Abstract
The U.S. NHANES included chemosensory assessments in the 2011–2014 protocol. We provide an overview of this protocol and 2012 olfactory exam findings. Of the 1818 NHANES participants aged ≥40 years, 1281 (70.5 %) completed the exam; non-participation mostly was due to time constraints. Health technicians administered an 8-item, forced-choice, odor identification task scored as normosmic (6–8 odors identified correctly) versus olfactory dysfunction, including hyposmic (4–5 correct) and anosmic/severe hyposmic (0–3 correct). Interviewers recorded self-reported smell alterations (during past year, since age 25, phantosmia), histories of sinonasal problems, xerostomia, dental extractions, head or facial trauma, and chemosensory-related treatment and changes in quality of life. Olfactory dysfunction was found in 12.4 % (13.3 million adults; 55 % males/45 % females) including 3.2 % anosmic/severe hyposmic (3.4 million; 74 % males/26 % females). Selected age-specific prevalences were 4.2 % (40–49 years), 12.7 % (60–69 years), and 39.4 % (80+ years). Among adults ≥70 years, misidentification rates for warning odors were 20.3 % for smoke and 31.3 % for natural gas. The highest sensitivity (correctly identifying dysfunction) and specificity (correctly identifying normosmia) of self-reported olfactory alteration was among anosmics/severe hyposmics (54.4 % and 78.1 %, respectively). In age- and sex-adjusted logistic regression analysis, risk factors of olfactory dysfunction were racial/ethnic minority, income-to-poverty ratio ≤ 1.1, education <high school, and heavy drinking. Moderate-to-vigorous physical activity reduced risk of impairment. Olfactory dysfunction is prevalent, particularly among older adults. Inexpensive, brief odor identification tests coupled with questions (smell problems past year, since age 25, phantosmia) could screen for marked dysfunction. Healthcare providers should be prepared to offer education on non-olfactory avoidance of hazardous events.
Keywords: Olfaction disorders, Epidemiology, Taste, Risk factors, Public health surveillance, Health status
1 Introduction
The National Health and Nutrition Examination Survey (NHANES) is part of the United States public health surveillance, the continuous, systematic collection, analysis, and interpretation of health-related data conducted by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC). NHANES data are used to clarify the prevalence of health problems for setting priorities, public health policy and strategies, and research needs. NHANES is administered to a nationally-representative sample of approximately 5000 civilian, non-institutionalized persons of all ages from 15 randomly selected counties/other similar geographic jurisdictions each year [1].
NHANES participants first take part in a health interview in their home, followed by clinical tests, a dietary interview, and physical exams in the mobile examination centers (MECs). Two teams of interviewers/examiners are in the field simultaneously, conducting operations for approximately six weeks in each of the randomly selected “stands” in targeted counties. Using a fully computerized data collection system and standardized measures, trained health technicians collect information on a wide variety of diseases, medical conditions, and health indicators, including diabetes, cardiovascular disease, sexual behavior, physical activity, dietary intake, and environmental exposures. NHANES has a core battery of tests and questions (e.g., household composition, annual household income, dietary intake, and behavioral questions) administered every year. The content and measurement procedures in NHANES, however, are updated and revised frequently to meet new and emerging public health needs in the United States [1].
For the first time since the U.S. national health examination surveys began in 1959, the NHANES 2011–2014 protocol included a chemosensory (taste and smell) component in its home questionnaire and battery of exams. The overall goals of the NHANES taste and smell component were: 1) to generate normative data for chemosensory function and nationally representative estimates for the prevalence of taste and smell dysfunction or alterations in U.S. adults 40 or more years of age, 2) to evaluate risk factors and co-morbidities that may be related to chemosensory impairment, including age, health history, obesity, life style factors (e.g., cigarette smoking and alcohol consumption), and 3) to determine how variation in chemosensory function may be associated with differences in dietary behaviors, including consumption of nutrients that influence health and chronic disease risk (e.g., consumption of salt, alcoholic beverages, vegetables).
This paper provides an overview of the NHANES taste and smell protocol, including a historical framework leading up to national health objectives for chemosensory disorders within Healthy People 2020. We report on the initial results from the limited access 2012 NHANES olfactory test data, including the prevalence of olfactory dysfunction, comparison of self-reported to measured olfactory dysfunction, and demographic and clinical predictors of olfactory dysfunction.
2 Historical framework
Despite significance from a public health and public safety point of view, knowledge about the prevalence and treatment of smell and taste disorders in the general population has been limited. Since only a few epidemiological studies had been conducted in this field, the National Institute on Deafness and Other Communications Disorders (NIDCD) proposed collaborating with the NHANES Division, NCHS to obtain normative reference data for the ability to taste and smell in the U.S. population. For several years, the NHANES efforts were suspended due to lack of Congressional authorization and funding. In 1997, when it became known that the U.S. Congress would reauthorize and fund NHANES, NIDCD convened a meeting of expert consultants in its mission areas (hearing, balance, smell/taste, voice, speech and language) to plan examination components. Further refinement of goals was accomplished during the 2005 NIDCD Workshop on Epidemiology of Communication Disorders [2]. A chemosensory protocol was developed by NIDCD and consultants for NHANES 2005–2006, but was not implemented due to concerns about exam length, practical difficulties with obtaining uniform taste/smell stimuli, and lack of prior experience in using the proposed protocol in population-based studies. The initiative was re-launched in 2010 as a result of intervening progress in the development and standardization of epidemiological tools to assess taste and smell. Concurrently, the Federal Interagency Workgroup for Healthy People 2020 endorsed the NIDCD proposal to add taste and smell disorder-related goals to its surveillance monitoring agenda, including a goal to increase the proportion of adults with chemosensory disorders who have seen a health care provider about their disorder and, also, to increase the proportion of adults who have tried recommended methods of treatment [3].
3 Chemosensory protocol – rationale and background
The NHANES 2011–2014 Chemosensory protocol [4] was developed through consultation with an expert team of researchers, clinicians, and epidemiologists in chemosensation. The primary challenge was to design a protocol that was brief to fit within the NHANES time constraint (<15 min), while providing an adequate amount of information on taste and smell. To meet the NHANES eligibility criteria, the chemosensory measures also had to be reliable, valid, and minimally burdensome to participants. The protocol was designed to address the goals of the taste and smell component, and to be consistent and feasible within the NHANES testing framework. Priority was given to taste and smell measures that covered the full range of chemosensory function (both clinical and normal variation) and were relevant to health and nutritional outcomes collected as a part of NHANES.
In designing the protocol, the NHANES chemosensory team built on the work of the NIH Toolbox project [5] – an initiative that identified and assembled brief, reliable measurement tools in the domains of cognition, emotion, sensation and motor function. Rapid taste and smell assessments [6, 7], developed and standardized in the NIH Toolbox, were modified, adapted, and standardized for NHANES use; they were pilot tested in two NHANES stands during 2010–2011. The final NHANES taste and smell protocol [4] was deemed highly acceptable by NHANES technicians and participants. The NHANES health technicians were provided significant training and retraining on the taste and smell protocol and their performance was checked and monitored at frequent intervals. Standardized scripts were developed and used for each exam component. The data collection system was computerized and included rigorous quality control and quality assurance procedures.
The NHANES taste and smell protocol [4] was administered to participants 40 years and older in the 2011–2014 data cycles and consisted of self-reported answers to a chemosensory questionnaire (CSQ) [8] that was administered in the respondents’ homes, followed by a brief battery of smell and taste exams in the NHANES mobile exam centers (MEC). The CSQ asked about perceived smell and taste problems during the past 12 months, distortions, and diminished abilities since age 25 (collectively termed “alterations”). Also, conditions known or thought to be associated with chemosensory alterations were included in the CSQ. In the MEC, the taste and smell exam protocol was operationalized in a manner similar to that used in the NIH Toolbox protocol, with an odor identification test to assess smell function [7] and a spatial or regional taste test of the perceived intensity of bitter and salt solutions to assess taste function [6]. The CSQ was implemented in NHANES at the outset of the 2011–2012 data cycle, and both the CSQ and taste and smell exams were performed throughout 2012–2014. The present study describes the selection, standardization, and implementation of the chemosensory measures included in the 2011–2014 NHANES taste and smell protocol. The prevalence and risk factors of self-reported olfactory alterations from the NHANES CSQ 2011–2012 have been reported previously [9]. In the present paper, we report the prevalence and risk factors of measured olfactory dysfunction, as well as sensitivity and specificity estimates for the self-reported olfactory measures from the 2012 NHANES data.
4 The chemosensory questionnaire (CSQ) for NHANES, 2011–2014
The CSQ was developed and standardized specifically for use in the NHANES home interview. The questionnaire was adapted from previous research, including a community-based study of self-rated olfaction in older adults [10], the olfaction and taste questionnaire used in the Beaver Dam Studies [11, 12], and the self-reported chemosensory questionnaire used in the Disability Supplement to the 1994 National Health Interview Survey [13]. The CSQ was content-validated by a nationwide team of chemosensory experts and cognitively tested for response problems and cultural appropriateness to ensure that respondents could understand, process, and interpret the questions in a consistent manner as intended. Test-retest reliability study of the CSQ items has shown good-to-excellent agreement across a six month interval [14].
The CSQ was constructed to be brief, with single questions capturing perceived taste and smell problems within the past 12 months; perceived distortions or phantom sensations in taste (dysgeusia and ‘quality’ of the dysgeusia, e.g., “metallic,” “foul,” “bitter,” etc.), oral somatosensation (burning, tingling) and distortions or phantom sensations in smell (parosmia, phantosmia); perceived changes since age 25 in overall smell function, ability to perceive specific taste qualities (sweet, salty, sour, or bitter), and the ability to “taste” food flavors. The CSQ was constructed with skip patterns; if the participants reported problems with smell or taste, they were prompted with additional questions about these problems. Follow-up questions about the frequency and time frame of chemosensory-related problems were included to capture transient or intermittent taste and smell problems.
To provide information for the Healthy People 2020 chemosensory objectives, participants were asked about the effects of chemosensory-related problems on their health and quality of life, and whether or not they sought medical attention and/or treatment for these problems; these questions were included to provide baseline data from which to track increases or decreases in the treatment and health burden of these disorders in the U.S. population. The CSQ also included additional questions on the history of some common conditions or exposures that could potentially affect taste and smell that were not being captured in other NHANES questionnaire or exam components. For example, a history of frequent nasal congestion, tonsillectomy, and loss of consciousness from head injury were included in the CSQ. In 2011–2014 data cycles, NHANES participants 40 years or older were administered the CSQ in their homes by trained interviewers, using a Computer-Assisted Personal Interviewing (CAPI) system.
The CSQ was designed to supplement the NHANES taste and smell exams as well as provide comparison between self-reported and measured chemosensory function in U.S. adults. Self-reported smell function typically has not been considered to be a sensitive indicator of measured impairment [10]. However, previously reported low sensitivities of self-reported smell function may have resulted from the use of a single question, which simply asked participants to rate their current smell ability [11, 15]. In a home-based community study [10], self-reported indices of olfactory function that included the CSQ question on smell loss with age (in addition to ‘current smell ability’) showed better sensitivity and specificity to measured function than had been previously reported. Another laboratory-based study [14] showed that individuals reporting smell problems or smell loss with age in the CSQ scored significantly lower in odor identification tests. Nonetheless, the high discrepancy between the prevalence rates of self-reported and measured olfactory function in population-based studies [11, 16, 17] suggests that a majority of individuals with smell problems are unaware of their deficits, and hence go undiagnosed and untreated. This further underscores the timeliness of Healthy People 2020 chemosensory objectives aimed at improving assessment and treatment rates for chemosensory disorders in U.S. adults. Still, subjective assessment tools like the CSQ are necessary to understand the perceived health impact of chemosensory disorders and capture intermittent losses and distortions (dysgeusia, phantosmia) that may be missed by chemosensory exams.
Previously released data from the NHANES 2011–2012 CSQ (n = 3603 adults, aged ≥40 years) revealed the prevalence of reported problems in the past year as 10.6 % for smell and 5.2 % for taste [9]. These prevalence estimates are comparable to recent population-based studies of adults for self-reported smell problems [11, 15, 18] and somewhat below those reported for taste problems [19, 20]. The proportion of adults in NHANES 2012 who noticed a reduction in smell and taste with aging was 17 % and 5 %, respectively, while the perception of a phantom odor was 5 % and dysgeusia was 5 % as well. The percent of NHANES participants who reported seeking treatment for either taste or smell disorders was less than 5 %.
5 Taste exam measures in NHANES
The NHANES chemosensory tests used regional and whole mouth taste intensities of bitter and salt tastants as measures of taste function, which were similar to those implemented in the NIH Toolbox norming study [6]. These NHANES taste measures were selected based on their ability to capture genetic and environmentally mediated variation in taste and for their potential relevance to diet and health.
Participants reported the taste intensity on the general Labeled Magnitude Scale (gLMS) – a fast, reliable, and well-validated method for assessing and comparing perceived sensory intensities across individuals or groups of individuals. The gLMS scale provides data similar to magnitude matching, the gold standard procedure for assessing perceived intensity [21], is reliable [10, 22] while enjoying the advantage of being much quicker and adaptable to population-based samples. The gLMS has been used in multiple clinical [23, 24] and population-based studies [25, 26] to study variation in taste perception. The gLMS taste intensity measure captures a range of taste perception, from low to heightened taste perception (i.e., “supertasting”) [21], and allows the assessment of taste phenotype-genotype [27–29] and taste-diet-health [30–33] associations.
In the NHANES taste protocol, participants were first oriented to the gLMS scale and encouraged to practice using the scale by rating the brightness of two LED-generated light stimuli. Next, they were asked to rate three additional LED-generated light stimuli (low, medium, and high luminance). These three light ratings permit cross-modality comparisons in analyses and, also, were used to efficiently identify participants who did use the scale accurately. Participants who did not rank the intensities of the three lights in the correct order (low < medium < high), following the initial practice opportunity, were considered to have not understood the scale, and hence were excluded from the taste test.
Spatial testing measures taste intensity sensations at regions of the tongue that are innervated by different cranial nerves. This is a common technique used in laboratory and clinical settings to evaluate regional taste losses and to detect altered patterns of oral sensations [34–36]. Since regional taste loss from the anterior tongue is fairly common, NHANES taste assessments included perceived intensities of bitter and salt tastants applied regionally on the tongue tip and also sampled with the whole mouth using a swish-and-spit procedure. Regional taste loss on the tongue tip may result from chorda tympani nerve damage and usually goes unnoticed due to release of inhibition from other taste-related cranial nerves [37]. Additionally, this disinhibitory feedback may cause changes in taste, flavor, touch and irritation sensations from foods and beverages or cause oral problems such as phantom sensations and dysgeusia [35, 37]. Some studies have shown that diminished taste intensity on the tongue tip, either measured directly, or as a ratio to whole mouth intensity, is associated with dietary behaviors that may contribute to chronic disease risk. Diminished taste sensations on the tongue tip, for example, have been associated with differences in preference and intake of vegetables [30], alcohol [38] and sweet/fatty foods [37].
6 Taste testing stimuli
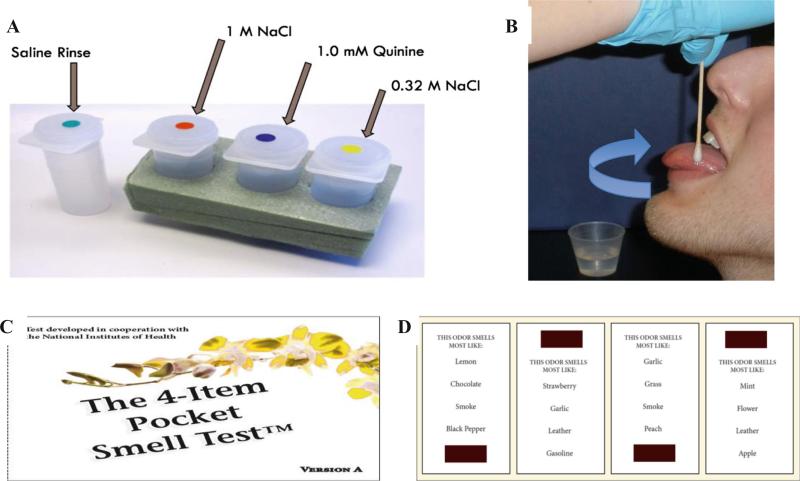
Concentrated aqueous solutions of quinine (1 mM) and NaCl (1 M) were selected as probes of regional taste function applied to the tongue tip [6], and these two tastants plus 0.32 M NaCl were also sampled with the whole mouth (Fig. 1). Quinine, an FDA-approved flavor additive found in tonic water, was selected since it is a widely used prototypical tastant for bitter sensations, and has been previously used in assessing regional taste damage [30, 34, 35, 38]. Quinine is broadly tuned to multiple bitter taste receptors [39], and age-related differences in both threshold and suprathreshold intensities of quinine have been reported previously [40]. Bitter taste perception was selected for inclusion in NHANES due to its influence on palatability and consumption of important food and beverage groups, such as fruits, vegetables, and alcohol. The salt stimuli were selected for their dual role as a taste and an irritant, plus the potential relevance of excessive salt consumption to health risks. These regional and whole mouth taste measures (excluding 0.32 M NaCl) were also selected for use in the NIH Toolbox taste protocol norming study [6].
Fig. 1.
a Depiction of the aqueous tastants (provided by the Laboratory of Dr. John Hayes, Pennsylvania State University, PA) and b application to the tongue tip (top panels) in the taste testing. c One of the two 4-item, odor identification tests (Pocket Smell Tests™, Sensonics, Inc., Haddon Heights, NJ) and d four microencapsulated scent strips (with odor choices for each of them) to illustrate the materials used in olfactory testing in the NHANES 2012–2014
In addition to quinine, 6-n-propylthiouracil (PROP) was initially included as another bitter taste measure in NHANES. PROP and phenylthiouracil (PTC) had been used in prior genetic epidemiological studies (e.g., [32, 41]), and are widely used phenotypic markers for genetic variation in taste [42, 43]. PROP is a commonly used medication to manage hyperthyroidism. While the clinical treatment dosage is much higher than the exposure during the swish-and-spit taste test procedure, given the potential chance of encountering an NHANES participant who had previously developed a treatment-related side effect to these medications [44] and the extended time required to fully inform participants of the risks of these medications, PROP taste testing was removed from the NHANES protocol during pilot testing.
Validation studies have indicated that NHANES (and NIH Toolbox) taste measures correspond well with traditional assessments and can identify population level differences in taste function [6, 14]. In our laboratory-based study [14], whole-mouth intensity of quinine correlated well with all taste qualities (1 M Sucrose, 32 mM Citric Acid, 1 M NaCl) including PROP, supporting its utility as a marker for broader taste functioning. Studies have also shown that NHANES taste measures have moderate-to-good test-retest reliability [14, 45]. In the NHANES, a replicate test for whole-mouth salt intensity (randomized between 1 M NaCl or 0.32 M NaCl) was also included in the protocol to demonstrate within session test-retest reliability of this whole mouth taste measure.
7 Assessing olfactory dysfunction in NHANES
Smell function in NHANES was assessed with an 8-item, odor identification test (Pocket Smell Tests™, Sensonics, Inc., Haddon Heights, NJ). Of many available psychophysical measures of olfactory function, odor identification tests are the most widely used in epidemiological and clinical settings, as they are quick, relatively inexpensive and easy to administer. The odor identification test corresponds well with odor threshold tasks as well as other suprathreshold olfactory measures (e.g., discrimination, odor intensity), and hence is considered to be a rapid and accurate method for detecting olfactory dysfunction [46–48].
The NHANES Pocket Smell Test (PST) was constructed based on the University of Pennsylvania Smell Identification Test (UPSIT) [49], which is considered one of the gold standards for measuring olfactory function. Prior to being implemented in the 2012–2014 NHANES protocol, the PST underwent significant cognitive and pilot testing to identify potential response problems and to ensure cultural appropriateness. The type and number of odorants included in the original odor identification test underwent several modifications for NHANES during the extensive review and pilot-testing process. For example, “banana,” which was initially included as one of the test odorants, was later removed. Since banana was not introduced on a broad scale into Korea until the 1990s, it was decided that this test item might pose problems for some older native Koreans and Korean-Americans. The odor items included in the final, modified version of the PST were carefully selected to include food odors (strawberry, chocolate, onion, grape) relevant to diet and nutrition, two warning odors (natural gas, smoke) and two common household odors (leather, soap). Natural gas and smoke were included, since these two odorants are important from a public safety point of view; onion was included as a trigeminal probe. Consistent with the precedent set by use of the San Diego Odor Identification Test (SDOIT) in the Beaver Dam studies [11, 12], it was decided that eight odorants could serve as an adequate number of test items needed to accurately characterize olfactory function, while minimizing the time needed to administer the test. Olfactory function categories were formed from the PST following the findings based on the Beaver Dam studies with the SDOIT, an approach which differed from that used in the National Social Life, Health, and Aging Project [11, 50].
The odorants in the NHANES PST are microencapsulated and positioned on scent strips at the bottom or top of each test card (Fig. 1). The odors were released when the NHANES health technician scratched the strips with a plastic stylus tip from left to right in a “Z” pattern.
Participants were asked to sniff or smell each odor, and identify the odor, selecting one of the four choices provided. The forced-choice design of the PST required the participant to choose an answer from one of the four alternatives even if no odor was perceived. Olfactory function score is based on the number of correct identifications (total scores range from 0 to 8). Incorrect identification of three or more odors (resulting in total scores 0–5) is classified as olfactory dysfunction. Olfactory dysfunction is sub-classified into anosmia/severe hyposmia (scores 0 to 3) and hyposmia (scores 4 to 5). This classification was adapted from the well-validated eight-item SDOIT used in the Beaver Dam studies [11, 12].
These cut-off points selected for the PSTalso aligned with the percentages calculated from the UPSIT scoring classification. In a laboratory-based study, the NHANES PST showed 100 % sensitivity and 98 % specificity for detecting moderate to severe dysfunction, when compared with a 40-item olfactometer-based identification test [14]. The NHANES PST also showed good test-retest reliability over a two week interval, for both olfactory function classification and overall identification scores [14].
8 Olfactory dysfunction analysis based on NHANES 2012
The NHANES 2012 data analyzed for this report were made available through secure, on-site access at the NCHS Research Data Center. The 2012 NHANES data will not be released publicly since it is NCHS policy not to release data based on a sample collected from only a single year of the NHANES, due to confidentiality considerations and concerns. We have analyzed the limited access NHANES 2012 data – the first available based on a nationally-representative sample of U.S. adults aged 40 or more years – to report on the prevalence of olfactory dysfunction based on the PST objective scoring algorithm, to examine the sensitivities and specificities of self-reported smell alterations from the CSQ with respect to the measured PST scores, and to examine some of the health and demographic and clinical factors associated with increased olfactory dysfunction.
8.1 Materials and methods
Study population
The NHANES 2012 involved the collection of questionnaire and clinical measures from a nationally-representative sample of the non-institutionalized, civilian U.S. population. An oversample of the following subgroups of the population was used to improve the reliability and precision as well as to increase the diversity in estimates of health status [51]: Hispanic, non-Hispanic (NH) black, and NH Asian; NH white (plus “other” race/ethnicity) below 130 % of the poverty level and/or aged 80 years and above. Data collection for NHANES 2012, which included the chemosensory protocol, was approved by the NCHS Research Ethics Review Board. Analysis of de-identified data from the survey is exempt from the federal regulations for the protection of human research participants. Analysis of restricted data through the NCHS Research Data Center is also approved by the NCHS IRB/ERB (Institutional Review Board/ Ethics Review Board). Pregnant or lactating women were excluded from the entire taste and smell exam in the MEC. There were no exclusions for the CSQ in the home interview. All participants provided written informed consent. The study complied with the Declaration of Helsinki for medical research involving human subjects.
The analytic sample in this report consisted of 1281 adults, aged ≥40 years, who participated in the PST along with the CSQ; this was 70.5 % of the NHANES 2012 cohort (N = 1818). Reasons for non-participation in the PST were that the participant came late/left early from the MEC (63.9 %), the participant had a time constraint (13.3 %), was with a child (6.1 %) or had a language barrier (5.9 %). Only 2 % refused to participate in the PST.
At the end of the exam, the NHANES health technician rated the participant's understanding of the protocol and cooperation during the exam as either very good, good, fair, poor, or unable to cooperate. After completing the chemosensory protocol, the participant was given a computer-generated report of findings, which included the exam results pertaining to his/her ability to identify the odors of smoke and natural gas, as well as their ability to identify salty and bitter tastes.
Measured olfactory function in the MEC
Prior to testing, participants were screened for exclusion criteria (pregnant/lactating, allergies to quinine), and asked to report any current nasal symptoms (e.g., sneezing frequently, blocked-up nose, sinus pain, or runny nose) that could alter or influence the results of their taste and smell exam. Data on current nasal symptoms were collected not for participant exclusion, but to identify transient problems with taste and smell that might prove useful in subsequent statistical analyses. Participants were then administered the 8-item PST, during which they were asked to smell each odor (released when the experimenter scratched the odor strips with a pointed stylus), and identify the odor as one of the four choices provided for each item.
Self-reported smell function and smell alteration index
The CSQ was administered by trained interviewers in the respondent's home—a complete codebook and data from NHANES 2011–2012 are publicly available online (CDC 2013c). For the analysis here, we have analyzed responses to the question on “a problem with the ability to smell within the past year,” as well as the smell alteration index [9], which categorizes an alteration as a “ yes” to any one of the following: a problem within the past year, a worse sense of smell since age 25, or phantosmia. Participants also answered questions on common conditions (e.g., sinonasal, head/orofacial injury) that have been previously shown to increase risk of chemosensory alterations. Validity and reliability estimates of some CSQ questions have been reported from two studies with community-based samples [10, 14].
Socio-demographic and health-related risk factors
Demographic, socio-economic, medical and health behavior variables from the CSQ and other self-reported questionnaires were used to evaluate associations with self-reported and measured olfactory function. These questionnaires and the corresponding data and codebooks are available online for public use [52]. Marital status [married or not (widowed/divorced/ separated/never married/living with partner)] and educational attainment (not having completed high school versus high school graduate or above) were dichotomized. Income-to-poverty ratio (family income divided by federal poverty threshold [53]) provided a measure of socio-economic status and was analyzed by quartiles [54]. Self-rated general health status was categorized (excellent/very good/good/fair/poor). Interview questions also asked about xerostomia (persistent dry mouth) during the past year, and lifetime histories of the following: tonsillectomy; loss of consciousness from head injury; broken nose or other serious injury to face/skull; heavy drinking (≥4/5 drinks daily); multiple (≥3) ear infections; and insertion of tympanostomy tubes to drain fluid from ear(s). Cigarette smoking was categorized as never, current, or past smoker. Participants were defined as physically active if they reported doing at least ten continuous minutes of vigorous- or moderate-intensity activity ≥3 days a week.
8.2 Statistical analysis
Analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, NC), and SUDAAN™, version 11.0 (Research Triangle Institute [RTI] International, Research Triangle Park, NC), to calculate national estimates, standard deviations (SDs), and 95 % confidence intervals (CIs) while taking into account the NHANES complex sampling design. Two-sided tests and 5 % significance levels were used in the analysis. Relationships between self-reported or measured ol-factory function and socio-demographic, health-related risk factors were assessed using Chi-square or two-tailed t-tests. Age- and sex-adjusted logistic regression models were used to assess associations between olfactory dysfunction and selected risk factors. The jackknife method with replicate weights was used to estimate variance. Results are reported as odds ratios (ORs) with 95 % CIs. The multiple imputation procedure and the fully conditional specification (FCS) method were used to impute missing data to minimize the bias associated with list-wise deletion of participants with missing values. Five imputations per missing observation were performed and the average measure was calculated using SUDAAN.
The sensitivity and specificity of the self-reported olfactory measures were calculated as follows: Sensitivity = TP/(TP + FN) and Specificity = TN/(TN + FP), where TP, TN, FP, and FN are true positive (measured dysfunction), true negative (measured normosmia), false positive (incorrectly classified as dysfunction), and false negative (incorrectly classified as normosmia), respectively.
8.3 Results
Socio-demographic, lifestyle behaviors, and health conditions of the study participants and the nationally weighted prevalence estimates of self-reported olfactory and measured dysfunction by these characteristics are shown in Table 1. Of particular importance to public safety, 9.5 % and 14.4 % were unable to correctly identify smoke and natural gas, respectively (data not shown). Sex and age differences also were observed for correct identification of these odors—males were less able to identify smoke and natural gas odors than were females (11.1 % vs. 8.1 % and 15.6 vs. 13.3 %, respectively). Especially vulnerable were participants 70 years and older; 20.3 % were unable to identify smoke correctly and 31.3 % were unable to identify natural gas correctly.
Table 1.
Nationally weighted prevalence (%) estimates of self-reported and measured smell dysfunction by socio-demographic and health characteristics in the 2012 NHANES adults aged 40+ years (N = 1281)
| sample size (n) | Any smell alterations (self-report) |
Smell problem during last year (self-report) |
Measured smell dysfunction (No. correct ≤5) |
||||
|---|---|---|---|---|---|---|---|
| Nationally weighted % | P-valueb | Nationally weighted % | P-valueb | Nationally weighted % | P-valueb | ||
| All participants | 1281 | 25.1 | 12.0 | 12.4 | |||
| Age, yearsa, Mean (SD) | 61.0 (11.7) | 62.0 (11.6) | 65.9 (11.7) | ||||
| Age in decades | <0.001 | <0.001 | <0.001 | ||||
| 40–19 years | 317 | 22.1 | 10.2 | 4.2 | |||
| 50–59 years | 335 | 24.2 | 10.0 | 10.5 | |||
| 60–69 years | 320 | 31.7 | 18.2 | 12.7 | |||
| 70–79 years | 190 | 22.3 | 9.0 | 25.3 | |||
| 80+ years | 119 | 25.0 | 13.3 | 39.4 | |||
| Sex | 0.643 | 0.776 | 0.036 | ||||
| Male | 647 | 24.7 | 12.2 | 14.1 | |||
| Female | 634 | 25.5 | 11.8 | 10.7 | |||
| Race/ethnicity | <0.001 | <0.001 | <0.001 | ||||
| Mexican American | 81 | 27.9 | 10.5 | 21.6 | |||
| Other Hispanic | 130 | 18.2 | 12.1 | 8.5 | |||
| Non-Hispanic (NH) white | 577 | 26.2 | 13.0 | 11.2 | |||
| NH black | 352 | 20.2 | 4.4 | 18.0 | |||
| NH Asian | 114 | 11.2 | 5.7 | 17.1 | |||
| Other races | 27 | 45.1 | 28.9 | 14.7 | |||
| Marital status | 0.047 | 0.190 | 0.024 | ||||
| Married | 764 | 23.2 | 11.7 | 11.8 | |||
| Not married | 517 | 28.7 | 12.6 | 13.6 | |||
| Education | 0.020 | 0.018 | 0.004 | ||||
| < High school | 289 | 27.4 | 8.8 | 21.4 | |||
| ≥ High school | 991 | 24.6 | 12.6 | 10.6 | |||
| Income-to-poverty ratio | 0.002 | 0.082 | <0.001 | ||||
| IPR ≤1.1 | 264 | 32.8 | 13.7 | 18.2 | |||
| 1.1 > IPR ≤2.0 | 315 | 23.1 | 11.7 | 13.3 | |||
| 2.0 > IPR ≤4.2 | 348 | 24.3 | 12.1 | 10.8 | |||
| IPR >4.2 | 353 | 23.9 | 11.4 | 10.9 | |||
| General health, self-rated | <0.001 | <0.001 | <0.001 | ||||
| Excellent | 126 | 6.3 | 4.5 | 14.1 | |||
| Very good | 361 | 25.0 | 10.5 | 9.7 | |||
| Good | 486 | 26.8 | 15.1 | 9.3 | |||
| Fair | 262 | 35.9 | 13.9 | 22.3 | |||
| Poor | 46 | 23.6 | 12.4 | 25.4 | |||
| Physical activity | 0.688 | 0.124 | <0.001 | ||||
| Yes | 800 | 25.0 | 11.3 | 8.9 | |||
| No | 481 | 25.4 | 13.6 | 20.1 | |||
| Smoking exposure | 0.099 | 0.935 | 0.055 | ||||
| None | 639 | 23.8 | 12.1 | 13.4 | |||
| Current smoker | 242 | 29.0 | 12.2 | 11.0 | |||
| Ever smoked | 399 | 24.7 | 11.7 | 11.6 | |||
| Heavy drinking, 4 to 5+ drinks/day | 218 | 39.6 | 0.003 | 17.7 | 0.003 | 17.6 | 0.004 |
| “YES,” EVER HAD... | |||||||
| Loss of consciousness due to head injury | 178 | 38.2 | 0.002 | 18.3 | <0.001 | 10.1 | 0.076 |
| Broken nose/serious injury to face/skull | 203 | 33.3 | 0.004 | 19.5 | <0.001 | 10.0 | 0.097 |
| 2+ sinus infections | 396 | 28.9 | 0.019 | 13.5 | 0.024 | 9.6 | 0.003 |
| Wisdom teeth removed | 880 | 24.8 | 0.639 | 12.4 | 0.235 | 13.2 | 0.035 |
| Tonsils removed | 366 | 32.9 | 0.006 | 17.4 | 0.001 | 17.6 | 0.002 |
| Ear infections, 3+ times | 259 | 29.3 | 0.002 | 11.3 | 0.145 | 12.5 | 0.878 |
| Ear tube placed to drain fluid | 48 | 26.4 | 0.768 | 5.1 | 0.001 | 6.1 | 0.034 |
| “YES”, IN LAST 12 MONTHS HAD... | |||||||
| Cold/flu ≥ 1 month | 67 | 30.1 | 0.232 | 22.3 | 0.028 | 9.8 | 0.177 |
| Persistent dry mouth | 172 | 37.2 | 0.004 | 19.9 | 0.002 | 17.9 | 0.012 |
| Frequent nasal congestion due to allergies | 326 | 33.6 | 0.011 | 16.5 | 0.004 | 11.6 | 0.155 |
| Current cold/flu symptoms – while being examined | 258 | 28.1 | <0.001 | 14.7 | 0.016 | 13.5 | 0.053 |
Value expressed as mean (SD, Standard Deviation)
Two-sided P-values for χ2
There are a number of similarities and differences to note about self-reported versus measured olfactory dysfunction. For instance, nearly equal proportions of participants reported “smell problems in the last year” and had “measured smell dysfunction,” yet double the rate had dysfunction by the smell alteration index, which combines responses of “smell problems in the last year” with “sense of smell is worse now compared to age 25” and “phantom odors – sometimes smelling an unpleasant, bad or burning odor when nothing is there.” While significant age differences were found for each of the self-reported outcomes, only measured smell dysfunction demonstrated a monotonically increasing prevalence with age. Furthermore, sex differences were observed only for measured smell dysfunction. Comparison of prevalence estimates by race/ethnicity revealed quite different patterns for each of the three outcomes. Increased prevalence of olfactory dysfunction, either measured or self-reported smell alteration index, was seen with lower educational attainment, the lowest quartile of income-to-poverty ratio, and fair or poor self-rated health status. In terms of lifestyle behaviors, heavy drinking was associated with a greater prevalence of olfactory dysfunction (measured and self-reported smell problem) whereas participating in physical activity showed a strong protective effect (measured smell dysfunction only).
Many of the chemosensory-related health conditions and exposures shown at the bottom of Table 1 have been previously reported as being related to olfactory function. In the NHANES 2012, many of these health conditions and exposures were found to be associated with increased prevalence of olfactory dysfunction, and many of them showed fairly consistent results across self-rated and measured olfactory dysfunction, including tonsillectomy and oral health problems. History of loss of consciousness from head injury, sinonasal problems, or serious nose/facial injury, on the other hand, were associated positively and significantly with the prevalence of self-reported smell alteration index and smell problems during the last year, but were inversely associated with measured smell dysfunction.
Self-reported smell function and smell alteration index
The frequency of NHANES 2012 participants reporting smell problems within the past year was 12.0 % (95 % CI: 7.7 %–18.3 %) (see Table 1), with 18 % reporting loss since age 25 (95 % CI: 12.5 %–25.2 %) and 7.1 % (95 % CI: 4.4 %–11.2 %) experiencing phantom odors. Considering responses to all three questions, the prevalence of any self-reported smell alteration was 25.1 % (95 % CI: 19.2 %–32.1 %) (Table 1). As reported earlier for the 3603 participants in NHANES 2011–2012 [9], all three questions contributed unique components to the combined smell alteration index.
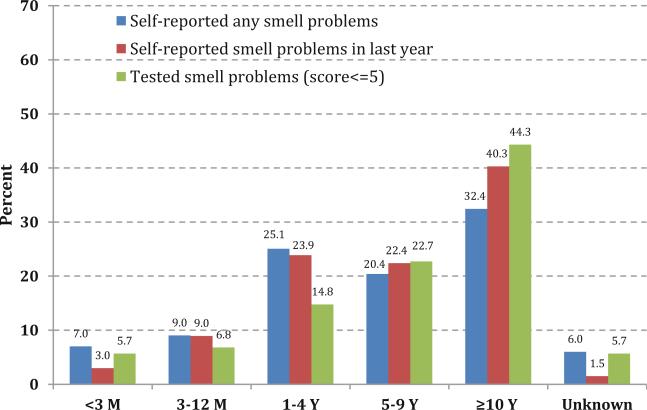
Of those noticing a change in their sense of smell, most were willing to report when they noticed a change in ability to smell (Fig. 2).
Fig. 2.
Distribution of the length of time elapsed since respondents experienced a change in their ability to smell (< 3 months [M], 3–12 M, 1–4 years [Y], 5–9 Y, 10 Y or more, unknown), based on whether they reported: a any alteration in their ability to smell (includes during last 12 months, since age 25, and/or phantosmia), b a smell problem in the last year, or c demonstrated a smell problem based on the measured exam results. This question on length of time was asked only if subjects self-reported an alteration in their sense of smell during the home interview prior to the olfactory exam in the MEC
There was consistency between the lengths of time reported since respondents first experienced a change in olfaction in Fig. 2, regardless of whether the olfactory dysfunction was self-reported or a measured (and self-reported) smell problem. The distributions by length of time were similar, averaging within the time frames shown of <3 months (5.2 %), 3 to 12 months (8.3 %), 1 to 4 years (21.3 %), 5 to 9 years (21.8 %), for ≥10 years (39.0 %), and for unknown (4.4 %).
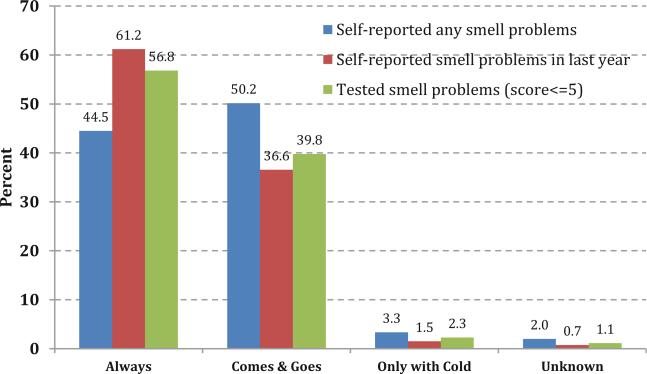
There also were similar distributions in terms of how frequently the participants experienced the problems for self-reported smell problems and measured smell dysfunction. As shown in Fig. 3, for adults with any self-reported smell alterations, including measured smell dysfunction, the distribution on average of each category was: “always present” (54.2 %), “the problem comes and goes” (42.2 %), “only with a cold” (2.4 %), and “unknown” (1.3 %).
Fig. 3.
Reported frequency of smell-related problems based on whether they reported: a any alteration in their ability to smell (includes during last 12 months, since age 25, and/or phantosmia), b a smell problem in the last year, or c demonstrated a smell problem based on the measured exam results. This question about constant versus fluctuating smell problems was asked only if subjects self-reported an alteration in their sense of smell during the home interview prior to the olfactory exam in the MEC
Comparing self-report with measured dysfunction
The sensitivity and specificity of self-report against measured smell dysfunction across the entire study sample is shown in Table 2. The highest accuracy (maximal sensitivity and specificity) was achieved through the smell alteration index of three questions (problems with smell during the past year, smell loss since age 25, and phantosmia). Although the individual questions achieved excellent specificity (correct identification of normosmia) at >85 %, they had poor sensitivity (correct identification of dysfunction) at <33 % (data not shown). The specificity of the smell alteration index varied only 3.4 % (77.3 % to 80.7 %) depending on the number correct on the NHANES PST. Conversely, the sensitivity varied nearly 24 % (30.6 % to 54.4 %) across the number correct on the PST. The highest sensitivity was dysfunction defined as ≤3 correct (anosmia/severe hyposmia) versus including the higher scores (i.e., hyposmia and normosmia). Based on maximal sensitivity and specificity, the best accuracy for the self-reported smell alteration index was found by classifying olfactory dysfunction as anosmia/severe hyposmia (≤3 on the PST).
Table 2.
Comparison of self-reported Smell Alteration Index (smell problem in the past year, smell loss since age 25, and phantoosmia) to the 8-item Pocket Smell Test, as scored by cut-points for measured olfactory dysfunction
| Cut-point (PST score) | Sample size, n (weighted %) | Specificity (%) | Sensitivity (%) | Accuracy (%) |
|---|---|---|---|---|
| ≤2 | 32 (2.4) | 77.3 | 53.6 | 76.8 |
| ≤3 | 62 (4.6) | 78.1 | 54.4 | 77.0 |
| ≤4 | 124 (9.2) | 78.5 | 43.2 | 75.5 |
| ≤5 | 251 (18.7) | 79.9 | 38.2 | 72.4 |
| ≤6 | 489 (36.4) | 80.7 | 30.6 | 62.8 |
9 Prevalence and risk factors associated with measured olfactory dysfunction
The NHANES 2012 sample sizes and the U.S. population estimates (nationally weighted percentages) for the distribution of anosmia/severe hyposmia, hyposmia, and normosmia by age, sex and race/ethnicity are shown in Table 3. Based on the number correctly identified on the olfaction test, 3.2 % (3.4 million) of U.S. adults ≥40 years of age were anosmic/severe hyposmic (scores 0–3), 9.2 % (9.8 million) were hyposmic (scores 4–5), and 87.6 % were normosmic (scores 6–8). Altogether the estimated prevalence of measured olfactory dysfunction (scores <6) was 12.4 % (13.2 million) with smell impairment, that is, measured smell dysfunction.
Table 3.
Nationally weighted prevalence (%) estimates of measured smell dysfunction (hyposmia and anosmia/severe hyposmia) and normal smell function (normosmia) defined by the Pocket Smell Test scores in the 2012 NHANES, adults aged 40+ years (N = 1281)
| Anosmia/Severe Hyposmia scores 0–3 (n = 58) |
Hyposmia scores 4–5 (n = 170) |
Normosmia scores 6–8 (n = 1054) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size (n) |
Population estimates (millions) |
Percent Distribution (%) |
Prevalence (%) |
Population estimates (millions) |
Percent distribution (%) |
Prevalence (%) |
Population estimates (millions) |
Percent distribution (%) |
Prevalence (%) |
|
| Age (years) | ||||||||||
| 40–49 | 317 | 0.1 | 3.2 | 0.3 | 1.2 | 12.3 | 3.7 | 31.9 | 33.2 | 96.0 |
| 50–59 | 335 | 0.8 | 23.0 | 2.5 | 2.6 | 26.2 | 8.1 | 29.3 | 30.4 | 89.2 |
| 60–69 | 320 | 0.5 | 14.2 | 2.0 | 2.7 | 27.0 | 10.8 | 22.1 | 22.9 | 87.2 |
| 70–79 | 190 | 1.3 | 37.1 | 9.9 | 2.0 | 20.0 | 15.5 | 9.7 | 10.1 | 73.7 |
| 80+ | 119 | 7.7 | 22.7 | 14.1 | 1.4 | 14.5 | 25.9 | 3.3 | 3.5 | 56.9 |
| Sex | ||||||||||
| Male | 647 | 2.5 | 73.5 | 4.8 | 4.8 | 48.9 | 9.3 | 44.5 | 47.4 | 85.8 |
| Females | 634 | 0.9 | 26.5 | 1.6 | 5.0 | 51.0 | 9.1 | 49.4 | 52.6 | 89.3 |
| Race/Ethnicity | ||||||||||
| Mexican American | 81 | 0.05 | 1.5 | 1.2 | 0.8 | 8.1 | 19.2 | 3.3 | 3.5 | 79.6 |
| Other Hispanic | 130 | 0.20 | 4.5 | 2.9 | 0.3 | 3.1 | 5.7 | 4.9 | 5.2 | 91.4 |
| NHa white | 577 | 2.60 | 77.1 | 3.2 | 6.4 | 65.5 | 7.9 | 72.2 | 76.9 | 88.8 |
| NHa black | 352 | 0.40 | 13.1 | 4.0 | 1.6 | 16.0 | 14.1 | 9.1 | 9.7 | 81.9 |
| NHa Asian | 114 | 0.06 | 1.9 | 1.9 | 0.5 | 5.2 | 15.3 | 2.8 | 3.0 | 82.7 |
| Other race | 27 | 0.07 | 2.0 | 3.6 | 0.2 | 2.1 | 11.1 | 1.6 | 1.7 | 85.3 |
| Total | 1281 | 3.40 | 100.0 | 3.2 | 9.8 | 100.0 | 9.2 | 93.9 | 100.0 | 87.6 |
NH = non-Hispanic
The prevalence of smell dysfunction increased with age, from lowest among the 40–49 year-olds with 4.0 % (96 % were normosmic) to highest in the ≥80 year-olds with 43.1 % (only 56.9 % were normosmic). Males were 3 times more likely to have anosmia/severe hyposmia than females, 4.8 % versus 1.6 %. The frequency of measured olfactory dysfunction was highest in Mexican American, NH black, and NH Asian, with the biggest difference in the classification of hyposmia. However, since the population distribution of age and sex varies across racial and ethnic groups in the U.S., we adjusted for age and sex before examining the race/ethnicity effects in logistic models (Table 4). The significant race/ethnicity associations increased in magnitude in the ageand sex-adjusted logistic model; this model may provide a truer assessment of the association between racial/ethnic groups and measured smell dysfunction.
Table 4.
Odds ratios (OR) and 95 % confidence intervals (CI) of measured olfactory dysfunction (PST scores ≤5) in NHANES 2012 with respect to selected, self-reported chemosensory alterations, other characteristics, and actions taken or quality of life experiences
| Univariate Model |
Age- and Sex-Adjusted Model |
|||
|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | |
| Age (years) | ||||
| 40–49 | 1 | – | 1 | – |
| 50–59 | 2.80 | 0.87–9.05 | 2.95 | 0.90–9.67 |
| 60–69 | 3.45 | 0.78–15.18 | 3.77 | 0.86–16.59 |
| 70–79 | 8.02 | 3.24–19.82 | 9.41 | 3.62–24.46 |
| 80+ | 15.57 | 6.44–37.61 | 19.93 | 7.85–50.62 |
| Male sex (vs. female) | 1.38 | 0.91–2.10 | 1.44 | 0.86–2.42 |
| Race/ethnicity | ||||
| Mexican American | 2.04 | 1.10–3.77 | 3.28 | 1.44–7.50 |
| Other Hispanic | 0.74 | 0.23–2.35 | 0.92 | 0.30–2.84 |
| Non-Hispanic (NH) white | 1 | – | 1 | – |
| NH black | 1.75 | 1.25–2.45 | 2.24 | 1.57–3.20 |
| NH Asian | 1.64 | 1.06–2.56 | 2.57 | 1.38–4.78 |
| Other race (includes multiracial) | 1.37 | 0.32–5.93 | 1.90 | 0.49–7.27 |
| Self-reported chemosensory function—derived from the Smell Alteration Index | ||||
| A. Self-reported smell problem last year | 4.09 | 2.18–7.68 | 4.68 | 2.29–9.59 |
| B. Ability to smell odors is worse now vs. age 25 (B. excludes A. smell problem during last year) | 1.50 | 0.90–2.53 | 1.43 | 0.72–2.84 |
| C. Had phantom odor only (C. excludes A. & B. smell problem last year or worse since age 25) | 1.93 | 0.43–8.68 | 2.70 | 0.72–10.16 |
| Self-reported chemosensory function—other characteristics | ||||
| Some odors bother you, but not other people | 0.75 | 0.42–1.35 | 0.99 | 0.61–1.60 |
| Self-reported taste problem last year | 2.17 | 0.80–5.90 | 1.91 | 0.65–5.58 |
| Ability to taste food flavors not as good as when he/she was 25 years old | 2.75 | 1.24–6.10 | 2.52 | 1.05–6.06 |
| Persistent taste in mouth last year (dysgeusia) | 1.65 | 0.58–4.71 | 1.88 | 0.59–6.03 |
| Actions taken or experiences in terms of quality of life due to taste or smell problems | ||||
| Ever discussed any taste or smell problem with, or change in ability to taste or smell problem, with a healthcare provider? | 1.22 | 0.48–3.14 | 1.10 | 0.48–2.51 |
| Tried any treatment recommended to improve your ability to taste or smell? | 1.70 | 0.06–48.46 | 1.83 | 0.05–61.88 |
| Experienced any problems with your general health, at work or in your environment because of a problem with your ability to taste or smell? | 4.16 | 0.30–58.72 | 3.67 | 0.40–33.36 |
NHANES National Health and Nutrition Examination Survey
a Univariate or unadjusted model: logistic regression model for each variable analyzed separately as a “predictor” of measured smell dysfunction
b Age- and sex-adjusted model: the effect of each variable is estimated in a logistic model that includes age and sex
Based on several questions asked on the CSQ, we assessed if measured olfactory dysfunction was associated with or “predicted” by self-reported chemosensory function responses in the univariate and age-and sex-adjusted logistic models (Table 4). A significant association was seen with one component of the smell alteration index (“smell problem during the past year”). Statistically removing the effects of this question rendered no significant effect of the self-report variable, “a worse sense of smell since the age 25.” Similarly, “smelling phantom odors” was not a significant factor after removing subjects who had reported either of the other two smell alteration index components. In other words, “reported smell problem during the past year” was the most proximal component of the smell alteration index associated with measured smell dysfunction. For the other questions on the CSQ, while many had increased odds ratios, the only other question that achieved significance was if the respondent said his/her ability to taste food flavors (perhaps reflecting retronasal olfaction) was worse compare to when younger (age 25). Adjusting for age and sex altered some of the odds ratios but did not change the significance of any of the CSQ questions shown in Table 4.
Finally, the “report of diminished quality of life due to chemosensory-related problems” had greatly increased odds ratios with respect to measured smell dysfunction in both un-adjusted and adjusted models in Table 4 (OR = 4.16 in the univariate model and OR = 3.67 after adjusting for age and sex). While the magnitude of these odds ratio estimates is impressive, the confidence intervals are too wide for even approaching statistical significance. This may well be due to the fact than only about one-fourth of respondents with measured smell dysfunction answered this question (only participants self-reporting smell or taste alterations were asked this question) and, in turn, the sample numbers for those responding affirmatively are relatively small. Both of these features contributed to a lack of statistical power.
Table 5 shows odds ratios (ORs) with 95 % CIs for risk (and protective) factors associated with measured smell dys-function in “univariate” analysis (each factor individually) and, also, in an age- and sex-adjusted analysis (showing the contribution of each factor, after accounting for age and sex). The variables significant in the univariate analysis included risks for olfactory dysfunction (older adults, aged 70–79 or 80+ years; race/ethnicity, Mexican American, NH black, and NH Asian compared to NH white; less than a high school education; xerostomia—persistent dry mouth; heavy alcohol drinking ever during respondents’ lifetime; ever had a tonsillectomy) and, also a protective factor for olfactory dysfunction (participating in moderate-to-vigorous physical activity ≥3 days in a typical week). Allowing for the effects of age and sex, additional variables as risks for olfactory dysfunction included: lowest IPR quartile (≤ 1.1). Associations of history of xerostomia and tonsillectomy with olfactory dysfunction lost significance in the age- and sex-adjusted analyses.
Table 5.
Odds ratios (ORs) and 95 % confidence intervals (CIs) for potential risk and protective factors associated with measured smell dysfunction, 2012 NHANES, adults aged 40+ years (N = 1281)
| Measured Smell Dysfunction (PST scores ≤5) |
||||
|---|---|---|---|---|
| Univariate Modela |
Age- and Sex-Adjusted Modelb |
|||
| Variables | OR | 95 % CI | OR | 95 % CI |
| Age (years) | ||||
| 40–49 | 1 | – | 1 | – |
| 50–59 | 2.80 | 0.87–9.05 | 2.95 | 0.90–9.67 |
| 60–69 | 3.45 | 0.78–15.18 | 3.77 | 0.86–16.59 |
| 70–79 | 8.02 | 3.24–19.82 | 9.41 | 3.62–24.46 |
| 80+ | 15.57 | 6.44–37.61 | 19.93 | 7.85–50.62 |
| Male sex (vs. female) | 1.38 | 0.91–2.10 | 1.44 | 0.86–2.42 |
| Race/ethnicity | ||||
| Mexican American | 2.04 | 1.10–3.77 | 3.28 | 1.44–7.50 |
| Other Hispanic | 0.74 | 0.23–2.35 | 0.92 | 0.30–2.84 |
| Non-Hispanic (NH) white | 1 | – | 1 | – |
| NH black | 1.75 | 1.25–2.45 | 2.24 | 1.57–3.20 |
| NH Asian | 1.64 | 1.06–2.56 | 2.57 | 1.38–4.78 |
| Other race (includes multiracial) | 1.37 | 0.32–5.93 | 1.90 | 0.49–7.27 |
| Married (vs. unmarried) | 0.86 | 0.48–1.55 | 0.84 | 0.44–1.59 |
| Income-to-poverty ratio (IPR) | ||||
| IPR ≤ 1.1 | 1.67 | 0.97–2.88 | 1.82 | 1.11–2.98 |
| 1.1 > IPR ≤ 2.0 | 1.10 | 0.43–2.82 | 0.81 | 0.37–1.79 |
| 2.0 > IPR ≤ 4.2 | 0.95 | 0.44–2.07 | 0.86 | 0.44–1.68 |
| IPR > 4.2 | 1 | – | 1 | – |
| Less than high school education | 2.29 | 1.37–3.83 | 2.01 | 1.11–3.63 |
| General health status, self-reported | ||||
| Excellent | 1 | – | 1 | – |
| Very good | 0.64 | 0.28–1.48 | 0.67 | 0.25–1.74 |
| Good | 0.60 | 0.24–1.46 | 0.61 | 0.23–1.65 |
| Fair | 1.76 | 0.53–5.82 | 1.78 | 0.50–6.35 |
| Poor | 2.03 | 0.38–10.88 | 2.18 | 0.36–13.25 |
| Moderate-to-vigorous physical activity during a typical week | 0.39 | 0.23–0.64 | 0.46 | 0.29–0.74 |
| Cigarette smoking | ||||
| Never | 1 | 1 | ||
| Current smoker | 0.79 | 0.47–1.36 | 0.94 | 0.47–1.85 |
| Ever smoked | 0.86 | 0.51–1.44 | 0.62 | 0.40–0.97 |
| Heavy drinking, 4 to 5+ drinks/day | 1.66 | 1.01–2.73 | 1.78 | 1.04–3.03 |
| Xerostomia (persistent dry mouth) | 1.65 | 1.05–2.59 | 1.30 | 0.73–2.31 |
| Loss of consciousness from head injury | 0.76 | 0.34–1.72 | 0.77 | 0.36–1.64 |
| Broken nose/other serious facial or skull injury | 0.75 | 0.30–1.88 | 0.84 | 0.34–2.12 |
| Wisdom teeth removed, ever had | 1.30 | 0.77–2.19 | 1.12 | 0.69–1.81 |
| Tonsillectomy, ever had | 1.94 | 1.13–3.31 | 1.54 | 0.84–2.84 |
| Multiple ear infections, ever had 3+ | 1.03 | 0.61–1.76 | 1.25 | 0.78–2.01 |
| Persistent cold/flu last 12 months | 0.76 | 0.09–6.32 | 0.86 | 0.08–9.05 |
| Frequent nasal congestion from allergies last 12 months | 0.90 | 0.55–1.48 | 1.01 | 0.63–1.63 |
NHANES National Health and Nutrition Examination Survey
Univariate or unadjusted model: logistic regression model for each variable analyzed separately as a “predictor” of measured smell dysfunction
Age- and sex-adjusted model: the effect of each variable is estimated in a logistic model that includes age and sex
10 Conclusion
The NHANES taste and smell protocol culminated from sustained efforts to integrate laboratory- and clinical-based understanding of taste and smell measures into population-based studies in order to capture variation in chemosensory function that may be associated with dietary and health outcomes. Building on previous research and the NIH Toolbox taste and smell assessments [6, 7], the NHANES protocol was designed to be brief, valid, reliable, and to minimize participant burden. With a computerized data collection system and standardized scripts, trained technicians administered the protocol to a nationally-representative sample of adults aged 40 or more years in the NHANES 2011–2012 and 2013–2014 data cycles. The initial public release of the 2011–2012 NHANES chemosensory data (N = 3603) included only self-report of chemosensory problems or alterations. An earlier report based on this initial public release found that 23 % self-reported smell alterations and 19 % self-reported taste alterations [9] in this nationally-representative sample of U.S. adults 40+ years of age.
This report is based on the limited access measured olfactory function data from the first year the chemosensory examination was implemented, NHANES 2012. The overall prevalence of measured olfactory dysfunction was 12.4 % in adults 40 or more years of age. The prevalence rate increased monotonically with each additional decade of age until reaching 39.4 % for participants 80 or more years of age. These findings showing steeply increasing prevalence with age parallel early reports from a largely community-based sample [55], the Baltimore Longitudinal study [56], and the National Geographic Smell Survey [57].
The overall rate reported for NHANES 2012 is higher than the prevalence of 3.8 % (n = 2838) reported in the Beaver Dam Offspring Study [12, 58] for younger participants, aged 21 to 84 years (mean age = 49 years), which utilized a technician-administered odor identification task, but less than the 19.1 % (n = 1387) for the Skövde population-based study [16] of subjects aged 20+ years, which also was technician-administered, the 22.1 % (n = 1277) for Dortmund Health Study [59] of subjects aged 25 to 75 years, which used an odor identification task, and the 20 % (n = 10,783) from the OLFACAT study [60] of subjects aged 15+ years, which used a combined newspaper-solicited survey and self-administered procedures.
The NHANES 2012 prevalence of measured olfactory dysfunction among older adults (≥70 years old) was 30 %, which is considerably lower than the approximately 50 % prevalence rates reported for older adults in the Rush Memory and Aging Project [61] (n = 481) and the Mayo Clinic Study of Aging [62] (n = 1430), but consistent with the nationally-representative sample in the National Social Life, Health, and Aging Project [63] (n = 1436) and the Epidemiology of Hearing Loss Study [11] (n = 2491).
The inability to identify the warning odors of smoke and natural gas, which was 20.3 % and 31.3 %, respectively, among adults ≥ 70 years in this study, is a major public health concern. Individuals with olfactory dysfunction are 2 to 3 times more likely to experience a hazardous event than individuals with normosmia [64], including burning or starting a fire while cooking, inability to smell smoke or a natural gas leak, or ingesting spoiled foods or toxic substances. Preventive efforts that can minimize these hazardous events among individuals with olfactory dysfunction include using smoke detectors and creating fire escape plans, dating foods, reading food labels, as well as following safe food handling to avoid ingesting spoiled foods.
Olfactory testing is not routine in primary care or specialized geriatric health care. Olfactory testing is recommended, for example, in the battery of neurological tests for Parkinson's Disease [65] as well as in the primary care for patients who present with an olfactory disorder, including suggested olfactory testing (e.g., [66]) for specific disease classifications by the International Classification of Diseases-10 codes [67] and referral if the patient reports changes in quality of life [68]. Self-report questions are used in primary care and gerontology to screen for sensory changes with aging, for example, with respect to changes in vision and hearing; however, testing is preferred [69]. The results from the current analysis suggest that a series of questions on ol-factory function within the last year, diminished ability to smell with aging, and presence of a phantom odor has reasonable sensitivity and specificity to identify individuals with severe hyposmia/anosmia as assessed by the PST with 8 odors. The utility of a series of questions to correctly self-identify smell dysfunction also was found among individuals with sinonasal disease [70]. Fluctuations in the sense of smell may partially explain the weak association between measured and self-rated olfactory ability [70]. Relatively poor association between measured and self-reported olfactory functioning also can result from using only a single question that fails to capture perceived change with aging [71], in part, because people may respond to this question from their current frame of reference to similarly (older) aged colleagues/peers. Although the overall prevalence of self-reported smell alteration was double that of measured dysfunction, a longitudinal study of olfactory loss suggests that cross-sectional measures underestimate olfactory losses with aging [63] and measured olfaction may not detect phantosmia. Finally, the associations between self-reported health, adverse exposures and function may vary by race/ethnicity [72].
Beyond advancing age, the other demographic risk factors for olfactory dysfunction in this study are consistent with previous reports. The present analysis showed that men were 3 times more likely than women to have test outcomes of anosmia/severe hyposmia but were equal in frequency for hyposmia. The sex difference with women outperforming men in odor identification and olfactory functioning has been reported in a number of epidemiological studies [62, 73–76] and, in a longitudinal study, olfactory decline with aging was greater among men than women [63]. Women may be more likely to notice and report changes in chemosensory problems and pathologies associated with impairment [77], which may partially explain why we did not observe a sex-difference in self-reported smell alteration [9].
Lower educational achievement also was reported as a risk factor of smell dysfunction in the National Social Life, Health and Aging Project, and explained some of racial/ethnicity differences between the sample of non-Hispanic whites and Hispanics [63]; similar associations were observed in the OLFACAT study, a large cohort study in Spain [60]. Low-income status, which is associated with poorer educational attainment, was reported in the Beaver Dam Offspring study [12] as a risk for olfactory dysfunction, which is consistent with the present findings.
Since the 2012–2014 NHANES oversampled Hispanic, NH black, and NH Asian [51] racial/ethnic groups, this provided a unique opportunity to examine olfactory dysfunction across sub-groups in the U.S. population. Although we did not observe racial/ethnic differences in self-reports of olfactory dysfunction [9], we observed a greater risk of measured olfactory dysfunction among adults who were Mexican Americans, NH black and NH Asian. These differences could have resulted from procedure effects, although the NHANES pilot testing and modifications of the PST to ensure cultural appropriateness aimed to minimize procedural differences. The parent forms of the PST, the 12-item B-SIT and 40-item UPSIT, have been shown to perform well in testing olfaction across diverse groups including Hispanics [74, 78] and Asians [79]. Other population-based studies also have reported differences in olfactory testing across race/ethnic groups [63, 80].
Consistent with our findings, NH blacks had lower olfactory functioning and a greater risk of loss of olfactory functioning with age than participants who were white or of European descent in the National Social Life, Health and Aging Project [63, 80]. One source of data on Asian Americans comes from the Honolulu-Asian Aging Study [81], an off-shoot of the Honolulu Heart Program, which included olfactory identification testing on over 2220 older men of Japanese heritage. Approximately half of the men aged 71 to 95 had olfactory dysfunction. This rate falls in the middle of that reported for older cohorts but above that reported in the current study (30 %). In another study utilizing a 40-item odor identification task, Korean Americans outperformed NH black and NH white adults, while Native Japanese scored the lowest [79]. Although we found greater risk of olfactory dysfunction among NH Asians, the NHANES 2012 data with relatively small numbers of Asians does not permit analyses by country of origin. Moreover, familiarity may influence performance on the odor identification task, particularly among adults who were raised in Asian countries with a different odor repertoire [82, 83]. It is noteworthy, however, that risk factors of self-reported olfactory dysfunction for Koreans, based on the Korean National Health and Nutrition Examination Survey [18], are similar to those reported for adults in the United States [9].
Reported history of smell-related exposures to clinical risk factors as well as lifestyle behaviors was associated with the risk of measured olfactory dysfunction in the univariate or unadjusted analyses yet some lost significance in the ageand sex-adjusted models. In unadjusted models only, reported histories of tonsillectomy and xerostomia increased the risk of olfactory dysfunction. Although currently the primary reasons for getting tonsillectomy are tonsillitis and chronic problems breathing during sleeping [84], this surgical procedure was common during the time when adults in the NHANES cohort were children. This was particularly the case among children who had medical insurance, were from upper income families, sought the care of older general practitioners or family physicians, and suffered from frequent upper respiratory tract infections [85], the latter of which is common in olfactory dysfunction [86]. We did not find that frequent ear infections (otitis media) and sinonasal symptoms, complications of upper respiratory tract infections for children [87] and adults [88] were positively associated with olfactory dysfunction. Our finding of increased risk of olfactory dysfunction in those with xerostomia is consistent with published findings [89, 90].
The present analysis did not find significant associations between measured olfactory dysfunction and some previously identified risk factors including head trauma [91] and sinonasal problems [92]. These risk factors did associate with the risk of self-reported smell alterations in our previous analysis of over 3600 participants of NHANES 2011–2012 [9]. In the univariate or multivariable analyses, head trauma, sinonasal problems, and xerostomia were independently associated with increased risk of self-reported smell alterations while the increasing age effects were not [9]. The lack of association between head trauma and measured olfaction in the present study is perplexing. The question asked in order to identify head trauma specified loss of consciousness but did not inquire about how recently the event had occurred or the frequency of occurrence (repeated events). The type of head trauma associated with olfactory dysfunction [91] may involve an interaction between physical activity, head trauma, and olfactory dysfunction. In addition, sinonasal problems can cause fluctuations in olfactory function that may not have been detected in the single NHANES olfactory test but may still have been recalled and mentioned in self-reports.
In agreement with findings from the Beaver Dam studies [93] and previously observed associations with self-reported olfactory alterations in NHANES 2011–2012 [9], we found that heavy drinking was associated with a greater risk of measured olfactory dysfunction. Alcoholism impairs the cognitive component of olfactory function that is tested by olfactory identification [94]. Heavy drinking also impairs the immune system [95], increasing the risk of infections that can damage the peripheral olfactory system. Smoking in this sample of older U.S. adults, however, was not identified as a risk factor for olfactory dysfunction; the logistic regression unexpectedly showed that past smoking, after adjusting for age and sex, was associated with decreased risk of olfactory dysfunction. The correction for age and sex, may be calling attention to the importance of quitting smoking for an extended period of time, which may allow for the recovery from the effects of past smoking. In population-based studies with cross-sectional analysis, smoking has been associated with greater risk of olfactory dysfunction [11, 59] as well as no association with olfactory dysfunction [16] or as a small protective factor [60]. Differences in findings across these population-based studies could have result from how smoking was defined. The current analysis did not consider additional information on the level of exposure to cigarette smoking, including amount smoked per day and number of years smoking, the recency of quitting, consideration of biomarkers of smoking (e.g., cotinine), and level of nicotine dependence, which may be better measures to capture associations between smoking behaviors and negative health outcomes [96]. Preliminary data from our laboratory suggests that chronic smokers have higher rates of olfactory dysfunction (assessed by odor identification) than reported in this NHANES analysis [97] and that heavy drinking and chronic smoking interact to explain risk of self-reported olfactory dysfunction [98]. Reduced risk of olfactory dysfunction was found with physical activity in this report and in the Epidemiology of Hearing Loss study [73]. Reported physical activity may be a proxy as well for an overall high level of health across aging [99].
A number of limitations of the current analysis need to be acknowledged. First, the cross-sectional design only allows reporting of associations; causal relations cannot be established. The sample size is limited for detailed population sub-group analysis, particularly with all of the risk factors and the racial/ethnic sub-groups available, as well as limited for multi-variation analyses. Future release of the larger NHANES 2013–2014 dataset will allow additional sensitivity and stratification analyses to further explore risk factors for olfactory dysfunction.
With regard to study procedures, the olfactory test administered did not repeat missed odors after feedback to minimize non-olfactory reasons for incorrect identification such as familiarity or testing situation [100]. The 8-item odor identification test (Pocket Smell Test) provided a screening for olfactory dysfunction that has reasonable sensitivity and specificity [14, 78], although has limitations with respect to detection of mild microsmia/hyposmia. Cognitive function was not examined as a risk factor for olfactory dysfunction in this report, which is a limitation, particularly as neurodegenerative disorders [101] increase the risk of olfactory dysfunction. Another limitation is self-report of studied risk factors, such as sinonasal problems and head trauma. Future analysis should also consider the pathways of the observed associations, including variables that mediate and moderate with regard to risk or protection (risk reduction) against olfactory dysfunction.
Despite these shortcomings, there are a number of strengths with regard to the findings presented in this paper. Aweighted analysis was used of a large representative national sample, increasing the generalizability of the findings to the current civilian, non-institutionalized U.S. population 40 or more years of age. The sample represented diversity in demographic factors including race/ethnicity, as well as education and especially income, which often are not possible to include in smaller scale population-based or community-based studies. The smell measures that were standardized and implemented in NHANES 2012 should have important implications for informing clinical practice in chemosensation, including how to screen for severe olfactory dysfunction.
These findings and future analyses of the NHANES taste and smell data may help to inform physicians and gerontologists about the likelihood and co-morbidities of chemosensory impairments, including appropriate counseling needed to mitigate the health and safety risks of chemosensory impairments in their patients. That 30 % of older adults appear unable to identify the natural gas odor underlines the importance of having working detectors in homes and the workplace, including the use of detectors for smoke, carbon monoxide and natural gas.
In support of Healthy People 2020 chemosensory objectives [3], findings from the 2013–2014 NHANES taste and smell protocol will become easily available via the internet to researchers, policy makers, and the general public. Findings from this first NHANES chemosensory protocol will have important implications in terms of informing Federal recommendations, programs and policies to increase research, education and healthcare services directed at improving prevention, diagnosis and treatment of chemosensory disorders in the United States. Future analyses of the chemosensory measures with dietary and other health measures concurrently collected in the NHANES will allow researchers to explore the effects of taste and smell alterations on dietary behaviors and diet-related health outcomes, such as excess adiposity, hypertension and hyperlipidemia. In addition, these NHANES data may contribute to identifying additional modifiable and non-modifiable risk factors of chemosensory disorders.
Acknowledgments
The authors wish to thank the study participants and the dedicated staff who helped organize and conduct the chemosensory (taste and smell) interview and examination components in the National Health and Nutrition Examination Survey (NHANES). We also wish to acknowledge the valuable contribution of our colleagues, Dr. Richard Doty (University of Pennsylvania), Dr. John Hayes (Pennsylvania State University), and Dr. Charles Dillon (Project Officer for the 2011-2014 NHANES Chemosensory Protocol, NCHS/CDC), as well as several experts in chemosensation science who provided critical counsel and encouragement over sustained periods, Dr. Linda Bartoshuk, Dr. Susan Coldwell, Dr. Beverly Cowart, Dr. Karen Cruickshanks, Dr. Pamela Dalton, Dr. Barry Davis [deceased], Dr. Claire Murphy, and multiple others. Also, Dr. Shristi Rawal was partially supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Preliminary analyses of the findings were presented at an invited Clinical Symposium for the annual meeting of the Association for Chemoreception Sciences meeting in Bonita Springs, Florida, April 9-12, 2014. The chemosensory component of NHANES 2011–2014 was supported by Interagency Agreement (Y1-DC-0013) between the National Institute on Deafness and Other Communication Disorders (NIDCD), National Institutes of Health (NIH) and the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC).
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Research Data Center, NCHS, CDC, or of the NIDCD and NICHD, National Institutes of Health, or of the University of Connecticut.
Footnotes
Compliance with ethical standards
Conflict of interest None of the authors has any conflict of interest to declare.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) [May 20, 2016];National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010. Available from: http://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf.
- 2.National Institutes on Deafness and Other Communication Disorders [May 20, 2016];NIDCD Workshop on Epidemiology of Communication Disorders. 2005 Available from: http://www.nidcd.nih.gov/funding/programs/pages/episummary.aspx.
- 3.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion [May 20, 2016];Healthy People 2020 Topics & Objectives: Hearing and Other Sensory or Communication Disorders. Available from: http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=20.
- 4.CDC. NCHS [May 20, 2016];National Health and Nutrition Examination Survey (NHANES) Taste and Smell Examination Component Manual. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/Taste_Smell.pdf.
- 5.NIH Blueprint for Neuroscience Research [May 20, 2016];NIH Toolbox For the Assessment of Neurological and Behavioral Function. Available from: http://www.nihtoolbox.org/WhatAndWhy/Sensation.
- 6.Coldwell SE, Mennella JA, Duffy VB, Pelchat ML, Griffith JW, Smutzer G, et al. Gustation assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S20–4. doi: 10.1212/WNL.0b013e3182872e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton P, Doty RL, Murphy C, Frank R, Hoffman HJ, Maute C, et al. Olfactory assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S32–6. doi: 10.1212/WNL.0b013e3182872eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NHANES. CDC. NCHS [May 20, 2016];2011–2012 Data Documentation, Codebook, and Frequencies: Taste and Smell Disorders [internet] Available from: http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/CSQ_G.htm.
- 9.Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, Duffy VB. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011-2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses. 2016;41(1):69–76. doi: 10.1093/chemse/bjv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawal S, Hoffman HJ, Chapo AK, Duffy VB. Sensitivity and specificity of self-reported olfactory dysfunction in a home-based study of independent-living. Healthy Older Women Chemosens Percept. 2014;7(304):108–16. doi: 10.1007/s12078-014-9170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy C, Schubert C, Cruickshanks K, Klein B, Klein R, Nondahl D. Prevalence of olfactory impairment in older adults. J Am Med Assoc. 2002;288(18):2307–12. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 12.Schubert CR, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, et al. Olfactory impairment in an adult population: the Beaver Dam Offspring Study. Chem Senses. 2012;37(4):325–34. doi: 10.1093/chemse/bjr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman H, Ishii E, MacTurk R. Age-related changes in the prevalence of smell/taste problems among the United States adult population. Results of the 1994 Disability Supplement to the National Health Interview Survey (NHIS). Ann N Y Acad Sci. 1998;855:716–22. doi: 10.1111/j.1749-6632.1998.tb10650.x. [DOI] [PubMed] [Google Scholar]
- 14.Rawal S, Hoffman HJ, Honda M, Huedo-Medina TB, Duffy VB. The taste and smell protocol in the 2011-2014 U.S. National Health and Nutrition Examination Survey (NHANES): test-retest reliability and validity testing. Chemosens Percept. 2015;8(3):138–48. doi: 10.1007/s12078-015-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wehling E, Nordin S, Espeseth T, Reinvang I, Lundervold AJ. Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch Clin Neuropsychol. 2011;26(3):260–9. doi: 10.1093/arclin/acr019. [DOI] [PubMed] [Google Scholar]
- 16.Bramerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: the Skovde population-based study. Laryngoscope. 2004;114(4):733–7. doi: 10.1097/00005537-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Shu CH, Hummel T, Lee PL, Chiu CH, Lin SH, Yuan BC. The proportion of self-rated olfactory dysfunction does not change across the life span. Am J Rhinol Allergy. 2009;23(4):413–6. doi: 10.2500/ajra.2009.23.3343. [DOI] [PubMed] [Google Scholar]
- 18.Lee WH, Wee JH, Kim DK, Rhee CS, Lee CH, Ahn S, et al. Prevalence of subjective olfactory dysfunction and its risk factors: Korean National Health and Nutrition Examination Survey. PLoS One. 2013;8(5):e62725. doi: 10.1371/journal.pone.0062725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michikawa T, Nishiwaki Y, Takebayashi T. Are you conscious of any age-related taste impairment? Prevalence of and factors associated with taste impairment in Japan. J Am Geriatr Soc. 2011;59(5):951–3. doi: 10.1111/j.1532-5415.2011.03397.x. [DOI] [PubMed] [Google Scholar]
- 20.Welge-Lussen A, Dorig P, Wolfensberger M, Krone F, Hummel T. A study about the frequency of taste disorders. J Neurol. 2011;258(3):386–92. doi: 10.1007/s00415-010-5763-5. [DOI] [PubMed] [Google Scholar]
- 21.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, et al. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82(1):109–14. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Galindo-Cuspinera V, Waeber T, Antille N, Hartmann C, Stead N, Martin N. Reliability of threshold and suprathreshold methods for taste phenotyping: characterization with PROP and sodium chloride. Chemosens Percept. 2009;2(4):214–28. doi: 10.1007/s12078-009-9059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, Klein S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring) 2014;22(5):E13–20. doi: 10.1002/oby.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelletier CA, Steele CM. Influence of the perceived taste intensity of chemesthetic stimuli on swallowing parameters given age and genetic taste differences in healthy adult women. J Speech Lang Hear Res. 2014;57(1):46–56. doi: 10.1044/1092-4388(2013/13-0005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAnally HM, Poulton R, Hancox RJ, Prescott J, Welch D. Psychosocial correlates of 6-n-propylthiouracil (PROP) ratings in a birth cohort. Appetite. 2007;49(3):700–3. doi: 10.1016/j.appet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Cruickshanks KJ, Schubert CR, Snyder DJ, Bartoshuk LM, Huang GH, Klein BE, et al. Measuring taste impairment in epidemiologic studies: the Beaver Dam Offspring Study. Ann N YAcad Sci. 2009;1170:543–52. doi: 10.1111/j.1749-6632.2009.04103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias AG, Rousseau D, Duizer L, Cockburn M, Chiu W, Nielsen D, et al. Genetic variation in putative salt taste receptors and salt taste perception in humans. Chem Senses. 2013;38(2):137–45. doi: 10.1093/chemse/bjs090. [DOI] [PubMed] [Google Scholar]
- 28.Rawal S, Hayes JE, Wallace MR, Bartoshuk LM, Duffy VB. Do polymorphisms in the TAS1R1 gene associate with broader differences in human taste intensity? Chem Senses. 2013;38(8):719–28. doi: 10.1093/chemse/bjt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer ME, Cruickshanks KJ, Pankow JS, Pankratz N, Schubert CR, Huang GH, et al. The associations between 6-n-propylthiouracil (PROP) intensity and taste intensities differ by TAS2R38 haplotype. J Nutrigenet Nutrigenomics. 2014;7(3):143–52. doi: 10.1159/000371552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinehart ME, Hayes JE, Bartoshuk LM, Lanier SL, Duffy VB. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol Behav. 2006;87(2):304–13. doi: 10.1016/j.physbeh.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Hayes JE, Sullivan BS, Duffy VB. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol Behav. 2010;100(4):369–80. doi: 10.1016/j.physbeh.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer ME, Cruickshanks KJ, Schubert CR, Pinto A, Huang GH, Klein BE, et al. The association of taste with change in adiposity-related health measures. J Acad Nutr Diet. 2014;114(8):1195–202. doi: 10.1016/j.jand.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simchen U, Koebnick C, Hoyer S, Issanchou S, Zunft HJ. Odour and taste sensitivity is associated with body weight and extent of misreporting of body weight. Eur J Clin Nutr. 2006;60(6):698–705. doi: 10.1038/sj.ejcn.1602371. [DOI] [PubMed] [Google Scholar]
- 34.Kveton J, Bartoshuk L. The effect of unilateral chorda tympani damage on taste. Laryngoscope. 1994;104(1):25–9. doi: 10.1288/00005537-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Yanagisawa K, Bartoshuk LM, Catalanotto FA, Karrer TA, Kveton JF. Anesthesia of the chorda tympani nerve and taste phantoms. Physiol Behav. 1997;63(3):329–35. doi: 10.1016/s0031-9384(97)00423-x. [DOI] [PubMed] [Google Scholar]
- 36.Sipiora ML, Murtaugh MA, Gregoire MB, Duffy VB. Bitter taste perception and severe vomiting in pregnancy. Physiol Behav. 2000;69(3):259–67. doi: 10.1016/s0031-9384(00)00223-7. [DOI] [PubMed] [Google Scholar]
- 37.Bartoshuk LM, Catalanotto F, Hoffman H, Logan H, Snyder DJ. Taste damage (otitis media, tonsillectomy and head and neck cancer), oral sensations and BMI. Physiol Behav. 2012;107(4):516–26. doi: 10.1016/j.physbeh.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Duffy VB, Peterson J, Bartoshuk LM. Associations between taste genetics, oral sensations and alcohol intake. Physiol Behav. 2004;82(2–3):435–45. doi: 10.1016/j.physbeh.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 39.Reed DR, Zhu G, Breslin PA, Duke FF, Henders AK, Campbell MJ, et al. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum Mol Genet. 2010;19(21):4278–85. doi: 10.1093/hmg/ddq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowart B, Yokomukai Y, Beauchamp G. Bitter taste in aging: compound-specific decline in sensitivity. Physiol Behav. 1994;56(6):1237–41. doi: 10.1016/0031-9384(94)90371-9. [DOI] [PubMed] [Google Scholar]
- 41.Campbell MC, Ranciaro A, Froment A, Hirbo J, Omar S, Bodo JM, et al. Evolution of functionally diverse alleles associated with PTC bitter taste sensitivity in Africa. Mol Biol Evol. 2012;29(4):1141–53. doi: 10.1093/molbev/msr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartoshuk LM, Duffy VB, Miller IJ., Jr PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav. 1994;56(6):1165–71. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 43.Tepper BJ. Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu Rev Nutr. 2008;28:367–88. doi: 10.1146/annurev.nutr.28.061807.155458. [DOI] [PubMed] [Google Scholar]
- 44.Akmal A, Kung J. Propylthiouracil, and methimazole, and carbimazole-related hepatotoxicity. Expert Opin Drug Saf. 2014;13(10):1397–406. doi: 10.1517/14740338.2014.953796. [DOI] [PubMed] [Google Scholar]
- 45.Coldwell S, Duffy V, Bartoshuk L, Griffith J, Hoffman H. The NIH Toolbox brief gustation assessment protocol (abstract).. Abstracts from the 35th Annual Meeting of AChemS—Chem Senses.2013. p. A114. [Google Scholar]
- 46.Doty R, Frye R. Influence of nasal obstruction on smell function. Otolaryngol Clin N Am. 1989;22:397–441. [PubMed] [Google Scholar]
- 47.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 48.Cain WS, Rabin MD. Comparability of two tests of olfactory functioning. Chem Senses. 1989;14(4):479–85. [Google Scholar]
- 49.Doty R, Shaman P, Dann M. Development of the University of Pennsylvania smell identification test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;34:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 50.Schumm LP, McClintock M, Williams S, Leitsch S, Lundstrom J, Hummel T, et al. Assessment of sensory function in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i76–85. doi: 10.1093/geronb/gbp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.CDC. NCHS [May 20, 2016];NHANES 2011–2012 Overview. 2015 Available at: http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/overview_g.htm.
- 52.CDC [May 20, 2016];2011-2012 National Health and Nutrition Examination Survey (NHANES): Survey Operations Manuals. Available from URL: http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/manuals11_12.htm.
- 53.U.S. Census [May 20, 2016];Poverty-Definitions. Available at: https://www.census.gov/hhes/www/poverty/methods/definitions.html.
- 54.Sabanayagam C, Shankar A. Income is a stronger predictor of mortality than education in a national sample of US adults. J Health Popul Nutr. 2012;20:82–6. doi: 10.3329/jhpn.v30i1.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441–2. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 56.Ship J, Weiffenbach J. Age, gender, medical treatment and medication effects on smell identification. J Gerontol. 1993;48:M26–32. doi: 10.1093/geronj/48.1.m26. [DOI] [PubMed] [Google Scholar]
- 57.Wysocki CJ, Gilbert AN. The National Geographic Smell Survey: effects of age are heterogeneous. Ann N Y Acad Sci. 1989;561:12–28. doi: 10.1111/j.1749-6632.1989.tb20966.x. [DOI] [PubMed] [Google Scholar]
- 58.Schubert CR, Cruickshanks KJ, Fischer ME, Huang GH, Klein R, Tsai MY, et al. Carotid intima media thickness, atherosclerosis, and 5-year decline in odor identification: the Beaver Dam Offspring Study. J Gerontol A Biol Sci Med Sci. 2015;70(7):879–84. doi: 10.1093/gerona/glu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vennemann M, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255(8):1121–6. doi: 10.1007/s00415-008-0807-9. [DOI] [PubMed] [Google Scholar]
- 60.Mullol J, Alobid I, Marino-Sanchez F, Quinto L, de Haro J, Bernal-Sprekelsen M, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ Open. 2012;2(6) doi: 10.1136/bmjopen-2012-001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson RS, Arnold SE, Tang Y, Bennett DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26(2):61–7. doi: 10.1159/000090250. [DOI] [PubMed] [Google Scholar]
- 62.Roberts RO, Christianson TJ, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, et al. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 2016;73(1):93–101. doi: 10.1001/jamaneurol.2015.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. The rate of age-related olfactory decline among the general population of older U.S. adults. J Gerontol A Biol Sci Med Sci. 2015;70(11):1435–41. doi: 10.1093/gerona/glv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pence TS, Reiter ER, DiNardo LJ, Costanzo RM. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol Head Neck Surg. 2014;140(10):951–5. doi: 10.1001/jamaoto.2014.1675. [DOI] [PubMed] [Google Scholar]
- 65.Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ, et al. Practice parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66(7):968–75. doi: 10.1212/01.wnl.0000215437.80053.d0. [DOI] [PubMed] [Google Scholar]
- 66.Aetna [May 20, 2016];Smell and Taste Disorders: Diagnosis. 2015 Available at: http://www.aetna.com/cpb/medical/data/300_399/0390.html.
- 67.ICD10Data.com Symptoms and signs involving cognition, perception, emotional state and behavior. [May 20, 2016];ICD-10-CM Diagnosis Codes. 2016 Available at: http://www.icd10data.com/ICD10CM/Codes/R00-R99/R40-R46/R43-.
- 68.Malaty J, Malaty IA. Smell and taste disorders in primary care. Am Fam Physician. 2013;88(12):852–9. [PubMed] [Google Scholar]
- 69.Eekhof JA, De Bock GH, Schaapveld K, Springer MP. Screening for hearing and visual loss among elderly with questionnaires and tests: which method is the most convincing for action? Scand J Prim Health Care. 2000;18(4):203–7. doi: 10.1080/028134300448751. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen AD, Shenton ME, Levitt JJ. Olfactory dysfunction in schizophrenia: a review of neuroanatomy and psychophysiological measurements. Harv Rev Psychiatry. 2010;18(5):279–92. doi: 10.3109/10673229.2010.511060. [DOI] [PubMed] [Google Scholar]
- 71.Knaapila A, Tuorila H, Kyvik KO, Wright MJ, Keskitalo K, Hansen J, et al. Self-ratings of olfactory function reflect odor annoyance rather than olfactory acuity. Laryngoscope. 2008;118(12):2212–7. doi: 10.1097/MLG.0b013e3181826e43. [DOI] [PubMed] [Google Scholar]
- 72.Kuk JL, Ardern CI. The influence of ethnicity and gender on the association between measured obesity and cardiorespiratory fitness with self-rated overweight, physical activity and health. Perspect Public Health. 2014;134(1):38–43. doi: 10.1177/1757913913480751. [DOI] [PubMed] [Google Scholar]
- 73.Schubert CR, Cruickshanks KJ, Nondahl DM, Klein BE, Klein R, Fischer ME. Association of exercise with lower long-term risk of olfactory impairment in older adults. JAMA Otolaryngol Head Neck Surg. 2013;139(10):1061–6. doi: 10.1001/jamaoto.2013.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menon C, Westervelt HJ, Jahn DR, Dressel JA, O'Bryant SE. Normative performance on the brief smell identification test (BSIT) in a multi-ethnic bilingual cohort: a project FRONTIER study. Clin Neuropsychol. 2013;27(6):946–61. doi: 10.1080/13854046.2013.796406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boesveldt S, Lindau ST, McClintock MK, Hummel T, Lundstrom JN. Gustatory and olfactory dysfunction in older adults: a national probability study. Rhinology. 2011;49(3):324–30. doi: 10.4193/rhino10.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doty RL, Petersen I, Mensah N, Christensen K. Genetic and environmental influences on odor identification ability in the very old. Psychol Aging. 2011;26(4):864–71. doi: 10.1037/a0023263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez-Martin P, Falup Pecurariu C, Odin P, van Hilten JJ, Antonini A, Rojo-Abuin JM, et al. Gender-related differences in the burden of non-motor symptoms in Parkinson's disease. J Neurol. 2012;259(8):1639–47. doi: 10.1007/s00415-011-6392-3. [DOI] [PubMed] [Google Scholar]
- 78.Krantz EM, Schubert CR, Dalton DS, Zhong W, Huang GH, Klein BE, et al. Test-retest reliability of the San Diego odor identification test and comparison with the brief smell identification test. Chem Senses. 2009;34(5):435–40. doi: 10.1093/chemse/bjp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doty RL, Applebaum S, Zusho H, Settle RG. Sex differences in odor identification ability: a cross-cultural analysis. Neuropsychologia. 1985;23(5):667–72. doi: 10.1016/0028-3932(85)90067-3. [DOI] [PubMed] [Google Scholar]
- 80.Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. Racial disparities in olfactory loss among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2014;69(3):323–9. doi: 10.1093/gerona/glt063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, et al. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann Neurol. 2008;63(2):167–73. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- 82.Shiga H, Yamamoto J, Kitamura M, Nakagawa H, Matsubasa T, Seo A, et al. Combinations of two odorants of smell identification test for screening of olfactory impairment. Auris Nasus Larynx. 2014;41(6):523–7. doi: 10.1016/j.anl.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 83.Ogihara H, Kobayashi M, Nishida K, Kitano M, Takeuchi K. Applicability of the cross-culturally modified University of Pennsylvania Smell Identification Test in a Japanese population. Am J Rhinol Allergy. 2011;25(6):404–10. doi: 10.2500/ajra.2011.25.3658. doi:10.2500/ajra.2011.25.3658. [DOI] [PubMed] [Google Scholar]
- 84.Patel HH, Straight CE, Lehman EB, Tanner M, Carr MM. Indications for tonsillectomy: a 10 year retrospective review. Int J Pediatr Otorhinolaryngol. 2014;78(12):2151–5. doi: 10.1016/j.ijporl.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 85.Grob GN. The rise and decline of tonsillectomy in twentieth-century America. J Hist Med Allied Sci. 2007;62(4):383–421. doi: 10.1093/jhmas/jrm003. [DOI] [PubMed] [Google Scholar]
- 86.Holbrook EH, Leopold DA. An updated review of clinical olfaction. Curr Opin Otolaryngol Head Neck Surg. 2006;14(1):23–8. doi: 10.1097/01.moo.0000193174.77321.39. [DOI] [PubMed] [Google Scholar]
- 87.Revai K, Dobbs LA, Nair S, Patel JA, Grady JJ, Chonmaitree T. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics. 2007;119(6):e1408–12. doi: 10.1542/peds.2006-2881. [DOI] [PubMed] [Google Scholar]
- 88.Linder JA, Singer DE. Health-related quality of life of adults with upper respiratory tract infections. J Gen Intern Med. 2003;18(10):802–7. doi: 10.1046/j.1525-1497.2003.21246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takano K, Yamamoto M, Kondo A, Takahashi H, Himi T. A clinical study of olfactory dysfunction in patients with Mikulicz's disease. Auris Nasus Larynx. 2011;38(3):347–51. doi: 10.1016/j.anl.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 90.Kamel UF, Maddison P, Whitaker R. Impact of primary Sjogren's syndrome on smell and taste: effect on quality of life. Rheumatology (Oxford) 2009;48(12):1512–4. doi: 10.1093/rheumatology/kep249. [DOI] [PubMed] [Google Scholar]
- 91.Coelho DH, Costanzo RM. Posttraumatic olfactory dysfunction. Auris Nasus Larynx. Apr. 2016;43(2):137–43. doi: 10.1016/j.anl.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Stuck BA, Hummel T. Olfaction in allergic rhinitis: a systematic review. J Allergy Clin Immunol. 2015;136(6):1460–70. doi: 10.1016/j.jaci.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 93.Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Olfactory impairment in older adults: five-year incidence and risk factors. Laryngoscope. 2011;121(4):873–8. doi: 10.1002/lary.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maurage P, Callot C, Chang B, Philippot P, Rombaux P, de Timary P. Olfactory impairment is correlated with confabulation in alcoholism: towards a multimodal testing of orbitofrontal cortex. PLoS One. 2011;6(8):e23190. doi: 10.1371/journal.pone.0023190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pasala S, Barr T, Messaoudi I. Impact of alcohol abuse on the adaptive immune system. Alcohol Res. 2015;37(2):185–97. doi: 10.35946/arcr.v37.2.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Selya AS, Oancea SC, Thapa S. Time to first cigarette, a proxy of nicotine dependence, increases the risk of pulmonary impairment, independently of current and lifetime smoking behavior. Nicotine Tob Res. 2016;18(6):1431–9. doi: 10.1093/ntr/ntv291. [DOI] [PubMed] [Google Scholar]
- 97.Glennon S-G, Litt M, Duffy VB. Olfactory but not taste dysfunction among chronic smokers: baseline results from an e-cigarette intervention.. Association for Chemoreception Sciences Annual Meeting; Bonita Springs, FL: Chemical Senses. 2016. in press (abstract) [Google Scholar]
- 98.Glennon S-G, Rawal S, Hoffman H, Duffy V. Chronic cigarette exposure associates with self-reported smell alterations: findings from the U.S. National Health and nutrition examination survey (NHANES) 2011-2012.. Association for Chemoreception Sciences Annual Meeting; Bonita Springs, FL: Chemical Senses. 2015. pp. 624–5. [Google Scholar]
- 99.Fishman EI, Steeves JA, Zipunnikov V, Koster A, Berrigan D, Harris TA, et al. Association between objectively measured physical activity and mortality in NHANES. Med Sci Sports Exerc. 2016 doi: 10.1249/MSS.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cain WS, Gent JF, Goodspeed RB, Leonard G. Evaluation of olfactory dysfunction in the Connecticut chemosensory clinical research center. Laryngoscope. 1988;98:83–8. doi: 10.1288/00005537-198801000-00017. [DOI] [PubMed] [Google Scholar]
- 101.Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014;5:20. doi: 10.3389/fpsyg.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]