Abstract
Objective
Chocó is a state located on the Pacific coast of Colombia that has a majority Afro-Colombian population. The objective of this study was to characterize the genetic ancestry, admixture and diversity of the population of Chocó, Colombia.
Methodology
Genetic variation was characterized for a sample of 101 donors (61 female and 40 male) from the state of Chocó. Genotypes were determined for each individual via the characterization of 610,545 single nucleotide polymorphisms genome-wide. Haplotypes for the uniparental mitochondrial DNA (female) and Y-DNA (male) chromosomes were also determined. These data were used for comparative analyses with a number of worldwide populations, including putative ancestral populations from Africa, the Americas and Europe, along with several admixed American populations.
Results
The population of Chocó has predominantly African genetic ancestry (75.8%) with approximately equal parts European (13.4%) and Native American (11.1%) ancestry. Chocó shows relatively high levels of three-way genetic admixture, and far higher levels of Native American ancestry, compared to other New World African populations from the Caribbean and the United States. There is a striking pattern of sex-specific ancestry in Chocó, with Native American admixture along the female lineage and European admixture along the male lineage. The population of Chocó is also characterized by relatively high levels of overall genetic diversity compared to both putative ancestral populations and other admixed American populations.
Conclusion
These results suggest a unique genetic heritage for the population of Chocó and underscore the profound human genetic diversity that can be found in the region.
Keywords: Admixture, Afro-Colombian, Colombia, Genetic ancestry, Genetic diversity, Human genome
Introduction
Chocó, Colombia
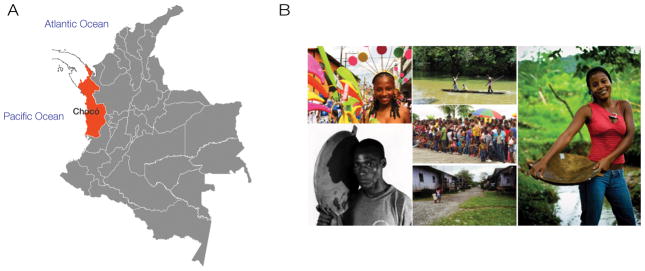
Chocó is a Colombian administrative department (i.e., a state) located along the country’s Pacific coast (Figure 1A). Chocó ranges from the Panamanian border in the north to the Cauca Valley in the southwest region of Colombia. The state of Chocó is recognized worldwide as a hotspot of biodiversity (http://www.eoearth.org/view/article/150631/). A biodiversity hotspot is defined as a specific geographic region with a large amount of endemic biodiversity that is threatened by human activity (Zachos and Habel 2011). In order for an area to be officially recognized as a biodiversity hotspot, at least 0.5% of its vascular plant species (or 1,500 species) must be characterized as endemics, which are defined as species found uniquely within a pro-scribed geographic region or habitat type. There are 25 global regions that qualify as biodiversity hotspots according to this criterion, and together these areas are home to almost 60% of the world’s known species of plants, birds, reptiles, amphibians and mammals. The entire area of Chocó is contained within the so-called Chocó-Darién biodiversity hotspot, also known as the Tumbes-Chocó-Magdalena biodiversity hotspot (http://tmalliance.org/about/where-we-work/biodiversity-hotspot/).
Figure 1.
Human biodiversity in Chocó. (A) The state of Chocó on the Pacific coast of Colombia. (B) The people of Chocó.
The Chocó-Darién biodiversity hotspot extends along the Pacific coast from the Panamá Canal region in the north, through the Darién Gap and Chocó wet rain forests in Panamá-Colombia, passing completely through Ecuador before ending in the coastal dry forests of northern Perú. This area encompasses a wide variety of diverse habitats including the wettest rain forests in the world, which are found in Chocó. The Colombian portion of this biodiversity hotspot in Chocó is relatively preserved compared to the Ecuadorean zone where 98% of the native forest has been cleared. The Chocó-Darién biodiversity hotspot supports ~10,000 species of vascular plants along with 600 species of birds, 235 species of mammals, 350 species of amphibians and 210 species of reptiles.
Human biodiversity in Chocó
Although Chocó is widely recognized as a hotspot of biodiversity, one critical aspect of the region’s biodiversity remains largely unexplored, the diversity of its human population (Figure 1B). The population of Chocó has a uniquely African genetic heritage with admixture from the Americas and Europe. The vast majority of the population is Afro-Colombian (82.1%) but there are also substantial numbers of Native Americans (12.7%) and individuals with primarily European ancestry (5.2%) (Hernández Romero 2005). There are anywhere from 9 to 20 million Afro-descendants in Colombia, making it the country with the third most Afro-descendants in the Americas. Despite the presence of such a large population of Afro-descendants in Colombia, there is a vast under-representation of genetic studies of Afro-Colombians (Rishishwar et al. 2015a). Previous Colombian genetic ancestry studies have dealt mainly with Mestizo genomes that have primarily European and secondary Native American ancestry (Carvajal-Carmona et al. 2000, Carvajal-Carmona et al. 2003, Bedoya et al. 2006, Wang et al. 2008, Bryc et al. 2010, Córdoba et al. 2012, Ruiz-Linares et al. 2014, Rishishwar et al. 2015b). Given the high percentage of Afro-Colombians living in Chocó, genetic studies of this population are ideally suited to uncover the as yet untapped African dimension of Colombian ancestry and human biodiversity.
Colombian individuals have three-way genetic admixture patterns that result from ancestral contributions to the modern population from Africa, the Americas and Europe (Rishishwar et al. 2015b). The story of every Colombian’s ancestry, along with their specific admixture patterns, is written in the sequence of their genome. Thus, genome sequence analysis can be used to infer genetic ancestry and admixture patterns for individuals and for the population as a whole. For individuals, the total overall proportions of African, Native American and European ancestry can be inferred along with both locus-specific and sex-specific patterns of ancestry and admixture. Genetic ancestry can be explored at both the continental level to uncover the broad regions of origin for Colombians and at the sub-continental level to explore the specific ancestral regions and countries from which individuals’ ancestors originate. At the population level, locus-specific admixture patterns can reveal whether natural selection has enriched for specific ancestry along particular genomic segments. Population level inference can also be used to assess whether there are sex-specific differences in ancestry that result from differential ancestry contributions along maternal versus paternal lineages. These kinds of genomic research approaches will be applied in order to elucidate the patterns of genetic ancestry and admixture of the population of Chocó under the auspices of a newly formed collaborative research project ChocoGen (http://www.chocogen.com/).
ChocoGen project
An exploration of human genetic biodiversity in Chocó is being conducted via the collaborative ChocoGen research project in an effort to value, conserve and utilize this precious resource. The ChocoGen research project has two overarching goals:
to characterize the genetic diversity and ancestry of the population of Chocó, and
to create a health profile of the region based on the genetic diversity of its people.
Research and development activities in support of both of these goals are being conducted in such a way as to develop the local human capacity in Chocó for research and education in genetic health and medicine. This project is a collaboration between Universidad Tecnológica del Chocó (UTCH) in Colombia, principle investigator Dr. Miguel A. Medina Rivas, and the Georgia Institute of Technology in the USA, principle investigator Dr. I. King Jordan. Bioinformatics analysis and interpretation of human genome sequences from the population of Chocó are being further supported by the National Center for Biotechnology Information (NCBI) in the USA, and the Colombian National Center for Bioinformatics and Computational Biology (BIOS) in Manizales.
Researchers from the ChocoGen project are conducting analysis of genomic sequences sampled from volunteers from the population of Chocó to characterize:
their genetic ancestry,
the quantity and nature of genetic admixture between ancestral populations, and
the possible relationship between genetic ancestry, admixture and determinants of health and disease.
The results of this project will serve as a resource for the development and application of genetic approaches to healthcare in the Pacific region of Colombia and help to position UTCH as a leader in this area of applied research. In this report, we present results of the first round of analyses of the genetic ancestry of 101 individuals sampled from the population of Chocó.
Methodology
Sample donors and genotyping
ChocoGen volunteer DNA sample donors were recruited at UTCH. Donors were selected in an effort to include representative samples of different geographic regions of Chocó (Atrato, Baudó, Atlantic coast, Pacific coast, San Juan), as well as an approximately equal representation of males and females, and donors were asked to self-identify their ethnic origins. Donors contributed DNA using a non-invasive saliva sampling method. All donors signed informed consent documents indicating their understanding of the potential risks of the project along with how their data would be handled and how their identity would be protected. Collection, genotyping and comparative analyses of human DNA samples were conducted with the approval of the ethics committee of UTCH. Donor DNA samples were genotyped using the Illumina HumanOmniExpress-24 single nucleotide polymorphism (SNP) chip.
Comparative genomic data sources
The genotypes of ChocoGen donors were compared to whole genome sequence data from the 1000 Genomes Project (1000G) (Genomes Project et al. 2010, Genomes Project et al. 2015) and genotype data from the Human Genome Diversity Project (HGDP) (Cann et al. 2002, Li et al. 2008) (Table 1). Genotypes from the ChocoGen donors, along with genotypes from the 1000G and HGDP projects, were all mapped to the coordinate space of the February 2009 human genome reference sequence version GRCh37/hg19 (Lander et al. 2001, Kent et al. 2002) for subsequent analysis. The program PLINK (Purcell et al. 2007) was used for genotype quality control and to extract autosomal genotyped positions (i.e., single nucleotide polymorphisms or SNPs) common to all three genotype sources to yield a final merged genotype dataset. For quality control, only individual SNP positions with a genotyping rate >98% were retained for subsequent analysis.
Table 1.
Human populations analyzed in this study*
| Dataset | Color | Short | Full Description | n |
|---|---|---|---|---|
|
Non-admixed Ancestral Populations
| ||||
| 1000G African (n=292) | LWK | Luhya in Webuye, Kenya | 99 | |
| MSL | Mende in Sierra Leone | 85 | ||
| YRI | Yoruba in Ibadan, Nigeria | 108 | ||
| 1000G European (n=198) | GBR | British in England and Scotland | 91 | |
| IBS | Iberian Population in Spain | 107 | ||
| HGDP Native American (n=70) | KRT | Karitiana in Brazil | 24 | |
| PMA | Pima in Mexico | 25 | ||
| SUR | Surui in Brazil | 21 | ||
|
| ||||
|
Admixed American Populations
| ||||
| 100G Admixed American (n=251) | ACB | African Caribbean in Barbados | 96 | |
| ASW | Americans of African Ancestry in SW USA | 61 | ||
| CLM | Colombians from Medellin, Colombia | 94 | ||
|
| ||||
| Chocó (n=101) | CHO | Colombians from Chocó, Quibdó, Colombia | 101 | |
The sources of the data-1000 Genomes Project (1000G) or Human Genome Diversity Project (HGDP) -are shown along with the population color codes, short abbreviations, full descriptions and numbers (n) of individuals analyzed. Populations are organized according to whether they represent non-admixed ancestral populations from Africa, Europe and the Americas or admixed American populations from the Caribbean, USA and Colombia. Chocó refers to the population sample studied here.
Ancestry and admixture inference
The program PLINK was used to prune the final merged genotype dataset by removing correlated sets of SNPs. Genomic distances were computed as pairwise allele sharing distances between all individual pruned genotypes using PLINK. The resulting pairwise distance matrix was projected onto two-dimensions with principal component analysis (PCA) using the prcomp function from the R package for statistical computing (Team 2008). The program ADMIXTURE (Alexander et al. 2009) was run on the genotype dataset to infer individual ancestry components. The resulting data was used with the nnls package from R to implement a non-negative least squares method to estimate the fractions of African, Native American and European ancestry for each individual from Chocó. For each individual, the entropy (H) of the admixture was calculated as
where pi is the ancestry fraction for population i. Sex-specific ancestry in the population of Chocó was determined via analysis of uniparental haplotypes: mitochondrial DNA (mtDNA) for the maternal lineage and Y chromosomal DNA (Y-DNA) for the paternal lineage.
The relative genetic diversity levels of the populations analyzed here were measured via the total amount of observed pairwise allele sharing distance and genome-wide heterozygosity. The total amount of observed pairwise allele sharing distance within each population was computed by fitting a minimum spanning ellipse to the individual genotype points of the population projected onto the first two principal components of the PCA analysis using the ellipsoidhull function in R. The areas (A) of the population-specific ellipses were computed using the lengths of the major (x) and minor (y) axes scaled to the principal component weights:
Heterozygosity was measured as the fraction of all genotype positions that are heterozygous within an individual using the program PLINK. To do this, we analyzed SNPs with minor allele frequency >25% in order allow for comparison between SNPs called from genome sequences versus SNPs called from genotype arrays, which are biased to high minor allele frequencies and European populations.
Results and discussion
Genetic characterization of the population of Chocó
Volunteer DNA sample donors were solicited on the main campus of UTCH located in the capital city of Quibdó; 101 volunteers (61 females and 40 males) provided DNA samples for genetic characterization along with answers to a series of questions related to their ethnic self-identity and family history. DNA samples were characterized in order to determine the specific identity of genetic sequence variants at 610,545 loci across the genome. Genetic variants are referred to here as single nucleotide polymorphisms (SNPs), and the specific identity of the DNA sequence residues that correspond to a genome-wide collection of SNPs is referred to as a genotype. The specific identify of the DNA sequence residues for a set of genetically linked SNPs is referred to as a haplotype. For the purposes of this study, donors’ genotypes were characterized for the entire set of human autosomes, and haplotypes were determined for uniparental mitochondrial DNA (mtDNA) and Y-DNA chromosomes. Chocó genotypes were compared to genotypes for a variety of human populations (Table 1), in order to make inferences about the genetic ancestry and diversity of the population. Chocó mtDNA and Y-DNA haplotypes were compared to known global distributions for haplotypes of these chromosomes in order to make inferences about female-specific (mtDNA) and male-specific (Y-DNA) genetic ancestry of the population.
Genetic ancestry and admixture of Chocó
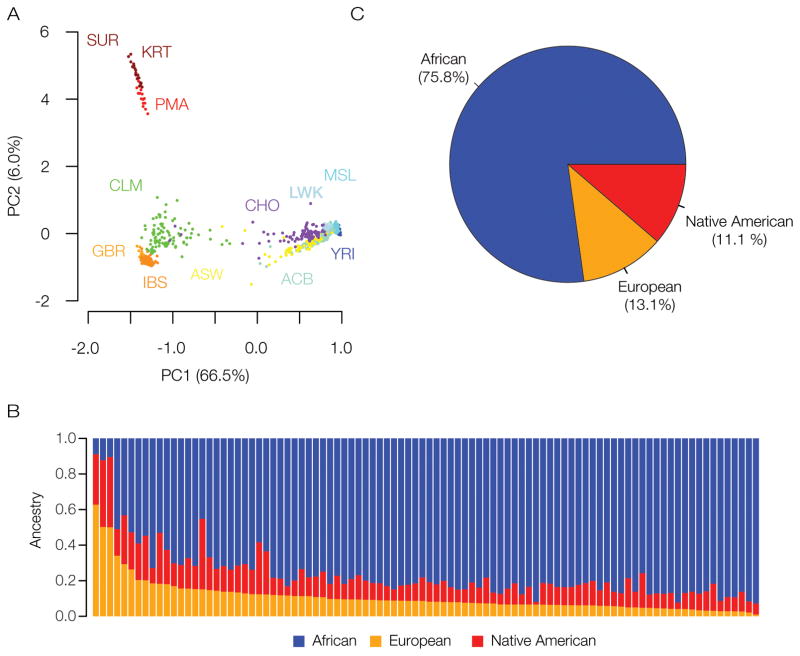
The Colombian population has a mixture of genetic ancestry from African, European and Native American populations, owing to the historical patterns of conquest and colonization in the New World (Markham 1912, Mann 2013). Thus, Chocó genotypes were compared to genotypes characterized from individuals sampled from representative populations of these regions (Table 1) in order to infer their overall genetic ancestry and admixture. The genetic relationships among individuals from the population of Chocó, along with individuals from the other global populations, are visually represented in Figure 2A. This panel shows a two-dimensional principal component analysis (PCA) projection of the pairwise genetic distances between all of the genotypes analyzed here, where the distance between each dot corresponds to the distance between each individual genotype. The main component of human genetic diversity in this representation is projected along the x-axis (PC1=66.5% of the diversity) and the secondary component is shown on the y-axis (PC2=6%). African, European and Native American populations occupy the three poles of human genetic diversity in this plot, whereas admixed American genomes, including Chocó (CHO) individuals, occupy intermediate positions between these three ancestral, and relatively non-admixed, population groups. The relative positions of the admixed American populations compared to the three ancestral groups gives an indication of their admixture proportions. For example, the Colombian population from Medellín (CLM) shows evidence of more European admixture compared to the Chocó population, which is located in much closer proximity to the African populations. Two other New World African populations (ACB and ASW) are also located in close proximity to the putative ancestral populations from the African continent, but occupy different positions than the Chocó population.
Figure 2.
Genetic ancestry and admixture in Chocó. (A) PCA plot showing the genetic relationships among individuals from different human populations compared to the population of Chocó. Each dot corresponds to a single individual, and the distances between dots correspond to the genetic distances between individuals. Populations are colored coded and labeled as shown in Table 1; individuals from Chocó are labeled with CHO and shown in purple. The principal components are labeled (PC1 and PC2) and shown along with the amounts of genetic variation captured by each component. (B) Admixture bar chart showing the percentage of African (blue), European (orange) and Native American (red) ancestry for each individual from population of Chocó. (C) Chocó population-average values for African (blue), European (orange) and Native American (red) ancestry.
These same pairwise genetic distances can be used to quantify the amount of genetic ancestry that any admixed individual shows from the putative ancestral African, European and Native American populations. The results of this kind of analysis are shown in Figure 2B and 2C. African ancestry represents the dominant admixture component for the vast majority of individuals from the population of Chocó analyzed here. The maximum fraction of African ancestry seen for any individual is 92.8%, and the average African ancestry for Chocó population is 75.8%. Nevertheless, there are substantial fractions of European and Native American ancestry seen for many of these individuals as well. The maximum fraction of European ancestry seen for any individual is 62.8%, and the average European ancestry is 13.1%. The maximum fraction of Native American ancestry is 39.6%, and the average Native American ancestry is 11.1%. The broad range of individual admixture percentages point to the diversity of the Chocó population.
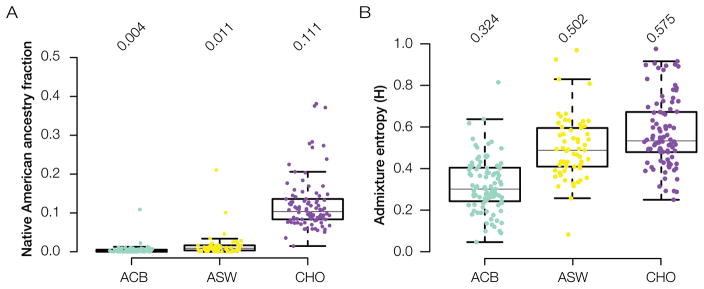
The genetic ancestry of the population of Chocó shows some interesting differences compared to the genetic ancestry of the two other New World African populations analyzed here, the ACB population from Barbados in the Caribbean and the ASW population from the USA, despite the fact that all three populations show similarly high levels of overall African ancestry (~75–80%). First of all, the Chocó population (CHO) has substantially higher levels of Native American ancestry compared to the Afro-Caribbean (ACB) or African-American (ASW) populations (Figure 3A). Chocó has 11.1% average Native American ancestry, whereas the Afro-Caribbean and African-American populations have 0.4% a 1.1% average Native American ancestry, respectively. Second, the Chocó population shows higher levels of three-way genetic admixture, as measured by Admixture entropy (H), compared to the other two New World African populations (Figure 3B). This reflects the fact that in Chocó the non-African ancestry component is relatively evenly divided between European and Native American ancestry, whereas almost all non-African ancestry in the Caribbean and US populations is European. This pattern is indicative of longer and more sustained contact between Afro-descendants and Indigenous communities in Chocó compared to what occurred in the Caribbean or the United States.
Figure 3.
Distinct admixture characteristics of Chocó. Admixture patterns are compared among Chocó (CHO) and other New World African populations: ACB from Barbados in the Caribbean and ASW from the United States. (A) Native American ancestry and (B) admixture distributions for the three new world African populations; average values are shown for each distribution.
Sex-specific genetic ancestry
Mitochondrial DNA (mtDNA) and Y-DNA chromosomes are referred to as uniparental ancestry markers since they are inherited strictly along the maternal (for mtDNA) and paternal (Y-DNA) lineages. This means that mtDNA haplotypes can be used to infer female-specific ancestry and admixture, and Y-DNA haplotypes can be used to infer male-specific ancestry and admixture. The global origins of the mtDNA and Y-DNA haplotypes characterized from the population of Chocó show striking evidence of sex-specific ancestry in this population (Figure 4). The majority of mtDNA (82.1%) and Y-DNA (77.8%) haplotypes have African origins, consistent with the overall genetic ancestry of the population. However, the non-African ancestry components differ markedly for the female (mtDNA) versus male (Y-DNA) lineages. All of the non-African mtDNA haplotypes (17.9%) have Native American origins, whereas all of the non-African Y-DNA haplotypes have European (16.7%) or Middle Eastern (5.6%) origins. This sex-specific pattern of genetic ancestry may be linked to the unique historical conditions under which the state of Chocó was founded and populated (Wade 1995).
Figure 4.
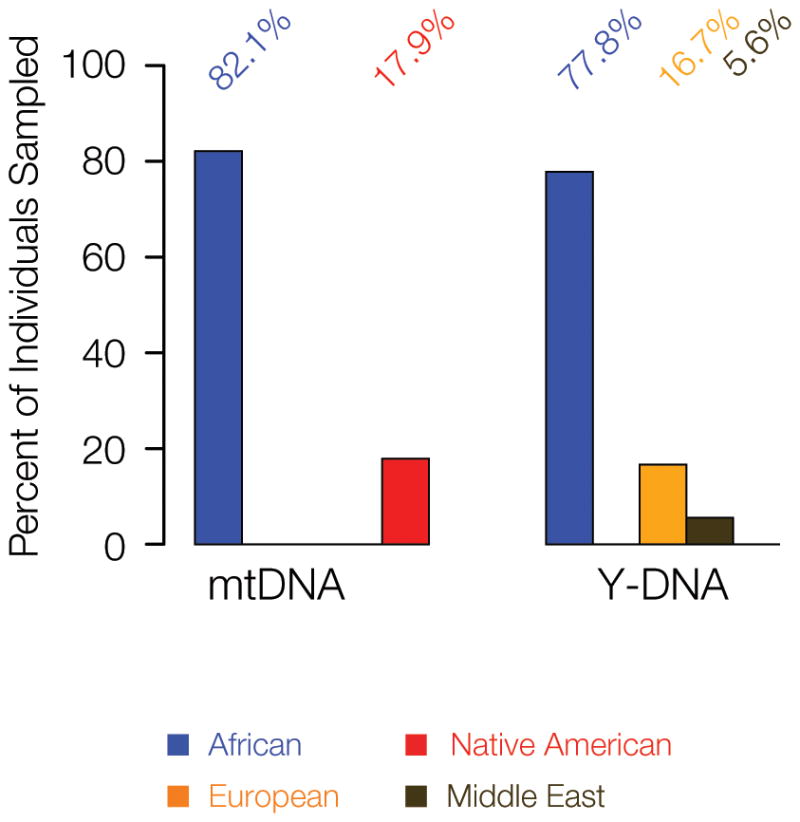
Sex-specific ancestry and admixture in Chocó. (A) Relative counts of African (blue), Native American (red), European (orange) and Middle Eastern (brown) mtDNA (maternal) and Y-DNA (paternal) haplotypes from the population of Chocó.
Genetic diversity levels in Chocó
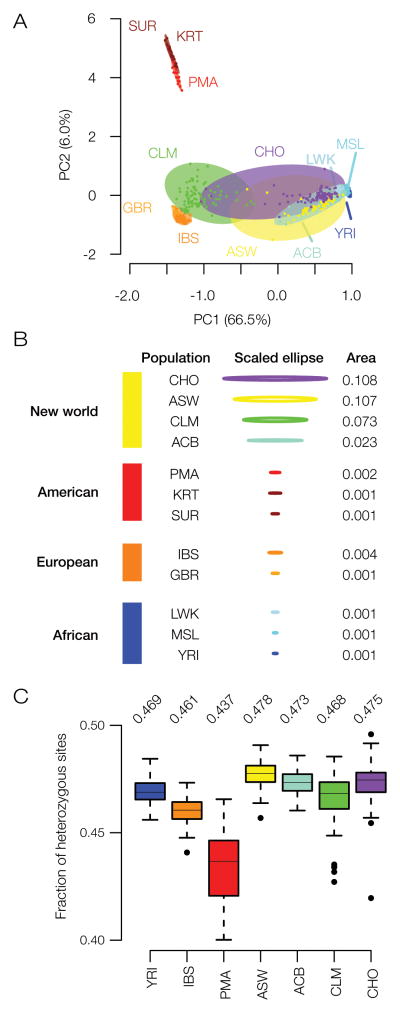
As previously noted, based on its distinct population demographics, we propose that the state of Chocó is a rich source of human biodiversity. To evaluate this proposition with respect to the genetic ancestry of the population, we compared the genetic diversity levels found in Chocó to levels of diversity seen for putative ancestral, non-admixed populations as well as other admixed American populations. The results of this analysis are shown in Figure 5. We evaluated genetic diversity in two ways: 1) via the overall scope of genetic distances between individuals in a population and 2) via the average genome-wide heterozygosity levels for all individuals in a population. The overall genetic diversity for each population was inferred by fitting a minimal spanning ellipse to the populations’ pairwise genetic distance projection on the PCA plot (Figure 5A). The areas of the population-specific minimum spanning ellipses were then determined and used to quantify the populations’ genetic diversity (Figure 5B). The Chocó population (CHO) has the highest level of overall genetic diversity calculated in this way for any of the populations analyzed here. The Chocó population also has high average heterozygosity levels compared to the other populations, second only to the other New World African population ASW (Figure 5C). Interestingly, the other admixed Colombian population from Medellín (CLM) also shows relatively high genetic diversity levels in these analyses despite the fact that it has a very different genetic ancestry profile (i.e., largely European ancestry) compared to the population of Chocó.
Figure 5.
Genetic biodiversity in Chocó. (A) PCA plot showing the genetic relationships among individuals from the different human populations analyzed here (as shown and described for Figure 1), with each population bounded by a minimum spanning ellipse. (B) Scaled areas of the population-specific minimum spanning ellipses are used to quantify the overall genetic diversity of each population. (C) Distributions of the genome-wide heterozygosity values are shown for the populations analyzed here; average values are shown for each distribution.
Conclusions
The ChocoGen collaborative research project has the joint aims of 1) characterizing the ancestry and genetic diversity of the population of Chocó, and 2) creating a genetic health profile of the population based on the diversity of its people. Investigators from UTCH and the Georgia Institute of Technology are collaborating to these ends, and this manuscript reports some of the first results of the project. The initial phase of the project is focused on ancestry analysis, and the results from this first phase will be used to inform the second health-related part of the effort. All of this work is being done in such a way as to develop the local human capacity for research in genetic ancestry and human health in the state of Chocó.
The analyses reported here indicate that the population of Chocó has an overwhelmingly African genetic ancestry, which is of course not surprising. Nevertheless, the Chocó population shows interesting differences, compared to other admixed American populations with similar levels of African ancestry, which likely reflect its distinct historical and cultural traditions (Wade 1995). In particular, individuals from Chocó show higher levels of three-way genetic admixture than other New World African populations, and this pattern can be largely attributed to the higher levels of Native American ancestry seen in Chocó. The population of Chocó also shows striking patterns of sex-specific ancestry, whereby non-African maternal ancestry is exclusively Native American, and non-African paternal ancestry is almost entirely European. This ancestry pattern may represent distinct admixture dynamics that characterized early (European admixture) from later (Native American admixture) historical periods in Chocó, and we plan to explore this idea further in subsequent studies.
Finally, the results on genetic ancestry and diversity obtained in this study underscore the extent to which Chocó represents a hotspot of human biodiversity. We hold that the human biodiversity of Chocó is an under-appreciated dimension of the area’s well known biodiversity, and one that should be equally valued and fully developed for its potential.
Literature cited
- Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655–64. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya G, Montoya P, García J, Soto I, Bourgeois S, Carvajal L, et al. Admixture dynamics in Hispanics: a shift in the nuclear genetic ancestry of a South American population isolate. Proc Natl Acad Sci USA. 2006;103(19):7234–9. doi: 10.1073/pnas.0508716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryc K, Vélez C, Karafet T, Moreno-Estrada A, Reynolds A, Auton A, et al. Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8954–61. doi: 10.1073/pnas.0914618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann HM, de Toma C, Cazes L, Legrand MF, Morel V, Piouffre L, et al. A human genome diversity cell line panel. Science. 2002;296(5566):261–2. doi: 10.1126/science.296.5566.261b. [DOI] [PubMed] [Google Scholar]
- Carvajal-Carmona LG, Ophoff R, Service S, Hartiala J, Molina J, Leon P, et al. Genetic demography of Antioquia (Colombia) and the Central Valley of Costa Rica. Hum Genet. 2003;112(5–6):534–41. doi: 10.1007/s00439-002-0899-8. [DOI] [PubMed] [Google Scholar]
- Carvajal-Carmona LG, Soto ID, Pineda N, Ortiz-Barrientos D, Duque C, Ospina-Duque J, et al. Strong Amerind/white sex bias and a possible Sephardic contribution among the founders of a population in northwest Colombia. Am J Hum Genet. 2000;67(5):1287–95. doi: 10.1016/s0002-9297(07)62956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Córdoba L, García J, Hoyos LS, Duque C, Rojas W, Caravajal S, et al. Composicion genética de una población del suroccidente de Colombia. Rev Colomb Antropol. 2012;48(1):21–48. [Google Scholar]
- Genomes Project C. Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genomes Project C. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández Romero A. La visibilización estadística de los grupos étnicos colombianos. Bogotá: Departamento Administrativo Nacional de Estadística (DANE); 2005. [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Li JZ, Absher DM, Tang H, Southwick AM, Casto AM, Ramachandran S, et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 2008;319(5866):1100–4. doi: 10.1126/science.1153717. [DOI] [PubMed] [Google Scholar]
- Mann CC. 1493: Uncovering the new world Columbus created. New York: Alfred A. Knopf; 2013. [Google Scholar]
- Markham C. The conquest of New Granada. New York: EP Dutton and Company; 1912. [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishishwar L, Conley AB, Vidakovic B, Jordan IK. A combined evidence Bayesian method for human ancestry inference applied to Afro-Colombians. Gene. 2015a;574(2):345–51. doi: 10.1016/j.gene.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Rishishwar L, Conley AB, Wigington CH, Wang L, Valderrama-Aguirre A, Jordan IK. Ancestry, admixture and fitness in Colombian genomes. Sci Rep. 2015b;5:12376. doi: 10.1038/srep12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Linares A, Adhikari K, Acuna-Alonzo V, Quinto-Sánchez M, Jaramillo C, Arias W, et al. Admixture in Latin America: geographic structure, phenotypic diversity and self-perception of ancestry based on 7,342 individuals. PLoS Genet. 2014;10(9):e1004572. doi: 10.1371/journal.pgen.1004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RDC. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Wade P. Blackness and race mixture: the dynamics of racial identity in Colombia. Balitmore: JHU Press; 1995. [Google Scholar]
- Wang S, Ray N, Rojas W, Parra MV, Bedoya G, Gallo C, et al. Geographic patterns of genome admixture in Latin American Mestizos. PLoS Genet. 2008;4(3):e1000037. doi: 10.1371/journal.pgen.1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos FE, Habel JC. Biodiversity hotspots: distribution and protection of conservation priority areas. Vienna: Springer Science & Business Media; 2011. [Google Scholar]