Abstract
During exercise, there is a progressive reduction in the ability to produce muscle forces. Processes within the nervous system, as well as within the muscles contribute to this fatigue. In addition to impaired function of the motor system, sensations associated with fatigue, and impairment of homeostasis can contribute to impairment of performance during exercise. This review discusses some of the neural changes that accompany exercise and the development of fatigue. The role of brain monoaminergic neurotransmitter systems in whole-body endurance performance is discussed, particularly with regard to exercise in hot environments. Next, fatigue-related alterations in the neuromuscular pathway are discussed in terms of changes in motor unit firing, motoneuron excitability and motor cortical excitability. These changes have mostly been investigated during single-limb isometric contractions. Finally, the small-diameter muscle afferents that increase firing with exercise and fatigue are discussed. These afferents have roles in cardiovascular and respiratory responses to exercise, and in impairment of exercise performance through interaction with the motor pathway, as well as providing sensations of muscle discomfort. Thus, changes at all levels of the nervous system including the brain, spinal cord, motor output, sensory input and autonomic function occur during exercise and fatigue. The mix of influences and the importance of their contribution varies with the type of exercise being performed.
Keywords: central fatigue, monoaminergic, Motor unit, motoneuron, motor cortex, muscle afferent
Introduction
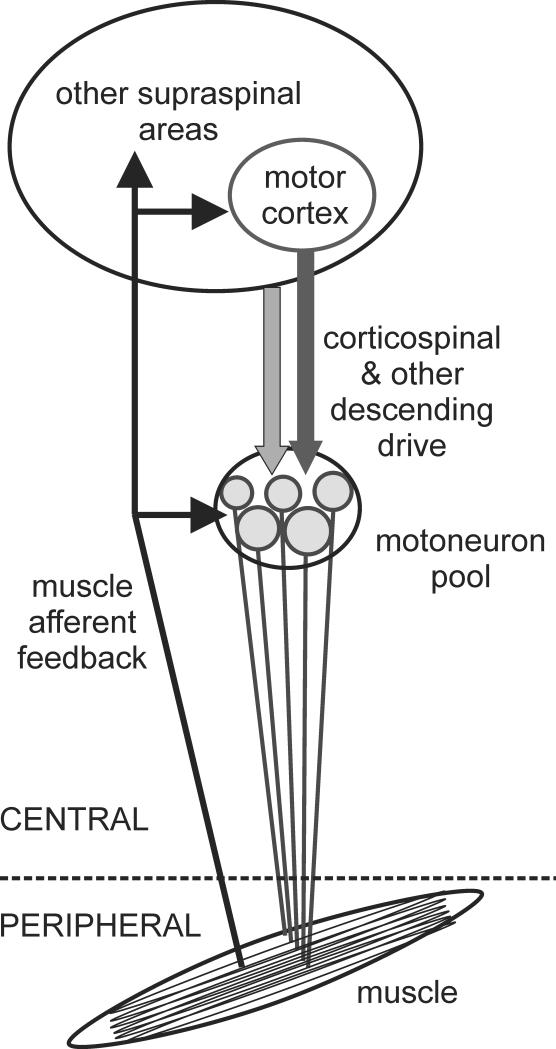
In humans, muscle fatigue can be defined as any exercise-induced reduction in the ability to produce force or power with a muscle or muscle group. Ultimately the production of force or power depends on contractile mechanisms within skeletal muscle fibres. However, a chain of processes in the nervous system and the muscle precede voluntary muscle contraction and changes at any level of this pathway could also impair force or power generation. It is well recognised that fatigue of the muscle itself occurs through multiple mechanisms related to both the contractile apparatus and how it is engaged through depolarisation of the muscle fiber membrane. Commonly, fatigue through processes at or distal to the neuromuscular junction is known as peripheral fatigue (e.g.14, 31). However, neural drive to the muscle determines if, when and to what degree muscle fibers are activated. Hence, processes within the central nervous system (CNS) that reduce neural drive to the muscle can also contribute to the decline in force or power and compromise performance. This phenomenon is known as central fatigue (Figure 1) and applies to single-joint exercise (e.g. elbow flexion) involving a relatively small muscle mass, but also to multiple-joint and whole body exercise (e.g. cycling) involving a large muscle mass
Figure 1. Schematic of neural contributions to muscle fatigue in single joint exercise.
Peripheral fatigue is attributed to processes at or distal to the neuromuscular junction whereas central fatigue is attributed to processes within the nervous system. In the neuromuscular pathway, force is generated by contraction of the muscle fibers. Strength and timing of contraction are controlled by the firing of the motoneurons. Motoneuron firing is influenced by the properties of the motoneurons, feedback from sensory input and by descending drive. There are fatigue-related changes at all of these levels (Changes in the neuromuscular pathway with fatiguing exercise). As well as influencing the neuromuscular pathway, group III/IV muscle afferent feedback interacts with cardiovascular and respiratory processes via the autonomic nervous system (Feedback from fatigue-sensitive muscle afferents). In addition, departures from homeostasis during whole body exercise influence performance through supraspinal mechanisms (Brain changes associated with performance).
In health, muscle fatigue limits athletic performance and other strenuous or prolonged activity. When it impacts on everyday tasks, such as carrying a heavy shopping bag or a child, or climbing flights of stairs, it is managed by swapping from one set of muscles to another or by periods of rest or reduced activity. However, in many disorders, muscle fatigue is increased and restricts daily life. Such disorders include neurological, muscular, cardiovascular and respiratory diseases but also with aging and frailty, and any disorder that enforces inactivity and leads to deconditioning.
Fatigue can alter overt performance, such that the task is performed more slowly or clumsily or even cannot be performed successfully, or it can alter the neuromuscular activity required to perform the task and this may be evident as increased electrical activity of the muscle (electromyogram, EMG). Additionally, there are sensations that accompany muscle fatigue, such as muscle pain or discomfort and perception of increased effort. In whole body exercise, disturbances to homeostasis of multiple systems provide signals that impact directly or indirectly on the motor system. Thus, there are multiple neural alterations that are associated with exercise-induced fatigue. Some of these alterations represent processes that reduce voluntary muscle force and so contribute to fatigue. In contrast, others reflect compensation to allow successful task performance despite impairment elsewhere in the neuromuscular system.
This review will discuss some of the neural changes that occur with fatiguing exercise. It will start with the influence of different neurotransmitter systems on whole-body exercise performance and the development of fatigue. Next, it will focus on changes within the direct neuromuscular pathway by describing the behaviour of motor units, motoneurons and the motor cortex during fatiguing contractions. Finally, it will discuss the contribution of small-diameter muscle afferents which fire with fatigue to impairment of exercise performance.
Brain changes associated with performance
Neurotransmitters dictate and create the communication between neurons in different brain regions and neuronal pathways. Generally speaking, nerve cells in the brain have a tendency to fire all the time. There is an incessant, irregular discharge from billions of nerve cells, giving a massive ‘background noise’. Probably none of the neurons in the brain are exposed only to excitation, and certainly no nerve cells are affected solely by inhibitory signals. Therefore, understanding the function of various neurotransmitters is important in understanding their role during whole body exercise and fatigue. The monoamines serotonin (5-Hydroxitryptamine; 5-HT), dopamine (DA) and noradrenaline (NA) play a key role in signal transduction between neurons, and exercise-induced changes in the concentrations of these neurotransmitters (especially 5-HT and DA) have been linked to central fatigue That is, fatigue that arises from changes within the central nervous system (or proximal to the neuromuscular junction; 14) In 1987, Newsholme and his co-workers proposed the so-called “Central Fatigue Hypothesis”, which stated that fatigue was caused by an increase in brain 5-HT concentration, which induced negative effects on arousal, lethargy, sleepiness and mood (65). It was proposed that this mechanism could influence the perception of effort and therefore, fatigue (65). Many studies have challenged the ‘central fatigue’ hypothesis. Some studies observed, in accordance with the hypothesis, a negative effect of an increase in 5-HT concentration on performance, whereas others could not confirm that finding (for review see 81). The contrasting findings in literature probably result from the complexity of the 5-HT neurotransmitter system, as many different receptors and receptor subtypes have been identified, each with different functions and interactions. The lack of consensus amongst studies that have tried to manipulate the neurotransmitter system suggest that 5-HT is not the key-factor in the development of central fatigue; it also indicates that the results could also be ‘drug-specific’.
Mixed effects on performance are also observed for drugs that influence the catecholamines NA and DA, with different results from drugs that manipulate NA or DA or both neurotransmitters at the same time. The NA reuptake inhibitor reboxetine has been shown to exert no effect or negative effects on endurance exercise performance in normal ambient temperature (52, 72, 79). However, it is not certain that this is solely through central mechanisms, as reboxetine can also have peripheral effects through the sympathetic system, such as vasodilatation and increased heart rate, and an influence of these on peformance cannot be ruled out. DA can be manipulated by methylphenidate, which both increases release and inhibits reuptake of DA, and thus, creates a large increase in the extracellular concentration of DA in the synapse. Methylphenidate improved exercise performance, with a longer time to task failure and a higher power output during cycling at a fixed rating of perceived exertion (90). On the other hand, it did not improve self-paced time trial performance in 18°C (80). This contrast suggests that the exercise protocol used to induce fatigue may also influence the outcome. Finally, after administration of a dual DA/NA reuptake inhibitor (bupropion), subjects finished a predetermined amount of work in the same time (89 min) as after administration of a placebo (71), illustrating that even when a combined reuptake inhibitor is used, it can be difficult to alter exercise performance through neurotransmitter manipulation. In a different protocol (97) using the same drug, bupropion, subjects first cycled for one hour at 55% of maximal workload, immediately followed by a time trial to measure performance. Again, there was no difference in performance in a normal environmental temperature, but compared to placebo, core temperature was significantly elevated during the time trial with no changes in the subjects’ ratings of perceived exertion and thermal stress scale (97). This increase in temperature indicates that the manipulation of the catecholaminergic system could cause a perturbation of thermoregulation. Fatigue during whole body exhaustive exercise is sometimes influenced by disturbances of neurotransmitter homeostasis, however, in ‘normal’ temperature it seems difficult to challenge fatigue mechanisms and perturb performance through drugs that influence neurotransmitter systems.
Fatigue in high environmental temperature
Neurotransmitters play a key role in the control of thermoregulation and are thought to mediate thermoregulatory responses. Serotonergic (5-HT), NA and DA pathways to the hypothalamus indicate their key role in temperature regulation (81). Therefore, shifts in the extracellular concentrations of these neurotransmitters could be expected to contribute to changes in thermal regulation and consequently to the onset of fatigue, specifically when exercise is undertaken in a warm (e.g. 30°C) environment (81). In a series of experiments the influence of neurotransmission on endurance performance in the heat was examined (for a recent review see 77). This paradigm was chosen because in the previous experiments with bupropion, core temperature was elevated by ~0.3° throughout the experiment compared to the placebo situation. Thus exercise in a hot environment in combination with manipulation of neurotransmitter systems could be a way to explore the brain mechanisms that limit performance in the heat.
When the 5-HT reuptake inhibitor citalopram was used to increase the brain content of 5-HT (78), no significant changes in endurance performance were detected. Also other studies (88, 89), were not able to influence performance and therefore fatigue during exercise in a hot environment. Thus, 5-HT is certainly not exclusively responsible for the onset of central fatigue during prolonged exercise in normal or high environmental temperature.
As in the normal temperature environment, the NA and DA neurotransmitter systems may have different effects on performance in a warm environment. NA reuptake inhibition by administration of reboxetine reduced performance in the heat. Subjects took 20% longer to complete a cycling time trial after reboxetine administration compared to the placebo administration (79).
Administration of methylphenidate, which increases DA in synapses, improved time trial performance in 30°C by 16%, an outcome that coincided with an average maximal core temperature of 40±0.6°C, much higher than in the placebo trial (39.1±0.7°C). Strikingly, both the ratings of perceived exertion and the thermal sensation were not different from the placebo. This indicates that subjects did not feel they were producing more power and consequently more heat. The authors concluded that the ‘safety switch’ or the mechanisms existing in the body to prevent harmful effects, are overridden by the drug administration (80). Taken together, these data indicate strong ergogenic effects of an increased DA concentration in the brain, without any change in the perception of effort.
Similarly, Watson et al. (97) examined the combined effects of DA and NA (by use of bupropion) on time trial performance in the heat, and found a significant improvement compared to placebo. Coinciding with this ergogenic effect the authors observed core temperatures that were significantly higher compared to the placebo situation (bupropion 40.0±0.3°C, placebo 39.7±0.3°C, P=0.017). Like methylphenidate (80), bupropion may also dampen or override inhibitory signals arising from the central nervous system to cease exercise due to hyperthermia, and enable an individual to continue to maintain a high power output (97). This outcome may suggest that after acute administration of bupropion the initial central influence originates from the DA neurotransmitter system, rather than from the NA system. Possible underlying mechanisms were explored in an animal study which showed that acute intraperitoneal bupropion injection produced increases in brain and core temperature and a decrease in tail temperature (heat loss mechanism) through an increase in NA and DA in the pre-optic area of the anterior hypothalamus (40). Thus it seems that the manipulation of neurotransmission in the ‘thermoregulation center’ of the brain (pre-optic anterior hypothalamus) is one of the mechanisms that negatively influences the rise in core temperature, therefore disturbing the adaptation to a homeostatic challenge.
Manipulation of neurotransmitter systems during exercise in the heat can also affect the pacing strategies subjects use during time trials (76). That is, there are effects on how subjects expend effort over the duration of a time trial (or race) and hence, effects on the time distribution of speed, power output or use of energetic reserves. After DA reuptake inhibition compared with placebo, subjects are able to maintain a higher power output throughout a time trial. Manipulation of serotonin and, especially, NA, have the opposite effect and force subjects to decrease power output early in the time trial. Interestingly, after manipulation of brain serotonin, subjects are often unable to perform an end sprint, indicating an absence of a reserve capacity or motivation to increase power output. Taken together, it appears that many factors, such as ambient conditions and manipulation of brain neurotransmitters, have the potential to influence power output during exercise, and might thus be involved as regulatory mechanisms in the complex skill of pacing (76).
Brain mechanisms responsible for fatigue during whole body exercise are complex, and are likely to involve integrative pathways. In normal ambient temperature, manipulation of monoaminergic neurotransmitters during exercise gives equivocal results. Effects become clearer with exercise in high ambient temperatures, and the thermoregulatory system may have an important influence on performance. As shown by changes in perceived exertion and pacing strategy, as well as body temperature, it seems that not only motor and thermoregulatory pathways, but also sensory and cognitive pathways work in concert to control power output and the development of fatigue during self-paced whole body exercise.
Changes in the neuromuscular pathway with fatiguing exercise
Voluntary movements and muscle forces are generated by muscle fibers which are controlled by the firing of spinal motoneurons. These receive sensory and descending signals from multiple sources. The corticospinal tract from the primary motor cortex to the spinal cord is especially important for the control of voluntary movement in humans. During fatiguing exercise, changes occur at each level of this neuromuscular pathway (Figure 1).
Motor unit firing in fatiguing contractions
The intimate connection between the central nervous system and the muscle is captured by the definition of the motor unit (MU); the spinal motoneuron and the muscle fibers innervated by its axon. The MU is the functional (controllable) or elemental portion of the neuromuscular system involved in motor output. The central nervous system through a variety of excitatory and inhibitory inputs and intrinsic properties of the motoneuron, ultimately activates MUs to achieve force output; Sherrington's final common pathway. The tight link and high fidelity of the muscle fiber's response to motoneuron output allows insights into spinal motoneuron function from electromyographic recordings of the peripheral muscle.
For the activation of muscle fibers, MUs are recruited or derecruited in a usually robust orderly fashion based on motoneuron size (see 41), essentially controlling the amount of muscle tissue being activated. Once a MU is recruited, force output can be further modulated by the rates of action potentials arriving at the muscle fiber. Muscle fibers are extremely responsive to relatively small variations in MU firing rates, taking advantage of the ‘active state’ processes (41). When first recruited in a healthy system, MUs usually fire at 5-8 Hz (41), although in certain tasks some MUs may initially fire doublets (2-3 action potentials at 100 Hz or more) to rapidly enhance rates of force development (39).
During brief non-fatiguing voluntary contractions in humans, mean MU firing rates as high as 50-60 Hz have been recorded from different (usually limb) muscles (27). Therefore, the working mean firing rate range varies a remarkable 8-10 fold, although muscle seems most responsive to rates between 10 and 40 Hz (30), and these are the more frequently recorded rates from large numbers of MUs.
In fatiguing contractions, MU recruitment has been less well-studied than firing rates, but it seems that recruitment order is not changed although recruitment thresholds may be altered depending on the task (2, 17, 28). Additionally during long endurance lower intensity tasks, new MUs are likely recruited and active MUs may drop-out (derecruited) for a period of time and then become activated again (67). This process is referred to as MU rotation or substitution (41). Changes in MU firing rate during a variety of fatiguing tasks, albeit studied mostly under isometric contractions, have been well described in many human muscles. Because of the muscle's responsiveness to changes in rates of excitation and the large range of rates recorded from human MUs, it is perhaps not surprising to expect rates to be modulated when the system is challenged or stressed with repetitive activation. But similar to other portions of the system described herein, the type of fatiguing task is a key factor.
During isometric contractions, the most consistent finding is that MU rates decline during intermittent or sustained maximal voluntary contractions (MVC) perhaps by as much as 50% compared with initial values regardless of their starting rate (12, 31, 99) - however there are rare exceptions to this observation (59). As discussed below, fatigue induced reductions in firing rates are due to one or a combination of a decline in neural drive, local intrinsic adaptations of the motoneuron (41), or to peripheral inhibitory feedback mechanisms. The relative contributions of these effects probably depend on the muscle studied and the duration of the high-intensity task. Studies in healthy aged adults with an altered MU system (42) expressing lower initial firing rates show the same response during high intensity tasks (21). This indicates that a reduction in firing rate is a fundamental response of the system's output. However, during submaximal fatiguing tasks MU firing rates show extremely variable responses that are not easy to categorize. At moderate intensity (40-50% MVC) during intermittent actions, first recruited MUs usually show declines in firing rates but units recruited during the task may increase their rates (17, 50) presumably in an effort to sustain the force. During contractions below 30-35% MVC, MU rate changes vary among units. No change, increases in rates, decreases in rates, or variable changes among MUs within the same experiment have been reported (reviewed in 37). At intensities of 20% or less of MVC during long duration contractions, units may show no change in rates but increases in variability in inter-discharge intervals as task failure approaches (67). Indeed, many factors including whether sustained or intermittent tasks, muscles of upper limb or lower limb, proximal or distal muscles, the composition and architecture of the muscle in terms of muscle fiber types and MU numbers, and training status among others can all be reasonably speculated to variably influence rate changes with submaximal fatiguing contractions. Thus, many potential factors need to be considered or carefully controlled when interpreting and comparing results across studies. Furthermore, unlike at higher contractile intensities, the opportunity for changes in recruitment and de-recruitment of MUs is much greater in fatiguing submaximal tasks, which can have an impact on discharge rates. However, relatively few studies have assessed concurrently the interaction between recruitment and rate coding during fatiguing tasks (61). Finally, interpretations of fatigue-related changes in MU output in many studies cited here have necessarily been directed by findings obtained from single MUs, but from a functional aspect the system utilizes groups of concurrently activated MUs. Therefore, further understanding of these processes would benefit from assessments of multiple MU pools during fatiguing contractions.
Dynamic contractions have been less well-studied due to the technical challenges of making reliable recordings of individual MUs during muscle length and architectural changes associated with joint rotations. Although initial MU firing rates can be higher due to greater central drive or other facilitatory processes related to fast shortening contractions when compared with isometric contractions (38), during dynamic fatiguing shortening contractions either at submaximal (37) or high intensity levels (19), maximal rates decline and with greater declines for the higher intensity task. Similar to isometric studies, MU firing rates show variable or no changes following a submaximal fatiguing shortening task (35, 37). Following muscle-damaging eccentric contractions, when strength was reduced immediately and for the subsequent 24h, the very few studies indicate that MU firing rates are slightly increased in relation to substantially lower recruitment thresholds compared with before the eccentric tasks (82 for review). With continuing improvements and validation of high-density surface electrodes (28) and miniaturization of intramuscular multi-channel electrode arrays (64), recording of MU properties may be forthcoming from more complicated dynamic fatiguing tasks involving multiple joint actions such as walking or cycling.
Motoneuron excitability during fatiguing contractions
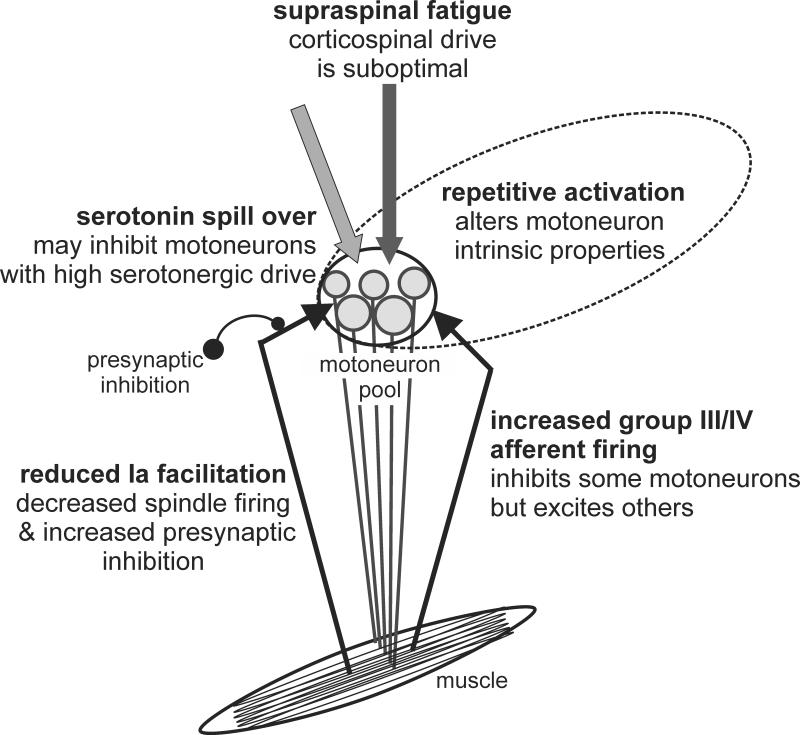
The one-to-one relationship of the firing of a motoneuron and the muscle fibers that it innervates means that the behavior of MUs (see above) represents the output of the motoneurons during fatiguing contractions. This output is influenced by the intrinsic properties of the motoneurons, the effects of neuromodulators such as serotonin and noradrenaline, and synaptic input from sensory feedback and descending drive (Figure 2). All of these influences can change with fatiguing exercise and these changes vary with task.
Figure 2. Influences on the firing of spinal motoneurons during fatiguing maximal contractions.
During fatiguing maximal contractions, motoneuron firing rates decrease. There are multiple influences on the motoneurons. Those described in the text are illustrated. First, repetitive activation (repeated firing) of motoneurons makes them less excitable, but the precise mechanism is not known. Second, group III and IV muscle afferents are mechanically and/or chemically sensitive, and increase their firing during fatiguing contractions. These afferents inhibit some motoneuron pools but excite others. Third, group Ia muscle afferents (from muscle spindles) are thought to decrease their firing and also to undergo additional presynaptic inhibition. This represents a decrease in excitatory drive to the motoneurons. Fourth, supraspinal fatigue, measured during maximal isometric contractions, suggests that excitatory drive from the motor cortex is also lower than it could be. Finally, it has been proposed that high serotonergic drive from the medulla may result in inhibition of motoneurons through activation of extrasynaptic receptors.
Several lines of evidence suggest that repetitive activation of motoneurons leads to a reduction in their excitability or response to excitatory synaptic input. First, in animal preparations, an individual motoneuron activated by current injection fires repetitively but this firing slows with time (for review 15). Second, in humans, feedback of the firing rate of a motoneuron can be provided through single MU recording. When subjects voluntarily hold this firing rate steady over several minutes, additional MUs are progressively recruited. This implies that the target motoneuron requires progressively more descending drive to maintain the same output (46). Third, muscle responses to stimulation of corticospinal axons at the cervicomedullary junction (cervicomedullary motor evoked potentials; CMEPs) are reduced when measured from the elbow flexor muscles during sustained MVCs (see 92). As direct cortical projections to motoneurons lack presynaptic inhibition, the reduction in CMEP suggests a reduction in motoneuron excitability. Furthermore, this reduction is magnified if the influence of descending drive on the motoneurons is temporarily removed by preceding transcranial magnetic stimulation (TMS), which briefly inhibits voluntary output from motor cortex. Without ongoing descending drive, motoneurons are profoundly less responsive after only 15 s of maximal activity (63). Finally, if similar stimuli (CMEP in the period of EMG silence following TMS) are delivered during a sustained submaximal voluntary contraction, CMEPs are also progressively reduced in size. However, small CMEPs are more affected than larger CMEPs (62). Because smaller, lower-threshold motoneurons are recruited before larger motoneurons (i.e. size principle), both in voluntary contractions and in responses to corticospinal stimulation, the differential effect suggests a reduction in the excitability of the motoneurons that fired repetitively in the sustained voluntary contraction with little effect on the larger, higher-threshold motoneurons (see 17). If repetitively active motoneurons are specifically reduced in excitability compared to non-active or less active motoneurons in the same pool, this suggests a change in intrinsic properties as the underlying mechanism, because other influences such as altered afferent or descending input would affect motoneurons across the pool. The specific intrinsic properties that change with repetitive activation are as yet unknown.
Descending neuromodulatory systems are likely to be important for exercise (48). For example, in reduced preparations, monoamines such as serotonin, noradrenaline and dopamine, promote locomotion or influence its rhythmicity through diverse actions on neurons in the locomotor circuits (e.g. 83). As yet, the integration of these systems into the control of voluntary movement is not well understood, but the serotonergic system has recently been proposed to contribute to fatigue (18). The actions of serotonin on motoneurons are complex (68). Descending serotonergic neurons synapse onto the dendrites and soma of motoneurons, where serotonin acts via 5-HT2 receptors to increase the excitability of the motoneurons (e.g. 48, 68). Such excitatory actions should aid in motor output rather than contribute to fatigue. Indeed, during rhythmic movements such as walking, the presence of neuromodulators may counteract the changes in intrinsic properties which lead to reduced excitability with repetitive firing of motoneurons (16). However, in addition to the 5-HT2 receptors, inhibitory 5-HT1A receptors have been identified on the axon initial segment of motoneurons. In turtle spinal cord, a high level of descending serotonergic drive results in spillover of serotonin from the synapses on the dendrites to the receptors on the axon initial segment and inhibits motoneuron firing (18 ). During motor tasks, serotonin is thought to be released relatively diffusely in the spinal cord (48). Studies in animals suggest more release with increased speed of treadmill running, but an eventual decrease with prolonged exercise (29, 34). Indirect measures in humans suggest that release is graded with the strength of voluntary contraction (98). Thus, the descending serotonergic system could reduce motoneuron excitability and contribute to fatigue either through withdrawal of its facilitatory actions at the dendrites, as may happen with prolonged exercise, or through spill over and activation of its inhibitory extrasynaptic receptors with strong contractions. So far, there is no direct evidence of serotonin's role at the motoneurons in fatigue although 5-HT1A receptors are present on human motoneurons (53) and human motoneuron excitability can be reduced by ingestion of a 5-HT1A agonist (20).
In addition to intrinsic changes of the motoneuron properties with repetitive activity and through neuromodulatory systems, the excitability of the motoneuron pool can be modulated by the afferent feedback (26, 92). The ionotropic synaptic input received by the motoneuron during fatiguing contractions comprises concurrent increases in excitatory (descending drive and muscle spindle afferents) and inhibitory (group Ib, group III and IV and Renshaw cell) afferent feedback. The inhibitory influence of Golgi tendon organs (group Ib afferent) and Renshaw cells should not play a substantial role as their activity is mainly diminished during fatigue (31). In contrast, firing of group III and IV muscle afferents is increased and it is well accepted that this leads to reduced motoneuron firing. The exact mechanisms of this reduction remain debated but likely include direct inhibition of some motoneuron pools, presynaptic inhibition of Ia afferents and supraspinal effects that reduce descending drive (25, 43, 60, 70, 99). The widespread influences of group III and IV muscle afferents on muscle fatigue are further discussed below (see Feedback from fatigue-sensitive muscle afferents).
Among the sensory receptors, muscle spindles have a facilitatory effect on the motoneuron pool. This facilitation can be modulated by change in the transduction properties of the sensory receptor and in the transmission of the Ia pathway. A pioneering study using microneurography reported a progressive decline in the discharge of muscle spindle afferents from the human pretibial muscles during a sustained (~1 min) submaximal isometric contraction (≤30% MVC) (58). This observation suggested that a progressive disfacilitation of the alpha motoneuron pool, associated with depressed gamma motoneuron activation and hence, decreased spindle firing, may contribute to the decline in MU discharge rate during a sustained contraction (58).
However, the facilitatory effect of muscle spindle output on the motoneuron pool can also be modulated by altered transmission prior to the motoneuron (i.e. presynaptic inhibitory mechanisms). This has been studied by the measurement of changes in short- and long-latency reflexes evoked by weak electrical stimulation of the Ia fibres of the corresponding muscle (23, 25). The short-latency reflex (Hoffmann or H reflex) comprises a spinal loop through largely monosynaptic input to the motoneurons, whereas the long-latency reflex involves transmission of the induced activity to supraspinal centers to evoke cortical output, which in turn reaches the motoneurons in the spinal cord.
When subjects sustained an MVC with the abductor pollicis brevis until force declined to 50% of maximum, the amplitude of the H reflex decreased progressively by 30% without any change in the amplitude of the long-latency reflex (25). As both reflex responses share the same motoneuron pool, the differential changes indicated a reduction in Ia afferent input by mechanism(s) located prior to the motoneuron. This implies that, not only may spindle firing be decreased as noted above, but that the efficacy of Ia input to facilitate the motoneuron pool is also progressively reduced. A comparable decrease in H-reflex amplitude was also seen in fatiguing submaximal (25% and 50% MVC) contractions held to failure (23). Furthermore, when normalized to the duration of the contraction, the rate of change of the H reflex was similar regardless of the intensity and duration of the contraction.
In contrast to the consistent reduction in H-reflex amplitude for the different intensities and durations of contraction, the amplitude of the long-latency reflex varied with the target force. Although, the long-latency reflex was unchanged at the end of the sustained MVC, it exhibited a progressive decline in amplitude for submaximal contractions (25% and 50% MVC) (23). This reduction was greater for the lower target force and longer duration of contraction (25% MVC). Because the long-latency reflex follows a supraspinal pathway that does not involve presynaptic inhibitory mechanisms, these results suggest that the reduced amplitude may reflect intrinsic changes of the motoneuron properties and the effect of neuromodulatory systems. Control of excitatory synaptic input at a presynaptic level has also been observed for the antagonist muscles. During a sustained submaximal contraction of the elbow flexors, the H-reflex response in triceps brachii decreased, whereas the amplitude of the CMEP, which is not influenced by presynaptic inhibition, increased (54). This specific modulation likely allows a precise control of co-activation during submaximal fatiguing contractions (24). Regardless of the exact mechanisms, these observations indicate a subtle control of the afferent feedback to the motoneuron pool of both agonist and antagonist muscles during fatiguing tasks.
Together, the results of these studies suggest that changes in the intrinsic properties of active motoneurons and disfacilitation of the motoneuron pool both occur during prolonged fatiguing contractions. In addition, an influence of descending neuromodulatory systems is probable. Thus, multiple mechanisms likely explain why spinal motoneurons become progressively harder to activate.
Motor cortical drive and excitability during fatiguing contractions
During a sustained MVC, the surface EMG activity drops progressively because motoneurons a) become less responsive to synaptic input, b) receive decreased afferent feedback from muscle spindles and c) receive insufficient descending drive due to supraspinal fatigue (Figure 2; 92). In contrast, during a submaximal task sustained for a long period of time surface EMG activity increases progressively. In sustained isometric contractions, this is associated with an increase in size of MEPs evoked by transcranial magnetic stimulation (TMS) (e.g. 51, 54, 87). Thus, augmented excitatory descending drive is usually thought to represent a compensation mechanism for contractile failure and the loss of spinal excitability (44, 51, 54, 87). Indeed, intramuscular EMG recordings indicate that although some MUs can stop discharging during the course of a sustained submaximal fatiguing contraction about a single joint (17, 69), enhanced descending drive recruits additional MUs (17, 33). Furthermore, the discharge rate of the newly activated MUs progressively increases after their recruitment before a later decline, as is the case for MUs activated from the beginning of the task (17, 75).
The intensification of descending drive is, however, limited in capacity and time, and a progressive reduction of the ability of the brain to drive the muscle maximally can be observed during and shortly after both maximal and submaximal single-limb and whole-body exercise. Stimulation of the motor cortex with a single TMS pulse during a brief MVC provides a method to assess the maximality of the output from the brain in humans (32). The presence of a twitch-like response on the force signal indicates that the voluntary output of the brain is not maximal because extra output can be evoked, and also that it is suboptimal to activate all MUs at their full capacity despite the subject's maximal effort. This may not necessarily indicate a reduction in motor cortical output or excitability, as the same descending drive could become less effective with reduced motoneuron excitability. However, as output remains untapped by voluntary effort, it does suggest a failure to employ all resources to generate output from the motor cortex (32, 93). Thus, an exercise-related increase of the superimposed twitch during an MVC is a classical marker of supraspinal fatigue. Using this method, supraspinal fatigue is relatively modest (accounting for ~25% of the loss of force) for short-duration sustained MVCs (~2 min) and most of the fatigability encountered is located in the muscle (32). In contrast, for long-duration submaximal contractions (≤15% MVC), the reduced ability of the brain to drive the muscle develops incrementally during the effort and contributes to a greater extent (50-66%) to the whole level of fatigability than during a brief MVC (92). Similarly, greater supraspinal fatigue also occurs with lower intensity, but longer duration, whole body exercise (94).
Despite the development of supraspinal fatigue, EMG responses to TMS suggest increased excitability of the motor cortex during fatiguing single-limb contractions. During submaximal contractions, increases in MEP size are associated with increases in voluntary EMG and at least partly reflect augmented cortical excitability associated with more voluntary cortical output (51, 53, 87). When the submaximal task requires EMG to be maintained steady, MEPs do not increase, but subcortically-evoked CMEPs decrease, with the comparison again suggesting an increase in cortical excitability (62). Similarly, during sustained maximal contractions or in brief MVCs performed in the midst of a submaximal task, MEPs increase in size despite reductions in ongoing EMG. However, some inhibitory responses to TMS also increase during fatiguing efforts, so that changes in the primary motor cortex are not as simple as just extra excitation (for review 36, 92). Furthermore, during cycling to task failure, MEPs in the knee extensors do not increase, raising the possibility that neural changes with fatigue differ in single-limb and locomotor exercise (84).
Imaging studies also offer insight into the activity of the motor cortex and other areas of the brain during fatiguing single-limb contractions. During prolonged submaximal contractions with hand muscles, as EMG increases, the fMRI BOLD signal increases progressively in the contralateral sensorimotor cortex, as well as in other motor areas, including the supplementary motor area, cingulate motor area and the ipsilateral sensorimotor cortex (57, 95). In contrast, although activation in cortical motor areas also increases during sustained maximal efforts, EMG and voluntary activation decrease, demonstrating that the increases in brain activity are not sufficient to maintain neural drive to the muscle (74). While the increases in the BOLD signal in the motor cortex may suggest increases in motor cortical output during both submaximal and maximal tasks, the interpretation of such changes is complex. Indeed, both excitatory and inhibitory activity could contribute to the extra BOLD response, along with recruitment of neurons engaging muscles not critical to the task and the effects of changing sensory feedback (74). Therefore, it is unclear how much of the increased response reflects an increase in descending drive to the fatigued muscle (74). For submaximal efforts, increasing voluntary EMG indicates increased excitatory input to the motoneuron pool and strongly suggests that motor cortical output is increasing. However, for maximal efforts, fMRI does not distinguish whether supraspinal fatigue is associated with a decrease in motor cortical output or an unchanged or increased output that has become less effective.
Cortical and spinal contributions to task failure
To determine the relative influence of spinal and cortical mechanisms on the endurance time, Klass and colleagues (51) compared two tasks that differed by the type of load used. The tasks consisted of sustaining a submaximal contraction at 20% maximum with the elbow flexor muscles while subjects either supported an inertial load (position task) or produced an equivalent constant torque against a rigid restraint (force task). Despite the similar load torque in both conditions, the time to task failure was about half as long for the position task as for the force task (see also 44, 51). At the end of the two tasks, when the subject was unable to continue, the superimposed twitch evoked by TMS, reflecting supraspinal fatigue, was increased similarly. The level of peripheral fatigue, assessed by the muscle twitch evoked by a single maximal electrical stimulation of the motor nerve, was also similar in both conditions. In contrast, the time course of change for the H-reflex response, induced by weak electrical stimulus of the Ia fibres in the brachial plexus, differed between the two tasks. It declined more quickly and to a greater extent for the position task, which suggested that spinal mechanisms constrained the endurance time in the position task compared to the force task (51).
Another approach to examine the contribution of the reduced output from the brain on the performance of a motor task is to manipulate pharmacologically the concentration of some brain neurotransmitters by the administration of drugs that inhibit specifically the reuptake of these neurotransmitters (see Brain changes associated with performance). Klass et al. (52) compared the influence of reuptake inhibitors for NA (reboxetine) or DA (methylphenidate) with a placebo on the performance of well-trained subjects on a 30-min time trial performed on a cycle ergometer at ~75% of maximum power production. With inhibition of NA reuptake, average power produced during the time trial was reduced compared to the other two conditions. As a consequence, the time to complete the time trial was comparable for the placebo and DA conditions, but ~12 % longer for the NA condition. Interestingly, the level of voluntary activation, assessed by TMS at 10 min after the time trial, was also different from pre-exercise values (~97%) only for the NA group (~8% reduction). Consistent with these observations, the time to complete a reaction time test, that assessed psychomotor vigilance, remained unchanged after the time trial for the placebo group, was faster with inhibition of DA reuptake, and was slower with inhibition of NA reuptake. In conclusion, manipulation of the brain neurotransmitter NA, but not DA, compromised neural adjustments during the time trial in well-trained individuals.
Together, these findings indicate that the endurance time of a fatiguing task is reduced by a depressed synaptic input from Ia afferent feedback and a decline in the capacity of the central nervous system to provide maximal excitation to the motoneurons. The rate-limiting adjustments of these two factors that constrain muscle function during fatiguing contractions are, however, task-dependent.
Feedback from fatigue-sensitive muscle afferents
As noted above, muscle contractions activate various receptors linked with sensory neurons innervating skeletal muscle. This stimulation changes the discharge frequency of these nerves and consequently their feedback to the central nervous system (CNS) including the brain and the spinal cord. Sensory nerves can be separated by their function, diameter, and conduction velocity (with the latter two depending on the degree of myelination) and have been classified as group I – IV. The small-diameter muscle afferents (i.e. groups III and IV) are most closely related to the changes in neural activity observed during fatiguing contractions . Most of the thinly myelinated group III afferents are mechanically sensitive and respond to muscle contraction and/or stretch, whereas group IV (and some group III) muscle afferents and associated receptors (see below) are sensitive to various intramuscular metabolites and metabolic changes within the contracting muscle as well as noxious levels of mechanical strain.
Recent findings in animals (45, 56); and humans (73) suggest the existence of two subgroups of metabosensitive group III/IV muscle afferents characterized by anatomical and functional differences (5). One subtype, the so-called metabo- or ergoreceptors, respond to innocuous levels of intramuscular metabolites (lactate, ATP, protons) associated with ‘normal’ (i.e. freely perfused and predominantly aerobic) exercise (11, 55) up to strenuous intensities. In contrast, the other subtype, the so-called metabo-nociceptors, only respond to higher (and concurrently noxious) levels of metabolites present in muscle during ischaemic contractions or following hypertonic saline infusions – but not to non-noxious metabolite concentrations associated with normal exercise (45, 56, 73). The specific phenotypical distinction of metaboreceptors vs metabo-nociceptors remains elusive to date. It is, however, recognized that molecular differences between the two subtypes includes the differential expression of purinergic receptors (P2X2,3,4), transient receptor potential vanilloid type 1 and/or 2 (TRPV1/2), and acid-sensing ion current 1, 2, and 3 (ASIC 1-3) (45, 56). Although the two different subtypes of group III/IV muscle afferents project to the same location in the superficial dorsal horn (45), it is currently unknown to what degree each subtype is anatomically linked to lamina I neurons which have direct projections to various supraspinal sites.
Group III and IV muscle afferents have been documented to substantially influence the development of peripheral and central fatigue during both single-joint and whole body exercise.
Role of Afferents in Peripheral Fatigue
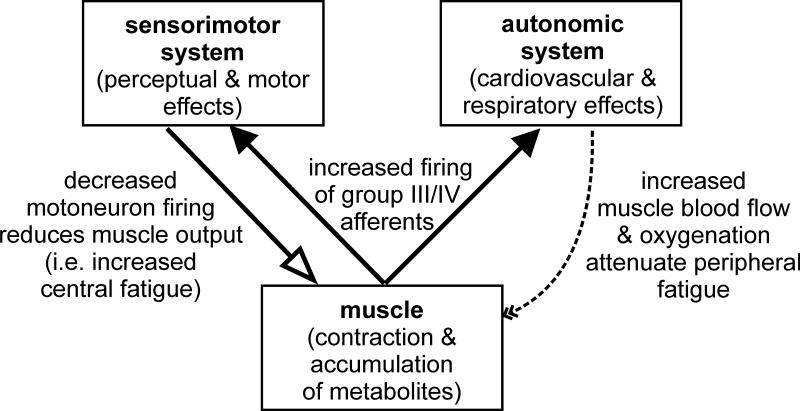
Group III/IV muscle afferents affect the development of peripheral fatigue through their involvement in the regulation of the cardiovascular, hemodynamic, and ventilatory response to exercise (Figure 3). The very rapid increases in these parameters after the onset of exercise are substantially influenced by group III/IV-mediated feedback from the working muscle (49) and central command (i.e. a feedforward signal originating within the brain and related to motor output) (96). Exercise-induced increases in ventilation and central (i.e. cardiac output) and peripheral (i.e. limb blood flow) hemodynamics promote optimal arterial oxygenation and muscle perfusion which together assure that O2 demand and delivery are matched in working muscles (22). Important in this context is the fact that muscle blood flow and O2 delivery depict key components in the rate of development of peripheral fatigue during both single joint exercise and activities including a large muscle mass (e.g. whole body exercise) (12). Specifically, decreases in blood flow/O2 delivery exacerbate this rate, while increases attenuate this rate (4). Consequently, by facilitating the circulatory and ventilatory response to exercise, group III/IV muscle afferents ensure adequate muscle blood flow/O2 delivery and thereby prevent premature fatigue of the contracting muscle (3).
Figure 3. Group III and IV muscle afferents increase central fatigue but attenuate peripheral fatigue.
Firing of group III and IV muscle afferents increases during fatiguing contractions. During exercise, these afferents produce reflex increases in heart rate, blood pressure and respiration to improve muscle blood flow and oxygenation. This slows the development of fatigue of the muscle itself (peripheral fatigue). At the same time, the afferent firing also leads to a reduction in voluntary neural drive to the muscle. That is, it contributes to central fatigue. The precise pathway for this effect is not known. The afferents evoke sensations of muscle discomfort and fatigue, increase supraspinal fatigue, presynaptically inhibit Ia input to motoneurons, and have differing actions on different motoneuron pools.
Aging might shift this positive influence of muscle afferent feedback on the development of peripheral fatigue through altering the role of these neurons in determining central and peripheral hemodynamics. Briefly while group III/IV-mediated feedback plays a clear role in facilitating limb blood flow and therefore O2 delivery during exercise in the young, these afferents seem to actually reduce limb vascular conductance in the elderly and may explain the limited exercise-induced peripheral vasodilation / limb blood flow often associated with aging (86). While this would suggest that group III/IV muscle afferents may actually facilitate the development of peripheral fatigue during exercise in the elderly, there is currently no data confirming this hypothesis.
Heart failure clearly exacerbates the group III/IV-mediated impact on vascular conductance and muscle blood flow / O2 delivery seen in healthy older individuals and exacerbates the development of fatigue during physical activity (10). Patients with heart failure are characterized by exaggerated group III and/or IV-mediated afferent feedback, which is thought to account for the exaggerated sympathoexcitation (10, 66). Recent data demonstrate that when heart failure patients perform exercise with pharmacologically (lumbar intrathecal fentanyl) blocked group III/IV muscle afferents, sympathetic outflow is attenuated and leg blood flow/O2 delivery significantly increased compared to control exercise. Importantly, this blockade-induced increase also attenuated the development of fatigue (as assessed via the pre- to post-exercise decrease in MVC) in these patients (10). Therefore, the abnormally elevated neural feedback (i.e., related to group III/IV muscle afferents) associated with heart failure exacerbates the rate of development of fatigue in patients with heart failure.
Role of Muscle Afferents in Central Fatigue
Feedback from group III/IV muscle afferents during fatiguing exercise directly or indirectly impairs the output from spinal motoneurons, which can compromise voluntary muscle activation and consequently exercise performance (Figure 3; 7, 13, 32, 91). This interaction has been demonstrated during single joint exercise where the output from motoneurons (i.e. surface EMG) and voluntary muscle activation (estimated from superimposed twitches elicited by TMS) was found to progressively decrease during a 2 min maximal, isometric elbow flexion but to recover to pre-exercise baseline levels within minutes after the cessation of exercise. However, when, at the end of exercise, a blood pressure cuff was inflated around the arm to trap metabolites within the elbow flexors and therefore to maintain the firing of group III/IV muscle afferents during the post-exercise rest period, motoneuronal output and voluntary muscle activation remained low and did not recover until the cuff was deflated, circulation restored, and group III/IV-mediated feedback recovered (32). As noted above, reduced motoneuron output has been variously attributed to direct inhibition of the motoneurons for some muscles, a reduction in excitatory input through presynaptic inhibition of Ia afferents and reduction of descending drive.
Group III/IV muscle afferents also exert inhibitory influences on the output from motoneurons during whole-body exercise. This was shown in a series of studies where subjects performed high-intensity cycling exercise to exhaustion under control conditions and with pharmacologically blocked (lumbar intrathecal fentanyl) afferent feedback from locomotor muscle. The unanimous observation was that output from spinal motoneurons (estimated via surface EMG) is less restricted and significantly higher during exercise with blocked group III/IV locomotor muscle afferents compared to the identical exercise performed with intact feedback (3, 6, 7). Interestingly, this inhibitory influence on motoneuronal output is not only specific to the working and fatiguing locomotor muscle, but can also cross-over to affect muscles / muscle groups not directly involved in the locomotor task (85). Together, these observations highlight the significant involvement of group III/IV muscle afferents in the development of central fatigue during intense whole body endurance exercise.
Investigating the effect of group III/IV muscle afferents on performance during longer duration, predominantly aerobic, exercise is challenging. The difficulty arises from the twofold role these neurons play in an exercising human. Specifically, although they limit motoneuronal output and consequently voluntary muscle activation, their contribution to attenuate the development of peripheral fatigue (by facilitating circulation and respiration during exercise, see above) is essential. Therefore, manipulating muscle afferents during exercise affects both central and peripheral mechanisms of fatigue and the net effect depends on how one effect outweighs the other. In a recent study designed to circumvent this caveat, subjects performed dynamic single leg knee-extensor exercise to task failure (inability to maintain 85% of peak power output) in one leg immediately followed by the identical task in the other leg (9). The goal was to determine whether afferent feedback arising from the knee-extensor, which was first exercised/exhausted, limits endurance exercise performance of the consecutively exercising contralateral knee-extensor. Various control experiments allowed the exclusion of other limiting influences on the endurance performance of the consecutively exercising contralateral leg (e.g. no peripheral fatigue, uncompromised ventilatory and circulatory responses). Afferent feedback associated with exercise to exhaustion in the first leg (~9 min) reduced endurance time to exhaustion of the consecutively exercised contralateral leg by nearly 50%. It was concluded that group III/IV muscle afferent feedback associated with exhaustive endurance exercise has an inhibitory effect on the CNS which limits output from motoneurons and therefore endurance performance (9). A similar cross-over effect on motoneuronal output and associated impact on exercise performance was also documented to occur from muscles in the upper body (fatiguing arm cranking) to the legs (cycling) (47).
Conclusion
During fatiguing exercise there are many changes in the nervous system. Some of these contribute to fatigue and others compensate to allow continued task performance despite reduced muscle forces. In the neuromuscular pathway, slowing or cessation of MU firing contributes to the loss of force that marks fatigue, whereas recruitment of additional MUs can compensate for it. These changes in MU firing result from a combination of influences on the motoneurons including alterations in intrinsic properties, afferent input, and descending neuromodulatory and synaptic inputs. In turn, each of the inputs to the motoneurons are susceptible to fatigue-related effects, which also depend on the specific exercise being performed. An important sensory signal of fatigue comes from the firing of group III/IV muscle afferents. This afferent signal interacts with the autonomic nervous system (8), as well as with various levels of the motor system (31), and also contributes to conscious sensations of muscle discomfort and fatigue (73). Finally, modulation of brain neurotransmitters can alter endurance performance. Such changes are exacerbated by exercise in the heat and may represent the interaction of homeostatic functions, such as temperature regulation, with the motor system and conscious sensations of fatigue. Thus, changes in the neuromuscular, sensory and homeostatic systems can all contribute to fatigue with exercise. The mix of influences on exercise performance will depend on the task and the conditions under which it is performed.
Acknowledgements
The results of the present study do not constitute endorsement by ACSM.
Source of Funding.
JLT is supported by a Research Fellowship and grant from the National Health and Medical Research Council of Australia. MA is supported by the US National Institute of Health (HL-103786 and HL-116579) and a Veterans Affairs grant (1I21RX001572). CLR is supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Footnotes
Conflicts of Interest
Other authors have nothing to declare.
References
- 1.Abbiss CR, Peiffer JJ, Meeusen R, Skorski S. Role of ratings of perceived exertion during self-paced exercise: what are we actually measuring? Sports Med. 2015;45(9):1235–43. doi: 10.1007/s40279-015-0344-5. [DOI] [PubMed] [Google Scholar]
- 2.Adam A, De Luca CJ. Recruitment order of motor units in human vastus lateralis muscle is maintained during fatiguing contractions. J Neurophysiol. 2003;90(5):2919–27. doi: 10.1152/jn.00179.2003. [DOI] [PubMed] [Google Scholar]
- 3.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high intensity endurance exercise performance in humans. J Physiol. 2011;589:5299–309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol. 2008;104(3):861–70. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- 5.Amann M, Light AR. From Petri dish to human: new insights into the mechanisms mediating muscle pain and fatigue, with implications for health and disease. Exp Physiol. 2015;100(9):989–90. doi: 10.1113/EP085328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol. 2008;105(6):1714–24. doi: 10.1152/japplphysiol.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587(Pt 1):271–83. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amann M, Sidhu SK, Weavil JC, Mangum TS, Venturelli M. Autonomic responses to exercies: group III/IV muscle afferents and fatigue. Auton Neurosci. 2015;188:19–23. doi: 10.1016/j.autneu.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amann M, Venturelli M, Ives SJ, et al. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol (1985) 2013;115(3):355–64. doi: 10.1152/japplphysiol.00049.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amann M, Venturelli M, Ives SJ, et al. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol. 2014;174:368–75. doi: 10.1016/j.ijcard.2014.04.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangsbo J, Johansen L, Graham T, Saltin B. Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J Physiol. 1993;462:115–33. doi: 10.1113/jphysiol.1993.sp019546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barclay JK. A delivery-independent blood flow effect on skeletal muscle fatigue. J Appl Physiol. 1986;61(3):1084–90. doi: 10.1152/jappl.1986.61.3.1084. [DOI] [PubMed] [Google Scholar]
- 13.Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–9. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigland-Ritchie B, Jones DA, Hosking GP, Edwards RH. Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin Sci Mol Med. 1978;54(6):609–14. doi: 10.1042/cs0540609. [DOI] [PubMed] [Google Scholar]
- 15.Brownstone RM. Beginning at the end: repetitive firing properties in the final common pathway. Prog Neurobiol. 2006;78(3-5):156–72. doi: 10.1016/j.pneurobio.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brownstone RM, Krawitz S, Jordan LM. Reversal of the late phase of spike frequency adaptation in cat spinal motoneurons during fictive locomotion. J Neurophysiol. 2011;105(3):1045–50. doi: 10.1152/jn.00411.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol. 2001;534(Pt 3):903–12. doi: 10.1111/j.1469-7793.2001.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotel F, Exley R, Cragg SJ, Perrier JF. Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc Natl Acad Sci U S A. 2013;110(12):4774–9. doi: 10.1073/pnas.1216150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowling B, Harwood B, Copithorne DB, Rice CL. Changes in anconeus motor unit firing rates during high-intensity dynamic elbow extensor fatiguing contractions. Med. Sci. Sports and Ex. 2015;47(Suppl 1)(5):322. [Google Scholar]
- 20.D'Amico JM, Butler AA, Butler JE, Gandevia SC, Taylor JL. Activation of 5HT1A receptors: a plausible contributor to central fatigue.. Congress of the European College of Sport Science; Malmo, Sweden. 2015. [Google Scholar]
- 21.Dalton BH, Harwood B, Davidson AW, Rice CL. Recovery of motoneuron output is delayed in old men following high-intensity fatigue. J Neurophysiol. 2010;103(2):977–85. doi: 10.1152/jn.00908.2009. [DOI] [PubMed] [Google Scholar]
- 22.Dempsey JA, Johnson JM, Wagner PD. Control of respiratory and cardiovascular system. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology Section 12: Exercise: Regulation and Integration of Multiple Systems. Oxford University Press; New York: 1996. pp. 331–838. [Google Scholar]
- 23.Duchateau J, Balestra C, Carpentier A, Hainaut K. Reflex regulation during sustained and intermittent submaximal contractions in humans. J Physiol. 2002;541(Pt 3):959–67. doi: 10.1113/jphysiol.2002.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duchateau J, Baudry S. The neural control of coactivation during fatiguing contractions revisited. J Electromyogr Kinesiol. 2014;24(6):780–8. doi: 10.1016/j.jelekin.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Duchateau J, Hainaut K. Behaviour of short and long latency reflexes in fatigued human muscles. J Physiol. 1993;471:787–99. doi: 10.1113/jphysiol.1993.sp019928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enoka RM, Baudry S, Rudroff T, Farina D, Klass M, Duchateau J. Unraveling the neurophysiology of muscle fatigue. J Electromyogr Kinesiol. 2011;21(2):208–19. doi: 10.1016/j.jelekin.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Enoka RM, Fuglevand AJ. Motor unit physiology: some unresolved issues. Muscle Nerve. 2001;24(1):4–17. doi: 10.1002/1097-4598(200101)24:1<4::aid-mus13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG: an update. J Appl Physiol (1985) 2014;117(11):1215–30. doi: 10.1152/japplphysiol.00162.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornal CA, Martin-Cora FJ, Jacobs BL. “Fatigue” of medullary but not mesencephalic raphe serotonergic neurons during locomotion in cats. Brain Res. 2006;1072(1):55–61. doi: 10.1016/j.brainres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Fuglevand AJ, Lester RA, Johns RK. Distinguishing intrinsic from extrinsic factors underlying firing rate saturation in human motor units. J Neurophysiol. 2015;113(5):1310–22. doi: 10.1152/jn.00777.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–89. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 32.Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490(Pt 2):529–36. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garland SJ, Enoka RM, Serrano LP, Robinson GA. Behavior of motor units in human biceps brachii during a submaximal fatiguing contraction. J Appl Physiol (1985) 1994;76(6):2411–9. doi: 10.1152/jappl.1994.76.6.2411. [DOI] [PubMed] [Google Scholar]
- 34.Gerin C, Becquet D, Privat A. Direct evidence for the link between monoaminergic descending pathways and motor activity. I. A study with microdialysis probes implanted in the ventral funiculus of the spinal cord. Brain Res. 1995;704(2):191–201. doi: 10.1016/0006-8993(95)01111-0. [DOI] [PubMed] [Google Scholar]
- 35.Griffin L, Ivanova T, Garland SJ. Role of limb movement in the modulation of motor unit discharge rate during fatiguing contractions. Exp Brain Res. 2000;130(3):392–400. doi: 10.1007/s002219900253. [DOI] [PubMed] [Google Scholar]
- 36.Gruet M, Temesi J, Rupp T, Levy P, Millet GY, Verges S. Stimulation of the motor cortex and corticospinal tract to assess human muscle fatigue. Neuroscience. 2013;231:384–99. doi: 10.1016/j.neuroscience.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 37.Harwood B, Choi I, Rice CL. Reduced motor unit discharge rates of maximal velocity dynamic contractions in response to a submaximal dynamic fatigue protocol. J Appl Physiol (1985) 2012;113(12):1821–30. doi: 10.1152/japplphysiol.00879.2012. [DOI] [PubMed] [Google Scholar]
- 38.Harwood B, Davidson AW, Rice CL. Motor unit discharge rates of the anconeus muscle during high-velocity elbow extensions. Exp Brain Res. 2011;208(1):103–13. doi: 10.1007/s00221-010-2463-4. [DOI] [PubMed] [Google Scholar]
- 39.Harwood B, Rice CL. Short interspike intervals and double discharges of anconeus motor unit action potentials for the production of dynamic elbow extensions. J Neurophysiol. 2014;111(10):2039–46. doi: 10.1152/jn.00412.2013. [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa H, Piacentini MF, Sarre S, Michotte Y, Ishiwata T, Meeusen R. Influence of brain catecholamines on the development of fatigue in exercising rats in the heat. J Physiol. 2008;586(1):141–9. doi: 10.1113/jphysiol.2007.142190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heckman CJ, Enoka RM. Motor unit. Compr Physiol. 2012;2(4):2629–82. doi: 10.1002/cphy.c100087. [DOI] [PubMed] [Google Scholar]
- 42.Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol. 2015 Oct; doi: 10.1113/JP270561. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilty L, Lutz K, Maurer K, et al. Spinal opioid receptor-sensitive muscle afferents contribute to the fatigue-induced increase in intracortical inhibition in healthy humans. Exp Physiol. 2011;96(5):505–17. doi: 10.1113/expphysiol.2010.056226. [DOI] [PubMed] [Google Scholar]
- 44.Hunter SK, Enoka RM. Changes in muscle activation can prolong the endurance time of a submaximal isometric contraction in humans. J Appl Physiol (1985) 2003;94(1):108–18. doi: 10.1152/japplphysiol.00635.2002. [DOI] [PubMed] [Google Scholar]
- 45.Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol. 2013;109(9):2374–81. doi: 10.1152/jn.01067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson KV, Edwards SC, Van Tongeren C, Bawa P. Properties of human motor units after prolonged activity at a constant firing rate. Exp Brain Res. 2004;154(4):479–87. doi: 10.1007/s00221-003-1678-z. [DOI] [PubMed] [Google Scholar]
- 47.Johnson MA, Sharpe GR, Williams NC, Hannah R. Locomotor muscle fatigue is not critically regulated after prior upper body exercise. J Appl Physiol (1985) 2015;119(7):840–850. doi: 10.1152/japplphysiol.00072.2015. [DOI] [PubMed] [Google Scholar]
- 48.Johnson MD, Heckman CJ. Gain control mechanisms in spinal motoneurons. Front Neural Circuits. 2014;8:81. doi: 10.3389/fncir.2014.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology Section 12: Exercise: Regulation and Integration of Multiple Systems. Oxford University Press; New York: 1996. pp. 381–447. [Google Scholar]
- 50.Kelly LA, Racinais S, Cresswell AG. Discharge properties of abductor hallucis before, during, and after an isometric fatigue task. J Neurophysiol. 2013;110(4):891–8. doi: 10.1152/jn.00944.2012. [DOI] [PubMed] [Google Scholar]
- 51.Klass M, Lévénez M, Enoka RM, Duchateau J. Spinal mechanisms contribute to differences in the time to failure of submaximal fatiguing contractions performed with different loads. J Neurophysiol. 2008;99(3):1096–104. doi: 10.1152/jn.01252.2007. [DOI] [PubMed] [Google Scholar]
- 52.Klass M, Roelands B, Lévénez M, et al. Effects of noradrenaline and dopamine on supraspinal fatigue in well-trained men. Med Sci Sports Exerc. 2012;44(12):2299–308. doi: 10.1249/MSS.0b013e318265f356. [DOI] [PubMed] [Google Scholar]
- 53.Laporte AM, Doyen C, Nevo IT, Chauveau J, Hauw JJ, Hamon M. Autoradiographic mapping of serotonin 5-HT1A, 5-HT1D, 5-HT2A and 5-HT3 receptors in the aged human spinal cord. J Chem Neuroanat. 1996;11(1):67–75. doi: 10.1016/0891-0618(96)00130-5. [DOI] [PubMed] [Google Scholar]
- 54.Lévénez M, Garland SJ, Klass M, Duchateau J. Cortical and spinal modulation of antagonist coactivation during a submaximal fatiguing contraction in humans. J Neurophysiol. 2008;99(2):554–63. doi: 10.1152/jn.00963.2007. [DOI] [PubMed] [Google Scholar]
- 55.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol. 2003;95(2):577–83. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- 56.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100(3):1184–201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu JZ, Shan ZY, Zhang LD, Sahgal V, Brown RW, Yue GH. Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: an FMRI study. J Neurophysiol. 2003;90(1):300–12. doi: 10.1152/jn.00821.2002. [DOI] [PubMed] [Google Scholar]
- 58.Macefield G, Hagbarth KE, Gorman R, Gandevia SC, Burke D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macefield VG, Fuglevand AJ, Howell JN, Bigland-Ritchie B. Discharge behaviour of single motor units during maximal voluntary contractions of a human toe extensor. J Physiol. 2000;528(Pt 1):227–34. doi: 10.1111/j.1469-7793.2000.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci. 2006;26(18):4796–802. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McManus L, Hu X, Rymer WZ, Lowery MM, Suresh NL. Changes in motor unit behavior following isometric fatigue of the first dorsal interosseous muscle. J Neurophysiol. 2015;113(9):3186–96. doi: 10.1152/jn.00146.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol. 2011;589(Pt 14):3533–44. doi: 10.1113/jphysiol.2011.207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McNeil CJ, Martin PG, Gandevia SC, Taylor JL. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol. 2009;587(Pt 23):5601–12. doi: 10.1113/jphysiol.2009.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muceli S, Poppendieck W, Negro F, et al. Accurate and representative decoding of the neural drive to muscles in humans with multi-channel intramuscular thin-film electrodes. J Physiol. 2015;593(17):3789–804. doi: 10.1113/JP270902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newsholme EA, Acworth I, Blomstrand E. Amino acids, brain neurotransmitters and a functional link between muscle and brain that is important in sustained exercise. In: Benzi G, editor. Advances in Myochemistry. John Libbey Eurotext; London: 1987. pp. 127–33. [Google Scholar]
- 66.Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001;280(3):H969–76. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- 67.Pascoe MA, Holmes MR, Stuart DG, Enoka RM. Discharge characteristics of motor units during long-duration contractions. Exp Physiol. 2014;99(10):1387–98. doi: 10.1113/expphysiol.2014.078584. [DOI] [PubMed] [Google Scholar]
- 68.Perrier JF, Rasmussen HB, Christensen RK, Petersen AV. Modulation of the intrinsic properties of motoneurons by serotonin. Curr Pharm Des. 2013;19(24):4371–84. doi: 10.2174/13816128113199990341. [DOI] [PubMed] [Google Scholar]
- 69.Peters EJ, Fuglevand AJ. Cessation of human motor unit discharge during sustained maximal voluntary contraction. Neurosci Lett. 1999;274(1):66–70. doi: 10.1016/s0304-3940(99)00666-7. [DOI] [PubMed] [Google Scholar]
- 70.Pettorossi VE, Della Torre G, Bortolami R, Brunetti O. The role of capsaicin-sensitive muscle afferents in fatigue-induced modulation of the monosynaptic reflex in the rat. J Physiol. 1999;515(Pt 2):599–607. doi: 10.1111/j.1469-7793.1999.599ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piacentini MF, Meeusen R, Buyse L, De Schutter G, De Meirleir K. Hormonal responses during prolonged exercise are influenced by a selective DA/NA reuptake inhibitor. Br J Sports Med. 2004;38(2):129–33. doi: 10.1136/bjsm.2002.000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piacentini MF, Meeusen R, Buyse L, et al. No effect of a noradrenergic reuptake inhibitor on performance in trained cyclists. Med Sci Sports Exerc. 2002;34(7):1189–93. doi: 10.1097/00005768-200207000-00021. [DOI] [PubMed] [Google Scholar]
- 73.Pollak KA, Swenson JD, Vanhaitsma TA, et al. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol. 2014;99.2:368–80. doi: 10.1113/expphysiol.2013.075812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Post M, Steens A, Renken R, Maurits NM, Zijdewind I. Voluntary activation and cortical activity during a sustained maximal contraction: an fMRI study. Hum Brain Mapp. 2009;30(3):1014–27. doi: 10.1002/hbm.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riley ZA, Maerz AH, Litsey JC, Enoka RM. Motor unit recruitment in human biceps brachii during sustained voluntary contractions. J Physiol. 2008;586(8):2183–93. doi: 10.1113/jphysiol.2008.150698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roelands B, de Koning J, Foster C, Hettinga F, Meeusen R. Neurophysiological determinants of theoretical concepts and mechanisms involved in pacing. Sports Med. 2013;43(5):301–11. doi: 10.1007/s40279-013-0030-4. [DOI] [PubMed] [Google Scholar]
- 77.Roelands B, De Pauw K, Meeusen R. Neurophysiological effects of exercise in the heat. Scand J Med Sci Sports. 2015;25(Suppl 1):65–78. doi: 10.1111/sms.12350. [DOI] [PubMed] [Google Scholar]
- 78.Roelands B, Goekint M, Buyse L, et al. Time trial performance in normal and high ambient temperature: is there a role for 5-HT? Eur J Appl Physiol. 2009;107(1):119–26. doi: 10.1007/s00421-009-1109-3. [DOI] [PubMed] [Google Scholar]
- 79.Roelands B, Goekint M, Heyman E, et al. Acute norepinephrine reuptake inhibition decreases performance in normal and high ambient temperature. J Appl Physiol (1985) 2008;105(1):206–12. doi: 10.1152/japplphysiol.90509.2008. [DOI] [PubMed] [Google Scholar]
- 80.Roelands B, Hasegawa H, Watson P, et al. The effects of acute dopamine reuptake inhibition on performance. Med Sci Sports Exerc. 2008;40(5):879–85. doi: 10.1249/MSS.0b013e3181659c4d. [DOI] [PubMed] [Google Scholar]
- 81.Roelands B, Meeusen R. Alterations in central fatigue by pharmacological manipulations of neurotransmitters in normal and high ambient temperature. Sports Med. 2010;40(3):229–46. doi: 10.2165/11533670-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 82.Semmler JG. Motor unit activity after eccentric exercise and muscle damage in humans. Acta Physiol (Oxf) 2014;210(4):754–67. doi: 10.1111/apha.12232. [DOI] [PubMed] [Google Scholar]
- 83.Sharples SA, Koblinger K, Humphreys JM, Whelan PJ. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits. 2014;8:55. doi: 10.3389/fncir.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sidhu SK, Cresswell AG, Carroll TJ. Motor cortex excitability does not increase during sustained cycling exercise to volitional exhaustion. J Appl Physiol (1985) 2012;113(3):401–9. doi: 10.1152/japplphysiol.00486.2012. [DOI] [PubMed] [Google Scholar]
- 85.Sidhu SK, Weavil JC, Venturelli M, et al. Spinal mu-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol. 2014;592(Pt 22):5011–24. doi: 10.1113/jphysiol.2014.275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sidhu SK, Weavil JC, Venturelli M, et al. Aging alters muscle reflex control of autonomic cardiovascular responses to rhythmic contractions in humans. Am J Physiol Heart Circ Physiol. 2015;309(9):H1479–89. doi: 10.1152/ajpheart.00433.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sogaard K, Gandevia SC, Todd G, Petersen NT, Taylor JL. The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol. 2006;573(Pt 2):511–23. doi: 10.1113/jphysiol.2005.103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strachan AT, Leiper JB, Maughan RJ. Paroxetine administration failed [corrected] to influence human exercise capacity, perceived effort or hormone responses during prolonged exercise in a warm environment. Exp Physiol. 2004;89(6):657–64. doi: 10.1113/expphysiol.2004.027839. [DOI] [PubMed] [Google Scholar]
- 89.Strachan AT, Leiper JB, Maughan RJ. Serotonin2C receptor blockade and thermoregulation during exercise in the heat. Med Sci Sports Exerc. 2005;37(3):389–94. doi: 10.1249/01.mss.0000155397.42481.53. [DOI] [PubMed] [Google Scholar]
- 90.Swart J, Lamberts RP, Lambert MI, et al. Exercising with reserve: exercise regulation by perceived exertion in relation to duration of exercise and knowledge of endpoint. Br J Sports Med. 2009;43(10):775–81. doi: 10.1136/bjsm.2008.056036. [DOI] [PubMed] [Google Scholar]
- 91.Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490(Pt 2):519–28. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol (1985) 2008;104(2):542–50. doi: 10.1152/japplphysiol.01053.2007. [DOI] [PubMed] [Google Scholar]
- 93.Taylor JL, Todd G, Gandevia SC. Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol. 2006;33(4):400–5. doi: 10.1111/j.1440-1681.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 94.Thomas K, Goodall S, Stone M, Howatson G, St Clair Gibson A, Ansley L. Central and peripheral fatigue in male cyclists after 4-, 20-, and 40-km time trials. Med Sci Sports Exerc. 2015;47(3):537–46. doi: 10.1249/MSS.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 95.van Duinen H, Renken R, Maurits N, Zijdewind I. Effects of motor fatigue on human brain activity, an fMRI study. Neuroimage. 2007;35(4):1438–49. doi: 10.1016/j.neuroimage.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 96.Waldrop TG, Eldridge FL, Iwamoto GA, Mitchell JH. Central neural control of respiration and circulation during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology Section 12: Exercise: Regulation and Integration of Multiple Systems. Oxford University Press; New York: 1996. pp. 333–80. [Google Scholar]
- 97.Watson P, Hasegawa H, Roelands B, Piacentini MF, Looverie R, Meeusen R. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol. 2005;565(Pt 3):873–83. doi: 10.1113/jphysiol.2004.079202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wei K, Glaser JI, Deng L, et al. Serotonin affects movement gain control in the spinal cord. J Neurosci. 2014;34(38):12690–700. doi: 10.1523/JNEUROSCI.1855-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Woods JJ, Furbush F, Bigland-Ritchie B. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol. 1987;58(1):125–37. doi: 10.1152/jn.1987.58.1.125. [DOI] [PubMed] [Google Scholar]