Abstract
The spectrum of alcoholic liver disease (ALD) is a major cause of mortality with limited therapies available. Because alcohol targets numerous signaling pathways in hepatocytes and in immune cells, the identification of a master regulatory target that modulates multiple signaling processes is attractive. In this report, we assessed the role of spleen tyrosine kinase (SYK), a non-receptor tyrosine kinase, which has a central modulatory role in multiple pro-inflammatory signaling pathways involved in the pathomechanism of ALD.
Using mouse disease models that represent various phases in the progression of human ALD, we found that alcohol, in all of these models, induced SYK activation in the liver, both in hepatocytes and liver mononuclear cells. Further, significant SYK activation also occurred in liver samples and peripheral blood mononuclear cells of patients with ALD/alcoholic hepatitis compared to controls. Functional inhibition of SYK activation in vivo abrogated alcohol-induced hepatic neutrophil infiltration, resident immune cell activation, as well as inflammasome and ERK1/2-mediated NF-κB activation in mice. Strikingly, inhibition of SYK activation diminished alcohol-induced hepatic steatosis and IRF3-mediated apoptosis.
Conclusion
Our data demonstrate a novel, functional, and multicellular role for SYK phosphorylation in modulating immune cell-driven liver inflammation, hepatocyte cell death, and steatosis at different stages of ALD. These novel findings highlight SYK as a potential multifunctional target in the treatment of alcoholic steatohepatitis.
Keywords: alcoholic hepatitis, non-receptor tyrosine kinase, inflammasome, neutrophils
Introduction
Approximately 3.3 million annual mortalities worldwide are a result of alcohol misuse, with alcoholic liver disease (ALD) accounting for the majority of these deaths (1). The clinical pathophysiology of ALD involves a progressive spectrum of steatosis, steatohepatitis, fibrosis, cirrhosis, and increased risk of hepatocellular carcinoma (HCC) (2). Currently, there is no definitive treatment for progressive ALD, except for abstinence (3). The current standard of care with prednisolone for acute alcoholic hepatitis (AAH) has limited efficacy, many contraindications, and side effects (4–6). Recent studies revealed that pentoxyphiline alone or in combination with steroids failed to provide benefits in AAH (4–6). Recent pre-clinical investigations and reports have deciphered the numerous, diverse, and cumulative signaling pathways that drive the complex molecular pathophysiology of ALD (7–9).
The pathomechanism of ALD has been attributed to direct effects of alcohol and its metabolites inducing steatosis, cellular injury, and activation of hepatic cells through reactive oxygen species and proinflammatory cytokine induction in the liver (7–11). Chronic alcohol use is linked to increased gut permeability (12–16) leading to increased hepatic levels of endotoxins that induce the production of proinflammatory cytokines by activated immune cells (12–16).
Spleen tyrosine kinase (SYK) is an important regulator and treatment target against hepatitis C virus infection of hepatocytes (17). SYK belongs to the Src-family and is a 72 kDa non-receptor tyrosine kinase that was initially identified in hematopoietic cells (18). However, numerous studies, including ours, have shown that SYK is expressed in the cytoplasm and (to a small extent) in the nucleus of other cell types (17, 19). SYK functions mainly in the transmission of inflammatory downstream signals via an immunoreceptor tyrosine-based activation motif (ITAM) or Toll-like receptors (TLRs) which are the first to be activated in response to pathogen-associated molecular patterns (PAMP) (18–20). Upon activation, SYK modulates downstream signaling events that drive inflammatory pathways of both the innate and adaptive immune systems. Functional inhibition or deletion of SYK in macrophages significantly reduced FcγR, ITAM-coupled C-type lectins, or TLR4-ligand-induced IL-6, MCP-1, active IL-1β, and TNF-α production (20, 21). A prominent feature of alcoholic hepatitis (AH) (22) is neutrophil infiltration of the liver. SYK induces β2 integrin-mediated respiratory burst, spreading, and site-directed migration of neutrophils to inflammatory lesions (23). Given the broad cellular expression pattern of SYK (17, 18, 24) as well as its diverse cellular regulatory role of signaling pathways associated with ALD (8, 18, 20, 24), we hypothesized that SYK may constitute a master regulator in the pathophysiology of ALD and that functional inhibition of SYK may serve as a novel therapeutic target.
To assess the role of SYK in ALD, we took advantage of mouse disease models that mimic molecular pathophysiologic features of acute and chronic ALD in humans (22, 25, 26). The moderate alcoholic steatohepatitis model (NIAAA model) induces significant hepatic steatosis, injury, and hepatic neutrophil infiltration but is of a relatively short duration, and lacks significant hepatic macrophage infiltration/activation compared to that observed with the chronic five weeks alcohol Lieber-DeCarli model (7, 26). Using these mouse disease models of ALD that replicate key features of human disease, we found that chronic and moderate ASH alcohol feedings led to a significant increase of activated hepatic SYK (pSYKY525/526)(27). Strikingly, patients with AH showed high levels of activated pSYKY525/526 expression in liver samples and blood-derived immune cells compared to healthy controls. Functional inhibition of SYK in mice, using a potent chemical inhibitor, decreased hepatic SYK activation, ameliorated liver injury and inflammation, and reduced hepatic steatosis induced by alcohol. Our novel observations show for the first time that SYK may constitute a novel master regulatory therapeutic target in ALD.
Methods
Healthy subjects and patients with diagnosed AH or ALD
Subjects were recruited from the Hepatology clinic at the University of Massachusetts Medical School (UMMS) under an approved protocol reviewed by the UMMS Committee for the Protection of Human Subjects in Research (IRB #2284). All subjects who donated samples for this project provided signed written informed consent. Healthy blood donor volunteers were recruited from UMMS labs, hospital staff, graduate and medical students using advertisements. Subjects recruited for healthy individuals were defined as being free of any systemic and non-systemic diseases based on patients’ history and routine laboratory findings identified by their primary care physician. These individuals provided history of “only social” alcohol consumption. Subjects with AH were recruited from Gastroenterology clinics under institutional approved research protocols. Diagnosis of AH was based on the American College of Gastroenterology guidelines (28). An expert clinician performed the diagnosis based on medical history, physical examination, and laboratory data. Basic clinical parameters for patients are detailed in Table 1.
Table 1. Patient Sample Details.
Control liver samples were from donors who died from intracranial hemorrhage, self-inflicted gunshot wound, motor vehicle accident and cerebrovascular accident.
| Patients with cirrhosis and superimposed alcoholic hepatitis | Mean (STDV)/Description |
|---|---|
| Age, years | 50.7 (± 8.5) |
| Collection type | Transplant |
| Sex (Number) | M(7) F(1) |
| MELD | 31.2 (± 0.6) |
| Prothrombin Time (INR) | 2.4 (± 5.6) |
| Total Bilirubin | 7.6 (± 0.3) |
| Direct Bilirubin Delta | 1.7 (± 1.0) |
| Creatinine | 2.3 |
| Aspartate aminotransferase AST IU/L | 205.2 (±432.1) |
| Alkaline phosphatase | 174.4 (± 77.3) |
| Albumin | 3.32 (± 0.6) |
| Primary Diagnosis | Cirrhosis with superimposed alcoholic hepatitis |
| Patients with Alcoholic Liver Disease n=30 (M=17, F=13) | Mean ±SEM |
|---|---|
| Age, years | 47.00 (± 1.75) |
| Child-Pugh score | 7.52 (± 0.46) |
| Maddrey’s discriminant function | 16.49 (± 4.15) |
| MELD | 14.52 (± 1.38) |
| Creatinine, mg/dL | 0.92 (± 0.11) |
| Aspartate aminotransferase (AST), IU/L | 92.15 (± 15.03) |
| Alanine aminotransferase (ALT), IU/L | 35.2 (± 0.8) |
| ALT/AST ratio | 0.38 |
| Albumin, g/L | 3.25 (± 0.16) |
| Bilirubin, mg/dL | 8.51 (± 1.99) |
| Alkaline phosphatase, IU/L | 166.00 (± 24.10) |
| White blood cell count, 103/mm3 | 8.88 (± 1.01) |
| Neutrophils, 103/mm3 | 17.42 (± 9.07) |
| Lymphocytes >1600, n (%) | 8 (72.73%) |
| Ascites, n (%) | 12 (52.17%) |
| n=5 (male=4, female=1) | Healthy Blood Donors |
| Age, years | 31 (±3.2) |
Animals
10–12 week old C57BL/6 female mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and housed at the UMMS animal facility. All animals received humane care in accordance with institutional guidelines for the care and use of animals in research. All animals used for the two experimental models detailed below had unrestricted access to water at all times throughout the entire experimental period.
Chronic alcohol feeding model: (n=8–12 mice per group). Mice were fed the Lieber-DeCarli ad libitum diet (BioServ) with 5% (vol/vol) ethanol (36% ethanol-derived calories) for 5 weeks; pair-fed control mice were fed a calorie-matched dextran-maltose diet. Intragastric administration of ethanol (5 g/kg body weight), or isocaloric dextran-maltose, was performed in some mice using 22-gauge stainless steel feeding tubes. Some mice were treated with the SYK inhibitor R406 (Selleckchem cat # S2194) (daily i.p. injections 10mg/kg) from day 5 on 5% ethanol diet until day 15. R406 was reduced to 5mg/kg (daily i.p. injections) for the final 10 days of the 5% alcohol diet. Control mice received vehicle treatment i.p. every day for the entire experimental period.
Moderate alcoholic steatohepatitis (ASH) ethanol feeding model: (n=8–12 mice per group): Mice for this experimental group were fed a control or ethanol diet according to the NIAAA model as recently described (22). Some mice received daily i.p. injections of R406 (10mg/kg) after 5 days on a 5% ethanol diet and established liver injury based on serum ALT. Control mice received vehicle treatment i.p. daily for the entire experimental period. After 10 days on 5% alcohol diet mice received a single gavage of 5g/kg body weight (20% vol/vol in sterile phosphate buffer saline) or sucrose solution gavage for mice in the control group.
At the end of all animal experiments, cheek blood samples were collected in serum collection tubes (BD Biosciences, San Jose, CA USA) and processed within the hour. Following blood collections, mice were euthanized and liver samples harvested and stored appropriately for further analysis.
Please see supplementary information for additional methods.
Results
Alcohol increases activated SYK (pSYKY526/526) levels in the liver in mice and circulating mononuclear cells in ALD and AH patients
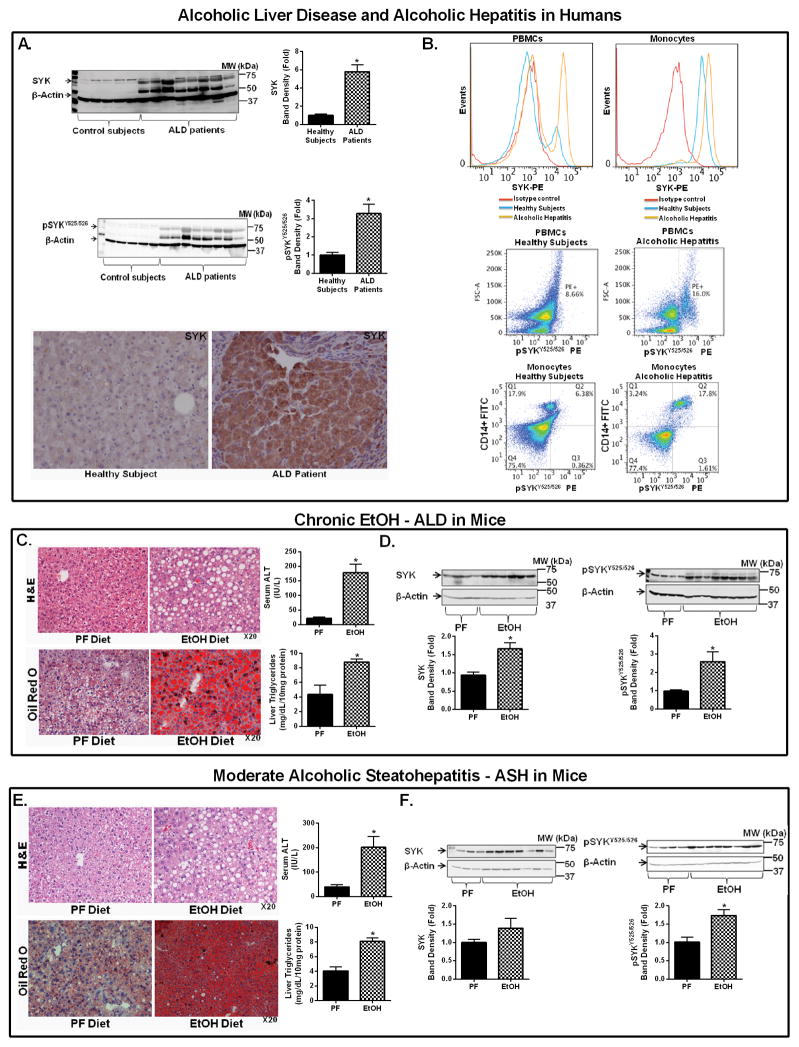
Previous reports demonstrated that functional inhibition of SYK reduces the severity of inflammatory diseases (18). Here we evaluated the role of SYK in the molecular pathomechanism of ALD. The potential human relevance of this hypothesis was confirmed by testing SYK expression in patients with ALD. We found a striking increase in total SYK and activated SYK [pSYKY525/526] expression in the liver of ALD patients compared to controls (Fig. 1A). Further, AH patients had higher SYK and activated pSYKY525/526 expression in circulating peripheral blood mononuclear cells (PBMCs) and monocytes (Fig. 1B & Sup. Fig. 1A,B) compared to healthy controls.
Figure 1. Increased SYK and pSYKY525/526 expression in mouse disease models and ALD and AH patients.
Total liver protein extracted from control subjects and ALD patients was analyzed for total SYK and activated pSYKY525/526 expression by western blot using β-actin as a loading control (A). Liver samples from control subjects were analyzed by immunohistochemistry for SYK expression (A). Flow cytometry was used to assess SYK and pSYKY525/526 expression in total PBMCs and monocytes from healthy control individuals and AH patients (B). Liver samples from C57BL/6 mice that received chronic EtOH feeding (C & D) or moderate ASH EtOH feeding (E & F) were stained by H&E or Oil-red-O, and liver injury and steatosis were quantified by measuring serum ALT and liver triglycerides, respectively (C & E). Total liver protein was analyzed for total SYK and activated pSYKY525/526 (A, D & F) expression by western blot using β-actin as a loading control. Human liver sample data is representative of 5 control donors and 8 patients with cirrhosis and superimposed alcoholic hepatitis. Flow cytometry analysis is representative of 5 healthy donors and 5 treatment naïve patients with diagnosed alcoholic hepatitis. Mice experiments are representative of 7–10 mice per experimental group. *p<0.05 compared to baseline was considered statistically significant by ANOVA.
Using two mouse models, the five-week chronic Lieber-Decarli model and the moderate ASH model (22, 25), we assessed potential differences between chronic and moderate ASH alcohol effects. Histopathological analysis revealed that both models induced liver damage (H&E), serum alanine aminotransferase (ALT) increase, and steatosis (Oil Red O staining and liver triglycerides) compared to control mice (Fig. 1C,E). Pro-inflammatory cytokine mRNA (TNF-α, MCP-1 and IL-1β) levels were also increased in the livers of alcohol-fed but not control mice (Sup. Fig. 1C). In chronic alcohol feeding, both total and activated SYK [pSYKY525/526] (Fig. 1D) were significantly up-regulated in the liver compared to isocaloric pair-fed controls. Alcohol feeding in the moderate ASH model increased total SYK levels and significantly increased activated pSYKY525/526 (27) levels (Fig. 1F).
In vivo SYK inhibitor treatment attenuates ERK1/2 phosphorylation, NF-κB activation, and proinflammatory cytokines in ALD
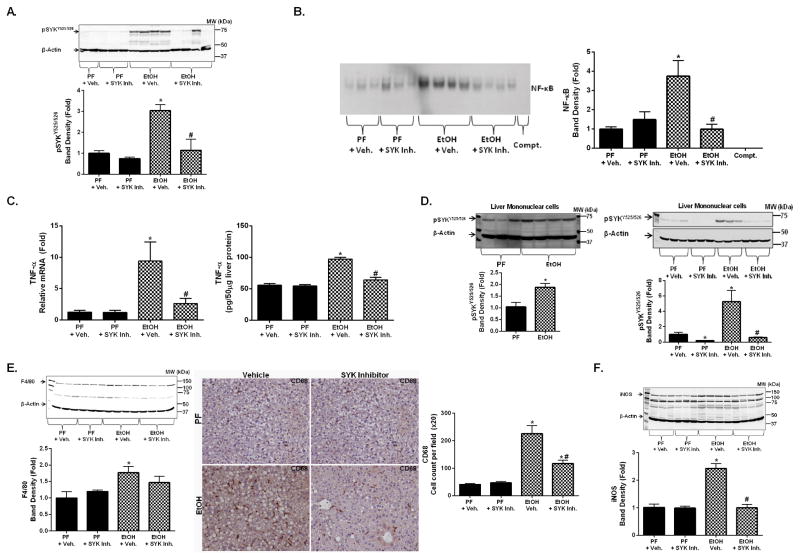
Given our observations that alcohol-induced liver injury was associated with elevated levels of activated pSYKY525/526 in patient samples and mice (Fig. 1), we next tested the effect of a SYK inhibitor, R406 (29), on proinflammatory cytokine secretion. In preliminary experiments we tested the SYK inhibitor in macrophages stimulated with TLR ligands that are known inducers of SYK-mediated proinflammatory cytokine secretion (20). We found that SYK inhibitor treatment (0.5 and 1μM) for 1h prior to TLR ligand activation (TLR4 [lipopolysaccharide, LPS], TLR8 [CLO75] and TLR7 [R837]) resulted in a significant decrease in TNF-α secretion and inhibition of pSYKY525/526 compared to ligand-activated controls without SYK inhibitor treatment (Sup. Fig. 2A,B). Next we tested the utility of in vivo SYK inhibition in ALD in the chronic alcohol feeding model (Sup. Fig. 2C). In vivo administration of the SYK inhibitor prevented SYK activation (pSYKY525/526 levels) induced by chronic alcohol feeding (Fig. 2A) (45).
Figure 2. Functional inhibition of SYK inhibits alcohol-induced pSYKY525/526, NF-κB nuclear binding, and pro-inflammatory mediators.
C57BL/6 mice were fed a chronic alcohol or an isocaloric pair fed (PF) diet with SYK inhibitor (SYK Inh.) treatment or vehicle treatment (+ Veh.). Total liver protein was analyzed for activated pSYKY525/526 by western blot (A). NF-κB nuclear binding was assessed from liver nuclear extracts by electrophoretic mobility shift assay (B). Total liver RNA and protein was assessed by RT-qPCR and ELISA for TNF-α mRNA and protein, respectively (C). Total liver mononuclear cell protein was analyzed for activated pSYKY525/526 by western blot (D). Total liver protein and liver samples were analyzed for F4/80 by western blot (E), CD68 by immunohistochemistry (E) and iNOS protein by western blot (F). A p-value <0.05 was considered statistically significant by ANOVA for 7–10 mice per experimental group (*, p<0.05 compared to baseline; #, p<0.05 compared to the EtOH + vehicle group).
SYK is an upstream regulator of multiple inflammation-associated signaling pathways (18). We found decreased NF-κB nuclear binding in acute or chronic alcohol-treated mice with SYK inhibition compared to without SYK inhibition (Fig. 2B). Alcohol-induced ERK1/2 phosphorylation in the liver was also inhibited by the SYK inhibitor (Sup. Fig. 2D). SYK inhibitor administration also reduced the alcohol-related increases in TNF-α both at mRNA and protein levels (Fig. 2C). These observations suggested that functional SYK inhibition prevented downstream signaling events that drive hepatic proinflammatory cytokine production in ALD.
Chronic alcohol-induced hepatic macrophage activation is significantly reduced with SYK inhibitor treatment
Evidence from clinical and experimental models show that chronic alcohol use results in significant hepatic macrophage infiltration and activation (12). We found that isolated liver mononuclear cells from mice with chronic alcohol feeding had increased activated pSYKY525/526 levels compared to controls (Fig. 2D) and this finding mirrored SYK activation seen in circulating human immune cells (Fig. 1A). In the chronic alcohol mouse model, SYK inhibitor treatment prevented the alcohol-induced increase of F4/80 (a Kupffer cell/macrophage marker) expression at the mRNA and protein levels in the liver (Fig. 2E). Furthermore, SYK inhibitor treatment decreased activated hepatic macrophages (Fig. 2E), CD68 immunohistochemistry, and liver iNOS protein (Fig. 2F) expression, indicative of macrophage activation. Together, these observations suggested that SYK inhibitor treatment can reduce chronic alcohol-induced hepatic macrophage activation.
Alcohol-induced hepatic neutrophil infiltration is ameliorated by pharmacological inhibition of SYK activation
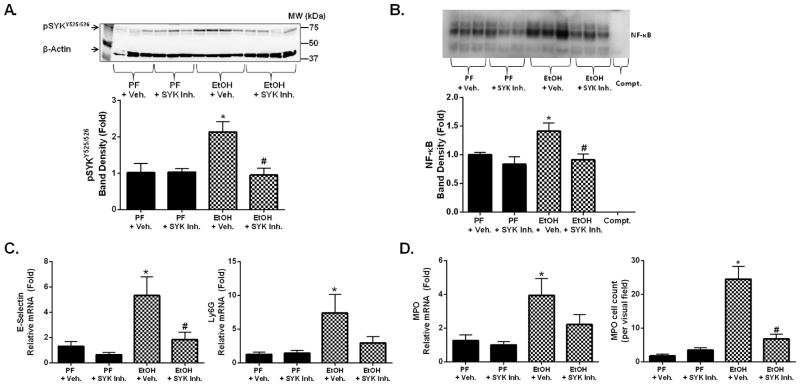
AAH is associated with a significant upregulation in hepatic neutrophil infiltration (22) and SYK modulates tissue neutrophil infiltration/activation. To assess the role of SYK in hepatic neutrophil infiltration, we used the moderate ASH model that induces significant hepatic neutrophil infiltration/activation that is not very prominent in the chronic alcohol feeding model in mice (22). We found that moderate ASH (Sup. Fig. 3A) resulted in a significant increase in hepatic pSYKY525/526 (Fig. 3A), pERK1/2 (Sup. Fig. 3B), and NF-κB nuclear binding (Fig. 3B) that were all suppressed by SYK inhibitor treatment. Further, moderate ASH resulted in a significant increase in the liver expression of neutrophil markers including E-selectin and Ly6G (Fig. 3C), and myeloperoxidase (MPO) (Fig. 3D) mRNA expression. Hepatic neutrophil infiltration was also demonstrated by MPO staining on liver histology (Fig. 3D & Sup. Fig. 3C)(22). SYK inhibitor treatment significantly decreased hepatic E-selectin, Ly6G (Fig. 3C), MPO mRNA expression and hepatic neutrophil infiltration (Fig. 3D & Sup. Fig. 3C).
Figure 3. Functional inhibition of SYK suppressed alcohol-induced hepatic SYK activation and hepatic neutrophil infiltration.
C57BL/6 mice were fed a chronic alcohol diet for 10 days with a single ethanol gavage at the end (NIAAA model) or an isocaloric pair fed (PF) diet with a single sugar gavage and either SYK inhibitor (SYK Inh.) or vehicle treatment (+ Veh.). Total liver protein was analyzed for activated pSYKY525/526 by western blot (A). NF-κB nuclear binding was assessed from liver nuclear extracts by electrophoretic mobility shift assay (B). Total RNA was extracted from mouse livers and assessed by RT-qPCR for E-Selectin (C), Ly6G (C), and MPO (D). Liver samples were assessed by immunohistology for neutrophil infiltration by MPO staining (D). A p-value <0.05 was considered statistically significant by ANOVA for 6–8 mice per experimental group (*, p<0.05 compared to baseline; #, p<0.05 for EtOH + vehicle group).
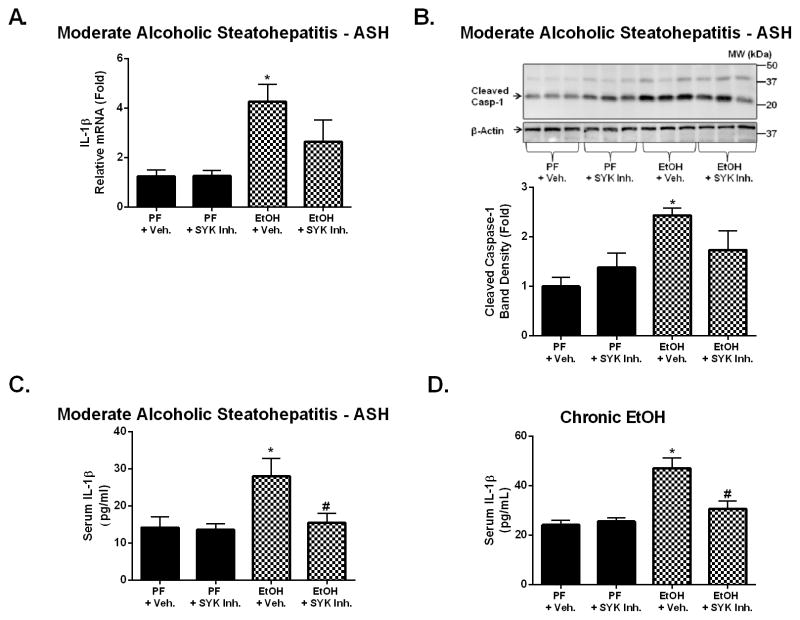
SYK inhibition prevents alcohol-induced inflammasome activation and IL-1β production
AAH results in significant increases in serum IL-1β expression (11) and we previously demonstrated that the inflammasome-caspase-1-IL-1β axis is also activated in chronic alcohol feeding (11). SYK regulates inflammasome activation leading to the production of active IL-1β and IL-18 (21, 30). Here we determined that moderate ASH was also associated with significant increases in IL-1β mRNA expression (Fig. 4A), caspase-1 activation (Fig. 4B), and serum IL-1β protein production (Fig. 4C) indicating inflammasome activation. All of these indicators of inflammasome activation were reduced by SYK inhibitor treatment (Fig. 4A–C). In addition, SYK inhibitor treatment significantly reduced serum IL-1β levels following chronic alcohol feeding (Fig. 4D) suggesting that SYK inhibition is a potent intervention to prevent IL-1β production in different stages of ALD similar to its role in other inflammatory conditions (21).
Figure 4. Inhibition of SYK activation suppressed alcohol-induced inflammasome activation and IL-1β production.
C57BL/6 mice were fed using the moderate ASH (A–C) or chronic (D) alcohol feeding model with SYK inhibitor (SYK Inh.) or vehicle treatment (+ Veh.). Total RNA was extracted from mouse livers and assessed by RT-qPCR for IL-1β mRNA (A). Liver protein was assessed by western blot for cleaved caspase-1 with β-actin as a protein loading control (B). Serum samples from mice were analyzed by ELISA for IL-1β (C&D). A p-value <0.05 was considered statistically significant by ANOVA for 6–8 mice per experimental group (*, p<0.05 compared to baseline; #, p<0.05 compared to EtOH + vehicle group).
Alcohol-induced liver injury and cell death are ameliorated by SYK inhibitor treatment
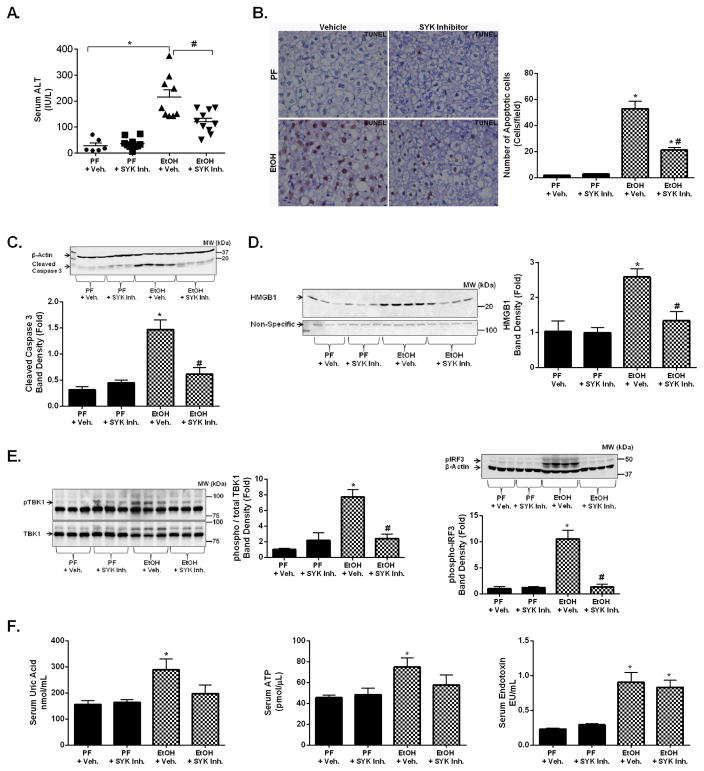
We found that alcohol-induced liver injury indicated by increased serum ALT levels (Fig. 5A) and cell death by TUNEL IHC (Fig. 5B) was attenuated by the in vivo SYK inhibitor treatment in the moderate ASH model, as well as in the chronic alcohol model (Sup. Fig. 4A). Consistent with the reduced liver injury, SYK inhibitor treatment significantly reduced caspase-3 cleavage (Fig. 5C) and serum HMGB1 (Fig. 5D) in alcohol-fed mice compared to vehicle-treated controls. We previously demonstrated that mitochondrial injury and cell death in alcohol-exposed hepatocytes is mediated by TBK1/IRF3 activation (31). Strikingly, SYK inhibitor treatment resulted in a significant reduction in liver phospho-TBK1 and phospho-IRF3 (Fig. 5E) levels induced by alcohol feeding. The alcohol-induced increase of downstream IFN-β expression was significantly reduced with SYK inhibitor treatment in livers from alcohol-fed mice (Sup. Fig. 4B). Alcohol-induced hepatocyte damage leads to release of endogenous danger molecules including uric acid and ATP (12, 14). We found that in vivo SYK inhibitor treatment prevented the alcohol-induced significant increases in serum uric acid and ATP levels compared to pair-fed mice (Fig. 5F). Finally, we found that gut-derived LPS that drives inflammatory cell activation in ALD, was increased by alcohol feeding; however, this was not affected by SYK inhibitor treatment (Fig. 5F). Together, these observations indicated a protective role of SYK inhibition in alcohol-induced hepatic cell death and release of sterile danger signals.
Figure 5. Alcohol-induced hepatic cell death is reduced with SYK inhibitor treatment.
C57BL/6 mice were fed a moderate ASH alcohol or an isocaloric pair fed (PF) diet with SYK inhibitor (SYK Inh.) or vehicle treatment (+ Veh.) as indicated. Serum samples from mice were analyzed for serum ALT (A). Total liver was analyzed for cell death using TUNEL immunohistochemistry (B). Total liver protein was analyzed by western blot for cleaved caspase-3 using β-Actin as a loading control (C). Serum samples from mice were analyzed for cell free HMGB1 by western blot with a non-specific as loading control (D). Total liver protein was analysed for phospho-TBK protein and phospho-IRF3 protein with total TBK1 and total β-Actin used as a loading control (E). Serum samples were used to determine uric acid, ATP, and endotoxin levels (F). A p-value <0.05 was considered statistically significant by ANOVA for 6–10 mice per experimental group (*, p<0.05 compared to baseline; #, p<0.05 compared to EtOH + vehicle group).
Pharmacological inhibition of SYK activation ameliorates alcohol-induced hepatic steatosis
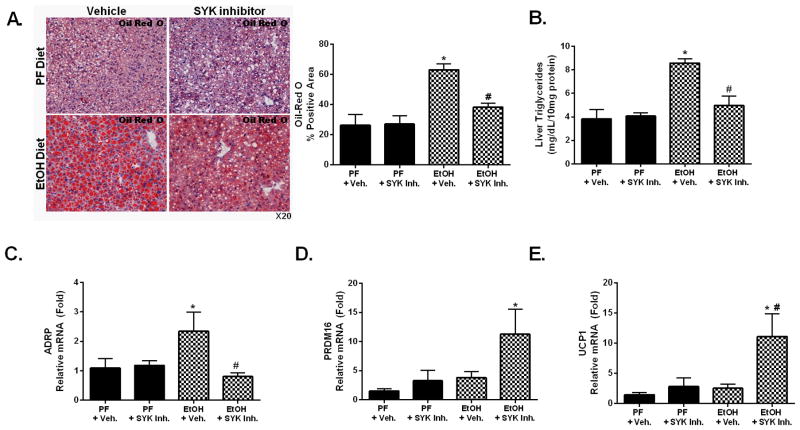
Excess alcohol consumption leads to hepatic steatosis caused by the direct effect of alcohol and alcohol metabolites modulating hepatic fatty acid synthesis and reducing fatty acid oxidation (32). While SYK activation was shown to regulate both innate and adaptive immune responses, its effect on hepatic steatosis is unknown. SYK plays a crucial role in LPS-TLR4-mediated macrophage responses to minimally oxidized low-density lipoprotein in atherosclerosis (20). Given these observations, we surmised that SYK may play a role in alcohol-induced liver steatosis. A five-week chronic alcohol diet resulted in a significant increase in hepatic steatosis as demonstrated by Oil Red O liver histology (Fig. 6A) and liver triglycerides (Fig. 6B). Further, alcohol feeding increased liver expression of adipose differentiation-related protein (ADRP) which induces lipid biogenesis (33) (Fig. 6C). We found that SYK inhibitor treatment significantly decreased steatosis in the chronic alcohol (Fig. 6A–C) and the moderate ASH mouse models (Sup. Fig. 5). Strikingly, SYK inhibitor treatment resulted in a significant increase in PRDM16 (PR-domain containing 16) (Fig. 6D & Sup. Fig. 5C) and UCP1 (uncoupling protein 1) (Fig. 6E & Sup. Fig. 5D) mRNA levels in all alcohol steatohepatitis models compared to the ethanol alone fed group.
Figure 6. Inhibition of SYK activation suppresses alcohol-induced liver steatosis.
C57BL/6 mice were fed a chronic alcohol or an isocaloric pair fed (PF) diet with SYK inhibitor (SYK Inh.) or vehicle treatment (+Veh.) as indicated. Liver steatosis was evaluated by Oil-RedO staining (A) and liver triglyceride assay (B). Liver RNA was analyzed for ADRP (C), PRDM16 (D) and UCP1 (E). A p-value <0.05 was considered statistically significant by ANOVA for 8–10 mice per experimental group (*, p<0.05 compared to baseline; #, p<0.05 compared to EtOH + vehicle group).
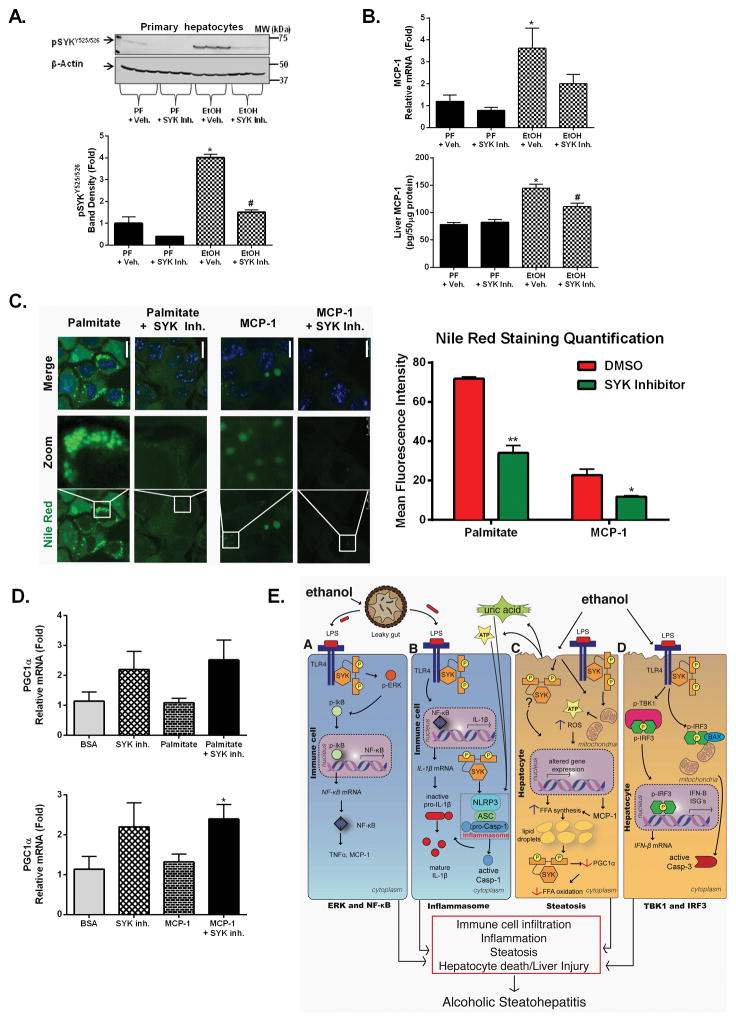
To further dissect the cell specificity of alcohol-induced SYK activation in the liver, we also evaluated SYK expression in hepatocytes isolated after alcohol use (Fig. 7A) and found robust upregulation of activated pSYKY525/526 levels that was prevented by in vivo SYK inhibitor treatment (Fig. 7A). This SYK activation correlated with increased liver steatosis after alcohol feeding (Sup. Fig. 6A&B). Liver steatosis is induced by multiple factors including the pro-inflammatory chemokine, monocyte chemotactic protein 1 (MCP1), which was increased after chronic alcohol feeding in the liver (Fig. 7B). In vivo SYK inhibitor treatment attenuated the alcohol-induced increases of MCP1 mRNA and protein levels (Fig. 7B). Finally, to further assess the mechanistic role of SYK in the regulation of hepatic steatosis, we treated Hepa1-6 cells with MCP1 and palmitate (a free fatty acid) to mimic lipid biogenesis/droplet formation (34) and lipid storage, respectively (Fig. 7C). We found that MCP1 treatment triggered some lipid accumulation while free fatty acid treatment resulted in more significant lipid accumulation in Hepa1-6 cells (Fig. 7C). The SYK inhibitor prevented cellular lipid accumulation after treatment with either free fatty acid or MCP1 (Fig. 7C) in hepatocytes suggesting that SYK activation directly regulates lipid accumulation in hepatocytes. Additionally, we found that SYK inhibitor treatment increased PGC1α (Fig. 7D) suggesting a direct role of SYK in modulating fatty acid oxidation
Figure 7. Functional inhibition of alcohol-induced SYK activation inhibited MCP1-mediated lipid biogenesis and prevented cellular lipid accumulation.
C57BL/6 mice were fed a chronic alcohol or an isocaloric pair fed (PF) diet with SYK inhibitor (SYK Inh.) or vehicle treatment (+ Veh.) as indicated. Total protein was isolated from hepatocytes and analyzed by western blot for pSYKY525/526(A). Liver MCP-1 mRNA and protein levels were analyzed by RT-qPCR or western blot, respectively (B). Hepa1-6 cells were treated with MCP-1 or palmitate with SYK inhibitor or vehicle treatment and lipid accumulation analyzed by Nile red (green) and dapi (blue) for nuclear staining using fluorescence microscopy (C). Hepa1-6 cells were treated with MCP-1 or palmitate with SYK inhibitor or vehicle treatment and total RNA extracted from cells and analyzed for PGC1α by RT-qPCR using 18s as a normalization control (D). In vitro studies are representative of three independent repeat experiments. Illustration summarizing our findings of the multiple and diverse signaling regulations by SYK during progressive alcoholic steatohepatitis (E). A p-value <0.05 was considered statistically significant by ANOVA for 8–10 mice per experimental group (*, p<0.05 compared to baseline; #, p<0.05 compared to EtOH + vehicle group).
The pharmacodynamics of the SYK inhibitor treatment was also monitored (food consumption, body weight, serum ALT, liver/body weight ratio and survival) for both the chronic and moderate ASH ALD mouse models (Sup. Fig. 6 & 7). High doses of SYK inhibitor (10mg/kg) were tolerated for short periods (up to 15 days) after which a spike in serum ALT was detected suggesting possible off-target toxicity effects when associated with alcohol use (Sup. Fig. 6C). A low dose of SYK inhibitor (5mg/kg) in the moderate ASH model and a 50% dose reduction for the chronic ALD models was well tolerated.
Discussion
Despite its high mortality, therapeutic options in AAH are limited and insufficient. The broad spectrum of deregulated signaling events that characterizes the molecular pathomechanism of ALD presents a major challenge to developing new therapeutic approaches. Here, we show for the first time that SYK, a multifunctional non-receptor cellular kinase, modulates critical aspects in the molecular development of ALD. We found that in vivo SYK inhibition can prevent and/or attenuate alcohol-induced liver inflammation, cell death, and steatosis in various phases of ALD including AH and chronic ALD. Our observation that alcohol feeding results in increased circulatory endotoxin in absence of SYK inhibitor together with previous reports that SYK constitutively binds to TLR4 (35) suggest that inhibition of SYK downstream of TLR4 is an attractive target for evaluation in ALD. SYK modulates numerous diseases characterized by sustained inflammation, tissue injury, and lipid accumulation and functional inhibition of SYK has anti-inflammatory effects in animal models and patients suffering from inflammatory diseases including rheumatoid arthritis, systemic lupus erythematosus, allergic rhinitis, ischemic reperfusion injury, and atherosclerosis (20, 36–39). Our study is the first report on the role of SYK in ALD and extends the potential clinical benefits of SYK inhibitors to ALD.
Chronic ALD is characterized by increased hepatic gut-derived LPS that, via TLR4, activates liver immune cells leading to the production of pro-inflammatory cytokines including TNF-α, MCP1, and IL-1β (12, 14). SYK constitutively binds to TLR4 (20). Following TLR4 recognition of LPS, which occurs during ALD, there is increased SYK binding to TLR4, and SYK phosphorylation in neutrophils and monocytes leads to pro-inflammatory cytokine production including TNF-α and MCP1 (20). We found that chronic alcohol feeding resulted in increased SYK phosphorylation in the liver in both hepatocytes and mononuclear cells in mice. This observation was validated in human disease in patients with AH/cirrhosis where total SYK and activated pSYKY525/526 expression was significantly increased in the liver, circulating blood monocytes, and PBMCs. These observations indicate that increased hepatic SYK activation has a major role in ALD. Furthermore, our data raise the possibility that activated pSYKY525/526 expression in circulating PBMCs and monocytes could be exploited as a potential biomarker of ALD.
SYK activation via TLR2/4-dependent mechanisms induces downstream activation of ERK1/2 and NF-κB proinflammatory cytokine production (18, 40) by activated immune cells, which are important features in ALD. Similar to previous reports (18, 40) we found that SYK inhibition with R406, a small molecule ATP-competitive inhibitor of SYK activation (29), resulted in a significant decrease of alcohol-induced activated pSYKY525/526 that ultimately resulted in decreased inflammation observed by lower NF-κB nuclear binding and decreased mRNA and protein levels of phospho-ERK-1/2, MCP1, and TNF-α. We also found that decreased hepatic inflammation with SYK inhibitor treatment was associated with significantly reduced hepatic monocytes/macrophages (F4/80) and activated macrophages (CD68 immunohistochemistry [IHC] and iNOS protein expression), which is a key feature of chronic AH (12). These novel observations suggest that pharmacological inhibition of SYK prevents alcohol-induced activation of key cellular pro-inflammatory pathways in hepatic immune cells and ultimately results in suppression of liver disease due to alcohol. We also found that total liver SYK protein expression increased significantly following chronic alcohol feeding in mice and liver samples from patients with cirrhosis and superimposed alcoholic hepatitis. The increased levels of total SYK may represent an increased pool for SYK phosphorylation or result from compensatory upregulation of SYK in response to increased SYK phosphorylation in the alcoholic livers. It is notable that an increase in SYK expression has been associated with the development of some cancers. Given that chronic ALD can progress to HCC, the role of SYK in hepatic tumorigenesis deserves future evaluation.
Several lines of evidence also suggest that SYK pathway activation can induce NLRP3 inflammasome activation and IL-1β and IL-18 maturation (11, 21) that are hallmarks of ALD (11, 41). We recently demonstrated the treatment potential of IL-1R inhibition during inflammasome-dependent ASH (11). The role of SYK in inflammasome activation during ALD has not been demonstrated. SYK inhibitor treatment also led to a reduction in alcohol-induced pro-IL-1β mRNA and cleaved caspase-1 as well as a significant decrease in alcohol-induced serum IL-1β suggesting a role for SYK in inflammasome activation during ALD.
Neutrophils and macrophages can produce IL-1β in response to TLR4 activation. Neutrophil infiltration in the liver in the moderate ASH ALD model represents human AH although without significant macrophage activation in mice (22, 25). Using the moderate ASH ALD mouse model we found that alcohol use was associated with a significant increase in hepatic E-selectin, Ly6G, MPO, TNF-α, and MCP1 mRNA levels and an increase in MPO positive cells (7). Importantly, SYK inhibitor treatment led to a reduction in all the molecules associated with hepatic neutrophil activation and recruitment suggesting that functional SYK inhibition can attenuate AH by decreasing hepatic neutrophil infiltration in mice. These novel observations in ASH are consistent with previous reports where SYK was found to modulate neutrophil mediated inflammation (24, 42). Due to embryonic lethality, studies with SYK knockouts are limited (43). The available SYK conditional knockout mice with inducible SYK inactivation by tamoxifen (44) also has limitations for ALD given the known adverse effects of tamoxifen on the liver (45).
ALD is also associated with significant hepatocyte cell death in both humans and mouse disease models (46). We found that SYK inhibitor treatment resulted in a significant decrease in liver cell death as demonstrated by a significant decrease in serum ALT. Recently, we showed that STING and IRF3 modulate hepatocyte cell death during ALD thereby linking ER stress signaling with the mitochondrial pathway (31, 47). We found that alcohol feeding resulted in increased activation of TBK1 and IRF3 as well as a significant increase in IFN-β which was significantly reduced with SYK inhibitor treatment. Ultimately, SYK inhibitor treatment resulted in decreased hepatocyte cell death based on significantly lower serum ALT, cleaved caspase-3 and cell free HMGB1 in alcohol fed mice treated with SYK inhibitor compared to vehicle treated mice. These observations suggest that SYK modulates hepatic cell death involving at least IRF3 signaling. Our observations suggest a role for SYK in cell death as recently reported (48).
In addition to hepatic inflammation, moderate ASH and chronic alcohol use led to significant hepatic steatosis. The role of SYK in hepatic steatosis has not been demonstrated. However, fostamatinib, a SYK inhibitor, was reported to attenuate atherosclerosis in mice fed on a high cholesterol diet (49) suggesting that SYK can modulate lipid homeostasis. These observations led us to surmise that SYK might play a modulatory role in lipid accumulation. Indeed, we found that SYK inhibitor treatment also blocked and prevented progressive steatosis by suppressing lipid biogenesis and increasing lipid energy dissipation in both in vitro cell culture systems and in vivo in ALD mouse models including moderate ASH and chronic alcohol drinking.
In summary, our novel observations as schematically illustrated (Fig. 7E), demonstrate a novel functional role of SYK in modulating liver inflammation, cell death, and steatosis due to alcohol. Further, this study highlights SYK inhibitors as potential multifunctional therapies for ALD capable of suppressing inflammation, hepatic injury, and steatosis by suppressing MCP1-mediated lipogenesis and enhancing PRDM16/UCP1-mediated lipid energy dissipation. Our data indicates that SYK inhibitors should be explored in therapeutic discoveries in ALD. Notwithstanding, given that SYK modulates numerous and diverse signaling pathways, it is possible that SYK inhibitor treatment might potentially lead to off-target consequences, and immune suppression with increased risk for infections. Such potential adverse effects need to be considered in evaluation of the therapeutic potential of SYK inhibition and could be managed with organ/cell specific delivery of a SYK inhibitor with a shorter serum half-life (50) and improved functional potency.
Supplementary Material
Acknowledgments
Human liver samples were provided by the NIH-Funded Liver Tissue Procurement and Cell Distribution System (N01-DK-7-0004/HHSN26700700004C).
Financial Support: This work was supported by NIH grant AA017729, U01 translational (AA021907) and U01 clinical AA021893 grants to GS. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
List of Abbreviations
- ALD
alcoholic liver disease
- SYK
spleen tyrosine kinase
- HCC
hepatocellular carcinoma
- AAH
acute alcoholic hepatitis
- ITAM
immunoreceptor tyrosine-based activation motif
- TLR
Toll-like receptor
- PAMP
pathogen-associated molecular patterns
- AH
alcoholic hepatitis
- NIAAA model
moderate ASH alcoholic hepatitis model
- PBMCs
peripheral blood mononuclear cells
- ALT
alanine aminotransferase
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- ADRP
adipose differentiation-related protein
- PRDM16
PR-domain containing 16
- UCP1
uncoupling protein 1
- MCP1
monocyte chemotactic protein 1
- PGC1α
peroxisome proliferator-activated receptor-γ coactivator 1α
Footnotes
Competing interest: The authors declare that they have no competing interests.
Authors’ contributions:
T.N.B. conceived the project idea, performed the experiments, analyzed the data and wrote the manuscript; A.I.V. performed some biochemical experiments, assisted in animal experiments and illustrated signaling graphics for the manuscript; BS performed flow cytometry; A.A., A.S., B.G., and P.L. performed some animal experiments; D.C. and K.K. performed some animal experiments and real time qPCR experiments; G.S. conceived the idea, supervised the project, wrote the manuscript and obtained the funding. All the authors read and approved the final manuscript.
References
- 1.Organization WH. Global status report on alcohol and health. (2014) 2014;XIV [Google Scholar]
- 2.Schwartz JM, Reinus JF. Prevalence and natural history of alcoholic liver disease. Clin Liver Dis. 2012;16:659–666. doi: 10.1016/j.cld.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Garcia ML, Blasco-Algora S, Fernandez-Rodriguez CM. Alcohol liver disease: A review of current therapeutic approaches to achieve long-term abstinence. World J Gastroenterol. 2015;21:8516–8526. doi: 10.3748/wjg.v21.i28.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abenavoli L, Milic N, Rouabhia S, Addolorato G. Pharmacotherapy of acute alcoholic hepatitis in clinical practice. World J Gastroenterol. 2014;20:2159–2167. doi: 10.3748/wjg.v20.i9.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amini M, Runyon BA. Alcoholic hepatitis 2010: a clinician’s guide to diagnosis and therapy. World J Gastroenterol. 2010;16:4905–4912. doi: 10.3748/wjg.v16.i39.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 7.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AM, Horiguchi N, Jeong WI, Radaeva S, Gao B. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35:787–793. doi: 10.1111/j.1530-0277.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathews S, Gao B. Therapeutic potential of interleukin 1 inhibitors in the treatment of alcoholic liver disease. Hepatology. 2013;57:2078–2080. doi: 10.1002/hep.26336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrasek J, Csak T, Szabo G. Toll-like receptors in liver disease. Adv Clin Chem. 2013;59:155–201. doi: 10.1016/b978-0-12-405211-6.00006-1. [DOI] [PubMed] [Google Scholar]
- 14.Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract. 2010;2010 doi: 10.1155/2010/710381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goel A, Gupta M, Aggarwal R. Gut microbiota and liver disease. J Gastroenterol Hepatol. 2014;29:1139–1148. doi: 10.1111/jgh.12556. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Schnabl B. Host-microbiome interactions in alcoholic liver disease. Gut Liver. 2014;8:237–241. doi: 10.5009/gnl.2014.8.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bukong TN, Kodys K, Szabo G. Human ezrin-moesin-radixin proteins modulate hepatitis C virus infection. Hepatology. 2013;58:1569–1579. doi: 10.1002/hep.26500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulathu Y, Grothe G, Reth M. Autoinhibition and adapter function of Syk. Immunol Rev. 2009;232:286–299. doi: 10.1111/j.1600-065X.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- 20.Miller YI, Choi SH, Wiesner P, Bae YS. The SYK side of TLR4: signalling mechanisms in response to LPS and minimally oxidized LDL. Br J Pharmacol. 2012;167:990–999. doi: 10.1111/j.1476-5381.2012.02097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 22.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 24.Ozaki N, Suzuki S, Ishida M, Harada Y, Tanaka K, Sato Y, Kono T, et al. Syk-dependent signaling pathways in neutrophils and macrophages are indispensable in the pathogenesis of anti-collagen antibody-induced arthritis. Int Immunol. 2012;24:539–550. doi: 10.1093/intimm/dxs078. [DOI] [PubMed] [Google Scholar]
- 25.Brandon-Warner E, Schrum LW, Schmidt CM, McKillop IH. Rodent models of alcoholic liver disease: of mice and men. Alcohol. 2012;46:715–725. doi: 10.1016/j.alcohol.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathews S, Xu M, Wang H, Bertola A, Gao B. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306:G819–823. doi: 10.1152/ajpgi.00041.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Billingsley ML, Kincaid RL, Siraganian RP. Phosphorylation of Syk activation loop tyrosines is essential for Syk function. An in vivo study using a specific anti-Syk activation loop phosphotyrosine antibody. J Biol Chem. 2000;275:35442–35447. doi: 10.1074/jbc.M004549200. [DOI] [PubMed] [Google Scholar]
- 28.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105:14–32. doi: 10.1038/ajg.2009.593. quiz 33. [DOI] [PubMed] [Google Scholar]
- 29.Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, Qu K, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 30.Laudisi F, Vigano E, Mortellaro A. Tyrosine kinases: the molecular switch for inflammasome activation. Cell Mol Immunol. 2014;11:129–131. doi: 10.1038/cmi.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrasek J, Iracheta-Vellve A, Csak T, Satishchandran A, Kodys K, Kurt-Jones EA, Fitzgerald KA, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A. 2013;110:16544–16549. doi: 10.1073/pnas.1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, Catalano D, et al. Hepatocyte-specific hypoxia-inducible factor-1alpha is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526–1537. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacheco P, Vieira-de-Abreu A, Gomes RN, Barbosa-Lima G, Wermelinger LB, Maya-Monteiro CM, Silva AR, et al. Monocyte chemoattractant protein-1/CC chemokine ligand 2 controls microtubule-driven biogenesis and leukotriene B4-synthesizing function of macrophage lipid bodies elicited by innate immune response. J Immunol. 2007;179:8500–8508. doi: 10.4049/jimmunol.179.12.8500. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhary A, Fresquez TM, Naranjo MJ. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol Cell Biol. 2007;85:249–256. doi: 10.1038/sj.icb7100030. [DOI] [PubMed] [Google Scholar]
- 36.Pamuk ON, Lapchak PH, Rani P, Pine P, Dalle Lucca JJ, Tsokos GC. Spleen tyrosine kinase inhibition prevents tissue damage after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2010;299:G391–399. doi: 10.1152/ajpgi.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol. 2014;176:1–10. doi: 10.1111/cei.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorarensen A, Kaila N. New spleen tyrosine kinase inhibitors: patent applications published during 2011–2013. Pharm Pat Anal. 2014;3:523–541. doi: 10.4155/ppa.14.34. [DOI] [PubMed] [Google Scholar]
- 39.Choi SH, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, et al. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moretto AF, Dehnhardt C, Kaila N, Papaioannou N, Thorarensen A. The 2010 patent landscape for spleen tyrosine kinase inhibitors. Recent Pat Inflamm Allergy Drug Discov. 2012;6:97–120. doi: 10.2174/187221312800166895. [DOI] [PubMed] [Google Scholar]
- 41.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12:387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 42.Van Ziffle JA, Lowell CA. Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection. Blood. 2009;114:4871–4882. doi: 10.1182/blood-2009-05-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 44.Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, Adachi T, et al. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4:274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 45.Jena SK, Suresh S, Sangamwar AT. Modulation of tamoxifen-induced hepatotoxicity by tamoxifen-phospholipid complex. J Pharm Pharmacol. 2015;67:1198–1206. doi: 10.1111/jphp.12422. [DOI] [PubMed] [Google Scholar]
- 46.Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szabo G, Mandrekar P. Focus on: alcohol and the liver. Alcohol Res Health. 2010;33:87–96. [PMC free article] [PubMed] [Google Scholar]
- 48.Lee CK, Yang Y, Chen C, Liu J. Syk-mediated tyrosine phosphorylation of mule promotes TNF-induced JNK activation and cell death. Oncogene. 2015 doi: 10.1038/onc.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilgendorf I, Eisele S, Remer I, Schmitz J, Zeschky K, Colberg C, Stachon P, et al. The oral spleen tyrosine kinase inhibitor fostamatinib attenuates inflammation and atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1991–1999. doi: 10.1161/ATVBAHA.111.230847. [DOI] [PubMed] [Google Scholar]
- 50.Baluom M, Grossbard EB, Mant T, Lau DT. Pharmacokinetics of fostamatinib, a spleen tyrosine kinase (SYK) inhibitor, in healthy human subjects following single and multiple oral dosing in three phase I studies. Br J Clin Pharmacol. 2013;76:78–88. doi: 10.1111/bcp.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.