Abstract
♦ Introduction:
This study was carried out to investigate the center effect on the risk of peritoneal dialysis (PD) failure within the first 6 months of therapy using a multilevel approach.
♦ Methods:
This was a retrospective cohort study based on data from the French Language Peritoneal Dialysis Registry. We analyzed 5,406 incident patients starting PD between January 2008 and December 2012 in 128 PD centers. The end of the observation period was December 31, 2013.
♦ Results:
Of the 5,406 patients, 415 stopped PD within the first 6 months. There was a significant heterogeneity between centers (variance of the random effect: 0.10). Only 3% of the variance of the event of interest was attributable to differences between centers. At the individual level, only treatment before PD (odds ratio [OR]: 1.93 for hemodialysis and OR: 2.29 for renal transplantation) and underlying nephropathy (p < 0.01) were associated with early PD failure. At the center level, only center experience was associated (OR: 0.78) with the risk of PD failure. Center effect accounted for 52% of the disparities between centers.
♦ Conclusion:
Center effect on early PD failure is significant. Center experience is associated with a lower risk of transfer to hemodialysis.
Keywords: Peritoneal dialysis, center effect, multilevel modelling
Reducing technique failure is a major objective for nephrologists in charge of peritoneal dialysis (PD) patients. It is well established that many factors can contribute to PD failure (1). Patient characteristics associated with technique failure can be used to predict the risk of transfer to hemodialysis (HD) but are non-modifiable factors. It has been established that center organization, center size, and center experience affect technique survival (2–6). Nevertheless, to estimate whether changes at the center level must be implemented, studies are needed to evaluate the magnitude of the center effect per se and to identify which organizations are significantly associated with the risk of technique failure. Furthermore, provision of resources for PD centers should be based on their effect on patient outcome. Center organization requirements have been proposed to ensure the success of PD programs (4). To our knowledge, there is no study available about the magnitude of the center effect on the risk of PD failure. Hierarchical modelling produces an estimate of the center contribution to the risk of the event of interest. Early PD failure leading to a transfer to HD is a frequent event (7,8,9). Early PD failure can be defined as a transfer to HD within the first 6 months of PD, regardless of PD duration during the time period. It is accepted that the causes of early PD cessation are different than the cause of late PD failure. Identification of center characteristics associated with a lower risk of early failure may help nephrologists to implement changes in the center organization. In France, structural organizations differ between PD centers, which could affect the risk of transfer to HD.
This study, based on a multilevel analysis, was carried out to evaluate the magnitude of the center effect on early PD failure. The objective was also to identify center organization associated with the risk of early transfer to HD.
Patients and Methods
Study Population
This was a retrospective study using data from the French Language Peritoneal Dialysis Registry (RDPLF). The RDPLF was set up in 1986. In France, 82% of all PD patients are registered in the RDPLF. Participating centers provide information regarding their patients every 3 months. Details about the registry management have been published (10). Patients older than 18 years old starting PD in France between January 1, 2008, and December 31, 2012, were included in the study. The end of the observation period was December 31, 2013, and only patients starting PD were included in the study. From the original dataset, we excluded 13 centers that stopped collecting data regularly during the study period. To conduct a multilevel modelling, we excluded 4 centers having only 1 patient starting PD during the study period. There were 5,406 patients from 128 centers in the final dataset.
Definition of Variables
Patient characteristics (level 1 covariates) were extracted from the database of the RDPLF. Covariates extracted from the registry were gender, comorbidities, diabetes mellitus, and underlying nephropathy, PD modality at dialysis initiation (automated PD [APD] or continuous ambulatory PD [CAPD], assisted PD (family assistance, nurse assistance), starting PD after transplantation failure). To assess patient comorbidities we extracted the Charlson comorbidity score (CCI) from the database, and we calculated the modified CCI by subtracting the age sub-score. Sub-optimal PD initiation was defined by a time spent on HD before PD of less than 1 month (11).
To obtain data about center organization and center characteristics (level 2 covariates), an additional questionnaire was sent to each PD facility. Subsequently, the information about the centers was collected by interviewing the head nurse of each PD center. Covariates used to describe center organization were: full-time nurse (nurses dedicated only to PD), part-time nurse (nurses specialized in PD but also involved in other activities), nephrologists specialized in PD, and home visits by PD nurses. The number of nephrologists and nurses by PD center were also noted. As centers were characterized by the number of incident PD patients per center and per year, no caregiver ratio was calculated to avoid colinearity between these 2 covariates. Centers were also separated by category (academic center, community center, non-profit center, and private center).
Statistical Analysis
Categorical covariates were expressed as frequencies and percentages; continuous covariates were expressed as the median (first and third quartile).
The event of interest was early PD failure, defined by a transfer to HD for more than 2 months within the first 6 months of PD, regardless of PD duration during the time period. Renal transplantation and death during the first 6 months on PD were censored. Fine and Gray survival model with random effect was not available. Estimation of the random effect using a Cox model is controversial. The aim of the study was not to evaluate PD duration during the first 6 months of dialysis. Logistic regression model enables risk estimation at a specific time point. Therefore a mixed logistic model, which provides a robust estimation of the center effect, was used for the statistical analysis. To explore the association between each covariate and the event of interest, a bivariate analysis was performed with a logistic regression model that estimates crude odds ratios (ORs) with their 95% confidence interval (CI). Covariates were selected for the multivariate analysis when the p value was lower than 0.20 in the bivariate analysis. Variance inflation factor was utilized to assess whether there was colinearity between 2 covariates.
In order to take into account hierarchical data, a multilevel modelling approach was used for the multivariate analysis. A logistic regression modelling was performed with center as a random effect, patient characteristics (level 1), center organizations, and center characteristics (level 2) as fixed effects (12). To detect heterogeneity between centers, we set up an empty model (model 0), with only center as a random effect. Model 0 was compared with a traditional logistic empty model by the likelihood ratio test that was considered to be significant if the p value was below 0.05. To quantify the heterogeneity between centers, we calculated the intraclass correlation coefficient (ICC) also called variance partition coefficient (VPC). Intraclass correlation coefficient represents the proportion of the total variance of the outcome attributed to the center level. Intraclass correlation coefficient was estimated with the latent variable method, that is ICC=VA/(VA+π2/3), where VA is the variance of the random effect and π2/3 is the variance of the underlying standard logistic error (13). Thereafter, individual covariates (level 1) were included in the model (model 1) to investigate whether center heterogeneity was explained by the patient composition of the center. Subsequently, center covariates (level 2) were included in the model (model 2) to investigate whether center effect was influenced by center characteristics and organization. Model 1 was compared with model 2 by analysis of variance. To estimate the contribution of the covariates introduced in each model, proportional change in variance (PCV) was calculated [PCV = (VN1-VN2)/VN1, where VN1 is the variance of random effect of the empty model and VN2 is the level-2 variance of the model 1 or of the model 2] (14).
Statistical analyses were performed with R 3.1.2. (R Foundation for Statistical Computing, Vienna, Austria) including the lme4 package.
The RDPLF has the approval of the French national ethics committee (“Commission Nationale de l'Informatique et des Libertés”). This study took place within the framework of this authorization.
Missing Data
The rate of missing data was inferior to 15% for each covariate of the dataset; a multiple imputation by chained equation was performed for all missing data.
Results
Patient Characteristics
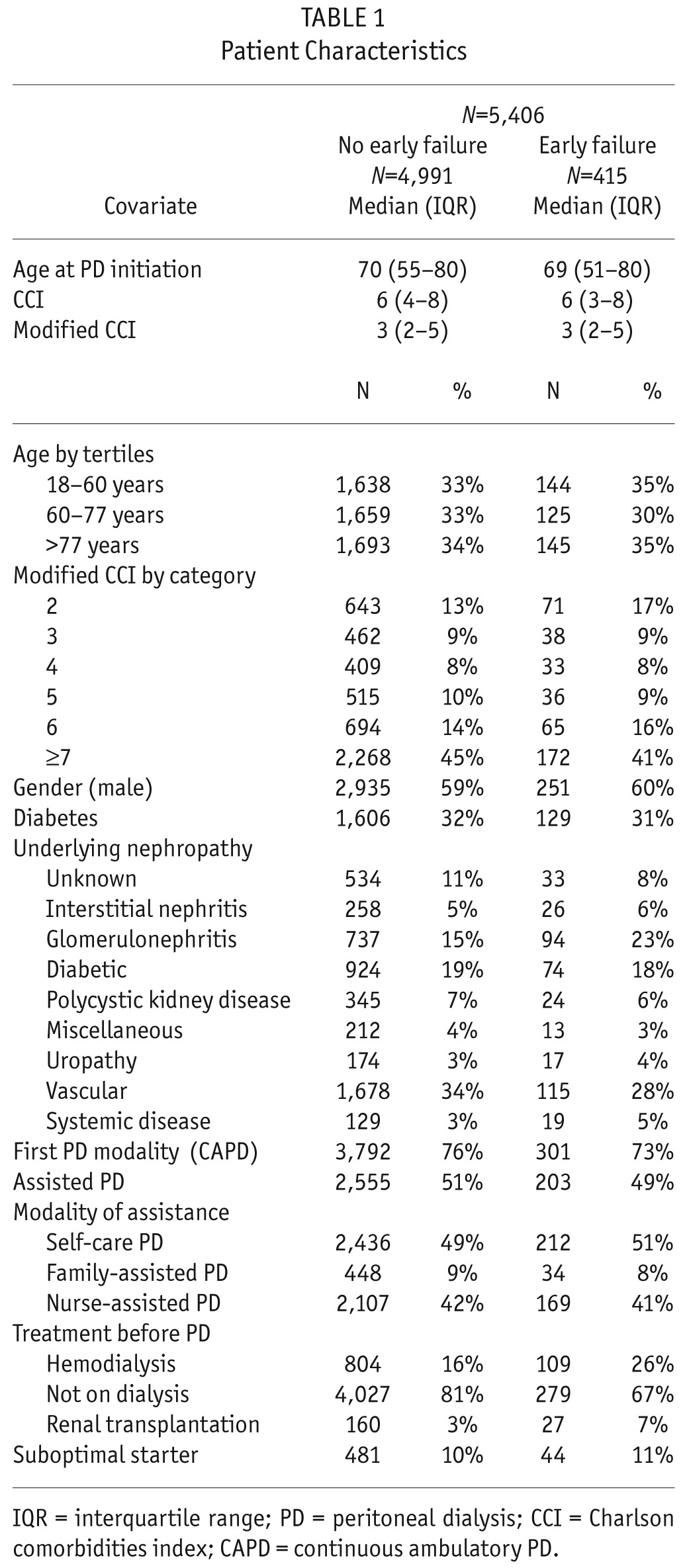
Of the 5,406 patients, 415 stopped PD and transferred to HD within the first 6 months of PD. The median age was 70 years (interquartile range [IQR] = 55 – 80), the median CCI was 6 (IQR: 4 – 8), the gender ratio (M/F) was 3,186/2,220, and there were 1,735 diabetic patients (32%). Of the 5,406 patients, 2,758 were on assisted PD (family-assisted: 482, nurse-assisted: 2,276). Causes of PD cessation were peritonitis (40), catheter dysfunction (82), dialysis adequacy (58), psychosocial problems (47), ultrafiltration failure (39), malnutrition (6), and other related to PD (143). Patient characteristics are presented in Table 1.
TABLE 1.
Patient Characteristics
Center Characteristics
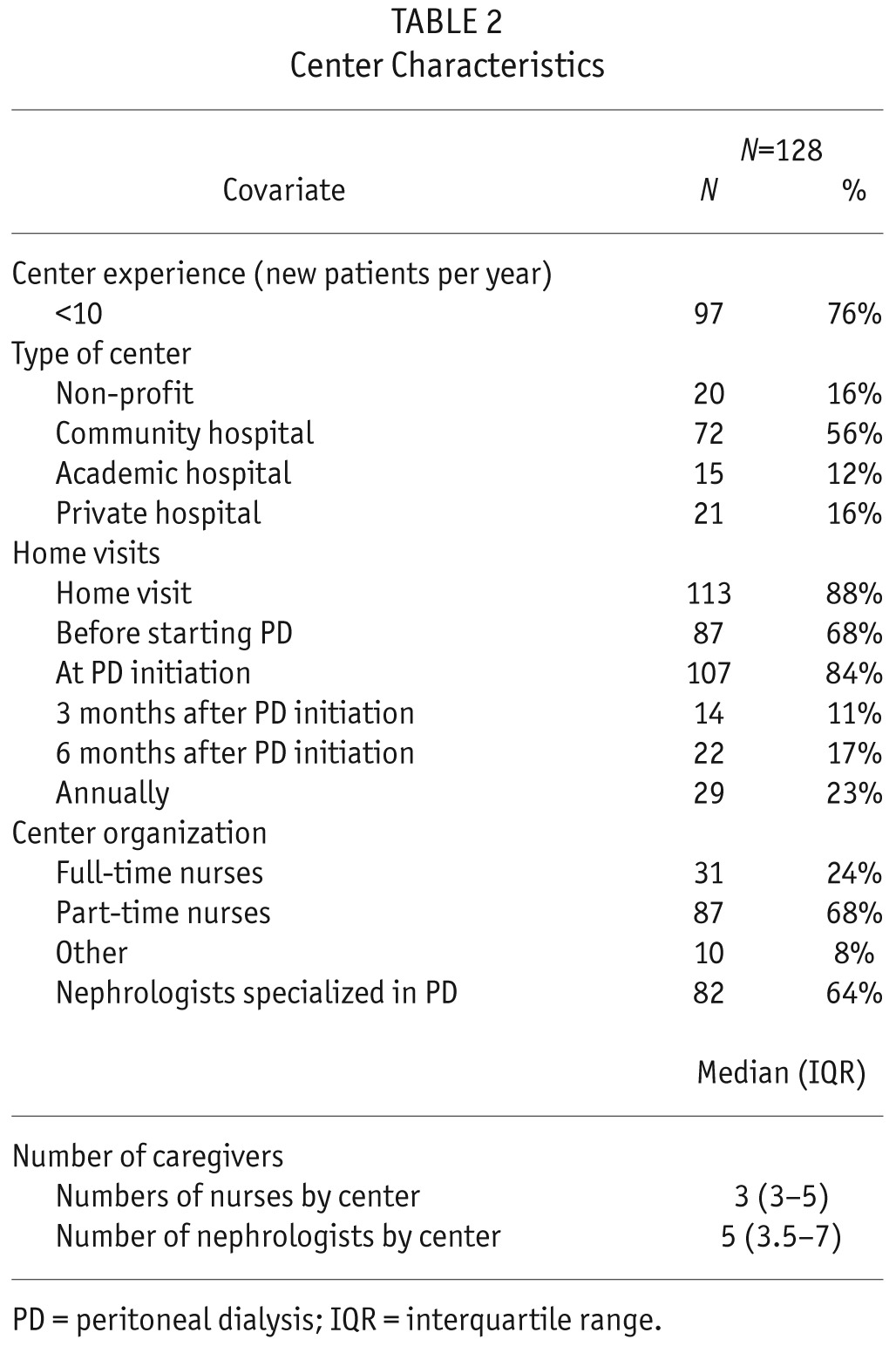
Among the 128 centers, there were 72 (56%) community hospital centers, 20 (16%) non-profit centers, 21 (16%) private centers, and 15 (12%) academic centers. Of these 128 centers, 97 (76%) treated less than 10 new PD patients per year. Nurse home visits were performed by 113 (88%) centers. Among those 113 centers, 87 (68%) provided a home visit before PD start, 107 (84%) at PD initiation, 14 (11%) 3 months after PD initiation (11%), and 22 centers 6 months after PD initiation (17%). There were part-time nurses in 87 centers (68%) and full-time nurses in 31 centers (24%). Center characteristics are presented in Table 2.
TABLE 2.
Center Characteristics
Bivariate Analysis
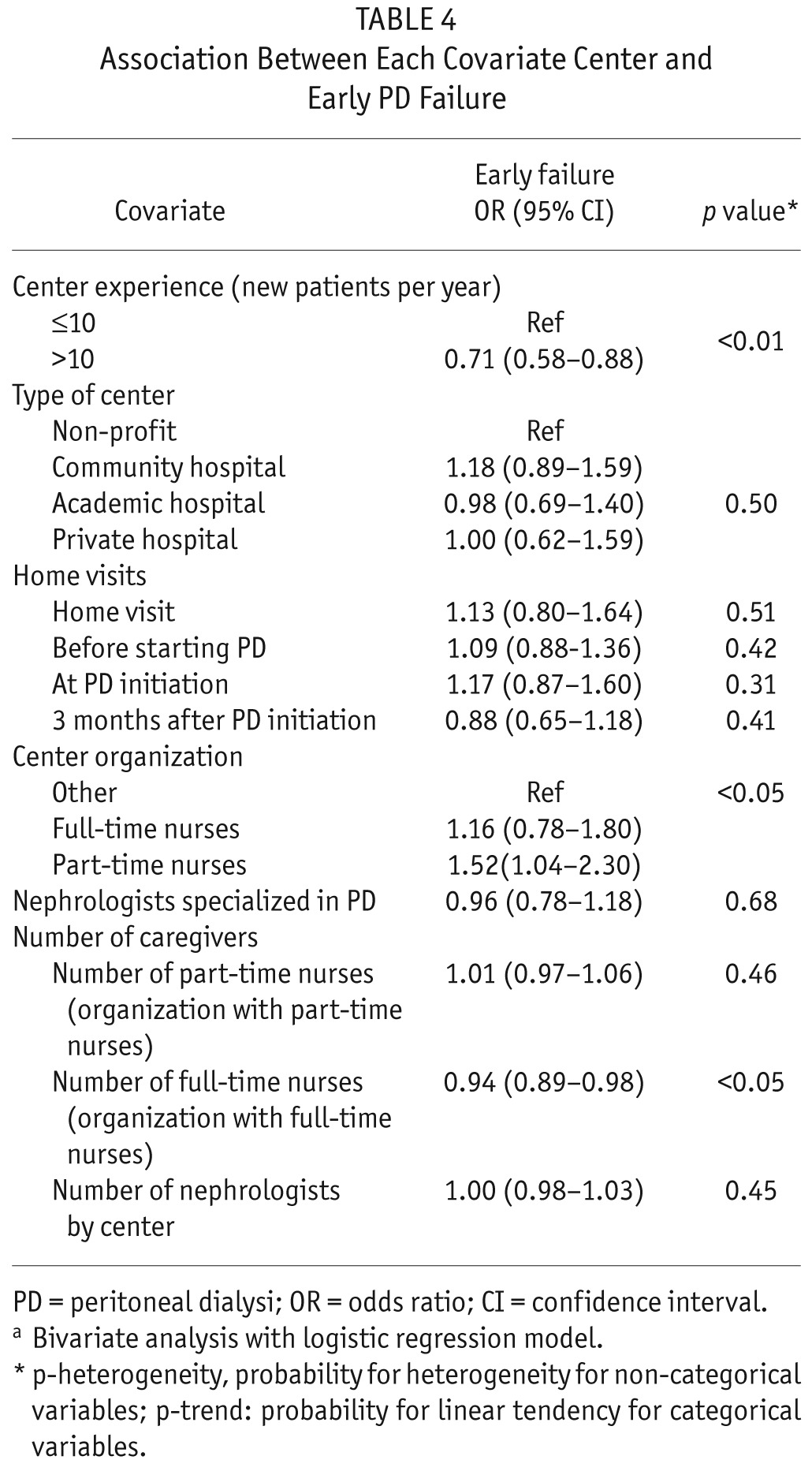
In the bivariate analysis, patient age (OR: 0.94, CI: 0.89 – 0.99), CCI (OR: 0.96, CI: 0.92 – 1.00), and modified CCI (OR: 0.94, CI: 0.89 – 0.99) were associated with a lower risk of early technique failure. There was also a significant association between the underlying nephropathy and the risk of transfer to HD (p < 0.001). Patients treated with HD (OR: 1.96, CI: 1.54 – 2.47) or starting PD after transplantation failure (OR: 2.43, CI: 1.56 – 3.66) had a higher risk of transfer to HD. Patients in centers that belonged to the group treating more than 10 new patients per year had a lower risk of early PD failure (OR: 0.71, CI: 0.58 – 0.88). There was no significant relationship between center experience and the causes of early PD failure. There was no association between center category, home visits, full-time nurse, part-time nurse, nephrologists specialized in PD, and transfer to HD. Results of the bivariate analysis are presented in Tables 3 and 4.
TABLE 3.
Association Between Each Covariate Patient and Early PD Failurea
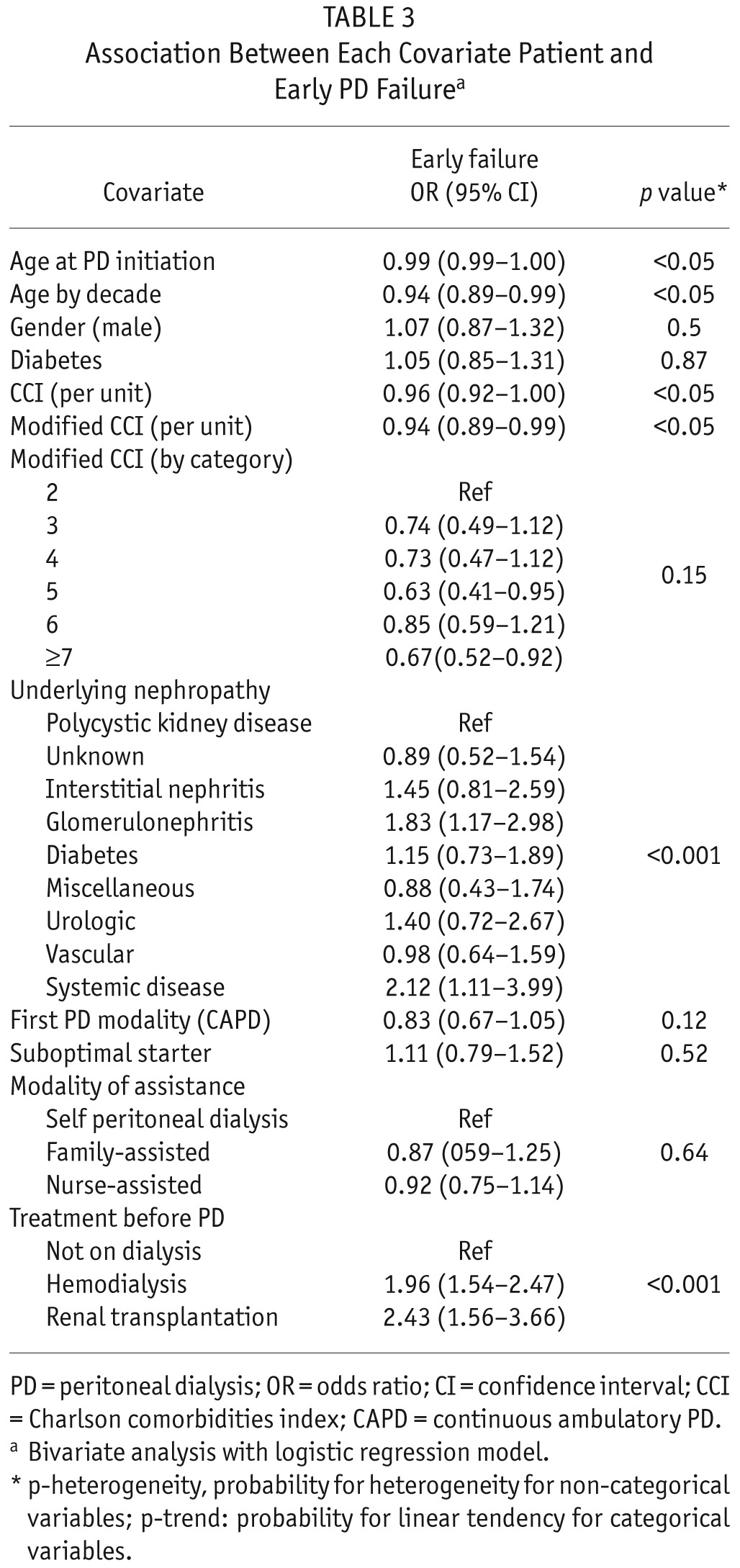
TABLE 4.
Association Between Each Covariate Center and Early PD Failure
Multilevel Analysis
Variance of the random effect was 0.10 and there was a significant variation across PD centers (Table 5). The ICC was 0.03 showing that only 3% of the variance of the event of interest was attributable to differences between centers.
TABLE 5.
Multivariate Mixed Logistic Regression of Factors Associated with Early PD Failure
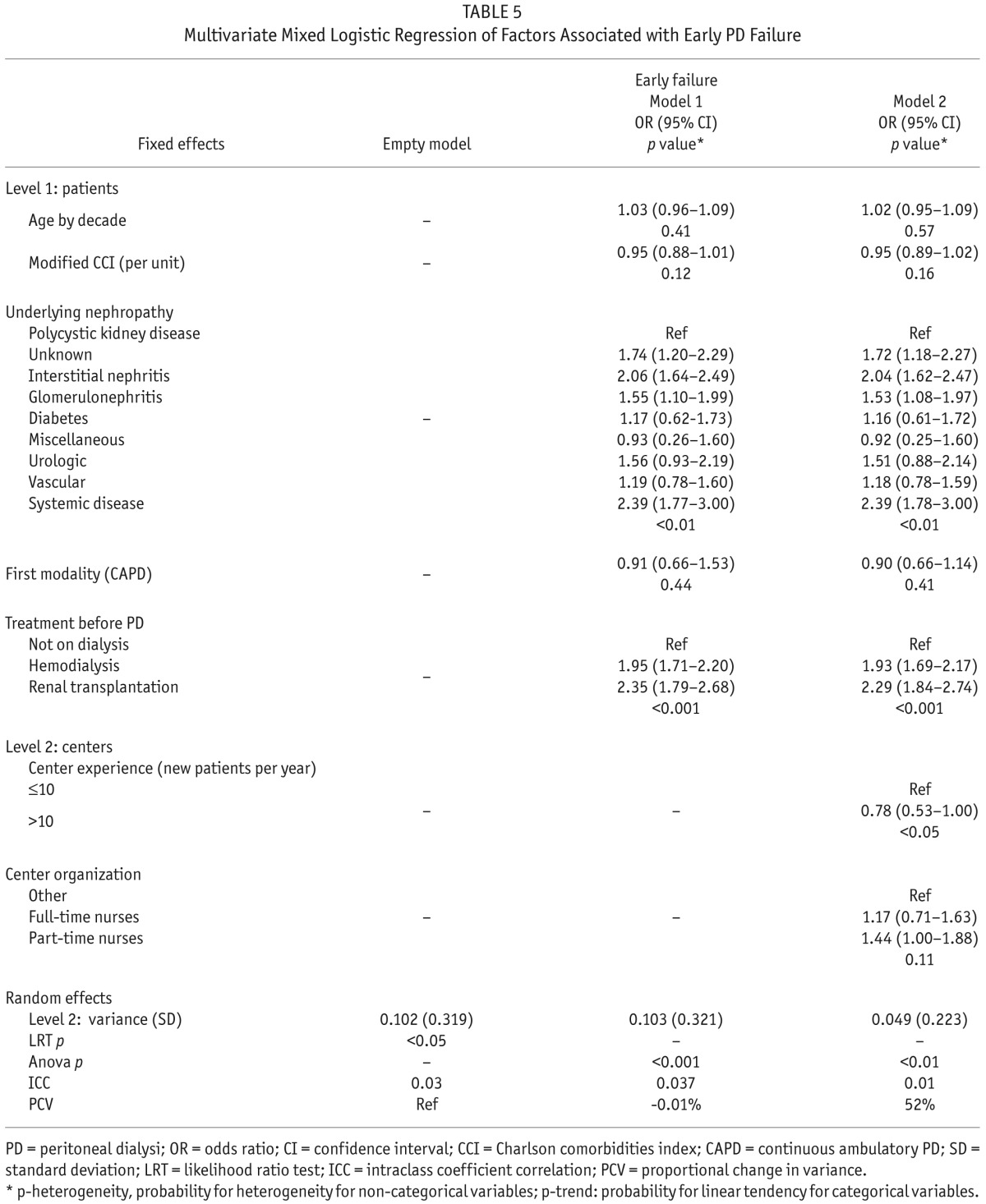
At the individual level (level 1), only treatment before PD (OR: 1.93, CI: 1.69 – 2.17 for hemodialysis and OR: 2.29, CI: 1.84 – 2.74 for renal transplantation) and underlying nephropathy were associated with early PD failure. Variance of the random effect increased by 0.01% after adjustment on patient characteristics. In other words, center effect was not due to differences in the patient characteristics between centers.
At the center level (level 2), only center experience was associated with the risk of PD failure. Variance of the random effect decreased by 52% after adjustment for patient characteristics, center organization, and center experience. That meant that center effect accounted for 52% of the disparities between centers.
Discussion
Although technique failure is a frequent event in PD, there are limited data about center effect on transfer to HD. Center effect should be investigated as it would allow modifications at the center level that could improve technique survival. To our knowledge, the studies available about center effect have utilized traditional measures of association between the event of interest and center size (3,5,6). Traditional regression analysis provides estimates, such as an odds ratio or hazard ratio, which measure the link between center characteristics and the probability of the event of interest. Multilevel logistic regression considers that individual probability of the event of interest is also dependent on the PD center where patients are treated (15). Intraclass coefficient correlation, estimated by the latent variable method, can provide an estimation of the magnitude of the center effect on the outcome of interest (15,16). Intraclass coefficient correlation does not depend on the event prevalence. Our study showed that there was significant heterogeneity between centers. However, the ICC estimation showed that only 3.5% of the total variance of the outcome could be attributed to the center level. In view of the proportional change in variance after adjustment for patient level covariates, heterogeneity between centers could not be explained by differences in patient characteristics between centers. The multivariate analysis showed that underlying nephropathy and treatment before PD were associated with the risk of early transfer to PD. These findings confirmed the results of a previous study about early failure of PD from our group that took into account competing events (6). The final model showed that the effect of the PD center cannot be fully explained by center experience and by patient characteristics. These results suggest that center practice may partially explain early transfer to HD. In support of this, one recent study has documented that training patterns were associated with peritonitis incidence (17). A negative correlation between nurse experience and the risk of gram-positive peritonitis was documented in a study from Hong Kong (18). As peritonitis is one of the leading causes of PD failure, these findings suggest that center experience and practice could affect technique survival.
Nephrologist experience with PD may also partially explain center effect. Among 91 French nephrologists who were asked to give their opinion about the optimal rate of PD, 23% felt that limited training and experience about PD was a barrier to PD development (19). Until recently, there was no core curriculum for PD in French academic hospitals. Only center experience was associated with a lower risk of transfer to HD in our study. This finding is consistent with the results of other studies. In Canada, center experience, measured by the cumulative number of patients treated by PD and the proportion of end-stage renal disease patients treated by PD was a predictor of technique failure (20). Huisman et al., in the Netherlands, found that patients treated in centers with less than 20 patients on PD had a higher risk of transfer to HD (2). In 1 study from the United States, technique failure was higher in centers caring for less than 25 patients (3). Patients treated in PD centers initiating PD in less than 10 patients per year had a greater risk of technique failure in studies taking into account competing risks (6,7). None of these studies have evaluated the magnitude of the center effect or the potential role of center characteristics on technique failure. Surprisingly, there was no link between center organization and the risk of early PD failure in our study. Because our study has only focused on the transfer to HD within the first 6 months of renal replacement, one cannot exclude an effect of center organization on technique survival.
In addition, the aim of our study was to evaluate the risk of transfer to HD; it is obvious that there are other events that may occur on PD, which could be influenced by center organization. As an example, it has been demonstrated that, in France, nurse home visits are associated with a lower risk of peritonitis in patients treated by nurse-assisted PD (21). It has been suggested recently that assessing and training patients at home could improve patients' knowledge (22). Furthermore, nurse-assisted PD patients had a lower risk of PD failure in 1 study from France (7). In France, there are differences between centers in assisted PD utilization, meaning that center practice could impact technique survival (unpublished data). Assisted-PD patients had a similar risk of early peritoneal failure in our study, which finding is in line with a recent study from our group (6).
Our study has several limitations. There were residual confounders, such as body mass index (BMI) or detailed PD prescription, not captured in the registry. In addition, one may argue that differences between centers regarding PD catheter insertion technique could influence PD success. Nevertheless, in France, the vast majority of PD catheters are placed by surgeons under laparotomy (2,409/2,731 PD catheters inserted during the study period – unpublished data from the “catheter section” of the RDPLF).
In conclusion, there is a significant center effect on the probability of early transfer of PD patients to HD. Among center characteristics, only center experience was associated with the risk of PD failure. Further studies are needed to estimate the center effect on other events that may occur during PD.
REFERENCES
- 1. Lobbedez T, Boissinot L, Ficheux M, Castrale C, Ryckelynck JP. How to avoid technique failure in peritoneal dialysis patients? Contrib Nephrol 2012; 178:53–7. [DOI] [PubMed] [Google Scholar]
- 2. Huisman RM, Nieuwenhuizen MGM, Th de Charro F. Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in The Netherlands. Nephrol Dial Transplant 2002; 17:1655–60. [DOI] [PubMed] [Google Scholar]
- 3. Afolalu B, Troidle L, Osayimwen O, Bhargava J, Kitsen J, Finkelstein FO. Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit Dial Int 2009; 29:292–6. [PubMed] [Google Scholar]
- 4. Figueiredo AE, de Moraes TP, Bernardini J, Poli-de-Figueiredo CE, Barretti P, Olandoski M, et al. Impact of patient training patterns on peritonitis rates in a large national cohort study. Nephrol Dial Transplant 2015; 30:137–42. [DOI] [PubMed] [Google Scholar]
- 5. Evans D, Lobbedez T, Verger C, Flahaut A. Would increasing centre volumes improve patient outcomes in peritoneal dialysis? A registry-based cohort and Monte Carlo simulation study. BMJ Open 2013; 3:e003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bechade C, Guittet L, Evans D, Verger C, Ryckelynck JP, Lobbedez T. Early failure in patients starting peritoneal dialysis: a competing risks approach. Nephrol Dial Transplant 2014; 29:2127–35. [DOI] [PubMed] [Google Scholar]
- 7. Lobbedez T, Verger C, Ryckelynck J-P, Fabre E, Evans D. Is assisted peritoneal dialysis associated with technique survival when competing events are considered? Clin J Am Soc Nephrol 2012; 7:612–8. [DOI] [PubMed] [Google Scholar]
- 8. Bernardini J, Price V, Figueiredo A, Riemann A, Leung D. International survey of peritoneal dialysis training programs. Perit Dial Int 2006; 31:614–30. [PubMed] [Google Scholar]
- 9. Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye WC, et al. ISPD position statement on reducing the risks of peritoneal dialysis–related infections. Perit Dial Int 2006; 26:625–32. [DOI] [PubMed] [Google Scholar]
- 10. Verger C, Ryckelynck J-Ph, Duman M, Veniez G, Lobbedez T, Boulanger E, et al. French Peritoneal Dialysis Registry. Outcome and main results. Kidney Int 2006; 103:S12–20. [DOI] [PubMed] [Google Scholar]
- 11. Lobbedez T, Verger C, Ryckelynck JP, Fabre E, Evans D. Outcome of the sub-optimal dialysis starter on peritoneal dialysis. Report from the French Language Peritoneal Dialysis Registry. Nephrol Dial Transplant 2013; 28:1276–83. [DOI] [PubMed] [Google Scholar]
- 12. Snidjers TAB, Bosker RJ. Multilevel analysis: an introduction to basic and advanced multilevel modeling. London: Sage publications; 1999. [Google Scholar]
- 13. De Leew J, Meijer E, eds. Handbook of multilevel analysis, Springer; 2008. [Google Scholar]
- 14. Merlo J, Yang M, Chaix B, Lynch J, Rastam L. A brief conceptual tutorial on multilevel analysis in social epidemiology: investigating contextual phenomena in different groups of people. J Epidemiol Community Health 2005; 59:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006; 60:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol 2005; 161:81–8. [DOI] [PubMed] [Google Scholar]
- 17. Figueiredo AE, De Moraes TP, Bernardini J, Poli-de-Figueiredo CE, Barretti P, Olandoski M, et al. BRAZPD Investigators Impact of patient training patterns on peritonitis rates in a large national cohort study. Nephrol Dial Transplant 2015; 30:137–42. [DOI] [PubMed] [Google Scholar]
- 18. Chow KM, Szeto CC, Law MC, Fun Fung JS, Li PK. Influence of peritoneal dialysis training nurses' experience on peritonitis rates. Clin J Am Soc Nephrol 2007; 2:647–52. [DOI] [PubMed] [Google Scholar]
- 19. Bouvier N, Durand PY, Testa A, Albert C, Planquois V, Ryckelynck JP, et al. Regional discrepancies in peritoneal dialysis utilization in France: the role of the nephrologist's opinion about peritoneal dialysis. Nephrol Dial Transplant 2009; 24:1293–7. [DOI] [PubMed] [Google Scholar]
- 20. Schaubel DE, Blake PG, Fenton SS. Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 2001; 60:1517–24. [DOI] [PubMed] [Google Scholar]
- 21. Verger C, Duman M, Durand PY, Veniez G, Fabre E, Ryckelynck JP. Influence of autonomy and type of home assistance on the prevention of peritonitis in assisted automated peritoneal dialysis patients. An analysis of data from the French Language Peritoneal Dialysis Registry. Nephrol Dial Transplant 2007; 22:1218–23. [DOI] [PubMed] [Google Scholar]
- 22. Ozturk S, Yucel L, Guvenc S, Ekiz S, Kazancioglu R. Assessing and training patients on peritoneal dialysis in their own homes can influence better practice. J Ren Care 2009; 35:141–6. [DOI] [PubMed] [Google Scholar]


