis.
Biomechanical parameters
The parameters recorded throughout each sample in this study were the force and displacement. From the force and displacement graph, the peak force (N) and the excursion of peak force (mm) were noted. Each load–displacement curve was plotted in Excel, and a range of the earliest visually linear portion of the curve had linear regression applied, ie, if R2>0.95, that slope was used as the structural stiffness (N/mm). From the ultimate load, the ultimate stress (MPa) was calculated by dividing the load by the cross-sectional area of the graft, which is calculated from the double loop diameter.
Statistical analysis
Student’s t-test was used to compare the matched pairs of allografts. Threshold P-value was set at 0.05.
Results
Peroneus brevis
Three pairs of PB tendons were statistical outliers due to the failure of the tendons at the grip or pulling through the grip. Thus, only seven pairs of PB were included. The mean ultimate load for the NI (right side) PB allografts was 1,485.7±209.3 N, while for the IR PB allografts (left side) it was 1,318.4±296.9 N (Figure 1). The mean ultimate stress for the NI PB allografts was 82.4±19.0 MPa, while for the IR PB allografts it was 72.5±16.6 MPa. The elongation at the peak load was 10.3±2.3 mm for the NI side and 13.5±3.3 mm for the IR side. This strain amounts to 25.8%±5.6% for the NI side vs 33.7%±8.3% for the IR side (Figure 2). None of these differences were statistically significant. The structural stiffness was 232.1±45.7 N/mm for NI and 161.9±74.0 N/mm for IR, and this difference was statistically significant (P=0.034). Table 1 summarizes these findings.
Figure 1.
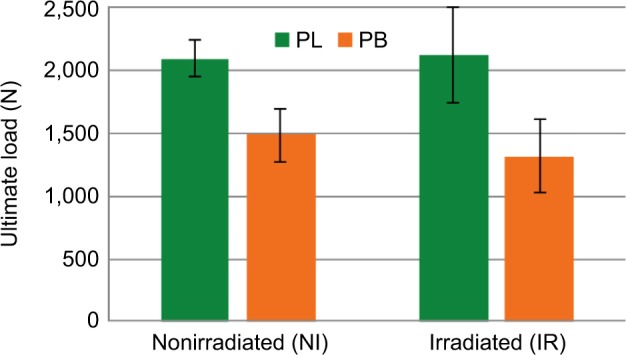
Mean ultimate load (N) comparison between NI and IR peroneus tendons.
Notes: The mean ultimate load for the NI PB allografts was 1,485.7±209.3 N, while for the IR PB allografts it was 1,318±296.9 N. The mean ultimate load for the NI PL allografts was 2,091.6±148.7 N, while for the IR PL allografts it was 2,122.8±380.0 N.
Abbreviations: IR, irradiated; NI, nonirradiated; PB, peroneus brevis; PL, peroneus longus.
Figure 2.
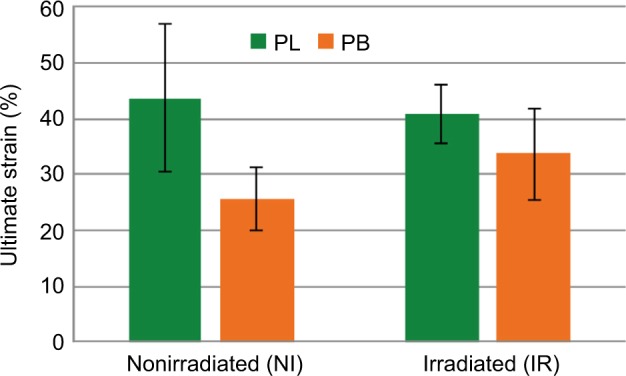
Mean ultimate strain (%) comparison between NI and IR peroneus tendons.
Notes: The ultimate strain of PB was of 25.8%±5.6% for the RI side vs 33.7%±8.3% for the NI side. The ultimate strain of PL was of 43.6%±13.2% for the NI side vs 40.8%±5.1% for the IR side.
Abbreviations: IR, irradiated; NI, nonirradiated; PB, peroneus brevis; PL, peroneus longus.
Table 1.
Summary of the peroneus brevis results
| PB
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NI
|
IR
|
||||||||||
| Diameter (mm) |
Ultimate load (N) | Stress(Mpa) | Elongation (mm) |
Ultimate strain (%) | Stiffness (N/mm) |
Diameter (mm) |
Ultimate load (N) | Stress (Mpa) |
Elongation (mm) |
Ultimate strain (%) | Stiffness (N/mm) |
| 7.0 | 1,457 | 75.7 | 8.6 | 22.0 | 225.0 | 7.0 | 936 | 48.6 | 18.5 | 46.0 | 65.5 |
| 6.0 | 1,323 | 93.6 | 8.6 | 22.0 | 250.0 | 5.5 | 1,087 | 91.5 | 12.2 | 31.0 | 131.6 |
| 8.0 | 1,545 | 61.5 | 8.0 | 20.0 | 245.5 | 7.5 | 1,480 | 67.0 | 10.3 | 26.0 | 262.5 |
| 7.5 | 1,513 | 68.5 | 13.1 | 33.0 | 218.2 | 7.0 | 1,545 | 80.3 | 10.7 | 27.0 | 235.7 |
| 6.5 | 1,910 | 115.1 | 12.0 | 30.0 | 303.0 | 8.0 | 1,771 | 70.5 | 14.0 | 35.0 | 187.5 |
| 6.0 | 1,318 | 93.2 | 8.9 | 22.0 | 233.3 | 6.0 | 1,308 | 92.5 | 11.2 | 28.0 | 168.4 |
| 7.0 | 1,334 | 69.3 | 12.9 | 32.0 | 150.0 | 7.0 | 1,102 | 57.3 | 17.4 | 44.0 | 82.3 |
| 6.9 (0.7) | 1,485.7 (209.3) | 82.4 (19.0) | 10.3 (2.3) | 25.8 (5.6) | 232.1 (45.7) | 6.9 (0.9) | 1,318.4 (296.9) | 72.5 (16.6) | 13.5 (3.3) | 33.7 (8.3) | 161.9 (74.0) |
Notes: The values in bold represent mean (SD).
Abbreviations: PB, peroneus brevis; NI, nonirradiated; IR, irradiated; SD, standard deviation.
Peroneus longus
Two pairs of PL tendons were statistical outliers due to the failure of the tendons at the grip or pulling through the grip. Thus, only five pairs of PL were included. The mean ultimate load for the NI PL allografts was 2,091.6±148.7 N, while for the IR PL allografts it was 2,122.8±380.0 N (Figure 1). The mean ultimate stress for the NI PL allografts was 90.3±11.3 MPa, while for the IR PL allografts it was 94.8±21.0 MPa. The elongation at the peak load was 17.4±5.3 mm for the NI side and 16.3±2.0 mm for the IR side. The ultimate strain was 43.6%±13.2% for the NI side vs 40.8%±5.1% for the IR side (Figure 2). The structural stiffness was 216.1±59.0 N/mm for the NI side and 195.7±51.4 N/mm for the IR side. None of these differences were statistically significant. Table 2 summarizes these findings.
Table 2.
Summary of the peroneus longus results
| PL
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NI
|
IR
|
||||||||||
| Diameter (mm) |
Ultimate load (N) | Stress (Mpa) |
Elongation (mm) |
Ultimate strain (%) | Stiffness (N/mm) |
Diameter (mm) |
Ultimate load (N) | Stress (Mpa) |
Elongation (mm) |
Ultimate strain (%) | Stiffness (N/mm) |
| 7.5 | 2,106 | 95.3 | 18.6 | 46.5 | 189.7 | 7.5 | 1,864 | 84.4 | 13.8 | 34.5 | 193.3 |
| 8.0 | 1,889 | 75.2 | 11.1 | 27.7 | 316.7 | 7.5 | 2,201 | 99.6 | 19.0 | 47.5 | 183.3 |
| 7.5 | 2,307 | 104.4 | 24.0 | 60.0 | 166.7 | 7.5 | 2,756 | 124.8 | 15.4 | 38.5 | 283.3 |
| 7.5 | 2,064 | 93.4 | 20.4 | 51.0 | 190.5 | 7.0 | 1,872 | 97.3 | 17.7 | 44.2 | 153.3 |
| 8.0 | 2,092 | 83.2 | 13.1 | 32.7 | 216.7 | 8.5 | 1,921 | 67.7 | 15.6 | 39.0 | 165.1 |
| 7.7 (0.3) | 2,091.6 (148.7) | 90.3 (11.3) | 17.4 (5.3) | 43.6 (13.2) | 216.1 (59.0) | 7.6 (0.5) | 2,122.8 (380.0) | 94.8 (21.0) | 16.3 (2.0) | 40.8 (5.1) | 195.7 (51.4) |
Notes: The values in bold represent mean (SD).
Abbreviations: PL, peroneus longus; NI, nonirradiated; IR, irradiated; SD, standard deviation.
Discussion
The possibility of irradiation affecting the biomechanical properties of PL and PB allografts for the use in ACL reconstruction is still a concern. Our study aimed at investigating the difference in ultimate load, ultimate stress, elongation, ultimate strain, and structural stiffness between the NI and IR PL and PB. Numerous groups have studied biomechanical differences between NI and IR tendons.3,6,12,15–17,21,22
Our statistical comparisons found no significant differences for ultimate load, ultimate stress, elongation, and ultimate strain between IR and NI PB and PL tendons when sterilized with 1.5–2.5 Mrad of gamma irradiation. Only the structural stiffness of PB tendons was significantly reduced upon irradiation.
The main limitation of this study is that only one dose of gamma irradiation was used for all the allografts tested.
Conclusion
The dose range used in this study sufficiently inactivates nonencapsulated pathogens, for example Porcine parvovirus.23 We are unsure if this dose range would inactivate encapsulated organisms such as Clostridium. However, tissues with such pathogens would not be used for allograft transplantation. Future studies are needed to determine adequate dosages to inactivate encapsulated pathogens so that more allografts can possibly be transplanted. The observed differences between IR and NI peroneus tendons are, for the most part, not statistically significant, thus increasing the source of available allografts for ACL reconstruction.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.McLean . 2007 American Association of Tissue Banks (AATB) Annual Survey of Accredited Tissue Banks in the United States. McLean, VA: 2007. VA: AATB 2010. [Google Scholar]
- 2.Friedberg R. Anterior Cruciate Ligament Injury. 2015. UpToDate. [Google Scholar]
- 3.Reid J, Sikka R, Tsoi W, et al. Sterilization effects on the mechanical properties of human bone-patellar tendon-bone allografts. Orthopedics. 2010 2010 Apr 16;33(4) doi: 10.3928/01477447-20100225-06. Epub. [DOI] [PubMed] [Google Scholar]
- 4.Management of anterior cruciate ligament injuries: evidence-based clinical practice guidelines; Poster presented at: American Academy of Orthopaedic Surgeons; March 11–15; 2014; New Orleans, Louisiana. [Google Scholar]
- 5.West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13(3):197–207. doi: 10.5435/00124635-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Allografts for ACL Reconstruction Survey Report; Presented at: The American Orthopaedic Society for Sport Medicine, AAOS Department of Research and Scientific Affairs; July 01; 2013. [Google Scholar]
- 7.Chan DB, Temple HT, Latta LL, Mahure S, Dennis J, Kaplan LD. A biomechanical comparison of fan-folded, single-looped fascia lata with other graft tissues as a suitable substitute for anterior cruciate ligament reconstruction. Arthroscopy. 2010;26(12):1641–1647. doi: 10.1016/j.arthro.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Delcroix GJ-R, Barton MD, Qureshi A, et al. Pretensioning of anterior tibialis tendons and fan-folded iliotibial bands. CellR4. 2015;3(5):e1673. [Google Scholar]
- 9.Delcroix GJ, Kaimrajh DN, Baria D, et al. Histologic, biomechanical, and biological evaluation of fan-folded iliotibial band allografts for anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(4):756–765. doi: 10.1016/j.arthro.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Kerimoglu S, Aynaci O, Saracoglu M, Aydin H, Turhan AU. Anterior cruciate ligament reconstruction with the peroneus longus tendon. Acta Orthop Traumatol Turc. 2008;42(1):38–43. doi: 10.3944/aott.2008.038. [DOI] [PubMed] [Google Scholar]
- 11.Moore MA, Wolf C. Peroneus Longus and Posterior Tibialis Bio-Implants in Knee Reconstruction. Lifenet Health; Mar, 2010. [Google Scholar]
- 12.Hoburg A, Keshlaf S, Schmidt T, et al. Fractionation of high-dose electron beam irradiation of BPTB grafts provides significantly improved viscoelastic and structural properties compared to standard gamma irradiation. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1955–1961. doi: 10.1007/s00167-011-1518-9. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt T, Hoburg A, Broziat C, et al. Sterilization with electron beam irradiation influences the biomechanical properties and the early remodeling of tendon allografts for reconstruction of the anterior cruciate ligament (ACL) Cell Tissue Bank. 2012;13(3):387–400. doi: 10.1007/s10561-011-9289-6. [DOI] [PubMed] [Google Scholar]
- 14.Health USFaDACfDaR . Medical Devices: Updated 510(k) Sterility Review Guidance K90-1. Final Guidance for Industry and FDA; Silver Spring, MD: 2002. [Google Scholar]
- 15.Vangsness CT, Jr, Triffon MJ, Joyce MJ, Moore TM. Soft tissue for allograft reconstruction of the human knee: a survey of the American Association of Tissue Banks. Am J Sports Med. 1996;24(2):230–234. doi: 10.1177/036354659602400221. [DOI] [PubMed] [Google Scholar]
- 16.Fideler BM, Vangsness CT, Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23(5):643–646. doi: 10.1177/036354659502300521. [DOI] [PubMed] [Google Scholar]
- 17.Yanke AB, Bell R, Lee A, et al. The biomechanical effects of 1.0 to 1.2 Mrad of gamma irradiation on human bone-patellar tendon-bone allografts. Am J Sports Med. 2013;41(4):835–840. doi: 10.1177/0363546512473816. [DOI] [PubMed] [Google Scholar]
- 18.Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone-patellar tendon-bone allografts. J Orthop Res. 1995;13(6):898–906. doi: 10.1002/jor.1100130614. [DOI] [PubMed] [Google Scholar]
- 19.Haut RC, Powlison AC. Order of irradiation and lyophilization on the strength of patellar tendon allografts. Trans Orthop Res Soc. 1989;14:514. [Google Scholar]
- 20.Nguyen H, Morgan DA, Forwood MR. Sterilization of allograft bone: is 25 kGy the gold standard for gamma irradiation? Cell Tissue Bank. 2007;8(2):81–91. doi: 10.1007/s10561-006-9019-7. [DOI] [PubMed] [Google Scholar]
- 21.Currey JD, Foreman J, Laketic I, Mitchell J, Pegg DE, Reilly GC. Effects of ionizing radiation on the mechanical properties of human bone. J Orthop Res. 1997;15(1):111–117. doi: 10.1002/jor.1100150116. [DOI] [PubMed] [Google Scholar]
- 22.Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653–1658. doi: 10.1177/0363546507302926. [DOI] [PubMed] [Google Scholar]
- 23.Miekka SI, Forng RY, Rohwer RG, et al. Inactivation of viral and prion pathogens by gamma-irradiation under conditions that maintain the integrity of human albumin. Vox Sang. 2003;84(1):36–44. doi: 10.1046/j.1423-0410.2003.00256.x. [DOI] [PubMed] [Google Scholar]


