Abstract
Acetaminophen (APAP) hepatotoxicity is characterized by an extensive mitochondrial oxidant stress. However, its importance as a drug target has not been clarified. To investigate this, fasted C57BL/6J mice were treated with 300 mg/kg APAP and the mitochondria-targeted antioxidant Mito-Tempo (MT) was given 1.5h later. APAP caused severe liver injury in mice, as indicated by the increase in plasma ALT activities and centrilobular necrosis. MT dose-dependently reduced the injury. Importantly, MT did not affect APAP protein adducts formation, glutathione depletion or c-jun N-terminal kinase activation and its mitochondrial translocation. In contrast, hepatic glutathione disulfide and peroxynitrite formation were dose-dependently reduced by MT, indicating its effective mitochondrial oxidant stress scavenging capacity. Consequently, mitochondrial translocation of Bax and release of mitochondrial intermembrane proteins such as apoptosis-inducing factor were prevented and nuclear DNA fragmentation was eliminated. To demonstrate the importance of mitochondria-specific antioxidant property of MT, we compared its efficacy with Tempo, which has the same pharmacological mode of action as MT but lacks the mitochondria targeting moiety. In contrast to the dramatic protection by MT, the same molar dose of Tempo did not significantly reduce APAP hepatotoxicity. In contrast, even a 3h post-treatment with MT reduced 70% of the injury, and the combination of MT with N-acetylcysteine (NAC) provided superior protection than NAC alone. We conclude that MT protects against APAP overdose in mice by attenuating the mitochondrial oxidant stress and preventing peroxynitrite formation and the subsequent mitochondrial dysfunction. MT is a promising therapeutic agent for APAP overdose patients.
Keywords: acetaminophen hepatotoxicity, mitochondria, oxidant stress, antioxidant, Mito-TEMPO
INTRODUCTION
Acetaminophen (APAP) hepatotoxicity is the leading cause of acute liver failure in the US and many Western countries (Budnitz et al. 2011; Manthripragada et al. 2011). Its toxicity is initiated by the formation of a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI) (Nelson 1990). Excess NAPQI after APAP overdose depletes glutathione (GSH) and binds to cellular proteins (Cohen et al. 1997). Mitochondrial protein adducts disturb mitochondrial respiration and enhance the formation of reactive oxygen species (ROS) and peroxynitrite (Cover et al. 2005; Jaeschke 1990; Meyers et al. 1988). The oxidative stress activates MAP kinases ultimately resulting in the phosphorylation of c-jun N-terminal kinase (JNK), which then translocates to mitochondria and further amplifies the mitochondrial oxidant stress (Hanawa et al. 2008; Saito et al. 2010a). The enhanced mitochondrial oxidant stress triggers the opening of the mitochondrial permeability transition (MPT) pore and cell necrosis (Kon et al. 2004; LoGuidice and Boelsterli 2011; Ramachandran et al. 2011a). N-acetylcysteine (NAC) was introduced in the 1970s as the clinical antidote for APAP poisoning (Prescott et al. 1977) and until today it is still the only available treatment for APAP overdose. NAC promotes the re-synthesis of GSH, which can scavenge more NAPQI and prevent protein adduct formation (Corcoran et al. 1985). Consequently, NAC is most effective for early presenting APAP overdose patients. However, in reality most APAP overdose patients seek medical attention relatively late; i.e. around or after the peak of the injury (Larson 2007). Therefore, a drug that is effective for late presenting patients is needed.
Mitochondrial oxidant stress has been suggested to be critical in the progression of the injury (Jaeschke et al. 2012). Evidence for a selective APAP-induced mitochondrial oxidant stress was the specific increase of mitochondrial GSSG levels (Jaeschke 1990; Knight et al. 2001) and the selective formation of nitrotyrosine protein adducts in mitochondria in vivo (Cover et al. 2005). In addition, the specific MitoSox Red staining in isolated hepatocytes supports the conclusion of a mitochondrial oxidant stress (Yan et al. 2010). The pathophysiological relevance of this oxidant stress was documented by the protective effect of promoting hepatic GSH synthesis (Du et al. 2014; James et al. 2003; Knight et al. 2002; Saito et al. 2010b), which will result in enhanced mitochondrial GSH levels and an improved capacity to scavenge ROS and peroxynitrite. Furthermore, mice with partial MnSOD-deficiency or inactivation of MnSOD resulted in increased liver injury, suggesting that impairment of mitochondrial antioxidant defense enhances the hepatocyte’s susceptibility to oxidative injury (Agarwal et al. 2011; Fujimoto et al. 2009; Ramachandran et al. 2011b). Together these data support the critical role of a mitochondrial oxidant stress in APAP hepatotoxicity. However, a therapeutic approach that specifically targets mitochondrial ROS has not been tested in this model.
Mito-Tempo (MT) was recently reported as a mitochondria-targeted antioxidant (Trnka et al. 2008). This compound combines the antioxidant piperidine nitroxide (Tempo) with the lipophilic cation triphenylphosphonium (TPP+) (Trnka et al. 2008). Tempo is a SOD mimetic that dismutases superoxide in the catalytic cycle, while TPP is a membrane permeant cation that accumulates several hundred-fold within mitochondria driven by the membrane potential (Trnka et al. 2008). This combination creates a mitochondria-targeted chemical with effective superoxide scavenging properties. Interestingly, this compound has been shown to protect against oxidative injury in various pathologies, such as endotoxin-induced liver injury, sepsis-induced acute kidney injury, hypertension or colitis (Choumar et al. 2011; Dikalova et al. 2010; Patil et al. 2014; Wang et al. 2014). Therefore, the objective of the present study was to test whether MT could also protect against APAP hepatotoxicity by alleviating mitochondrial oxidant stress and dysfunction, which are critical events in the pathophysiology of APAP hepatotoxicity. In addition, we also assessed its therapeutic potential for APAP overdose by comparing its effect with NAC treatment, the current standard of care antidote.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice (8–12 weeks old) purchased from Jackson Laboratories (Bar Harbor, ME) were kept in an environmentally controlled room with a 12h light/dark cycle. All animals were accustomed at least 3 days before experiments with free access to food and water. All experimental protocols followed the criteria of the National Research Council for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center.
Experimental design
Mice were fasted overnight and treated i.p. with 300 mg/kg APAP (Sigma-Aldrich, St. Louis, MO) dissolved in warm saline. Mito-Tempo (10 or 20 mg/kg) or Tempo (6.1 or 100 mg/kg) (Sigma-Aldrich) dissolved in saline (10 ml/kg) was i.p. administered 1.5h after APAP. To test the therapeutic potential of MT, some mice were treated i.p. with MT or NAC (500 mg/kg dissolved in saline) or both at 3h post-APAP. The number of mice used in our experiments was based on our extensive experience with the APAP model. In our study we used 3–7 mice/group (Suppl. Table 1). Generally, more mice were applied when liver injury was expected to be more variable (e.g. NAC-treated mice), while less mice were used when the results were consistent and the difference was obvious (e.g. MT-treated mice). Mice were euthanized at 3, 6 or 12h post-APAP; under isoflurane anesthesia blood was drawn into a heparinized syringe and livers were harvested. Blood was centrifuged to obtain plasma for ALT determination. The liver tissue was cut into pieces, and then used for mitochondrial isolation as described (Du et al. 2015a), or fixed in phosphate-buffered formalin for histology analyses, or was flash frozen in liquid nitrogen and subsequently stored at −80°C.
Biochemical assays
Plasma ALT activities were measured using an ALT assay kit (Pointe Scientific, MI). GSH and GSSG measurement were performed using a modified method of the Tietze assay as described in detail (Jaeschke and Mitchell 1990; McGill and Jaeschke 2015). Hepatic APAP-protein adducts were measured as described previously (Muldrew et al. 2002) with modifications as described (Ni et al. 2012). In short, liver homogenates were filtered through Bio-Spin 6 columns (Bio-Rad, Hercules, CA) that were pre-washed with 10 mM sodium acetate buffer (pH 6.5) to separate cellular proteins from small molecules. The protein samples were digested overnight with proteases to liberate APAP-CYS from APAP protein adducts. The debris was then pelleted with 40% trichloroacetic acid and supernatants were filtered. APAP-CYS was measured using an HPLC method with electrochemical detection (Muldrew et al., 2002; Ni et al., 2012).
Histology and western blotting
Formalin-fixed tissue samples were embedded in paraffin, cut in 5 µm sections and stained with hematoxylin and eosin (H&E) for necrosis evaluation (Gujral et al. 2002). Cell necrosis was identified by morphological criteria in high resolution microscopic fields: cell swelling, vacuolization, karyorrhexis and karyolysis. The percentage of necrosis was estimated by comparing the necrosis area to the entire section. The pathologist (A. Farhood) evaluated all histological sections in a blinded manner. Nitrotyrosine staining was performed to assess nitrotyrosine (NT) protein adducts as described (Knight et al. 2002), using the Dako LSAB peroxidase kit (Dako, Carpinteria, CA) and a rabbit polyclonal anti-nitrotyrosine antibody (Life Technologies, Grand Island, NY; Cat. # A-21285). Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay was performed for DNA strand break assessment with the In Situ Cell Death Detection Kit, AP (RocheDiagnostics, Indianapolis, IN) following manufacturer’s instructions. The positive NT and TUNEL cells were identified by the dark brown staining around the centrilobular areas. In general, the positive staining was first estimated at low power (100×); questionable areas were evaluated at higher magnification (200× or 400×). Western blotting was performed as described (Bajt et al. 2000). The primary antibodies (1:1000 dilutions) included a rabbit anti-JNK antibody (Cat. # 9252) and a rabbit anti-phospho-JNK antibody (Cat. # 4668), rabbit anti-Bax polyclonal antibody (Cat. # 2772), rabbit anti-AIF antibody (Cell Signaling Technology, Danvers, MA; Cat # 5318) and mouse anti-Smac/DIABLO (BD Biosciences, San Diego, CA; Cat # 612245). A horseradish peroxidase-coupled anti-rabbit or anti-mouse IgG (Santa Cruz) was used as secondary antibody (1:5000 dilutions). Proteins were visualized by enhanced chemilumines-cence (GE Bioscience) and quantified by densitometric analysis of the x-ray film (Kodak, Rochester, NY).
Statistics
All results were expressed as mean ± SEM. Statistical significance between two groups was evaluated using the Student's t-test, while comparisons of multiple groups was assessed by one-way analysis of variance (ANOVA), followed by Student-Newman-Keul’s test. For non-normally distributed data, ANOVA was performed on ranks, followed by Dunn’s multiple comparisons. P < 0.05 was considered significant.
RESULTS
Mito-Tempo protects against APAP-induced liver injury
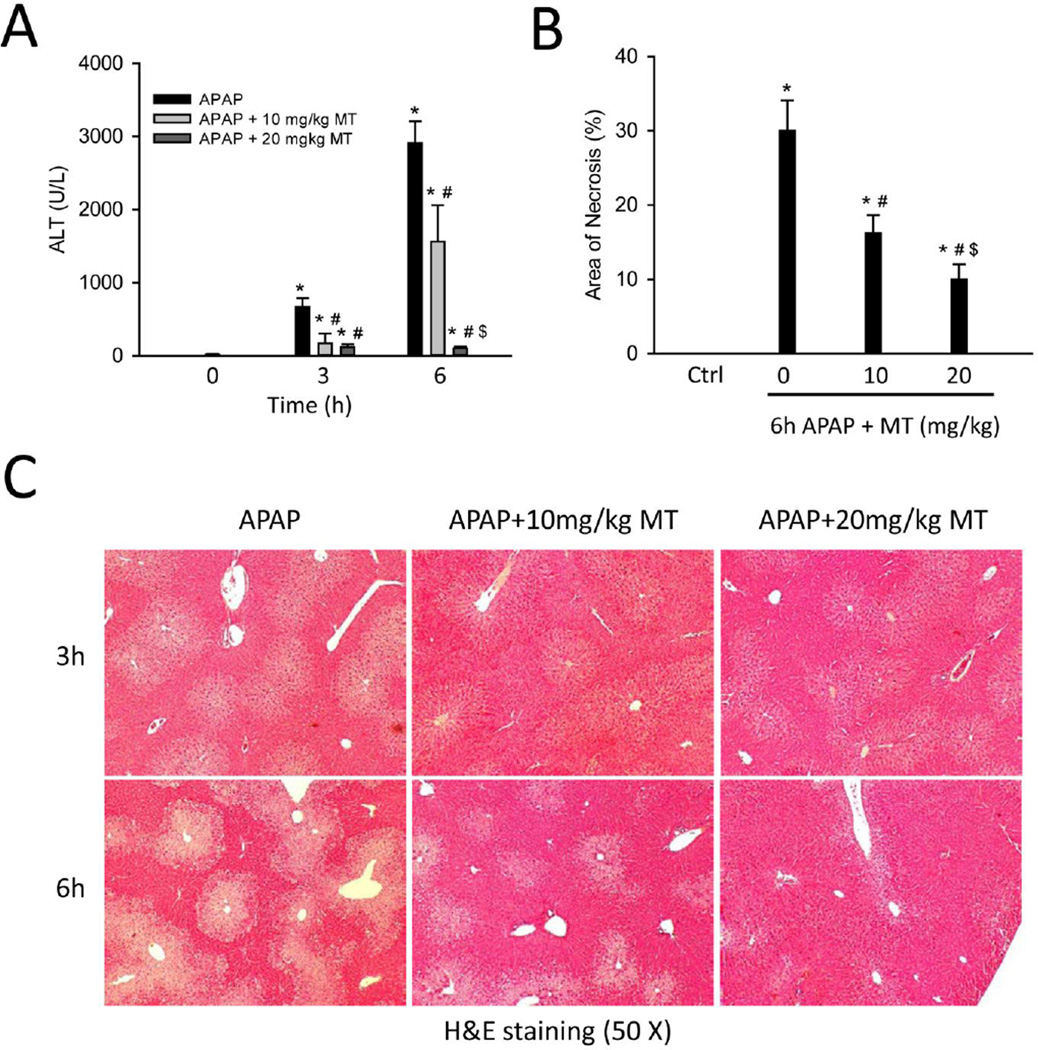
Mice treated with 300 mg/kg APAP developed severe liver injury at 3 h and 6 h post-APAP, as indicated by increased plasma ALT activities (Fig. 1A) and extensive centrilobular necrosis in H&E-stained histology sections (Fig. 1C). Quantification of the areas of necrosis by the pathologist (A.F.) in a blinded manner at 6h confirmed these results (Fig. 1B). Treatment with MT (10 or 20 mg/kg) 1.5h post-APAP dose-dependently attenuated the increase in ALT activities (Fig. 1A) and significantly reduced the areas of necrosis at both time points (Fig. 1B, C), suggesting that MT effectively protected against APAP-induced liver injury.
Figure 1. MT protected against APAP hepatotoxicity.
Mice were treated with 300 mg/kg APAP, and 10 or 20 mg/kg MT or saline (10 ml/kg) was given 1.5 h later. Blood and liver tissue were harvested at 3 or 6 h post-APAP. (A) Plasma alanine aminotransferase (ALT) activity. (B) Area of necrosis at 6 h (%). (C) H&E stained liver sections. Bars represent means ± SEM for n = 4 – 7 mice. *p<0.05 vs. 0h. #p<0.05 vs. APAP. $p<0.05 vs. APAP+10 mg/kg MT.
Mito-Tempo does not inhibit the metabolic activation of APAP
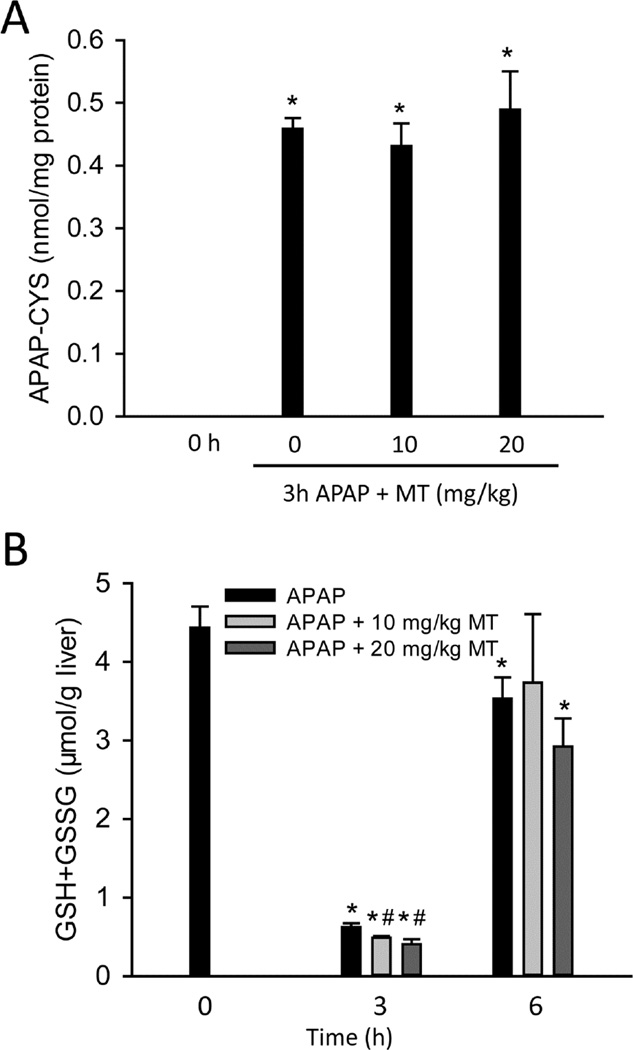
To determine whether MT affects the metabolic activation of APAP, APAP-protein adducts were measured at 3h post-APAP. MT did not affect APAP-protein adducts formation at either dose (Fig. 2A), indicating that MT did not inhibit reactive metabolite formation when it was given 1.5h after APAP, i.e. when APAP metabolic activation is almost completed. Consistent with this, GSH depletion at 3h was not inhibited by MT (Fig. 3B). In addition, there was no significant difference in GSH recovery among the groups at 6h post-APAP (Fig. 2B), suggesting that the protection by MT is neither caused by an effect on protein adduct formation nor hepatic GSH recovery.
Figure 2. MT did not inhibit APAP metabolic activation.
Mice were treated with 300 mg/kg APAP, and 10 or 20 mg/kg MT or saline (10 ml/kg) was given 1.5 h later. (A) Total liver APAP-cysteine adducts. (B) Time course of hepatic GSH levels. Bars represent mean ± SEM for n = 4 – 7 mice. *p<0.05 vs. 0h. #p<0.05 vs. APAP.
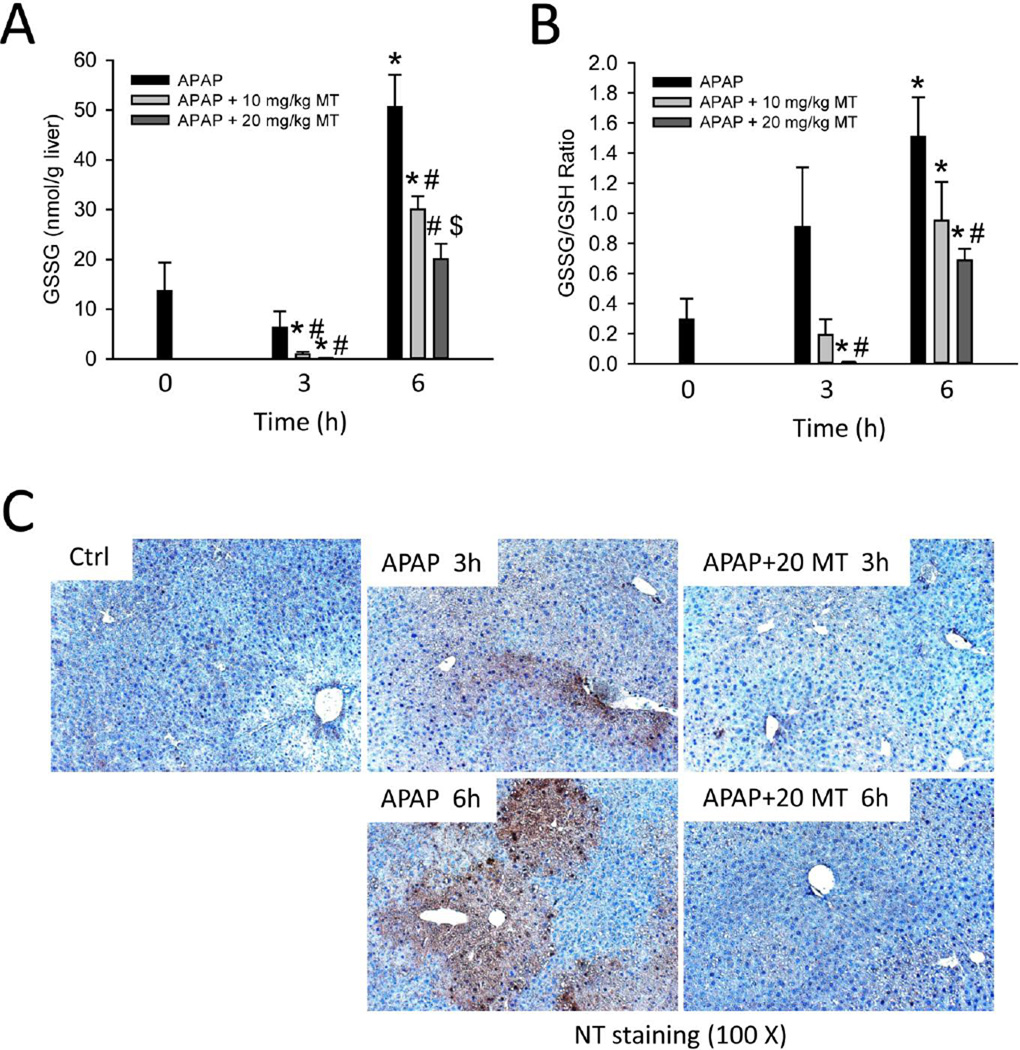
Figure 3. MT dose-dependently attenuated mitochondrial oxidant stress.
Mice were treated with 300 mg/kg APAP, and 10 or 20 mg/kg MT or saline (10 ml/kg) was given 1.5 h later. (A) Total liver GSSG levels. (B) GSSG-to-GSH ratios. (C) Nitrotyrosine staining of representative liver sections. Bars represent mean ± SEM for n = 4 – 7 mice. *p<0.05 vs. 0h. #p<0.05 vs. APAP. $p<0.05 vs. APAP+10 mg/kg MT.
Mito-Tempo attenuates APAP-induced mitochondrial oxidant stress in a JNK-independent manner
Next, we tested whether MT can alleviate the mitochondrial oxidant stress as was hypothesized based on the mode of action of the drug. Formation of reactive oxygen species was assessed by GSSG levels and the GSSG-to-GSH ratio (Fig. 3A, B). In support of the protection by MT (Fig. 1), GSSG levels and the GSSG-to-GSH ratio were significantly lower in MT-treated mice in a dose-dependent manner at both 3h and 6h (Fig. 3A, B). Superoxide reacts with nitric oxide (NO) to form peroxynitrite (ONOO−), a highly potent oxidant and nitrating species that has been shown to be formed during APAP toxicity (Hinson et al. 1998). Consistent with previous findings (Hinson et al. 1998; Knight et al. 2001), there was extensive staining for nitrotyrosine protein adducts in the centrilobular areas (Fig. 3C). Importantly, MT treatment almost eliminated APAP-induced nitrotyrosine staining (Fig. 3C), consistent with the hypothesis that the effective dismutation of superoxide can prevent peroxynitrite formation.
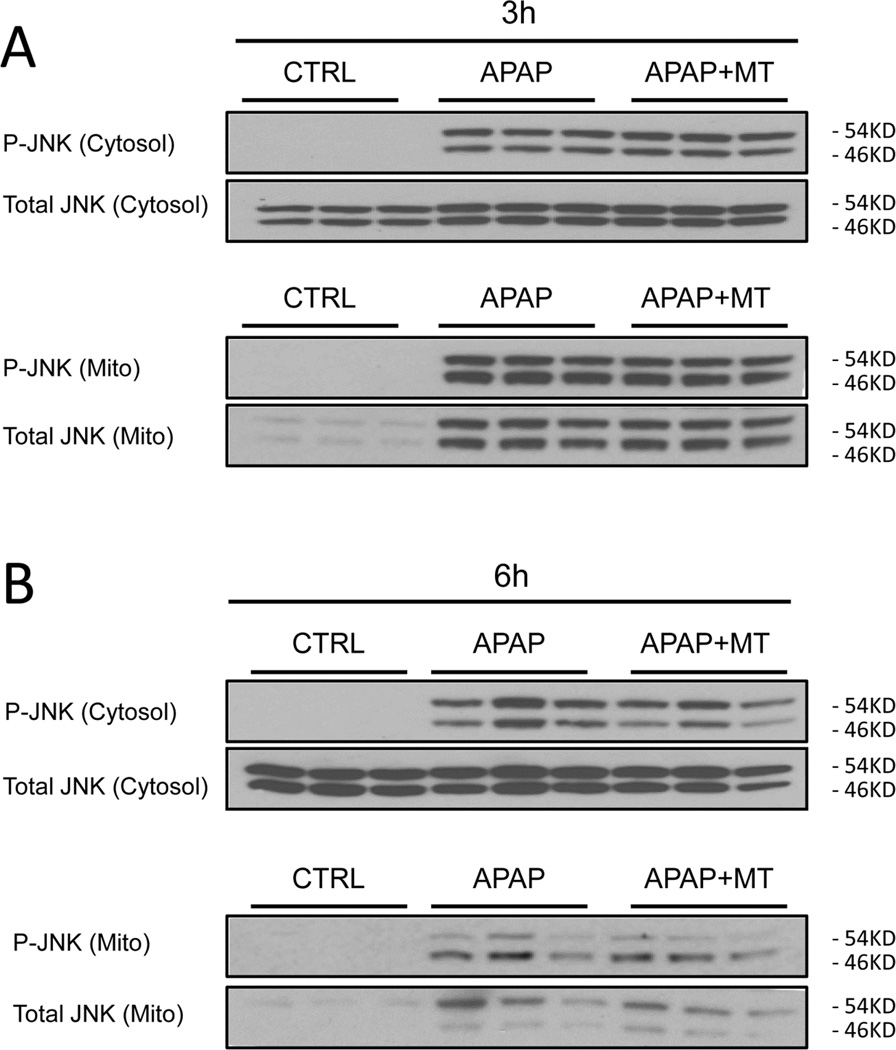
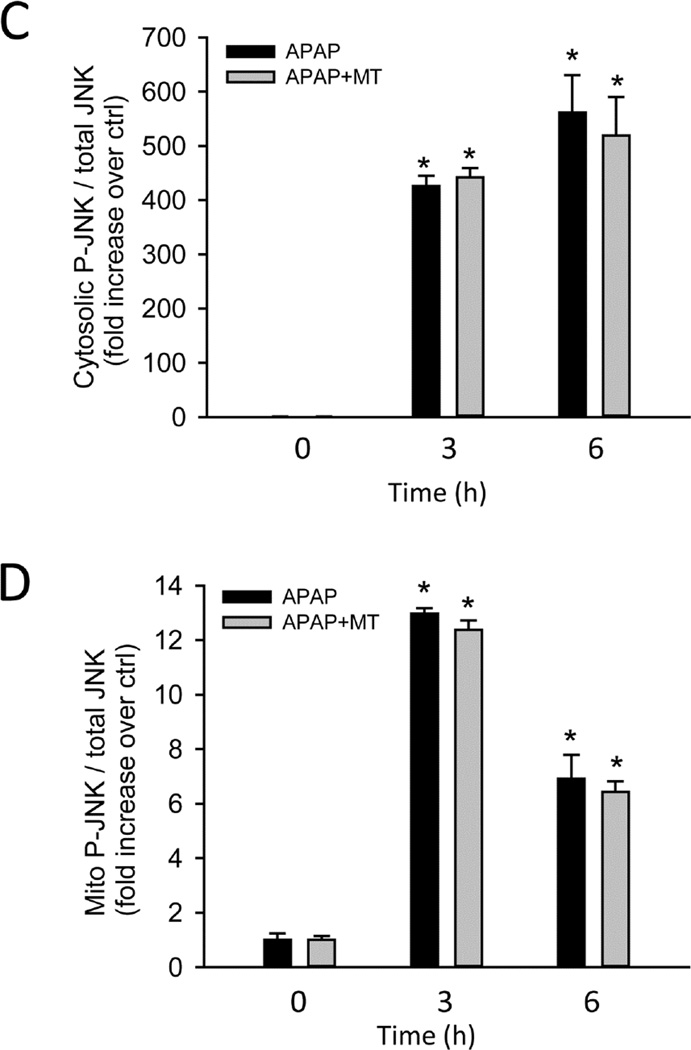
It has been hypothesized that the initial oxidant stress after APAP overdose is responsible for JNK activation, and it translocation to mitochondria where it further amplifies the mitochondrial oxidant stress (Hanawa et al. 2008; Saito et al. 2010a). Consistent with the previous studies, our western blots showed that APAP overdose induced JNK activation (phosphorylation) in the cytosol, and activated P-JNK translocated to the mitochondria as early as 3h (Fig. 4A); cytosolic JNK activation and to a lesser degree mitochondrial JNK translocation was maintained up to 6h (Fig. 4B). However, neither JNK activation nor the mitochondrial translocation was affected by MT treatment (Fig. 4A,B). Densitometric analysis of these western blots further confirmed these findings (Fig. 4C,D). This indicated that the protection by MT is independent of the activation of the JNK signaling pathway.
Figure 4. MT did not inhibit JNK activation or mitochondrial translocation.
Mice were treated with 300 mg/kg APAP, and 20 mg/kg MT or saline (10 ml/kg) was given 1.5 h later. Total JNK and P-JNK were measured in cytosolic and mitochondrial liver fractions at 3 h (A) or 6h (B) post-APAP. Densitometric analysis of P-JNK / JNK in the cytosolic (C) and mitochondrial (D) fractions. Bars represent means ± SEM for 3 mice. *p<0.05 vs. Ctrl.
Mito-Tempo attenuates APAP-induced mitochondrial dysfunction
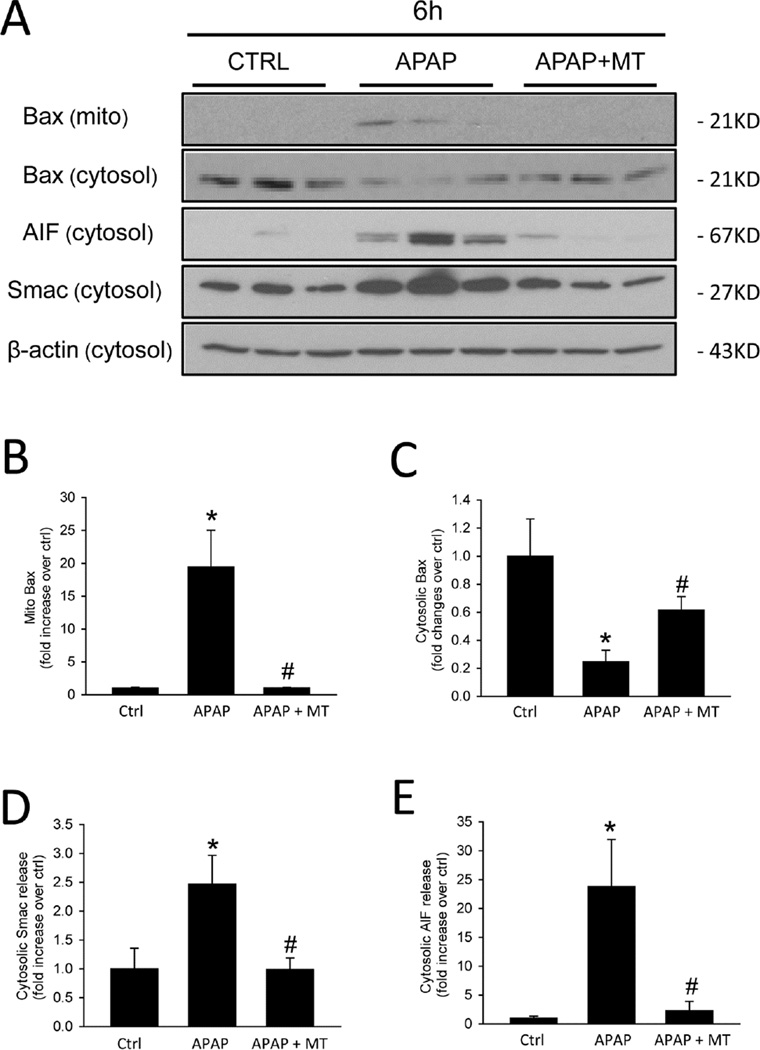
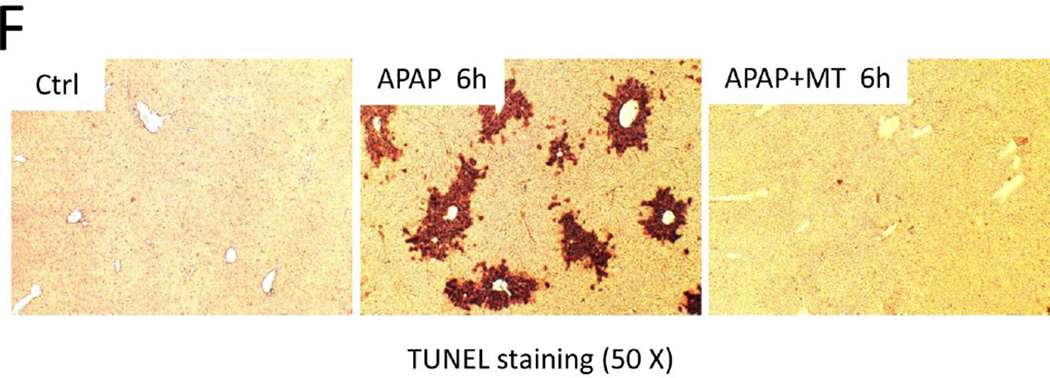
Bax was shown to translocate to mitochondria and to form pores in the mitochondrial outer membrane, resulting in the release of intermembrane proteins such as Smac and AIF after APAP overdose (Bajt et al. 2006; 2008). Consistent with these previous studies, we observed Bax translocation to mitochondria after APAP, as indicated by the appearance of Bax in the mitochondrial fraction and a reduction in the cytosolic fraction (Fig. 5A), which was confirmed by the corresponding densitometric analysis (Fig. 5B,C). MT almost completely prevented Bax translocation (Fig. 5A–C). As a result, the release of mitochondrial intermembrane proteins such as Smac and AIF was also completely eliminated (Fig. 5A,D,E). The corresponding DNA fragmentation as indicated by the TUNEL assay (Fig. 5F) is caused by release of mitochondrial endonucleases such as AIF and endonuclease G (Bajt et al. 2006; 2011; Cover et al. 2005). Consistent with the elimination of mitochondrial intermembrane protein release, MT treatment also prevented DNA fragmentation as indicated by the elimination of TUNEL-positive cells (Fig. 5F).
Figure 5. MT protected against mitochondrial dysfunction.
Mice were treated with 300 mg/kg APAP, and 20 mg/kg MT or saline (10 ml/kg) was given 1.5 h later. (A) Mitochondrial Bax and cytosolic Bax, Smac, AIF and β-actin measured by western blot at 6 h post-APAP. Densitometric analysis of mitochondrial Bax (B) and cytosolic Bax (C), Smac (D) and AIF (E) with β-actin as the loading control. (F) TUNEL staining of representative liver sections. Bars represent means ± SEM for 3 mice. *p<0.05 vs. Ctrl. #p<0.05 vs. APAP.
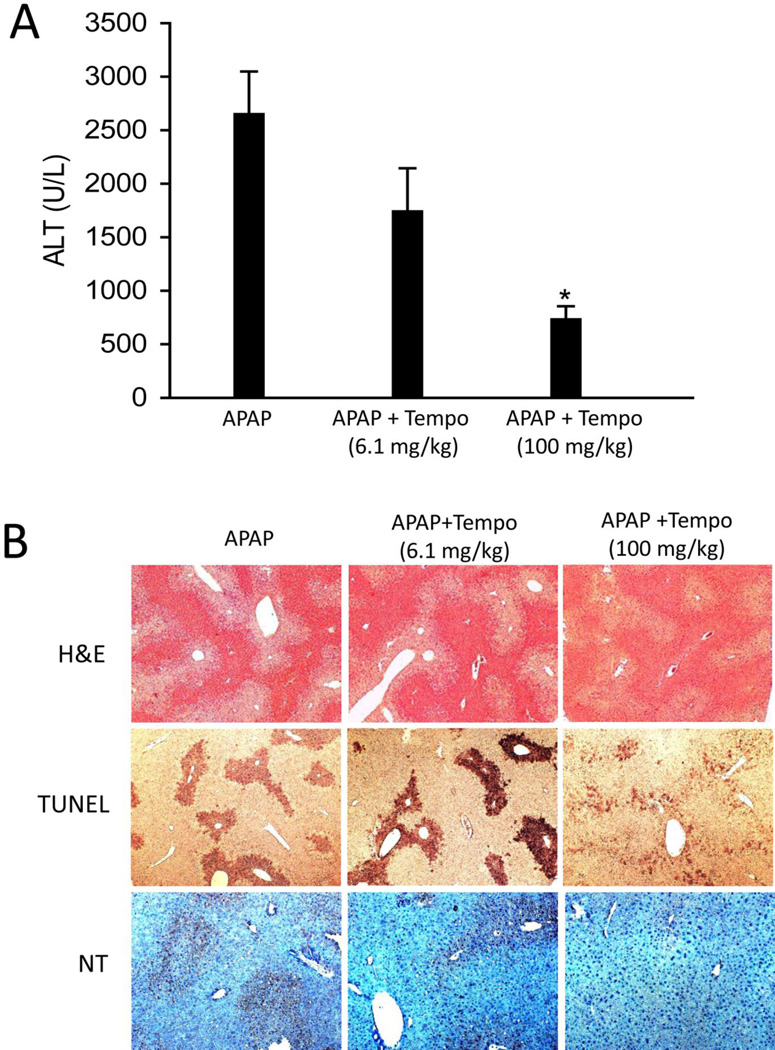
Protection by Tempo was less effective than Mito-Tempo
To investigate whether the mitochondria targeting property of MT is critical for its protection, we compared its efficacy with its analog Tempo, which retains the ROS scavenging moiety piperidine nitroxide of MT but lacks the part of the molecule (TPP+) that is responsible for targeting mitochondria. Considering the differences in molecular weight between MT (510.03 g/mol) and Tempo (156.25 g/mol), the amount of Tempo in 20 mg/kg MT is 6.1 mg/kg on a molar basis, which was subsequently used in our experiments. In contrast to the dramatic protection by MT, 6.1 mg/kg Tempo did not afford significant protection to APAP toxicity, as indicated by the plasma ALT activities (Fig. 6A) and H&E-stained sections and the TUNEL assay (Fig. 6B). NT staining was also not affected by the lower dose of TEMPO (Fig 6B). Interestingly, Tempo did significantly protect against APAP toxicity and reduced NT staining when a much higher dose (100 mg/kg) was administered (Fig. 6A, B), although this protection was still less when compared to a much lower dose of MT (20 mg/kg) (Fig. 1A, B).
Figure 6. Protection by Tempo was less effective than MT.
Mice were treated with 300 mg/kg APAP, and 6.1 or 100 mg/kg Tempo or saline (10 ml/kg) was given 1.5 h later. (A) Plasma ALT activity at 6 h post-APAP. (B) Representative liver sections (6 h APAP) were stained with H&E (50×), TUNEL (50×) and Nitrotyrosine (100×). Bars represent means ± SEM for n = 4 mice. *p<0.05 vs. APAP.
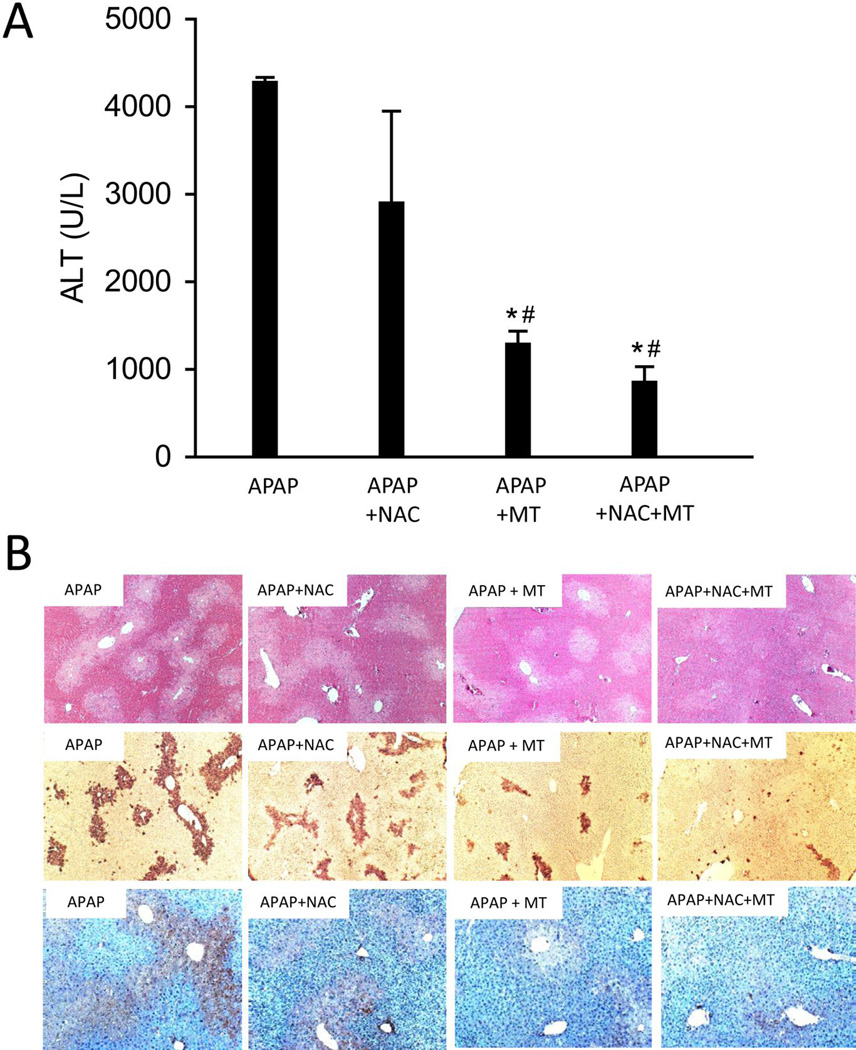
Therapeutic potential of Mito-Tempo against APAP hepatotoxicity
Until today, N-acetylcysteine (NAC) is the only available clinically approved antidote against APAP poisoning. However, it has to be given shortly after APAP overdose to achieve its greatest effect, while in reality most APAP overdose patients present for medical care relatively late (Larson 2007). Therefore, a pharmacological intervention that still works after the onset of liver injury would greatly benefit the late presenting patients. Our data showed that NAC as a 3h post-treatment provided only marginally lower average ALT values at 12h after APAP treatment (Fig. 7A,B). In contrast, MT administration 3h after APAP treatment significantly reduced liver injury as indicated by 70% lower ALT activities, less necrosis and less TUNEL-positive cells (Fig. 7A,B). Interestingly, the mice receiving both MT and NAC showed greater protection than those with NAC alone (Fig. 7A,B). These results indicate that MT can improve the beneficial effect of NAC, the current standard of care in the clinic. The significant protection by MT against APAP hepatotoxicity, especially when given as a late post-treatment, indicates its therapeutic potential for APAP overdose patients.
Figure 7. MT seemed to be a promising therapeutic agent for APAP poisoning.
Mice were treated with 300 mg/kg APAP, and saline (10 ml/kg), 20 mg/kg MT or 500 mg/kg NAC or MT+NAC was given 3 h later, plasma and liver tissue were harvested at 12 h post-APAP. (A) Plasma ALT activity. (B) Representative liver sections (12 h APAP) were stained with H&E (50×), TUNEL (50×) and Nitrotyrosine (100×). Bars represent means ± SEM for n = 3 – 7 mice. *p<0.05 vs. APAP. #p<0.05 vs. APAP + NAC.
DISCUSSION
The objective of this study was to evaluate the efficacy of the mitochondria-targeted antioxidant Mito-Tempo in a murine model of APAP hepatotoxicity. Our data demonstrated that MT attenuated the mitochondrial oxidant stress and subsequent mitochondrial dysfunction and thus protected against APAP hepatotoxicity. In addition, MT did not affect the metabolic activation of APAP, GSH recovery or the JNK signaling pathway. More importantly, MT showed a superior protective effect compared to NAC as a late post-treatment alone or together with NAC, suggesting that MT has therapeutic potential for APAP overdose patients.
Critical role of mitochondrial oxidant stress in APAP hepatotoxicity
Our current study demonstrated that MT, when administered 1.5 h after an overdose of APAP, dose-dependently attenuated liver injury. The protection was independent of any impact on the metabolic activation of APAP, the critical initiating step in the pathophysiology, as indicated by the lack of an effect on APAP-protein adducts formation. This result is not surprising as a dose of 300 mg/kg is largely metabolized and the peak of adduct formation is reached 1.5 h after APAP treatment (McGill et al. 2013). In addition, MT did not affect JNK activation and the translocation of activated (phosphorylated) JNK to the mitochondria. Since the initial mitochondrial dysfunction is thought to be responsible for activation of MAP kinases, which ultimately result in JNK activation (reviewed in Du et al. 2015b), the fact that MT did not prevent JNK activation suggests that MT did not affect these early signaling events. In contrast, MT eliminated NT staining and attenuated GSSG formation. It is known that NT protein adducts (Cover et al. 2005) and GSSG (Jaeschke 1990) are accumulating almost exclusively in mitochondria after APAP overdose. Thus, the elimination of NT staining is consistent with the effective promotion of superoxide dismutation by MT and therefore the prevention of nitrotyrosine formation. On the other hand, dismutation of superoxide yields oxygen and hydrogen peroxide, which can be detoxified by glutathione peroxidase resulting in formation of GSSG. This explains why MT was more effective in preventing the formation of NT adducts than GSSG. Nevertheless, since peroxynitrite is the more potent oxidant compared to hydrogen peroxide, the shift away from the more aggressive oxidant resulted in effective attenuation of the injury. These data are consistent with previous findings where the accelerated recovery of hepatic and mitochondrial GSH levels improved the scavenging capacity for peroxynitrite and hydrogen peroxide with similar protection (James et al. 2003; Knight et al. 2002; Saito et al. 2010b). In addition, mice with partial deficiency of SOD2, which resides in mitochondria, showed enhanced toxicity after APAP overdose (Fujimoto et al. 2009; Ramachandran et al. 2011). Since the mitochondrial oxidants generated during APAP toxicity inactivate SOD2 (Agarwal et al. 2011), this explains why an improved SOD activity in the mitochondria by MT treatment effectively prevents mitochondrial dysfunction and cell necrosis.
The mitochondria-targeted antioxidant is most effective against APAP hepatotoxicity
When MT was compared to Tempo, the identical antioxidant lacking a mitochondrial target signal, it was obvious that on a molar basis, MT was considerably more effective in protecting against the mitochondrial oxidant stress during APAP overdose. This supports the conclusion that the active transport of MT into mitochondria achieves a higher concentration in the target organelle and thus higher efficacy. It actually required 20-times the dose of Tempo to cause significant but still less protection than MT. Together these results indicate the high efficacy of a SOD-mimetic that can be targeted to mitochondria in protecting against the selective mitochondrial oxidant stress during APAP-induced liver injury.
MT prevents mitochondrial dysfunction and DNA fragmentation
DNA fragmentation is a hallmark of APAP hepatotoxicity (Lawson et al. 1999; Ray et al. 1990). Previous studies have shown that nuclear DNA damage is related to mitochondrial dysfunction (Cover et al. 2005), which causes the release and nuclear translocation of mitochondrial intermembrane proteins AIF and endonuclease G (Bajt et al. 2006). The release of AIF and endonuclease G is caused initially by formation of a bax pore in the outer mitochondrial membrane and later, after the MPT, by rupture of the outer membrane due to matrix swelling (Bajt et al. 2008). Since MT prevented mitochondrial bax translocation, the release of intermembrane proteins such AIF and nuclear DNA strand breaks as indicated by the TUNEL assay, these findings are consistent with the attenuation of mitochondrial dysfunction by MT.
Overall, the data suggest that preventing peroxynitrite formation by MT prevents the oxidant stress-mediated MPT and as a consequence all down-stream events such as intermembrane protein release, their nuclear translocation and nuclear DNA damage. Ultimately, the severe mitochondrial dysfunction and the nuclear DNA fragmentation are the cause of necrotic cell death (Jaeschke et al. 2012).
MT is more effective than N-acetylcysteine
NAC is currently the only approved clinical antidote against APAP overdose (Polson and Lee 2005). NAC treatment supports hepatic GSH synthesis and protects against APAP toxicity by providing GSH to scavenge the reactive metabolite and preventing protein adduct formation (Corcoran et al. 1985). Thus, NAC is most effective when administered during the metabolism phase of APAP (Xie et al. 2015; Smilkstein et al. 1988). Unfortunately, many patients present after the metabolism is over and the injury is already in progress (Larson 2007). During this time, NAC is still somewhat effective (Smilkstein et al. 1988). However, GSH is now being used to scavenge peroxynitrite and reactive oxygen in mitochondria (James et al. 2003; Knight et al. 2002; Saito et al. 2010b). Furthermore, some of the excess NAC is being converted to Krebs cycle intermediates supporting mitochondrial energy metabolism (Saito et al. 2010b). Although the mouse model of APAP overdose mimicks closely events in humans (McGill and Jaeschke 2014), overall the time to liver injury is shorter in mice. Thus, the therapeutic window for NAC in mice is between 0–2 h after APAP and the protective effect of NAC disappears by 3 h (James et al. 2003; Knight et al. 2002). Interestingly, MT administered 3 h after APAP was still highly effective making it a better therapeutic intervention than NAC at later time points. Furthermore, there was some additive effect between MT and NAC. This is possible because the mechanism of protection is different. MT prevents peroxynitrite formation and NAC, after GSH synthesis in the cytosol and uptake into mitochondria, can scavenge peroxynitrite and hydrogen peroxide.
NAC is administered intravenously (i.v.) in clinical practice. In the mouse model of APAP hepatotoxicity, both i.v. and i.p. injection of NAC have been shown to effectively protect against APAP toxicity (Saito et al., 2010b; James et al., 2003). Since i.p. injection is less stressful to the animal, both NAC and MT were injected i.p. in our study, which may need to be considered when translating these findings to the human pathophysiology.
Another issue to consider is the difference in the time course of APAP hepatotoxicity in mice and humans. It is known that the development of liver injury after APAP overdose is much more delayed in humans compared to mice. This fact has to be taken into account when defining "late post-treatment" therapy. In humans, the liver injury is typically first seen around 24h with peak injury at 48–96h after APAP overdose (Larson, 2007; McGill et al., 2012; Rumack, 1983); this time course of cell death can also be reproduced in primary human hepatocytes exposed to APAP (Xie et al., 2014). In mice, the injury normally begins at 3h and peaks around 12h post-APAP (McGill et al., 2013). The reason for this delay in humans may be related to the delayed mitochondrial protein binding and delayed JNK activation as we have shown in primary human hepatocytes (Xie et al., 2014). Consequently, NAC is very effective in patients when administered within 8h of APAP ingestion (before the injury starts) (Smilkstein et al., 1988). Again, this can be reproduced in human hepatocytes where NAC is 100% effective at 6h and partially effective at 15h after APAP exposure (Xie et al., 2014). In contrast, NAC is only highly effective up to 2h after APAP in mice (James et al., 2003; Knight et al., 2002; Saito et al., 2010b). When administered at 3h after APAP, the “protection” (if any) is very marginal and variable because the injury is already in progress. In this scenario, treatment of NAC or MT at 3h post-APAP can be considered as a "late post-treatment" in mice comparable to a 24h time point in humans. Since MT is still effective in mice when given at 3h post-APAP while NAC is not, MT could be regarded as a promising therapeutic agent for late-presenting patients in the clinic.
Summary and Conclusions
In summary, we demonstrated that MT protected against APAP hepatotoxicity in mice. It did not inhibit metabolic activation of APAP but dose-dependently attenuated mitochondrial oxidant stress and prevented the following mitochondrial dysfunction. Comparison of the protection by MT to its analog Tempo highlights the importance of mitochondrial oxidant stress in the development of APAP toxicity. Our study also demonstrated that MT as a treatment alone or together with NAC offers a better protection than NAC alone, which supports MT as a therapeutic option for treatment of APAP overdose or acute liver failure in patients.
Supplementary Material
Acknowledgments
This investigation was supported in part by the National Institutes of Health grant R01 DK102142 and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health. Additional support came an award from the Biomedical Research Training Program (BRTP) from the University of Kansas Medical Center (to K.D.).
List of Abbreviations
- AIF
apoptosis-inducing factor
- ALT
alanine aminotransferase
- APAP
acetaminophen
- CYP
cytochrome P450
- GSH
glutathione
- GSSG
glutathione disulfide
- HPLC-ECD
high-pressure liquid chromatography with electrochemical detection
- MPT
mitochondrial permeability transition
- MT
Mito-Tempo
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- ROS
reactive oxygen species
- Smac
second mitochondria-derived activator of caspases
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Compliance with ethical standards
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther. 2011;337:110–118. doi: 10.1124/jpet.110.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40:585–592. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Choumar A, Tarhuni A, Lettéron P, Reyl-Desmars F, Dauhoo N, Damasse J, Vadrot N, Nahon P, Moreau R, Pessayre D, Mansouri A. Lipopolysaccharide-induced mitochondrial DNA depletion. Antioxid Redox Signal. 2011;15:2837–2854. doi: 10.1089/ars.2010.3713. [DOI] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Corcoran GB, Racz WJ, Smith CV, Mitchell JR. Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J Pharmacol Exp Ther. 1985;232:864–872. [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly PJ, Walker RM, Racz WJ. Inhibition of mitochondrial respiration in vivo is an early event in acetaminophen-induced hepatotoxicity. Arch Toxicol. 1994;68:110–118. doi: 10.1007/s002040050043. [DOI] [PubMed] [Google Scholar]
- Du K, McGill MR, Xie Y, Jaeschke H. Benzyl alcohol protects against Acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes but causes mitochondrial dysfunction and cell death at higher doses. Food Chem Toxicol. 2015a;86:253–261. doi: 10.1016/j.fct.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Xie Y, McGill MR, Jaeschke H. Pathophysiological significance of c-jun N-terminal kinase in acetaminophen hepatotoxicity. Expert Opin Drug Metab Toxicol. 2015b;11:1769–1779. doi: 10.1517/17425255.2015.1071353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Williams CD, McGill MR, Jaeschke H. Lower susceptibility of female mice to acetaminophen hepatotoxicity: Role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol Appl Pharmacol. 2014;281:58–66. doi: 10.1016/j.taap.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol. 2009;37:193–200. doi: 10.1177/0192623308329282. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol. 1998;11:604–607. doi: 10.1021/tx9800349. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- James LP, McCullough SS, Lamps LW, Hinson JA. Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci. 2003;75:458–467. doi: 10.1093/toxsci/kfg181. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62(2):212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–548. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999 May 1;156(3):179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- LoGuidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology. 2011 Sep 2;54(3):969–978. doi: 10.1002/hep.24464. [DOI] [PubMed] [Google Scholar]
- Manthripragada AD, Zhou EH, Budnitz DS, Lovegrove MC, Willy ME. Characterization of acetaminophen overdose-related emergency department visits and hospitalizations in the United States. Pharmacoepidemiol. Drug Saf. 2011;20:819–826. doi: 10.1002/pds.2090. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Mechanistic biomarkers in acetaminophen-induced hepatotoxicity and acute liver failure: from preclinical models to patients. Expert Opin Drug Metab Toxicol. 2014 Jul;10(7):1005–1017. doi: 10.1517/17425255.2014.920823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine and N-ethylmaleimide. Toxicol Mech Methods. 2015 Oct;25(8):589–595. doi: 10.3109/15376516.2015.1094844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002 Apr;30(4):446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding WX. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol Sci. 2012;127:438–450. doi: 10.1093/toxsci/kfs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306:F734–F743. doi: 10.1152/ajprenal.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson J, Lee WM American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;27:432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011a;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2011b;251:226–233. doi: 10.1016/j.taap.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SD, Sorge CL, Raucy JL, Corcoran GB. Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 1990;106:346–351. doi: 10.1016/0041-008x(90)90254-r. [DOI] [PubMed] [Google Scholar]
- Rumack BH. Acetaminophen overdose. Am J Med. 1983;75:104–112. doi: 10.1016/0002-9343(83)90240-1. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010a;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010b;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- Trnka J, Blaikie FH, Smith RA, Murphy MP. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic Biol Med. 2008;44:1406–1419. doi: 10.1016/j.freeradbiomed.2007.12.036. [DOI] [PubMed] [Google Scholar]
- Wang A, Keita AV, Phan V, McKay CM, Schoultz I, Lee J, Murphy MP, Fernando M, Ronaghan N, Balce D, Yates R, Dicay M, Beck PL, MacNaughton WK, Söderholm JD, McKay DM. Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis. Am J Pathol. 2014;184:2516–2527. doi: 10.1016/j.ajpath.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Cook SF, Sharpe MR, Winefield RD, Wilkins DG, Rollins DE, Jaeschke H. Time course of acetaminophen-protein adducts and acetaminophen metabolites in circulation of overdose patients and in HepaRG cells. Xenobiotica. 2015;45:921–929. doi: 10.3109/00498254.2015.1026426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol. 2014;279:266–274. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HM, Ramachandran A, Bajt ML, et al. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol Sci. 2010;117:515–523. doi: 10.1093/toxsci/kfq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.