Abstract
Objectives
To detail the development of ester-free thiol-ene dental resins with enhanced mechanical performance, limited potential for water uptake/leachables/degradation and low polymerization shrinkage stress.
Methods
Thiol-terminated oligomers were prepared via a thiol-Michael reaction and a bulky tetra-allyl monomer containing urethane linkages was synthesized. The experimental oligomers and/or monomers were photopolymerized using visible light activation. Several thiol-ene formulations were investigated and their performance ranked by comparisons of the thermo-mechanical properties, polymerization shrinkage stress, water sorption/solubility, and reactivity with respect to a control comprising a conventional BisGMA/TEGDMA dental resin.
Results
The ester-free thiol-ene formulations had significantly lower viscosities, water sorption and solubility than the BisGMA/TEGDMA control. Depending on the resin, the limiting functional conversions were equivalent to or greater than that of BisGMA/TEGDMA. At comparable conversions, lower shrinkage stress values were achieved by the thiol-ene systems. The polymerization shrinkage stress was dramatically reduced when the tetra-allyl monomer was used as the ene in ester-free thiol-ene mixtures. Although exhibiting lower Young’s modulus, flexural strength, and glass transition temperatures, the toughness values associated with thiol-ene resins were greater than that of the BisGMA/TEGDMA control. In addition, the thiol-ene polymerization resulted in highly uniform polymer networks as indicated by the narrow tan delta peak widths.
Significance
Employing the developed thiol-ene resins in dental composites will reduce shrinkage stress and moisture absorption and form tougher materials. Furthermore, their low viscosities are expected to enable higher loadings of functionalized micro/nano-scale filler particles relevant for practical dental systems.
Keywords: photopolymerization, dental resin, thiol-ene, shrinkage stress
1. Introduction
Thiol-ene reactions have many attributes identified as a part of the “click” reaction paradigm [1]. These include, among others, insensitivity to oxygen inhibition, high conversions coupled with fast kinetics, and mild reaction conditions [2–5]. These robust reactions are very attractive in many potential applications including surface modification, [6] biomaterials [5,7,8], polymer functionalization [9,10], photolithography [11,12], and nano- and micro-particle synthesis [13,14].
In the past decade, thiol-ene photopolymerization has gained significant attention as a candidate for dental restorative materials [15–18]. Still, despite their high efficiency and many desirable properties, methacrylate-based resins continue to dominate the dental restoratives market. A widely utilized dental resin consists of 2,2-bis[p-(3-methacryloxy-2-hydroxypropoxy)phenyl]propane (BisGMA) and triethylene glycol dimethacrylate (TEGDMA), which is present as a reactive diluent. BisGMA is an aromatic monomer with a rigid core structure that provides low monomer volatility and high polymer modulus. The high viscosity of BisGMA conveys several positive attributes but limits the amount of filler loading as well as the ultimate conversion. As a result, TEGDMA is incorporated as a reactive diluent, which at higher concentrations negatively affects shrinkage and the mechanical performance. Another disadvantage of using these monomers is the significant water sorption, and in consequence the potential for hydrolytic or enzymatic degradation, in particular of the esters. Degradation of monomers in conventional dimethacrylate-based dental restorative materials has been indicated in several adverse phenomena that result in reduced service lifetimes [19–26]. Free-radical photopolymerization of dimethacrylates provides very rapid, on-demand curing under mild conditions. However, this polymerization exhibits a chain-growth propagation mechanism and is accompanied by substantial volume shrinkage and the associated shrinkage stress development that may cause clinical failure due to disruption of the bonded interface or deformation of the surrounding tooth structure [27–29].
Several studies have investigated the feasibility of formulating novel restorative materials that utilize the thiol-ene photopolymerization reactions [15–18]. Due to the step-growth evolution of the network, the thiol and ene monomers are quickly consumed while low molecular weight species dominate in the early stages of the polymerization. This mechanism causes a delayed gel point conversion and lower polymerization induced shrinkage and shrinkage stress as opposed to chain-growth homopolymerizations. These previous studies have used primarily commercially available multifunctional thiols, mainly pentaerythritol tetramercaptopropionate (PETMP). The photopolymerization of PETMP and triallyl–1,3,5–triazine–2,4,6–(1H, 3H, 5H)–trione (TTT) was shown to occur at a much higher rate than BisGMA/TEGDMA cured under the same conditions. Most importantly, the maximum shrinkage stress achieved with the thiol-ene system was only 14% of the maximum shrinkage stress of the dimethacrylate system [16]. This result motivated studies on alternative dental resin compositions such as oligomeric thiol-ene or ternary thiol-ene-methacrylate systems [17,18].
Recently we demonstrated that by eliminating the ester moieties from thiol monomers, non-degradable thiol-ene polymeric materials are effectively created with improved thermal and mechanical behavior. These materials also demonstrated extraordinary stability in acidic and basic solutions [30]. Based on another study, we found that introducing a vinyl sulfone group as an ene moiety significantly enhanced the mechanical properties of the material [31]. This array of approaches to improving mechanical and degradation properties was the motivation for the development of ester-free thiol-ene dental resins.
In this paper, we present ester-free thiol-ene resins with enhanced properties that will meet the strict requirements for dental materials, such as high functional group conversions, good mechanical behavior, low shrinkage and stress, and also with the unique benefit of the complete elimination of hydrolytically or enzymatically reactive chemical moieties. Thiol-functionalized oligomers were formed via thiol-Michael addition reactions using the synthesized tetra(2-mercaptoethyl)silane (SiTSH) and divinyl sulfone (DVS) monomers. Then, the resulting oligomers were photopolymerized with equimolar ene groups of trimethylpropane diallyl ether (TTT). In the second approach, monomeric thiol-ene resin mixtures were formed by using SiTSH, TTT, and a synthesized bulky tetra-allyl monomer containing urethane linkages. Polymerization induced shrinkage stress, reaction kinetics, mechanical properties, water sorption and solubility were evaluated for both the oligomeric and monomeric thiol-ene systems. Their performance was compared with a conventional BisGMA/TEGDMA dental resin as a control.
2. Materials and methods
2.1. Materials
Triethylamine (TEA), TTT, trimethylolpropane diallyl ether (DEA), tetravinylsilane, propylamine, thioacetic acid, and hexamethylene diisocyanate (HMDI) were purchased from Sigma-Aldrich. Divinyl sulfone (DVS) was purchased from Oakwood Chemicals. Irgarcure 819 (bis-(2,4,6-trimethylbenzoyl)-phenylphosphine oxide-BPO) was obtained from BASF. BisGMA/TEGDMA Solution Lot: 795-07 was purchased from ESSTECH. All chemicals were used as received. SiTSH and 1,3,5-tris-(3-mercaptopropyl)-1,3,5-triazine-2,4,6-trione (TTTSH) were synthesized according to a previously reported procedure [30,32,33]. The tetra-allyl monomer (TENE) was synthesized upon treatment of an alcohol with an isocyanate to form urethane linkages [34]. The structures of the thiol and vinyl monomers are depicted in Fig. 1.
Figure 1.
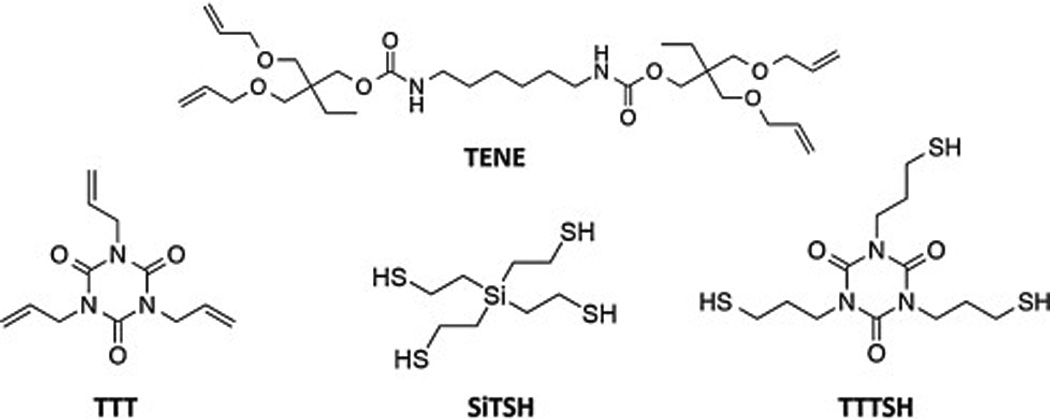
Thiol and allylic monomers utilized in this study.
2.2. Monomer Synthesis
2.2.1. Synthesis of SiTSH
Thioacetic acid (28.8 g) was added slowly to a flask with tetravinyl silane (10.0 g). The flask was kept in an ice-bath. Azobisisobutylronitrile (AIBN) (0.776 g) was used as a radical-generating thermal initiator. The solution was stirred at 65°C for 36 h. After this first synthesis step was completed, thioacetic acid was distilled under vacuum at 65°C. Without performing any purification, methanol (50 mL) and hydrochloric acid (20 mL) were added to a flask with the crude product from the thiol-ene reaction step. The solution was stirred at 60°C for 12 h. After the second step was completed, the solvent was distilled and the product washed twice with 5 wt.% sodium bicarbonate solution. Finally, the crude product was purified by column chromatography (silica gel, dichloromethane (DCM)/hexane 1:2) to give a colorless liquid.
2.2.2. Synthesis of TTTSH
To a flask with TTT (10.0 g), thioacetic acid (14.4 g) was added slowly in an ice-bath. After addition of AIBN (0.776 g), the solution was stirred at 65°C for 36 h. After the reaction was completed, thioacetic acid was distilled under vacuum at 65°C. No further purification was performed on the crude product. Methanol (50 mL) and hydrochloric acid (20 mL) were added to a flask with the crude product from the first step. The solution was stirred at 60°C for 12 h. After hydrolysis was complete, the solvent was distilled and the crude product was washed twice with 5 wt.% sodium bicarbonate solution. Finally, the crude product was purified by column chromatography (silica gel, ethyl acetate/hexane gradient from 0:1 to 4:1) to give a colorless viscous liquid.
2.2.3. Synthesis of TENE
The monomer was prepared by reacting HMDI with DAE in a NCO/OH = 1/1 stoichiometric ratio. The addition was carried out in a 100 ml round-bottom flask equipped with a magnetic stirrer and nitrogen gas inlet at ambient temperature for 24 h. The isocyanate was added dropwise to DEA containing one/two drops of dibutyltin dilaurate as a catalyst. The reaction was considered quantitative when the NCO IR signal at 2250 cm−1 disappeared completely. Then, the monomer was dissolved in DCM and washed through a silica plug. After solvent removal under vacuum, the monomer was used without any further purification.
2.3. Oligomer Preparation
The gel point conversion of step-growth polymerizations is determined using the following equation [35]:
where fA and fB are the functionalities of the two monomers and r is the molar ratio of monomers present such that it is always less than or equal to 1.0. Monomers with a stoichiometric imbalance for which alpha is less that one will form a crosslinked polymer when reacted via a step-growth mechanism. Theoretically, to form non-gelled oligomers at full conversion, the gel point conversion (i.e., alpha) has to be greater than one. Hence, by reacting thiol and vinyl sulfone monomers with a sufficient excess of a thiol monomer will produce thiol-terminated oligomers with full incorporation of sulfone groups within the oligomer structure. More specifically, a 3:1 and 4:1 molar ratio of thiol-to-ene functional groups in the trithiol:divinyl sulfone and tetrathiol: divinyl sulfone mixtures, respectively, were used to form the oligomers. These oligomers were synthesized via thiol-Michael addition in the presence of a solvent (DCM). After forming a homogenous mixture of the comonomers, 1 wt% TEA was added to initiate the thiol-Michael addition reaction, which proceeds exclusively via a step-growth mechanism. The resulting multifunctional thiol-terminated oligomers were used for further kinetic and mechanical evaluation in thiol-ene reactions with TTT.
2.5. Fourier transform infrared spectroscopy (FT-IR)
The real-time conversion during photopolymerization was measured via Fourier Transform Infrared Spectroscopy (Nicolet 6700 FT-IR) combined with a vertical light cable. The mixtures were cured in the FT-IR chamber using irradiation intensities of 30 mW cm−2 or 50 mW cm−2 (400–500 nm filter was used) at the surface of the sample. The visible light intensity was monitored by a radiometer (model IL1400A equipped with a GaAsP detector and a quartz diffuser). Near-IR was utilized to evaluate the functional group conversions during curing of 1 mm thick samples sandwiched between glass slides separated by appropriate spacers. More specifically, the first C=C overtone signal at 6160 cm−1 was monitored during the FT-IR measurements.
2.6. Shrinkage stress measurement
Simultaneous conversion and polymerization shrinkage stress measurements were conducted using a tensometer (American Dental Association Health Foundation), which measures polymerization-induced stress using cantilever beam deflection theory. The sample is clamped between two silanized quarts rods. The tensile force generated by the bonded shrinking sample causes the cantilever beam to deflect. The deflection of the beam is measured with a linear variable differential transformer (LVDT) and translated into the appropriate force based on the calibrated beam constant. To calculate the appropriate stress, the force corresponding to the beam deflection is divided by the cross-sectional sample area. Simultaneous conversion measurements are facilitated using near-IR (as described before) transmitted through the polymer sample via fiber optic cables. A more detailed description of the tensometer and conversion measurement can be found elsewhere [36].
2.7. Water sorption/solubility test
Water sorption tests were performed using disc-shaped specimens of 1mm in thickness and 15 mm in diameter. The curing was performed in an IR chamber according to the methodology described above. The samples were dried in an oven at 37°C to constant initial mass (mi). The thickness and diameter of each sample film were measured with calipers at 4–5 points, and the initial volume (Vi) was calculated. The samples were then placed in distilled water at 37°C. At regular time intervals (~12 h), the samples were removed from the water and with paper towel the excess water was blotted away. After their mass was recorded, the samples were returned to water conditioning. The measurements were repeated until there was no significant change in mass. This final mass is referred to as the equilibrium saturation mass (ms). After determining the equilibrium saturation mass, the dimensions of each sample were measured again and the saturation volume was calculated (Vs). Finally the samples were dried in an oven at 37°C for 24-hour cycles until the measured mass remained constant. After determining the desorption mass (md), the thickness of each sample was measured with calipers at 4–5 points, and the desorption volume (Vd) was calculated. The equilibrium solubility limit, s, and the equilibrium water sorption, w, were calculated according to the following equations [37,38]:
2.8. Dynamic mechanical analysis
A DMA Q800 (TA instruments) was utilized for dynamic mechanical analysis (DMA). Sample specimens with 1 × 4 x 10 mm rectangular dimensions were tested in multifrequency strain mode by applying a sinusoidal stress of 1 Hz frequency with the temperature ramping at 3°C min−1. The glass transition temperature was determined as the maximum of the tan delta curve.
2.9. Flexural tests and conversion analysis
Mechanical properties (Flexural strength, Young’s Modulus, Toughness, etc.) were measured via a three-point bending test (MTS 858 Mini Bionix II). The sample dimensions were 2 mm x 2 mm x 10 mm. The samples were irradiated on one side for 5 minutes and immediately inverted and irradiated under similar conditions from the opposite side. The light intensity used was 50 mW/cm2 with wavelength range from 400–500 nm as selected by wavelength selective filters.
2.10. Viscosity measurement
The resin viscosities were measured via a TA instruments ARES rheometer. The resins were placed between 20 mm quartz plates with a gap spacing of 0.4 mm. All resins were Newtonian fluids, and the reported viscosity values were recorded at a shearing rate of 63 1/s.
2.11. Statistical analysis
One-way analysis of variance (ANOVA) was used for the statistical analysis of the experimental results. All experiments used these n-number of repetitions: FT-IR (n=5), shrinkage stress (n=3), viscosity (n=3), flexural modulus and strength testing (n=5), water sorption and solubility (n=5), and DMA (n=3). Furthermore, multiple pair-wise comparisons were conducted using Tukey’s test with a significance level of 0.05.
3. Results and discussion
Several oligomeric and monomeric thiol-ene mixtures were prepared to assess the resin polymerization kinetics upon photocuring, the resulting mechanical properties of the crosslinked materials formed, polymerization induced shrinkage stress, and water sorption/solubility. The composition and description of the oligomeric resins used is shown in Table 1, and the monomer structures are presented in Figure 1. Basically, the thiols were pre-reacted with DVS by means of the base-catalyzed thiol-Michael addition reaction, and then the thiol excess was compensated for by adding the triallyl monomer to obtain a final, stoichiometric resin. The control dimethacrylate mixture was composed of BisGMA and TEGDMA monomers at a ratio of 70 to 30 wt %. As depicted in Table 1, the varied degree of oligomerization resulted in thiol-ene mixtures with different viscosities. Although in two of the four thiol-ene resins, the viscosities were of the same order as that of the control, the viscosity values were found to be statistically different.
Table 1.
Composition of BisGMA/TEGDMA and thiol-ene oligomeric mixtures and their viscosity values. Standard deviation (SD) is given in parentheses. Values followed by the same letter in the column are not significantly different.
| Resin | Composition | Viscosity (Pa · s) |
|---|---|---|
| BisGMA/TEGDMA | Mixture of BisGMA and TEGDMA at a 70/30 wt% ratio. | 1.47 (0.01)A |
| TEO 01 | Thiol-Michael oligomer formed by 3 mol TTTSH and 1 mol DVS was photopolymerized with 2.33 mol TTT. | 31.1 (0.2)B |
| TEO 02 | Thiol-Michael oligomer formed by 4 mol SiTSH and 1 mol DVS was photopolymerized with 4.67 mol TTT. | 0.33 (0.01)C |
| TEO 03 | Thiol-Michael Oligomer formed by 3 mol SiTSH, 2 mol TTTSH and 2 mol DVS was photopolymerized with 4.67 mol TTT. | 4.9 (0.2)D |
| TEO 04 | Thiol-Michael Oligomer formed by 6 mol SiTSH, 3 mol TTTSH, and 3 mol DVS was photopolymerized with 9 mol TTT. | 2.09 (0.02)E |
In Figure 2 the plots of conversion vs. time and shrinkage stress vs. conversion are depicted for the various oligomeric resins. The ultimate values of conversion and shrinkage stress are also summarized in Table 2. In general, radically initiated thiol-ene reactions are a very rapid processes. In the present study, the reaction rates of the oligomeric thiol-ene mixtures are significantly higher than for the BisGMA/TEGDMA control (Fig. 2a). It is presumed that the increase in the reaction rate observed in oligomeric mixtures originates from increased functionality due to the presence of the thiol-Michael oligomers. One possible cause could be the intra/inter-molecular hydrogen bonding interactions between the oxygen in the sulfone groups and the hydrogen of the thiol groups, which can weaken the bond between the sulfur and hydrogen, thus facilitating the thiol-ene chain-transfer (hydrogen-abstraction) step. Supportive of this hypothesis is the broadening and shifting of the thiol peak in the infrared spectra towards shorter wavelengths caused by the sulfone presence in oligomeric thiols as compared with the sulfone-free thiol monomer (see supporting information Fig. S1).
Figure 2.
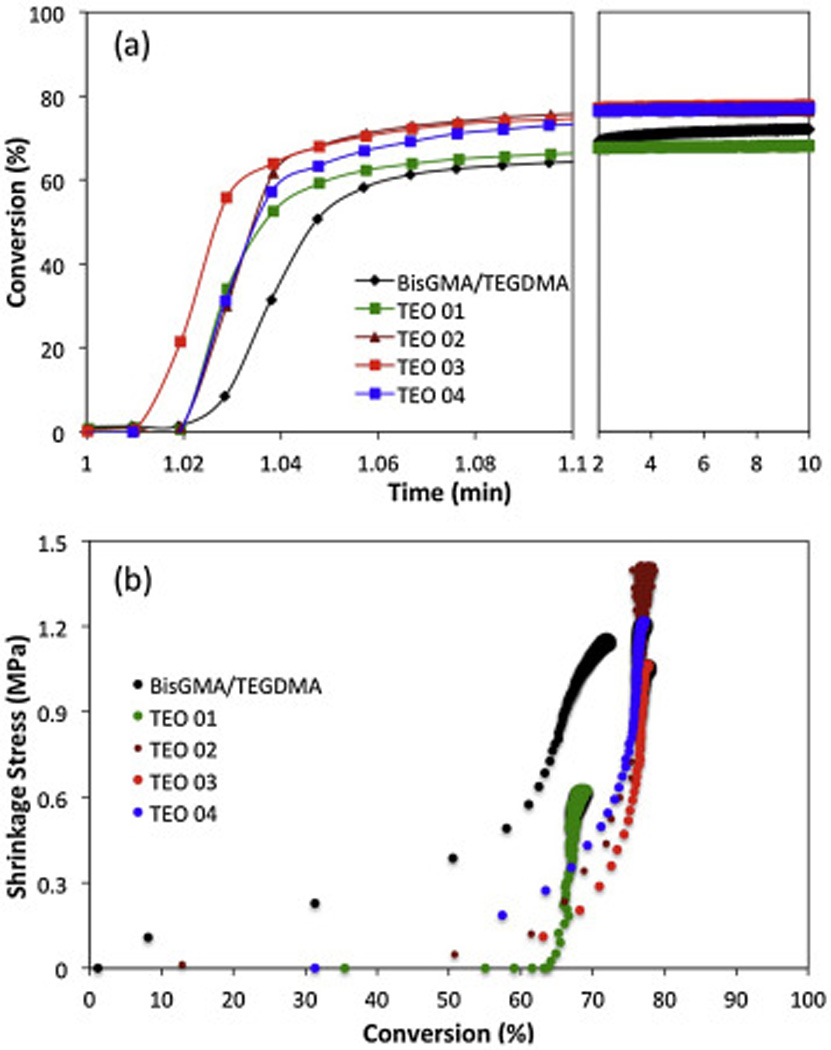
(A) FT-IR kinetic profiles for oligomeric thiol-ene and BisGMA/TEGDMA mixtures cured with 1 wt% IR 819 and 50 mW/cm2 of 400–500 nm wavelength at ambient conditions. (B) Polymerization induced shrinkage stress vs. double bond conversion for thiol-ene and BisGMA/TEGDMA mixtures cured with 1wt% IR 819 and 30 mW/cm2 of 400–500 nm wavelength at ambient conditions.
Table 2.
The final shrinkage stress values with the respective conversion values for the BisGMA/TEGDMA and oligomeric thiol-ene mixtures (SD in parentheses). Values followed by the same letter in the same row are not significantly different.
| Samples | BisGMA/ TEGDMA |
TEO 01 | TEO 02 | TEO 03 | TEO 04 |
|---|---|---|---|---|---|
| Final Conversion (%) | 70 (1)B | 68 (1)C | 77 (1)A | 78 (1)A | 78 (1)A |
| Final Shrinkage Stress (MPa) | 1.16 (0.03)B | 0.48 (0.14)C | 1.47 (0.10)A | 1.02 (0.04)B | 1.24 (0.03)A,B |
Further, it is evident from Fig. 2B, that at fixed functional group conversions, the thiol-ene polymerizations are, with no exceptions, associated with lower shrinkage stress due to the delayed gel point conversions when compared to the dimethacrylate polymerization.
However, as shown in Table 2, the oligomeric thiol-ene systems achieve in most cases comparable level of ultimate shrinkage stress as the BisGMA/TEGDMA control, albeit at higher conversions. The formation of a thiol-ene network with high elastic modulus, functional conversion, and fast reaction kinetics can lead to similar shrinkage stress. Although delayed gelation supports the previous literature reports [15–18] on stress development in thiol-ene vs. dimethacrylate resins, the examples at hand reveal the important balance that has to be considered between elastic modulus (and stress) development and conversion in highly converted thiol-ene glasses.
Conventional dimethacrylate-based dental materials have a high ester content. Esters are known to degrade through hydrolysis catalyzed by acids or bases as well as various enzymatic cleavage processes. Also, by having affinity to water and being able to associate with water through hydrogen bonding, ester-containing networks usually swell quite considerably [30]. As shown in Table 3, the BisGMA/TEGDMA polymer has a significantly higher degree of water sorption and solubility compared to any of the thiol-ene systems tested. The thiol-ene polymers swell two (or more) times less in water than the BisGMA/TEGDMA control polymer.
Table 3.
The results from water sorption tests of BisGMA/TEGDMA and oligomeric thiol-ene systems. Standard deviations in parentheses. Values followed by the same letter in the same row are not significantly different.
| Samples | BisGMA/ TEGDMA |
TEO 01 | TEO 02 | TEO 03 | TEO 04 |
|---|---|---|---|---|---|
| Solubility (µg/mm3) | 7.0 (2.3)A | 3.9 (1.1)B | 0.8 (0.9)C | 2.1 (0.4)B | 2.8 (1.1)B |
| Sorption (µg/mm3) | 39 (2)A | 16 (1)B | 11 (4)C | 16 (1)B | 11 (1)C |
Therefore, this study points out another important benefit of using ester-free thiol-ene networks that significantly reduces moisture absorption. The swelling in water (like in any other solvent) causes a decrease in mechanical performance by lowering the modulus and strength, and when some of the network crosslinks degrade, it affects the crosslinking density and further weakens the overall network mechanics.
By properly designing oligomeric thiol-ene systems, our approach was to improve the mechanical properties through elimination of the flexible ester-containing moieties and simultaneously introducing secondary interactions via sulfone groups as an auxiliary reinforcement mechanism to counteract the lower covalent crosslink density. Moreover, reactive oligomers offer an alternative solution to the polymerization shrinkage problem owed to a decrease in the initial reactive group concentration [17]. The rigid backbone structure of the BisGMA monomer enhances the mechanical properties of BisGMA/TEGDMA leading to high Young’s modulus and flexural strength. However, the brittleness of this material caused by its heterogeneous nature and high crosslink density results in lower toughness than what is observed in oligomeric thiol-ene systems. Relevant thermo-mechanical properties of the oligomeric thiol-ene polymer networks are summarized in Table 4. Importantly, all thiol-ene mixtures resulted in tough glasses when polymerized at ambient conditions. Even though the initial values of strength and modulus are lower for thiol-ene materials we predict that these values would improve in glass filled composites, and above all after conditioning in a humid environment.
Table 4.
Mechanical properties for the oligomeric thiol-ene and BisGMA/TEGDMA samples cured with 1 wt% IR 819, and 50 mW/cm2 of 400–500 nm wavelength at ambient conditions. Standard deviations are given in parentheses. Values followed by the same letter in the same column are not significantly different.
| Sample | Toughness (J·m−3·104) |
Young’s Modulus (GPa) |
Flexural Strength (MPa) |
Tg (°C) |
|---|---|---|---|---|
| BisGMA/TEGDMA | 288 (129)A | 4.3 (0.2)A | 135 (22)A | 134 (1)A |
| TEO 01 | 2566 (173)B | 3.4 (0.1)D | 110 (4)B | 73 (5)B |
| TEO 02 | 690 (102)C | 2.8 (0.1)B,C | 99 (1)B | 89 (2)C |
| TEO 03 | 470 (50)A,C | 2.6 (0.3)B | 92 (10)B | 79 (4)B,C |
| TEO 04 | 512 (10)C | 3.1 (0.1)C | 104 (3)B | 81 (4)B,C |
Further, the ultimate Tg values at the post-cured limiting conversion of the oligomeric thiol-ene systems are lower than the BisGMA/TEGDMA system but significantly above that of body temperature. However, these values do not represent the Tg values that will be achieved under clinically restricted photocuring conditions. The Tg achieved at high conversion at ambient temperature within the thiol-ene materials may be equal to the Tg achieved by BisGMA/TEGDMA under the same conditions even though the post-cured Tg values differ considerably. Due to the chain-growth polymerization, the BisGMA/TEGDMA copolymer has a very broad glass transition range. DMA results are detailed in the, supporting information Fig. S2. In addition, we expect the mechanical properties of the BisGMA/TEGDMA network to deteriorate with time due to its high water sorption/solubility and hydrolytic degradation. Water induced plasticization is known to be a practical concern for mechanical property decline even without network hydrolysis.
Despite the benefits of ester-free thiol-ene resins pointed out above, such as rapid kinetics, high elastic modulus and low moisture absorption, there are still obvious drawbacks that have to be addressed. These include prominent shrinkage stress (though generated at higher conversions) compared to the relevant data of the BisGMA/TEGDMA control. To address this issue we adopted a strategy that had already been proven to work well for dimethacrylate polymerizations, i.e. reduction in the functional group density in the initial resin. In order to implement such an approach, we synthesized a high molecular weight urethane-based tetra-allyl monomer. This monomer introduces urethane moieties, and its backbone is readily modified with alternative chemical structures (aliphatic, cycloaliphatic or aromatic moieties) to tune the final material properties. The modified thiol-ene formulations that were investigated here included SiTSH, TTT, and TENE whose amount was increased incrementally from 10 to 45 wt% to test the effects of its presence on the mechanical performance of the final polymers. Thus, the second series of thiol-ene materials that were tested was based on neat monomeric (thiol and ene) mixtures. The description of the monomeric resins used is shown in Table 5.
Table 5.
Composition of thiol-ene monomeric mixtures and their viscosity values. Standard deviations are in parentheses. Values followed by the same letter in the same column are not significantly different.
| Resin | Composition | Viscosity (Pa · s) |
|---|---|---|
| BisGMA/TEGDMA | Mixture of BisGMA and TEGDMA at a 70/30 wt% ratio respectively. | 1.50 (0.01)A |
| TER 01 | Stoichiometric mixture of SiTSH and TTT | 0.045 (0.010)B |
| TER 02 | Stoichiometric mixture of SiTSH, TTT, and 10 wt% TENE | 0.054 (0.005)B |
| TER 03 | Stoichiometric mixture of SiTSH, TTT, and 18 wt% TENE | − |
| TER 04 | Stoichiometric mixture of SiTSH, TTT, and 26 wt% TENE | 0.068 (0.004)C |
| TER 05 | Stoichiometric mixture of SiTSH, TTT, and 34 wt% TENE | 0.071 (0.002)C |
| TER 06 | Stoichiometric mixture of SiTSH, TTT, and 42 wt% TENE | − |
When compared with the oligomeric thiol-enes, the water sorption and solubility results for some exemplary monomeric TER systems (depicted in Table S2) follow the same trend, i.e. the water swelling is significantly reduced with regard to the dimethacrylate control.
An important difference manifests itself when comparing the kinetic profiles of the monomeric thiol-ene systems plotted in Figs. 2A and 3A. It becomes evident, that the neat monomer formulations are characterized by lower polymerization rates than the BisGMA/TEGDMA control and the oligomeric thiol-ene resins. As further depicted in Fig. 3 and Table S1 (supporting information), the addition of TENE reduced the final shrinkage stress without compromising the final conversion values. Contrary to thiol-ene resins formulated in oligomeric form, the TENE-containing systems are characterized by significantly lower ultimate shrinkage stress values comparing to BisGMA/TEGDMA (Fig. 3b).
Figure 3.
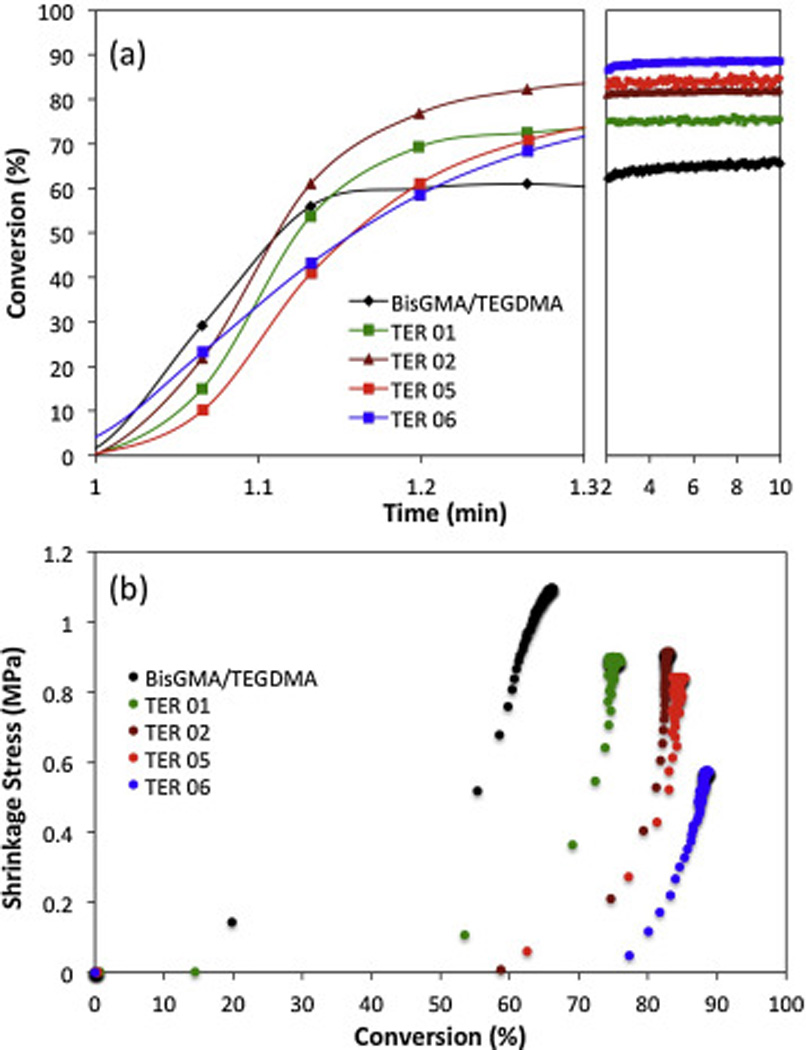
(A) FT-IR kinetic profiles for exemplary thiol-ene and BisGMA/TEGDMA mixtures cured with 1 wt% IR 819 and 30 mW/cm2 of 400–500 nm wavelength at ambient conditions. (B) Polymerization induced shrinkage stress vs. double bond conversion for exemplary thiol-ene and BisGMA/TEGDMA mixtures cured with 1 wt% IR 819, 50 mW/cm2, and 400–500 nm wavelength at ambient conditions.
However, the improved functional group conversion and lower shrinkage stress results are accompanied by somewhat diminished mechanical performance. It can be seen from Fig. S3 (supporting information) that the increased amount of TENE in a thiol-ene composition corresponds with lower glass transition temperatures, as the Tg values decrease from 113°C (no TENE loading) to 45°C (42 wt % of TENE) with intermediate values achieved for intermediate compositions.
The thiol-ene data obtained from the DMA measurements (Fig. S2 and S3) points out the need for further resin optimization, with the right balance in monomer compositions still to be achieved to afford simultaneously excellent conversion, shrinkage stress and mechanical behavior.
So as to determine which set of thiol-ene materials would fit the requirements for dental applications better, it is necessary to assess their properties in glass filled composites. Some selected thiol-ene compositions, DVS-based and neat monomeric mixtures, are therefore assessed as resin matrices in filled composites as detailed in a subsequent report [39].
4. Conclusion
The need to restore teeth will always exist because of time-dependent failure or degradation of conventional restorative materials. Scientific reports will continue to bring valuable information necessary for the development of ideal restorative dental materials which would be biocompatible; bind permanently to the tooth structure or bone; be esthetically appealing; and exhibit properties similar to those of tooth enamel. Herein, based on a comprehensive structure-property study, we introduced potential dental resins based on thiol-ene step-growth reactions devoid of ester moieties. The oligomeric mixtures presented possess enhanced mechanical properties, faster reaction kinetics, reduced thiol odor, and high functional conversion. On the other hand, the monomeric thiol-ene systems tested present a viable way to achieve higher functional conversion and lower shrinkage stress than the conventional dimethacrylate-based dental material. In addition, all the formulations eliminate degradable moieties and reduce the extent of any potentially toxic elutable materials. Also, resins with low viscosities will likely allow for higher filler loadings in practical dental composite systems.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the National Institutes of Health for support of this research through the 1U01DE023777-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chemie (International Ed) 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Hoyle CE, Lowe AB, Bowman CN. Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chem Soc Rev. 2010;39:1355–1387. doi: 10.1039/b901979k. [DOI] [PubMed] [Google Scholar]
- 3.Hoyle CE, Lee TY, Roper T. Thiol-enes: Chemistry of the past with promise for the future. J Polym Sci Part A Polym Chem. 2004;42:5301–5338. [Google Scholar]
- 4.Hoyle CE, Bowman CN. Thiol-ene click chemistry. Angew Chemie (International Ed) 2010;49:1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 5.Kade MJ, Burke DJ, Hawker CJ. The Power of Thiol-ene Chemistry. J Polym Sci Part A Polym Chem. 2010;48:743–750. doi: 10.1002/pola.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harant AW, Khire VS, Thibodaux MS, Bowman CN. Thiol-ene photopolymer grafts on functionalized glass and silicon surfaces. Macromolecules. 2006;39:1461–1466. [Google Scholar]
- 7.Gupta N, Lin BF, Campos LM, Dimitriou MD, Hikita ST, Treat ND, et al. A versatile approach to high-throughput microarrays using thiol-ene chemistry. Nat Chem. 2010;2:138–145. doi: 10.1038/nchem.478. [DOI] [PubMed] [Google Scholar]
- 8.Schreck KM, Leung D, Bowman CN. Hybrid organic/inorganic thiol-ene-based photopolymerized networks. Macromolecules. 2011;44:7520–7529. doi: 10.1021/ma201695x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo SPS, Stamenovic MM, Prasath RA, Inglis AJ, Prez FEDu, Barner-Kowollik C, et al. Limitations of radical thiol-ene reactions for polymer-polymer conjugation. J Polym Sci Part A Polymer Chem. 2010;48:1699–1713. [Google Scholar]
- 10.Connal LA, Kinnane CR, Zelikin AN, Caruso F, Am FJ. Stabilization and Functionalization of Polymer Multilayers and Capsules via Thiol - Ene Click Chemistry polymer multilayered thin films and capsules. Chem Mater. 2009;21:576–578. [Google Scholar]
- 11.Polizzotti BD, Fairbanks BD, Anseth KS. Three-dimensional biochemical patterning of click-based composite hydrogels via thiolene photopolymerization. Biomacromolecules. 2008;9:1084–1087. doi: 10.1021/bm7012636. [DOI] [PubMed] [Google Scholar]
- 12.Besson E, Gue AM, Sudor J, Korri-Youssoufi H, Jaffrezic N, Tardy J. A novel and simplified procedure for patterning hydrophobic and hydrophilic SAMs for microfluidic devices by using UV photolithography. Langmuir. 2006;22:8346–8352. doi: 10.1021/la053303l. [DOI] [PubMed] [Google Scholar]
- 13.Amato DV, Amato DN, Flynt aS, Patton DL. Functional, sub-100 nm polymer nanoparticles via thiol–ene miniemulsion photopolymerization. Polym Chem. 2015 doi: 10.1039/C4PY01449A. [Google Scholar]
- 14.Wang SJ, Meng YZ, Hlil AR, Hay AS. Synthesis and Characterization of Phthalazinone Containing Poly(arylene ether)s, Poly(arylene thioether)s, and Poly(arylene sulfone)s via a Novel N – C Coupling Reaction. Macromolecules. 2004;37:60–65. [Google Scholar]
- 15.Reinelt S, Tabatabai M, Moszner N, Fischer UK, Utterodt A, Ritter H. Synthesis and photopolymerization of thiol-modified triazine-based monomers and oligomers for the use in thiol-ene-based dental composites. Macromol Chem Phys. 2014;215:1415–1425. [Google Scholar]
- 16.Lu H, Carioscia JA, Stansbury JW, Bowman CN. Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dent Mater. 2005;21:1129–1136. doi: 10.1016/j.dental.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Carioscia JA, Lu H, Stanbury JW, Bowman CN. Thiol-ene oligomers as dental restorative materials. Dent Mater. 2005;21:1137–1143. doi: 10.1016/j.dental.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Beigi S, Yeganeh H, Atai M. Evaluation of fracture toughness and mechanical properties of ternary thiol-ene-methacrylate systems as resin matrix for dental restorative composites. Dent Mater. 2013;29:777–787. doi: 10.1016/j.dental.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Sideridou I, Tserki V, Papanastasiou G. Study of water sorption , solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials. 2003;24:655–665. doi: 10.1016/s0142-9612(02)00380-0. [DOI] [PubMed] [Google Scholar]
- 20.Craig RG, O’ Brien W, Power J. Dental materials: properties and manipulations. 5th ed. St. Louis, MO: 1992. [Google Scholar]
- 21.Anseth KS, Newman SM, Bowman CN. Polymeric dental composites: Properties and reaction behavior of multimethacrylate dental restorations. Adv Polym Sci. 1995;122:177–217. [Google Scholar]
- 22.Moszner N, Salz U. New developments of polymeric dental composites. Prog Polym Sci. 2001;26:535–576. [Google Scholar]
- 23.Stansbury JW. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent Mater. 2012;28:13–22. doi: 10.1016/j.dental.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res. 2011;90:402–416. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charton C, Falk V, Marchal P, Pla F, Colon P. Influence of Tg, viscosity and chemical structure of monomers on shrinkage stress in light-cured dimethacrylate-based dental resins. Dent Mater. 2007;23:1447–1459. doi: 10.1016/j.dental.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Asmussen E, Peutzfeldt A. Influence of UEDMA , BisGMA and TEGDMA on selected mechanical properties of experimental resin composites. Dent Mater. 1998;14:51–56. doi: 10.1016/s0109-5641(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 27.Kleverlaan CJ, Feilzer AJ. Polymerization shrinkage and contraction stress of dental resin composites. Dent Mater. 2005;21:1150–1157. doi: 10.1016/j.dental.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Braga RR, Ferracane JL. Contraction Stress Related to Degree of Conversion and Reaction Kinetics. J Dent Res. 2002;81:114–118. [PubMed] [Google Scholar]
- 29.Braga RR, Ballester RY, Ferracane JL. Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dent Mater. 2005;21:962–9670. doi: 10.1016/j.dental.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Podgórski M, Becka E, Chatani S, Claudino M, Bowman CN. Ester-free thiol-X resins: new materials with enhanced mechanical behavior and solvent resistance. Polym Chem. 2015;6:2234–2240. doi: 10.1039/C4PY01552E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Podgórski M, Chatani S, Bowman CN. Development of Glassy Step-Growth Thiol- Vinyl Sulfone Polymer Networks. Macromol Rapid Commun. 2014;35:1497–1502. doi: 10.1002/marc.201400260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Podgórski M, Nair DP, Chatani S, Berg G, Bowman CN. Programmable mechanically assisted geometric deformations of glassy two-stage reactive polymeric materials. ACS Appl Mater Interfaces. 2014;6:6111–6119. doi: 10.1021/am405371r. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki Y, Liu J, Nakamura Y, Shibasaki Y, Ando S, Ueda M. Synthesis of Highly Refractive and Transparent Polyimides Derived from 4,4′-[p-Sulfonylbis(phenylenesulfanyl)]diphthalic Anhydride and Various Sulfur-containing Aromatic Diamines. Polym J. 2008;40:414–420. [Google Scholar]
- 34.Senyurt AF, Hoyle CE, Wei H, Piland SG, Gould TE. Thermal and mechanical properties of cross-linked photopolymers based on multifunctional thiol-urethane ene monomers. Macromolecules. 2007;40:3174–3182. [Google Scholar]
- 35.Odian G. 4th ed. New York: Wiley-Interscience; 2004. [Google Scholar]
- 36.Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Probing the origins and control of shrinkage stress in dental resin-composites: I. Shrinkage stress characterization technique. J Mater Sci Mater Med. 2004;15:1097–1103. doi: 10.1023/B:JMSM.0000046391.07274.e6. [DOI] [PubMed] [Google Scholar]
- 37.Sideridou I, Achilias DS, Spyroudi C, Karabela M. Water sorption characteristics of light-cured dental resins and composites based on Bis-EMA/PCDMA. Biomaterials. 2004;25:367–376. doi: 10.1016/s0142-9612(03)00529-5. [DOI] [PubMed] [Google Scholar]
- 38.McCabe JF, Rusby S. Water absorption, dimensional change and radial pressure in resin matrix dental restorative materials. Biomaterials. 2004;25:4001–4007. doi: 10.1016/j.biomaterials.2003.10.088. [DOI] [PubMed] [Google Scholar]
- 39.Podgórski M, Becka E, Claudino M, Shah P, Stansbury JWBC. Ester-free Thiol-ene Dental Restoratives Part B: Composite Development. Dent Mater. doi: 10.1016/j.dental.2015.08.147. n.d.:submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


