Abstract
Obese and sedentary persons have an increased risk for cancer, but underlying mechanisms are poorly understood. Angiogenesis is common to adipose tissue formation and remodeling, and to tumor vascularization. 439 overweight/obese, healthy, postmenopausal women (body mass index (BMI)>25 kg/m2) aged 50-75 years, recruited between 2005-2008 were randomized to a 4-arm 12-month randomized controlled trial, comparing a caloric restriction diet arm (goal: 10% weight-loss, N=118), aerobic exercise arm (225 min/week of moderate-to-vigorous activity, N=117), a combined diet+exercise arm (N=117), or control (N=87) on circulating levels of angiogenic biomarkers. Vascular endothelial growth factor (VEGF), plasminogen activator inhibitor-1 (PAI-1); and pigment epithelium-derived factor (PEDF) were measured by immunoassay at baseline and 12-months. Changes were compared using generalized estimating equations, adjusting for baseline BMI age, and race/ethnicity. Participants randomized to the diet+exercise arms had statistically significantly greater reductions in PAI-1 at 12-months compared to controls (-19.3% vs. +3.48% respectively, P<0.0001). Participants randomized to the diet and diet+exercise arms had statistically significantly greater reductions in PEDF (-9.20%, -9.90% respectively, both P<0.0001) and VEGF (-8.25%, P=0.0005; -9.98%, P<0.0001, respectively) compared to controls. There were no differences in any of the analytes in participants randomized to the exercise arm compared to controls. Increasing weight-loss was statistically significantly associated with linear trends of greater reductions in PAI-1, PEDF and VEGF. Weight-loss is significantly associated with reduced circulating VEGF, PEDF and PAI-1, and could provide incentive for reducing weight as a cancer prevention method in overweight and obese individuals.
Keywords: Angiogenesis, exercise, weight-loss VEGF, PAI-1, PEDF
Introduction
Overweight and obesity is associated with increased risk of a variety of cancers,(1) and regular physical activity is associated with reduced cancer risk.(2) A largely unexplored mechanism that could link obesity with cancer risk is angiogenesis, a process where new blood vessels form from pre-existing vessels allowing tissues to expand, regulated by maintaining a balance between pro- and anti-angiogenic factors.(3) Angiogenesis plays an important role in obesity; adipose tissue is highly plastic, and requires vascularization in order to expand.(4). Here, we investigated the pro-angiogenic Vascular Endothelial Growth Factor (VEGF) and Plasminogen Activator Inhibitor type-1 (PAI-1), and the anti-angiogenic Pigment Epithelium-Derived Factor (PEDF), as they have been extensively studied in overweight/obesity, and have also been implicated in tumorigenesis in both animal models and in epidemiological studies, extending our previous investigation of physical activity on these biomarkers.(5)
Angiogenesis blockade is an active area of clinical and translational research.(6,7) Most of the developed drugs target VEGF and its related pathways, including bevacizumab, a monoclonal antibody that inhibits VEGF-A, which has been approved for use by the FDA for treatment of a number of cancers.(8) However, given the potential adverse effects of these compounds,(9) they have not been proposed for the cancer prevention setting. Low-risk and low-cost methods for reducing angiogenesis, therefore, could have important public health benefits.
VEGF is a key regulator of angiogenesis and vascular permeability.(10) VEGF is elevated in the obese state (11,12), although mice overexpressing VEGF were protected against diet-included obesity and insulin resistance.(13) VEGF can also promote the growth, survival, migration and invasion of cancer cells.(14) PAI-1 is a serine protease inhibitor (serpin) and is elevated in overweight and type-2 diabetic patients.(15,16) It promotes angiogenesis,(17,18) and is a prognostic marker for poor outcome in a number of cancers including breast.(19) PEDF, an adipokine and serpin, has anti-angiogenic properties and is active against a wide range of angiogenic stimuli, including VEGF.(20) It has broad anti-tumor activity, and reduced levels of PEDF are associated with a worse prognosis in a variety of different cancers.(21) However, it is also associated with the presence of the metabolic syndrome, and contributes to the development of insulin resistance in obesity.(22,23)
Excessive accumulation of adipose tissue creates a pro-tumorigenic environment, characterized by inflammation, macrophage invasion and increased angiogenesis.(24) In this environment, tumor cells can circumvent inhibitory signals and harness these dysregulated processes to proliferate, and form new blood vessels resulting in inappropriate growth and tissue invasion.(24)(25) Further expansion of established dormant avascular tumors requires initiation of angiogenesis, or the ‘angiogenic switch’, allowing the tumor to transition to exponential growth.(26)
Previously, we found that women who lost at least 1.85% of baseline fat-mass (corresponding to median levels) in a 12-month exercise intervention experienced significant reductions in some biomarkers of angiogenesis,(5) compared with sedentary controls. To extend this research, we investigated the independent and combined effects of dietary weight-loss and exercise on circulating levels of VEGF, PAI-1 and PEDF, in the context of a completed 12-month randomized controlled trial, the Nutrition & Exercise for Women (NEW) trial. We randomly assigned 439 postmenopausal overweight/obese women to a reduced calorie dietary weight-loss program, an aerobic exercise program, a combined dietary weight-loss plus exercise program, or to a control arm. We hypothesized that women randomized to dietary weight-loss, with or without exercise, would have significant decreases in VEGF and PAI-1, and increases in PEDF, compared with controls.
Methods
This study is ancillary to the NEW (www.clinicaltrials.gov NCT00470119) study, a 12-month randomized controlled trial that test the effects of caloric restriction and/or exercise on circulating sex steroid hormones in healthy overweight postmenopausal women. The study was carried out in the FHCRC, Seattle, Washington, USA, and performed with the approval of the FHCRC Institutional Review Board, in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services. Written informed consent was obtained from each participant.
Study Population
The trial is described in detail elsewhere.(27) Briefly, 439 postmenopausal, healthy overweight (BMI>25 kg/m2), sedentary women, aged 50-75 years, not taking hormonal therapy, were recruited through media and mass mailings and were enrolled in the study between 2005-2008; 12-month follow-up for all participants was completed in 2009. Eligible participants were randomly assigned to a (i) reduced-calorie dietary modification intervention (N=118); (ii) moderate-to-vigorous intensity aerobic exercise intervention (N=117); (iii) combined diet and exercise intervention (N=117); or (iv) control (no intervention) (N=87). Exclusion criteria included: >100 min/week of moderate physical activity; diagnosed serious medical condition(s); postmenopausal hormone use; consumption of >2 alcoholic drinks/day; current smoking; participation in another structured weight-loss program; contraindication to participation (e.g. abnormal exercise tolerance test). Permuted block randomization was used to achieve a proportionally smaller control group, stratified according to BMI (≥/<30 kg/m2) and race/ethnicity. The random assignment was generated by a computerized program, written by the study statistician, and run by the study manager to assign eligible participants to study arms. Investigators and laboratory staff were blinded to randomization arm.
Interventions
The dietary intervention was a modification of the Diabetes Prevention Program and LookAHEAD lifestyle behavior-change programs with goals of 1200-2000 kcal/day, <30% daily calories from fat, 10% weight-loss by 6-months, and weight maintenance thereafter. Participants had at least two individual meetings with a dietician followed by weekly group meetings for 6-months; thereafter they attended monthly, with biweekly phone/email contact. Intervention adherence was defined by percent of in-person nutrition session attendance.
The exercise intervention goal was 45 minutes of moderate-to-vigorous (≥4 metabolic equivalents [METs]) intensity exercise at a target heart rate of 70-85% observed maximum, 5 days/week by week 7. Participants attended three facility-based supervised sessions/week and exercised 2 days/week at home. They recorded exercise mode, duration, peak heart-rate, and perceived exertion at each session. Activities of ≥4 METs(28) counted towards the prescribed target.
Controls were asked not to change their diet or exercise habits.
Blood Specimen Collection and Processing
Fasting (12 hours) venous blood samples (50 mL) were collected during clinic visits at baseline (pre-randomization) and 12-months, when the study was completed. Participants refrained from alcohol (48 hours), vigorous exercise or NSAID use (24 hours) prior to fasting venous blood collection (50mL) at baseline and 12-months. Blood was processed within 1 hour, and stored at -70°C.
Assays
VEGF, PEDF (serum) and PAI-1 (plasma) were assayed at the Clinical and Epidemiologic Research Laboratory, at the Department of Laboratory Medicine, Boston Children's Hospital, Boston, MA, using Enzyme Linked Immunosorbent Assays from R&D Systems (Minneapolis, MN). Duplicate pooled blood samples were included for quality assurance (QA) purposes and to assess inter and intra-assay coefficient of variation (CV). Baseline and 12-month samples from each individual were included in the same batch, and participants' samples were randomly placed across batches. Laboratory personnel were blinded with regard to subject and QA sample identity. Inter- and intra-assay CVs for each assay were: VEGF 7.3% and 5.9%; PAI-1 8.2% and 7.9%; and PEDF 9.3% and 6.0%.
Covariates
All study measures were obtained at baseline and 12 months by trained personnel blinded to participants' randomization status. Height and weight were measured and body mass index (BMI, kg/m2) calculated. Body composition (fat mass and percent body fat) was measured by DXA (Dual-energy X-ray absorptiometry) whole-body scanner (GE Lunar, Madison, WI). Cardiorespiratory fitness (VO2max) was assessed using a maximal graded treadmill test according to a modified branching protocol.(29)
Questionnaires collected information on demographics, medical history, dietary intake, supplement use and physical activity patterns. Other covariates including adipokines, sex steroid hormones, biomarkers of inflammation, lipids, blood counts, and telomere length were measured as described previously. (30-35)
Statistical Analyses
Partial Pearson correlation coefficients were calculated between baseline biomarker measures, with Bonferroni correction for multiple testing (0.05/99=significant at P<0.0005).
Descriptive data are presented as geometric means (95% confidence intervals (CI)). Mean changes in analytes from baseline to 12-months, stratified by arm, were computed. The intervention effects on these variables were examined based on the assigned treatment at randomization, regardless of adherence or study retention (i.e., intent-to-treat). Mean 12-month changes in the intervention arm were compared to controls using the generalized estimating equations modification of linear regression to account for intra-individual correlation over time. Intervention effects are presented as both absolute and relative change. Bonferroni correction adjusted for multiple comparisons (2-sided α=0.05/3=0.02 for 3 comparisons) for the primary analysis. We used the method of last observation carried forward (LOCF) to deal with missing data at 12-months.
Changes in body composition and VO2max levels were calculated and used to stratify observed changes in analytes between arms at 12 months. Weight-loss was categorized as no change/gained any weight (referent); lost <5% of baseline weight; and lost >5% of baseline weight. Participants with missing 12-month data were categorized as no change/gained weight. Changes in VO2max were calculated and categorized as tertiles (increased <3.5%; increased >3.5-14.3%; increased >14.3%); participants with missing VO2max data at 12 months were classified as increased <3.5%. Percent fat loss was categorized as: no change/gaining any fat; decreasing <2.6%; decreasing 2.6-6.4%; and decreasing >6.4%, corresponding to tertiles of change. Participants missing 12-month percent body-fat, were categorized as no change/gaining fat. Fat loss, weight change and VO2max levels in the control group were added as separate categories.
In secondary analyses, we examined changes in analytes for each intervention arm in subgroups of participants compared to controls by the above categories of weight-loss, change in VO2max, and change in % body fat.
All models were adjusted for age, baseline BMI (<30kg/m2, >30kg/m2) and race/ethnicity. All statistical tests were two sided. Statistical analyses were performed using SAS software (version 8.2, SAS Institute Inc., Cary, NC).
Results
Participants
At 12-months, 399 of 439 participants completed physical exams and provided a blood sample, 397 underwent a DXA scan, and 371 completed a VO2max test; 39 did not complete the study (Fig. 1). One participant randomized to diet+exercise was excluded from analysis due to missing baseline blood measures. At baseline, participants were on average 57.9 years, with an average BMI of 30.9 kg/m2, and were predominantly non-Hispanic Whites; (Table 1).
Figure 1. CONSORT diagram of the Nutrition and Exercise for Women (NEW) trial.
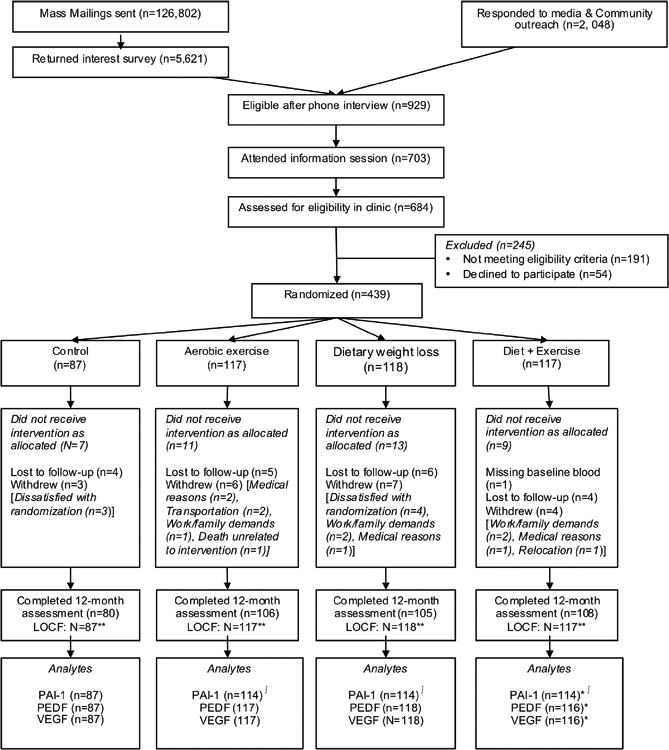
*Missing baseline blood sample (N=1): omitted from analysis
∫Missing 12-month plasma sample (N=10 in total)
**LOCF: Missing data at 12 months were imputed using the method last observation carried forward for VO2max, percent body fat, and weight
Table 1. Baseline characteristics of NEW study participants.
| Control (N=87) | Diet (N=118) | Exercise (N=117) | Diet+Exercise (N=117) | All Participants (N=438) | |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | |||||
|
| |||||
| Age (years) | 57.4 (4.4) | 58.1 (6.0) | 58.1 (5.0) | 58.0 (4.5) | 57.9 (5.0) |
|
| |||||
| BMI (kg/m2) | 30.7 (3.9) | 31.1 (3.9) | 30.7 (3.7) | 31.0 (4.3) | 30.9 (4.0) |
|
| |||||
| Waist circumference (cm) | 94.83 (10.2) | 94.61 (10.2) | 95.05 (10.1) | 93.71 (9.9) | 94.5 (10.1) |
|
| |||||
| VO2max (kg/mL/min)) | 23.1 (4.1) | 22.7 (3.8) | 22.5 (4.1) | 23.6 (4.1) | 22.9 (4.03 |
|
| |||||
| Usual physical activity (min/wk) | 23.8 (41.2) | 33.6 (45.5) | 37.7 (43.7) | 32.4 (42.9) | 32.4 (43.6) |
|
| |||||
| Total calories (kcal/d) | 1988 (669) | 1884 (661) | 1986 (589) | 1894 (638) | 1935 (637.9) |
|
| |||||
| N (%) | |||||
|
| |||||
| Race/Ethnicity | |||||
| Non-Hispanic White | 74 (85.1) | 101 (85.6) | 98 (83.8) | 100 (85.4) | 372 (84.9) |
| African American | 6 (6.9) | 9 (7.6) | 15 (12.8) | 5 (4.3) | 35 (8.0) |
| Hispanic/Latino | 3 (3.4) | 2 (1.7) | 2 (1.7) | 5 (4.3) | 12 (2.7) |
| Other | 4 (4.6) | 6 (5.1) | 2 (1.7) | 7 (6.0) | 19 (4.3) |
|
| |||||
| Education | |||||
| College Graduate and Above | 59 (67.8) | 76 (64.4) | 70 (59.8) | 81 (69.8) | 286 (65.3) |
|
| |||||
| Smoker (ever) | 32 (36.8) | 55 (46.6) | 47 (40.2) | 47 (40.5) | 181 (41.3) |
|
| |||||
| Mean (SD) | |||||
|
| |||||
| PAI-1 (ng/mL) | 8.9 (4.6) | 8.1 (5.0) | 7.6 (4.3) | 7.9 (4.9) | 8.1 (4.7) |
|
| |||||
| PEDF (μg/mL) | 10.9 (1.9) | 10.9 (2.2) | 10.6 (1.8) | 10.7 (2.3) | 10.8 (2.1) |
|
| |||||
| VEGF (pg/mL) | 391.4 (248.2) | 369.7 (255.6) | 377.3 (229.9) | 393.9 (266.0) | 382.5 (249.7) |
Intervention Fidelity
Data on intervention adherence, weight-loss, and body composition changes in this trial have been previously reported.(27) The mean weight change was -2.4% (p=0.03; exercise arm), -8.5% (p<0.001; diet), and -10.8% (p<0.001; diet+exercise) vs. -0.8% among controls. Women in all intervention groups significantly reduced % body fat (all p<0.001) compared to controls.
Percent of daily calories from fat decreased in both the diet and diet+exercise arms (-6.7% and -8.0%, respectively). In both diet groups, women attended an average of 27 diet counseling sessions (86%). Women randomized to exercise participated in moderate-to-vigorous activity for a mean (SD) of 163.3 (70.6) minutes/week, while women randomized to diet+exercise participated for 171.5 (62.9) minutes/week. Both groups significantly increased average pedometer steps/day and VO2max compared to baseline.
Baseline Correlations
After Bonferroni correction, both PAI-1 and PEDF correlated strongly and statistically significantly (hereon ‘significantly’) (all P<0.0005) with the majority of markers associated with overweight (anthropometrics), triglyceride levels and insulin dysregulation, and negatively with adiponectin, ghrelin and HDL cholesterol (Table 2). PEDF correlated positively and significantly with CRP and SAA. Both had significant and negative associations with SHBG; with red blood cell counts and hematocrit levels (PAI-1 only); and with white blood cell counts (PEDF only); all P<0.001. VEGF did not correlate significantly with any baseline variables, with the exception of blood platelets (P<0.0001).
Table 2. Baseline correlations between angiogenesis biomarkers and other study covariates.
| PAI-1* | PEDF** | VEGF** | ||||
|---|---|---|---|---|---|---|
| Rho | P-value | Rho | P-value | Rho | P-value | |
| Anthropometrics | ||||||
| Percent body-fat | 0.13 | 0.007 | 0.20 | <0.0001 | 0.06 | 0.22 |
| BMI | 0.29 | <0.0001 | 0.36 | <0.0001 | 0.07 | 0.19 |
| Weight | 0.24 | <0.0001 | 0.33 | <0.0001 | 0.09 | 0.09 |
| Adipokines/Insulin etc. | ||||||
| Homeostatic Model Assessment | 0.40 | <0.0001 | 0.36 | <0.0001 | -0.05 | 0.33 |
| C-Peptide | 0.48 | <0.0001 | 0.45 | <0.0001 | -0.02 | 0.65 |
| Adiponectin | -0.23 | <0.0001 | -0.16 | 0.0009 | -0.04 | 0.44 |
| Ghrelin | -0.27 | <0.0001 | -0.20 | <0.0001 | -0.13 | 0.009 |
| Leptin | 0.25 | <0.0001 | 0.38 | <0.0001 | 0.11 | 0.03 |
| Insulin-like grown factor-1 | -0.04 | 0.38 | -0.06 | 0.22 | -0.07 | 0.17 |
| IGF- binding protein-3 | 0.20 | <0.0001 | 0.13 | 0.007 | 0.02 | 0.72 |
| Inflammation associated biomarkers | ||||||
| C-reactive protein (CRP) | 0.14 | 0.004 | 0.26 | <0.0001 | 0.08 | 0.12 |
| Serum amyloid protein A (SAA) | 0.07 | 0.15 | 0.17 | 0.0004 | 0.02 | 0.66 |
| TNF-alpha | 0.05 | 0.34 | 0.07N=437 | 0.12 | 0.02 N=437 | 0.73 |
| IL-10 | -0.07 | 0.19 | -0.07N=435 | 0.17 | -0.02 N=435 | 0.73 |
| IL-6 | 0.04 | 0.38 | 0.15 | 0.002 | 0.06 | 0.19 |
| Lipoproteins and triglycerides | ||||||
| Triglycerides | 0.32 | <0.0001 | 0.26 N=434 | <0.0001 | 0.02 N=434 | 0.69 |
| Low density lipoprotein | 0.03 | 0.66 | -0.04 N=434 | 0.47 | -0.07 N=434 | 0.12 |
| High density lipoprotein (HDL) | -0.21 | <0.0001 | -0.20 N=434 | <0.0001 | -0.02 N=434 | 0.69 |
| Oxidized LDL | 0.09 | 0.07 | -0.04 N=434 | 0.46 | -0.05 N=434 | 0.33 |
| Sex steroid hormones | ||||||
| Androstenedione | 0.08 | 0.12 | 0.02 | 0.63 | 0.06 | 0.19 |
| Estrone | 0.13 | 0.004 | 0.19 | 0.0001 | 0.09 | 0.05 |
| Estradiol | -0.01 | 0.77 | 0.07 | 0.15 | 0.06 | 0.19 |
| Testosterone | -0.07 | 0.15 | -0.02 | 0.61 | 0.01 | 0.80 |
| Sex hormone binding globulin | -0.28 | <0.0001 | -0.24 | <0.0001 | -0.11 | 0.02 |
| Blood cell counts and indices | ||||||
| Hematocrit | 0.23 N=427 | <0.0001 | 0.11 N=436 | 0.02 | -0.01 N=436 | 0.92 |
| Mean corpuscular volume | -0.10 | 0.03 | -0.18 | 0.0002 | 0.01 | 0.88 |
| Red blood cell count | 0.27 N=428 | <0.0001 | 0.22 N=437 | <0.0001 | -0.01 N=437 | 0.97 |
| White blood cell count | 0.12 | 0.02 | 0.17 | 0.0004 | 0.16 | 0.0009 |
| Lymphocytes | 0.04 N=428 | 0.39 | -0.03 N=437 | 0.48 | -0.02 N=437 | 0.61 |
| Mean corpuscular hemoglobin | -0.12 N=428 | 0.02 | -0.14 | 0.004 | -0.04 | 0.36 |
| Platelets | 0.11 N=428 | 0.03 | 0.02 N=437 | 0.78 | 0.23 N=437 | <0.0001 |
| Mean corpuscular hemoglobin concentration | -0.07 | 0.16 | 0.04 | 0.36 | -0.14 | 0.003 |
| Telomere length | ||||||
| Telomere length | 0.04N=427 | 0.48 | 0.03N=436 | 0.56 | 0.01 N=436 | 0.78 |
Correlations between continuous variables were estimated using the Pearson correlation. Significance was set at P<0.0005 after Bonferroni correction (P=0.05/99, for 33 × 3 comparisons).
N=429, except where indicated by superscript
All N=438 except where indicated by superscript
Intervention effects
Participants randomized to the diet+exercise arms had statistically significantly greater reductions in PAI-1 at 12-months compared to controls (-19.3% vs. +3.48% respectively, P<0.0001; Table 3). Participants randomized to the diet (-9.20%) and diet+exercise (-9.90%) arms had significantly greater reductions in PEDF compared to those randomized to the control arm (+0.18%), all P<0.0001. Finally, participants randomized to the diet (-8.25%, P=0.0005) and diet+exercise (-9.98%, <0.0001) arms had significantly greater reductions in VEGF compared to those randomized to the control arm (-1.21%). There were no statistically significant differences in any of the analytes in participants randomized to the exercise arm, compared to controls.
Table 3. Effects of dietary weight-loss, exercise, and combined dietary weight-loss and exercise on PAI-1, PEDF and VEGF in overweight and obese postmenopausal women.
| Biomarker | Study Arm | Time-point | Change | P-valuea | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Month | Absolute change (%) | Relative change 12MΔ (I-C)c | Unadjusted | Adjustedb | ||||
| N | Mean (95% CI) | N | Mean (95% CI) | P | P | ||||
| PAI-1 (ng/mL) | Control | 87 | 7.88 (7.09-8.76) | 87 | 8.15 (7.30-9.11) | 0.27 (3.48) | |||
| Diet | 114 | 6.97 (6.28-7.73) | 114 | 6.32 (5.71-7.01) | -0.65 (-9.31) | -0.92 | 0.04 | 0.04 | |
| Exercise | 114 | 6.49 (5.82-7.24) | 114 | 7.03 (6.371-7.75) | 0.53 (8.23) | 0.26 | 0.45 | 0.59 | |
| Diet+Ex | 114 | 6.74 (6.08-7.47) | 114 | 5.45 (4.87-6.07) | -1.30 (-19.3) | -1.58 | <0.0001 | <0.0001 | |
| PEDF (μg/mL) | Control | 87 | 10.75 (10.35-11.17) | 87 | 10.77 (10.37-11.20) | 19.63 (0.18) | |||
| Diet | 118 | 10.68 (10.31-11.07) | 118 | 9.70 (9.33-10.08) | -0.98 (-9.20) | -1.00 | <0.0001 | <0.0001 | |
| Exercise | 117 | 10.46 (10.16-10.79) | 117 | 10.20 (9.89-10.52) | -0.27 (-2.59) | -0.29 | 0.12 | 0.07 | |
| Diet+Ex | 116 | 10.44 (10.02-10.88) | 116 | 9.41 (9.04-9.79) | -1.03 (-9.90) | -1.05 | <0.0001 | <0.0001 | |
| VEGF (pg/mL) | Control | 87 | 325.0 (284.6-371.1) | 87 | 321.0 (279.3-369.0) | -3.92 (-1.21) | |||
| Diet | 118 | 294.4 (259.7-333.7) | 118 | 270.1 (238.6-305.8) | -24.3 (-8.25) | -20.4 | 0.0004 | 0.0005 | |
| Exercise | 117 | 307.5 (272.1-347.5) | 117 | 297.9 (263.7-336.5 | -9.65 (-3.14) | -5.73 | 0.33 | 0.24 | |
| Diet+Ex | 116 | 310.9 (272.2-355.1) | 116 | 279.9 (244.7-320.2) | -31.0 (-9.98) | -27.1 | <0.0001 | <0.0001 | |
P: p-values for comparing the 12-month changes in intervention groups vs. Control group.
GEE models adjusted for age, baseline BMI (<30kg/m2, >30kg/m2) and race/ethnicity
Relative difference of absolute change at 12 months from baseline between intervention and control arms. P-values<0.008 are considered significant
There was a statistically significant linear trend of reductions in PAI-1, PEDF and VEGF with increasing weight-loss among participants randomized to both the diet and diet+exercise arm: PAI-1, Ptrend=0.0003; Ptrend<0.0001 respectively; PEDF both Ptrend<0.0001; and VEGF both Ptrend<0.0001 (Table 4). In addition, participants who were randomized to the exercise arm had a statistically significant linear trend of reductions in PEDF with increasing levels of weight-loss (Ptrend=0.002), but not in PAI-1 or VEGF.
Table 4. Change in PAI-1, VEGF, and PEDF by percent weight-loss in intervention groups compared with controls.
| Analyte and Weight change categoriesb |
Diet | Exercise | Diet+Exercise | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | 12 Month | Abs. Chang e (%) |
Pc | N | Baseline | 12 Month | Abs. Chang e (%) |
Pc | N | Baseline | 12 Month | Abs. Chang e (%) |
Pc | |
| GMa (95% CI) |
GMa (95% CI) |
GMa (95% CI) |
GMa (95% CI) |
GMa (95% CI) |
GMa (95% CI) |
||||||||||
| PAI-1 | |||||||||||||||
| Control | 87 | 7.88 (7.09-8.76) | 8.15 (7.29---9.11) | 0.27 (3.5) | 87 | 7.88 (7.09-8.76) | 8.15 (7.29-9.11) | 0.27 (3.5) | 87 | 7.88 (7.09-8.76) | 8.15 (7.29-9.11) | 0.27 (3.5) | |||
| No Change/Gained Weight | 22 | 7.05 (5.61---8.87) | 8.29 (6.75---10.18) | 1.24 (17.6) | 0.15 | 41 | 5.87 (4.88-7.05) | 6.67 (5.69-7.82) | 0.81 (13.7) | 0.20 | 12 | 6.39 (5.22-7.84) | 6.87 (5.43-8.69) | 0.48 (7.4) | 0.68 |
| Lost ≥5% | 18 | 7.72 (5.58---10.69) | 8.56 (6.78---10.80) | 0.84 (10.8) | 0.56 | 44 | 6.66 (5.65-7.85) | 7.46 (6.38-8.72) | 0.80 (12.0) | 0.38 | 14 | 8.12 (6.20-10.63) | 7.55 (5.72-9.96) | -0.57 (-7.0) | 0.28 |
| Lost >5% | 74 | 6.78 (6.00---7.66) | 5.42 (4.80---6.12) | -1.36 (-20.0) | 0.0002 | 29 | 7.22 (5.73-9.09) | 6.91 (5.61-8.53) | -0.30 (-4.2) | 0.32 | 88 | 6.59 (5.83-7.45) | 5.00 4.40-5.68 | -1.59 (-24.2) | <0.0001 |
| dPtrend | 0.0003 | 0.79 | <0.0001 | ||||||||||||
| PEDF | |||||||||||||||
| Control | 87 | 10.75 (10.35---11.17) | 10.77 (10.37---11.20) | 19.63 (0.2) | 87 | 10.75 (10.35-11.17) | 10.77 (10.37-11.20) | 0.02 (0.2) | 87 | 10.75 (10.35-11.17) | 10.77 (10.37-11.20) | 0.02 (0.2) | |||
| No Change/Gained Weight | 23 | 11.84 (11.12---12.60) | 12.21 (11.47---13.00) | 373.6 (3.2) | 0.33 | 41 | 10.46 (9.97-10.97) | 10.58 (10.09-11.05) | 0.09 (0.9) | 0.88 | 12 | 9.36 (7.88-11.11) | 9.48 (8.06-11.16 | 0.13 (1.3) | 0.87 |
| Lost <5% | 19 | 11.22 (10.25---12.29) | 10.72 (9.83---11.70 | -0.50 (-4.5) | 0.16 | 46 | 10.44 (9.95-10.96) | 10.28 (9.82-10.76) | -0.164 (-1.6) | 0.27 | 14 | 11.08 (10.03-12.23) | 10.61 (9.84-11.45 | -0.47 (-4.2) | 0.18 |
| Lost ≥5% | 76 | 10.23 (9.79---10.68) | 8.82 (8.49, 9.16) | -1.40 (-13.7) | <0.0001 | 30 | 10.51 (9.84-11.23) | 9.60 (8.92-10.33) | -0.91 (-8.7) | 0.001 | 90 | 10.50 (10.03-10.99) | 9.23 (8.82-9.64) | -1.27 (-12.1) | <0.0001 |
| dPtrend | <0.0001 | 0.002 | <0.0001 | ||||||||||||
| VEGF | |||||||||||||||
| Control | 87 | 325.0 (284.6---371.1) | 321.0 (279.3---369.0) | -3.92 (-1.2) | 87 | 325.0 (284.6-371.1) | 321.0 (279.3-369.0) | -3.92 (-1.2) | 87 | 325.0 (284.6-371.1) | 321.0 (279.3-369.0) | -3.92 (-1.2) | |||
| No Change/Gained Weight | 23 | 288.9 (220.7---378.0) | 292.8 (222.9---384.6) | 3.97 (1.4) | 0.13 | 41 | 265.9 (212.9-332.2 | 262.2 (211.0-325.9) | -3.69 (-1.4) | 0.89 | 12 | 381.8 (282.6-515.8) | 377.6 (269.0-530.0) | -4.21 (-1.1) | 0.86 |
| Lost <5% | 19 | 296.4 (227.4---386.3) | 284.2 (219.3---368.3) | -12.2 (-4.1) | 0.36 | 46 | 302.6 (253.4-361.4 | 292.0 (243.8-349.6) | -10.7 (-3.5) | 0.39 | 14 | 369.4 (257.6-529.7) | 337.4 (229.0 497.0) | -32.0 (-8.7) | 0.07 |
| Lost ≥5% | 76 | 295.6 (250.5,348.7) | 260.2 (221.1---306.2) | -35.3 (-12.0) | <0.0001 | 30 | 384.3 (304.2-485.6 | 365.5 (287.7-464.4) | -18.8 (-4.9) | 0.25 | 90 | 294.5 (251.9-344.2) | 261.2 (223.8 (304.9) | -33.3 (-11.3) | <0.0001 |
| dPtrend | <0.0001 | 0.20 | <0.0001 | ||||||||||||
GM: geometric mean
Analyses stratified by weight-loss percentage and using all available data. All models adjusted for age, baseline BMI (<30kg/m2, >30kg/m2), race/ethnicity
Pc: p-value obtained from GEE model comparing the difference in change of the biomarkers from baseline to 12-month in intervention group vs control within strata of percent weight-loss.
dPtrend: p-value obtained from GEE model testing the linear trend in the change of the biomarkers from baseline to 12-month from Control through all levels of percent weight-loss.
Participants randomized to the diet+exercise arm and who increased their VO2max levels by any level (Table 5) had significantly greater decreases in PAI-1 compared to controls (Ptrend=0.0006). Similar trends were seen for PEDF and VEGF (both Ptrend<0.0001). There were no statistically significant changes in any analyte in participants who were randomized to the exercise arm only and who increased their VO2max compared to controls.
Table 5. Change in PAI-1, VEGF, and PEDF by tertiles of percent change in VO2max levels (mL/kg/min).
| Analyte and Change in VO2max, stratified by tertilesb | Exercise | Diet+Exercise | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-month | Abs. Change (%)e | P** | Baseline | 12-month | Abs. Change (%)e | P c | |||||
| N | GMa (95% CI) | N | GMa (95% CI) | N | GMa (95% CI) | N | GMa (95% CI) | |||||
| PAI-1 | ||||||||||||
| Control | 87 | 7.88 (7.09-8.76) | 87 | 8.15 (7.29-9.11) | 0.27 (3.5) | 87 | 7.88 (7.09-8.76) | 87 | 8.15 (7.29-9.11 | 0.27 (3.5) | ||
| Increased <3.5% | 47 | 6.20 (5.28-7.27) | 47 | 6.59 (5.62-7.74) | 0.40 (6.4) | 0.71 | 52 | 6.86 (5.76-8.14) | 52 | 5.77 (4.93-6.76 | -1.08 (-15.7) | 0.005 |
| Increased ≥3.5-14.3% | 35 | 7.45 (6.09-9.10) | 35 | 7.49 (6.24-8.98) | 0.04 (0.6) | 0.75 | 31 | 6.68 (5.58-7.99) | 31 | 4.78 (3.70-6.18 | -1.90 (-28.4) | 0.0001 |
| Increased≥14.3% | 32 | 5.99 (4.83-7.43) | 32 | 7.20 (6.08-8.52) | 1.21 (20.3) | 0.10 | 31 | 6.63 (5.62-7.84) | 31 | 5.60 (4.74-6.61) | -1.04 (-15.6) | 0.02 |
| Ptrendd | 0.24 | 0.0006 | ||||||||||
| PEDF | ||||||||||||
| Control | 87 | 10.75 (10.35-11.17) | 87 | 10.77 (10.37-11.20) | 1.96 (0.2) | 87 | 10.75 (10.35-11.17) | 87 | 10.77 (10.37-11.20) | 19.63 (0.2) | ||
| Increased <3.5% | 47 | 10.78 (10.32-11.25) | 47 | 10.52 (10.04-11.02) | -0.25 (-2.4) | 0.13 | 53 | 10.45 (9.87-11.07) | 53 | 9.52 (9.00-10.07) | -0.93 (-8.9) | <0.0001 |
| Increased ≥3.5-14.3% | 37 | 10.49 (9.92-11.09) | 37 | 10.17 (9.63-10.73) | -0.32 (-3.1) | 0.19 | 32 | 10.55 (9.80-11.34) | 32 | 9.41 (8.73-10.14) | -1.14 (-10.8) | 0.0002 |
| Increased≥14.3% | 33 | 10.02 (9.43-10.64) | 33 | 9.78 (9.20,10.41) | -0.24 (-2.3) | 0.31 | 31 | 10.32 (9.37-11.36) | 31 | 9.22 (8.45-10.05) | -1.11 (-10.7) | <0.0001 |
| Ptrendd | 0.18 | <0.0001 | ||||||||||
| VEGF | ||||||||||||
| Control | 87 | 325.0 (284.6-371.1) | 87 | 321.0 (279.3,369.0) | -3.92 (-1.2) | 87 | 325.0 (284.6-371.1) | 87 | 321.0 (279.3-369.0) | -3.92 (-1.2) | ||
| Increased <3.5% | 47 | 347.2 (285.0-423.1) | 47 | 340.6 (281.5-412.0) | -6.66 (-1.9) | 0.66 | 53 | 323.5 (258.4-404.9) | 53 | 301.8 (242.5-375.7) | -21.6 (-6.7) | 0.07 |
| Increased ≥3.5-14.3% | 37 | 333.0 (273.9-404.9) | 37 | 324.6 (263.2-400.2) | -8.43 (-2.5) | 0.81 | 32 | 272.4 (218.2-340.0) | 32 | 237.5 (188.6-299.1) | -34.9 (-12.8) | <0.0001 |
| Increased≥14.3% | 33 | 236.5 (188.1-297.5) | 33 | 223.5 (179.7-278.0) | -13.0 (-5.5) | 0.07 | 31 | 333.0 (267.7-414.4) | 31 | 291.5 (230.2-369.0 | -41.6 (-12.5) | 0.0009 |
| Ptrendd | 0.15 | <0.0001 | ||||||||||
GM: geometric mean.
Analyses stratified by change in VO2max by tertiles, using all available data, adjusted for age, baseline BMI (<30kg/m2, >30kg/m2), race/ethnicity
Pc: p-value obtained from GEE model comparing difference in change of the biomarkers from baseline to 12-month in intervention group vs control within tertiles of % change in VO2max.
dPtrend: p-value obtained from GEE model testing the linear trend in the change of the biomarkers from baseline to 12-month from Control through all tertiles of %change in VO2max.
Finally, similar to weight-loss, increasing quantities of percent body-fat loss were associated with a statistically significant linear trend in reductions in PAI-1 in the diet and diet+exercise arms (both Ptrend<0.0001); in PEDF (Ptrend<0.0001 (diet); Ptrend=0.009 (exercise); Ptrend<0.0001 (diet+exercise)); and in VEGF in the diet and diet+exercise arms (both Ptrend<0.0001; Supplementary Table 1).
Discussion
This study compared the effects of dietary weight-loss, exercise, or their combination on circulating levels of regulators of angiogenesis, in a large sample of healthy, overweight/obese postmenopausal women. While correlation does not imply causality, the strong and statistically significant associations between PAI-1, PEDF and the majority of the anthropometric markers and circulating adipokines, suggest that they are interlinked with other factors associated with overweight and obesity. Of interest, despite PEDF's categorization as an anti-angiogenic factor, all of the correlations were in the same direction as for PAI-1, confirming similar results from a study in 125 men.(36) In contrast, VEGF levels did not correlate significantly with any variable, with the exception of platelet levels and white blood cell counts. Platelets are themselves regulators of angiogenesis; have a proliferative effect on cancer cells both in vitro and in vivo, (37) and can guide formation of early metastatic niches.(38)
Women randomized to the diet and diet+exercise arms had statistically significant reductions in PEDF (-9.20% and -9.90% respectively) and VEGF (-8.35% and -9.98% respectively); and statistically significant reductions in PAI-1 in women randomized to the diet+exercise arm only (-19.3%). Weight-loss appeared to account for the majority of these changes, with increasing quantities of weight-loss and reductions in percent body-fat both associated with statistically significant linear decreases in all analytes. This effect was seen only in women randomized to the diet and diet+exercise arms for PAI-1 and VEGF, but in all 3 intervention arms for PEDF. However, changes in VO2max in the exercise arm alone were not associated with statistically significant reductions in any analyte. We confirmed that despite being an anti-angiogenic factor, weight-loss is significantly associated with reductions in PEDF.
While some studies have also reported effects of exercise and weight-loss on these angiogenic factors,(39,40) the studies have been small,(41,42) cross-sectional,(43) or limited to men.(44) In one study, 79 participants were randomized to a 12-week trial comparing an exercise intervention, a hypocaloric diet, and a combined arm, found that VEGF-A was non-significantly reduced by 10-22% in the weight-loss groups. However, the study lacked a control arm.(39) In our previous exercise trial in overweight/obese postmenopausal women, a 12 month exercise intervention produced a significantly greater reduction in PEDF levels (-3.7%), compared to control condition (+3.0%; P=0.009), and that above-median loss of body-fat was associated with greater reduction in PEDF.(5) The reasons for this difference are unclear – baseline levels of analytes in the present study were similar to those in our earlier investigation (444.9 pg/mL, VEGF; 11.8 μg/mL, PEDF; and 6.1 ng/mL, PAI-1).(5) Dietary weight-loss was not tested in that trial. A small study of 33 obese men and women also reported that weight-loss was statistically significantly associated with reductions in circulating PEDF levels.(36) A variety of studies have characterized the anti-tumorigenic effects of PEDF, (21) so the reductions in PEDF in response to weight-loss appear to be counterintuitive. However, its actions appear to be tissue-specific, having for example, anti-inflammatory effects in the retina, and pro-inflammatory effects in adipose tissue and macrophages.(21)
In our previous studies investigating the role of weight-loss and exercise on circulating biomarkers, the majority of the biomarkers which were reduced (or in some cases elevated) in response to weight-loss were also reduced or elevated – albeit to a lesser degree- by exercise alone.(30-32,34,35) Our findings here indicate that exercise alone – either by intervention arm, or when stratified by changes in VO2max – had no effect on PEDF, PAI-1 or VEGF. With the exception of PEDF, weight-loss in the exercise arm was not associated with alterations in levels of these analytes. Exercise increases both skeletal muscle mass and circulation; both processes require upregulation of angiogenesis. It is unclear why the exercise intervention or increases in VO2max in the exercise arm alone, did not affect circulating levels of VEGF, PAI-1 or PEDF. However while there are few data on this topic in the literature, it appears that expression of angiogenic factors may be localized to muscle tissue, and may contribute little to circulating levels. A small RCT of 79 obese men and women randomized to a diet, exercise or control arm found no statistically significant differences in circulating VEGF levels in the exercise arm at 6-months.(39) Finally the degree of weight-loss experienced by participants in our study in the exercise arm (-2.4%) compared to -8.5% in the diet arm, and -10.8% in the diet + exercise arm,(27) may have been insufficient to influence circulating levels of these analytes. While it is impossible to ascertain whether reductions in circulating levels of VEGF and other angiogenic factors could impact tumor-level angiogenesis, systemic reductions in these markers might conceivably be associated with a less favorable milieu for tumor growth and proliferation. It has been hypothesized that, as a consequence of metabolic syndrome, up-regulation of PAI-1 expression predisposes breast cancer to more aggressive stages, partially by affecting angiogenesis.(45,46) Pro-angiogenic factors such as VEGF secreted by adipose stem cells have been implicated in tumor growth by promoting vascularization.(47,48) Indeed a review suggested that a chemopreventive approach targeting both angiogenesis and inflammation in healthy individuals (termed angioprevention) may prevent tumor cell growth and progression by blocking vascularization of indolent tumors.(6) Suggested interventions include metabolic regulators such as metformin, anti-inflammatory agents, and a variety of phytochemicals and their derivatives.(6) However many anti-angiogenic drugs –including metformin (49)- have significant side effects. Weight-loss may represent a safe and effective method of improving the angiogenic profile in both cancer patients and in healthy individuals.
Study strengths include the randomized trial design, the inclusion of dietary weight-loss, exercise, and combined weight-loss and exercise interventions, the excellent adherence to interventions, and high quality and valid biomarker assays. Limitations include the relatively homogenous population which may limit generalizability, assays of only a select number of angiogenesis markers, and measurement of angiogenesis only in blood rather than in target tissue. Linear analyses may potentially incorporate observational study weaknesses, such as confounding. However we used the GEE modification of linear regression analyses, which has been demonstrated to overcome potential observational study weaknesses such as confounding, effect modification, and correlation within individuals over time.
In conclusion, we report that weight-loss reduced circulating VEGF, PEDF, and PAI-1, and suggest that weight-loss in overweight or obese postmenopausal women may reduce risk for cancer in part through altering angiogenesis. Further investigations into the unexpected reductions of PEDF levels with weight-loss are warranted.
Supplementary Material
Acknowledgments
CD and AMcT had full access to all data and take responsibility for its integrity and accuracy of the data analysis
Financial Support: This work was supported by grants from the NCI at the NIH:R01 CA105204-01A1 and U54-CA116847, and from the Breast Cancer Research Foundation.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to report.
Trial registration: Clinicaltrials.gov identifier NCT00470119
References
- 1.Renehan AG, T M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 2.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2011;188:125–39. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 3.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10(7):505–14. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117(9):2362–8. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duggan C, Xiao L, Wang CY, McTiernan A. Effect of a 12-month Exercise Intervention on Serum Biomarkers of Angiogenesis in Postmenopausal Women: a randomized controlled trial. CEBP. 2014;23:648–57. doi: 10.1158/1055-9965.EPI-13-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albini A, Tosetti F, Li VW, Noonan DM, Li WW. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012;9(9):498–509. doi: 10.1038/nrclinonc.2012.120. [DOI] [PubMed] [Google Scholar]
- 7.El-Kenawi AE, El-Remessy AB. Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. British journal of pharmacology. 2013;170(4):712–29. doi: 10.1111/bph.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Cancer Treatment: A to Z List of Cancer Drugs. National Cancer Institute at the National Institutes of Health; 2014. FDA Approval for Bevacizumab. 12/7/2015. Available at: http://wwwcancergov/about-cancer/treatment/drugs/fda-bevacizumab#Anchor-Cerv. 12/7/2015. [Google Scholar]
- 9.Gacche RN, Meshram RJ. Angiogenic factors as potential drug target: efficacy and limitations of anti-angiogenic therapy. Biochimica et biophysica acta. 2014;1846(1):161–79. doi: 10.1016/j.bbcan.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 11.Doupis J, Rahangdale S, Gnardellis C, Pena SE, Malhotra A, Veves A. Effects of diabetes and obesity on vascular reactivity, inflammatory cytokines, and growth factors. Obesity (Silver Spring) 2011;19(4):729–35. doi: 10.1038/oby.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29(11):1308–14. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 13.Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61(7):1801–13. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871–82. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juhan-Vague I, Roul C, Alessi MC, Ardissone JP, Heim M, Vague P. Increased plasminogen activator inhibitor activity in non insulin dependent diabetic patients--relationship with plasma insulin. Thromb Haemost. 1989;61(3):370–3. [PubMed] [Google Scholar]
- 16.De Pergola G, De Mitrio V, Giorgino F, Sciaraffia M, Minenna A, Di Bari L, et al. Increase in both pro-thrombotic and anti-thrombotic factors in obese premenopausal women: relationship with body fat distribution. Int J Obes Relat Metab Disord. 1997;21(7):527–35. doi: 10.1038/sj.ijo.0800435. [DOI] [PubMed] [Google Scholar]
- 17.Isogai C, Laug WE, Shimada H, Declerck PJ, Stins MF, Durden DL, et al. Plasminogen activator inhibitor-1 promotes angiogenesis by stimulating endothelial cell migration toward fibronectin. Cancer Res. 2001;61(14):5587–94. [PubMed] [Google Scholar]
- 18.McMahon GA, Petitclerc E, Stefansson S, Smith E, Wong MK, Westrick RJ, et al. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J Biol Chem. 2001;276(36):33964–8. doi: 10.1074/jbc.M105980200. [DOI] [PubMed] [Google Scholar]
- 19.Look MP, van Putten WL, Duffy MJ, Harbeck N, Christensen IJ, Thomssen C, et al. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. Journal of the National Cancer Institute. 2002;94(2):116–28. doi: 10.1093/jnci/94.2.116. [DOI] [PubMed] [Google Scholar]
- 20.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285(5425):245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 21.Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat Rev Cancer. 2013;13(4):258–71. doi: 10.1038/nrc3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamagishi S, Adachi H, Abe A, Yashiro T, Enomoto M, Furuki K, et al. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2006;91(6):2447–50. doi: 10.1210/jc.2005-2654. [DOI] [PubMed] [Google Scholar]
- 23.Crowe S, Wu LE, Economou C, Turpin SM, Matzaris M, Hoehn KL, et al. Pigment Epithelium-Derived Factor Contributes to Insulin Resistance in Obesity. Cell Metabolism. 2009;10(1):40–47. doi: 10.1016/j.cmet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Lemoine AY, Ledoux S, Larger E. Adipose tissue angiogenesis in obesity. Thromb Haemost. 2013;110(4):661–8. doi: 10.1160/TH13-01-0073. [DOI] [PubMed] [Google Scholar]
- 25.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17(3):320–9. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 27.Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012;20(8):1628–38. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 29.Pate R, Blair S, Durstine J. Guidelines for Exercise Testing and Prescription. Philadelphia, Pa: Lea & Febinger; 1991. pp. 70–72. [Google Scholar]
- 30.Mason C, Foster-Schubert KE, Imayama I, Kong A, Xiao L, Bain C, et al. Dietary weight-loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41(4):366–75. doi: 10.1016/j.amepre.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imayama I, Ulrich CM, Alfano CM, Wang C, X L, Wener MH, et al. Effects of a caloric restriction weight-loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Research. 2012;72:2314–26. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbenhardt C, McTiernan A, Alfano CM, Wener MH, Campbell KL, Duggan C, et al. Effects on adiponectin and leptin after individual and combined diet and exercise interventions in postmenopausal women. J Intern Med. 2013;274(2):163–75. doi: 10.1111/joim.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mason C, Risques RA, Xiao L, Duggan C, Imayama I, Campbell KL, et al. Independent and Combined Effects of Dietary Weight-loss and Exercise on Leukocyte Telomere Length in Post-menopausal Women. Obesity. 2013;21(12):E549–54. doi: 10.1002/oby.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mason C, Xiao L, Duggan C, Imayama I, Foster-Schubert KE, Kong A, et al. Effects of Dietary Weight-loss and Exercise on Insulin-Like Growth Factor-1 and Insulin-Like Growth Factor Binding Protein-3 in Postmenopausal Women. Cancer Epidemiology, Biomarkers and Prevention. 2013;22(8):1457–63. doi: 10.1158/1055-9965.EPI-13-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell KL, Foster-Schubert KE, Alfano CM, Wang CC, Wang CY, Duggan CR, et al. Reduced-calorie dietary weight-loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J Clin Oncol. 2012;30(19):2314–26. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabater M, Moreno-Navarrete JM, Ortega FJ, Pardo G, Salvador J, Ricart W, et al. Circulating pigment epithelium-derived factor levels are associated with insulin resistance and decrease after weight-loss. J Clin Endocrinol Metab. 2010;95(10):4720–8. doi: 10.1210/jc.2010-0630. [DOI] [PubMed] [Google Scholar]
- 37.Cho MS, Bottsford-Miller J, Vasquez HG, Stone R, Zand B, Kroll MH, et al. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120(24):4869–72. doi: 10.1182/blood-2012-06-438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A. 2014;111(30):E3053–61. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cullberg KB, Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Effect of weight-loss and exercise on angiogenic factors in the circulation and in adipose tissue in obese subjects. Obesity (Silver Spring) 2013;21(3):454–60. doi: 10.1002/oby.20060. [DOI] [PubMed] [Google Scholar]
- 40.Makey KL, Patterson SG, Robinson J, Loftin M, Waddell DE, Miele L, et al. Increased plasma levels of soluble vascular endothelial growth factor receptor 1 (sFlt-1) in women by moderate exercise and increased plasma levels of vascular endothelial growth factor in overweight/obese women. Eur J Cancer Prev. 2013;22(1):83–9. doi: 10.1097/CEJ.0b013e328353ed81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joham AE, Teede HJ, Hutchison SK, Stepto NK, Harrison CL, Strauss BJ, et al. Pigment epithelium-derived factor, insulin sensitivity, and adiposity in polycystic ovary syndrome: impact of exercise training. Obesity (Silver Spring) 2012;20(12):2390–6. doi: 10.1038/oby.2012.135. [DOI] [PubMed] [Google Scholar]
- 42.DeSouza CA, Jones PP, Seals DR. Physical activity status and adverse age-related differences in coagulation and fibrinolytic factors in women. Arterioscler Thromb Vasc Biol. 1998;18(3):362–8. doi: 10.1161/01.atv.18.3.362. [DOI] [PubMed] [Google Scholar]
- 43.Wilund KR, Tomayko EJ, Evans EM, Kim K, Ishaque MR, Fernhall B. Physical activity, coronary artery calcium, and bone mineral density in elderly men and women: a preliminary investigation. Metabolism. 2008;57(4):584–91. doi: 10.1016/j.metabol.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 44.Rokling-Andersen MH, Reseland JE, Veierod MB, Anderssen SA, Jacobs DR, Jr, Urdal P, et al. Effects of long-term exercise and diet intervention on plasma adipokine concentrations. Am J Clin Nutr. 2007;86(5):1293–301. doi: 10.1093/ajcn/86.5.1293. [DOI] [PubMed] [Google Scholar]
- 45.Beaulieu LM, Whitley BR, Wiesner TF, Rehault SM, Palmieri D, Elkahloun AG, et al. Breast cancer and metabolic syndrome linked through the plasminogen activator inhibitor-1 cycle. Bioessays. 2007;29(10):1029–38. doi: 10.1002/bies.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leik CE, Su EJ, Nambi P, Crandall DL, Lawrence DA. Effect of pharmacologic plasminogen activator inhibitor-1 inhibition on cell motility and tumor angiogenesis. J Thromb Haemost. 2006;4(12):2710–5. doi: 10.1111/j.1538-7836.2006.02244.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Bellows CF, Kolonin MG. Adipose tissue-derived progenitor cells and cancer. World J Stem Cells. 2010;2(5):103–13. doi: 10.4252/wjsc.v2.i5.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freese KE, Kokai L, Edwards RP, Philips BJ, Sheikh MA, Kelley J, et al. Adipose-Derived Stems Cells and Their Role in Human Cancer Development, Growth, Progression, and Metastasis: A Systematic Review. Cancer Res. 2015;75(7):1161–68. doi: 10.1158/0008-5472.CAN-14-2744. [DOI] [PubMed] [Google Scholar]
- 49.Dallaglio K, Bruno A, Cantelmo AR, Esposito AI, Ruggiero L, Orecchioni S, et al. Paradoxic effects of metformin on endothelial cells and angiogenesis. Carcinogenesis. 2014;35(5):1055–66. doi: 10.1093/carcin/bgu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


