Abstract
♦ Background and objective:
Residual renal function (RRF) correlates with mortality and morbidity rates in patients receiving peritoneal dialysis (PD). We examined the effect of a biocompatible PD solution (Gambrosol Trio; Gambro Lundia AB, Lund, Sweden) with lower concentrations of glucose degradation products on rates of decline in RRF.
♦ Design, setting, participants, and measurements:
Incident patients at 2 centers in Canada and 1 in Hong Kong were randomized (by minimization) in an open-label parallel group trial to receive Gambrosol Trio or standard PD solution (Dianeal; Baxter Healthcare, Mississauga, Canada) for 2 years. Primary outcome was slope of RRF. Secondary outcomes were urine volumes, fluid and nutrition indices, PD and membrane characteristics, peritonitis rates, adverse events, and PD technique survival.
♦ Results:
Residual renal function declined by 0.132 mL/minute/1.73 m2/month in 51 patients allocated to biocompatible, and 0.174 mL/minute/1.73 m2/month in 50 patients allocated to standard PD solution (difference 0.042 mL/minute/1.73 m2/month, p = 0.001). Urine volume, body mass index, normalized protein catabolic rates, and fat mass were higher; total body water, peritoneal ultrafiltration, and D/P creatinine did not differ; and serum phosphate, rates of icodextrin, and automated cycler use were lower with Gambrosol Trio use. There were more peritonitis events with Gambrosol Trio use, while PD technique survival did not differ between groups.
♦ Conclusions:
The use of the biocompatible PD solution Gambrosol Trio was associated with slower rates of decline in RRF, fluid and nutrition benefits, and increased peritonitis rates. Trial Number: ISRCTN26252543
Keywords: Peritoneal dialysis, biocompatible PD solutions, residual renal function, peritonitis
Residual renal function (RRF) is associated with lower mortality rates in dialysis-dependent patients (1,2). It is also linked to lower rates of left ventricular hypertrophy, lower indices of inflammation, and improved nutritional status, blood pressure control, and quality-of-life scores (1,2). Finally, RRF is a key contributor to peritoneal dialysis (PD) technique survival (1,2). Modifiable factors linked to faster declines in RRF include extracellular fluid depletion and previous hemodialysis treatments, while slower declines are associated with the use of inhibitors of the renin-angiotensin-aldosterone system, and possibly with the use of biocompatible PD solutions (1,3).
Biocompatible PD solutions contain lower levels of glucose degradation products that, in animal models, are nephrotoxic both directly and after conversion to advanced glycation end-products (1,4). However, these solutions are significantly more expensive than standard PD solutions, and studies have been inconsistent with regard to their effect on RRF (4,5,6). The largest and most recent was the balANZ trial, where Johnson et al. randomized 185 incident patients with glomerular filtration rates (GFRs) greater than 5 mL/min/1.73 m2 in Australia, New Zealand, and Singapore to 2 years of treatment with a biocompatible (Balance; Fresenius Medical Care North America, Waltham, MA, USA) or standard (Stay•Safe; Fresenius Medical Care North America, Waltham, MA, USA) PD solution. No difference was seen in RRF decline rates between groups (p value 0.06 for difference in rates of decline over the 2-year study period) (7). However the study enrolled only 55% of the intended 336 patients, and had a drop-out rate of 54%. It also included patients who had previously been on hemodialysis, a potentially important confounder that was not evenly distributed between groups (13 [14.3%] in the biocompatible arm and 4 [4.4%]) in the standard arm) at randomization (7). Other studies have shown variable effects of biocompatible solutions on RRF, and have been hampered by methodological issues such as shorter durations of follow-up, study design limitations, small numbers of enrolled patients, and inclusion of prevalent patients (4). None of the previous studies were conducted in North America or China, where differences in cultural or ethnic make-up and practice patterns may be important.
We examined RRF decline rates in incident patients in 2 centers in Toronto and 1 in Hong Kong in a 2-year, open-label, parallel group, randomized controlled trial.
Material and Methods
Approval to conduct the study was obtained from the Ethics Review Boards at our respective hospitals. The protocol was registered at controlled-trials.com/isrctn/pf/26252543 (ISRCTN26252543).
Subjects were recruited from pre-dialysis outpatient clinics at The Scarborough Hospital in Scarborough, Ontario, the Credit Valley Hospital in Mississauga, Ontario, and the Princess Margaret Hospital in Hong Kong. Eligible patients were new to any renal replacement therapy, older than 18 years, and had urine volumes greater than 100 mL/day with GFRs of at least 1 mL/min/1.73 m2. They were excluded if it was unlikely that PD would be continued for at least 6 months.
Sample size calculations requiring at least 3 RRF measurements per patient suggested that samples of 49 patients in each arm (with allowance for a study drop-out rate of 20%) would achieve 80% power to detect a difference in slope of RRF of 0.15 mL/min/1.73 m2/month at a significance level of 0.05.
Patients were allocated to receive Gambrosol Trio (Gambro Lundia AB, Lund, Sweden) or Dianeal (Baxter Healthcare, Mississauga, Canada) by the study statistician using a computerized algorithm. The treating nephrologists (but not the patients, nurses, or outcome assessors) were blinded to treatment allocation, with decisions regarding PD modality choice and PD prescription adjustments being made by the nephrologists and according to the usual practice at each centre. No target Kt/Vs were pre-specified, and results from bioimpedendance tests were not accessible for clinical decision-making purposes. Home use of icodextrin and cyclers was not available to patients in Hong Kong.
Twenty-four-hour urine specimens to calculate urea and creatinine clearances were collected every 6 weeks for assessment of the primary outcome. To minimize the impact any inadvertent extracellular fluid volume depletion may have had on RRF and urine volume, a repeat 24-hour urine collection (after correction of any volume depletion) was obtained and recorded for study purposes 1 week later if less than 100 mL/24 hours was measured. Standardized 4-h peritoneal equilibration tests (using 2 L of 2.5% Dianeal solution), bioimpedence measurements (BC-418 Segmental Body Composition Analyzer; Tanita Corporation of America Inc., Arlington Heights, IL, USA), and subjective global assessments (SGAs) were conducted at baseline and every 6 months. Peritonitis was defined using International Society for Peritoneal Dialysis criteria and treated empirically with intra-peritoneal administration of cefazolin and tobramycin until causative organisms and sensitivities were identified. Relapsing peritonitis events were excluded from analyses determining peritonitis incidence and impact on technique survival rates. Any patients who required admission to hospital during the study period received conventional PD solutions while admitted, resuming their study dialysis solution upon discharge.
Statistical Methods
Randomization by minimization was performed to ensure that the baseline variables of diabetes mellitus, history of congestive heart failure, and center were balanced between treatment groups. Patients were censored at study completion or if they were lost to follow-up, withdrew from the study, transferred to hemodialysis, underwent renal transplantation or died. Data were stored in Microsoft Access, prepared in Microsoft Excel, and analyzed with Stata (StataCorp, College Station, TX, USA). Statistical analyses were conducted on an intention-to-treat basis for all patients with 3 or more GFR determinations. Baseline variables were described using descriptive statistics: continuous normally distributed variables with means and standard deviations, non-normally distributed variables with medians and ranges, and categorical variables with frequencies. Generalized mixed modeling analysis was employed to test the effect of PD solution on RRF. Non-independence between repeated measures was assumed by using an unstructured covariance matrix. Residual renal function was empirically determined to exhibit a linear trend. It was then assessed for each patient in a mixed model that counted time in days, center, presence or absence of diabetes, presence or absence of a history of congestive heart failure, PD solution, and interaction of PD solution with time as fixed-effects explanatory variables. Patient identification number was the only random-effect covariate included in the model, allowing for different starting GFRs for each patient.
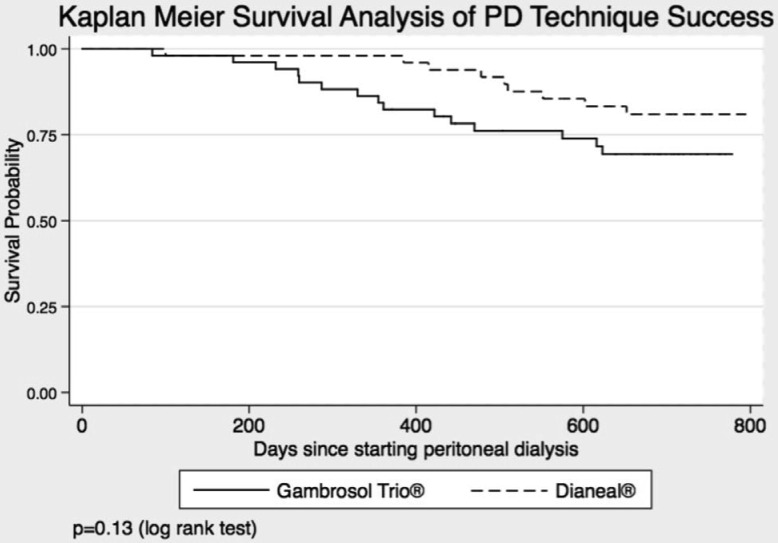
Urine volume, 4-hour peritoneal ultrafiltration volume, D/P creatinine, body mass index, serum albumin, bio-impedance scores of total body water and fat, serum phosphate, C-reactive protein, plasma phosphate and sodium were each also assessed using mixed modeling analysis, employing the same covariance structure and mix. Frequencies of group peritonitis episodes and SGA scores were compared using Fischer's or chi-square tests as appropriate. Kaplan-Meier curves with log-rank testing and Cox proportional hazards modeling were used to assess PD technique survival. Peritoneal dialysis technique survival was defined as remaining on PD at completion of the study (even if censored before completing 2 years of follow-up), at time of transfer to another institution, or at time of renal transplant. All others were considered to have failed.
Results
Patient Characteristics
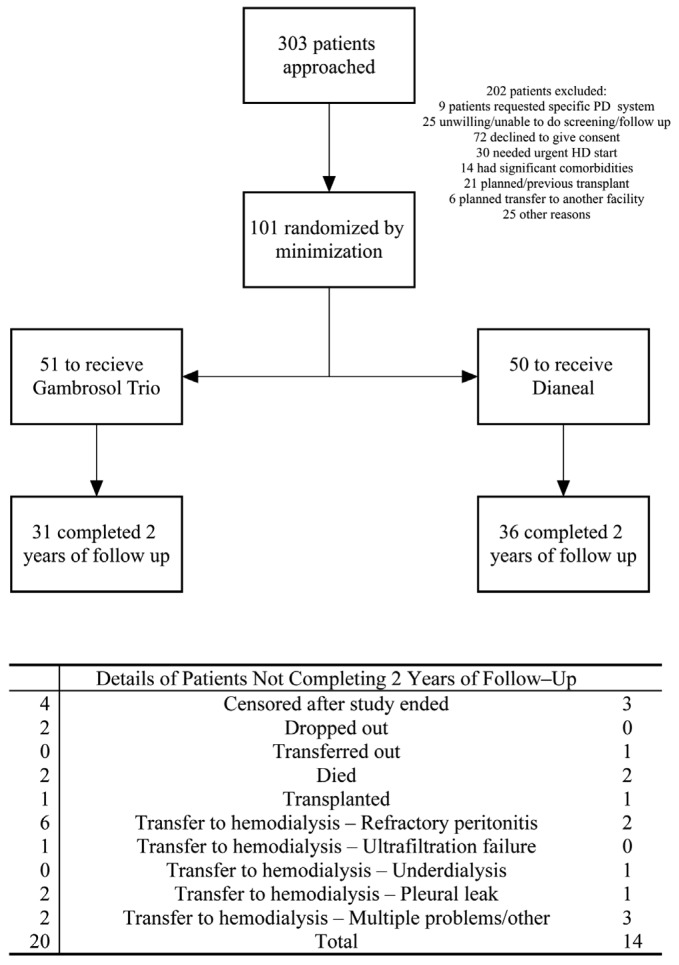
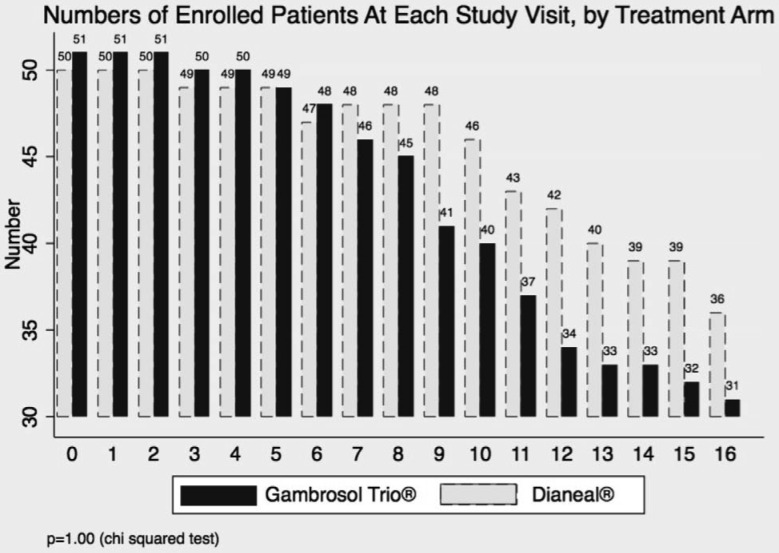
Three hundred-three patients were screened from August 2005 until July 2010 (Figure 1). One hundred-one eligible patients completed 3 or more GFR determinations for primary outcome analysis, 51 allocated to the Gambrosol Trio, and 50 to the Dianeal arm. Baseline characteristics were balanced with no significant differences between groups (Table 1). Median follow-up was 685 days per patient (inter-quartile range [IQR] 523 – 713 days). Thirty-four patients had less than 2 years of follow-up, including 7 censored when the study closed April 13, 2011 (Figure 1), and 31 patients in the Gambrosol Trio and 36 in the Dianeal group completed all 2 years of follow-up (Figure 2).
Figure 1 —
Flow of patients through the Trio Trial. PD = peritoneal dialysis; HD = hemodialysis.
TABLE 1.
Baseline Characteristics of 101 Trio Trial Participants
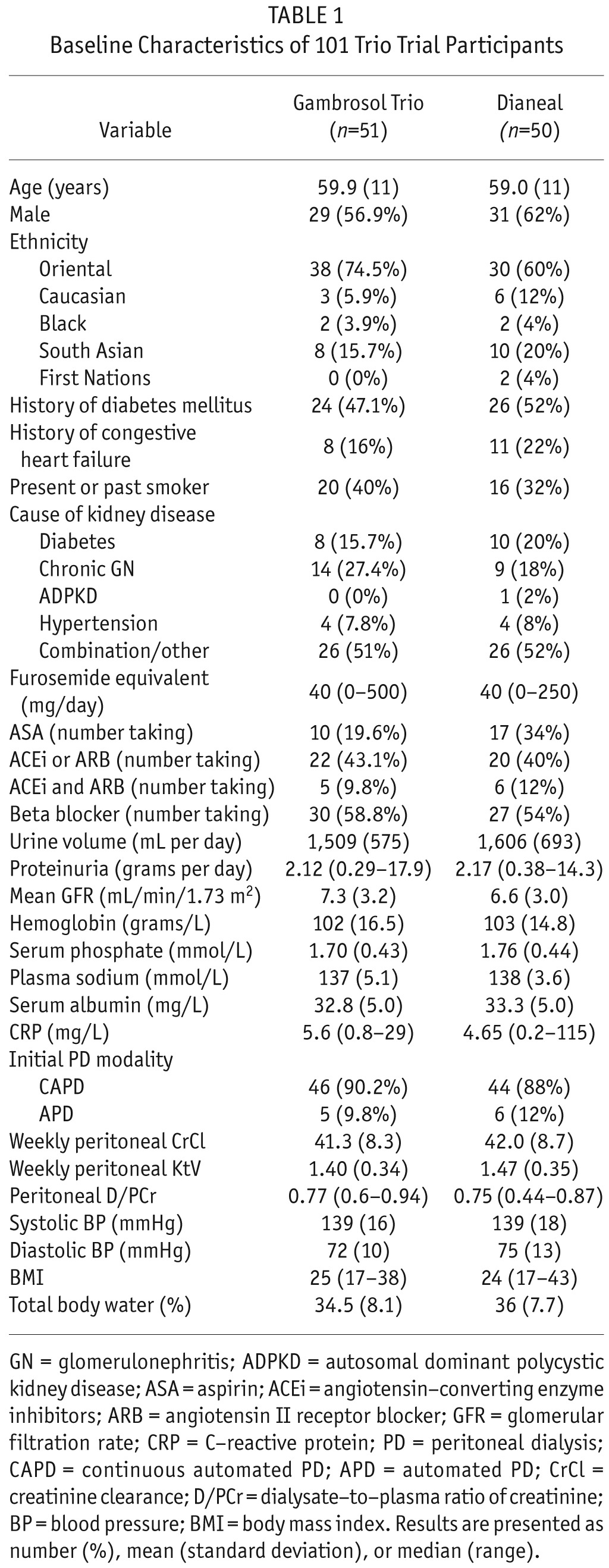
Figure 2 —
Numbers of participants in Trio Trial, by study visit and treatment.
Residual Renal Function
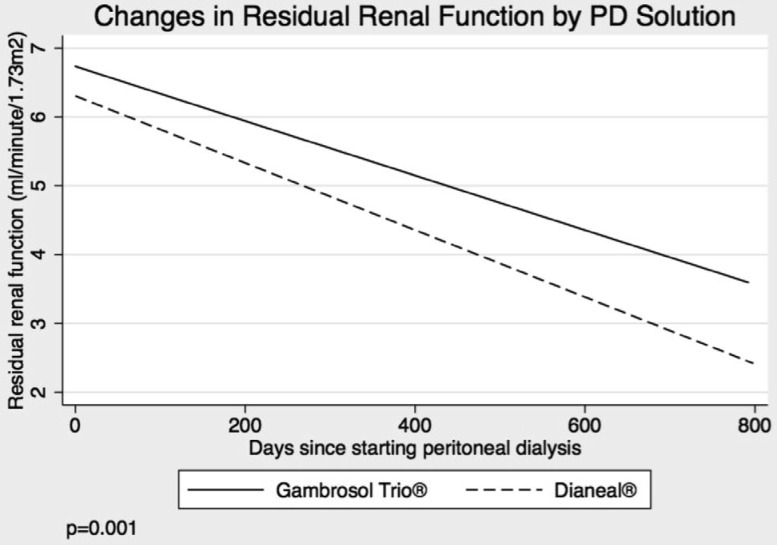
Residual renal function declined by 0.132 and 0.174 mL/min/1.73 m2/month in the Gambrosol Trio and Dianeal arms, respectively. The difference of 0.042 mL/min/1.73 m2/month was significant at p = 0.001 (Figure 3). Rates of decline were lower in the biocompatible group regardless of initial RRF or starting PD modality.
Figure 3 —
Primary outcome of the Trio Trial. PD = peritoneal dialyis.
Urine Output
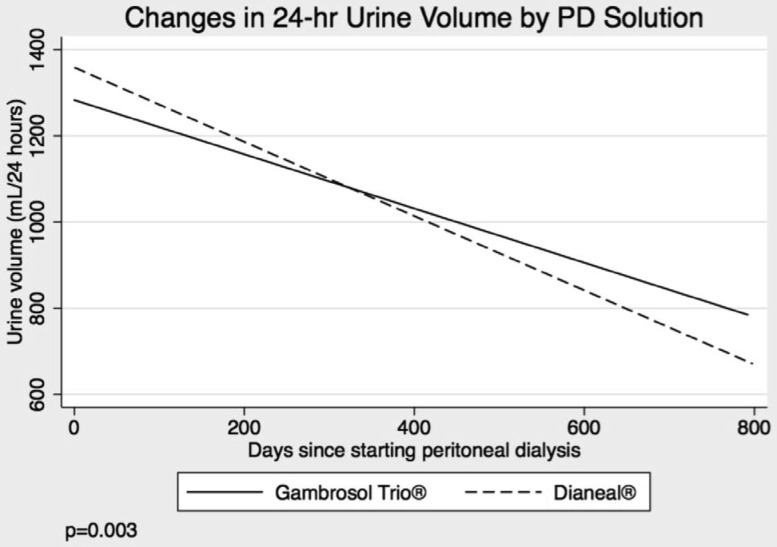
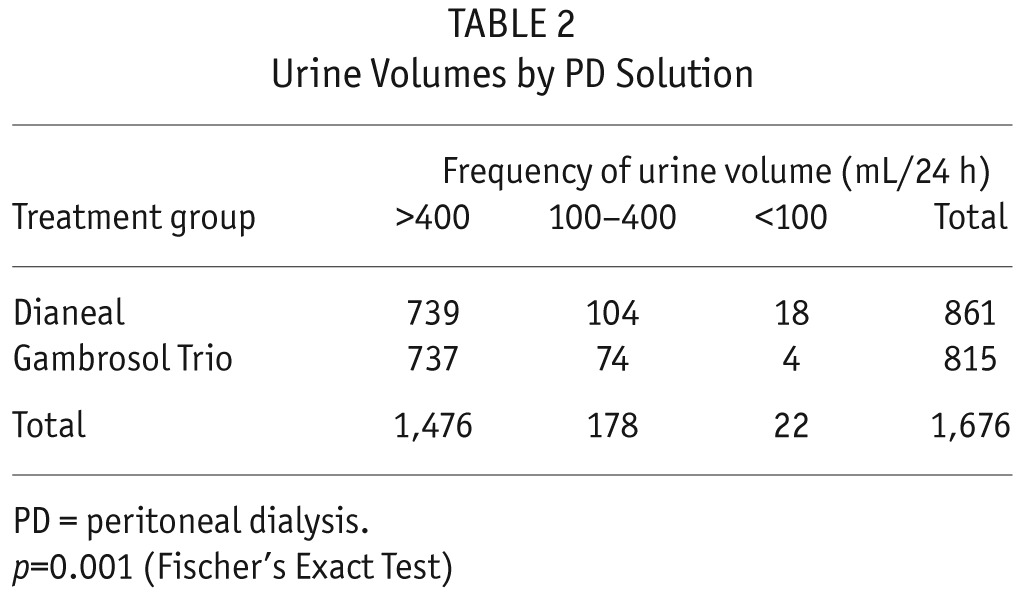
Urine volume declined by 30 and 39 mL/month in the Gambrosol Trio and Dianeal arms, respectively (p = 0.003) (Figure 4). Oligoanuria was observed less frequently in the biocompatible group (p = 0.001) (Table 2). Time to anuria was not calculated as 4 of 9 patients with daily urine volumes under 100 mL produced greater than 100 mL on subsequent collections. Mean furosemide use was similar between groups at baseline (63 [38 – 89] mg/day in Gambrosol Trio and 55 [38 – 73] mg/day in Dianeal groups, p = 0.60). Furosemide use increased during the course of the study to a mean of 87 (79 – 95) mg/day and 98 (91 – 106) mg/day in the Gambrosol Trio and Dianeal arms, respectively (p = 0.036).
Figure 4 —
Urine volumes by PD solution. PD = peritoneal dialysis.
TABLE 2.
Urine Volumes by PD Solution
Bioimpedence and Nutrition Indices
Body mass indices rose by 0.89 and 0.62 in the biocompatible and standard groups at 1 year (p = 0.003). Serum albumin and SGA scores did not differ between groups. Mean protein catabolic rates normalized for nitrogen appearance declined by 0.04 in the standard and rose by 0.02 in the biocompatible arms (p = 0.023) after 1 year (Supplemental Figure 1). Serum phosphate levels were lower (1.57 vs 1.64 mmol/L, p = 0.018) and plasma sodium levels higher (138 vs 137 mmol/L, p = 0.003) after 1 year in the Gambrosol Trio group. Bioimpedance analysis showed no difference in total body water stores between groups, while fat mass rose more in the biocompatible group (2.8% vs 1.7% per year, p = 0.046) (Supplemental Figures 2 and 3).
Peritoneal Dialysis and Membrane Characteristics
Peritoneal ultrafiltration volume increased and D/P creatinine decreased during the study, but did not differ between groups (Supplemental Figures 4 and 5). Peritoneal Kt/V declined (p = 0.042), while urine Kt/V increased (p = 0.029) in the biocompatible group over the study period. Urine weekly creatinine clearances declined at a slower rate in the biocompatible arm (p = 0.024), while peritoneal weekly creatinine clearances did not differ between groups.
Icodextrin and Automated Cycler use
Icodextrin was prescribed in 3 of 29 (10.3%) vs 13 of 33 (39%) of the Canadian patients receiving biocompatible and standard solutions, respectively (p = 0.01). Icodextrin use was associated with faster declines in RRF (0.193 vs 0.149 mL/min/1.73 m2/month [p = 0.04]) and urine volume (40 vs 24 mL/month [p = 0.001]). When icodextrin and the interaction term of icodextrin and time were added as fixed effects to the mixed model, PD solution no longer had a significant effect on RRF (p = 0.198) or urine volume (p = 0.112). However, RRF and urine volume rates still declined at slower rates in the 46 Canadian patients who did not receive icodextrin if allocated to the Gambrosol Trio arm: (0.113 vs 0.162 mL/min/1.73 m2/month [p = 0.013] and 16 vs 27 mL/month [p = 0.006]) (Supplemental Figure 6). Thirty-two (52%) of the Canadian patients were prescribed an automated cycler at some point in the study, 11 in the biocompatible and 21 in the standard arms (p = 0.043). Automated cycler use did not significantly associate with RRF.
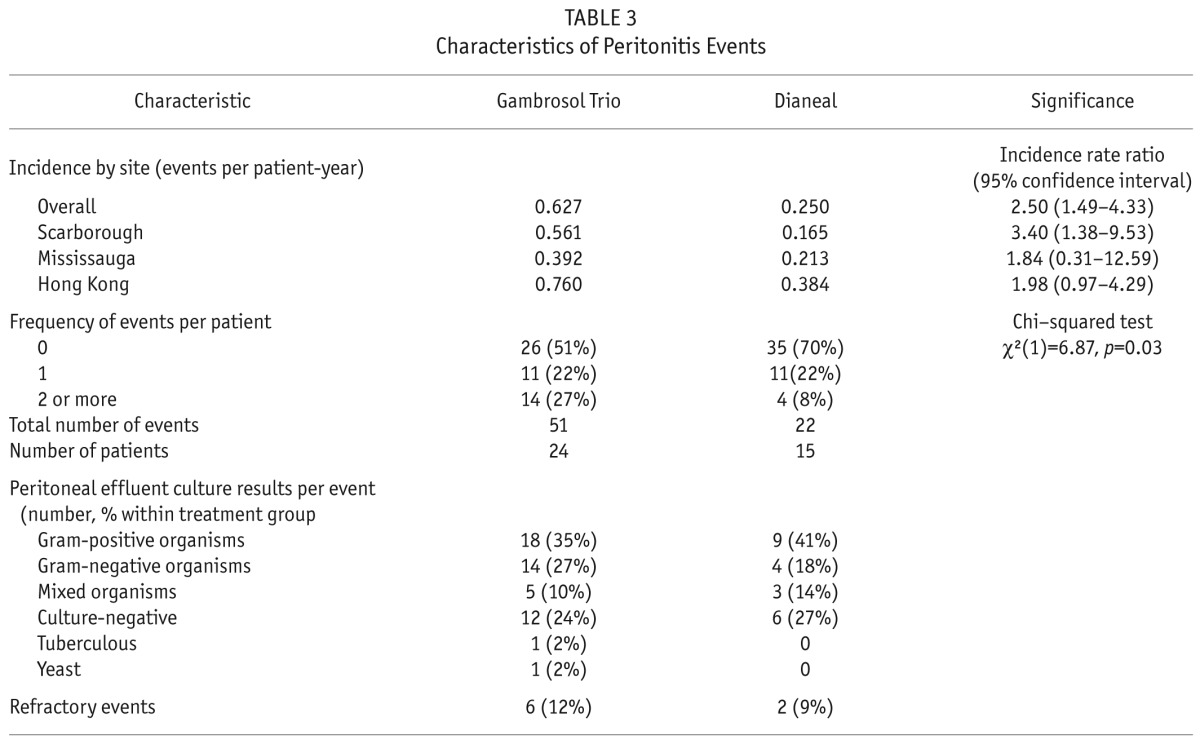
Peritonitis Rates
Peritonitis rates were significantly higher in the biocompatible group (Table 3). They were not associated with initial PD prescription or subsequent PD modality changes and did not change the finding of slower RRF decline rates in patients treated with the biocompatible solution (Supplemental Figures 7a and 7b). C-reactive protein levels did not differ between groups.
TABLE 3.
Characteristics of Peritonitis Events
Adverse Events
There were no differences between groups in the number of deaths (2 in each group) or hospitalizations (24 [54%] in Gambrosol Trio and 21 [46%] in Dianeal arms, p = 0.48).
Survival Analyses
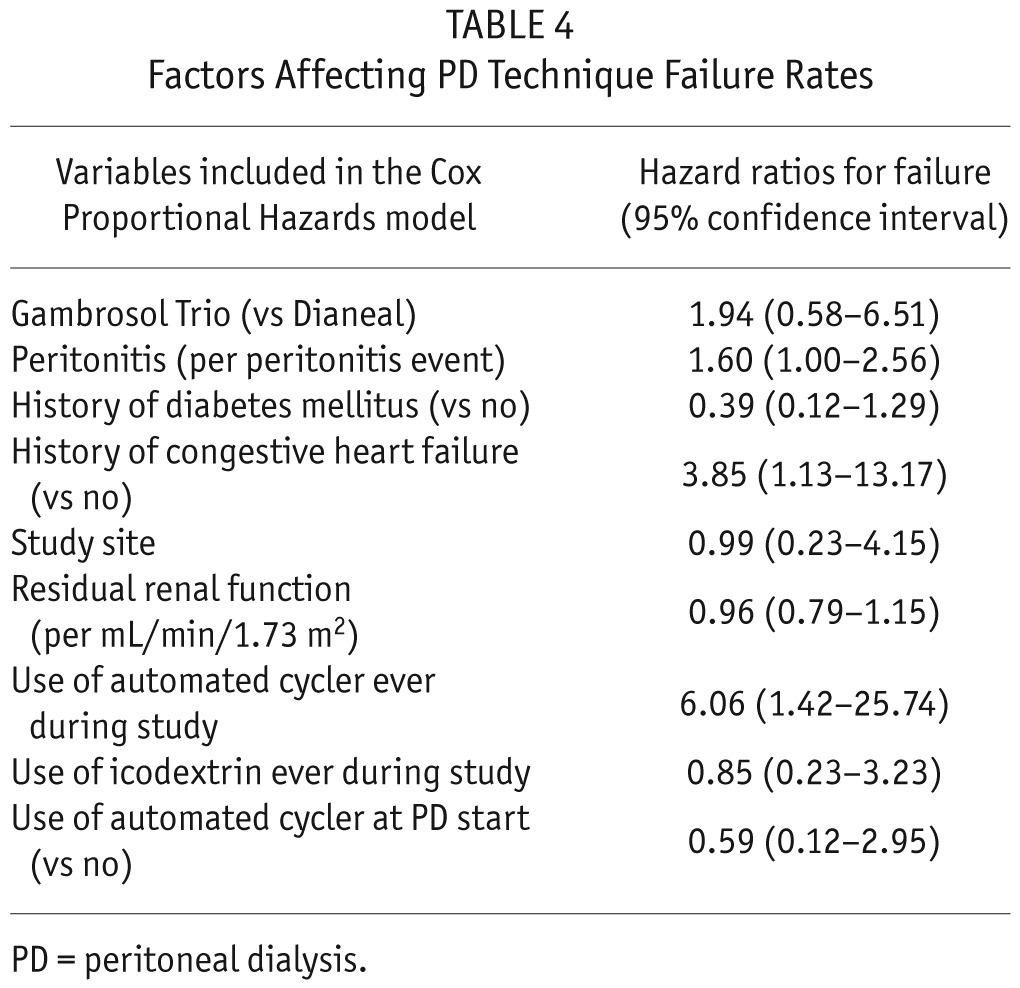
There was no difference between treatment groups in peritoneal technique survival rates (p = 0.13) (Figure 5). Cox proportional regression analysis showed survival to be negatively affected by peritonitis events (hazard ratio [HR] 1.60 (95% confidence interval [CI] 1.00 – 2.56), a history of congestive heart failure (HR 3.85 (95% CI 1.13 – 13.17), and use of an automated cycler (HR 6.06 (95% CI 1.42 – 25.74) (Table 4).
Figure 5 —
PD technique survival by PD solution. PD = peritoneal dialysis.
TABLE 4.
Factors Affecting PD Technique Failure Rates
Discussion
Residual renal function declined at slower rates in incident patients who received the biocompatible PD solution Gambrosol Trio. This finding contrasts with that of the balANZ study, and is notable given otherwise remarkable similarities between the 2 studies. For example, study design, methods, and statistical analyses were all comparable. Mean differences in 2-year overall GFR decline rates between treatment groups of 0.035 mL/min/1.73 m2/month in that study and 0.042 mL/min/1.73 m2/month in this study were in close agreement (7). And although there were more patients enrolled in the balANZ study, higher drop-out rates (54% vs 34% in this study) meant that numbers of patients completing each study were similar (43 vs 31 in the biocompatible arms of the balANZ and this study, and 48 vs 36 in the standard arms of the studies, respectively).
One potentially relevant difference between study designs was the frequency of primary outcome measurements: every 6 weeks in the current study and every 12 weeks in the balANZ study. To assess its potential impact, we replicated the balANZ study frequency by dropping alternate GFR values for each patient, and emulated the balANZ mixed effects model by adding initial PD modality to site, time, presence or absence of diabetes (instead of diabetic nephropathy), PD solution, and interaction of PD solution with time as fixed-effect covariates (7). After applying the balANZ mixed effects model to our original and modified datasets we obtained p values of 0.001 and 0.072, respectively, the latter being similar to the p value of 0.06 reported in the balANZ study. The results suggest that outcome measurement frequency was a key difference between the 2 studies.
Another notable difference was the significantly greater use of icodextrin and automated cyclers in patients allocated to receive standard solution in the current study. Changes in PD prescriptions were left to the treating teams in both studies. One of the indications for automated cycler use would have been patient preference for nocturnal dialysis. The main indication for and benefit of cycler and icodextrin use, however, is management of fluid overload (8). Given that patients in the standard group had reduced urine volumes (despite increased use of furosemide), it stands to reason that peritoneal ultrafiltration volumes would have had to be increased to treat or prevent volume overload. Our demonstration of similar total body water stores and ultrafiltration capacity suggests that PD prescription changes (such as the addition of icodextrin) may have been what effectively offset the reduced urine output in the standard solution group. The balANZ study participants who received standard solutions had shorter times to anuria and trended to reduced urine volumes, but use of icodextrin or automated cyclers did not differ between groups (7). However, bioimpedence analyses and standardized ultrafiltration tests were not performed, and it is difficult to objectively ascertain whether there were any differences between groups in total body water or ultrafiltration capacity.
We were surprised to see that icodextrin use was associated with faster declines in RRF. To our knowledge, this has not previously been reported. We believe the likeliest explanation is that icodextrin use is a marker of decline in renal function and urine output. The greater need for icodextrin therapy in the standard group would then be consistent with the reduced renal function and urine output observed in these patients. Similarly, while the absolute observed differences between groups may not have been clinically significant, the reduced fat mass, normalized protein catabolic rates, and higher serum phosphate levels in the standard group are each also consistent with reduced RRF (9,10,11). The results provide further rationale against the notion that expanded plasma volume (on the basis of reduced peritoneal ultrafiltration volumes) is the reason for reduced rates of decline in RRF in patients receiving biocompatible solutions (6,12).
Peritonitis rates were significantly higher in the Gambrosol Trio arm, driven by greater numbers of patients with 2 or more peritonitis events. While a sensitivity analysis did not reveal any effect on RRF decline, peritonitis events contributed to PD technique failure. Cho et al. also reported higher peritonitis rates in the 157 of all 2,245 incident PD patients in Australia who received biocompatible solutions of various kinds over a 4-year period (13). Other studies including balANZ, however, found lower rates of peritonitis in patients receiving biocompatible solutions (7,14). Given that the solutions were produced by different manufacturers, it is conceivable that differences in their respective connectologies may account for some of these discrepancies. However we were unable to demonstrate more infections with skin contaminants, and it is possible that other as yet unknown mechanisms contributed to the increased peritonitis rates observed in patients treated with Gambrosol Trio.
The strengths of this study include its design, size, frequency of outcome measurements, duration of follow-up, and ethnic make-up. Our inclusion of incident patients who had GFRs as low as 1 mL/min allowed us to examine patients starting PD across a broader range of GFRs than in some other studies. It is the first to provide data involving patients from North America and Hong Kong and to examine changes in body composition in relation to urine and peritoneal ultrafiltration volumes measured using serial bioimpedance analyses and standardized peritoneal equilibrium testing.
Potential weaknesses of this study include the fact that only about 1/3 of eligible patients consented to participate in the study. This has potential implications for the generalizability of our results. We also had higher drop-out rates and fewer subjects than anticipated completing 2 years of follow-up. Although not statistically significant, there was a trend to greater and earlier drop-outs in the Gambrosol group. This resulted in fewer patients being available for primary and secondary outcome measurements later in the course of the study and the attendant risk of informative censoring biased in favor of RRF preservation in the Gambrosol group. Also, while we endeavored to keep treating physicians blinded to treatment allocation, we could not guarantee that this was maintained throughout the study, and we were unable to mask trial participants and treating nurses. We are therefore unable to rule out bias in decisions regarding icodextrin and automated cycler use, potentially important given that icodextrin use appeared to be detrimental to renal function and was less commonly prescribed in the Gambrosol arm. We also did not collect information on PD glucose prescriptions. While differences in dialysate glucose concentrations would not have affected the results of standardized peritoneal equilibration testing, they could potentially have affected the results of our 24-hour urine collections via effects on peritoneal ultrafiltration volumes. This, in turn, might have impacted results of RRF and urine volume measurements. Another limitation was that our bioimpedence testing did not allow for measurements of extracellular fluid volume. This was because the bioimpedence machines available to us at the time of study initiation did not measure this variable. We thus chose to measure total body water instead, recognizing the limitations of this as a surrogate marker of extracellular fluid volume. Finally, having different manufacturers of dialy-sate solutions meant different systems and connectologies between treatment groups, a factor that may have independently influenced peritonitis rates. Although this was a major finding of our study, the increased peritonitis rates did not (in common with other published reports) impact the primary outcome of RRF (15).
Summary
Incident PD patients treated with the biocompatible PD solution Gambrosol Trio had slower rates of decline in RRF. Its use was associated with better-preserved urine volumes, less icodextrin and automated cycler use, and more favorable serum phosphate and nutrition profiles. However, use of Gambrosol Trio was also associated with higher rates of peritonitis, a finding that requires further investigation and precaution before wider-spread use of Gambrosol Trio can be recommended.
Disclosures
GW, MT, and PT have each received speakers' honoraria from, and DO was a consultant for, multiple dialysis providers including Gambro, Baxter, and Fresenius. All the other authors had no competing interests to declare.
Supplementary Material
Acknowledgments
The late Professor Dimitrios Oreopoulos played an integral role in the design and conduct of this study. He touched almost every one of our study team members as a scholar, mentor, colleague and friend, and is deeply missed.
The Trio Trial was partially funded by an unrestricted grant from Gambro Lundia AB, Lund, Sweden. TS, GW, MT, DO, and PT conceived, designed, and supervised the Trio Trial. TS, SM, AS, and MA conducted the statistical analysis. TS wrote the manuscript and, after review and approval from all coauthors, submitted it for consideration of publication.
Footnotes
Supplemental material available at www.pdiconnect.com
REFERENCES
- 1. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis 2009; 53:1068–81. [DOI] [PubMed] [Google Scholar]
- 2. Diaz-Buxo JA, White SA, Himmele R. The importance of residual renal function in peritoneal dialysis patients. Adv Perit Dial 2013; 29:19–24. [PubMed] [Google Scholar]
- 3. Jansen MA, Termorshuizen F, Korevaar JC, Dekker FW, Boeschoten E, Krediet RT, NECOSAD Study Group Predictors of survival in anuric peritoneal dialysis patients. Kidney Int 2005; 68:1199–205. [DOI] [PubMed] [Google Scholar]
- 4. Cho Y, Johnson DW, Badve SV, Craig JC, Strippoli GF, Wiggins KJ. The impact of neutral-pH peritoneal dialysates with reduced glucose degradation products on clinical outcomes in peritoneal dialysis patients. Kidney Int 2013; 84:969–79. [DOI] [PubMed] [Google Scholar]
- 5. Qayyum A, Oei EL, Paudel K, Fan SL. Increasing the use of biocompatible, glucose-free peritoneal dialysis solutions. World J Nephrol 2015; 4:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blake PG, Jain AK, Yohanna S. Biocompatible peritoneal dialysis solutions: many questions but few answers. Kidney Int 2013; 84:864–6. [DOI] [PubMed] [Google Scholar]
- 7. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, balANZ Trial Investigators et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cho Y, Johnson DW, Craig JC, Strippoli GF, Badve SV, Wiggins KJ. Biocompatible dialysis fluids for peritoneal dialysis. The Cochrane Database of Systematic Reviews 2014; 3, CD007554. [DOI] [PubMed] [Google Scholar]
- 9. Rroji M, Seferi S, Cafka M, Petrela E, Likaj E, Barbullushi M, et al. Is residual renal function and better phosphate control in peritoneal dialysis an answer for the lower prevalence of valve calcification compared to hemodialysis patients? Int Urol Neph 2014; 46:175–82. [DOI] [PubMed] [Google Scholar]
- 10. Kang SH, Cho KH, Park JW, Yoon KW, Do JY. Change in body composition in accordance with residual renal function in patients on peritoneal dialysis. J Renal Nut 2013; 23:438–44. [DOI] [PubMed] [Google Scholar]
- 11. López-Menchero R, Miguel A, García-Ramón R, Pérez-Contreras J, Girbés V. Importance of residual renal function in continuous ambulatory peritoneal dialysis: its influence on different parameters of renal replacement treatment. Nephron 1999; 83:219–25. [DOI] [PubMed] [Google Scholar]
- 12. McCafferty K, Fan S, Davenport A. Extracellular volume expansion, measured by multifrequency bioimpedance, does not help preserve residual renal function in peritoneal dialysis patients. Kidney Int 2014; 85:151–7. [DOI] [PubMed] [Google Scholar]
- 13. Cho Y, Badve SV, Hawley CM, McDonald SP, Brown FG, Boudville N, et al. Association of biocompatible peritoneal dialysis solutions with peritonitis risk, treatment, and outcomes. Clin J Am Soc Nephrol 2013; 8:1556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmad S, Sehmi JS, Ahmad-Zakhi KH, Clemenger M, Levy JB, Brown EA. Impact of new dialysis solutions on peritonitis rates. Kidney Int 2006; 103:S63–6. [DOI] [PubMed] [Google Scholar]
- 15. Rivera-Gorrin M, Teruel-Briones JL, Rodríguez-Mendiola N, Díaz-Domínguez M, Ruiz-Roso G, Quereda-Rodríguez-Navarro I. Residual renal function in patients on peritoneal dialysis: effect of peritonitis episodes. Nefrología 2014; 34:802–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.