Abstract
Although increasingly global, data-driven genomics and other ‘omics’-focused research hold great promise for health discoveries, current research ethics review systems around the world challenge potential improvements in human health from such research. To overcome this challenge, we propose a ‘Safe Harbor Framework for International Ethics Equivalency’ that facilitates the harmonization of ethics review of specific types of data-driven international research projects while respecting globally transposable research ethics norms and principles. The Safe Harbor would consist in part of an agency supporting an International Federation for Ethics Review (IFER), formed by a voluntary compact among countries, granting agencies, philanthropies, institutions, and healthcare, patient advocacy, and research organizations. IFER would be both a central ethics review body, and also a forum for review and follow-up of policies concerning ethics norms for international research projects. It would be built on five principle elements: (1) registration, (2) compliance review, (3) recognition, (4) monitoring and enforcement, and (5) public participation. The Safe Harbor would create many benefits for researchers, countries, and the general public, and may eventually have application beyond (gen)omics to other areas of biomedical research that increasingly engage in secondary use of data and present only negligible risks.
Keywords: biomedical research, ethics, genomics, governance, harmonization, safe harbor
INTRODUCTION
We live in an age of global data and global research. The scale and intensity of researchers’ mobility and connectivity have reached an extraordinary level, contesting established ethical and legal boundaries between global and local research practice.1 In the health context, consider the explosive growth in biomedical research infrastructures—biobanks, genetic databases, and large genomics research consortia spanning multiple jurisdictions, such as the H3Africa Initiative,2 the 1000 Genomes Project,3 the International Cancer Genome Consortium,4 and the International Rare Diseases Research Consortium.5 Or consider the ever-growing volume of de-identified genomic sequence and clinical data being deposited into shared research databases such as the eMERGE Network,6 dbGaP,7 the European Bioinformatics Institute,8 or the DNA Data Bank of Japan.9 Regional and international organizations are working to foster broader biomedical research collaboration, as seen in the recent establishment of a European Research Infrastructure Consortium for the Biobanking and Biomolecular Resources Research Infrastructure,10 and the proposed Global Alliance for Genomics and Health (GA4GH) for sharing genomic and clinical data.11 Regulatory agencies are also supporting broader and more international collaboration, as seen in the US National Institutes of Health's (NIH) proposed genomic data sharing policy,12 the European Medicines Agency's current development of a policy on the proactive publication of clinical trial data,13 and the US Food and Drug Administration's proposal to make publicly available de-identified and masked non-summary safety and efficacy data derived from medical product applications.14
Both inside and outside the health research context, data now flows unconstrained in all directions.15 This is opening up cultural shifts in current research and clinical practice. For example, coupled with ever-increasing advances in digital technologies and user-friendly tools such as social networking sites, individuals can exercise greater control over their data and further engage with researchers in novel ways, such as becoming active partners in the research process. This new level of interaction may blur the classic distinction between researcher, research participant, and patient.16
What we are witnessing, then, are two main developments in biomedical research: first, increasing connectivity and mobility of data, researchers, and participants, and second, fundamental changes in the nature of biomedical research. Biomedical research is vastly more varied (not to mention infinitely more voluminous) than the classic, physically risky specific disease studies on ‘human subjects’ that gave rise to the ethics codes of the mid-to-late 20th century.17 Today, it is less built around small-scale, de novo, single-site or one-country interventional studies, and more built around large-scale studies on stored materials or data. Researchers inductively ‘trawl’ through data to find patterns,18 but also engage in massive aggregation and analysis of data and samples that were initially collected for one disease and are now being used to study another.19 This includes consolidating prospective or retrospective population cohorts and pooling datasets, such as current and legacy collections of health, lifestyle, and environmental data, to facilitate international, large-scale, collaborative, longitudinal, or remote analyses of samples to better understand complex disease etiology.20
Multiple causes explain this ‘new normal’ of biomedical research, but principally they include the shift from hard-copy medical files to electronic health or medical records; the growth of information technology such as cloud computing, e-health, and social networks; and the emergence of hypothesis-generating rather than hypothesis-testing research through Big Data and high performance search algorithms and programs that mine through data to find genotypic-phenotypic connections and similarities across patient and participant health profiles.
Various governments, research institutions, and private entities are recognizing that health is best advanced in a collaborative, socially distributed system.21 That is, when multiple experts, including patients and participants, are afforded the opportunity to contribute to research by sharing data and knowledge over a prolonged period wherever they are situated in the world, there is a greater likelihood for translating basic research into beneficial innovations. Public and private entities alike are advancing this new paradigm that embraces the borderless use and sharing of data. Indeed, society is coming to recognize that sharing data and knowledge carries strategic benefits for the improvement of our collective wellbeing.
In the field of human genetics, the aggregation of massive amounts of data is particularly compelling. The development of the breakthrough breast cancer drugs tamoxifen and trastuzumab, leukemia drug imatinib, colorectal cancer drugs cetuximab and panitumumbab, and cystic fibrosis drug ivacaftor;22 the discovery of the connection between a breakdown of DNA repair pathways and aberrant methlylation of the MGMT gene causing glioblastoma tumors to acquire resistance to standard therapies;23 ongoing discoveries of new genetic loci associated with breast and ovarian cancer risk;24 and the recent Collaborative Oncological Gene-environment Study,25 which collected genomic sequence data from over 200,000 people to identify 74 new susceptibility loci for breast, ovarian, and prostate cancer, are tangible examples of why large-scale studies that combine database information with samples are crucial to advancing health.
If this be the global age, what to make of the national and multinational governance structures that support it? Specifically, let us reflect on the state of research ethics review. In health, is the individualist-based,26 geographically-siloed27 model of ethics review the optimal paradigm for advancing knowledge, health, and wealth in our globally networked information society? Is legal regulation that is nationally coherent but internationally disharmonized an optimal model to ensure the ethical conduct of research and the advancement of knowledge?
If these reflections lead to doubts, then, taking a further step back, what principles of regulatory design should an ethics review system follow? In other words, to what extent should legal regulation be used as a tool to protect humans and simultaneously advance knowledge, as opposed to regulation by peers or some combination thereof?28 And what should one make of the political and regulatory principle of subsidiarity, which states that a central authority should perform only those tasks which cannot be performed effectively at a more immediate or local level,29 in the context of health research with humans?
We think that the legal regulation of ethics review, when perceived or implemented as the sole regulatory tool to use, presents intractable problems for 21st century health research. Science, technology, and research practice shift too frequently and operate on a much too globally connected level to fit securely within the ambit of national laws that take years to enact and years to replace. A subsidiarity principle-based analysis of the current ethics review system offers the opposite conclusion normally reached within the rubric of that principle, ie a justification for more local performance of tasks. Subsidiarity is premised on the lowest reasonable level to solve a problem, and here, ethics review performance effectiveness appears to be negatively correlated to geographic immediacy, leading us to question the value of jurisdiction-based and site-specific ethics committees. In sum, the potential for progress in human health is seriously challenged by a disharmonized Westphalian system that enchains laws, policies, and culture to political boundaries.30
The ethics review process is a prime example. The structure within which biomedical research, including large-scale ‘omics’31 research, is conducted remains stuck in a siloed, single-site paradigm with laws restricting global communication.32 In most countries today, research involving identifiable natural persons or information requires the informed consent of research participants and approval from local institutional review boards (IRBs) or their national equivalent33 at each project site. IRBs function for at least three main reasons: first, to ensure that risks and burdens to actual or potential research participants do not outweigh the importance of the research objective; second, to strike a reasonable balance between the risks and benefits for actual or potential research participants; and third (and related to the first two), to safeguard their dignity, rights, safety, and well-being.34 These roles remain primary, but the current structure of site-specific ethics review disproportionately burdens projects that post only negligible risks to participants and makes little sense in an era marked by the massive aggregation and analysis of data.35
Unfortunately, we are now sailing in a Sargasso Sea of entangled ethics review that impedes improvements in human health, chokes data flows, and paradoxically undermines respect for persons who want to participate or have participated in research.36 An ethics review process stuck in a 20th century paradigm that is single site-specific and that is designed for potentially physically harmful interventional clinical trials hinders the promises of globally and socially distributed data and health sciences research. It is time to shift the paradigm.
Anticipation and exploration of the ethical, legal, and social implications (ELSI) of scientific developments has been at the heart of genomics since the launch of the Human Genome Project almost a generation ago. However, much of this research has been conducted in an uncoordinated fashion, and often at a high level of abstraction. As genomics and biomedical research has become more globally intertwined, and research design challenges have become more pressing, ELSI research must adapt accordingly. The ‘ELSI 2.0’ Initiative was recently launched with the support of the Public Population Project in Genomics and Society (P3G)37 to enable ELSI to become ‘more coordinated, responsive to societal needs, and better able to apply the research knowledge it generates at the global level.’38 In the spirit of this ELSI 2.0 Initiative, we intend to accelerate the translation of ELSI research findings into globally applicable practice and policy. This Article launches the process.
In Section II, we highlight recent troubling research findings and case studies regarding the ethics review process for multi-site studies. In Section III, we translate these findings into a globally applicable but flexible policy proposal that advocates structural governance reform. Specifically, we propose a ‘Safe Harbor Framework for International Ethics Equivalency’ that facilitates the harmonization of ethics review of specific types of data-driven international research projects while respecting globally transposable research ethics norms and principles. We thereby exclude from our purview clinical trials with pharmaceutical products or devices. We outline criteria to create a uniform process of ethics review that can be applied across various premises to reduce quality variations; ensure consistency of end results; reduce the development of new initiatives; and facilitate comparison and the sharing of data and samples.39 In Section IV, we discuss and refute possible objections to the Safe Harbor. Section V concludes.
THE STORMY SEAS OF THE CURRENT ETHICS REVIEW SYSTEM
Studies across the international ELSI landscape illustrate that the current system for ethics review of multi-site research projects, particularly with respect to cross-organizational collaboration, is deficient.40 Three principal sources explain this deficiency.
Presumed Participant Vulnerabilities
The first principal source is the heavy focus on presumed participant vulnerabilities. With the rise of Western individualism41 and the mounting influence of the civil rights movement in the second half of the last century,42 ethics review has adopted a more unilateral approach that is arguably disproportionally fixated on individual risks.43 Doing so undervalues the cognitive capacities of citizens,44 communal concerns, or alternative bioethical lenses such as solidarity and citizenry,45 that view community as a unit of identity and worthy of ethical concern. In fact, community plays a central role in non-interventional research that could only be fueled by trust, a shared belief in the common good and the importance of the contribution of all stakeholders involved.46 Ethics review that overlooks these new realities can only end up bogging down an efficient, equitable (and arguably ethical) accomplishment of international multi-site studies.
Limited Ethics Review Systems and Genomic Sovereignty
The second principal source is the limited ethics review system in certain countries. Here, the deficiency lies not in a morass of red tape that thwarts cross-border data flows or knowledge exchanges, but rather, in a void of sufficient support structures and resources that facilitate studies of local populations and health issues, particularly those in the ‘omics’ field.47 An unfortunate consequence is that countries, if not whole regions, can be neglected from engagement with the international research community. Swathes of the world with acute health problems, particularly in sub-Saharan Africa, may be under-researched because approvals cannot be obtained on the ground. At the same time, researchers in other, more developed regions may fear ethical and legal repercussions from venturing into ethically unchartered territory, or believe that the perceived risks of delay outweigh the perceived benefits. Certain countries in the developing world have fueled these perceptions by regarding genetic information as a matter of ‘genomic sovereignty’ to be protected. For example, countries such as Brazil, India, and Mexico also have in place a second layer of regimes with centralized review or approvals for genomics research that make international participation (even when approved by local IRBs) impossible or impracticable.48
Governments and international organizations are working towards improving the situation. In the past decade, several international genomics research consortia such as the 1000 Genomes Project49 and MalariaGEN50 have conducted studies in previously neglected regions to study health problems, though crossing ethics review hurdles has not been easy. For the MalariaGEN Consortium in Africa, Asia, and Oceania, over twenty ethics committees in sixteen countries reviewed and approved the study, with review taking up to at least a year to complete at some partner sites; some committees required multiple rounds of correspondence to clarify the study design and rationale.51 In Africa, it remains to be seen whether the H3Africa Initiative can streamline its ethics review processes.
The World Health Organization's (WHO) Strategic Initiative for Developing Capacity in Ethical Review52 is making good progress in assisting countries to build ethics review policies. But research projects and organizations like the WHO can only do so much if the underlying global governance structure remains unchanged and unfocused on the power of data. The often-discussed ‘10/90 gap’ between worldwide expenditure on health research and the problems that primarily affect the poorest 90 per cent of the world's population53 seems to us partly attributable to ethics governance weaknesses, if not failure, in promoting consistent multi-site reviews, training, and experience to identify and analyze the key ethical issues in ‘omics’ research and global sharing of data.
Anachronistic Ethics Review Systems
The third source is more common: countries may have constructed a comprehensive ethics review infrastructure, but it is now ossified and overly politicized. Regarding the latter critique, IRBs are a growth industry,54 with expertise in demand and members increasingly becoming professionalized and embedded within institutional structures that value power and fiefdoms over community norm-making and transparency. Jurisdictional battles for control over specific areas of work are inherent in almost all professions once a profession has developed its core expertise,55 but for the emerging professional ‘human subjects regulator’ (as opposed to amateur community volunteer, or even bioethicist), negative externalities are stark.
IRBs exercise more frequent and intense regulatory scrutiny over researchers than regulatory oversight bodies do over other professionals, including physicians. Sharing data or providing clarity over the chain of command may be deprioritized for the sake of self-protection, stability, or institutional liability.56 Academic centers may be keener to keep data confidential (without a legal basis) so as to continue a steady stream of research funding, while hospitals may be keener to promote ‘in-house’ medical researchers than open the black box of decision-making and command structure. One result of this may be that IRBs are reluctant to publish their decisions (even if anonymized), share decisions with other IRBs, or defer to their colleagues in other institutions, leading one scholar to describe the process of IRB ethics review as ‘both insular and secretive.’57 Lacking transparent ‘jurisprudence’ or published procedural norms, researchers find it hard to determine whether, how, and to whom they should appeal an ethics review decision.58 And as Heimer and Petty remark, ‘Rather than protecting research subjects from harm, [IRBs] now seem especially focused on protecting universities and research centers.’59
The other critique focuses on ossification. Simply put, the structure in many countries is anachronistic, characterized by guidelines and laws that paternalistically protect participants, fail to meet conditions of legal legitimacy, and unduly impede data sharing. What we now have in many jurisdictions around the world is fragmentation, duplication, and confusion. It is understandable from the viewpoint of sovereignty and diversity that a country would design a locally tailored ethics review system, but in the international consortia or ‘omics’ research ethics review context, a hyper-localized focus incites considerable drawbacks. It can significantly bog down the process of multi-site ethics review and approval, introduce recruitment and consent bias, raise transaction costs (for example, by restricting the use of a sample to one particular study), prevent transfer of samples or data abroad, create redundancies and arbitrary discrepancies, and cause confusion among researchers and participants alike.60 Additionally, as many commentators have written, IRB policies and regulations may fail fundamental conditions for legality and the rule of law, as most famously detailed in the work of legal scholar Lon Fuller: ‘that it be possible to follow the law, that those affected by a law be given some opportunity to comment on the rules, and that those administering the law should be reviewable.’61 Heimer and Petty note that ‘the decentralization of the IRB system is likely a disadvantage here because local IRBs mostly seem not to understand the principle that those affected must be consulted as laws are being formulated.’62
Moreover, in the context of large-scale, non-interventional, and data-driven studies that pose minimal risk to a participant, the imposition of universal, one-size-fits-all specific consent and ethics review at each study site is inefficient, costly, and unnecessarily dilatory.63 IRBs spend superfluous time reviewing research plans incommensurate with the level of risk presented by the proposed research, while data-driven ‘omics’ researchers must shoehorn protocols and consent forms into templates designed for physically interventional clinical trials. To be sure, data-driven research carries risks, but their kinds—mainly subjective, dignitary harms that are psychosocial and informational—are wholly distinct from physical risks due to the ingestion of a pharmacologic agent or the insertion of a device.64 In a costly system that regulates behavior to prevent harms before they have actually occurred—and may never occur—this seems particularly wasteful.
The evidence of systemic problems is telling. Some researchers have estimated that 17 per cent of the research budget of a multicenter medical study was spent on securing IRB approval,65 and that IRB work took researchers 30 hours per proposal, and that approval took more than three months to arrive.66 Another group of researchers examined the costs and effects of local IRB review of the consent form and protocol in a multi-center clinical trial of Parkinson's disease and found that 76 per cent of changes to the consent form reflected standard institutional language, with no substantive changes to the protocol. On average, the site and coordinating center staff spent 13.7 hours submitting each sites’ consent and protocol; the direct costs associated with local review and approval was $107,544: $82,610 in IRB fees and $24,934 in labor.67 These, of course, are studies of multi-site research protocols submitted for review and subsequently approved. It thus begs the question: what are the costs, in dollar amounts and to human health and knowledge production, of delayed or derailed research, that is, research that has been totally thwarted by the system? And what sort of chilling effect does the system impose on future research or researchers planning a large-scale, longitudinal study, especially one that is international?
Personal anecdotes, along with articles and reports by many commentators (not to mention regulators68), have noted that the system is insufficient and quickly falling out of step with the current nature of biomedical research. In an influential article from 2010, Jerry Menikoff, Director of the Department of Health and Human Services’ Office for Human Research Protections (OHRP), lamented that the current ethics framework of multi-IRB review for a single study ‘may actually reduce the likelihood that studies are in keeping with relevant ethical standards’69 since IRBs often fail to communicate their findings to one another (including changes to the consent forms), and no one IRB feels empowered to enact changes to protocols lest it fear stepping on the toes of another IRB—what Menikoff calls an ‘authority vacuum.’70
Anne Junker, Scientific Director of the Maternal Infant Child & Youth Research Network of Canada, has noted that in Canada, researchers or industry sponsors must pay thousands of dollars for institutions to conduct IRB reviews, complete applications that are not standardized across the country, acquire multiple signatures, often submit multiple copies, and respond to queries from IRBs.71 Moreover, in the clinical context, inconsistent IRB decisions in Canada are provoked by differences between provinces in standards of care and in legislation governing access and distribution of clinical information. In addition, IRBs do not get the same information on any given proposal, given the lack of standardization of content and format of application forms. Preliminary results of a survey that Junker sent to research networks to solicit their plans for multi-center research studies to be conducted in 2012 indicated that 16 networks planned for a total of 42 studies, but given the number of centers involved with each study and the current regulatory system, a total of 318 IRB reviews would be conducted.72 This would involve an immense amount of time and resources. Two Canadian researchers have estimated that for a 20-center clinical trial, managing ethics review can involve some 300–500 person-hours in total.73 Not surprisingly, Canadian bioethicists and legal scholars have found that researchers are dissatisfied and perceive the ethics review process as ineffective and in need of reform.74
Remaining in the Canadian but also international context, one of us (Bartha Knoppers) is involved in co-leading the ongoing international ‘Personalized Risk Stratification for Prevention and Early Detection of Breast Cancer’ project that will develop tools to allow for a personalized evaluation of breast cancer risk, using a variety of factors such as genetics, environmental, hormonal, and clinical data. This four-year endeavor encompasses 20 researchers from 10 universities across three countries. The genetic analyses will necessitate 82,000 samples already collected from more than 65 studies in 30 countries from the Breast Cancer Association Consortium.75 Part of the clinical data originates from a centralized databank located at the University of Cambridge. The samples and data will be exchanged several times between different universities in different countries because stages of the analysis will happen in different places. All the new genetic data generated will be added to the central database in the UK for future research. Quite clearly, these studies have been initiated at different times and in diverse legal, regulatory, and ethical contexts.
Before the project could begin, project leaders had to address multiple issues, including: (1) non-uniform local legal frameworks; (2) multiple languages; (3) varied ethical norms based on both countries and epochs; (4) the necessity of a uniform agreement to use a common databank; (5) diversified conditions for data and sample collection; (6) the large number of researchers, cohorts, and countries; (7) non-harmonized consent forms; (8) tight deadlines for starting the sequencing; (9) numerous ethical approvals required; (10) non-uniform material transfer agreements (MTAs); and (11) an imposed time limit on the research funding (with a possible claw back of funds beyond the deadline of the project by federal funding agencies).
Overcoming these issues required not only money, but also months of planning. The project hired a lawyer who worked closely with the scientific team for several months. The project then engaged in a multi-step process. First, a meeting of researchers and the core ethics committee was organized to allow them to better understand the project and to communicate to the project leaders any concerns they may have had relating to ethics. During this meeting, a strategy was adopted with the collaboration of the ethics committee chair of the principal investigator (PI) at Laval University in Quebec City. The project would be divided into eight parts, with each part submitted in succession to the ethical committee, and the members of the committee would be informed about the steps undertaken with the other ethical committees. Second, important preparatory work with regard to numerous agreements required for the project was undertaken, especially for sample and data sharing. The project leaders reached an agreement with the data repository leader (University of Cambridge) to have a uniform research agreement signed by all the data and sample providers. The project leaders also had an in-person meeting with University of Cambridge members involved in contract drafting in order to help develop these agreements and to ensure their conformity with ethical norms. Lastly, the project leaders developed tools (ie tables and diagrams) to help the main ethics review committee at Laval University follow the step-by-step evolution of all the ongoing ethical authorizations. These tools are also useful to different researchers to obtain ethical authorizations on a local level.
Although this multi-step process and overall strategy was ultimately successful in securing the first step in ethical approval and launching the project on time, the launching of the project does not imply that every part will be approved. Furthermore, the process required hundreds of hours of labor time and tens of thousands of dollars in resources. Unfortunately, these represent sums of time, energy, and dollars that cannot be directly invested into breast cancer prevention research.
Our anecdotal story is buttressed by comprehensive research. A UK-based study from 2006 found that the overall level of agreement regarding 18 protocols among three different research ethics committees was only slightly better than chance (kappa = 0.29).76 An analysis by Lidz et al. of 104 protocol reviews from 20 IRB meetings at 10 leading academic medical centers found that essential elements of human subjects protection, as required by the Common Rule,77 were not implemented uniformly.78 A Canadian study of IRB chair and administrator views on health data registries and biobanks found that there was a ‘significant degree of variation in how the sites in our survey indicated they would handle research proposals for creation and use of [registries].’79
In a US observational health services research study conducted by Green et al., which qualified under US government regulations for expedited review, approximately 4,680 hours of staff time over a 19-month period were devoted solely to the IRB process; 72 per cent (31/43) of IRBs required full board review, 28 per cent (12/43) requested changes that increased patient risk, and one IRB even rejected the protocol.80 Additionally, 53 per cent (23/43) required inapplicable sections in the consent form and five required Health Insurance Portability and Accountability Act (HIPAA) consent from physicians although no health information was asked of them.81 The process required from 52 to 798 (median 286) days to obtain approval at each site and 15 per cent of the IRBs required three to six revisions, most of which were editorial rather than substantive.82
A 2011 meta-review by Canadian researchers of IRB decisions on multi-site studies found that ‘variation in ethics review across multiple [IRB]s appears to be the rule rather than the exception. Studies from around the world have found substantial variation across [IRB]s, and even among members on the same [IRB], when reviewing the same protocol.’83 In a 2011 meta-review of 43 published empirical studies of IRBs, researchers found that ‘US IRBs differ in their application of the federal regulations, in the time they take to review studies, and in the decisions made,’ not to mention ‘evidence of variation in multicenter review, inconsistent or ambiguous interpretation of the federal regulations, and inefficiencies in review.’84 In another 2011 meta-review of 52 published US studies to identify all existing primary data on the costs of IRB review, researchers found that ‘IRBs operate at different levels of efficiency; that waiting to obtain IRB approval has, in some instances, delayed project initiation; that IRBs presented with identical protocols sometimes asked for different and even competing revisions; and that some decisions made (and positions held) by IRBs are not in accord with federal policy guidance.’85
Many researchers are dissatisfied with the current system. Even as IRBs are becoming professionalized, a recurring complaint is that members lack knowledge of formal guidelines or regulations specific to the domain of the project they are charged with applying, thereby leading to haphazard outcomes. A recent qualitative study of 46 investigator's experiences of IRBs in the US noted serious deficiencies and frustrations:
[Investigators] noted that institutions viewed risk differently and had different ethical concerns related to recruitment, consent, data collection, and data management. Further, investigators described areas of disagreement between IRBs on issues that had little to do with human subjects protections, such as which institution's name would appear on study letterhead. Investigators reported they were often the messengers between organizations’ IRB staff who should have instead talked directly with each other. Study delays could be significant in these situations.
Although investigators described their frustrations with institutional differences, they were generally unable to identify satisfactory strategies for avoiding or resolving differences unless there were ongoing, multi-site partnerships. One investigator, who regularly partnered with colleagues at another institution, indicated that colleagues completed all IRB documentation for their own sites rather than using a shared IRB application, which worked well. Most investigators expressed concerns that differing views of risk as well as logistical variations across IRBs discouraged multi-site research at a time when large samples are needed to advance science.86
Some countries have attempted to improve the status quo, albeit without fundamentally changing the underlying governance structure at a global level. Australia87 and New Zealand88 have launched national ethics review systems with national application forms. Several countries operate national ethics committees that can review protocols, including China, Denmark, Iceland, and South Africa;89 many more operate advisory national bioethics commissions.90 In France, the Commission nationale de l'informatique et des libertés is an independent national administrative authority that since 2004 determines, on a case by case basis, whether researchers can access personal health data for research purposes. Their committee of experts (all scientists) vets, among other criteria, the research organization's credibility, the researcher's legitimacy, and the data security measures in place.91 In the US, central IRBs for multi-site studies have been formed at the NIH's National Cancer Institute92 and National Institute of Child Health and Human Development,93 and the Department of Veteran Affairs.94 Canada's national ethics guidelines for federally funded studies, the Tri-Council Policy Statement (TCPS),95 acknowledges that modern research is not confined to one territory. In the interest of avoiding undue delays, since 2010 it has endorsed alternative ethics review models, including delegation of specialized content review to an external, multi-institutional ethics review body or a joint subcommittee of IRBs to facilitate appropriate deliberation on ethics review in order to provide flexibility and efficiency while avoiding unnecessary review duplication.96
Yet none of these countries contemplate the changes needed to truly modernize the ethics review framework for internationally collaborative data-driven research. For instance, Canada's TCPS stipulates that ‘for a Canadian research institution, review of the ethical acceptability of the research by the institution's REB [(research ethics board)] is required, in addition to ethics review by an REB … with jurisdiction at the research site elsewhere in Canada, or outside Canada, if any.’97 Similarly, the EU's policy regarding IRB review for clinical trials on medicinal products reflects only incremental reform by restricting each participating country to a ‘single opinion’ that represents the ethics review for that country, ‘notwithstanding the number of Ethics Committees’ involved.98 Certainly some of these and other reform proposals99 are an improvement and particularly appropriate in a non-interventional, data-driven research context. However, by still relying on a comprehensive ethics review in each country, the potential for global bottlenecks and incongruence remain, with multiple IRBs reviewing a research project that seeks to aggregate and use data on a global scale. Indeed, as noted in a much-discussed white paper by the Global Alliance for Genomics and Health, local consent and ethics approval may allow for data sharing in the same jurisdiction, but providing data to researchers in other institutions and jurisdictions can require additional approvals, even when participants have consented to such sharing and where foreign researchers intend to use the data in a protocol approved by their own local IRB.100
This situation must be rectified. As more formally or informally associated research projects are conducted by teams of researchers affiliated with different institutions or organizations, there should be gateways for researchers that ensure high standards and consistent application for non-interventional studies that test neither drugs nor devices, and that work toward the mutual recognition between countries of ethically equivalent approaches.
PILOTING THE ETHICS REVIEW PROCESS TO A SAFE HARBOR
All research with humans must confront the central ethical tension between promoting socially valuable knowledge and protecting research participants from exploitation and harm. But certain governance systems may be more adept than others at mediating this tension. As the previous section illustrated, commentators have exposed weaknesses in the current ethics review system, particularly for international research consortia. This sub-optimal state cannot continue if countries, to say nothing of global society, wish to maximize the potential of ethical biomedical research. As two science policymakers assert, ‘Harmonizing norms and standards may be the most pressing need for successful globalization.’101 Radical reform of the ethics review system, such as a conversion from judgment of documented anticipatory research plans to retrospective examination of records of compliance,102 or outright IRB abolition,103 is a formidable, possibly utopian, and arguably counter-productive endeavor. But we need not be barbarians at the gate. Bold, transnational reform proposals are possible without revolution.104 Indeed, it may be useful to acknowledge that in a world of Big Research and Big Data, Big Ethics is needed.105
So, in the ELSI 2.0 spirit,106 we pose two polar questions: given what we know from years of research, is it possible to design a modern, global system where certain types of international research projects can undergo comprehensive but streamlined ethics review that addresses and overcomes unalterably local ethical issues, laws, and regulations? Is it possible or desirable to have an independent body capable of tackling ‘the uncertainty inherent in research and the complexity and controversy implicit in moral decision making’?107 We believe the answer to these questions is ‘yes’: it is both possible and desirable, and that it is time to accelerate the ethics evaluative process in terms of a novel global policy proposal. One can design a system that respects national sovereignty, the rule of law, and varying risks or cultural practices in populations among research sites while promoting harmonization and a streamlined ethics review approach. We also believe it is possible to design a governance framework that balances competing goals and values so as to conduct research ethically across political borders, or what Rial-Sebbag and Cambon-Thomsen call an emerging ‘organizational ethics’,108 and what Dunn calls ‘adjudication between general principles upon which people can reasonably disagree.’109
Commentators have averred that a 21st century ethics system must promote both public beneficence and the centrality of respecting all persons.110 They have also promoted the principle of regulatory parsimony, which recommends ‘only as much oversight as is truly necessary to ensure justice, fairness, security, and safety’ of individuals while pursuing the public good of data sharing and biomedical research.111 Coupled to this is the consideration that an ‘integration of a plurality of regulatory tools, each designed to perform a content-specific normative function,’112 is most suitable for creating standardization between national ethics structures.
Armed with this insight and a desire to accelerate the ELSI policy agenda, we propose a ‘Safe Harbor Framework for International Ethics Equivalency’ that would maintain prospective ethics review, a consistent floor of ethical protections and accountability mechanisms, and promote socially valuable biomedical research.
A safe harbor is distinct from the accredited ‘safe haven’ models advocated in recent UK reports on data sharing113 and referenced in the UK's Health and Social Care Act 2012,114 which allow for approved (ie accredited) researchers to access and link personal data sets from more than one organization for a purpose other than direct care in a secure environment.115 Safe havens, unlike safe harbor models, are seen as accredited organizations with a secure electronic environment in which personal data and/or de-identified data can be obtained and made available to users, generally in some de-identified form.116 A safe harbor is not an organization per se that creates or manages storage facilities where personal data, anonymized or coded data, or samples are only disclosed for linkage in secure environments. Rather, it is more systemic and more complex.
The meaning of a safe harbor in the regulatory world is similar to its meaning in the seafaring world: protection from threatened loss. We define it as a process, system, or framework that allows a bona fide entity to perform certain actions in compliance with defined standards in exchange for mutual recognition of substantial equivalency in regulatory and ethical guidance. The defining feature of a safe harbor is its embrace of flexibility and interoperability. The goal is the harmonization of laws, policies, and guidelines, based on common principles and comparable protection. What brings multiple jurisdictions together in building a safe harbor is the recognition that different legal systems and political systems do not equate ipso facto to incompatible values, particularly in the advancement of human wellbeing.
Safe harbors have been implemented in numerous areas of the law and carry resonance in the field of health and data protection, as seen in the HIPAA ‘Safe Harbor’ technique that permits data sharing without patient consent or IRB approval if 18 patient identifiers are removed.117 The US-EU Safe Harbor Framework presents another good example.118 The EU Data Protection Directive, which came into force in 1998, allows free transfer of personal data across international borders, but only (among other exceptions) to countries deemed to have ‘adequate’ data protection regulation in place, that is, laws similar to those contained in European member states’ data protection statutes.119
From a European point of view, the US does not provide ‘adequate’ data protection regulation.120 In 2000, the European Commission and the US Department of Commerce agreed to ‘Safe Harbor Privacy Principles’ in response to European-led concern that the sectoral, market-driven regulatory approach to privacy by the US government would lead to a finding of inadequacy and stoppage of cross-border data transfer.121 The US-EU Safe Harbor Framework is a compromise solution that offers a streamlined approach to compliance with the EU Data Protection Directive, or more accurately, an equivalent substitute for ‘adequate’ privacy protection. The Framework comprises in part a list of seven general principles (notice, choice, onward transfer to third parties, access, security, data integrity, and enforcement) and further explanatory details attached to the instrument as ‘frequently asked questions’ or FAQs. American organizations voluntarily subscribe in order to receive European member states’ data. They can self-certify or be third-party certified as Safe Harbor compliant through an online registration form submitted to the US Department of Commerce that guarantees their adherence to ‘adequate’ privacy safeguards and enforcement by the Federal Trade Commission if they fail to adhere to the privacy obligations. Upon this registration, which is subject to obligatory annual renewal and affirmative representation of compliance, European data flows to these organizations can proceed.122
Similarly, in the commercial context, the Asia-Pacific Economic Cooperation (APEC) forum recently implemented its Cross Border Privacy Rules (CBPR) System that approves the transfer of personal data between all 21 APEC member countries.123 The CBPR system builds on the APEC Privacy Framework,124 a set of nine guiding principles and guidance in developing consistent domestic approaches to data protection laws, agreed to by the member countries in 2004, by providing a practical mechanism for companies in member countries to safely and efficiently transfer personal data in a cross-border context.
The CBPR System consists of several core documents.125 ‘Accountability Agents’ are approved by APEC to review, certify, monitor, and enforce the privacy practices of participating companies to ensure compliance with CBPR requirements. Once an Accountability Agent certifies a company, its privacy policies and practices become binding as to that company and will be enforceable by an appropriate authority, such as a regulator. Though a voluntary and self-regulatory initiative, an enforceable code of conduct governs the behavior of participating companies (for example, the Federal Trade Commission is the designated enforcement authority for the US). To date, the US and Mexico have been accepted as CBPR System participating economies.126
A safe harbor framework can also apply to research ethics review. For example, the US already has a safe harbor-type arrangement in its Common Rule: ‘[I]f a Department or Agency head determines that the procedures prescribed by the institution afford protections that are at least equivalent to those provided in this policy, the Department or Agency head may approve the substitution of the foreign procedures in lieu of the procedural requirements provided in this policy.’127 It is intriguing why this subsection has sat dormant since its implementation in 1991.128 According to the Secretary's Advisory Committee on Human Research Protections (SACHRP):
[T]here have been no determinations of equivalent protections, even as research has globalized and several countries have developed robust human subjects protection and regulatory mechanisms, consistent with their own national laws and cultural values, and requested that (the OHRP) deem their systems of protection to be equivalent. At the same time, FDA [the Food and Drug Administration] accepts foreign data developed in studies that are performed in compliance with foreign laws and standards if they are completed before the FDA application filing; the FDA thus tacitly accepts an equivalent standard (eg International Conference on Harmonisation (ICH) and Council for International Organizations of Medical Sciences) in its own approval process, in significant contrast to (the OHRPs) current stance on these ‘equivalence’ issues. The lack of determinations of ‘equivalence’—and of acceptable methods to determine ‘equivalence’—has led to circumstances in which US-based researchers and research institutions must insist on foreign entities’ and foreign researchers’ strict adherence to what can seem, to them, confusing and even impenetrable US regulations and guidance documents.129
Even though it remains dormant and calls for pilot testing have gone unheard,130 the Common Rule ‘equivalent protections’ subsection evidences the feasibility of a safe harbor framework for ethics review equivalency of international research projects. Of course, the provision reflects a desire by the US to make foreign countries ascribe to the ethics protections embodied in the Common Rule, that is, the law of one country (its own). The policy challenge is to scale up ‘ethics equivalency’ to the global stage. This requires not only the formulation of benchmark standards to which ethics protections of different countries can be compared, but also an international organization that can act like OHRP in terms of coordinating and implementing such a framework. Needless to say, this scaling up is impossible without political and regulatory will for interoperability and international cooperation.
The Safe Harbor Framework for International Ethics Equivalency
Our proposed Safe Harbor Framework for International Ethics Equivalency (hereinafter ‘Safe Harbor’) would consist of a new supranational agency built on five principle elements: (1) registration, (2) compliance review, (3) recognition, (4) monitoring and enforcement, and (5) public participation. The agency's mission would be to connect governments around the world to harmonize where possible ethics review guidelines and policies, increase ethical conduct, and ensure compliance for researchers involved in a clearly defined type of research project (Box 1). In recognition of the longstanding work occurring in related fields, clinical trials with pharmaceutical products or devices would remain excluded from the Safe Harbor and should remain subsumed within the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) framework.131
BOX 1: THRESHOLD CRITERIA TO ENGAGE IFER ETHICS REVIEW.
- Human Subjects Research
-
°The proposed project must be a systematic investigation designed to develop or contribute to generalizable knowledge and must involve data obtained through interaction with living or deceased natural persons.
-
°
- Scientific Validity
-
°The research project's design and aims must be well-founded, conform to generally accepted scientific principles, and be based on comprehensive knowledge of the scientific literature, as determined by funding or granting agencies.
-
°
- Consortia of International Scope
-
°The research project must be managed by a consortium or similar association comprised of member researchers or organizations from more than two countries. Specifically, the multinational scope of the project must involve researchers and data transfer from more than two countries.
-
°
- Genomics and Health Data-Focused
-
°The research project must integrate genomics or ‘omics’ data (ie proteomics, metabolomics, transcriptomics) of population cohorts into the study design, but may also involve other health-related data such as medical records, stored biological samples, biomarkers, phenotypic, environmental, epidemiological, and clinical trial data.
-
°
- Non-Interventional
-
°The research project must not involve direct physical interventions in a person, such as clinical trials involving pharmacologic agents or devices.
-
°
In the long run, we envision the Safe Harbor having the authority to handle a broad array of global biomedical research projects, though we believe that in the short term, the greatest chance of success necessitates a focus on just one critical area of data-driven research: genomics and related omics-focused research. As legal scholars and scientists recently noted in a study on the proposed revisions to the US Common Rule in the context of evolving large-scale research projects like the Human Microbiome Project (HMB), ‘While a change in the Common Rule to streamline IRB approval of multisite studies or mandate a single IRB for multisite studies would be a benefit to the HMP and other similar ‘big science’ research studies, it may make more sense to consider the type of research being proposed rather than to mandate this change for all multisite research studies.’132 We agree with this sentiment, but add that depending on its feasibility and viability, the Safe Harbor's scope could encompass all global biomedical research.
While the Safe Harbor does not aim to displace a country's domestic laws and regulations regarding ethics review or protection of research participants, it is possible that a country may need to modify its laws or adopt regulations under its laws to facilitate participation. Reviews of the legal and regulatory framework of human subjects research and data protection are first-step endeavors. The Safe Harbor therefore may need to develop capacity building activities at an early stage to help countries work towards appropriate domestic law or ethics guidance modification. Regardless, we think countries around the world share the same ultimate goal for ethics review: to respect persons while enhancing biomedical research to improve human health. In the envisioned Safe Harbor, a nimble and agile system supported by substantive principles (Box 2) and procedural mechanisms (Box 3) will guide the ethics review process (see Box 4 for key definitions).
BOX 2: TEN GUIDING PRINCIPLES OF A SAFE HARBOR FRAMEWORK FOR INTERNATIONAL ETHICS EQUIVALENCY.
- Respect for Persons
-
°Research participants should be treated with dignity and integrity. They should be respected both as beings who are capable of exercising decisions, and also as members in communities who make choices in the context of their relationships.
-
°
- Beneficence
-
°Researchers must have the welfare of research participants as a primary goal, particularly those who are vulnerable. Unnecessary or unjustified risks must be avoided throughout the course of the research project. Consideration should also be given to the interests of other parties, including future research participants or connected others such as family members or cultural or ethnic groups.
-
°
- Justice
-
°The benefits and burdens of the research project should be distributed equitably among all groups in society, taking into account age, gender, economic status, culture, and ethnic considerations, as well public goods and public harms.
-
°
- Social and Scientific Value
-
°Research projects must be designed to yield important information and new knowledge that has a positive impact for science and society. This information can consist of varying types, including disease trends and risk factors, treatment outcomes, and healthcare costs and use. In an environment that aims to promote solidarity and citizenry, research projects should aim to deliver new insights regarding health and disease and consequentially, improvements in human health.
-
°
- Proportionality
-
°Ethics review and oversight must be commensurate with the risks to and benefits for research participants. Review and oversight must be designed to achieve the necessary protection of research participants from harm in a reasonable way and with the least onerous measures to all stakeholders involved in the process.
-
°
- Procedural Fairness
-
°The process for ethics review of research projects must be conducted efficiently and consistently in accordance with principles of procedural fairness, namely the right for a research project's PIs to be heard and the right to be judged impartially.
-
°
- Transparency
-
°IFER-approved research projects must be publicly disclosed on the IFER website. The quality and type of disclosure should be current and consistent for ease of reference and searchability. It should include, in part, the nature of the project, the purposes for which data are being collected and used, the planned duration of the project, and the date of IFER approval. Contact information for research project organizers or PIs must also be posted so that the public can communicate with them.
-
°
- Security
-
°When reasonable, and whenever possible, state-of-the-art measures must be employed to minimize the risk of research projects’ data becoming lost, misused, or unjustifiably altered or destroyed.
-
°
- Data Integrity and Quality
-
°The data being collected, used, and transferred must be relevant to the research project's purpose(s). Data must be reliable, accurate, complete, and current. If samples are used in a research project, they must be collected, stored, and processed in a way that preserves their long-term stability, searchability, and integrity.
-
°
- Accountability
-
°Research projects and their PIs must be willing to be audited at any time and benchmarked to established standards and metrics of ethics protection. NCO screening determinations may also be periodically audited to ensure international consistency and avoid adverse ‘forum shopping’ by PIs. Both research participants and the public must have readily available independent recourse mechanisms to enforce the Safe Harbor standards, and have complaints investigated and resolved and penalties enforced when warranted (ie actions committed negligently or in bad faith). The public should be promptly notified on the IFER website of any established breach of the Safe Harbor's policies and standards.
-
°
BOX 3: LIST OF STANDARDS TO SATISFY A SAFE HARBOR FRAMEWORK FOR INTERNATIONAL ETHICS EQUIVALENCY.
- Self-Assessment, Registration, and Compliance
-
°The research purpose must be legitimate: researchers must intend to extend public knowledge through a disciplined inquiry or systematic investigation that is not in contravention of any applicable laws or fundamental human rights.
-
°All researchers and staff who are directly or indirectly involved in the research project must agree to not use research participants’ personal data in any way that deviates from the research plan, and must not share such data with third parties unless obligated by law.
-
°All research staff who directly handle personal data must certify that they are trained in security and privacy compliance, as determined by the jurisdiction in which they are situated.
-
°Personal data must not be stored on portable devices or storage media unless encrypted according to standards set by nationally or internationally recognized organizations/agencies that develop and apply measurements and standards (eg ISO).
-
°Researchers are responsible for ensuring that all downstream users of data within the project are in compliance with data security controls and ethics guidelines (including IFER's policies and standards) and laws in the jurisdiction(s) hosting the data or research team.
-
°All research projects that share data and/or samples with downstream users must use a simplified Access Agreement (eg P3G's Generic Access Agreement)134 to govern the responsible use of those data and/or samples and set out the enforceable rights and obligations of all parties.
-
°
- Dispute Resolution and Enforcement
-
°Research projects must adhere to IFER's dispute resolution system (eg appeals process, Compliance Branch, and Ombuds Office) to investigate and resolve complaints and procedures for verifying international and national compliance, in coordination with NCOs. Failure to comply with the Safe Harbor can lead to sanction by IFER, NCOs, and/or other governmental bodies.
-
°Research projects must be subject to ongoing assessment by IFER, with written attestation by the PIs and persons with requisite signing authority to affirm compliance with the periodic assessment and that the research project remains in accordance with the Safe Harbor.
-
°
BOX 4: KEY DEFINITIONS IN SAFE HARBOR FRAMEWORK FOR INTERNATIONAL ETHICS EQUIVALENCY.
Advisory Group: Group comprised of multiple organizations (eg NGOs, industry, technology research bodies) that would keep IFER Bureau abreast of the changing realities and needs of technology and data, as well as laws, regulations, and policies governing ethics review and human subjects research.
Bureau: Executive arm of IFER that consists of one Chairperson and a multidisciplinary panel of 12 independent experts from various geographic regions.
Compliance Branch: IFER branch responsible for managing the ethics review of research projects and ensuring ongoing and prospective compliance with the IFER-promulgated policies and standards.
Ethics Committee: Committee of 5 to 7 technical officers who are tasked with making a consensus decision (approved as submitted; conditional approval; deferred decision; or not approved) that reflects an ethical judgment about the permissibility of a research project.
External Ethics Appeal Board: An ad hoc committee of independent research ethics experts appointed by the Bureau who are tasked with making a final, binding ethics review upon appeal from re-review by the IFER Ethics Committee.
IFER: The International Federation for Ethics Review (IFER) is an international non-governmental organization, formed by a voluntary compact among countries, granting agencies, philanthropies, and healthcare, patient advocacy, and research organizations, that seeks to streamline and harmonize the ethics review process of specific types of research projects.
IT/Communications Branch: IFER branch responsible for maintaining the IFER website and access portals, as well as coordinating ethics educational and factual information dissemination and communication flows between researchers, NCOs, and the public.
NCO: A National Coordinating Office that is created or delegated by each country that signs the voluntary compact with IFER. It is charged with undertaking a timely and efficient screening of a research project application to ensure that it adheres to the mandatory legal and ethical standards of that country and ascribes to IFER's promulgated ethical principles and norms so as to ensure ethics equivalency.
Ombuds Office: IFER office responsible for receiving, investigating, and addressing complaints of both internal IFER concerns and research project ethics violations. The Office would report its findings and recommendations for changes to policies or procedures to the IFER Bureau.
Policies and Standards Branch: IFER branch responsible for creating, revising, and interpreting policies and standards that govern the ethics review process and related ethical issues, such as confidentiality, consent, and conflicts of interest.
Technical officers: IFER's professional ethics reviewers who come from around the world and who have a broad diversity of prior IRB experience and expertise. They are appointed by the IFER Bureau by consensus for uniform term limits, and are responsible for providing an independent ethics review opinion. Technical officers are situated within the Compliance Branch.
International Federation for Ethics Review
Harmonizing ethics review for international data-driven research projects requires international ethics governance reform. An individual country may work towards reducing redundancies in ethics review and aim to create efficiencies for multi-site studies, but usually such reform stops at the political boundary. National reform alone does not and cannot address international concerns. Policymakers, researchers, and other stakeholders who wish to remedy the systemic problems in ethics review could support an international organization that is capable of steering globally collaborative research projects to an ethical safe harbor. The chief component of the proposed Safe Harbor, therefore, is a newly constituted organization. In line with the goals of the Global Alliance for Genomics and Health,133 which promotes the responsible sharing of genomic and clinical data and international interoperability and harmonization, we suggest an International Federation for Ethics Review (IFER), formed by a voluntary compact among countries, granting agencies, philanthropies, institutions, and healthcare, patient advocacy, and research organizations.
As constituted by a foundational Charter and governed by internal Rules of Procedure, IFER would be both a central ethics review body engaged in deliberation of the possibly divergent ethical aims of funders, institutions, research organizations, and participants, and also a forum for review and follow-up of policies concerning ethics norms for international research projects. Oversight and accountability of IFER would be maintained through publicly accessible annual reports, public participation in annual or biannual meetings and online fora, as well as an Ombuds Office (discussed below).
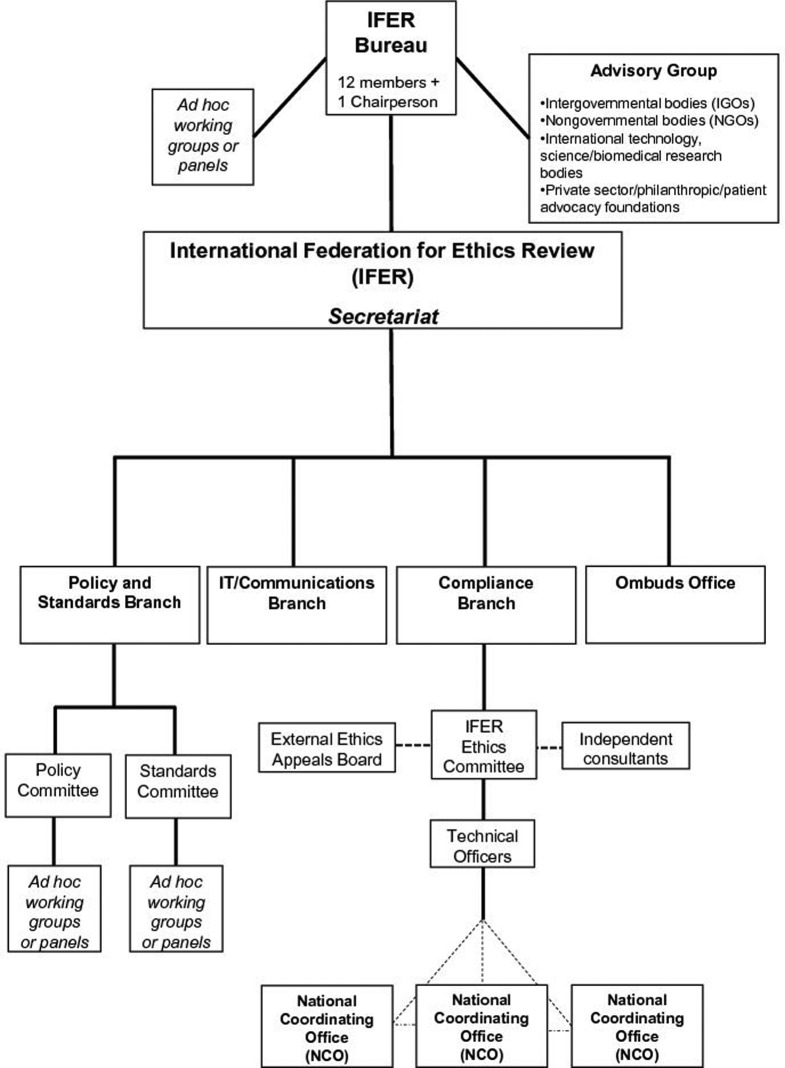
IFER would have a small, permanent Secretariat with staff responsible for day-to-day management. As depicted in Figure 1, the Agency would be comprised of several parts. A Bureau would serve as the executive arm and consist of one Chairperson and a multidisciplinary panel of 12 independent experts (ie in bioethics, social science, law, biomedical research, vulnerable populations, public involvement, and data protection), with two coming from each of the following geographic regions: Africa, Asia, Europe, North America, Near East, and one from both South-West Pacific and Latin America/Caribbean. The Chairperson and panel members could be nominated for a term of three years on a rotating basis among regions. Assisting the Bureau with its core functions would be an Advisory Group comprised of multiple types of organizations that would keep IFER abreast of the changing realities and needs of technology, data, as well as laws, regulations and policies governing ethics review and human subjects research.
Figure 1.
Organization chart of the Safe Harbor's primary component, an International Federation for Ethics Review (IFER). IFER would be constituted by a voluntary compact among countries, granting agencies, philanthropies, institutions, and healthcare, patient advocacy, and research organizations. The dotted lines in the figure represent ad hoc or external parts of IFER. In particular, the IFER Ethics Committee can call upon a standing list of independent consultants who could provide special expertise to the Committee on proposed research projects, be it in methodology, disease, or legal domain. Applicants whose first appeal is rejected by the IFER Ethics Committee may further appeal to the External Ethics Appeal Board. Additionally, NCOs are a key feature of the Safe Harbor but are external to IFER; they work with the technical officers and the Compliance Branch, and coordinate with each other for each research project, but are situated in their own country and are subject to their country's laws and regulations.
IFER would have four internal branches, with staff members appointed by the Board.135 An Ombuds Office would receive, investigate, and address complaints of both internal IFER concerns and research project ethics violations; it would report its findings and recommendations for changes to policies or procedures to the IFER Bureau. A Policy and Standards Branch would create, revise, and interpret policies and standards that govern the ethics review process and related ethical issues, such as confidentiality, consent, and conflicts of interest. Within this branch, the Policy Committee would be charged with the policy component of IFER, while the Standards Committee would be charged with developing standards for operationalizing IFER's policies. An IT/Communications Branch would maintain the IFER website and access portals; it would also coordinate ethics educative and factual information dissemination and communication flows between researchers, National Coordinating Offices (discussed below), and the public. Finally, a Compliance Branch would manage the ethics review of research projects and ensure ongoing and prospective compliance with the IFER-promulgated policies and standards.
The main component of the Compliance Branch would be a cadre of approximately 40–60 experienced, international professional ethics reviewers (ie technical officers) who are age, gender, and culturally balanced. These individuals will carry a broad diversity of prior IRB experience and have varied but defined standards of expertise, including knowledge of ‘omics’ research, bioethics, life sciences, public engagement, social science (eg sociology and anthropology), statistics, and privacy/data protection law. Each would be appointed by the Bureau by consensus for uniform term limits and would be responsible for providing an independent ethics review opinion that is free from political, institutional, professional, and market influences. While conflicts of interest, real or perceived, should be avoided, this may not always be possible if an individual has previous experience at a large funding agency or research organization. In such unavoidable instances, there should be transparency with regard to such experience and possible perceived interests.
Creating and maintaining IFER will involve sustainable funding commitments, even beyond those typically granted to an IRB, since this would be a fully functioning agency with a large staff compensated for their labor. It has been estimated that the annual costs of US IRB activities total between approximately $500,000 to almost $2 million per institution,136 and the average cost for full or expedited reviews is at least $1000 per protocol.137 Higher-volume institutions generally have lower costs, which is indicative of economies of scale.138 While it is difficult to transpose these estimates to an international agency, it is reasonable to assume that significant cost savings would be achieved by removing many costs borne by individuals and institutions and consolidating and streamlining the ethics review system.
IFER's budget could be maintained by requiring research projects or their funding agencies to pay for the registration submission and ethics review, and collecting dues from member countries based on their ability to pay. Average annual global R&D spending is around 2 per cent of gross domestic product (GDP).139 Setting aside even a portion of that, for example, 0.05 per cent of each member country's gross national income (GNI)140 per annum, could go a long way to improving R&D outcomes by streamlining and harmonizing the ethics review process through IFER. As an incentive, the six leading funding countries would have a permanent seat on the Bureau such that they can nominate a member each time the membership expires, while six seats would rotate every three years to ensure global representation, regardless of size or funding ability.
Element 1: Registration
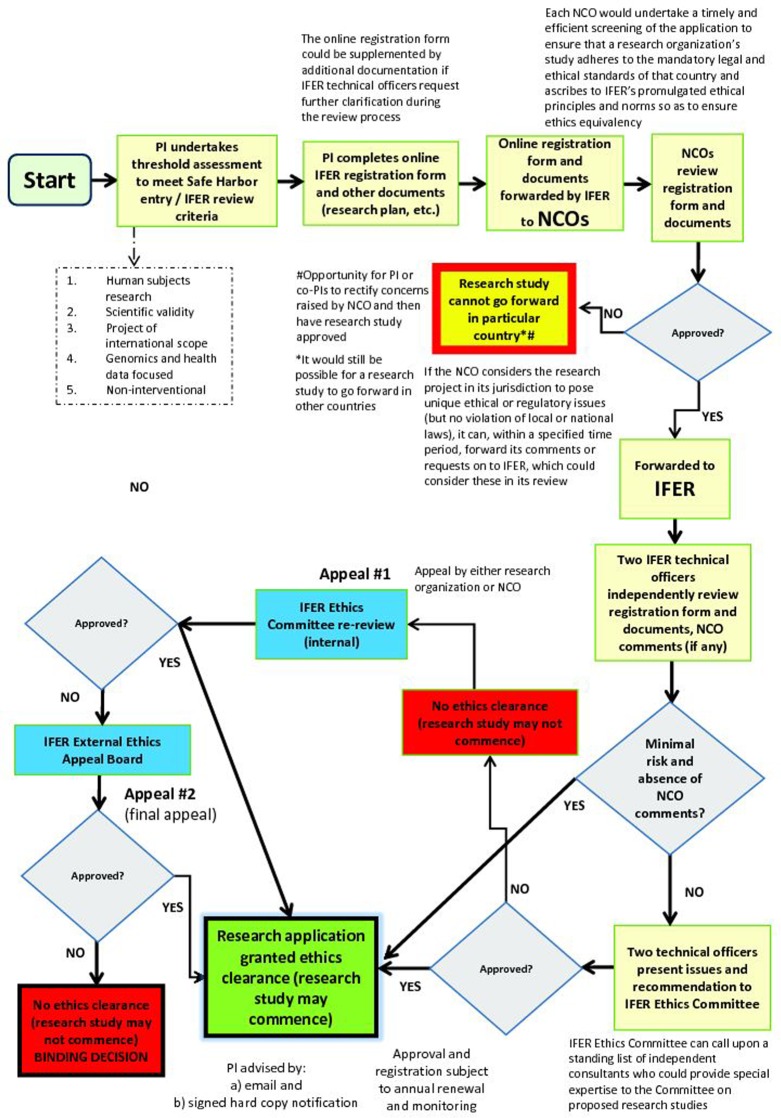
As depicted in the flow chart in Figure 2, the type of research project determines whether it can partake in the Safe Harbor. By participating in the Safe Harbor, healthcare, research, and disease advocacy organizations that plan to conduct an international, multi-site data-driven project whose primary purpose is presumptively scientifically valid141 and has a data-driven, ‘omics’ focus would avoid multiple IRB review within and between countries but still satisfy the local context concerns of the countries wherein the project is based. To do so, they must meet specified criteria (Box 3). The registration element would entail two main parts. First, researchers involved in a project would register on the IFER website's access portal so that their identity and bona fides can be checked. Second, upon confirmation, researchers (or specifically, the PI) would complete a standardized, publicly available online application form that requires several disclosures, including: (1) a comprehensive summary of the research project that conforms to a recommended format; (2) a brief summary of the main ethical issues the PI believes the project may raise; (3) all anticipated research procedures, benefits, risks, and burdens; (4) a plan for ensuring the confidentiality of research participants’ health information; and, if relevant, (5) a plan for maintaining the quality and security of data and/or biological materials; (6) how secondary or unsolicited and potentially clinically significant findings would be handled; and (7) plans for benefit sharing arrangements.
Figure 2.
Safe Harbor flow chart. Interested applicants who are undertaking an international, multi-site data-driven genomics project would be able to partake in the Safe Harbor Framework for International Ethics Equivalency, whose ethics review mechanism is represented in this flow chart. The process includes the PI(s) completing an online IFER registration form and other relevant documents (research plan, etc.), undergoing streamlined NCO screening and IFER review, and having the opportunity to appeal a decision.
The application form ideally could be submitted in several common languages, such as English, French, Spanish, German, Arabic, or Chinese. The criteria to be completed in this form, which could be framed as a comprehensive questionnaire, must be accurate and attested to by the PI as well as a signing party with the requisite authority to bind the research organization or institution. The application form could be supplemented by additional documentation if IFER technical officers request further clarification during the review process.
National Coordinating Offices (NCOs)
Before IFER technical officers undertake streamlined ethics review, each country that hosts a site (or sites) in a research project would have a vital role to play. Indeed, the formation of an international agency tasked with ethics review is impossible without the explicit buy-in of government bodies and institutions. But it also requires more than that. We envision a federated approach in the Safe Harbor whereby countries engage in a dialogue with each other and with IFER to work towards shared principles and norms, but also understanding of cultural specificities.
Each country's government or agency (and state or provincial equivalent) that is responsible for human subjects ethics review oversight would sign onto IFER via a revocable, voluntary agreement. Additionally, institutions could sign onto IFER, but as some may be more wary of potential liability issues than others, or simply reluctant to defer to a centralized IRB, IFER could agree that in exchange for joining, it would offer them greater liability protection, such as foregoing any third party claims against the institution if it (IFER) faces a lawsuit resulting from its activities.142 The agreement between IFER and a country would require the country to abide by an IFER Charter and Code of Conduct and to create a national coordinating office (NCO) for the specific types of research projects appertaining to the Safe Harbor. The agreement would mandate IFER to distribute the application form and additional documentation to the NCOs where a research project is planned. The NCOs would undertake a timely and efficient screening of the application to ensure that the research project adheres to the mandatory legal and ethical standards of that country (and/or province or state through communication with local agencies that are charged with human subjects research). These standards could range from laws and policies on human rights, privacy or data protection, to research involving humans or human biological materials.
In addition, NCOs would work towards achieving mutual recognition and coordination in the screening of a research study so as not to create a fragmented scenario of partial research study approval. Thus, NCOs would ensure that a research project within its jurisdiction ascribes to, at a minimum, IFER's promulgated ethical principles and norms (Box 2 and Box 3) so as to ensure ethics equivalency. Each NCO should have continuous, open communication channels with other NCOs and the IFER Secretariat, albeit in a way that protects the confidentiality of the research project and research participants where appropriate.
NCOs would also be responsible for coordinating the enforcement of the ethical obligations of the project in each country and would serve as the contact point for interested parties to direct questions and complaints regarding ethical issues of a project. Depending on the country's regulatory system and administrative/statutory authority, it may designate another enforcement authority to handle disputes (such as data protection authorities, health ministries, or an OHRP) or manage the disputes itself. Whatever the case may be, each NCO should endeavor to promote cross-border cooperation between enforcement authorities for global ethics protection of research participants.
Unlike the current IRB system, however, the NCO would not function as an IRB and engage in thorough, adjudicative ethics review. The emphasis in the Safe Harbor is on streamlined and efficient ethics review. At the NCO level, the benefit is reduced regulatory burden through coordinated screening at one central location, as opposed to burdensome review at multiple locations throughout a country, often at significant cost, delay, and uncertainty to researchers. To reiterate, the NCO's mandate would be to preliminarily screen the IFER application form and supplemental documentation to ensure that all necessary information has been submitted and that it meets local and national laws.
It is possible, of course, for a country to adopt or insist on more stringent standards, be it for data protection or modalities of consent, but the goal of IFER is to develop ethical best practices and interoperability for international research projects, so ideally such variation would be minimal. If the NCO considers the research project in its jurisdiction to pose unique ethical or regulatory issues, then it could, within a specified time period, notify IFER of particular requirements for ethically or legally conducting the project in its country (such as specific clauses in consent forms). This way, issues could be settled locally. Alternatively, the NCO could forward its comments or requests on to IFER, whose technical officers would consider them in conjunction with the IFER Ethics Committee.
Thus, IFER's final ethics review decision could be an approval of the project but with site-specific, tailored conditions that reflect unique circumstances. Additionally, it would be possible for an NCO to inform IFER that the project cannot go forward in its country because it violates local or national laws. But if the latter situation arises, there should be an opportunity for the PI (or co-PIs in that country) to rectify the concerns raised by the NCO within a reasonable period of time, assuming rectification is possible and the NCO specifies how its concerns could be rectified. For instance, if an NCO finds that a research project violates local data protection laws because personal data in that country will be transferred to another country without opt-out notification or adequate data protection laws, the PI or co-PIs should be informed of this concern (as should IFER and the NCO in the country of concern) and given a reasonable period of time to rectify it by formulating, for example, standard contractual clauses in an agreement between the study sites offering sufficient data protection safeguards.
While the one-NCO-per-country proposal improves the multiple-IRB-per-country situation, problems could still arise. In particular, because not all NCOs may be alike in the rigor they apply to application screenings, IFER's Compliance Branch should periodically audit NCO determinations (which should be documented and digitally archived on a secure IFER website portal) to assess their consistency across time and their variation among other NCOs. The Compliance Branch should also monitor the potential for any adverse ‘forum shopping’ that could arise where applicants design their projects to either take advantage of NCOs that are viewed as considerably less stringent in their screening process, or to bypass IFER review altogether by submitting applications to local IRBs.
Further compliance review by IFER's technical officers should assuage some of these concerns, but to take greater precaution, during the initial online registration stage, applicants should be required to disclose whether they have previously submitted the same research project proposal to any local or regional IRBs or NCOs, and if so, to disclose which IRBs or NCOs and the outcome of each review. Failure to disclose this information, and subsequent discovery by IFER of already-submitted applications with unfavorable ethics review outcomes, could lead to sanctions. Active disclosure of already-submitted applications with unfavorable ethics review outcomes could lead to a kind of ‘ethical estoppel’ of the research project, not to mention alleviate the risk of adverse forum shopping.
Element 2: Compliance Review
Once each NCO undertakes its preliminary screening, and assuming the NCO determines that the research project adheres to mandatory legal and ethical standards, it would then send its approval letter or comments on to IFER via a secure online portal. The application material would then be confidentially reviewed and benchmarked by the IFER technical officers against publicly available ethical norms and procedural safeguards established by IFER's Policy and Standards Branch that promote internationally consistent and substantially equivalent ethical assessment of large-scale, data-driven and genomics or ‘omics’-focused research projects (Box 2 and Box 3). While subject to ongoing revision and assessment, at the initial stage of IFER's creation, these norms would be procedural implementations of already established ethical principles espoused by documents such as the World Medical Association's Declaration of Helsinki143 and the International Ethical Guidelines for Biomedical Research Involving Human Subjects,144 albeit with modifications suitable for the type of research projects targeted by the Safe Harbor.
Initially, two technical officers would each have the responsibility for undertaking ethics review of the submitted forms. In order to provide wide latitude to regional variation, as expressed either by an NCO or the research project, IFER's reviewers would apply an in toto assessment for ensuring ethical protections, not necessarily letting one specific problematic ethical issue be the fatal blow for the project. For example, the inability of a research project to secure written informed consent of participants, if otherwise necessary, should not in itself prevent approval if the project site is in a location where obtaining written consent is challenging or culturally insensitive.
While the focus of the review should be on ethical issues, the widening division between ethics review and scientific review is too often exaggerated and counter-productive. As ethics committees now function like a regulatory authority,145 and as would especially be the case in an international organization like IFER, inevitably the committee will be tasked with both protecting participants and also promoting best practices and standards for research.146 Scientific review, therefore, should not be radically separated between funding agencies and IFER. Both should vet the research project to ensure that it meets standards for technically good science, particularly with respect to methodological issues.
At the same time, the non-interventional, minimal risk nature of research projects within IFER calls for a stronger deliberative platform for researchers and higher thresholds to require changes to a research project design.147 If both technical officers independently determine that the submitted application presents only minimal risk to research participants,148 and no NCO has submitted ethical issues of concern that require full IFER Ethics Committee deliberation, the application would be approved and exempted from further IFER review. Minor issues of concern raised by one or both technical officers should be resolved through negotiation with the PI and/or co-PIs. Notification of approval would immediately be sent to the PI and NCOs and disclosed on the IFER website.
After this first-stage review, for those likely few applications with NCO comments attached or presenting more than minimal risk as determined by at least one of the two technical officers, the two officers would then each present the research project, issues of concern, and his or her recommendation to a committee of five to seven technical officers (IFER Ethics Committee). At this stage, the PI should be provided the opportunity to be heard, and so he or she may choose to personally present the project proposal or elaborate on specific issues. Moreover, the IFER Ethics Committee should be able to call upon a standing list of independent consultants who could provide special expertise to the Committee on proposed research projects, be it in methodology, disease, or legal domain. These consultants can attend the meeting or provide written comments on a research project, provided they sign a confidentiality agreement.
Following a general discussion, the Committee would then make a consensus decision (approved as submitted; conditional approval; deferred decision; or not approved) in a timely manner that reflects an ethical judgment about the permissibility of the research project. Guiding the ethics review and discussion would be both the IFER baseline ethics equivalency norms and Rules of Procedure that govern the operation of IFER Ethics Committee and its meetings,149 the latter of which may take place on a monthly or bimonthly basis. Minutes of meetings would be memorialized and posted online, but anonymized when appropriate to protect the privacy of individuals and organizations.
The IFER Ethics Committee would be constituted and guided by four principles, namely independence, competence, pluralism, and transparency. Regarding the transparency principle, a mordant criticism of current ethics review governance is the absence of a published casework of precedents for IRB judgments and efficient means to appeal IRB decisions.150 In our view, since IFER would function quasi-judicially, as arguably IRBs currently do anyway,151 certain legal principles should follow. In addition to inviting the PI to take part in the IFER Ethics Committee deliberation of the issues presented so a more robust decision can be reached, the Ethics Committee should provide written reasons for its consensus decision and make these publicly available in a way that appropriately protects confidentiality.
While some may view this as an unnecessary cost, the number of times the IFER Ethics Committee would meet to adjudicate a research project proposing more than minimal risk would likely be small. More importantly, the purpose of such transparency is critically important: where an ethics review committee overrules the PI on an ethical point of contention, it should provide clear reasons for doing so. A database of written IFER Ethics Committee decisions offers precedents and the greater likelihood of consistency, rationality, and certainty in decision-making.152 The costs associated with written decisions are minor compared to the collateral benefits that could accrue to researchers and ethics reviewers alike, who would gain knowledge of carefully reasoned, principled decisions and the merits of the issues under consideration for a given research project. For researchers, this can only increase their trust in the legitimacy of the ethics review process. Additionally, in contrast to the status quo regulatory environment in many countries, we think there should be a natural presumption that research projects are ethical. The burden to stop or modify a research project should fall on IFER. Therefore, the obligation to provide written reasons for a decision better levels the playing field since it requires IFER to carefully deliberate over the ethical roadblocks to a project and to demand changes to a project or reject a project altogether.
Yet obligatory provision of written reasons alone is an insufficient regulatory feature of a Safe Harbor. There should also be a dispute resolution system to assuage concerns of concentrated power and allow for better appreciation of due process in the ethics review system, particularly the right to a fair hearing—a principle of natural justice known as audi alteram partem. If either the research project undergoing review or an NCO is dissatisfied with the IFER Ethics Committee decision, there can be a two-stage appeal. First, an internal appeals process would consist of re-review by the IFER Ethics Committee, wholly comprised of different ethics reviewers, who would adjudicate the proposed project de novo and allow the PI or NCO to submit new evidence and a brief that outlines why they believe the initial decision should be overturned. If upon re-review the IFER Ethics Committee reaches the same decision, the PI or NCO could appeal, requesting final, binding review by an ad hoc three person-committee of independent lawyers and research ethics experts (IFER External Ethics Appeal Board) appointed by the Bureau. The Appeal Board would review the research project and all the documents associated with the internal appeal, and upon clear and convincing evidence of incorrectly decided IFER Ethics Committee decisions, overturn the decisions by majority vote.
Compliance review also interacts with the issue of consent, particularly regarding two aspects: (1) initial consent as the research project unfolds (assuming it is prospective and not retrospective research), and (2) consent for secondary analyses of data generated in an IFER-approved project. First, at the initial stage of a project, each local site should, in consultation with the NCO, carry the obligation to notify research participants of the project and that their data (anonymized or coded) could be shared with other researchers confidentially and securely. When feasible, an option to opt-out of the research project should be provided to all participants in all countries, and there should also be an option to withdraw at any time, unless the data are anonymized.
Second, if a research project receives IFER approval, a question arises regarding what happens if another research project wishes to access the (personal) data for secondary analysis, and how the issue of consent would be addressed. One proposal could be a safe harbor within the Safe Harbor: IFER-approved research projects are permitted to disclose personal data without consent to ‘prescribed persons or entities’ (that is, certified researchers) for clearly defined and approved purposes, and which have in place privacy practices, policies, and procedures approved by IFER and the applicable NCO (ie countries where research participants and/or data are situated). These privacy practices, policies, and procedures should be publicly available, in addition to a description of the functions of the person or entity, so that any interested person may see whether they are satisfied with data being shared, and if they are a participant in the research project, whether they wish to remain a part of it. With respect to further downstream data use, the certified researchers may then disclose that data to other researchers for secondary analysis, but only under strict security measures, and only in either anonymized form or in coded form with approval of the applicable NCO and IFER. A key element of this mini safe harbor is that a Code of Conduct153 guides researchers from the IFER-approved study all the way downstream, and that there are meaningful penalties for researchers who abuse their access to personal data or wrongfully attempt to de-anonymize data.
Ideally, participants would be in control of their data: they would give consent for its use over a lifetime, but with a ‘live’ withdrawal option, unless their data is anonymized.154 Yet how data control mechanisms are designed varies, and some proposals may encounter practical problems. We question whether the majority of research participants, other than patients involved in disease-specific research, will desire constant engagement beyond monitoring of ongoing results, and whether any cafeteria-style panel of opt-ins/outs can make large-scale international research sustainable.155 So, short of wide-scale and immediate implementation of this approach, secondary use of personal information should be permitted only if both the NCO and IFER determine, based on an application for a consent waiver, that the secondary research use meets certain requirements such as minimal risk to participants and impracticability of obtaining new informed consent. A key component of this secondary use review is a proportionality test that considers the risks and benefits of the research in its particular context, the research purposes, with whom the study data and/or samples will or could be shared, the estimated time period of the project (including longitudinal), the likely effects on individuals and society, and the possible consequences of not approving the secondary use.
We champion informed consent as an ethical principle and manifestation of autonomy.156 But we also think that notions of solidarity in health research,157 ie that we all benefit from research using health information from current and past participants, impel reconsiderations of the need for specific, explicit consent in all instances, especially if a research participant faces almost no risk of direct physical harm, such as in the use of residual samples or longitudinal or observational studies.158 In particular, the Safe Harbor could consider the requirement of a new consent for supplementary use of previously collected personal data under a broad consent with monitoring and governance mechanisms to be unwieldy and unduly detrimental to large-scale data-based research. If: (1) a research project adheres to state-of-the-art data security standards, (2) original consent documents are sufficiently transparent about the current and future uses of data for biomedical research, (3) an option to withdraw is provided, and (4) ongoing ethics monitoring is present, the IFER Ethics Committee should look to the research project itself for determination of ethics and privacy protections, rather than the obtainment of fresh consent each and every time.
Element 3: Recognition
As e-governance is an emerging tool in the regulation of biomedical research,159 information technology should drive the Safe Harbor, ensuring adequate review, communication, oversight, and public participation. The IFER website, coordinated by the IT/Communications Branch, would contain separate portals for the public, projects and their institutions and funders, and governments, each with FAQs and additional information for educative purposes. It would maintain a publically accessible database of country (and state/provincial) laws relevant to reviewing human research protocols, and an up-to-date registry and digital archive of IFER-approved research projects, along with a lay summary of each project, the rationale for IFER's approval of the project so that other researchers may learn how to design an ethically valid project and promote continuous quality improvement, and site-specific contact information should participants or members of the public wish to obtain further information.
Element 4: Monitoring and Enforcement
IFER ethics approval would be subject to several conditions beyond satisfaction at the NCO level and adherence to IFER's ethics principles and procedural safeguards. The Safe Harbor requires a strong regulatory system that can receive and investigate complaints, monitor and evaluate compliance, ensure enforcement, and promote an international Code of Conduct for clear and consistent behavior by all actors within a well-defined scope. A Code of Conduct in the context of ethics review should include the PI's obligation to provide IFER's Compliance Branch with current information about the project and an agreement to be subject to occasional audits or ongoing assessments. It should also include mandatory notification by the PI to IFER and all applicable NCOs upon a data breach or other defined ethical lapse, clear statements of strict enforcement and penalties for data breaches or serious ethical lapses, and a prohibition on attempted individual re-identification or de-anonymization if personal data is coded or anonymized.
To further boost ethics monitoring and compliance, an independently functioning Ombuds Office would receive, investigate, and address complaints of possible ethics violations and report its findings and recommendations for changes to policies or procedures to the IFER Bureau. Such an office would also liaise with NCOs, who could investigate and address complaints at local project sites and pass along information to a research project's funders or institutional administrators. While there may be uneven local enforcement of IFER's international policies and standards, enforcement should be conducted, if it is to garner social and political legitimacy—foremost on a local level—with appropriate supervision and support by IFER.
Enforcement also requires looking inward. A non-profit agency is just as legally liable as a for-profit agency, particularly if it has legal personality through incorporation.160 IFER and its staff would be liable for professional misconduct or neglect of duties and breaches of the law. Further, as commentators have noted, IRB members face potential liability in negligence for issues such as failure to ensure proper informed consent, failure to address potential conflicts of interest, failure to ensure adequate screening of participants, negligent approval of study design, and procedural negligence.161 Should a research participant claim injury in the course of an IFER-approved research project (which presumably would be a psychosocial rather than physical injury, given the de minimis risks), that participant may attempt to recover compensation from multiple parties, including the NCO, IFER, and individual employees or agents.
One way to mitigate this risk is for IFER to include a term in its agreement with the NCO and a disclaimer on its website and online application form that it will not be personally liable for any injuries that may result from its review and that research projects, funding agencies, and participants agree to hold harmless IFER and each of its agents, employees, representatives, and volunteers from any and all liability, claims, losses, expenses, judgments, or demands, except for claims or litigation arising from gross negligence or willful misconduct. However, this mitigation strategy may face obstacles, including local enforcement and acceptance. IFER should therefore also consider indemnifying both internal and external ethics committee members and staff, and obtaining adequate liability insurance coverage for itself and its management and staff in case it faces lawsuits and participants are harmed by an IFER-approved study.
Element 5: Public Participation
While the Ethics Committee meetings should be conducted in camera to preserve the confidentiality of the information discussed and allow for uninhibited deliberation,162 IFER traverses the precincts of ethical deliberation and acts broadly as an international regulatory authority. Public accountability and trust in a regulatory system are best cultivated in an environment of participation and transparency.163 A possible criticism of the IFER Ethics Committee is that no so-called ‘layperson’ would sit on it. This is a deliberate choice. It is unfeasible and dangerously tokenistic to have one or two persons sitting on the Committee who are deemed to represent the interests and concerns of an undefined, protean ‘community.’ But what ‘laity’ is removed at the Ethics Committee level should be appended at the broader institutional level. Therefore, in addition to being transparent about its decisions (both for proposals approved and not approved) and the rationales for those decisions through annual reports and online disclosures, IFER should seek a plurality of views to prevent ethical and governance blind spots and encourage ‘ethical norm entrepreneurship.’164 This means it should embrace public participation in the development of ethics equivalency norms and related policies that set standards for research regulation.
However, public participation in itself does not necessarily lead to better decisions. Poor representation and legitimacy, cacophony, and short-sighted ethical and governance frameworks can quickly become a Lernaean Hydra for organizations that want to open up their governance.165 Coupled with a mindfulness of limited resources, the design principles for public participation in a regulatory system should ensure that a multiplicity of viewpoints is heard and legitimated, and that input from ‘experts in the wild’166 is translated into output that is more beneficial than it would be in a more classic, official expertise system.
Multiple avenues for meaningful public participation are possible. Resources and interest permitting, one possibility is for IFER to hold annual or biannual live-streaming conferences at the Secretariat that are open to all members of the public. The conferences could serve as a forum to review policies and standards and assess recent work done by IFER, including a review of Ethics Committee decisions. All participants would have opportunities to comment and possibly even vote on the review or adoption of new policies. By having publics come together and deliberate, the IFER Bureau and staff would be encouraged to continually reassess the Safe Harbor and scrutinize it in light of new information and diverse perspectives.
BENEFITS, OBJECTIONS, AND REFUTATIONS
Some questions that institutions and countries (not to mention critics) will ask first are: (1) what is the benefit of this Safe Harbor, (2) how is it better than the status quo, and (3) how much will it cost? Ascertaining exact financial outlays in the creation of the Safe Harbor is difficult as anticipated expenditures and cost savings depend on country and institutional support and economic studies of the proposed system. That said, there is likely to be a redistribution of costs from multiple local IRBs, paper generation, lawyer consultations, etc. to central digital document management and communication through IFER and NCOs. Economies of scale generally entail reductions in cost, including for IRBs.167 Thus, while countries, institutions, and research projects will have to politically and financially support the Safe Harbor, overall expenditures on the ethics review process should dissipate.
Benefits
In addition to anticipated cost savings, the benefits of the Safe Harbor for international ethics review harmonization are manifold. Generally speaking, research projects in the Safe Harbor would experience significant reduction in administrative hassle and redundant regulatory hurdles. Researchers could access data without undergoing multi-site IRB review that is wasteful, overly burdensome, and inconsistent. Because researchers and their institutions will be subject to a IFER Code of Conduct168 that requires NCOs or designated agencies with statutory authority to dole out strict sanctions for violations of the Safe Harbor policies and standards, countries, research participants, and members of the public can rest assured that a streamlined ethics review process does not equate to a reduction in oversight or enforcement. To the contrary, it means more efficient review and increased monitoring and sanctioning for ethical and legal transgressions. Moreover, countries can benefit from the Safe Harbor since it would allow their biomedical research sector to save money and time otherwise spent on multi-site ethics review. Countries will add value to their society through improvements in healthcare and public health, which not only have individual benefits but also collective economic benefits by way of increased economic development and productivity.169 Most importantly, society would enjoy the maximization of the potential benefits of biomedical research, which undoubtedly depends on continuing public support and trust in the integrity of the research ethics oversight system.
The Local Context Objection
Purported benefits aside, at least three main objections can be raised that challenge whether the Safe Harbor is better than the status quo. The first objection is that harmonization or standardization, be it in law or guidelines, wrongly effaces policies or rules that arise out of local tradition, culture, and knowledge. The argument is simple but powerful: local context matters.170 Some contend that what works in one location may not work in another, and that it is far from certain that harmonized guidelines reflect a fair representation of multiple areas rather than a ‘regionalism in disguise,’171 running roughshod over cultural sensitivities. It may be that, ‘It is a paradox of the harmonisation process that it aims at removing differences, but derives its acceptability from diversity.’172 Yet, it could also be, as others counter, that harmonization works towards substantial equivalency, that it seeks not to eliminate differences, but rather seeks to make differences compatible,173 and is a ‘process in which points of policy convergence are identified and nuances in their detailed provisions are accepted as part of the realities of policy making activities across sovereign states.’174
Those who object to a Safe Harbor based on the value of local context have an obligation to delineate local context specificities that make the status quo ethics review system function well. These specificities may be legal culture, unique interpretations of principles, traditional customary practices, or traits in the general environment. Each concept is different and must be treated as such.175 This differential treatment of each concept is important because as Coleman notes, ‘to the extent…differences continue to exist, they are more likely to be relevant to issues like subject recruitment or informed consent than to assessment of a protocol's underlying risks and benefits.’176 Further, documenting and determining what counts as local or traditional customary practice is challenging from a geographical, sociological, and historical perspective. For example, does local context reflect the general values of a community, or those of the majority? Many subgroups exist within communities but they may be transient in time, space, and social relationship. Those who take up the battle cry of local context run the risk of championing majority group cultural values over minority group values and presenting a monochromatic values mirage of a locality.177
This is not to deny the importance of local context. As many scholars note, our contemporary world is marked by an intermingling of the global and local (‘glocal’).178 What the Safe Harbor can and should do is be attentive to ‘pluralism [as] a basic fact in the interpretation and status’ of ethical norms and values.179 The heterogeneity of IFER's technical officers allows for a tailored approach to a research project that raises issues of concern to particular communities. But tailored approaches still rely on a bedrock of shared principles and norms, even if their interpretation or weight, and their accompanying detailed provisions, may vary. In this sense, accounting for the local preferences of each jurisdiction beyond acceptance of more general features is counter-productive and could potentially unravel the purpose of ethics equivalency, which is to enable researchers to engage in international research collaboration with the support of a modern ethics review structure.
The creation of a Safe Harbor differs from the imposition of a legal system, which should be adapted to its local environment, be it culture, infrastructure, institutions, complexity, or resources. The Safe Harbor is instead a flexible, nimble, and agile extra-legal policy approach that is the product of shared learning180 of regional differences and is the reflection of a synthesis of shared principles and norms attuned to a concern for humankind.181 Regional differences are respected in a system where the values language is common, yet spoken in different dialects. Indeed, nothing in the Safe Harbor design precludes obtaining an expert report from local ethicists on any matter where cultural sensitivity may be important for the ethical analysis. Through the diversity of IFER's technical officers, expert advisors, and independent consultants, and by incorporating NCOs into the system, local contexts—whatever they may be—can be preserved and respected within reasonable limits.
The Safe Harbor-as-Suboptimal Objection
Another possible objection is that safe harbor frameworks are suboptimal. The argument goes that not every country has an institution, or the resources to create an institution, that is capable of monitoring compliance and enforcing sanctions when lapses occur. Similar lines of argument critique the self-regulatory nature of some safe harbors. For example, several commentators, and the European Commission more recently, have criticized the US-EU Safe Harbor Framework for being ‘ineffective in practice’182 and express concerns about the effectiveness of industry self-regulation compared to an enforceable rights-based approach. Leathers notes that critics fault inadequate internal and external enforcement mechanisms,183 while Connolly's empirical assessment of 1,597 organizations in the Safe Harbor List exposed problems with compliance and false and/or misleading information.184
However, this criticism may be applicable only to certain models of safe harbor frameworks. The criticism overlooks the benefits a Safe Harbor can have for non-profit institutions that host or sponsor many research projects. Precisely because our proposed Safe Harbor facilitates a streamlined and flexible ethics review approach, research projects (and their institutions or funders) do not have to spend significant resources to provide effective ethics protection. But even within the safe harbor frameworks that have been criticized, such as the US-EU Safe Harbor Framework, enforcement has in fact been successful, witnessed by the recent FTC settlements with Google, Facebook, and MySpace.185 A leading privacy lawyer has remarked that a mutual recognition system can ‘cause higher regulatory standards from one system gradually to influence another system; for example, the author is aware from his personal experience of many companies based in the US that have adapted their privacy practices to become closer to EU standards after having joined the Safe Harbor mechanism.’186 The criticism also has not stopped the US and EU from jointly stating in 2012 that ‘over 3,000 companies have self-certified to the Framework to demonstrate their commitment to privacy protection and to facilitate transatlantic trade’ and jointly pledging to ‘remain dedicated to the operation of the Safe Harbor Framework.’187 One should be aware of the potential suboptimality of safe harbors, but that does not mean a safe harbor framework in general is problematic. Rather, it gives impetus to design—and openness to modifying—a system that mitigates risks and avoids pitfalls.
The Complex Bureaucracy and One-Size-Fits-All Objection
Finally, a third objection likely to be leveled at the proposed Safe Harbor is that it is too complex and bureaucratic. Between the capital investments, political obstacles, under-developed ethics infrastructure of many countries, and the formidable task of inventing a governance structure, one is tempted to dismiss the Safe Harbor as utopian. Further, some may find it incoherent to claim that a current problem with the ethics review system is the one-size-fits-all nature of local IRB review and yet propose an international, one-application-fits-all-countries approach.
Contrary to the latter critique, we see no incoherence. A problem with the current system is that PIs of international genomics research projects often must shoehorn their protocol into a standard consent form template for each IRB, which typically is designed for clinical trials or other studies for research on or with humans, not data. This can cause over-disclosure, under-disclosure, or nondisclosure of information that may be pertinent for an IRB review. Furthermore, the perspective from which IRB members may assess the research project may be template form or traditional research project-fixated, causing an overly burdensome review of research that does not fit inside the classic research-on-human-subjects box. True, the Safe Harbor would have a standard, universal application form, and review would be consolidated into one stream (coupled with NCO screening), but this is to promote much-needed international interoperability, and the application form itself would be designed specifically for the type of research falling within the Safe Harbor: genomics (and ideally in the future, biomedical research more generally). Thus, a one-application-fits-all-countries approach we think is entirely coherent in the quest for a functional and legitimate 21st century ethics review system for global biomedical research.
Undoubtedly, the proposal is bold, and the task daunting. The current system and other alternatives, however, are incapable of tackling the problem—and a problem it is. Serious capital and operating investments will be necessary to build the Safe Harbor, but as Coleman observes, this should not cause opprobrium:
[I]f we are serious about reforming the human subject protection system, we must accept the fact that doing so will cost money. … Moreover, paying for a more rigorous IRB review process can be seen as an investment in the future of biomedical research, to the extent it will help regain public trust in the oversight system's integrity.188
Consider also the claim of too much bureaucracy. The status quo system in fact is more bureaucratic than the Safe Harbor. Observes Widdows:
Rather than admit that current models do not work, the current [ethics governance] model simply adds more processes and tiers of bureaucracy in an attempt to improve current practices, for instance, by focusing on refining informed consent forms, as if further scrutiny will succeed in making this an adequate ethical system.189
The Safe Harbor streamlines the bureaucracy involved in ethics review by funneling the multiple channels into one.
Consider also the claim of too much complexity. The Common Rule's ‘equivalent protections’ provision190 signals the kind of assemblage needed for large-scale research projects to extricate themselves from the morass of multiple IRBs and disjointed ethics principles and standards. Yet we cannot design a system where it is one country in one region imposing its ethics norms on everyone else, with the expectation that those norms are the gold standard for all other countries to follow. Not only is that disingenuous and culturally hegemonic, it is unworkable at the international level.
To scale up the Common Rule's ‘equivalent protections’ provision to a level where researchers can collaborate in a seamless but pluralistic and ethically sound way, a global Safe Harbor is required. No extant international agency exists to design, coordinate, and promote a safe harbor for international ethics equivalency. Some might opine that the WHO is more than capable to coordinate ethics harmonization. We refute that contention on the grounds that the WHO's remit, based on its Constitution, is limited to international health work, which only tangentially relates to international ethics review.191
Policy complexity and some bureaucracy are inevitable when attempting to remedy a complex governance issue. But perplexity is not. For this reason, we think that the streamlined approach the Safe Harbor offers, as established by an international voluntary compact, gives it greater likelihood to gain international acceptance even if it entails initial heavy lifting. For it is heavy lifting shared by all, through international coordination at IFER and through local coordination at the NCOs. It is a system where all contribute to the betterment of human health by first identifying impediments and then rectifying them—constantly—by seeing what works and what does not. Unlike the status quo, the Safe Harbor is not inputs and processes-oriented. It is outputs and consequences-oriented. It respects national sovereignty while holding no single country's structure as the gold standard. It challenges governments to be as collaborative and intrepid as researchers. It asks publics to come to the table in making the system better, to ensure that the ethics policies and standards actually reflect the pluralist world in which we live. In short, the Safe Harbor is not utopian. It is pragmatic.
CONCLUSION
In this Article, we have sought to demonstrate the undue burdens borne by researchers, research participants, and society because of the current ethics review system. That system, particularly when it comes to multi-site research ethics review, unduly impedes advances in human health because it is costly, fragmented, inefficient, inadequate, and inconsistent. To accelerate the translation of ELSI research findings into practice and policy, and to maintain the public's trust in the integrity of the research ethics overview system, we have proposed a Safe Harbor that advocates structural global governance reform. This Safe Harbor, built around a voluntary compact signed by countries, institutions, funding agencies, philanthropies, and healthcare, patient advocacy, and research organizations, confronts the challenges we face in bridging 21st century data-driven biomedical research with an increasingly anachronistic ethics review system. Now is the time for the international community to come together and act with a unified voice.
Acknowledgments
The authors wish to thank the anonymous referees for their comments on this article. This work was supported by the Government of Canada through Genome Canada and the Canadian Institutes of Health Research, the Quebec Breast Cancer Foundation, and the Ministère de l'enseignement supérieur, de la recherche, de la science et de la technologie du Québec through Génome Québec.
Footnotes
Edward S. Dove, LL.M. (Columbia Law School), Academic Associate at the Centre of Genomics and Policy, McGill University, Montreal, Canada.
Bartha M. Knoppers, Ph.D. (Université de Paris I, Panthéon-Sorbonne), Director of the Centre of Genomics and Policy and Canada Research Chair in Law and Medicine. Admitted to the Bar of Québec in 1985. Centre of Genomics and Policy, McGill University and Génome Québec Innovation Centre, 740 Dr Penfield Avenue, Suite 5200, Montreal, Quebec, Canada, H3A 0G1. Tel: +1-514-398-8155; E-mail: bartha.knoppers@mcgill.ca.
Ma'n H. Zawati, LL.M. (Université de Montréal), lawyer, Academic Coordinator of the Centre of Genomics and Policy, Associate Member of the Biomedical Ethics Unit at McGill University.
Jonathan Adams, Collaborations: The Fourth Age of Research, 497 Nature 557, 559 (2013) (claiming that ‘we are entering a fourth age of research, driven by international collaborations between elite research groups’ and that ‘there is a growing divide between international and domestic research.’) [hereinafter, Adams, Collaborations]; Alan I. Leshner & Vaughan Turekian, Harmonizing Global Science, 326 Science 1459 (2009) (‘As more countries have invested in science and technology to advance their societies, high-quality science is increasingly being carried out in every part of the world. The scientific enterprise has become highly collaborative both within and across countries.’). Commentators have documented increasing amounts of cross-border scientific collaboration. See, eg Olle Persson, Wolfgang Glänzel & Rickard Danell, Inflationary Bibliometric Values: The Role of Scientific Collaboration and the Need for Relative Indicators in Evaluative Studies, 60 Scientometrics 421 (2004); Loet Leydesdorff et al., International Collaboration in Science: the Global Map and the Network, 22 El Profesional de la Informacion 87 (2013); Jonathan Adams et al., International Collaboration Clusters in Africa, 98 Scientometrics 547 (2014).
Human Heredity and Health in Africa (H3Africa) Initiative, http://www.h3africa.org/ (accessed 1 November 2013). H3Africa is an ongoing global effort to apply genomic science and associated technologies to further understand the biological and environmental determinants of common diseases with the goal of improving the health of African populations.
1000 Genomes Project, http://www.1000genomes.org (accessed 2 November 2013).
International Cancer Genome Consortium (ICGC), http://www.icgc.org (accessed 2 November 2013).
International Rare Diseases Research Consortium (IRDiRC), http://www.irdirc.org (accessed 2 November 2013).
Electronic Medical Records and Genomics (eMERGE) Network, http://emerge.mc.vanderbilt.edu/ (accessed 2 November 2013). See also Catherine A. McCarty et al., The eMERGE Network: A Consortium of Biorepositories Linked to Electronic Medical Records Data for Conducting Genomic Studies, 4 BMC Med. Genomics 13 (2011).
Database of Genotypes and Phenotypes (dbGaP), http://www.ncbi.nlm.nih.gov/gap/ (accessed 30 October 2013).
European Bioinformatics Institute, http://www.ebi.ac.uk/ (accessed 30 October 2013).
DNA Data Bank of Japan, http://www.ddbj.nig.ac.jp/ (accessed 30 October 2013).
European Research Infrastructure Consortia (ERIC) are entities with legal personality and full legal capacity established by participating EU member states. See Council Regulation (EC) No. 725/2009 of 25 June 2009 on the community legal Framework for a European research Infrastructure Framework (ERIC), 2009 OJ (L 206) 1. See also European Commission, European Research Infrastructure Consortium (ERIC), http://ec.europa.eu/research/infrastructures/index_en.cfm?pg=eric (accessed 7 January 2014).
White Paper, Creating a Global Alliance to Enable Responsible Sharing of Genomic and Clinical Data (3 June 2013), http://oicr.on.ca/files/public/White_paper_2013_06_03_FINAL.pdf (accessed 7 January 2014) [hereinafter, White Paper].
Nat'l Inst. Health, Draft NIH Genomic Data Sharing Policy Request for Public Comments, 78 Fed. Reg. 183 (proposed 20 September 2013).
Eur. Med. Agency, Draft Policy 70: Publication and Access to Clinical-Trial Data (proposed 24 June 2013), http://www.ema.europa.eu/docs/en_GB/document_library/Other/2013/06/WC500144730.pdf (accessed 7 January 2014).
Food & Drug Admin., Availability of Masked and De-identified Non-Summary Safety and Efficacy Data, 78 Fed. Reg. 107 (proposed 4 June 2013).
Viktor Mayer-Schönberger & Kenneth Cukier, Big Data: A Revolution that Will Transform How We Live, Work, and Think 8 (2013) (‘From the sciences to healthcare, from banking to the Internet, the sectors may be diverse yet together they tell a similar story: the amount of data in the world is growing fast, outstripping not just our machines but our imaginations.’)
Jane Kaye et al., From Patients to Partners: Participant-Centric Initiatives in Biomedical Research, 13 Nat. Rev. Genet. 371 (2012).
See, eg 2 Trials of War Criminals Before the Nuremberg Military Tribunals Under Control Council Law No. 10, at 181, 182 (U.S. GPO, 1946–1949) [hereinafter Nuremberg Code]; World Med. Ass’n, World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects (1964) (amended 2013), http://www.wma.net/en/30publications/10policies/b3/ (accessed 7 January 2014) [hereinafter Declaration of Helsinki]; The Nat'l Comm'n for the Prot. of Human Subjects of Biomedical & Behavioral Research, The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research (1979), http://www.hhs.gov/ohrp/humansubjects/guidance/belmont.html (accessed 7 January 2014). See also G.A. Res, 2200A (XXI), U.N. GAOR, 22nd Sess., Supp. No 16, U.N. Doc A/6316 at art. 7 (16 December 1966) (‘[N]o one shall be subjected without his free consent to medical or scientific experimentation.’).
Mollie R. Cummins, Nonhypothesis-Driven Research: Data Mining and Knowledge Discovery, in Clinical Research Informatics 277–91 (Rachel L. Richesson & James E. Andrews eds., 2012).
Bartha M. Knoppers, Ma'n H. Zawati & Emily S. Kirby, Sampling Populations of Humans Across the World: ELSI Issues, 13 Annu. Rev. Genom. Hum. Genet. 395 (2012).
Isabelle Budin-Ljøsne et al., Data Sharing in Large Research Consortia: Experiences and Recommendations from ENGAGE, Eur. J. Hum. Genet. (forthcoming, 2014), http://www.nature.com/ejhg/journal/vaop/ncurrent/full/ejhg2013131a.html (accessed 6 January 2014).
Adams, Collaborations, supra note 1; Kaye et al., supra note 16.
Seiichi Mori et al., Utilization of Genomic Signatures to Identify Phenotype-Specific Drugs, 4 PLoS One e6772 (2009), http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0006772 (accessed 7 January 2014); Geoffrey S. Ginsburg, Realizing the Opportunities of Genomics in Health Care, 309 JAMA 1463 (2013).
Cancer Genome Atlas Research Network, Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways, 455 Nature 1061 (2008).
Kyriaki Michailidou et al., Large-Scale Genotyping Identifies 41 New Loci Associated with Breast Cancer Risk, 45 Nat. Genet. 353 (2013); Cancer Genome Atlas Network, Comprehensive Molecular Portraits of Human Breast Tumours, 490 Nature 61 (2012); Cancer Genome Atlas Research Network, Integrated Genomic Analyses of Ovarian Carcinoma, 474 Nature 609 (2011).
Collaborative Oncological Gene-environment Study, http://www.cogseu.org/ (accessed 1 November 2013). See also Editorial, Open to Interpretation, 31 Nature Biotech. 661 (2013).
Heather Widdows, Between the Individual and the Community: The Impact of Genetics on Ethical Models, 28 New Genet. & Soc. 173, 175 (2009) (‘The ethical focus of bioethics is (or has been) almost exclusively the individual.’); Onora O’Neill, Broadening Bioethics: Clinical Ethics, Public Health and Global Health, Nuffield Council on Bioethics Lecture (24 May 2011), http://www.nuffieldbioethics.org/sites/default/files/files/Broadening_bioethics_clinical_ethics_public_health_&global_health.pdf (‘[M]odern medical ethics has been radically individualistic.’) (accessed 7 January 2014). Arguably, some elements of research ethics are attuned to group-based concerns, as reflected in international guidance documents such as the Declaration of Helsinki and the Council for International Organizations of Medical Sciences’ (CIOMS) International Ethical Guidelines for Biomedical Research Involving Human Subjects. However, it seems to us that the driving force behind much of Western research ethics as a discipline and concept has been an individualistic notion of autonomy, which can undermine trust relationships and stifle other principles that seek to widen the scope of ethical concern. See generally Martin Wilkinson, Individualism and the Ethics of Research on Humans, 16 HEC Forum 6 (2004); Jennifer Nedelsky, Law's Relations: A Relational Theory of Self, Autonomy, and Law (2011); Onora O’Neill, Autonomy and Trust in Bioethics (2002). Even the concept of justice (eg distributive justice), which at first glance seems to be a bulwark against individual autonomy, can in fact rest on libertarian values that exhort individuals to be responsible for their own health and well-being. See Rogeer Hoedemaekers, Bert Gordijn & Martien Pijnenburg, Solidarity and Justice as Guiding Principles in Genomic Research, 21 Bioethics 342, 344–46 (2007).
See, eg Piret Veerus, Joel Lexchin & Elina Hemminki, Legislative Regulation and Ethical Governance of Medical Research in Different European Union Countries, J. Med. Ethics (forthcoming, 2013) (‘Besides large variations in the legal regulation of medical research in the EU, our study found notable differences between countries concerning the type of research handled by [research ethics committees], which sometimes differed from that specified by national laws.’).
Carol A. Heimer & JuLeigh Petty, Bureaucratic Ethics: IRBs and the Legal Regulation of Human Subjects Research, 6 Annu. Rev. Law Soc. Sci. 601, 605 (2010).
See generally Comment, Re-Balancing State and Federal Power: Toward a Political Principle of Subsidiarity in the United States, 5 Am. U. L. Rev. 1421, 1446–56 (2005).
Anne-Marie Tassé, From ICH to IBH in Biobanking? A Legal Perspective on Harmonization, Standardization and Unification, 7 Stud. Ethics Law Technol. 1 (2013).
‘Omics’ is a term used to describe research on the ‘ome,’ that is, the nature of, a particular biomedical field. Examples include genomics (the quantitative study of genes, regulatory and non-coding sequences), transcriptomics (RNA and gene expression), proteomics (protein expression), metabolomics (metabolites and metabolic networks), and pharmacogenomics (the quantitative study on how genetics affects hosts’ responses to drugs). See also Monya Baker, Big Biology: The ’Omes Puzzle, 494 Nature 416 (2013).
Bartha M. Knoppers et al., Towards a Data Sharing Code of Conduct for International Genomic Research, 3 Genome Med. 46 (2011), http://genomemedicine.com//content/3/7/46 (accessed 7 January 2014).
In the context of research involving humans, jurisdictions contain different kinds of research ethics structures, but generally they are constituted in the form of an ethics review body. Ethics committees may also be called ethics review boards (ERBs), research ethics boards (REBs) or research ethics committees (RECs). While IRBs are institution-specific, ERBs, REBs, or RECs may not be. Instead, they may be charged with evaluating research projects within a specific geographic area. See, eg Søren Holm, How Many Lay Members Can You Have in Your IRB?: An Overview of the Danish System, 14 IRB: Ethics and Human Research 8 (1992). Countries have varied approaches to research ethics review. Some require research ethics committee approval for any research involving humans, while others only suggest it. Some research ethics committees are formal and statutory, while others are informal and voluntary. Research ethics committees can be located in many sectors, including public universities, hospitals, or the private sector.
See generally Eve Garrard & Angus Dawson, What is the Role of the Research Ethics Committee? Paternalism, Inducements, and Harm in Research Ethics, 31 J. Med. Ethics 419 (2005); Jean Philippe de Jong, Myra C.B. van Zwieten & Dick L. Willems, Ethical Review from the Inside: Repertoires of Evaluation in Research Ethics Committee Meetings, 34 Soc. Health & Illness 1039 (2012); Marilys Guillemin et al., Human Research Ethics Committees: Examining their Roles and Practices, 7 J. Empir. Res. Hum. Res. Ethics 38 (2012); Laura Stark, Behind Closed Doors: IRBs and the Making of Ethical Research (2012).
See Diane E. Hoffmann, J. Dennis Fortenberry & Jacques Ravel, Are Changes to the Common Rule Necessary to Address Evolving Areas of Research? A Case Study Focusing on the Human Microbiome Project, 41 J.L. Med. & Ethics 454, 455 (2013) (observing that new large-scale data-driven biomedical research projects present ‘a variety of nontraditional issues that were not present in the classic model of one research scientist or team working in a single lab or clinic and attempting to determine the effectiveness of a new drug or device’).
Mats G. Hansson et al., Patients Would Benefit From Simplified Ethical Review and Consent Procedure, 14 Lancet Oncol. 451 (2013); Tassé, supra note 30; Bartha M. Knoppers, Consent to ‘Personal’ Genomics and Privacy, 11 EMBO Rep. 416 (2010).
Public Population Project in Genomics and Society (P3G), http://www.p3g.org/ (accessed 3 November 2013).
Jane Kaye et al., ELSI 2.0 for Genomics and Society, 336 Science 673 (2012).
Kaye et al., supra note 16.
See, eg Budin-Ljøsne, supra note 20; Rashmi Kadam & Shashikant Karandikar, Ethics Committees in India: Facing the Challenges! 3 Perspect. Clin. Res. 50 (2012); En-chang Li et al., Chinese Ethics Review System and Chinese Medicine Ethical Review: Past, Present, and Future, 17 Chin. J. Integr. Med. 867 (2011).
James F. Childress, Who Should Decide? Paternalism in Health Care 66 (1982).
Suzanne Philips-Nootens, Pauline Lesage-Jarjoura & Robert P. Kouri Éléments de responsabilité civile médicale 139 (3rd edn, 2007) (Fr.).
See generally Heather Widdows, The Connected Self: The Ethics and Governance of the Genetic Individual (2013).
Witness, for example, the development of Reg4ALL (https://www.reg4all.org/), a website run by the non-profit Genetic Alliance that gives patients personal privacy controls and greater say in how their data is used for medical research.
Bartha M. Knoppers & Ruth Chadwick, Human Genetic Research: Emerging Trends in Ethics, 6 Nat. Rev. Genet. 75 (2005); Margit Sutrop, Changing Ethical Frameworks: From Individual Rights to the Common Good?, 20 Cambridge Q. Healthcare Ethics 533 (2011).
Amy A. Lemke et al., Public and Biobank Participant Attitudes toward Genetic Research Participation and Data Sharing, 13 Pub. Health Genomics 368, 374 (2010) (finding that participants in focus groups identified trust in a research organization ‘as a key positive influence to participation in genetic research.’)
Jantina de Vries et al., Ethical Issues in Human Genomics Research in Developing Countries, 12 BMC Med. Ethics 5 (2011), http://www.biomedcentral.com/1472-6939/12/5 (accessed 7 January 2014); Nancy E. Kass, The Structure and Function of Research Ethics Committees in Africa: A Case Study, 4 PLoS Medicine e03 (2007), http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.0040003 (accessed 7 January 2014).
Béatrice Séguin et al., Genomic Medicine and Developing Countries: Creating a Room of Their Own, 9 Nat. Rev. Genet. 487 (2008); Béatrice Séguin et al., Genomics, Public Health and Developing Countries: The Case of the Mexican National Institute of Genomic Medicine (INMEGEN), Suppl. 1 Nat. Rev. Genet. S5 (2008); Billie-Jo Hardy et al., From Diversity to Delivery: The Case of the Indian Genome Variation Initiative, Suppl. 1 Nat. Rev. Genet. S9 (2008); Ruha Benjamin, A Lab of Their Own: Genomic Sovereignty as Postcolonial Science Policy, 28 Pol. Soc. 341 (2009).
Supra note 3.
Malaria Genomic Epidemiology Network (MalariaGEN), http://www.malariagen.net/ (accessed 5 November 2013).
de Vries et al., supra note 47.
World Health Org., Strategic Initiative for Developing Capacity in Ethical Review, http://www.who.int/sidcer/en/ (accessed 1 November 2013).
Global Forum for Health Research, The 10/90 Gap in Health Research (2000).
Joseph A. Catania et al., Survey of U.S. Human Research Protection Organizations: Workload and Membership, 3 J. Empir. Res. Hum. Res. Ethics 57 (2008).
Andrew Abbott, The System of Professions: An Essay on the Division of Expert Labor (1988).
Scott Burris & Kathryn Moss, U. S. Health Researchers Review their Ethics Review Boards: A Qualitative Study, 1 J. Empir. Res. Hum. Res. 39 (2006).
Carl H. Coleman, Rationalizing Risk Assessment in Human Subject Research, 46 Ariz. L. Rev. 1, 14 (2004).
Bartha M. Knoppers, Challenges to Ethics Review in Health Research, 17 Health L. Rev. 47 (2009). However, it may also be that the underlying nature of research ethics review is an intuitive, fact-sensitive process that is inherently incapable of systematization. If this is so, it may also explain why there is absence of research ethics committee ‘jurisprudence.’ Yet, it remains a potent criticism that the IRB decision-making process around much of the world is unstructured and raises problems of legitimacy: members are not required to explain or justify the reasons for their decisions, and absent a coherent system of precedent, it encourages idiosyncratic, impressionist judgments, or ‘gut reasons.’ See Coleman, supra note 57, at 14.
Heimer & Petty, supra note 28, at 611. See also Guillemin et al., supra note 34 at 42, 43 (‘Some researcher participants suggested that ethics committees also adopted an additional role, which was to protect the institution's interests.’); Harold Edgar & David J. Rothman, The Institutional Review Board and Beyond: Future Challenges to the Ethics of Human Experimentation, 73 Milbank Q. 489, 493 (1995) (‘In effect, then, the regulations governing the IRB are, to say the least, a permeable shield, with no strong framework to ensure that subjects’ interests take precedence over institutional ones.’).
Murray Dyck & Gary Allen, Is Mandatory Research Ethics Reviewing Ethical?, 39 J. Med. Ethics 517 (2013).
Heimer & Petty, supra note 28, at 606.
Id.
See, eg Scott Kim, Peter Ubel & Raymond De Vries, Pruning the Regulatory Tree, 457 Nature 534 (2009).
Rosamond Rhodes et al., De Minimis Risk: A Proposal for a New Category of Research Risk, 11 Am. J. Bioethics 1, 3 (2011) (‘[M]ost of the sample collection and future use associated with microbiome and biobank genetic studies are likely to involve only minute physical risks, the kind of risks involved, say, in having a cheek swab. In most cases, the physical risks are so small compared with the risks of everyday life, that we consider them de minimis.’).
Keith Humphreys, Jodie Trafton & Todd H. Wagner, The Cost of Institutional Review Board Procedures in Multicenter Observational Research, 139 Ann. Intern. Med. 77 (2003).
William Burman et al., The Effects of Local Review on Informed Consent Documents From a Multicenter Clinical Trials Consortium, 24 Controlled Clin. Trials 245, 251 (2003).
Bernard Ravina et al., Local Institutional Review Board (IRB) Review of a Multicenter Trial: Local Costs Without Local Context, 67 Ann. Neurol. 258, 259 (2010).
Coleman, supra note 57 at 11 (‘A 1996 General Accounting Office study found that IRBs are overburdened, underfunded, insufficiently prepared … . Two years later, a report issued by the Office of the Inspector General of the Department of Health and Human Services … reached essentially the same conclusions.’)
Jerry Menikoff, The Paradoxical Problem with Multiple-IRB Review, 363 New Eng. J. Med. 1591 (2010).
Id. at 1592.
E-mail from Anne Junker, Sci. Dir., Maternal Infant Child & Youth Research Network of Canada (MICYRN), to authors (9 July 2013, 20:23 EST) (on file with authors).
Id.
Paul Hébert & Raphael Saginur, Research Ethics Review: Do It Once and Do It Well, 180 Can. Med. Ass'n J. 597 (2009).
Zubin Master, Nola M. Ries & Timothy Caulfield, Balancing Efficiency and the Protection of Research Participants: Canadian Allergy/Asthma Researchers’ Perspectives on the Ethics Review of Multi-Site Health Research, 2 J. Clinical Res. & Bioethics 104e (2011).
Breast Cancer Association Consortium, http://ccge.medschl.cam.ac.uk/consortia/bcac/ (accessed 31 October 2013).
Emma Angell et al., Consistency in Decision Making by Research Ethics Committees: A Controlled Comparison, 32 J. Med. Ethics 662, 663 (2006).
Common Rule, 45 C.F.R. pt. 46 (2012).
Charles W. Lidz et al., How Closely Do Institutional Review Boards Follow the Common Rule?, 87 Acad. Med. 1 (2012).
Elaine Gibson et al., Who's Minding the Shop? The Role of Canadian Research Ethics Boards in the Creation and Uses of Registries and Biobanks, 9 BMC Med. Ethics 17, 21 (2008), http://www.biomedcentral.com/1472-6939/9/17 (accessed 7 January 2014).
Lee A. Green et al., Impact of Institutional Review Board Practice Variation on Observational Health Services Research, 41 Health Services Res. 214, 219–21 (2006).
Id. at 221.
Id. at 220, 222.
Timothy Caulfield, Nola Ries & Graham Barr, Variation in Ethics Review of Multi-Site Initiatives, 3 Amsterdam L. Rev. 85, 86 (2011).
Lura Abbott & Christine Grady, A Systematic Review of the Empirical Literature Evaluating IRBs: What We Know and What We Still Need to Learn, 6 J. Empir. Res. Hum. Res. 3 (2011).
George Silberman & Katherine L. Kahn, Burdens on Research Imposed by Institutional Review Boards: The State of the Evidence and Its Implications for Regulatory Reform, 89 Milbank Q. 599 (2011).
Juliana C. Cartwright et al., Investigators’ Successful Strategies for Working With Institutional Review Boards, 36 Res. in Nursing & Health 478 (2013).
Austl. Gov’t, National Statement on Ethical Conduct in Human Research Chapter 5.3.1, 2 (2007) (amended 2013) (allowing research ethics committees to accept review by a single ethics review body). Australia's centralized National Health and Medical Research Council oversees local human research ethics committees (HRECs) and, through its Harmonisation of Multi-Centre Ethical Review (HoMER) initiative, offers a National Ethics Application form. See National Health and Medical Research Council, National Ethics Application Form, https://www.neaf.gov.au/default.aspx (accessed 7 January 2014). See also Mark A. Rosenthal et al., Ethics Committee Reviews and Mutual Acceptance: A Pilot Study, 35 Internal Med. J. 650 (2005) (discussing the development of a research ethics committee mutual acceptance model that aims to facilitate aspects of multi-site research studies that could increase the efficiency of the research ethics committee review process).
New Zealand's Health Research Council accredits Institutional Ethics Committees (IECs) and the four Health and Disability Ethics Committees (HDECs) in the country, the latter of which can also act as a multi-regional ethics committee. Health Research Council of New Zealand, http://www.hrc.govt.nz/ethics-and-regulatory (accessed 3 November 2013). Additionally, the National Ethics Advisory Committee independently advises the New Zealand Minister of Health on ethical issues related to health and disability research and services and determines nationally consistent ethical standards. National Ethics Advisory Committee, http://neac.health.govt.nz/ (accessed 1 November 2013). Applying for HDEC review requires the completion of a national application form. See Health and Disability Ethics Committees, HDEC Form, http://ethics.health.govt.nz/system/files/documents/pages/HDEC-form.doc (accessed 6 January 2014).
Eric M. Meslin & Summer Johnson, National Bioethics Commissions and Research Ethics, in The Oxford Textbook of Clinical Research Ethics 192–94 (Ezekiel J. Emanuel et al. eds., 2008). Some commentators have advocated the creation of a national ethics committee in the U.K. See, eg Susan M.C. Gibbons, Are UK Genetic Databases Governed Adequately? A Comparative Legal Analysis, 27 Legal Stud. 312 (2007); Jean V. McHale, Accountability, Governance and Biobanks: The Ethics and Governance Committee as Guardian or as Toothless Tiger?, 19 Health Care Analysis 231 (2011).
In 2005, at least 85 countries had a national bioethics commission. See Meslin & Johnson, supra note 89, at 188.
La Commission nationale de l'informatique et des libertés, http://www.cnil.fr/accessible/non/ (accessed 2 November 2013). See also Research Ethics Committees, Data Protection and Medical Research in European Countries 63 (Deryck Beyleveld, David Townend & Jessica Wright eds., 2005).
Michaele C. Christian et al., A Central Institutional Review Board for Multi-Institutional Trials, 346 New Eng. J. Med. 1405 (2002).
Keith Marsolo, Approaches to Facilitate Institutional Review Board Approval of Multicenter Research Studies, 50 Med. Care S77, S79 (2012).
Dep't Vet. Affairs, VA Central IRB, http://www.research.va.gov/vacentralirb/ (accessed 7 January 2014).
Can. Inst. Health Research, Natural Sci. & Eng'g Research Council of Canada & Soc. Sci. & Humanities Research Council of Canada, Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans, http://www.ethics.gc.ca/eng/policy-politique/initiatives/tcps2-eptc2/Default/ (accessed 7 January 2014) [hereinafter TCPS].
Id., at Art. 8.1.
Id., at Art. 8.3.
Directive 2001/20, of the European Parliament and of the Council of 4 April 2001 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use art. 7, 2001/20/EC, 2001 O.J. (L 121) 34. The EU clinical trials directive is currently undergoing revision. In addition to its likely conversion to a regulation, it may also address some of the criticisms raised here. For example, the proposal recommends a ‘single portal’ to submit an application for conducting a clinical trial. This portal would be managed by the European Commission and would be free of charge for sponsors. However, there are doubts as to whether the proposal would reform the ethics review system, as it does not intend to ‘regulate or harmonise the precise functioning of Ethics Committees, impose a systematic cooperation at an operational level between Ethics Committees in the EU, or limit the Ethics Committee's scope of the assessment to genuinely-ethical issue (science and ethics cannot be separated).’ See Proposal for a Regulation of the European Parliament and of the Council on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC, COM (2012) 369 final (17 July 2012).
See, eg Mary Warnock, A National Ethics Committee, 297 Brit. Med. J. 1626 (1988) (proposing a UK national ethics committee); Michael G. Gelder, A National Committee for the Ethics of Research, 16 J. Med. Ethics 146 (1990) (proposing a national research ethics committee in the UK for overseeing quality control, but leaving detailed monitoring of district committees at the regional level); Ezekiel J. Emanuel et al., Oversight of Human Participants Research: Identifying Problems To Evaluate Reform Proposals, 141 Ann. Intern. Med. 282 (2004) (outlining five fundamental components of a research ethics reform proposal); Jocelyn Downie, The Canadian Agency for the Oversight of Research Involving Humans: A Reform Proposal, 13 Acct. Res. 75 (2006) (proposing a Canadian national level agency that covers all research involving humans). See also Jane Kaye, Do We Need a Uniform Regulatory System for Biobanks Across Europe?, 14 Eur. J. Hum. Genet. 245 (2006) (proposing a uniform regulatory system for human biobanks used for genetic research purposes in Europe, including the possible establishment of an independent European body with enforcement powers).
White Paper, supra note 11, at 14.
Leshner & Turekian, supra note 1.
Robert Klitzman & Paul S. Appelbaum, To Protect Human Subjects, Review What Was Done, Not Proposed, 335 Science 1576 (2012).
Dyck & Allen, supra note 60.
See, eg Steven Joffe, Revolution or Reform in Human Subjects Research Oversight, 40 J.L. Med. & Ethics 922, 927 (2012) (arguing that ‘our oversight system needs major improvement, not radical revision.’).
Heimer & Petty, supra note 28, at 622 (‘[O]ne can plausibly argue that the IRB regulatory system, sometimes pejoratively labeled Big Ethics, is at least partly a response to the needs of Big Research.’).
Kaye et al., supra note 38.
David Hunter, How Not To Argue Against Mandatory Ethics Review, 39 J. Med. Ethics 521, 523 (2013).
Emmanuelle Rial-Sebbag & Anne Cambon-Thomsen, The Emergence of Biobanks in the Legal Landscape: Towards a New Model of Governance, 39 J.L. Soc. 113, 127–28 (2012) (‘[W]e are certainly facing a new form of ethics in the life sciences area; after what authors called ‘applied ethics’, a new ‘organizational ethics’ is emerging. … This new approach emphasizes the limits of legal harmonization because of the technical limitations posed by the legal instruments themselves, and it allows space for normative creativity. The only proposals that can be made for a ‘harmonization of ethics’, understood as a tool of governance, should be based on these common principles for the protection of participants…’).
Michael Dunn, Getting the Justification for Research Ethics Review Right, 39 J. Med. Ethics 527, 528 (2013).
Amy Gutmann & James W. Wagner, Found Your DNA on the Web: Reconciling Privacy and Progress, 43 Hastings Center Rep. 15, 17 (2012).
Presidential Comm'n for the Study of Bioethical Issues, Privacy and Progress in Whole Genome Sequencing 29 (2012).
Simone Penasa, Converging by Procedures: Assisted Reproductive Technology Regulation Within the European Union, 12 Med. Law Int'l 300, 327 (2012).
Richard Thomas & Mark Walport, Data Sharing Review Report 70, 71 (2008); Cabinet Office, Open Data White Paper: Unleashing the Potential 38 (2012); Fiona Caldicott, Information: To Share or not to Share? The Information Governance Review 66, 67 (2013) [hereinafter, Caldicott, Information].
Health and Social Care Act 2012, 2012 c. 7, §§ 252–273 (Eng.).
Fiona Stevenson et al., Use of Electronic Patient Records for Research: Views of Patients and Staff in General Practice, 30 Fam. Prac. 227, 228 (2013).
Caldicott, Information, supra note 113.
Health Insurance Portability and Accountability Act of 1996 Safe Harbor Method, 45 CFR § 164.514(b) (2012). See also Douglas Peddicord et al., A Proposal To Protect Privacy Of Health Information While Accelerating Comparative Effectiveness Research, 29 Health Aff. 2082, 2083 (2010).
See generally Rolf H. Weber, Transborder Data Transfers: Concepts, Regulatory Approaches and New Legislative Initiatives, Int'l Data Privacy L. 117, 125–26 (2013); Daniel R. Leathers, Giving Bite to the EU-U.S. Data Privacy Safe Harbor: Model Solutions for Effective Enforcement, 41 Case W. Res. J. Int'l L. 193, 198–208 (2009).
Council Directive 95/46/EC of the European parliament and of the Council on the protection of individuals with regard to the processing of personal data and on the free movement of such data, art. 25, 1995 O.J. (L 281) 31 (EC) [hereinafter EU Data Protection Directive]. The European Commission has the power to determine, on the basis of Article 25(6) of EU Data Protection Directive, whether a third (external) country ensures an adequate level of protection by reason of its domestic law or of the international commitments into which it has entered. See generally European Commission, Commission Decisions on the Adequacy of the Protection of Personal Data in Third Countries, http://ec.europa.eu/justice/data-protection/article-29/index_en.htm (accessed 5 November 2013). To date, the European Commission has recognized the adequacy of data protection laws of Andorra, Argentina, Australia, Canada, Switzerland, Faeroe Islands, Guernsey, State of Israel, Isle of Man, Jersey, the US Department of Commerce's Safe Harbor Privacy Principles, and the transfer of Air Passenger Name Record to the United States’ Bureau of Customs and Border Protection.
Eur. Parl. Comm. on Citizens’ Freedoms and Rights, Justice and Home Affairs, together with Committee on Legal Affairs and the Internal Market. Hearing on 22/23 February 2000, http://www.europarl.europa.eu/hearings/20000222/libe/default_en.htm (accessed 7 January 2014).
Commission Decision 2000/520/EC Pursuant to Directive 95/46/EC of the European Parliament and of the Council on the Adequacy of the Protection Provided by the Safe Harbor Privacy Principles and Related Frequently Asked Questions Issued by the US Department of Commerce, 2000 O.J. (L 215) 7.
See generally Export.gov, http://export.gov/Safeharbor/ (accessed 5 November 2013).
APEC Elec. Commerce Steering Grp., APEC Cross-Border Privacy Rules System: Policies, Rules, Guidelines, http://www.apec.org/Groups/Committee-on-Trade-and-Investment/~/media/Files/Groups/ECSG/CBPR/CBPR-PoliciesRulesGuidelines.ashx (accessed 7 January 2014) [hereinafter APEC CBPR System].
APEC Privacy Framework, http://www.apec.org/Groups/Committee-on-Trade-and-Investment/~/media/Files/Groups/ECSG/05_ecsg_privacyframewk.ashx (accessed 7 January 2014).
See APEC CBPR System, supra note 123.
APEC Elec. Commerce Steering Grp., Consumer Protection in Asia-Pacific Gets Boost as Mexico Joins Privacy Regime, APEC (16 January 2013), http://www.apec.org/Press/News-Releases/2013/0116_cbpr.aspx. On 7 June 2013, the Japanese government submitted an application to participate in the CBPR System. See Toshio Aritake, Japan Ministry Files Application to Join APEC Cross-Border Privacy Rules System, Bloomberg BNA (18 June 2013), http://www.bna.com/japan-ministry-files-n17179874584/ (accessed 7 January 2014).
Common Rule, 45 C.F.R. § 46.101(h) (2012).
To date, OHRP has not deemed any country to have equivalent protections. E-mail from Michelle Feige, Pub. Health Analyst, Div. of Educ. and Dev., Off. for Hum. Res. Prot., to authors (7 August 2013, 12:44 EST) (on file with authors).
Sec'y Advisory Comm. on Human Research Protections, SACHRP Minutes (19–20 July 2011), http://www.hhs.gov/ohrp/sachrp/mtgings/mtg07-11/july2011minutes.pdf.pdf (accessed 7 January 2014).
James V. Lavery, Michael McDonald & Eric M. Meslin, Research Ethics Across the 49th Parallel: The Potential Value of Pilot Testing ‘Equivalent Protections’ in Canadian Research Institutions, 13 Health L. Rev. 86, 94 (2005) (‘[W]e believe that formal study of the topic should be undertaken to demonstrate the feasibility of an equivalent protections initiative in selected institutions in Canada as a test case for a broader international application of equivalent protections by OHRP.’). See also Jeremy Sugarman, Should the Gold Rule? Assessing ‘Equivalent Protections’ for Research Participants Across International Borders, 35 Hastings Ctr. Rep. 12 (2005).
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), http://www.ich.org (accessed 6 November 2013).
Hoffmann et al., supra note 35, at 462.
See, eg Bartha M. Knoppers et al., A P3G Generic Access Agreement for Population Genomic Studies, 31 Nature Biotech. 384 (2013).
White Paper, supra note 11.
Cf. Downie, supra note 99, at 84–88 (proposing a tri-partite national agency in Canada for human subjects research oversight, with a policy and standards branch, education branch, and compliance branch).
Jeanne L. Speckman et al., Determining the Costs of Institutional Review Boards, 29 IRB: Ethics & Hum. Res. 7, 10 (2007).
Jeremy Sugarman et al., The Cost of Institutional Review Boards in Academic Medical Centers, 352 New Eng. J. Med. 1825, 1826 (2005); Howard B. Dickler & David Korn, The Costs of Institutional Review Boards, 353 New Eng. J. Med. 315 (2005) (arguing that the data that Sugarman et al. cited in their study ‘seriously underestimate the current costs of human-research oversight’); Margaret Byrne et al., Variability in the Costs of Institutional Review Board Oversight, 81 Academic Medicine 708, 710 (2006).
Byrne et al., supra note 136, at 711.
Battelle Mem'l Inst., 2013 Global Funding R&D Forecast, http://www.battelle.org/docs/default-document-library/2013-R-and-D-Funding-Forecast.pdf?sfvrsn=4#page=3 (accessed 7 January 2014); The World Bank, 2013 States and Markets, World Development Indicators: Science and Technology, http://data.worldbank.org/products/wdi (accessed 7 January 2014).
According to the World Bank, gross national income (GNI) is the sum of value added by all resident producers plus any product taxes (fewer subsidies) not included in the valuation of output plus net receipts of primary income (compensation of employees and property income) from abroad. See World Bank, World Development Indicators, supra note 139.
As determined, via peer review, by the project's granting or funding agencies.
Emanuel et al., supra note 99, at 289 (‘The solution to the problem of repetitive and time-consuming review of multisite proposals … is the establishment of a system of single review of multisite research with liability protection for local institutions.’)
Declaration of Helsinki (2013), supra note 17.
Council Directive 2001/20/EC 2001 O.J. (L 121) 37 (EC), http://www.eortc.be/Services/Doc/clinical-EU-directive-04-April-01.pdf (accessed 7 January 2014).
Scott Burris, Regulatory Innovation in the Governance of Human Subjects Research: A Cautionary Tale and Some Modest Proposals, 2 Reg. & Gov. 65 (2008).
Sheelagh McGuinness, Research Ethics Committees: The Role of Ethics in a Regulatory Authority, 34 J. Med. Ethics 695 (2008).
Rhodes et al., supra note 64.
‘Minimal risk’ could be defined in accordance with established laws or guidelines, such as the Common Rule or Canada's TCPS, or through the formulation of a new definition or standard established by the IFER Policy and Standards Branch, Advisory Group, independent consultants, and/or the public.
Eg functions and duties of the committee, membership requirements, terms of appointment, conditions of appointment, internal procedures, quorum requirements.
Dyck & Allen, supra note 60, at 517; Coleman, supra note 57.
Coleman, supra note 57, at 4 (‘[T]his Article begins with the premise that IRBs are engaged in a process of legal decision-making, insofar as they interpret specific regulatory requirements pursuant to authority that has been delegated to them by administrative agencies.’). See also Michael Hadskis & Peter Carver, The Long Arm of Administrative Law: Applying Administrative Law Principles to Research Ethics Boards, 13 Health L. Rev. 19, 28 (2005) (arguing that policymakers should ‘consider administrative law principles when designing … the decision-making process to be employed in the ethical review of research involving human participants’).
See Coleman, supra note 57, at 42 (‘Incorporating written evaluations into IRB decision-making could improve risk assessment in a number of ways.’).
Bartha M. Knoppers et al., A Human Rights Approach to an International Code of Conduct for Global Genomic and Clinical Data Sharing (9 December 2013) (unpublished manuscript) (on file with authors).
This option has been proposed in John Wilbanks’ Portable Legal Consent model. See Consent to Research, http://weconsent.us/ (accessed 4 November 2013).
Knoppers et al., supra note 153.
Contra Tom Walker, Respecting Autonomy without Disclosing Information, 24 Bioethics 388 (2013) (arguing that autonomy can be respected without seeking informed consent).
See, eg Barbara Prainsack & Alena Buyx, A Solidarity-Based Approach to the Governance of Research Biobanks, 21 Med. L. Rev. 71 (2013); Hoedemaekers et al., supra note 26.
Gert Helgesson, In Defense of Broad Consent, 21 Cambridge Q. Healthcare Ethics 40 (2012); Mark Sheehan, Can Broad Consent be Informed Consent?, 4 Pub. Health Ethics 226 (2011).
Jane Kaye, From Single Biobanks to International Networks: Developing e-Governance, 130 Hum. Genet. 377 (2011).
Bruce R. Hopkins, Starting and Managing a Nonprofit Organization: A Legal Guide 7–12, 313–18 (6th edn, 2013).
Amy Zarzeczny & Timothy Caulfield, Legal Liability and Research Ethics Boards: The Case of Neuroimaging and Incidental Findings, 35 Int'l J.L. & Psychiatry 137 (2012); Leah E. Hutt, Protecting the Protectors: Indemnification Agreements for REB Members, 175 Can. Med. Ass'n J. 1229 (2006); Jennifer L. Gold, Watching the Watchdogs: Negligence, Liability, and Research Ethics Boards, 11 Health L.J. 153 (2003).
Mark Sheehan, Should Research Ethics Committees Meet in Public?, 34 J. Med. Ethics 631 (2008) (arguing that IRB meetings should not be fully public).
Jonathan G.S. Koppell, World Rule: Accountability, Legitimacy, and the Design of Global Governance 31–71 (2010). We do recognize, however, that a presumption of openness could be critiqued by some as a contemporary western liberal/Rawlsian sensibility. See Sheehan, supra note 161, at 632; Lemke et al., supra note 46, at 375 (‘Research participants’ need for names of responsible oversight individuals, as well as clear penalties for violators of policy, may be important elements for obtaining trust and participation in research.’).
Burris, supra note 144, at 71 (‘Ethicists act as norm entrepreneurs in formulating and disseminating new standards.’).
Milena I. Neshkova & Hai (David) Guo, Public Participation and Organizational Performance: Evidence from State Agencies, 22 J. Public Adm. Res. Theory 267, 270–74 (2012).
Michel Callon, Pierre Lascoumes & Yannick Barthe, Acting in an Uncertain World: An Essay on Technical Democracy 70–106 (Graham Burchell trans., MIT Press 2009) (2001).
Todd H. Wagner et al., Economies of Scale in Institutional Review Boards, 42 Med. Care 817 (2004).
Knoppers et al., supra note 153.
Rita Banzi et al., Conceptual Frameworks and Empirical Approaches Used to Assess the Impact of Health Research: An Overview of Reviews, 9 Health Res. Pol'y & Systems 26 (2011).
Adam M. Hedgecoe, Trust and Regulatory Organisations: The Role of Local Knowledge and Facework in Research Ethics Review, 42 Soc. Stud. Sci. 662, 668 (2012) (‘RECs are still deeply ‘localised’ in their decision-making, and local knowledge is still valuable for helping them think about applicants’ trustworthiness.’). See also Patricia Jaspers, Rob Houtepen & Klasien Horstman, Ethical Review: Standardizing Procedures and Local Shaping of Ethical Review Practices, 98 Soc. Sci. & Med. 311 (2013) (evidence from qualitative ethnographic-sociological study of three Dutch RECs suggests that specific local, institutional contexts offer useful resources for ethics review); Coleman, supra note 57, at 16 (‘The virtually unfettered discretion that IRBs currently exercise is partly an intentional result of the system's commitment to localized research oversight, in which responsibility for reviewing protocols rests primarily on the institution in which the research will be carried out.’); John E. Sidle et al., A Needs Assessment to Build International Research Ethics Capacity, 1 J. Empirical Res. Hum. Res. Ethics 23 (2006); Eric M. Meslin, Edwin Were & David Ayuku, Taking Stock of the Ethical Foundations of International Health Research: Pragmatic Lessons from the IU–Moi Academic Research Ethics Partnership, 28(Supp. 3) J. Gen. Internal Med. S639, S644 (2013) (describing the experience of an ultimately unsuccessful proposal to develop a joint ethics review committee between Indiana University and Moi University in Kenya and that ‘in our experience trying to establish a joint IRB, we learned that in spite of agreement at the local level to develop new procedures to enhance research, there may remain strongly held views about the impact of such innovation on other deeply held ethical values.’).
Jürgen Basedow, Worldwide Harmonisation of Private Law and Regional Economic Integration—General Report, 8 Unif. L. Rev. 31, 32, 33 (2003).
José A.E. Faria, Future Directions of Legal Harmonisation and Law Reform: Stormy Seas or Prosperous Voyage?, 14 Unif. L. Rev. 5, 25 (2009).
Tassé, supra note 30, at 11.
Rosario M. Isasi, Policy Interoperability in Stem Cell Research: Demystifying Harmonization, 5 Stem Cell Rev. & Rep. 108, 113 (2009).
Marcel Fontaine, Law Harmonization and Local Specificities—A Case Study: OHADA and the Law of Contracts, 18 Unif. L. Rev. 50, 62–64 (2013).
Coleman, supra note 57, at 44. Similarly, some scholars are quite critical of local IRBs. See, eg Edgar & Rothman, supra note 59, at 498 (‘[T]he localism of the IRB appears to generate more problems than it solves.’).
See Downie, supra note 99, at 94 (‘Geography as a proxy is both over- and underinclusive. … [A]n issue of particular concern to Orthodox Jews would cut across many small communities across Canada but may not be captured through any institutional review. No one REB can reflect all the subgroups in a community (and, indeed, REBs tend to reflect dominant culture rather than subcultures.’)).
Roland Robertson, Globalization: Social Theory and Global Culture (1992); Ulrich Beck, What is Globalization 46, 47 (2000); Belinda Bennett, Health Law's Kaleidoscope: Health Law Rights in a Global Age 10 (2008).
Jacob Dahl Rendtorff, Basic Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability—Towards a Foundation of Bioethics and Biolaw, 5 Med., Health Care & Phil. 235, 239 (2002).
See generally Tanya Heikkila & Andrea K. Gerlak, Building a Conceptual Approach to Collective Learning: Lessons for Public Policy Scholars, 41 Pol'y Stud. J. 484 (2013).
Michael Ignatieff, Reimagining a Global Ethic, 26 Ethics & Int'l Aff. 7, 16 (2012) (‘[A] global ethic defends the universal interests of mankind and the planet; its purpose is to engage all forms of ethical particularism in adversarial justification; and the rules of these encounters, flowing as they do from the starting premise of human equality, preclude coercion and mandate tolerance.’). Cf. Nitsan Chorev, Changing Global Norms through Reactive Diffusion: The Case of Intellectual Property Protection of AIDS Drugs, 77 Am. Soc. Rev. 831 (2012) (claiming that global norms change through local experiences accumulated across countries, which ‘reactively diffuse’ and eventually lead to new, globally accepted reinterpretations of the original norms).
See, eg Stuart Hargreaves, Inadequate: The APEC Privacy Framework & Article 25 of the European Data Protection Directive, 8 Can. J.L. & Tech. 1, 27 (2010); Hogan Lovells Privacy Team, US-EU Safe Harbor Under Pressure, Privacy Tracker (2 August 2013), https://www.privacyassociation.org/privacy_tracker/post/us_eu_safe_harbor_under_pressure (accessed 7 January 2014) (quoting Viviane Reding, European Commissioner for Justice, Fundamental Rights and Citizenship, as finding that the Safe Harbor ‘may not be so safe after all’ and that the European Commission plans to conduct a full review of Safe Harbor by the end of 2013). See also European Commission, Restoring Trust in EU-US data flows - Frequently Asked Questions (27 November 2013), http://europa.eu/rapid/press-release_MEMO-13-1059_en.htm (calling on U.S. authorities to implement 13 recommendations to improve the Safe Harbor scheme by summer 2014).
Leathers, supra note 118, at 220–30.
Chris Connolly, The US Safe Harbor—Fact or Fiction?, Privacy Laws & Bus. Int'l 1 (2008).
See export.gov, Federal Trade Commission Enforcement of Safe Harbor Commitments, http://export.gov/build/groups/public/@eg_main/@safeharbor/documents/webcontent/eg_main_052211.pdf (accessed 7 January 2014).
Christopher Kuner, Transborder Data Flows and Data Privacy Law 180 (2013).
Dep't of Commerce, U.S.-EU Joint Statement on Privacy from EU Commission Vice-President Viviane Reding and U.S. Commerce Secretary John Bryson (19 March 2012), http://www.commerce.gov/news/press-releases/2012/03/19/us-eu-joint-statement-privacy-eu-commission-vice-president-viviane-re (acces-sed 7 January 2014).
Coleman, supra note 57, at 49.
Widdows, supra note 43, at 175.
Supra note 127.
Constitution of the World Health Organization art. II, §§ a, b, 22 July 1946, 62 Stat. 2679, 14 U.N.T.S. 185, http://www.who.int/governance/eb/who_constitution_en.pdf (accessed 7 January 2014).