Abstract
Interference of conventional peritoneal dialysis fluids (cPDFs) with peritoneal membrane cell functions may be attributed to the dialysis fluid's low pH, high glucose concentration, and/or the presence of glucose degradation products (GDPs), the last of which leads to higher levels of advanced glycation end-products (AGEs). It has been suggested that the peritoneal membrane might be better preserved by using biocompatible solutions, including cancer antigetn 125 (CA125). This prospective, open-label, multicentre, randomized, controlled, cross-over phase IV study compared the in vivo biocompatibility of a neutral-pH, low-GDP peritoneal dialysis (PD) solution (balance) with a cPDF in automated PD (APD) patients. Our study revealed a significantly increased appearance rate and concentration of CA125 in the peritoneal effluent of APD patients treated with the neutral-pH, low-GDP solution balance versus a conventional PD solution.
Keywords: Biocompatibility, pH neutral, cancer antigen 125, residual renal function, peritoneal dialysis fluids, ultrafiltration
Interference of conventional peritoneal dialysis fluids (cPDFs) with peritoneal membrane cell functions (1) may be attributed to the dialysis fluid's low pH, high glucose concentration, and/or the presence of glucose degradation products (GDPs) (2), the last of which leads to higher levels of advanced glycation end-products (AGEs) (3). Previous continuous ambulatory peritoneal dialysis (CAPD) studies suggest a better preservation of the peritoneal membrane with the use of biocompatible solutions, in particular those using cancer antigen 125 (CA125) as a marker of mesothelial cell mass/turnover (3–5). It is noteworthy that the negative effects of the low pH and high glucose concentrations in cPDFs are most significant immediately after inflow. Therefore, one may assume that in automated PD (APD), with its more frequent exchanges, shorter dwell times, and larger solution volumes, the use of a bioincompatible solution is particularly relevant (6).
This prospective, open-label, multicentre, randomized, controlled, cross-over phase IV study compared the in vivo biocompatibility of a neutral-pH, low-GDP PD solution (balance) with a cPDF in APD patients. As a secondary aim, fluid overload (FO) was compared. Both solutions were from Fresenius Medical Care, Germany. The study was approved by the relevant ethics committees and/or national authorities, and written informed consent was obtained from all patients.
Subjects and Methods
Patients were randomized to 1 of the 2 treatment sequences of 8 weeks each. Prior dialysis schedule prescriptions were maintained throughout the study, unless changes were medically indicated.
At baseline (Visit 1) and during the control visits at the end of each treatment phase (Visits 2 and 3), samples from the pooled 24-hour dialysate and serum were collected for analysis of biocompatibility markers (CA125, hyaluronic acid [HA], carboxymethyllysine [CML], C-reactive protein [CRP] and interleukin-6 [IL-6]). Cancer antigen125 was expressed as CA125 appearance rate (appearance rate [ar]CA125) to account for different daily treatment times:
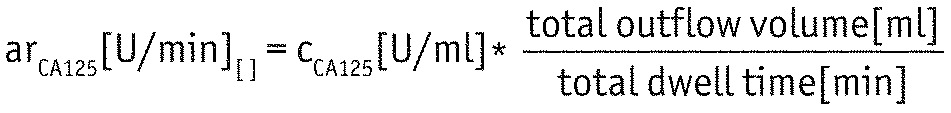 |
A parallel 24-hour urine collection was also performed. Fluid overload was assessed by bioimpedance spectroscopy using the Body Composition Monitor (BCM) (Fresenius Medical Care, Germany).
For primary analysis, an intention-to-treat analysis was applied, using baseline-adjusted mixed models with solution type (balance/cPDF), visit (Visit 2/Visit 3), randomization group and country as fixed effects, and patient as random effect. Two-sided 95% confidence intervals (CIs) were calculated from the corresponding mixed models for the least-square means in each solution type as well as for their difference. The primary parameters arCA125 and FO were tested in a hierarchical procedure: If arCA125 was significantly higher after treatment with balance compared to cPDF, the equivalence of FO in both solutions was to be tested with an equivalence limit of ±1 L.
Results
Twenty-five patients were enrolled. One patient dropped out due to a technical failure before study start. Two patients dropped out after Visit 2.
At baseline, 48% were male, mean age was 49.2 ± 13.7 years, and mean PD vintage was 17.6 ± 11.7 months. The majority of patients (54.2%) were treated with nightly intermittent PD (NIPD). The median (min, max) arCA125 was 281.81 U/min (89.39; 1,736.18) at baseline (cPDF), 252.15 U/min (92.61; 2,233.21) after treatment with cPDF, and 282.33 U/min (178.35; 2,922.52) after treatment with balance. Taking the covariates into account, arCA125 was significantly higher after treatment with balance compared to treatment with cPDF (Table 1). No significant differences were observed for HA, serum CML, IL-6, and CRP.
TABLE 1.
Estimate of Difference of Study Parameters Between Study Phases*
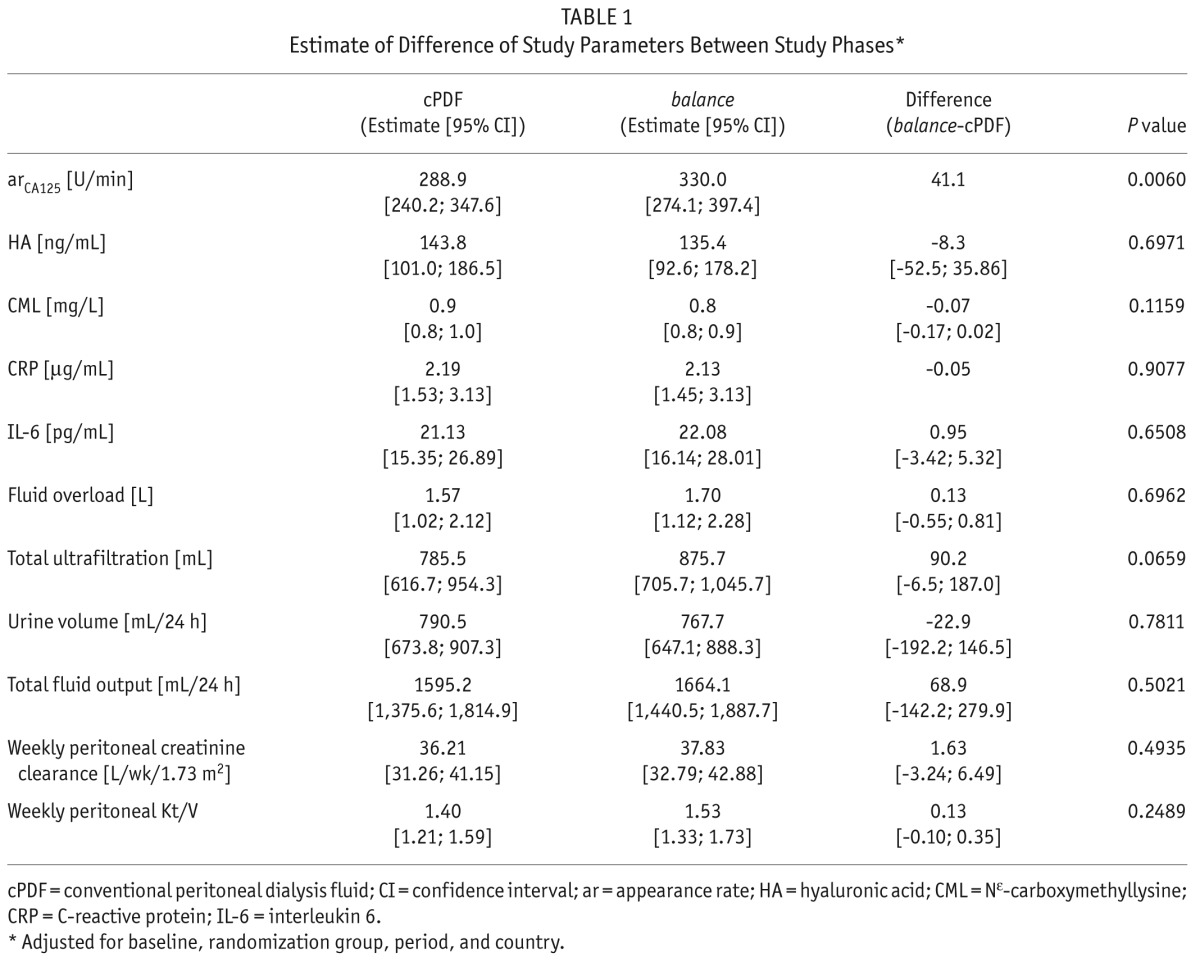
Fluid overload was 1.3 ± 1.2 L at baseline (cPDF), 1.6 ± 1.4 L after treatment with cPDF, and 1.8 ± 1.3 L after treatment with balance. The difference between the solutions remained non-significant after adjustment for the covariates (Table 1). Since arCA125 was significantly higher after treatment with balance than after treatment with cPDF, the equivalence of FO was tested in the second step of the hierarchical testing procedure. The 95% confidence interval for the FO-difference (Δ balance – cPDF estimate = 0.13 L) ranged from -0.55 L to 0.81 L, so that FO achieved with both solutions was equivalent with respect to the predefined equivalence margin of ±1 L. No significant differences in volumes of ultrafiltration (UF), urine, and total fluid output were observed after adjustment for covariates (Table 1). The achieved UF based on absorbed glucose was comparable for both solutions (cPDF: 13.7 ± 10.7 mL UF/g absorbed glucose; balance: 14.3 ± 9.8 mL UF/g absorbed glucose).
Peritoneal and total clearance of urea and creatinine did not differ significantly between the 2 solutions (Table 1). Neither did blood pressure nor hematology and clinical chemistry parameters (data not shown). No adverse drug reaction was recorded during the study. Five peritonitis episodes occurred, 3 during cPDF and 2 during balance applications.
Discussion
In the present study, arCA125 (a marker of peritoneal membrane integrity) was significantly elevated in the peritoneal effluent of APD patients applying a PD solution with neutral pH and low GDP content compared to those applying a conventional solution. Hyaluronic acid levels, an indication of inflammation, did not differ between the solution types. Superiority of biocompatible PD solutions compared with cPDF based on CA125 has been demonstrated in studies performed with CAPD patients (summarized in [7]). During APD treatment, the peritoneal membrane is usually exposed to larger volumes of solutions and more frequent exchanges (with shorter dwell times). The extent of the increase in arCA125 was lower than previously observed in CAPD patients (3,8,9). This difference could be explained by shortness of the study phases, being only 2 months for each PD solution. This period was chosen to limit time-dependent changes in patient characteristics but may result in less prominent arCA125 effects than after the longer study periods applied in other studies (3,10). Overall, the concentration of CA125 in the APD patient effluent was lower in our study than that reported elsewhere after 6 months of treatment with CAPD, suggesting that mesothelial cells are less viable in APD compared with CAPD (11). Furthermore, the empty abdomen during daily APD regimens means that the peritoneal membrane is exposed to less solution cytotoxicity, which might result in a recovery of cellular functions independent of the solution type (12, 13). In our study, more than half of the patients were treated with NIPD, i.e. a dry day. As CA125 has been shown to decrease with time on PD (14) in prevalent patients, this effect of overall time on PD might compete with a potentially beneficial effect of the PD fluid.
It is unclear whether elevated CA125 levels are caused by a higher mesothelial cell number or by an increased CA125 production per cell, maybe related to inflammatory processes (7). As we could not find differences in inflammatory markers, we cannot provide evidence for the latter hypothesis.
Previous studies reported effects of biocompatible solutions on UF (3,9,15) that may be explained by local effects on the peritoneal membrane and/or by better preservation of residual renal function (both suggested to be explained by less advanced glycation end-product [AGE] formation). There were no significant differences between the 2 solutions in our study regarding the inflammatory markers CRP and IL-6 or regarding CML as a marker of systemic glycation.
It has been questioned whether reduced UF, as observed in some studies, could result in fluid overload, which in turn might increase renal pressure and thus explain higher diuresis. However, using bioimpedance spectroscopy in our study, we could confirm that application of the 2 solutions did not result in different extents of overhydration.
Our study has some limitations. Despite proper sample size estimation, the number of patients was small and the time of exposure to each solution was short. Longer exposure times might more fully reveal differences between the PD solutions.
In conclusion, our study revealed a significantly increased appearance rate and concentration of CA125 in the peritoneal effluent of APD patients treated with the neutral-pH, low-GDP solution balance versus a conventional PD solution. As CA125 is a putative biomarker of increased mesothelial cell mass, this implies that balance solution had less impact on peritoneal membrane morphology than the conventional PD solution. No differences between the solutions were observed regarding UF and residual diuresis. With both solutions, equivalent management of hydration status could be achieved.
Disclosures
T. De los Ríos, C. Bohnhorst, and M. Lichodziejewska-Niemierko are employees of Fresenius Medical Care. The authors have no further conflicts of interest to declare.
Acknowledgments
We thank all nurses, monitors, and co-investigators who helped to collect the data and all the patients who took part in this study. The study was supported by Fresenius Medical Care.
REFERENCES
- 1. Schmitt CP, von HD, Rieger S, Arbeiter K, Bonzel KE, Fischbach M, et al. Reduced systemic advanced glycation end products in children receiving peritoneal dialysis with low glucose degradation product content. Nephrol Dial Transplant 2007; 22:2038–44. [DOI] [PubMed] [Google Scholar]
- 2. Wieslander A, Linden T, Kjellstrand P. Glucose degradation products in peritoneal dialysis fluids: how they can be avoided. Perit Dial Int 2001; 21(Suppl 3):S119–24. [PubMed] [Google Scholar]
- 3. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro-balance trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int 2004; 66:408–18. [DOI] [PubMed] [Google Scholar]
- 4. Rippe B, Simonsen O, Heimburger O, Christensson A, Haraldsson B, Stelin G, et al. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int 2001; 59:348–57. [DOI] [PubMed] [Google Scholar]
- 5. Jones S, Holmes CJ, Krediet RT, Mackenzie R, Faict D, Tranaeus A, et al. Bicarbonate/lactate-based peritoneal dialysis solution increases cancer antigen 125 and decreases hyaluronic acid levels. Kidney Int 2001; 59:1529–38. [DOI] [PubMed] [Google Scholar]
- 6. Fusshoeller A, Plail M, Grabensee B, Plum J. Biocompatibility pattern of a bicarbonate/lactate-buffered peritoneal dialysis fluid in APD: a prospective, randomized study. Nephrol Dial Transplant 2004; 19:2101–6. [DOI] [PubMed] [Google Scholar]
- 7. Perl J, Nessim SJ, Bargman JM. The biocompatibility of neutral pH, low-GDP peritoneal dialysis solutions: benefit at bench, bedside, or both? Kidney Int 2011; 79:814–24. [DOI] [PubMed] [Google Scholar]
- 8. Szeto CC, Chow KM, Lam CW, Leung CB, Kwan BC, Chung KY, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products—a 1-year randomized control trial. Nephrol Dial Transplant 2007; 22:552–9. [DOI] [PubMed] [Google Scholar]
- 9. Kim S, Oh J, Kim S, Chung W, Ahn C, Kim SG, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant 2009; 24:2899–908. [DOI] [PubMed] [Google Scholar]
- 10. Kim YL, Do J, Park SH, Cho K, Park J, Yoon K, et al. Low glucose degradation products dialysis solution modulates the levels of surrogate markers of peritoneal inflammation, integrity, and angiogenesis: preliminary report. Nephrology (Carlton) 2003; 8(Suppl):S28–32. [DOI] [PubMed] [Google Scholar]
- 11. Kanjanabuch T, Puttipittayathorn N, Leelahavanichkul A, Lieusuwan S, Katavetin P, Mahatanan N, et al. Exfoliated mesothelial cell and CA-125 in automated peritoneal dialysis (APD) and continuous ambulatory peritoneal dialysis (CAPD) patients. J Med Assoc Thai 2011; 94(Suppl 4):S119–25. [PubMed] [Google Scholar]
- 12. Wrenger E, Baumann C, Behrend M, Zamore E, Schindler R, Brunkhorst R. Peritoneal mononuclear cell differentiation and cytokine production in intermittent and continuous automated peritoneal dialysis. Am J Kidney Dis 1998; 31:234–41. [DOI] [PubMed] [Google Scholar]
- 13. Woodrow G. Nightly intermittent peritoneal dialysis prescription and power. Contrib Nephrol 1999; 129:109–14. [DOI] [PubMed] [Google Scholar]
- 14. Ho-dac-Pannekeet MM, Hiralall JK, Struijk DG, Krediet RT. Longitudinal follow-up of CA125 in peritoneal effluent. Kidney Int 1997; 51:888–93. [DOI] [PubMed] [Google Scholar]
- 15. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]


