Abstract
Objective
To compare the long-term outcomes among robotic, video-assisted thoracic surgery (VATS), and open lobectomy in stage I non-small cell lung cancer (NSCLC).
Summary Background Data
Survival comparisons between robotic, VATS, and open lobectomy in NSCLC have not yet been reported. Some studies have suggested that survival following VATS is superior, for unclear reasons.
Methods
Three cohorts (robotic, VATS, and open) of clinical stage I NSCLC patients were matched by propensity score and compared to assess overall survival (OS) and disease-free survival (DFS). Univariate and multivariate analyses were performed to identify factors associated with the outcomes.
Results
From January 2002 to December 2012, 470 unique patients (172 robotic, 141 VATS, and 157 open) were included in the analysis. The robotic approach harvested a higher number of median stations of lymph nodes (5 for robotic vs 3 for VATS vs 4 for open; P<0.001). Patients undergoing minimally invasive approaches had shorter median length of hospital stay (4 days for robotic vs 4 days for VATS vs 5 days for open; P<0.001). The 5-year OS for the robotic, VATS, and open matched groups were 77.6%, 73.5%, and 77.9%, respectively without a statistically significant difference; corresponding 5-year DFS were 72.7%, 65.5%, and 69.0%, respectively, with a statistically significant difference between the robotic and VATS groups (P=0.047). However, multivariate analysis found that surgical approach was not independently associated with shorter OS and DFS.
Conclusions
Minimally invasive approaches to lobectomy for clinical stage I NSCLC result in similar long-term survival as thoracotomy. Use of VATS and robotics is associated with shorter length of stay, and the robotic approach resulted in greater lymph node assessment.
INTRODUCTION
Despite the increasing use of minimally invasive procedures in recent years, thoracotomy remains the most common approach for lobectomy in the United States.1–3 However, multiple studies have demonstrated clear benefits of video-assisted thoracic surgery (VATS) over the traditional thoracotomy approach for early-stage NSCLC, including decreased length of hospital stay, decreased short-term postoperative pain, fewer complications,1–5 and even superior survival for unclear reasons.6
In recent years, robotic lobectomy has been increasingly used for early-stage NSCLC, owing to its advanced features, including three-dimensional visualization and small-wristed instruments, which can facilitate complex movements in a closed space. Technique feasibility,7–13 complications,14–20 and costs20–24 have been reported for robotic lobectomy. However, robust long-term data are lacking for robotic lobectomy used to treat NSCLC,25–27 and survival comparisons between robotic, VATS, and open lobectomy have not yet been reported.
In this study, we compare the outcomes among robotic, VATS, and open lobectomy in patients with clinical stage I NSCLC, with the purpose of evaluating the long-term overall survival (OS), disease-free survival (DFS), and perioperative outcomes of robotic lobectomy compared with propensity score matched groups of patients treated with VATS or open lobectomy.
METHODS
Patient Selection
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC). The study was conducted using data from a prospective database, comprising 2389 consecutive patients surgically treated for clinical stage I lung cancer at MSKCC between January 2002 and December 2012.
All patients included in the analysis fit the following criteria: (1) the disease was histologically defined NSCLC; (2) the disease was clinical stage I by the seventh American Joint Committee on Cancer (AJCC) staging system;28 (3) the patient underwent lobectomy; and (4) the resection was not preceded by preoperative induction therapy.
We excluded patients with a history of concurrent malignant disease or other previous primary cancers, patients with small cell lung cancer, and patients who had procedures other than lobectomy, such as wedge resection, segmentectomy, bilobectomy, pneumonectomy, or chest wall resection. Operative death was defined as death within 30 days of the operation or any time after the operation if the patient did not leave the hospital alive.
Patients were retrospectively classified into three groups on the basis of surgical approach: robotic lobectomy, VATS lobectomy, and thoracotomy lobectomy.
Surgical Procedures
This study covered a period of technology and technique transition at MSKCC. The choice of surgical approach of lobectomy was at the discretion of each individual surgeon. The details of the robotic,14,26 VATS, and open lobectomy procedures have been described previously.6 Overall, minimally invasive lobectomy (VATS or robotic) techniques conformed to the Cancer and Leukemia Group B (CALGB) 39802 consensus technique on VATS lobectomy.29 VATS lobectomy was performed via a 4-cm utility incision in the mid-axillary line, at the fourth or fifth intercostal space, without rib spreading. A port at the eighth intercostal space, at the anterior axillary line, was used for camera visualization, and a posterior port was used for lung retraction and stapler insertion. In the case of robotic lobectomy, a three-arm or four-arm approach utilizing similar incisions to the VATS approach and the da Vinci Robotic System (Intuitive Surgical, Mountain View, CA) were used. Thoracotomy lobectomy was performed through a posterolateral incision with either partial (serratus anterior) or full muscle sparing (both serratus anterior and latissimus dorsi). Systematic hilar and mediastinal lymph nodal dissection or sampling was performed in every case. Conversion was defined as the use of a rib-spreading thoracotomy at any point after initiation of a robotic or VATS dissection.
Follow up
Data regarding disease status and survival were recorded in the database from information provided at subsequent surveillance and treatment visits at our institution. For patients receiving additional treatment and/or follow-up outside of our center, information regarding their status was obtained by telephone follow-up or outside correspondence received by caregivers. Date and mode of death were obtained from various sources, including in-house deaths, Social Security Death Index updates, Medicare database searches, and death notifications from caregivers via physician offices to the Cancer Registry’s Death Notification mailbox.
Statistical Analysis
Patients were characterized by demographic and clinical variables, including age, sex, smoking history (current, former, or never), clinical stage (IA or IB), grade (well, moderately, poorly/undifferentiated, or unknown), forced expiratory volume in 1 second (FEV1), and spirometry diffusion capacity (DLCO). Differences in patient characteristics among the three surgery groups were evaluated using chi-squared tests for categorical variables and one-way ANOVA tests for continuous variables.
OS was defined as the time from surgery until death from any cause, with patients who did not die during the study period censored at the date of the last available follow-up. DFS was defined as the time from surgery until recurrence or death from any cause. OS and DFS were estimated using the Kaplan-Meier method and were compared across groups using univariate and multivariate Cox proportional hazards models in the full cohort.
Differences in patient characteristics among the surgical groups suggested that treatment assignment was subject to selection bias, which may not be completely accounted for in multivariate modeling. Therefore, we used a multinomial logistic model to construct propensity scores, an index for each patient representing the probability of receiving each type of surgical treatment. Each patient who underwent the robotic approach was matched with replacement with one VATS and one thoracotomy patient with similar propensity scores (within 3% probability of having a robotic procedure), resulting in surgical groups with similar probabilities of being assigned each type of surgery. The variables used for propensity-score matching were age, sex, smoking history, clinical stage, tumor cell differentiation grade, FEV1, diffusion, and pathology. Propensity score matching creates treatment groups in a way that approximates the effect of randomization, and therefore partially removes the bias that typically accompanies treatment assignment in non-randomized studies. It should be noted that because of matching with replacement, some patients who underwent VATS and thoracotomy were matched to more than one patient who underwent the robotic approach.
Univariate analyses for DFS and OS were repeated in the matched cohort using Cox models clustered by matched group (i.e., triplet of matched patients, one from each surgical group). The surgery effect for OS and DFS was then evaluated in the matched surgical groups using a multivariate Cox regression model adjusted for patient characteristics and clustered by matched group.
All statistical tests were two-tailed, and P<0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.2 (SAS Institute) and R, using the “survival,” “survcomp,” and “nnet” packages (version 3.1; R Development Core Team).
RESULTS
Patient Characteristics in the Unmatched Cohort
In total, 2132 cases fit the criteria for inclusion in this study: 184 robotic, 761 VATS, and 1187 open lobectomy. Almost two-thirds of patients treated with VATS were women (P=0.006); 80% of patients treated with robotic surgery or VATS were clinical stage IA, compared with only 60% of thoracotomy patients (P<0.001). The average pulmonary function (FEV1 percentage and DLCO percentage) was also slightly better in the robotic and VATS groups than in the open group (both P<0.001). Age, smoking history, tumor location, and tumor cell differentiation grade were similar among the three groups (Table 1).
Table 1.
Patient and Disease Characteristics from the Unmatched Database (n=2132)
| Characteristic | Approach
|
P | ||
|---|---|---|---|---|
| Robotic (n=184) |
VATS (n=761) |
Open (n=1187) |
||
| Age, mean (SD) | 68.2 (10.0) | 66.4 (10.3) | 67.0 (10.5) | 0.082 |
| Sex | 0.006 | |||
| Female | 104 (57) | 498 (65) | 699 (59) | |
| Male | 80 (43) | 263 (35) | 488 (41) | |
| Smokingc | 0.24 | |||
| Current | 24 (13) | 93 (12) | 166 (14) | |
| Former | 124 (67) | 505 (66) | 814 (69) | |
| Never | 36 (20) | 163 (21) | 206 (17) | |
| FEV1,a mean (SD) | 92.9 (19.4) | 93.2 (17.7) | 88.5 (22.1) | <0.001 |
| DLCO,a mean (SD) | 86.1 (25.0) | 88.1 (25.6) | 82.8 (22.3) | <0.001 |
| Clinical stage | <0.001 | |||
| IA | 147 (80) | 612 (80) | 730 (61) | |
| IB | 37 (20) | 149 (20) | 457 (39) | |
| Tumor site | 0.55b | |||
| RUL | 74 (40) | 288 (38) | 406 (34) | |
| RML | 5 (3) | 56 (7) | 94 (8) | |
| RLL | 39 (21) | 118 (16) | 211 (18) | |
| LUL | 48 (26) | 197 (26) | 298 (25) | |
| LLL | 18 (10) | 101 (13) | 177 (15) | |
| Cell differentiation | 0.58 | |||
| Well | 21 (11) | 97 (13) | 135 (11) | |
| Moderately | 98 (53) | 379 (50) | 563 (47) | |
| Poorly/undifferentiated | 41 (22) | 185 (24) | 315 (27) | |
| Unknown | 24 (13) | 99 (13) | 174 (15) | |
Data are no. (%) of patients, unless otherwise noted. DLCO, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in 1 second; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; SD, standard deviation; VATS, video-assisted thoracic surgery.
Percentage predicted. 3% of patients missing FEV1, and 7% missing DLCO.
Regrouped tumor site into left or right lobe.
One patient missing smoking history.
Survival Comparison in the Unmatched Cohort
A multivariate analysis of OS in the full cohort revealed associations with age, sex, tumor differentiation, and pulmonary function (Table 2). There was a trend of worse survival for thoracotomy patients, compared with robotic patients (HR=1.41; p=0.063), but there was no evidence of a significant difference between VATS patients and robotic patients.
Table 2.
Multivariate Analysis of Prognostic Factors for Death for the Unmatched Cohort (N=2132)
| Variable | Multivariate Analysis
|
||
|---|---|---|---|
| HR | 95% CI | P | |
| Age (continuous) | 1.04 | 1.03–1.05 | < 0.001 |
| Sex | |||
| Female | Reference | — | — |
| Male | 1.20 | 1.02–1.42 | 0.028 |
| Smoking | |||
| Never | Reference | — | — |
| Former | 1.09 | 0.84–1.40 | 0.53 |
| Current | 1.28 | 0.92–1.79 | 0.14 |
| Clinical stage | |||
| IA | Reference | — | — |
| IB | 1.40 | 1.18–1.67 | < 0.001 |
| Pathologic type | |||
| Adenocarcinoma | Reference | — | — |
| Squamous cell carcinoma | 1.03 | 0.83–1.29 | 0.78 |
| Other | 0.86 | 0.61–1.20 | 0.37 |
| Cell differentiation | |||
| Well | Reference | — | — |
| Moderately | 1.41 | 1.05–1.88 | 0.022 |
| Poorly/undifferentiated | 1.63 | 1.20–2.22 | 0.002 |
| Unknown | 1.66 | 1.09–2.52 | 0.018 |
| FEV1 (continuous) | 0.99 | 0.989–0.998 | 0.003 |
| DLCO (continuous) | 0.99 | 0.985–0.994 | <0.001 |
| Approach | |||
| Robotic | Reference | — | — |
| Open | 1.41 | 0.98–2.01 | 0.063 |
| VATS | 1.19 | 0.81–1.73 | 0.37 |
CI, confidence interval; DLCO, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in 1 second; HR, hazard ratio; VATS, video-assisted thoracic surgery.
Patient Characteristics of the Propensity Score Matched Patients
After propensity score matching, 172 cases were included in each surgical group (516 cases total). Because control subjects in the VATS and open groups were selected with replacement, the total number corresponds to 470 unique patients (172 of the 184 robotic patients, 141 of the 761 VATS patients, and 157 of the 1187 open patients were selected). Patient and disease characteristics were well-balanced among the three matched groups (Table 3).
Table 3.
Patient and Disease Characteristics of the Propensity Score Matched Groups (n=470)
| Characteristic | Approach
|
P | ||
|---|---|---|---|---|
| Robotic (n=172) |
VATS (n=141) |
Open (n=157) |
||
| Age, mean (SD) | 68.0 (10.2) | 67.5 (10.0) | 67.9 (9.6) | 0.89 |
| Sex | 0.22 | |||
| Female | 98 (57) | 88 (62) | 104 (66) | |
| Male | 74 (43) | 53 (38) | 53 (34) | |
| Smoking | 0.69 | |||
| Current | 24 (14) | 15 (11) | 25 (16) | |
| Former | 115 (67) | 100 (71) | 107 (68) | |
| Never | 33 (19) | 26 (18) | 25 (16) | |
| FEV1,a mean (SD) | 91.6 (17.4) | 90.3 (17.9) | 90.3 (18.3) | 0.75 |
| DLCO,a mean (SD) | 84.9 (23.1) | 85.4 (20.5) | 82.5 (22.9) | 0.46 |
| Clinical stage | 0.98 | |||
| IA | 139 (81) | 115 (82) | 128 (82) | |
| IB | 33 (19) | 26 (18) | 29 (18) | |
| Tumor site | ||||
| RUL | 69 (40) | 60 (43) | 55 (35) | 0.60b |
| RML | 5 (3) | 6 (4) | 10 (6) | |
| RLL | 36 (21) | 22 (16) | 27 (17) | |
| LUL | 46 (27) | 38 (27) | 45 (29) | |
| LLL | 16 (9) | 15 (11) | 20 (13) | |
| Cell differentiation | 0.82 | |||
| Well | 19 (11) | 23 (16) | 22 (14) | |
| Moderately | 91 (53) | 69 (49) | 73 (46) | |
| Poorly/undifferentiated | 39 (23) | 30 (21) | 40 (25) | |
| Unknown | 23 (13) | 19 (13) | 22 (14) | |
Data are no. (%) of patients, unless otherwise noted. DLCO, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in 1 second; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; SD, standard deviation; VATS, video-assisted thoracic surgery.
Percentage predicted.
Regrouped tumor site into left or right lobe.
Surgery-Related Outcomes of the Propensity Score Matched Patients
Table 4 summarizes surgery-related outcomes. Two patients died within 90 days of surgery, including 1 operative death (VATS group) because of pneumonia at day 50 after surgery. The other patient (open thoracotomy group) died of distant metastasis at day 89 after surgery. The rates of surgical complications were comparable among the three cohorts of patients (P=0.55), but the median length of hospital stay was shorter for the minimally invasive groups (P<0.001). The conversion rate from minimally invasive approaches to thoracotomy was comparable between the robotic group and the VATS group (P=0.32). The most frequent postoperative complications were arrhythmia, prolonged air leak, pneumonitis, pneumothorax, and atelectasis among the three cohorts of patients. The detailed types and grades of complications are listed in Table S1–3 (online only). The median number of stations of lymph nodes sampled in the robotic group was higher than that in the VATS or thoracotomy group (P<0.001). The migration from clinical stage I to more advanced pathologic stages after surgery was comparable among the three groups (P=0.13).
Table 4.
Surgery-Related and Postoperative Outcomes of the Propensity Score Matched Groups (n=470)
| Characteristic | Approach
|
P | ||
|---|---|---|---|---|
| Robotic (n=172) |
VATS (n=141) |
Open (n=157) |
||
| Mortality | 0 | 1 (1) | 0 | 0.30 |
| LOS, days, median (range) | 4 (1–32) | 4 (2–50) | 5 (2–29) | <0.001 |
| Conversion to open | 16 (9) | 8 (6) | — | 0.32 |
| Conversion for bleeding | 3 | 0 | — | |
| Conversion for other reasons | 13a | 8b | — | |
| Sampled LN stations, median (range) | 5 (0–8) | 3 (0–7) | 4 (1–8) | <0.001 |
| Cases with complications | 51 (29.7) | 35 (24.8) | 47 (29.9) | 0.55 |
| Resection completeness | 0.99 | |||
| R0 | 170 (99) | 141 (100) | 157 (100) | |
| R1 | 2 (1) | 0 | 0 | |
| Pathologic type | 0.82 | |||
| Adenocarcinoma | 19 (11) | 23 (16) | 22 (14) | |
| Squamous cell carcinoma | 91 (53) | 69 (49) | 73 (46) | |
| Carcinoid | 39 (23) | 30 (21) | 40 (25) | |
| Other | 23 (13) | 19 (13) | 22 (14) | |
| Pathologic N category | 0.64 | |||
| N0 | 145 (84) | 121 (86) | 137 (87) | |
| N1 | 20 (12) | 14 (10) | 11 (7) | |
| N2 | 7 (4) | 6 (4) | 9 (6) | |
| Pathologic stage | 0.13 | |||
| 0-Ic | 133 (77) | 114 (81) | 135 (86) | |
| II | 29 (17) | 21 (15) | 12 (8) | |
| III/IVd | 10 (6) | 6 (4) | 10 (6) | |
Data are no. (%) of patients, unless otherwise noted. LN, lymph node; LOS, length of stay in hospital; VATS, video-assisted thoracic surgery.
Due to extensive adhesion (5 cases), inadequate single lung ventilation (3 cases), technique inability to advance instrument through assistant ports (2 cases), poor visualization for delayed atelectasis and body habitus (1 case), technical problems with anesthesia machine (1 case), and enlarged but benign appearing hilar lymph nodes (1 case).
Due to extensive adhesion (1 case), inadequate single lung ventilation (2 cases), enlarged but benign appearing hilar lymph nodes (2 cases), unable to palpate lesions (2 cases), multiple lesions (1 case).
Two patients had stage 0 disease, both in the thoracotomy group.
One patient had stage IV disease (multiple pleural metastases) in the robotic group.
Survival Comparison in Propensity Score Matched Patients
The median follow-up time among survivors was 52.7 months for all the matched cases (open, 65.1 months [range, 0.3 to 147.8]; VATS, 52.7 months [range, 5.5 to 127.5]; robotic, 39.8 months [range, 0.6 to 137.9]). Recurrence occurred in 25 cases, 33 cases, and 37 cases for the robotic, VATS, and open matched groups, respectively. The 5-year OS for the robotic, VATS, and open matched groups were 77.6%, 73.5%, and 77.9%, respectively. There was no significant difference in OS among the three groups (Figure 1). Younger age, never smoking, clinical stage IA, adenocarcinoma, better pulmonary function, and well-differentiated tumor cells were prognostic factors favoring OS in univariate analysis (Table 5A). Only younger age, well-differentiated tumor cells, and better DLCO remained independently associated with better OS in multivariate analysis (Table 5B). Surgical approach was not associated with OS.
Figure 1.
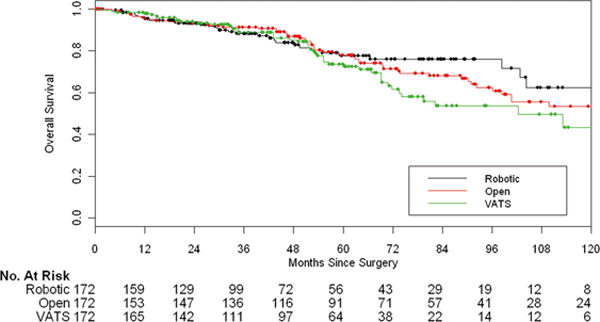
Overall survival in the matched cohort (n=516), by surgical approach (VATS vs robotic, P=0.10; open vs robotic, P=0.53).
Table 5A.
Univariate Analysis of Prognostic Factors for Death for the Propensity Score Matched Patients (n=516; 172 in each group)
| Variable | Univariate Analysis
|
|
|---|---|---|
| 5-year OS, % | Pa | |
| Age (continuous) | — | <0.001 |
| Sex | ||
| Female | 79.3 | — |
| Male | 71.5 | 0.33 |
| Smoking | ||
| Never | 86.9 | — |
| Former | 74.7 | 0.057 |
| Current | 72.5 | <0.001 |
| Clinical stage | ||
| IA | 79.0 | — |
| IB | 62.9 | 0.011 |
| Pathologic type | ||
| Adenocarcinoma | 78.3 | — |
| Squamous cell carcinoma | 63.4 | <0.001 |
| Other | 75.2 | 0.46 |
| Cell differentiation | ||
| Well | 95.0 | — |
| Moderately | 75.8 | 0.011 |
| Poorly/undifferentiated | 61.5 | <0.001 |
| Unknown | 81.3 | 0.20 |
| FEV1 (continuous) | — | <0.001 |
| DLCO (continuous) | — | <0.001 |
| Approach | ||
| Robotic | 77.6 | — |
| Open | 77.9 | 0.53 |
| VATS | 73.5 | 0.10 |
DLCO, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in 1 second; VATS, video-assisted thoracic surgery.
From univariate Cox regression models.
Table 5B.
Multivariate Analysis of Prognostic Factors for Death for the Propensity Score Matched Patients (516; 172 in each group)
| Variable | Multivariate Analysis
|
||
|---|---|---|---|
| HR | 95% CI | P | |
| Age (continuous) | 1.03 | 1.01–1.05 | 0.006 |
| Sex | |||
| Female | Reference | — | — |
| Male | 0.97 | 0.67–1.40 | 0.87 |
| Smoking | |||
| Never | Reference | — | — |
| Former | 1.15 | 0.61–2.19 | 0.66 |
| Current | 1.96 | 0.93–4.13 | 0.079 |
| Clinical stage | |||
| IA | Reference | — | — |
| IB | 1.33 | 0.82–2.16 | 0.24 |
| Pathologic type | |||
| Adenocarcinoma | Reference | — | — |
| Squamous cell carcinoma | 0.89 | 0.52–1.53 | 0.68 |
| Other | 0.73 | 0.37–1.44 | 0.37 |
| Cell differentiation | |||
| Well | Reference | — | — |
| Moderately | 2.19 | 1.32–3.63 | 0.003 |
| Poorly/undifferentiated | 2.77 | 1.53–5.01 | <0.001 |
| Unknown | 2.19 | 1.06–4.53 | 0.034 |
| FEV1 (continuous) | 1.00 | 0.99–1.01 | 0.42 |
| DLCO (continuous) | 0.98 | 0.97–0.99 | <0.001 |
| Approach | |||
| Robotic | Reference | — | — |
| Open | 1.07 | 0.62–1.83 | 0.82 |
| VATS | 1.36 | 0.80–2.33 | 0.26 |
CI, confidence interval; DLCO, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in 1 second; HR, hazard ratio; VATS, video-assisted thoracic surgery.
The robotic group had better DFS than the VATS group in univariate analysis (Figure 2), but this result was not confirmed by multivariate analysis. Younger age, never smoking, clinical stage IA, adenocarcinoma, better pulmonary function, and well-differentiated tumor cells (compared to poorly differentiated or undifferentiated) were also prognostic factors favoring DFS in univariate analysis (Table 6A). Younger age, never smoking (compared to current smokers), clinical IA, well-differentiated tumor cells, and better DLCO remained independently associated with better DFS in multivariate analysis (Table 6B).
Figure 2.
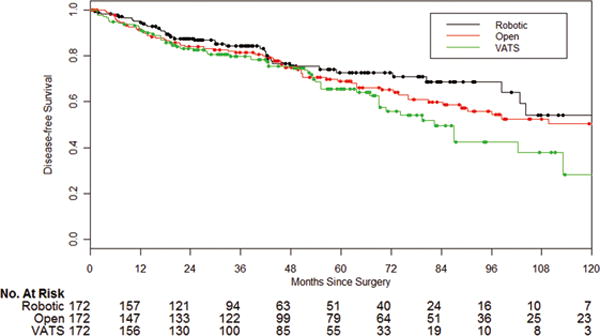
Disease-free survival in the matched cohort (n=516), by surgical approach (VATS vs robotic, P=0.047; open vs robotic, P=0.34).
Table 6A.
Univariate Analysis of Prognostic Factors for Recurrence or Death for the Propensity Score Matched Patients (516; 172 in each group)
| Variables | Univariate Analysis
|
|
|---|---|---|
| 5-year DFS, % | Pa | |
| Age (continuous) | — | <0.001 |
| Sex | ||
| Female | 71.3 | — |
| Male | 65.1 | 0.56 |
| Smoking | ||
| Never | 79.7 | — |
| Former | 68.2 | 0.044 |
| Current | 59.5 | <0.001 |
| Clinical stage | ||
| IA | 73.8 | — |
| IB | 44.3 | <0.001 |
| Pathologic type | ||
| Adenocarcinoma | 71.0 | — |
| Squamous cell carcinoma | 75.0 | <0.001 |
| Other | 50.9 | 0.26 |
| Cell differentiation | ||
| Well | 83.3 | — |
| Moderately | 71.5 | 0.13 |
| Poorly/undifferentiated | 45.7 | <0.001 |
| Unknown | 80.3 | 0.86 |
| FEV1 (continuous) | — | <0.001 |
| DLCO (continuous) | — | <0.001 |
| Approach | ||
| Robotic | 72.7 | — |
| Open | 69.0 | 0.34 |
| VATS | 65.5 | 0.047 |
DLCO, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in one second; VATS, video-assisted thoracic surgery.
From univariate Cox regression models.
Table 6B.
Multivariate Analysis of Prognostic Factors for Recurrence or Death for the Propensity Score Matched Patients (516; 172 in each group)
| Variable | Multivariate Analysis
|
||
|---|---|---|---|
| HR | 95% CI | P | |
| Age (continuous) | 1.03 | 1.01–1.04 | 0.002 |
| Sex | |||
| Female | Reference | — | — |
| Male | 0.83 | 0.59–1.16 | 0.27 |
| Smoking | |||
| Never | Reference | — | — |
| Former | 1.27 | 0.74–2.16 | 0.39 |
| Current | 1.99 | 1.07–3.71 | 0.031 |
| Clinical stage | |||
| IA | Reference | — | — |
| IB | 1.96 | 1.33–2.90 | < 0.001 |
| Pathologic type | |||
| Adenocarcinoma | Reference | — | — |
| Squamous cell carcinoma | 0.86 | 0.53–1.41 | 0.55 |
| Other | 0.80 | 0.43–1.49 | 0.48 |
| Cell differentiation | |||
| Well | Reference | — | — |
| Moderately | 1.60 | 1.02–2.50 | 0.042 |
| Poorly/undifferentiated | 2.61 | 1.55–4.40 | <0.001 |
| Unknown | 1.19 | 0.58–2.46 | 0.63 |
| FEV1 (continuous) | 1.00 | 0.99–1.01 | 0.88 |
| DLCO (continuous) | 0.985 | 0.975–0.995 | 0.002 |
| Approach | |||
| Robotic | Reference | — | — |
| Open | 1.12 | 0.73–1.74 | 0.60 |
| VATS | 1.44 | 0.92–2.26 | 0.11 |
CI, confidence interval; DFS, disease-free survival; DLCO, diffusing capacity of carbon monoxide; FEV1, forced expiratory volume in 1 second; HR, hazard ratio; VATS, video-assisted thoracic surgery.
DISCUSSION
In recent years, robotic lobectomy has been shown to be a feasible minimally invasive approach, compared with VATS and thoracotomy,30 but the long-term survival data for patients with NSCLC are lacking. In previous studies, we reported the long-term oncologic outcomes of robotic surgery for the treatment of early-stage NSCLC.25–27 However, these studies25–27 did not include a comparative arm of VATS or thoracotomy patients. In the current study, we compared long-term survival outcomes and postoperative outcomes among robotic, VATS, and open lobectomy approaches in patients with clinical stage I NSCLC by use of a propensity score matched database. Our results suggest that surgical approach is not associated with OS in patients with clinical stage I NSCLC. This is in contrast to previous publications suggesting a survival advantage to a VATS approach over thoracotomy that was independent of stage (5-year OS, 79% for VATS vs 75% for thoracotomy; P=0.08).6 The robotic approach resulted in more stations of lymph nodes sampled than the VATS and open approaches, and the MIS approaches were associated with shorter chest tube duration and length of hospital stay. This is consistent with prior, large series of VATS and robotic pulmonary resection. Patients who underwent the robotic approach had better DFS than the patients who underwent VATS, although this result was not confirmed by multivariate analysis.
A recent review of the State Inpatient Database demonstrated that robotic pulmonary resections have increased from 0.2% of all pulmonary resections in 2008 to 3.4% in 2010.3 In our study, in the raw, unmatched database, the robotic approach accounted for 8.6% (184/2132) of resections, which is much higher than the number in the national database; however, the higher percentage in our population is consistent with the fact that the robotic approach is used with a higher prevalence in large-volume hospitals.3 Whether the robotic or open approach results in less morbidity remains a matter of debate. Using the Nationwide Inpatient Sample, Paul et al. recently reported that robotic-assisted lobectomy was associated with a higher rate of intraoperative injury and bleeding, compared with thoracoscopic lobectomy.20 In the current study there was no difference in conversion rate to thoracotomy with either the robotic or VATS approaches. Three conversions in the robotic group were for bleeding, but none of those patients required transfusion or greater extent of resection as a result.
Some studies have suggested that the robotic approach is associated with less morbidity than the open approach,3,18 whereas others have suggested that morbidity is comparable between the two.17,19 We observed that complication rates and severity were comparable among the three cohorts while the length of hospital stay was shorter in the minimally invasive groups than in the open group. This is likely the result of more timely removal of chest tubes and quicker transition to oral pain medication. However, due to the retrospective nature of this study, there were no reliable data about the duration of chest tube drainage or the degree of postoperative pain in the medical record or the prospective database. The lack of these data is a limitation of the study.
Results of the American College of Surgery Oncology Group Z0030 Trial showed that there was no difference in the number of lymph nodes removed by VATS compared with open resections (median nodes removed: 15 vs 19, P=0.17).31 Owing to the advantages of three-dimensional optics, the stable camera platform, and the flexible instrumentation, one potential strength of the robotic approach is the thoroughness of the lymphadenectomy. Our results support this hypothesis, as more stations of lymph nodes were sampled in the robotic group than in the VATS or open groups. In addition, the proportion of pathological N1 category in the robotic group was higher than that in the VATS and open groups, indicating that the robotic approach might achieve more accurate nodal staging. Nevertheless, the difference of nodal categories and TNM upstaging was not statistically significant among the three groups. The small number of nodal positive cases may contribute to this result. We think this would change with the inclusion of more patients. Using the Society of Thoracic Surgeons database, Wilson et al. recently reviewed 302 patients with clinical stage I NSCLC after robotic lung resection, with the purpose of determining the rate of nodal upstaging.32 Their findings demonstrated that pathologic nodal upstaging occurred in 6.6% of patients who were pN1 and 4.3% of patients who were pN2.32 This finding was similar to ours, but, unfortunately, no comparison groups were included in their study.32 However, contrasting results have also been found. Using the Society of Thoracic Surgeons database, with a large volume of 11,531 cases, Boffa et al. reported that N1 upstaging was significantly lower in the VATS group than in the thoracotomy group (6.7% vs 9.3%; P< 0.001).33 However, 167 participants were involved in the study from Boffa et al.33 When only VATS-predominant participants were included in the analyses, the prevalence of upstaging from cN0 to pN1 was identical (8.7%) between the VATS group and the open group.33 At MSKCC, we also perform a large proportion of cases using minimally invasive approaches. From this perspective, our results are similar to those of Boffa et al.33 Licht et al. recently reported the outcomes of 1513 patients with clinical stage I NSCLC, using the Danish Lung Cancer Registry database; they found less upstaging in the VATS group than in the thoracotomy group, for both the N1 category (8.1% vs 13.1%; P<0.001) and the N2 category (3.8% vs 11.5%; P<0.001).34 Nevertheless, the national databases may suffer from selection bias, as the field of lymph node dissection and the individual surgeon’s skills and expertise may vary dramatically among different institutions. Similar to the current study, previous studies using data from single institutions have reported that the efficacy of lymph node dissection was comparable between VATS and open lobectomy.35,36
The current literature lacks long-term survival comparisons among robotic, VATS, and open lobectomy for patients with early-stage NSCLC. Wilson et al. reported only 2-year survival for patients treated with the robotic approach, owing to a short duration of follow-up; however, OS and DFS for robotic lobectomy in their study were comparable to those for VATS and thoracotomy in the historical data.32 We have previously reported the long-term survival prognosis of robotic surgery for the treatment of early-stage NSCLC, but comparison groups were not included.25–27 The current study is important because, to our knowledge, it is the first to compare long-term OS and DFS among robotic, VATS, and open lobectomy in the treatment of patients with clinical stage I NSCLC. In this study, we used propensity score matching to minimize the bias involved in treatment assignment. The use of propensity score matching and large sample sizes should increase the reliability of our results.
There were no significant differences in OS and DFS observed in our propensity matched cohorts of robotic, VATS and open lobectomy. On the basis of our findings, as well as the historical reports, we see promise in expanding patient access to robotic lung resections. A recent analysis using the State Inpatient Database found that, although the percentage of VATS lung resections has been increasing, thoracotomy was used in 56.6% of cases in 2010.3 The disadvantages of VATS, such as the two-dimensional view, camera tremor, and less freedom of instrumentation, result in a deep learning curve and, thus, hinder its widespread use.16
The limitations of this study must also be considered. First, although propensity score matching was performed to decrease selection bias among groups, bias might also exist due to the retrospective nature of the study. For example, the extent of lymph node removal might vary among cases. Nevertheless, since there are no clear standards on the surgical procedures for robotic and VATS lobectomy, and since the favored surgical approach varies dramatically among surgeons, it would likely be difficult to set up a randomized controlled clinical trial to compare the survival prognosis among robotic, VATS, and open approaches in patients with NSCLC. Second, because of the retrospective nature of this study, we did not compare costs, but the cost of robotic technology, especially in a time of increasing health care expenditures, may be a real issue. Nevertheless, we believe that with advances in the robotic technique, the device-related costs will decrease. Third, due to the retrospective nature, the time points for surveillance may not be necessarily consistent among groups, and this may impact the comparison of survival outcomes. In this study, some patients achieved long-term survival of over 10 years after surgery; however, the median follow-up time was 52.1 months for all the matched cases. We believe that a longer follow-up time is necessary to update the survival outcomes in the future. Lastly, the number of factors included in our propensity score model was limited by the data available for this cohort. An ideal model would include more factors.
In conclusion, after propensity score matching, there is no difference in OS between surgical approaches for lobectomy in the treatment of patients with clinical stage I NSCLC. Moreover, surgical approach is not independently associated with OS or DFS in contradistinction to suggestions of previous, single institution series of minimally invasive lobectomy for lung cancer. Minimally invasive approaches result in shorter length of hospital stay, compared with thoracotomy, and the robotic approach harvests more stations of lymph nodes than VATS or thoracotomy. Further studies comparing the benefits and indications for MIS approaches are warranted.
Supplementary Material
Acknowledgments
Hao-Xian Yang is supported, in part, by the State Scholarship Fund of the China Scholarship Council (201306385029). Kaitlin M. Woo and Camelia S. Sima are funded, in part, through the NIH Core Grant P30 CA00848.
Financial Support: This work was supported in part by National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Footnotes
All authors declare no conflicts of interest.
References
- 1.Paul S, Sedrakyan A, Chiu Y, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy:a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg. 2013;43:813–817. doi: 10.1093/ejcts/ezs428. [DOI] [PubMed] [Google Scholar]
- 2.Paul S, Altorki N, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: A propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–378. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg. 2014;97:236–242. doi: 10.1016/j.athoracsur.2013.07.117. [DOI] [PubMed] [Google Scholar]
- 4.Whitson BA, Andrade RF, Boettcher A, et al. Video-Assisted Thoracoscopic Surgery is More Favorable Than Thoracotomy for Resection of Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg. 2007;83:1965–1970. doi: 10.1016/j.athoracsur.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 5.Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg. 2014;148:637–643. doi: 10.1016/j.jtcvs.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 6.Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138:11–18. doi: 10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez JM, Humphries LA, Keeling WB, et al. Robotic lobectomy: fattening the learning curve. J Robotic Surg. 2012;6:41–45. doi: 10.1007/s11701-011-0275-6. [DOI] [PubMed] [Google Scholar]
- 8.Cerfolio RJ, Bryant AS. Robotic-assisted pulmonary resection - Right upper lobectomy. Ann Cardiothorac Surg. 2012;1:77–85. doi: 10.3978/j.issn.2225-319X.2012.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Augustin F, Bodner J, Maier H, et al. Robotic-assisted minimally invasive vs. thoracoscopic lung lobectomy: comparison of perioperative results in a learning curve setting. Langenbecks Arch Surg. 2013;398:895–901. doi: 10.1007/s00423-013-1090-5. [DOI] [PubMed] [Google Scholar]
- 10.Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot. 2012;8:448–452. doi: 10.1002/rcs.1455. [DOI] [PubMed] [Google Scholar]
- 11.Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg. 2012;94:929–934. doi: 10.1016/j.athoracsur.2012.04.086. [DOI] [PubMed] [Google Scholar]
- 12.Cerfolio RJ. Total port approach for robotic lobectomy. Thorac Surg Clin. 2014;24:151–156. doi: 10.1016/j.thorsurg.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Melfi FM, Fanucchi O, Davini F, et al. Robotic lobectomy for lung cancer: evolution in technique and technology. Eur J Cardiothorac Surg. 2014;46:626–630. doi: 10.1093/ejcts/ezu079. [DOI] [PubMed] [Google Scholar]
- 14.Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg. 2006;131:54–59. doi: 10.1016/j.jtcvs.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Gharagozloo F, Margolis M, Tempesta B. Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg. 2008;85:1880–1885. doi: 10.1016/j.athoracsur.2008.02.085. [DOI] [PubMed] [Google Scholar]
- 16.Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg. 2009;88:380–384. doi: 10.1016/j.athoracsur.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg. 2010;140:19–25. doi: 10.1016/j.jtcvs.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg. 2011;142:740–746. doi: 10.1016/j.jtcvs.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg. 2014;147:724–729. doi: 10.1016/j.jtcvs.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Paul S, Jalbert J, Isaacs AJ, et al. Comparative effectiveness of robotic-assisted vs thoracoscopic lobectomy. Chest. 2014;146:1505–1512. doi: 10.1378/chest.13-3032. [DOI] [PubMed] [Google Scholar]
- 21.Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin. 2008;18:297–300. doi: 10.1016/j.thorsurg.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Deen SA, Wilson JL, Wilshire CL, et al. Defining the cost of care for lobectomy and segmentectomy: a comparison of open, video-assisted thoracoscopic, and robotic approaches. Ann Thorac Surg. 2014;97:1000–1007. doi: 10.1016/j.athoracsur.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Nasir BS, Bryant AS, Minnich DJ, et al. Performing Robotic Lobectomy and Segmentectomy: Cost, Profitability, and Outcomes. Ann Thorac Surg. 2014;98:208–209. doi: 10.1016/j.athoracsur.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 24.Swanson SJ, Miller DL, McKenna RJ, Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier) J Thorac Cardiovasc Surg. 2014;147:929–937. doi: 10.1016/j.jtcvs.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 25.Park BJ. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg. 2012;1:24–26. doi: 10.3978/j.issn.2225-319X.2012.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park BJ. Robotic lobectomy for non-small cell lung cancer: long-term oncologic results. Thorac Surg Clin. 2014;24:157–162. doi: 10.1016/j.thorsurg.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg. 2012;143:383–389. doi: 10.1016/j.jtcvs.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 28.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 29.Swanson SJ, Herndon JE, 2nd, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802–a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25:4993–4997. doi: 10.1200/JCO.2007.12.6649. [DOI] [PubMed] [Google Scholar]
- 30.Iwata H. Minimally invasive pulmonary surgery for lung cancer, up to date. Gen Thorac Cardiovasc Surg. 2013;61:449–454. doi: 10.1007/s11748-013-0260-2. [DOI] [PubMed] [Google Scholar]
- 31.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141:662–770. doi: 10.1016/j.jtcvs.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg. 2014;97:1901–1906. doi: 10.1016/j.athoracsur.2014.01.064. [DOI] [PubMed] [Google Scholar]
- 33.Boffa DJ, Kosinski AS, Paul S, et al. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg. 2012;94:347–353. doi: 10.1016/j.athoracsur.2012.04.059. [DOI] [PubMed] [Google Scholar]
- 34.Licht PB, Jorgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg. 2013;96:943–949. doi: 10.1016/j.athoracsur.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Nomori H, Horio H, Naruke T, et al. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg. 2001;72:879–884. doi: 10.1016/s0003-4975(01)02891-0. [DOI] [PubMed] [Google Scholar]
- 36.Sagawa M, Sato M, Sakurada A, et al. A prospective trial of systematic nodal dissection for lung cancer by video-assisted thoracic surgery: can it be perfect? Ann Thorac Surg. 2002;73:900–904. doi: 10.1016/s0003-4975(01)03409-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


