Abstract
Most drugs of abuse lead to a general blunting of dopamine release in the chronic phase of dependence, which contributes to poor outcome. To test whether cannabis dependence is associated with a similar dopaminergic deficit, we examined striatal and extrastriatal dopamine release in severely cannabis dependent participants (CD), free of any comorbid conditions, including nicotine use. Eleven CD and twelve healthy controls (HC) completed two positron emission tomography scans with [11C]-(+)-PHNO, before and after oral administration of d-amphetamine. CD stayed inpatient for 5–7 days prior to the scans to standardize abstinence. Magnetic Resonance Imaging (MRS) measures of glutamate in the striatum and hippocampus were obtained in the same subjects. Percent change in [11C]-(+)-PHNO binding potential (ΔBPND) was compared between groups and correlations with MRS glutamate, subclinical psychopathological and neurocognitive parameters were examined. CD had significantly lower ΔBPND in the striatum (p=0.002, effect size (ES)=1.48), including the associative striatum (p=0.003, ES=1.39), sensorimotor striatum (p=0.003, ES=1.41), and the pallidus (p=0.012, ES=1.16). Lower dopamine release in the associative striatum correlated with inattention and negative symptoms in CD, and with poorer working memory and probabilistic category learning performance in both CD and HC. No relationships to MRS glutamate and amphetamine-induced subclinical positive symptoms were detected. In conclusion, this study provides evidence that severe cannabis dependence -without the confounds of any comorbidity- is associated with a deficit in striatal dopamine release. This deficit extends to other extrastriatal areas and predicts subclinical psychopathology.
Introduction
Cannabis dependence, now referred to as a cannabis use disorder1, is a relatively common disorder with an estimated prevalence as high as 8.3% in young adults in the United States 2. It is a public health concern as cannabis use has been associated with psychosis and depression3, 4. The exact effects of cannabis on the brain are not well understood, however, brain imaging studies show that it is associated with altered brain structure and function 5.
Cannabis exerts its effect on the brain primarily through the psychoactive component Δ9tetrahydrocannabinol (THC). THC is a partial agonist at the endocannabinoid receptor (CB1), which is widely expressed throughout the brain, with particularly high densities in the basal ganglia and substantia nigra pars reticulata 6. THC stimulates neuronal firing of mesolimbic dopamine neurons and elevates striatal dopamine levels in animals 7. In humans, acute THC induced striatal dopamine release in some 8–10, but not all studies 11, 12.
Dependence on dopamine-enhancing substances of abuse is associated with blunted striatal dopamine transmission 13, 14. Previously, we studied amphetamine-induced dopamine release in chronic cannabis users (CD) who were able to stay abstinent until THC was undetectable in their urine 15. Using positron emission tomography (PET) and [11C]raclopride, we found no differences in striatal amphetamine-induced dopamine release between CD and healthy controls (HC). Similarly, Mizrahi et al. found no difference between CD and HC in stress-induced dopamine release using [11C]-(+)-PHNO 16. However, Volkow et al. found deficits in methylphenidate-induced striatal dopamine release in CD with [11C]raclopride, when comparing across groups changes in distribution volumes (VT) but not in binding potential (BPND) 17. Studies in CD with comorbid psychotic symptoms also showed reduced dopamine synthesis capacity 18 and reduced stress-induced dopamine release 19. In summary, studies that included CD with psychotic symptoms suggest that chronic cannabis use is associated with reduced dopamine release; for users without psychotic symptoms, results are inconsistent. These inconsistencies could be related to recency of cannabis use, effects of concomitant use of other drugs either in the cannabis users or in the control groups, severity of cannabis use, which may vary across cohorts and/or methodological limitations of the quantification of dopamine changes with PET [11C]raclopride. The aim of our study was to measure striatal dopamine release capacity in CD who did not use other substances of abuse (including nicotine), whose abstinence period could be standardized, and who had no other major psychiatric illnesses or comorbid drug use, using a more sensitive PET radiotracer for quantification of dopamine changes. We examined amphetamine-induced dopamine release with PET and the dopamine D2/3 receptor (D2/3) agonist tracer [11C]-(+)-PHNO, which is more sensitive to the dopamine-releasing effects of amphetamine than the D2/3 antagonist tracer [11C]raclopride 20, 21 and allowed us to examine extrastriatal regions including midbrain, globus pallidus and thalamus. We hypothesized that the magnitude of striatal dopamine release would be reduced in CD compared to HC. Since deficits in neurocognitive function, including working memory, impulsivity and attention have previously been associated with cannabis use 22 and with decreased striatal dopamine function 23, 24, we also predicted that low DA release in CD would negatively correlate with neurocognitive function and positively correlate with psychosis-related symptoms.
In addition, since schizophrenia is associated with elevated glutamate levels in the hippocampus and striatum25, 26, and given that cannabis use is associated with psychosis27, we explored striatal and hippocampal glutamate levels and their relationship to striatal dopamine release in this cohort, using magnetic resonance spectroscopy (MRS). This was also motivated by reports showing that glutamate in the hippocampus modulates striatal dopamine release28 and that cannabis use may directly affect striatal glutamate 29, 30. Thus, we hypothesized that cannabis users would show altered glutamate levels in the hippocampus and striatum, as in patients with schizophrenia, and these alterations may correlate with dopamine release.
Methods
Study population
This study was approved by the Institutional Review Board of the New York State Psychiatric Institute (NYSPI) and Columbia University Medical Center (CUMC). All participants provided written informed consent. The inclusion criteria for the CD participants were: age 21–55 years; DSM-IV criteria for cannabis dependence or current marijuana use of average twice per day at least five days per week for the past 4 weeks; not currently seeking treatment; and currently using as evidenced by positive urine toxicology for cannabis at the time of recruitment and on admission to the inpatient unit. CD participants were excluded if they had any other Axis I diagnosis, including current and/or previous substance dependence or nicotine abuse. Only occasional cigarette smoking (<2 times per month) and past depressive episodes were allowed. HC participants had no current or past DSM-IV Axis I diagnosis, as determined by the DIGS 31. Participants were free of significant medical and neurological illnesses, did not use psychotropic medications or substances of abuse (confirmed with urine drug toxicology), had no clinically significant brain abnormalities on a T1-weighted MRI scan, and were not pregnant or nursing. Groups were matched for age, gender, ethnicity and parental socioeconomic status. Participants were recruited through advertisements, word of mouth, and referrals from other researchers.
Assessments
Cannabis dependence was confirmed using the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV (PRISM-IV) 32. CD participants estimated the number of joints, blunts, or bowls used each day for the prior 30 days using the Time Line Follow Back 33 and estimated the quantity of marijuana used in a joint, blunt or bowl as the equivalent amount of dried basil, which was then weighed by a member of the research team. The product of these two quantities provided an estimate of the amount of marijuana used by each CD participant each day. The day prior to the PET scan, CD participants completed the Marijuana Craving Questionnaire 34.
All participants completed additional assessments on the day prior to their PET scans, i.e. for CD after 5–7 days of abstinence from cannabis. These included the Barratt Impulsiveness Scale (BIS) 35, the Positive and Negative Syndrome Scale (PANSS) 36, the Hollingshead interview for socio-economic status (for participants and their parents) 37, and two computerized neurocognitive tests: the n-back task 38 and the weather prediction task 39. The n-back task assesses working memory. Outcome measure was the adjusted hit rate, i.e. the percent of properly identified targets corrected for false positives, in the 2-back and 3-back conditions. The weather prediction task assesses probabilistic category learning. Outcome measure was the percent optimal responses, i.e. the proportion of trials in which the high probability outcome (rain or sun) was selected.
The PANSS was repeated on the PET scan day two hours after amphetamine administration.
All clinical and neurocognitive assessments were administered by trained raters.
PET data acquisition
CD participants were admitted to the inpatient unit of NYSPI for 5–7 days directly prior to the PET scan procedures to ensure abstinence from cannabis during this period. Participants underwent two PET scans in one day with [11C]-(+)-PHNO (for detailed methods see Supplement). In short, a 120-min baseline scan was acquired, followed immediately by oral administration of amphetamine (0.5 mg/kg). A second 120-min scan was acquired 3-hours after amphetamine administration (5 hours between radiotracer injections). Data were acquired in list mode on a Biograph mCT PET-CT scanner (Siemens, Knoxville TN), binned into a sequence of frames of increasing duration and reconstructed by filtered back projection using manufacturer-provided software.
PET data analysis
PET data were motion corrected and registered to the individual’s T1-weighted MRI scan using SPM2 software. Regions of interest (ROIs) were drawn on each subject’s MRI and transferred to the coregistered PET data. Included ROIs were midbrain, thalamus, globus pallidus, associative striatum (AST), including pre-commissural dorsal caudate, post-commissural caudate and pre-commissural dorsal putamen, the limbic striatum (LST), which comprises the ventral striatum (VST), and the sensorimotor striatum (SMST), which comprises the post-commissural putamen as in 40. The cerebellum was included as a reference region.
Time activity curves were formed as the mean activity in each ROI in each frame. Data were analyzed using the simplified reference tissue model (SRTM)41 to determine the binding potential relative to the non-displaceable compartment (BPND).
The primary outcome measure was the relative reduction in BPND for [11C]-(+)-PHNO (ΔBPND), reflecting amphetamine-induced dopamine release, calculated according to:
Additionally, voxelwise analysis was performed (for details see Supplement). BPND was computed at each voxel to form BPND maps and these were nonlinearly transformed into MNI space. Statistical analysis was done by 2-group t-test for BPND and ΔBPND maps.
MRI/MRS data acquisition
MRI and MRS studies were performed using a 3.0T MRI system (Achieva, Philips, Best, The Netherlands) and a SENSE 8 channel head coil. Participants were instructed to lie still and close their eyes but stay awake. High-resolution T1-weighted images were acquired and used for PET coregistration and ROI delineation and to determine placement of MRS voxels in hippocampus and striatum (see supplemental methods for details of MRI and MRS acquisition and analysis). Outcome measures for MRS were concentration ratios of Glx (the combined concentration of glutamate and glutamine, which is more stable than glutamate alone) and Glutamate (Glu) to total Creatine (tCr, i.e. Creatine + Phospho-Creatine).
Statistical analyses
We used t-tests and Fisher’s exact tests for between-group comparisons of clinical, demographic and dopamine release measures. Voxelwise statistical analyses were performed using SPM12 software.
Relationships between dopamine release in the AST and clinical and cognitive measures were investigated on an exploratory basis in all participants by performing linear regressions with a clinical/cognitive measure as the dependent variable and 3 independent variables: group, AST ΔBPND and the interaction term. When the interaction term was not significant the model was rerun with only group and AST ΔBPND . The relationship between positive symptoms and AST ΔBPND was tested using Pearson correlation (only in CD due to lack of variability in HC).
For exploratory analyses, Pearson correlations were performed between ΔBPND in the functional striatal subregions and all cannabis-use-severity measures. For correlations between ΔBPND and age of onset and use/duration measures we used partial correlations including current age.
Results
Participants
Sixty-two cannabis-smoking participants were recruited for in-person screening. Of those, 21 were disqualified for Axis I diagnosis other than cannabis dependence, 9 for negative urine screen for cannabis, 8 for comorbid substance use, and one each for a neurological condition, a history of aggression, or risk factors for coronary artery disease. Eight CD qualified but decided not to participate. Thirteen CD and 15 HC participated in PET, however 2 participants (1 CD, 1 HC) did not complete the PET protocol due to radiochemistry failure and subsequent loss to follow-up, 2 participants’ (1 CD, 1 HC) PET data were not usable due to scan interruption for emesis, and 1 HC was excluded due to psychiatric comorbidity revealed during the study, but undetected at screening. Eleven CD and 12 HC participants completed all study procedures and were included in the analysis. Demographics and use history are displayed in Table 1. For each subject, both PET scans were acquired on the same day with 2 exceptions: two HC participants received their baseline and post-amphetamine scans on separate days due to [11C]-(+)-PHNO chemistry failure for the post-amphetamine scan on the initial day.
Table 1.
Demographics, History of Cannabis Use, Clinical and Neurocognitive Characteristics, PET Scan Parameters and Plasma Amphetamine Levels
| Healthy Controls N = 12 |
Cannabis Users N = 11 |
pa | |
|---|---|---|---|
| Demographics | |||
| Age | 28.3 ± 3.3 | 28.6 ± 5.1 | 0.843 |
| Sex (F/M) | 4/8 | 4/7 | 1.000 |
| Ethnicity (C/AA/Hisp/mixed) | 2/6/3/1 | 2/6/2/1 | 1.000b |
| Participant SES (scale range: 8–66) | 40.6 ± 13.4 | 33.8 ± 10.3 | 0.192 |
| Parental SES | 43.5 ± 10.0 | 41.9 ± 7.3 | 0.672 |
| Nicotine smoking | 0 | 0 | |
| History of Cannabis Use | |||
| Age of onset cannabis use (years) | - | 16.3 ± 3.2 | |
| Duration of use (years) | - | 11.3 ± 3.6 | |
| Age of onset dependence (years) | - | 20.8 ± 6.6 | |
| Duration of dependence (years) | - | 7.0 ± 4.0 | |
| Days used (past month) | - | 29.1 ± 3.6 | |
| Severity (estimated grams/month) | - | 79.2 ± 72.7 | |
| Marijuana Craving score (scale range: 1–7) | - | 4.4 ± 1.2c | |
| Clinical Characteristics | |||
| PANSS: | |||
| Positive symptoms at baseline | 7.2 ± 0.4 | 9.3 ± 2.3 | 0.002 |
| Negative symptoms at baseline | 8.8 ± 1.8 | 9.4 ± 2.5d | 0.503 |
| General symptoms at baseline | 17.5 ± 1.6 | 22.5 ± 5.6 | 0.009e |
| Positive symptoms, change post-amph | 0.36 ± 1.0 | 0.18 ± 2.7 | 0.841 |
| BIS: Inattention (scale range: 8–32) | 12.3 ± 3.4 | 16.2 ± 3.8d | 0.018 |
| Neurocognitive Tasks | |||
| Weather prediction (%optimal responses) | 61.6 ± 12.5c | 61.9 ± 6.8d | 0.95 |
| N-back (adjusted hit rate): | |||
| 2-back | 0.84 ± 0.19 | 0.83 ± 0.18 | 0.91 |
| 3-back | 0.54 ± 0.26b | 0.53 ± 0.40d | 0.92 |
| PET Scan Parameters and Plasma Amph | |||
| Baseline Injected Activity (MBq) | 236 ± 122 | 193 ± 80 | 0.336 |
| Post-amph Injected Activity (MBq) | 256 ± 75 | 195 ± 106 | 0.127 |
| Baseline Injected Mass (µg/kg) | 0.024 ± 0.007 | 0.024 ± 0.008 | 0.891 |
| Post-amph Injected Mass (µg/kg) | 0.028 ± 0.007 | 0.026 ± 0.007 | 0.464 |
| Plasma amph (ng/mL) | 63.2 ± 9.7 | 59.9 ± 7.8 | 0.399 |
independent samples t-tests for continuous variables, except for the comparison of PANSS positive symptoms baseline which was a Mann-Whitney test; Fisher’s exact for categorical.
dichotomized to African American vs. non-African American.
comparable to cannabis users in Heishman et al (2001)63.
significant correlations with ΔBPND in the associative striatum
log-transformed
M = male, F = female, C = Caucasian, AA = African American, Hisp = Hispanic, AST = associative striatum, LST = limbic striatum, SMST = sensorimotor striatum, PANSS = Positive And Negative Syndrome Scale, amph = amphetamine, BIS = Barratt Impulsiveness Scale.
PET Parameters, Regional Volumes and Plasma Amphetamine Levels
Neither the average injected [11C]-(+)-PHNO radioactivity, nor the injected PHNO mass per kilogram of body weight differed between groups or scans (Table 1). Regional volumes did not differ between groups (Supplemental Table S1), nor did plasma amphetamine levels (Table 1).
PET results
Baseline BPND did not differ between groups in any ROI. Amphetamine produced a robust decrease of BPND in all ROIs in all participants (Table 2 and Supplemental Figure S3). Comparison of ΔBPND between groups revealed less release in the CD group in the striatum as a whole (p=0.002, effect size (ES)=1.48) and in the AST (p=0.003, ES=1.39) and SMST (p=0.003, ES=1.41). Among extra-striatal ROIs, between-group differences reached statistical significance only in the pallidus (p=0.010, ES=1.16). Voxelwise results were similar to the ROI results, with no significant group differences in baseline BPND and large significant clusters of group differences in ΔBPND bilaterally in dorsal striatum (Figure 1 and Supplemental Figure S4).
Table 2.
BPND and Dopamine Release (ΔBPND)
| BPND Healthy Controls (n = 12) | BPND Cannabis Users (n = 11) | CD vs HC | ||||||
|---|---|---|---|---|---|---|---|---|
| ROI | Baseline | Post-amph | ΔBPND | Baseline | Post-amph | ΔBPND | p BPND |
p ΔBPND |
| Striatum | 2.46 ± 0.29 | 1.84 ± 0.22 | −24.9% ± 4.5% | 2.31 ± 0.30 | 1.89 ± 0.28 | −18.4% ± 4.3% | 0.234 | 0.002 |
| LST | 4.02 ± 0.39 | 2.73 ± 0.43 | −32.1% ± 8.4% | 4.01 ± 0.69 | 2.98 ± 0.46 | −26.2% ± 8.6% | 0.798 | 0.115 |
| AST | 2.33 ± 0.28 | 1.83 ± 0.23 | −21.1% ± 5.2% | 2.19 ± 0.31 | 1.87 ± 0.29 | −14.6% ± 4.1% | 0.280 | 0.003 |
| SMST | 2.31 ± 0.27 | 1.56 ± 0.16 | −32.3% ± 4.2% | 2.17 ± 0.27 | 1.63 ± 0.28 | −24.9% ± 6.1% | 0.223 | 0.003 |
| Pallidus | 4.43 ± 0.83 | 3.43 ± 0.76 | −22.6% ± 9.5% | 3.97 ± 0.58 | 3.45 ± 0.52 | −13.0% ± 6.8% | 0.141 | 0.012 |
| Thalamus | 0.61 ± 0.15 | 0.44 ± 0.10 | −26.5% ± 6.7% | 0.60 ± 0.16 | 0.45 ± 0.13 | −24.7% ± 16.0% | 0.917 | 0.720 |
| Midbrain | 0.76 ± 0.11 | 0.49 ± 0.07 | −33.6% ± 11.7% | 0.69 ± 0.13 | 0.51 ± 0.16 | −26.1% ± 19.6% | 0.244 | 0.277 |
AST = associative striatum, LST = limbic striatum, SMST = sensorimotor striatum, post-amph = post-amphetamine administration.
Figure 1. Voxelwise Analysis Comparing ΔBPND between Groups.
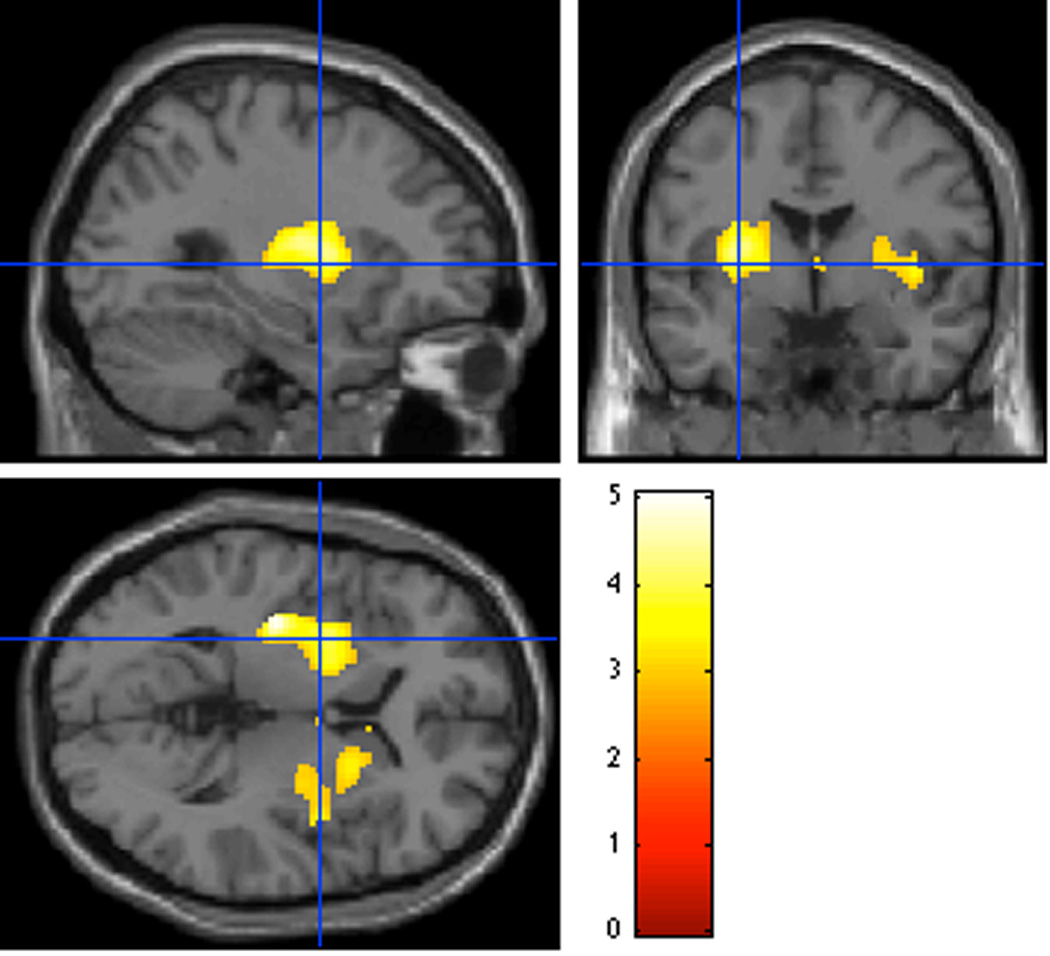
There were two clusters with significantly different ΔBPND between the CD and HC samples (i.e. larger displacement in HC than CD): one in the left putamen, FWE-corrected p=0.002, cluster size = 1403 voxels, peak voxel at MNI (−36,−18,2), and one mainly in right putamen with some overlap of right precommissural caudate, FWE-corrected p=0.02, cluster size = 711 voxels, peak voxel at MNI(40,−2,2). The peak voxel in the left cluster survived voxelwise FWE correction (p=0.022) but the right cluster had no single voxels that were significant after FWE correction. The color bar shows t21 values.
Clinical and cognitive measures and their correlation with dopamine release
Characterization of cannabis use severity in the CD group is shown in Table 1. Exploratory analyses showed no significant correlations between dopamine release and severity of cannabis use in any striatal subdivision.
CD had higher self-reported inattention symptoms on the BIS than HC (p=0.018; although this was not significant after correction for multiple comparisons), higher levels of baseline positive symptoms (p=0.002) and general symptoms (p=0.009) on the PANSS but the groups did not differ in negative symptoms or amphetamine-induced positive symptoms (Table 1). There was no significant relationship between positive symptoms and dopamine release in the AST in CD (p=0.31). Lower dopamine release in the AST was associated with greater negative symptoms in CD (standardized β=-1.09, p=0.012), but not in HC (standardized β=0.11, p=0.70, group-by-ΔBPND interaction standardized β =2.4, p=0.024); and with higher inattention scores in CD (standardized β=-0.83, p=0.029; although this was not significant after correction for multiple comparisons) but not in HC (standardized β=0.30 p=0.27); interaction standardized β=2.3, p=0.019; Figure 2).
Figure 2. Relationships between ΔBPND in the Associative Striatum and Psychopathology and Neurocognitive Parameters.
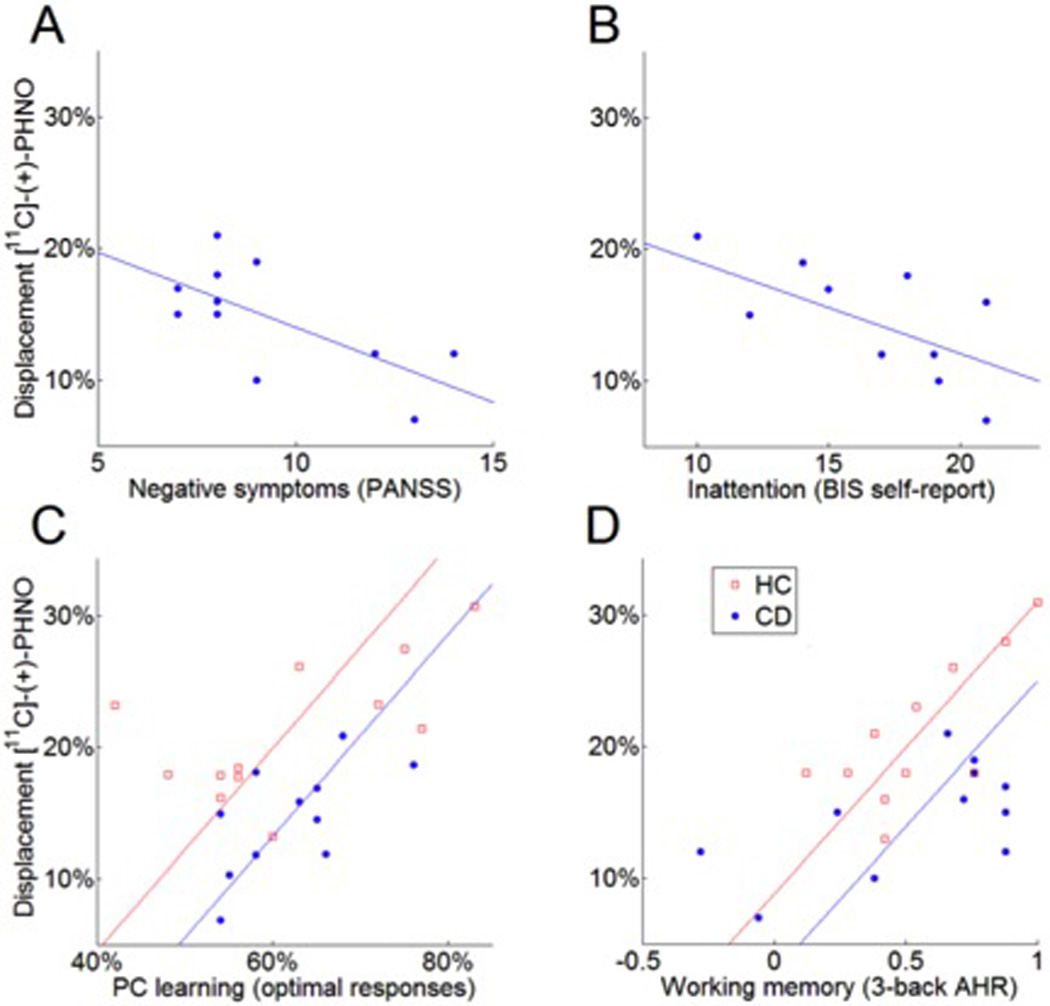
Blunted dopamine release in the associative striatum was associated with higher negative symptoms (A) and inattention symptoms (B) in CD and with poor probabilistic category learning (C) and working memory (D) performance in all participants. Clinical and cognitive measures were the dependent variable in regression analyses. β values relating dopamine release to clinical (A, B) and cognitive (C, D) measures had p<0.05 and p<0.01, respectively. AHR = adjusted hit rate, PC learning = probabilistic category learning.
The groups did not differ on working memory or probabilistic category learning performance (Table 1). Lower dopamine release in the AST was associated with lower performance on both tasks in the groups combined (working memory: standardized β=0.77 p=0.002; probabilistic category learning: standardized β=0.74 p=0.003, Figure 2). There was no significant group-by-performance interaction for either task (p-values>0.35).
MRS results
There were no significant differences between groups in Glx/tCr or Glu/tCr, nor were there significant correlations between dopamine release in any ROI with Glx/tCr or Glu/tCr in either MRS voxel. There were no group differences in tCr, grey and white matter fractions in the MRS voxels and no interaction of group by CSF portion on metabolite ratios (Supplemental Table S2).
Discussion
Our study provides evidence for a deficit in the capacity for striatal dopamine release in CD without the confounds of recent cannabis use or other comorbidities such as other substance use, tobacco smoking or psychiatric disorders. Lower striatal dopamine release also correlated with measures of psychopathology in CD. Baseline dopamine D2/3 BPND did not differ between CD and HC, in line with previous findings 15, 17, 42, 43, in contrast with other addictive substances of abuse 13, 14. MRS measures of glutamate in hippocampus and striatum did not differ between CD and HC and did not relate to dopamine release. Therefore this study does not provide evidence to support a role for glutamate in the dysregulation of dopamine in cannabis dependence.
The finding of dopamine deficit observed here contrasts with our previously published report 15, which showed no deficits in striatal dopamine release. There are several important differences between these two studies that may account for the divergent results. Our prior study required abstinence for 3 weeks on an outpatient basis, which by design excluded the more severely addicted subjects who were unable to abstain and thus dropped out of the abstinence phase, leaving us with a sample of users with less problematic use. Here by requiring a shorter inpatient abstinence period we were able to retain the more problematic severe users. To further explore this potential interpretation of the differences between the two studies, as illustrated in Figure 3, we combined data from both cohorts to show the severity of use versus dopamine release for each subject, normalized to the mean of their own healthy control comparison group. The plot suggests an inverse relationship between frequency of use and magnitude of amphetamine-induced dopamine release, supporting the interpretation that severity of dependence, indexed by frequency of use, is associated with dopamine deficits, and contributed to the different results across the two cohorts. Additionally, the longer abstinence (3 weeks vs 5 days) in our first cohort may have contributed to a normalization of any dopamine deficit that may have been present prior to or during the abstinence period. Reduced CB1 receptor availability in chronic heavy users begins to reverse after 4 weeks of abstinence 44, and cerebral blood volume partially normalizes after 4 weeks of abstinence 45. It is also possible that a state of relative withdrawal, after 5 days of abstinence, may have contributed to the reduced dopaminergic response we measured. One risk of shorter abstinence is the potential direct effect of cannabis on our outcome measure due to incomplete washout. We do not believe this is the case here because plasma levels of THC and its metabolites are very low after few days 46. Finally, other differences from our previous study 15 include the use of oral instead of intravenous amphetamine administration, although this is unlikely to have affected the outcome, and [11C]-(+)-PHNO, a radioligand that is more sensitive to the dopamine-releasing effects of amphetamine than [11C]raclopride, which may have allowed more power to detect differences among groups.
Figure 3. Cannabis Use and ΔBPND in the Associative Striatum (Normalized to HC Means).
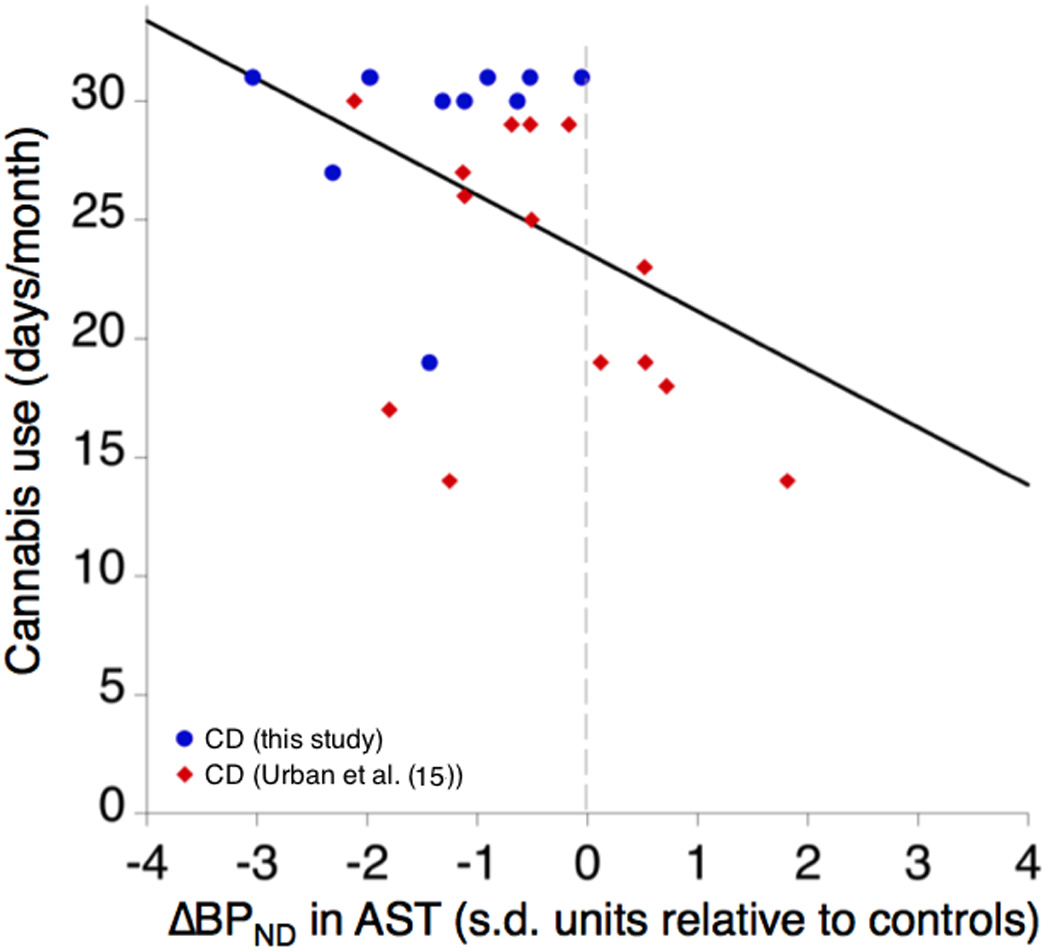
To compare between the current study and the earlier Urban et al. (2012) study 15, taking into account the globally different magnitude of ΔBPND between [11C]raclopride and [11C]-(+)-PHNO, the distance of AST ΔBPND in CD relative to the mean ΔBPND of the HC in each study is expressed in standard deviation units. Here, cannabis use frequency in days per month is plotted against this normalized ΔBPND to demonstrate the relationship between severity of use and amphetamine-induced dopamine release. ΔBPND = 0 represents the HC mean from each study.
A strength of this study, as opposed to previous reports 16–18, is the strict selection of CD with no psychiatric comorbidities to reduce confounding factors and isolate the effect of cannabis dependence. In particular, exclusion of nicotine dependence is important because tobacco smoking has substantial impact on the likelihood of cannabis relapse as measured in the human laboratory 47, and nicotine dependence is associated with decreased availability of striatal D2/3 48. These potentially confounding relationships highlight the importance of excluding chronic tobacco smokers when assessing effects of chronic cannabis use on striatal dopamine release.
Although we observed reduced dopamine release in the striatum as a whole in CD participants, the subregions of the striatum most affected were the AST and SMST, in contrast to other drug addictions, where deficits in LST dopamine release are greatest and predict craving 13, 14. A possible explanation could be the differential anatomical distribution of the CB1 receptor, the target of THC. CB1 levels are lower in the ventral striatum compared to the putamen (data in in human brain) 49 or dorsal striatum (data in rat brain) 50, 51. In addition, CB1 levels are higher in the pallidus compared to thalamus in humans 6, mirroring our findings of greater impact in pallidus compared to thalamus. This suggests a direct pharmacological effect of chronic, heavy cannabis use on dopamine release. Nevertheless, we cannot exclude the possibility that striatal dopamine release is also reduced in the LST, but difficult to detect due to greater variability in this ROI. The same reasoning could apply to the midbrain and thalamus.
Another factor to consider regarding the regional pattern of dopaminergic deficits is the differential distribution of dopamine D3 receptors (D3) across the ROIs we report on here, since [11C]-(+)-PHNO has higher affinity for D3 than D252, as does dopamine53. While this study was not designed to rule out possible differences in D3 availability between CD and HC, this appears to be a less parsimonious explanation than differences in dopamine release. We observed deficits in the SMST, which has been shown to have negligible D3, and in the AST, which has low D3, in HC 54, and therefore differences between groups in [11C]-(+)-PHNO binding are unlikely to be explained by higher D3 expression in the dorsal striatum of HC than CD. Conversely, we saw no group differences in several regions known to have high expression of D3 (LST, midbrain, thalamus) but a detectable difference in pallidus (although not significant in the voxelwise analysis), where D3 accounts for 60% or more of baseline [11C]-(+)-PHNO BPND, and where we did not detect any differences in baseline BPND. Therefore, while we cannot strictly rule out the possibility that group differences in D3 expression contributed to our results, a more plausible explanation is that the deficit is related to presynaptic dopamine storage and release capacity. The observed group differences are also unlikely to have been caused by mass carryover effects at D3 receptors where the affinity of [11C]-(+)-PHNO is high55–58, both because there is low to negligible D3 expression in the SMST and AST, where the strongest group differences were observed, and because there were five hours between the two PET injections, which has been shown to lead to negligible mass carryover in dorsal striatum59. To address the extent to which the small mass difference between groups in the post-amphetamine scans may have influenced our results, we performed simulations that show this mass difference would have negligible effect on ΔBPND (see supplement). A related phenomenon is the likelihood that tracer dose (peak radiotracer receptor occupancy ≤ 5%) is difficult to achieve with [11C]-(+)-PHNO at D3 receptors56, 59. This would have little effect on our results in SMST and AST, but may have affected ΔBPND in D3-rich regions (however, see simulation results in the supplement). This would not, however, explain the different between-group pattern observed across these regions, such as large group differences in globus pallidus and negligible differences in the thalamus.
It is notable that lower dopamine release in the AST predicted subclinical psychopathology in CD, specifically inattention and negative symptoms. CD also scored higher on these measures than HC. Although we cannot show a causal relation, these results indicate that lower dopamine release may contribute to the negative functional impact of chronic cannabis dependence, consistent with a previous finding linking deficits in dopamine release with apathy in chronic cannabis users 60. Similarly our findings of an association between inattention and lower dopamine release are consistent with previous findings in participant with attention deficit disorder in whom inattention was associated with blunted dopamine release in striatum61.
We also found that lower dopamine release in the AST correlated with poorer working memory performance and probabilistic category learning for both CD and HC. The groups did not differ in performance, although poorer working memory performance has been described in cannabis abuse 22. Similarly, previous studies found that higher striatal dopamine release predicts better working memory performance 23 and probabilistic category learning 24. Therefore, our results are consistent with these reports, in suggesting a positive relationship between striatal dopamine function and working memory and probabilistic category learning, shared across diagnoses, including cannabis use disorder.
Although there is an association between cannabis use and psychosis, we did not observe a significant correlation between dopamine release and amphetamine-induced positive symptoms. This may be related to the a priori exclusion of subjects with psychiatric comorbidities, thereby excluding subjects at high-risk for psychosis.
A limitation of our study is the relatively small sample size, which may have limited our ability to report conclusive evidence for smaller and noisier ROIs such as LST or midbrain, and may have limited our power for the correlational analyses and to detect alterations in MRS measures of glutamate. Unlike the ROI analysis, the voxelwise analysis did not detect a significant group difference in pallidus. Small sample size may have contributed to this result as ΔBPND values from individual voxels are considerably more variable than averages over many voxels in ROIs. Furthermore, it is possible that we did not find associations between cannabis use parameters and dopamine release in our sample due to homogeneity of use severity; pooling our CD samples provides a larger range of frequency of use and suggests that such a relationship is likely.
In conclusion, this study demonstrates that severe cannabis dependence, with no other psychiatric or drug comorbidities, is associated with deficits in amphetamine-induced dopamine release in the AST, SMST and globus pallidus. The lower dopamine release in the AST might contribute to the association between heavy cannabis use and psychopathology. These results are important in light of the steady increase in daily cannabis use in the U.S.62, along with continually increasing THC potency and the movement to legalize its use, which would expose a wider proportion of the population to the negative impact of cannabis use disorder. In particular, as most of our subjects here initiated cannabis use during their adolescent years, our study suggests that adolescent use of cannabis leading to dependence is associated with a compromised dopaminergic system that may have a negative impact on brain function.
Supplementary Material
Acknowledgments
Funding for this study was provided by grant R01 DA022455-01A1 from the National Institute on Drug Abuse. Dr. van de Giessen was supported by a Rubicon grant from the Netherlands Organisation for Scientific Research (825.12.009).
Footnotes
Conflicts of Interest
Dr. Haney has received partial salary support for investigator-initiated studies from Insys Therapeutics Inc, and Lifeloc Technologies and has served as a consultant to Aelis Farma and Health Advances LLC.
Dr. Kegeles has received research support from Amgen.
Dr. Slifstein has received research support from Forest Laboratories, Pierre-Fabre, CHDI, and Otsuka and has provided consultation for Amgen.
Dr. Abi-Dargham has received research support from Takeda and Forest Pharmaceuticals and has served on advisory boards for Roche, Forum, and Otsuka.
Drs. Van de Giessen, Weinstein, Cassidy, Dong, Ghazzaoui, Ojeil, Xu, Vadhan and Volkow report no competing interests.
References
- 1.Association AP. Diagnostic and statistical manual of mental disorders: DSM-5. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Haberstick BC, Young SE, Zeiger JS, Lessem JM, Hewitt JK, Hopfer CJ. Prevalence and correlates of alcohol and cannabis use disorders in the United States: results from the national longitudinal study of adolescent health. Drug and alcohol dependence. 2014;136:158–161. doi: 10.1016/j.drugalcdep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. The New England journal of medicine. 2014;370(23):2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James A, James C, Thwaites T. The brain effects of cannabis in healthy adolescents and in adolescents with schizophrenia: a systematic review. Psychiatry research. 2013;214(3):181–189. doi: 10.1016/j.pscychresns.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. British journal of pharmacology. 2004;143(2):227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34(3):759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- 9.Voruganti LN, Slomka P, Zabel P, Mattar A, Awad AG. Cannabis induced dopamine release: an in-vivo SPECT study. Psychiatry research. 2001;107(3):173–177. doi: 10.1016/s0925-4927(01)00104-4. [DOI] [PubMed] [Google Scholar]
- 10.Bossong MG, Mehta MA, van Berckel BN, Howes OD, Kahn RS, Stokes PR. Further human evidence for striatal dopamine release induced by administration of 9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology. 2015;232(15):2723–2729. doi: 10.1007/s00213-015-3915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stokes PR, Mehta MA, Curran HV, Breen G, Grasby PM. Can recreational doses of THC produce significant dopamine release in the human striatum? NeuroImage. 2009;48(1):186–190. doi: 10.1016/j.neuroimage.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Barkus E, Morrison PD, Vuletic D, Dickson JC, Ell PJ, Pilowsky LS, et al. Does intravenous Delta9-tetrahydrocannabinol increase dopamine release? A SPET study. Journal of psychopharmacology. 2011;25(11):1462–1468. doi: 10.1177/0269881110382465. [DOI] [PubMed] [Google Scholar]
- 13.Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2014;76 Pt B:498–509. doi: 10.1016/j.neuropharm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;(56 Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, et al. Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biological psychiatry. 2012;71(8):677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizrahi R, Suridjan I, Kenk M, George TP, Wilson A, Houle S, et al. Dopamine response to psychosocial stress in chronic cannabis users: a PET study with [11C]-+-PHNO. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(4):673–682. doi: 10.1038/npp.2012.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkow ND, Wang GJ, Telang F, Fowler JS, Alexoff D, Logan J, et al. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(30):E3149–E3156. doi: 10.1073/pnas.1411228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biological psychiatry. 2014;75(6):470–478. doi: 10.1016/j.biopsych.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Mizrahi R, Kenk M, Suridjan I, Boileau I, George TP, McKenzie K, et al. Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(6):1479–1489. doi: 10.1038/npp.2013.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tziortzi AC, Haber SN, Searle GE, Tsoumpas C, Long CJ, Shotbolt P, et al. Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb Cortex. 2014;24(5):1165–1177. doi: 10.1093/cercor/bhs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, et al. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. Journal of neurochemistry. 2006;97(4):1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- 22.Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, et al. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology. 2013;226(2):307–319. doi: 10.1007/s00213-012-2908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landau SM, Lal R, O’Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cerebral cortex. 2009;19(2):445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson L, Tai YF, Lin CS, Lagnado DA, Brooks DJ, Piccini P, et al. Probabilistic classification learning with corrective feedback is associated with in vivo striatal dopamine release in the ventral striatum, while learning without feedback is not. Human brain mapping. 2014;35(10):5106–5115. doi: 10.1002/hbm.22536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(9):1781–1791. doi: 10.1038/npp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA psychiatry. 2013;70(12):1294–1302. doi: 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. Journal of psychopharmacology. 2005;19(2):187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- 28.Cachope R, Cheer JF. Local control of striatal dopamine release. Frontiers in behavioral neuroscience. 2014;8:188. doi: 10.3389/fnbeh.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. Journal of neurophysiology. 2001;85(1):468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- 30.Sneider JT, Mashhoon Y, Silveri MM. A Review of Magnetic Resonance Spectroscopy Studies in Marijuana using Adolescents and Adults. Journal of addiction research & therapy. 2013;(Suppl 4) doi: 10.4172/2155-6105.S4-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- 32.Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. The American journal of psychiatry. 1996;153(9):1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 33.Sobell LC, Sobell MB. Timeline Followback. A calendar method for assessing alcohol and drug use. Toronto, Ontario: Addiction Research Foundation; 1996. [Google Scholar]
- 34.Heishman SJ, Singleton EG. Assessment of cannabis craving using the Marijuana Craving Questionnaire. Methods in molecular medicine. 2006;123:209–216. doi: 10.1385/1-59259-999-0:209. [DOI] [PubMed] [Google Scholar]
- 35.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. JClinPsychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 37.Hollingshead AB. Four factor index of social status. Working paper published by the author; New Haven, Connecticut: 1975. [Google Scholar]
- 38.Cohen JD, Forman SD, Braver TS, Casey BJ, Servan-Schreiber D, Noll DC. Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Human brain mapping. 1994;1(4):293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 39.Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning & memory. 1994;1(2):106–120. [PubMed] [Google Scholar]
- 40.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. JCerebBlood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4(3 Pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 42.Albrecht DS, Skosnik PD, Vollmer JM, Brumbaugh MS, Perry KM, Mock BH, et al. Striatal D(2)/D(3) receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug and alcohol dependence. 2013;128(1–2):52–57. doi: 10.1016/j.drugalcdep.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology. 2008;197(4):549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Molecular psychiatry. 2012;17(6):642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sneider JT, Pope HG, Jr, Silveri MM, Simpson NS, Gruber SA, Yurgelun-Todd DA. Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2008;18(8):612–619. doi: 10.1016/j.euroneuro.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huestis MA. Human cannabinoid pharmacokinetics. Chemistry & biodiversity. 2007;4(8):1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, et al. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biological psychiatry. 2013;73(3):242–248. doi: 10.1016/j.biopsych.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, et al. Smoking-induced ventral striatum dopamine release. The American journal of psychiatry. 2004;161(7):1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- 49.Ceccarini J, De Hert M, Van Winkel R, Peuskens J, Bormans G, Kranaster L, et al. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. NeuroImage. 2013;79:304–312. doi: 10.1016/j.neuroimage.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 50.Martin AB, Fernandez-Espejo E, Ferrer B, Gorriti MA, Bilbao A, Navarro M, et al. Expression and function of CB1 receptor in the rat striatum: localization and effects on D1 and D2 dopamine receptor-mediated motor behaviors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(7):1667–1679. doi: 10.1038/sj.npp.1301558. [DOI] [PubMed] [Google Scholar]
- 51.Van Waes V, Beverley JA, Siman H, Tseng KY, Steiner H. CB1 Cannabinoid Receptor Expression in the Striatum: Association with Corticostriatal Circuits and Developmental Regulation. Frontiers in pharmacology. 2012;3:21. doi: 10.3389/fphar.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, et al. Affinity and selectivity of [(1)(1)C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012;66(6):489–500. doi: 10.1002/syn.21535. [DOI] [PubMed] [Google Scholar]
- 53.Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS & neurological disorders drug targets. 2006;5(1):25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- 54.Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, et al. Imaging Dopamine D-3 Receptors in the Human Brain with Positron Emission Tomography, [C-11]PHNO, and a Selective D-3 Receptor Antagonist. Biological psychiatry. 2010;68(4):392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 55.Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, et al. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32(1):127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabiner EA, Laruelle M. Imaging the D3 receptor in humans in vivo using [11C](+)-PHNO positron emission tomography (PET) The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2010;13(3):289–290. doi: 10.1017/S1461145710000088. [DOI] [PubMed] [Google Scholar]
- 57.Searle GE, Beaver JD, Tziortzi A, Comley RA, Bani M, Ghibellini G, et al. Mathematical modelling of [(1)(1)C]-(+)-PHNO human competition studies. NeuroImage. 2013;68:119–132. doi: 10.1016/j.neuroimage.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 58.Rabiner E, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, et al. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)PHNO: studies in non-hman primates and transgenic mice. Synapse. 2009;63:782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- 59.Gallezot JD, Zheng MQ, Lim K, Lin SF, Labaree D, Matuskey D, et al. Parametric Imaging and Test-Retest Variability of (1)(1)C-(+)-PHNO Binding to D(2)/D(3) Dopamine Receptors in Humans on the High-Resolution Research Tomograph PET Scanner. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55(6):960–966. doi: 10.2967/jnumed.113.132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bloomfield MA, Morgan CJ, Kapur S, Curran HV, Howes OD. The link between dopamine function and apathy in cannabis users: an [18F]-DOPA PET imaging study. Psychopharmacology. 2014;231(11):2251–2259. doi: 10.1007/s00213-014-3523-4. [DOI] [PubMed] [Google Scholar]
- 61.Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Archives of general psychiatry. 2007;64(8):932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 62.SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Center for Behavioral Health Statistics and Quality: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- 63.Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96(7):1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


