Abstract
Polymeric delivery vehicles can improve the safety and efficacy of chemotherapy drugs by facilitating preferential tumor delivery. Polymer-drug conjugates are especially attractive carriers because additional formulation steps are not required during manufacturing, and drug release profiles can be altered based on linker choice. For clinical translation, these vehicles should also be reproducibly and controllably synthesized. Recently, we reported the development of a class of materials called “sunflower polymers,” synthesized by controlled radical polymerization of hydrophilic “petals” from a cyclic multimacroinitiator “core.” This synthesis strategy afforded control over the size of the polymer nanostructures based on their petal polymerization time. In this work, we demonstrate that particle size can be further tuned by varying the degree of polymerization of the cyclic core in addition to that of the petals. Additionally, we investigate the application of these materials for tumor-targeted drug delivery. We demonstrate that folate-targeted, doxorubicin-conjugated sunflower polymers undergo receptor-mediated uptake into cancer cells and pH-triggered drug release leading to cytotoxicity. These materials are attractive as drug carriers due to their discrete and small size, shielded drug cargo that can be triggered for release, and relative ease of synthesis.
Keywords: sunflower polymer, tumor penetration, doxorubicin delivery, pH-sensitive drug release, tumor targeting
Graphical Abstract
INTRODUCTION
Cytotoxic chemotherapy remains the preferred frontline strategy of treatment against most types of cancer but is associated with significant side effects such as major organ damage, infertility, immunosuppression, and nausea/vomiting that severely compromise patient quality of life.1,2 The development of improved chemotherapy formulations therefore remains an important research priority with significant medical impact. The adverse side effects associated with chemotherapy can be ameliorated by developing delivery formulations that rely on tumor localization via the “enhanced permeability and retention” (EPR) effect.3 These formulations are large enough to be retained in the circulation after injection, avoiding tissue distribution and rapid renal clearance, but remain small enough to extravasate from abnormal tumor vasculature into tumors.4,5 This paradigm is used by clinically-approved chemotherapy formulations such as liposomal anthracyclines (e.g. Doxil and Myocet) and albumin-bound paclitaxel (Abraxane) to offer improved safety profiles over equivalent solution formulations.
Still, drawbacks to these formulations remain. Multiple formulation steps are often required in manufacturing, increasing scale-up complexity.6 Interstitial tumor penetration is also reduced due to the larger size of these formulations (50–150 nm) compared to free drug.7–9 Finally, drug leakage from the vehicle prior to tumor localization can result in off-target effects.10,11
Polymer-drug conjugates are an alternative delivery technology in the development pipeline.12,13 The smaller size of these constructs compared to liposomal carriers and drug attachment via labile linkages allows for improved tumor penetration and controlled drug release. In contrast to polymeric carriers such as micelles or nanoencapsulates,14,15 polymer-drug conjugates do not require formulation steps during manufacturing. Dendrimers, star polymers, and cyclic polymers have prolonged circulation in vivo compared to linear polymers, an important property in tumor accumulation via EPR.16–18 Therefore, polymers with advanced architectures that can be reproducibly and controllably synthesized are of great interest as drug carriers.
We have recently described a strategy for synthesizing macrocyclic brush or “sunflower” polymers,19 using atom transfer radical polymerization (ATRP) and a “grafting-from” approach.20,21 We demonstrated that sunflower polymers could be synthesized with relatively low polydispersity and with control over their hydrodynamic size based on the polymerization time of their radiating “petals.” Because the folate receptor (FR) is commonly overexpressed on various cancer cell types,22,23 we also conjugated the petal termini with folate (FA) as a targeting ligand and the polymer “core” with fluorescein as a model drug to demonstrate receptor-mediated uptake in FR-positive cells. These sunflower polymers appeared to have an advantage over their linear analogues (comb-like polymers) which were shown to be taken up by non-specific mechanisms.
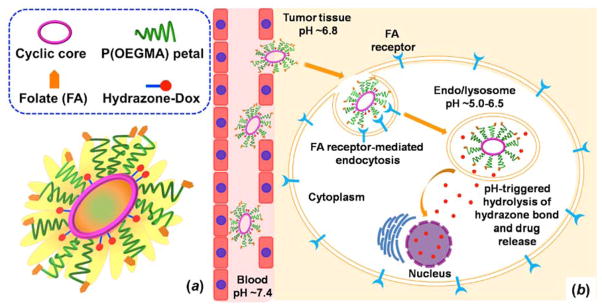
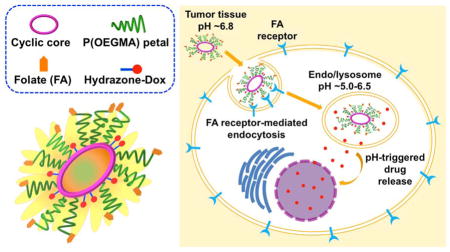
In this work, we have expanded on our platform by synthesizing sunflower polymers with two different core sizes and then applied these materials for targeted drug delivery through conjugation of folate to the sunflower petals and doxorubicin to the sunflower cores. Together with the petal polymerization time, controlling core size allows for greater control over the hydrodynamic size of the polymers. We investigate the uptake properties as compared to the previously reported small-core sunflowers. We further demonstrate that sunflower polymers can be used for targeted, intracellular drug delivery by conjugating the chemotherapy drug doxorubicin (Dox) via hydrazone bonds that release drug under acidic conditions (Figure 1). Finally, we investigate the cytotoxicity of the various polymer-Dox constructs synthesized.
Figure 1.
Schematic illustrations of (a) sunflower polymer containing folate targeting ligands and doxorubicin (Dox) drug and (b) proposed delivery mechanism: extravasation into tumor tissue by EPR effect, internalization by folate receptor-expressing cancer cells, and intracellular, pH-triggered Dox release.
EXPERIMENTAL SECTION
Materials
All chemicals were purchased from Sigma-Aldrich and used without further purification unless otherwise noted.
Preparation of P((HEMA-sunflower-OEGMA-folate)-st-(GMA-hydrazide)) (FA-SF-hydrazide)
All steps for the synthesis of the folate-targeted, hydrazide-containing sunflower polymer were performed as reported previously,19 but using a HEMA:initiator molar ratio of 100:1 in the synthesis of the linear P(HEMA-st-EGMA) precursor to yield sunflower polymers with a larger core.
Preparation of P((HEMA-sf-OEGMA-folate)-st-(GMA-fluorescein))
A 50 mL falcon tube equipped with a magnetic stirrer was charged with the polymer precursor P((HEMA-sf-OEGMA-folate)-st-(GMA-hydrazide)) (146.0 mg, 14.5 μmol of hydrazide), NHS-Fluorescein (Thermo Scientific, 13.7 mg, 29.0 μmol), and anhydrous methanol (4 mL). The tube was sealed, and the reaction mixture was placed in the dark at room temperature for 48 h. The resultant fluorescein-labeled sunflower polymer, P((HEMA-sf-OEGMA-folate)-st-(GMA-fluorescein)), was purified by extensive dialysis against distilled water (4 L) for 24 h and collected by freeze-drying. Yield: 67.0% (97.8 mg). The amount of fluorescein conjugated to the sunflower polymer was determined by 1H NMR analysis as described previously,19 with each polymer containing approximately 10 fluorescein molecules, a conjugation efficiency of 49% based on the theoretical polymer composition of 24 EGMA groups and 85% conversion of EGMA to hydrazide groups for fluorescein incorporation.
Preparation of P((HEMA-sf-OEGMA-folate)-st-(GMA-Dox))
A 50 mL falcon tube equipped with a magnetic stirrer was charged with the polymer precursor P((HEMA-sf-OEGMA-folate)-st-(GMA-hydrazide)) (180.0 mg, 29.2 μmol of hydrazide), doxorubicin hydrochloride (Dox•HCl, LC Laboratories, Woburn, MA) (33.8 mg, 58.4 μmol), a drop of acetic acid, and anhydrous methanol (6 mL). The tube was sealed, and the reaction mixture was placed in the dark at room temperature for 48 h. The resultant sunflower polymeric prodrug, P((HEMA-sf-OEGMA-folate)-st-(GMA-Dox)), was purified by extensive dialysis against anhydrous methanol (2 L) for 24 h, followed by dialysis against distilled water (4 L) for another 24 h, and collected by freeze-drying. Yield: 78.7% (155 mg). The amount of Dox conjugated to the sunflower polymer was determined by absorbance at 480 nm based on a standard curve obtained from free Dox•HCl in water.
Characterization of polymers
1H NMR spectra were recorded on a Bruker AV 500 nuclear magnetic resonance (NMR) instrument using DMSO-d6 as the solvent. Fourier transform infrared (FT-IR) spectra were recorded on a Bruker Vector 33 FT-IR spectrometer. Samples were pressed into potassium bromide (KBr) pellets for measurements. Gel permeation chromatography (GPC) was used to determine molecular weight and polydispersity (Mw/Mn, PDI) of polymer samples prepared. SEC Tosoh TSK-GEL R-3000 and R-4000 columns (Tosoh Bioscience, Montgomeryville, PA) were connected in series to a Agilent 1200 Series System (Agilent Technologies, Santa Clara, CA), Optilab-rEX refractometer and miniDAWN TREOS triple-angle static light scattering detector (Wyatt Technology, Santa Barbara, CA). HPLC-grade DMF containing 0.1 wt% LiBr at 60°C was used as the mobile phase at a flow rate of 1 mL/min. Absolute molecular weight averages and dn/dc were calculated using ASTRA software (Wyatt). Average dimensions of sunflower polymers in an aqueous phase were measured by dynamic light scattering (DLS) with a ZetaPALS (Brookhaven Instruments Corp.) at a detection angle of 90°. The measurements were performed in triplicate. The aqueous solution was passed through a 0.22 μm pore-sized syringe filter before measurements.
Quantification of Dox release
FA-SF-Dox polymer was dissolved in release buffer at different pHs (PBS at pH 7.4, phosphate buffer at pH 6.8, or citrate buffer at pH 5.5) at a concentration of 1 mg/mL. Spectra/Por Float-A-Lyzer G2 Dialysis Devices (MWCO: 8–10 kDa, Spectrum Laboratories) were pre-soaked in release buffer for 30 minutes, then loaded with 1 mL of polymer solution each. Each device was immersed in a 50 mL conical containing 12 mL of the same release buffer and incubated in the dark at 37°C with shaking at 125 rpm. At predetermined times, 1 mL of the dialysate was sampled and replaced with an equal volume of fresh buffer. Dialysate samples were stored at −20°C for later analysis. After all time points were collected, dialysate samples were analyzed for Dox fluorescence (excitation: 480 nm, emission: 580 nm) on a Tecan Safire2 plate reader (Männerdorf, Switzerland). The concentration of released Dox in each sample was calculated based on standard curves obtained from free Dox•HCl in the respective release buffer.
Cell culture
KB cells (ATCC CCL-17) and A549 cells (ATCC CCL-185) were maintained in folate-free RPMI 1640 (Life Technologies) and F-12K (Corning cellgro) media, respectively. Media was supplemented with 10% fetal bovine serum (FBS, HyClone) and 1% penicillin/streptomycin (HyClone). Cells were cultured as a monolayer in a 37°C, 5% CO2 environment. Medium was replaced every 2–3 days. Cells were passaged at ~70–80% confluence by incubation with Trypsin-EDTA, followed by resuspension in complete growth medium.
Serum stability of polymers
Sunflower polymers were incubated in cell culture medium (MEM supplemented with 10% FBS) for 4 h at 37°C. The resultant polymer was recovered by extraction using dichloromethane (DCM), vacuum dried, and analyzed by GPC.
Uptake and competition study by flow cytometry
Cells were seeded in 24-well plates at a density of 40,000 cells per well in 1 mL of complete growth medium and incubated in a 37°C, 5% CO2 environment for 24 h. Cells were rinsed once with PBS and incubated in 1 mL of supplemented folate-free RPMI 1640 medium +/− 2 mM folate competition for 1 h at room temperature. Polymer samples at varying concentrations were prepared in serial dilutions in water and then diluted 10-fold in supplemented folate-free RPMI 1640 medium +/− 2 mM folate. The media was aspirated from each well, and cells were incubated with 200 μL of polymer solution for 20 min at 37°C. After incubation, the polymer solutions were aspirated, and the cells were rinsed twice with PBS/1% BSA. Cells were then harvested by incubation with 200 μL of Trypsin-EDTA, followed by resuspension with 1 mL of complete growth medium. Cells were transferred to 1.5 mL microcentrifuge tubes and pelleted at 300 g for 5 min at 4°C. The supernatant was aspirated, and the cell pellets were resuspended in 200 μL of PBS/1% BSA containing 2 μg/mL propidium iodide (PI) as a marker for cell viability. Cells were analyzed for uptake of fluorescent polymer using a MACSQuant Analyzer flow cytometer (Miltenyi).
Confocal imaging
KB cells were seeded on poly-D-lysine-coated glass coverslips in 24-well plates at a density of 80,000 cells per well in 1 mL of complete growth medium and incubated in a 37°C, 5% CO2 environment for 12 h. Solutions of FA-SF-Dox polymer and free Dox were prepared in complete growth medium at concentrations equal to 25% of their respective IC50 values. Cells were treated with 400 μL of FA-SF-Dox or free Dox for 12 h at 37°C. Cells were rinsed 3 times with HBSS/1% BSA, fixed with 300 μL of 4% paraformaldehyde (PFA) solution for 15 min at room temperature, and rinsed twice with HBSS/1% BSA. Finally, cells were counterstained with 300 μL of 5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI) in HBSS/1% BSA for 5 min at room temperature and rinsed twice more. Coverslips were mounted onto glass slides with Fluoromount-G (eBioscience) and imaged using a Leica TCS SP8X confocal microscope.
Cell viability study
The cytotoxicities of various formulations were evaluated in vitro using the MTS assay. The cells were seeded in 96-well plates at a density of 2500 cells per well in 100 μL of complete growth medium and incubated in a 37°C, 5% CO2 environment for 24 h. Samples were prepared in serial dilutions in water and then diluted 10-fold in OptiMEM medium (Invitrogen). The cells were then rinsed once with PBS and incubated with 40 μL of the sample solutions with different polymer or Dox concentrations at 37°C for 4 h. Cells were then rinsed with PBS and the medium was replaced with 100 μL of culture medium. At 48 h, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS, Promega) reagent was added to each well. Cells were then incubated at 37°C, 5% CO2 for 3 h. The absorbance of each well was measured at 490 nm on a Tecan Safire2 plate reader (Männerdorf, Switzerland). Cell viability for each treatment condition was determined by normalizing to the cells only signal.
RESULTS AND DISCUSSION
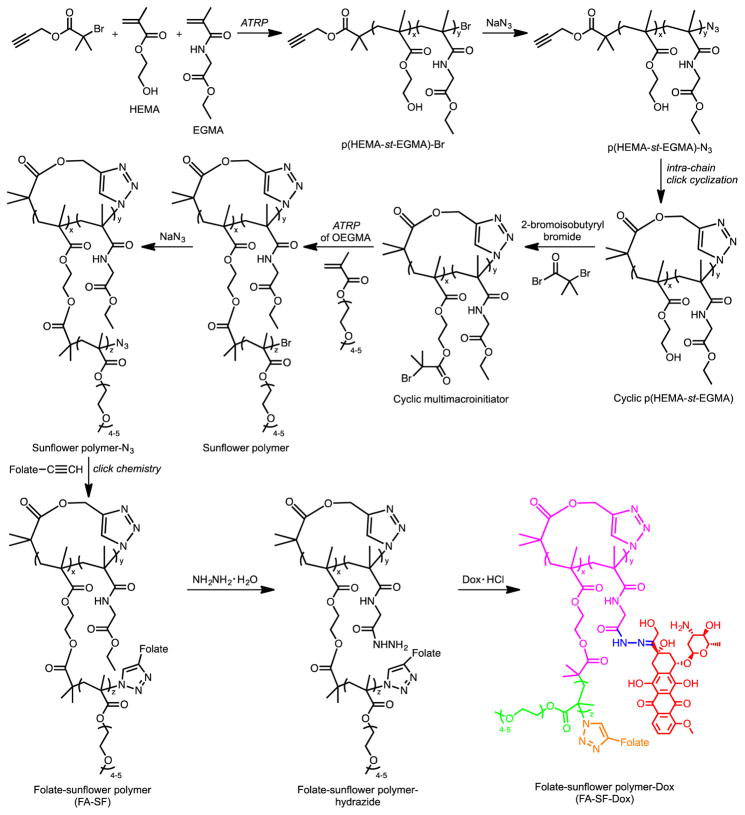
We have previously reported a strategy for synthesizing sunflower polymers using a combination of ATRP and click chemistry.19 The general steps of the synthesis are (i) synthesis of linear statistical copolymers of hydroxyethyl methacrylate (HEMA) and ethyl glycinate methacrylamide (EGMA), (ii) cyclization of the polymers by intra-chain coupling of the chain ends under highly diluted conditions, (iii) conversion of HEMA groups in the polymer core to alkyl halide ATRP initiators, and (iv) ATRP of oligoethylene glycol methacrylate (OEGMA) to generate petals that radiate from the polymer’s cyclic core (Scheme 1).
Scheme 1.
Synthesis of folate-sunflower polymer-Dox.
In this report, we synthesize sunflower polymers with two different core sizes and then investigate these materials for targeted anti-cancer drug delivery to cultured cells. Sunflower polymers with smaller cores were prepared as described previously; these polymers were determined to have, on average, a core composition of 53 monomers (~41 HEMA and ~12 EGMA) and are therefore referred to as core 53 polymers.19 Sunflower polymers with larger cores were prepared by first synthesizing the linear P(HEMA-st-EGMA) precursor using an alkyne-containing ATRP initiator and a target DP of 100. A 16 h polymerization time was used to achieve a conversion of 80% and an actual DP of ~80. Gel permeation chromatography (GPC) indicates that the resulting copolymer has a number-average molar mass (Mn) of 8.37 kDa and relatively low dispersity (Ð) of 1.33, demonstrating good control over the polymerization (Figure S1a). The molar ratio of HEMA and EGMA in the copolymer was determined to be 3.3:1 by 1H NMR spectroscopy, the same as for the linear P(HEMA-st-EGMA) precursor with a target DP of 50. The resulting copolymer was thus denoted P(HEMA80-st-EGMA24) and used to prepare core 104 sunflower polymers.
Following conversion of the terminal bromine of the polymer to an azide, the linear precursor was cyclized by an intra-chain copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction24 between the polymer chain ends.25–27 Cyclization was confirmed by a shift toward longer retention times on GPC relative to the linear precursor and by the disappearance of the characteristic azide signal (2100 cm−1) from the Fourier transform-infrared (FT-IR) spectrum (Figure S1).
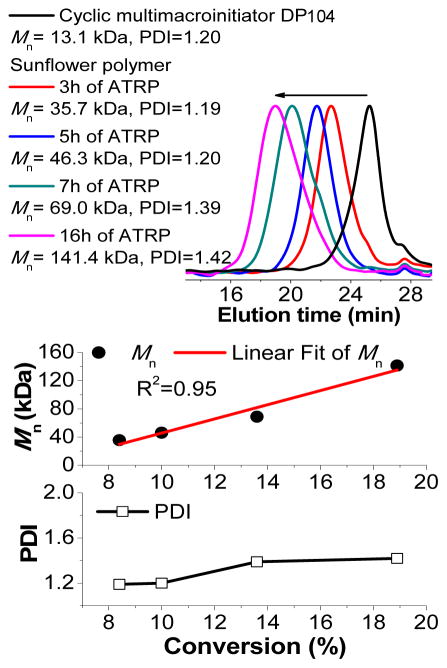
Cyclic multimacroinitiators were prepared by esterifying the HEMA units of cyclic P(HEMA-st-EGMA) with the reagent 2-bromoisobutyryl bromide as described previously.19 Finally, polymerization of OEGMA from the cyclic multimacroinitiator yielded large core sunflowers. As with the small core sunflowers from our earlier work, a kinetic study indicates an increase in molecular weight with petal polymerization time, pseudo-first-order kinetics of the polymerization process, and relatively low Ð (~1.2 when conversion is limited to ~10%) (Figure 2 and S2).
Figure 2.
ATRP kinetics of sunflower polymers prepared using cyclic multimacroinitiator of DP 104, with a target DP of 100 for each petal.
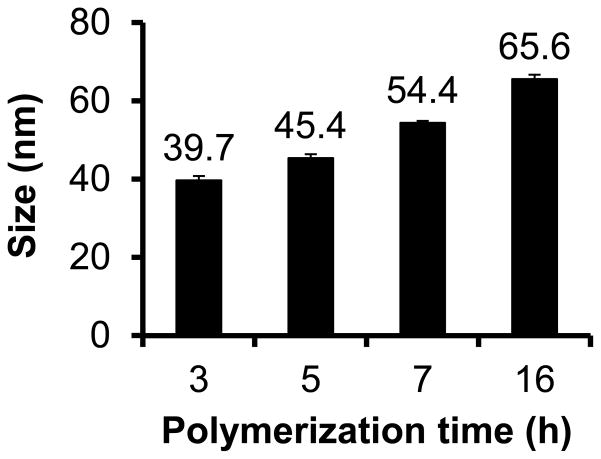
The z-average hydrodynamic diameters of the large core sunflower polymers as determined by dynamic light scattering (DLS) were also shown to increase with petal polymerization time and ranged from 39.7 to 65.6 nm for the panel (Figure 3). Particle size is a particularly important factor in tumor delivery applications. Prolonged circulation is achieved by increasing polymer size above the threshold for renal filtration and extravasation into normal tissue (>5 nm28 and >2–6 nm29, respectively), but the optimal particle size for maximizing tumor penetration is generally less than ~50 nm in diameter.30–32 The sizes of these sunflower polymers are therefore relevant for tumor targeted delivery.
Figure 3.
Z-average diameters of sunflower polymers with cyclic core of DP 104 and petals polymerized for different lengths of time.
Core 104 sunflower polymers with petals synthesized by 3 h ATRP of OEGMA were selected for biological evaluation. We first confirmed that sunflower polymers retain their structure in the presence of serum-containing cell culture media. Polymers were incubated with complete media for 4 h, recovered by extraction, and analyzed by size exclusion chromatography. Chromatography traces reveal that serum-treated polymers have elution times similar to those of untreated polymers (Figure S3), indicating that molecular weight and structure are not significantly changed by serum proteins which could potentially hydrolyze polymers during the course of the experiment. Therefore, polymers were conjugated with folate (FA)-alkyne targeting ligands at petal terminal azides (59% efficiency, determined by absorbance at 360 nm) and NHS-fluorescein at core hydrazides (49% efficiency, determined by 1H NMR). These constructs, named FA-SF-fluor, were then investigated for folate receptor (FR)-mediated uptake in KB (FR+) and A549 (FR−) cells. Although KB cells demonstrated higher normalized median fluorescence intensities (MFIs) than A549 cells at the same polymer concentrations, competition with free folate surprisingly did not reduce polymer uptake in either cell line. This indicates that substantial uptake of these large core sunflowers can occur by non-FR-mediated mechanisms (Figure 4). This is in contrast to the results observed for their small core counterparts, which undergo FR-mediated endocytosis specifically in KB cells.19 Based on this finding, we decided to focus on the small (DP 53) core sunflower polymers for targeted drug delivery applications.
Figure 4.
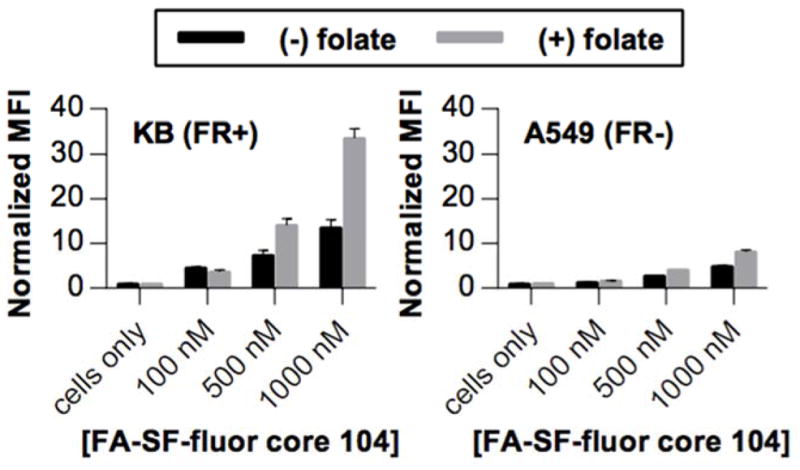
Uptake of FA-SF-fluor with DP 104 core in FR+ KB cells and FR− A549 cells in the absence (black bars) and presence (gray bars) of 2 mM competing free folate.
Ideally, polymer-drug conjugates for cancer therapy should remain stable during circulation and release drug in the tumor environment or after tumor cell uptake in order to minimize off-site exposure and toxicity. Doxorubicin (Dox) hydrochloride was therefore conjugated to the folate-targeted sunflower polymer (FA-SF) via an acyl hydrazone bond, shown previously to provide preferred in vivo release kinetics at tumor sites.33,34 EGMA side chains in the polymer core were converted from ethyl esters to hydrazides (85% efficiency, determined by trinitrobenzenesulfonic acid [TNBS] assay) by reaction with hydrazine hydrate. GPC analysis showed that polymer elution was not significantly changed by this reaction, confirming that the macrocyclic structure remains intact (Figure S4). Dox was then covalently linked via its hydroxyl ketone group to polymer hydrazides (38% efficiency, determined by absorbance at 480 nm; ~2.5 w/w%). Dox conjugation was also confirmed by an increased signal at 8.0 ppm in the 1H NMR spectrum of the targeted, drug-loaded sunflower polymers (FA-SF-Dox, Figure S5), and DLS sizing indicated minimal polymer aggregation (Figure S6).
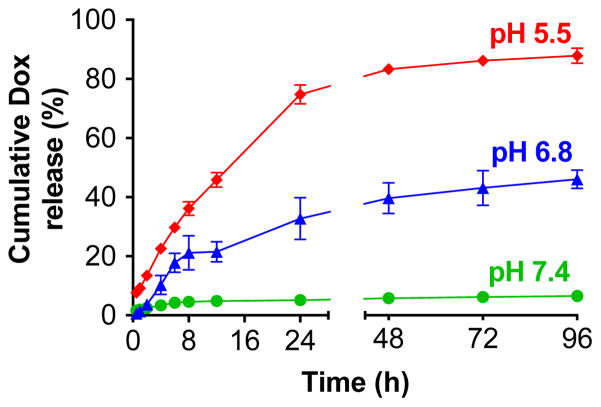
To investigate the pH-dependent kinetics of Dox release, FA-SF-Dox was incubated in PBS (pH 7.4), phosphate buffer (pH 6.8), or citrate buffer (pH 5.5) at 37°C and Dox release was quantified by dialysis method (Figure 5).35 The conjugates were highly stable under physiological conditions, with only 6.5% of the Dox being released over 4 d at pH 7.4. In contrast, incubation of the FA-SF-Dox at pH 5.5 (comparable to the pH of late endosomes and lysosomes) resulted in over 74% Dox release from the polymer after 24 h. Incubation at pH 6.8 (the pH of the tumor microenvironment)36 resulted in intermediate levels of hydrolysis, with 33% Dox release after 24 h.
Figure 5.
Release kinetics of Dox from FA-SF-Dox polymer at pH 7.4, 6.8, and 5.5.
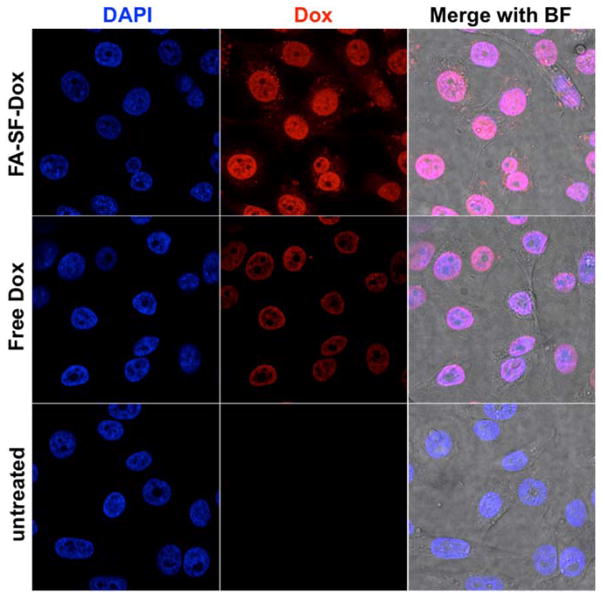
The intracellular trafficking of FA-SF-Dox in KB cells was then examined by confocal microscopy. Dox fluorescence was clearly seen in the nuclei of cells incubated with either FA-SF-Dox or free Dox for 12 h, suggesting that drug is released from the polymer and localizes to the nucleus, or that the entire polymer construct undergoes intracellular trafficking and nuclear localization in a pattern consistent with the free drug (Figure 6). Some punctate staining throughout the cytoplasm could also be observed in the FA-SF-Dox-treated cells, but not cells treated with free Dox, suggesting that the polymer traffics through an endocytic pathway, consistent with the internalization mechanism of other folate-conjugated carriers.23
Figure 6.
Confocal imaging of FA-SF-Dox and Dox (red) uptake in KB cells (nuclei stained blue with DAPI). Cells were treated with polymer or free drug at 25% of their respective IC50 values to minimize cell death. (BF: brightfield image.)
Finally, the cytotoxicity of sunflower polymer constructs to FR+ KB and FR− A549 cells was assessed by MTS cell viability studies (Table 1). The base sunflower (SF) and FA-modified SF (FA-SF) polymers were minimally toxic to cells (IC50, or concentration for 50% cell killing, at least 1 mg/mL), although cytotoxicity of polymers is increased by conjugation of FA, likely due to higher levels of polymer internalization mediated by the targeting ligand. In KB cells, the IC50 of free Dox and FA-SF-Dox were 0.92 and 2.47 μg/mL in Dox equivalents, respectively. The increased IC50 of FA-SF-Dox compared to free Dox is likely due to slower internalization mechanism (endocytosis vs. direct membrane permeation) and the release kinetics of free drug from polymer. In A549 cells, the IC50 of the FA-SF-Dox formulation is increased to an even greater extent relative to free Dox and is not significantly different from the untargeted SF-Dox formulation.
Table 1.
IC50 values and 95% confidence intervals for Dox and polymer formulations in KB (FR+) and A549 (FR−) cell lines.
| Cell line | SF | FA-SF |
IC50 in Dox equivalents (μg/mL) (95% CI)
|
|||
|---|---|---|---|---|---|---|
| Free Dox | SF-Dox | FA-SF-Dox | FA-comb-Dox | |||
| KB | N/A* (>5.5 mg/mL) | 3.59 mg/mL (3.40, 3.79) | 0.918 (0.866, 0.972) | 4.15 (3.93, 4.38) | 2.47 (2.22, 2.75) | 2.42 (2.22, 2.64) |
| A549 | N/A* (>5.5 mg/mL) | N/A* (>5.5 mg/mL) | 1.65 (1.47, 1.84) | 7.23 (6.79, 7.70) | 8.14 (7.64, 8.67) | 3.40 (3.07, 3.75) |
Cell survival >90% at the highest tested concentration.
The cytotoxicity results also confirm trends in uptake based on polymer architecture. FA-SF-Dox polymer, which was previously shown to undergo FR-dependent uptake, exhibited the best selectivity in cell killing for KB cells over A549 cells. Meanwhile, the analogous linear comb-like polymer, FA-comb-Dox, was similarly cytotoxic in both KB cells and non-target A549 cells. This non-specific cytotoxicity correlates well with the previous uptake data suggesting non-FR-mediated uptake of the comb polymer.19 It is possible that the constrained architecture of the sunflower polymer facilitates display of the folate targeting ligand and subsequent interaction with cell surface receptors; the mechanistic basis for this difference in uptake warrants further study.
CONCLUSIONS
In this work, we have extended the sunflower polymer platform by investigating the degree of polymerization of the core as an additional tunable parameter for synthesizing polymers with precise hydrodynamic sizes. Preliminary findings indicate that polymers of different core sizes have significantly different uptake behaviors; the mechanisms underlying this phenomenon may be interesting for further investigation. We have also demonstrated that drugs can be conjugated to the cyclic core buried within the polymer petals, thereby reducing polymer aggregation that has been observed in dendrimer-drug conjugates induced by hydrophobic drug-drug interactions.37 Using this platform, doxorubicin can be incorporated via a pH-sensitive linker and triggered for intracellular release of active drug. In future work, drug loading (currently 2–5 w/w%) might be increased by altering sunflower polymer structure, e.g. conjugating drugs onto petals instead of cores or encapsulating drugs by hydrophobic or ionic interactions, methods which have been shown to improve drug loading (15–50 w/w%).38,39
In summary, sunflower polymers represent a unique platform for targeted drug delivery. Sunflower polymers combine the structural complexity and nano-size of dendrimers with the relative ease of synthesis of traditional linear polymers. These polymers integrate multiple characteristics to address the challenges of drug delivery to tumors – such as appropriate size for tumor penetration, preferential drug release in the tumor microenvironment, and cell-specific delivery – into a single material.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (1R01CA177272), the National Science Foundation (DMR 1206426), a National Science Foundation Graduate Research Fellowship to C.E.W., and the National Natural Science Foundation of China (51473072) to H.W. Confocal imaging was completed at the UW Keck Microscopy Facility with help from Greg Martin.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest.
Supporting Information. Polymer yields and additional GPC, FT-IR, 1H NMR, and DLS characterizations. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Meirow D, Nugent D. Hum Reprod Update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 2.Singal PK, Iliskovic N. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura Y, Maeda H. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 4.Davis ME, Chen ZG, Shin DM. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 5.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 6.Desai N. AAPS J. 2012;14:282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyer I, Cao H, Persson J, Song H, Richter M, Feng Q, Yumul R, van Rensburg R, Li Z, Berenson R, et al. Clin Cancer Res. 2012;18:3340–3351. doi: 10.1158/1078-0432.CCR-11-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghimi SM, Szebeni J. Prog Lipid Res. 2003;42:463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 9.Sun X, Yan Y, Liu S, Cao Q, Yang M, Neamati N, Shen B, Niu G, Chen X. J Nucl Med. 2011;52:140–146. doi: 10.2967/jnumed.110.080606. [DOI] [PubMed] [Google Scholar]
- 10.Elzoghby AO, Samy WM, Elgindy NA. J Control Release. 2012;157:168–182. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Gabizon A, Shmeeda H, Barenholz Y. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 12.Du JZ, Du XJ, Mao CQ, Wang J. J Am Chem Soc. 2011;133:17560–17563. doi: 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- 13.Tong R, Cheng J. J Am Chem Soc. 2009;131:4744–4754. doi: 10.1021/ja8084675. [DOI] [PubMed] [Google Scholar]
- 14.Kataoka K, Harada A, Nagasaki Y. Advanced Drug Delivery Reviews. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 15.Tong R, Cheng J. Angew Chem. 2008;120:4908–4912. [Google Scholar]
- 16.Fox ME, Guillaudeu S, Fréchet JMJ, Jerger K, Macaraeg N, Szoka FC. Mol Pharm. 2009;6:1562–1572. doi: 10.1021/mp9001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaminskas LM, Boyd BJ, Karellas P, Henderson SA, Giannis MP, Krippner GY, Porter CJH. Mol Pharm. 2007;4:949–961. doi: 10.1021/mp070047s. [DOI] [PubMed] [Google Scholar]
- 18.Nasongkla N, Chen B, Macaraeg N, Fox ME, Fréchet JMJ, Szoka FC. J Am Chem Soc. 2009;131:3842–3843. doi: 10.1021/ja900062u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei H, Wang CE, Tan N, Boydston AJ, Pun SH. ACS Macro Lett. 2015:938–941. doi: 10.1021/acsmacrolett.5b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matyjaszewski K, Tsarevsky NV. J Am Chem Soc. 2014;136:6513–6533. doi: 10.1021/ja408069v. [DOI] [PubMed] [Google Scholar]
- 21.Neugebauer D, Zhang Y, Pakula T, Sheiko SS, Matyjaszewski K. Macromolecules. 2003;36:6746–6755. [Google Scholar]
- 22.Low PS, Henne WA, Doorneweerd DD. Acc Chem Res. 2008;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Low PS. Advanced Drug Delivery Reviews. 2012;64:342–352. doi: 10.1016/j.addr.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Laurent BA, Grayson SM. J Am Chem Soc. 2006;128:4238–4239. doi: 10.1021/ja0585836. [DOI] [PubMed] [Google Scholar]
- 26.Laurent BA, Grayson SM. J Am Chem Soc. 2011;133:13421–13429. doi: 10.1021/ja2024355. [DOI] [PubMed] [Google Scholar]
- 27.Laurent BA, Grayson SM. Polym Chem. 2012;3:1846–1855. [Google Scholar]
- 28.Duncan R. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 29.Jain RK. Cancer Metastasis Rev. 1987;6:559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- 30.Goodman TT, Olive PL, Pun SH. Int J Nanomedicine. 2007;2:265–274. [PMC free article] [PubMed] [Google Scholar]
- 31.Ng CP, Pun SH. Biotechnol Bioeng. 2008;99:1490–1501. doi: 10.1002/bit.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 33.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Fréchet JMJ, Dy EE, Szoka FC. Proc Natl Acad Sci USA. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padilla De Jesús OL, Ihre HR, Gagne L, Fréchet JMJ, Szoka FC. Bioconjug Chem. 2002;13:453–461. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 35.Kim D, Lee ES, Oh KT, Gao ZG, Bae YH. Small. 2008;4:2043–2050. doi: 10.1002/smll.200701275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerweck LE, Seetharaman K. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 37.Pu Y, Chang S, Yuan H, Wang G, He B, Gu Z. Biomaterials. 2013;34:3658–3666. doi: 10.1016/j.biomaterials.2013.01.082. [DOI] [PubMed] [Google Scholar]
- 38.Kataoka K, Matsumoto T, Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kwon GS. J Control Release. 2000;64:143–153. doi: 10.1016/s0168-3659(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 39.Kim JO, Kabanov AV, Bronich TK. J Control Release. 2009;138:197–204. doi: 10.1016/j.jconrel.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.