Abstract
Social support can attenuate the behavioral and stress hormone response to threat, a phenomenon called social buffering. The mother’s social buffering of the infant is one of the more robust examples; yet we understand little about the neurobiology. Using a rodent model, we explore the neurobiology of social buffering by assessing neural processing of the maternal odor, a major cue controlling social buffering in rat pups. We used pups before (postnatal day (PN) 7) and after (PN14, PN23) the functional emergence of social buffering. Pups were injected with 14C 2-deoxyglucose (2-DG) and presented with the maternal odor, a control preferred odor incapable of social buffering (acetophenone), or no odor. Brains were removed, processed for autoradiography and brain areas identified as important in adult social buffering were assessed, including the amygdala basolateral complex (Basolateral Amygdala [BLA]), medial prefrontal cortex (mPFC), and anterior cingulate cortex (ACC). Results suggest dramatic changes in the processing of maternal odor. PN7 pups show mPFC and ACC activation, although PN14 pups showed no activation of the mPFC, ACC, or BLA. All brain areas assessed were recruited by PN23. Additional analysis suggests substantial changes in functional connectivity across development. Together, these results imply complex nonlinear transitions in the neurobiology of social buffering in early life that may provide insight into the changing role of the mother in supporting social buffering.
Keywords: Social buffering, maternal odor, infant, amygdala, prefrontal cortex
Introduction
When threatened, social support can attenuate the stress response and reduce the release of stress hormones, a phenomenon referred to as social buffering (Levine, Johnson, & Gonzalez, 1985). This aspect of social support has been described in myriad species, including humans and rodents, and occurs throughout the lifespan (Ditzen & Heinrichs, 2014; Gee et al., 2014; Hennessy, Kaiser, & Sachser, 2009; Hennessy et al., 2015; Hostinar & Gunnar, 2013; Kikusui, Winslow, & Mori, 2006; Moriceau & Sullivan, 2006; Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996; Sanchez, 2006; Sanchez, McCormack, & Howell, 2015; Shionoya, Moriceau, Bradstock, & Sullivan, 2007; Stanton & Levine, 1990; Takahashi et al., 2013). Social buffering has profound beneficial effects on physiological processes and both short-term and long-term health (Hennessy et al., 2009; Uchino, Cacioppo, & Kiecolt-Glaser, 1996) and also impacts immediate behaviors through reducing the stress response, increasing prosocial behaviors, and decreasing threat/fear (Hennessy et al., 2015; Moriceau & Sullivan, 2006; Shionoya et al., 2007; Upton & Sullivan, 2010).
One of the more powerful demonstrations of social buffering is the mother’s social buffering of offspring (Hennessy et al., 2009; Hostinar, Sullivan, & Gunnar, 2014; Kikusui et al., 2006; Sanchez et al., 2015; Sullivan & Perry, 2015). Both human research and animal models have highlighted that maternal cues are important for infant stress buffering via multiple sensory systems (Gee et al., 2014; Iacobucci, Colonnello, Fuchs, D’Antuono, & Panksepp, 2013; Levine, 2001; McCormack, Newman, Higley, Maestripieri, & Sanchez, 2009; Sanchez, 2006; Stanton & Levine, 1990; Suchecki, Nelson, Van Oers, & Levine, 1995; Van Oers, De Kloet, Whelan, & Levine, 1998), and research has begun to explore the specific neural mechanisms involved in social buffering (Moriceau & Sullivan, 2006; Shionoya et al., 2007). This research has revealed that social buffering attenuates stress hormone release by the hypothalamic–pituitary–adrenal (HPA) axis at the level of the paraventricular nucleus (PVN) of the hypothalamus with maternal odor suppression of norepinephrine (NE) afferents from medullary A1/A2 noradrenergic neurons (Shionoya et al., 2007), which is the same as the HPA regulatory system identified in adults (Guillaume et al., 1987; Pacak et al., 1992; Walker et al., 2004). Additional work manipulating PVN NE and amygdala corticosterone (CORT) has suggested a causal relationship between stress hormone reduction and infants’ processing of trauma-induced stress during social buffering by the mother (Shionoya et al., 2007). Research also suggests that maternal buffering of children and rodents goes beyond reduction of the HPA axis by activating other brain areas, including the amygdala and medial prefrontal cortex (mPFC) (Gee et al., 2014; Hennessy et al., 2015).
Studies suggest that in humans, maternal buffering of the child is greatly attenuated with maturation, as children begin to gain some independence leading up to adolescence (Gee et al., 2014; Hostinar, Johnson, & Gunnar, 2015; Sanchez et al., 2015; Sandi & Haller, 2015). The rodent literature also suggests neurobehavioral changes in social buffering and the stress response throughout development (Hennessy et al., 2015; Moriceau & Sullivan, 2006; Shionoya et al., 2007). However, there seems to be a complex interaction between neurodevelopmental changes within the HPA axis and social buffering controlled by maternal cues. For example, in very young rodents, painful stimuli fail to activate the HPA system, a period of life termed the stress hyporesponsive period (SHRP) (Dallman, 2000; Stanton & Levine, 1985) and thus what is traditionally thought of as social buffering does not occur at this age. However, the SHRP is controlled by the relatively continuous sensory stimulation that pups receive from the mother, combined with the prolonged effects of this sensory stimulation on regulating pups’ physiology (Hofer, 1973, 1994; van Oers et al., 1998). Indeed, depriving pups of the mother’s sensory stimulation for at least 2 h produces a gradual rise in pups’ CORT levels—as is seen in the maternal deprivation/separation procedure (Lehmann & Feldon, 2000; Lehmann, Russig, Feldon, & Pryce, 2002; Maken, Weinberg, Cool, & Hennessy, 2010; Plotsky et al., 2005). This illustrates a unique, more enduring form of social buffering by the mother that is confined to early life. With maturation, at about postnatal day (PN) 10, painful stimuli (such as a mild electric shock) begins to produce a more immediate increase in pups’ CORT levels (Moriceau & Sullivan, 2006; Moriceau, Wilson, Levine, & Sullivan, 2006; Shionoya et al., 2007; Sullivan, Landers, Yeaman, & Wilson, 2000). At this point, the more adult-like social buffering of the pups’ HPA axes begins. Over the next 2 weeks, stress-induced activation of the HPA axis and CORT release greatly increases, with adult-like levels of CORT seen by around weaning age (PN23). The mother can socially buffer pups throughout this time (Stanton & Levine, 1990; Suchecki, Rosenfeld, & Levine, 1993; Upton & Sullivan, 2010). However, it should be noted that the relative effectiveness of maternal social buffering on the infant’s neurobehavioral response also shows developmental changes: the mother’s social buffering blocks the entire, yet small CORT release in PN10 pups, but by weaning age (PN23) social buffering only decreases the larger CORT release by about half (Levine, Stanton, & Gutierrez, 1988; Stanton & Levine, 1990; Suchecki et al., 1993; Upton & Sullivan, 2010). Thus, the interaction between the developing HPA axis and the developing social buffering system is complex.
The effect of social buffering on pup behavior also changes throughout development. Specifically, the ability of social buffering to change pups’ behavior greatly decreases with maturation (Barr et al., 2009; Shionoya et al., 2007; Upton & Sullivan, 2010). For example, maternal buffering of the infant stress response between the ages of PN10–15 blocks amygdala learning plasticity, such that pups do not learn to avoid an odor paired with shock if the mother is present (Moriceau & Sullivan, 2006; Shionoya et al., 2007; Upton & Sullivan, 2010). A causal relationship between social buffering and learning was demonstrated through intra-amygdala infusion/blockade of CORT and increases/decreases in HPA activation at the level of the PVN with infusion/blockade of NE (Barr et al., 2009; Moriceau & Sullivan, 2006; Moriceau et al., 2006; Roth & Sullivan, 2005; Shionoya et al., 2007; Stanton & Levine, 1990). We refer to this age range as the transitional sensitive period because maternal presence transiently returns the brain to the attachment-learning sensitive period (i.e., pups learn a preference to odors when paired with pleasant or aversive stimuli through maternal suppression of both amygdala plasticity and activation of HPA). Specifically, there is a profound neurobehavioral consequence of maternal presence on pup brain and behavior: maternal suppression of both the amygdala (controlled by CORT) and PVN (controlled by NE) result in attenuation of amygdala plasticity and blockade of HPA activation, so that pups’ cannot learn about threat/fear (Moriceau, Roth, Okotoghaide, & Sullivan, 2004; Moriceau & Sullivan, 2006; Shionoya et al., 2007).
In pups older than PN15, while the mother and her odor continue to socially buffer pups, her presence can no longer attenuate amygdala function (Landers & Sullivan, 2012; Sullivan & Holman, 2010; Sullivan & Perry, 2015; Upton & Sullivan, 2010). These data suggest that the maternal odor cue controlling social buffering has a profound effect on these developmental changes, yet we have little understanding of how maternal cues are processed by the brain throughout development and how the social buffering value changes. Thus, the purpose of this manuscript is to better understand rat pups’ developmental changes associated with neural processing of the maternal odor. We include ages that represent the SHRP (PN7), the developmental period when maternal presence/odors strongly socially buffer CORT release and have the ability to block amygdala learning plasticity (PN14), and the developmental time point where the maternal social buffering occurs but does not fully block CORT release nor control amygdala plasticity (PN23) (Moriceau & Sullivan, 2006; Upton & Sullivan, 2010). We focus our attention on assessment of maternal odor beyond the olfactory system (Dulac, 2006; Logan et al., 2012; Sullivan, Wilson, Wong, Correa, & Leon, 1990) and focus on brain areas previously implicated in social buffering and emotional processing, including subareas of the amygdala and prefrontal cortex (PFC).
In summary, maternal stimuli impact young pups’ neurobehavioral function through all functional sensory systems in early life (somatosensory, olfactory). We focus on the maternal odor because there is robust literature on both the importance of maternal odor and the neural circuitry supporting social odors. Additionally, using olfaction permits the comparison of a naturally preferred odor to the preferred maternal odor to help delineate the neural circuitry supporting the qualities of maternal odor that go beyond odor preference, such as its ability to socially buffer.
Animals and methods
Animals and housing conditions
Female and male Long-Evans rats were born and bred in our colony (originally from Harlan Laboratories). All animals were housed in standard polypropylene cages (34 × 29 × 17 cm) lined with an abundant amount of wood shavings for nest building. Temperature of all animal rooms was set at 20 ± 1°C and lit on a 12:12 light–dark cycle (light on at 6:00 am). Water and standard rodent pellets (Purina Lab Diet, #5001) were offered ad libitum. The day of parturition was considered as PN0. To standardize conditions of housing and of pup development, litters were culled to 12 pups (6 males and 6 females) on PN1 and pups were kept with their mother until time of testing. Only one male and one female were used per litter in each experimental condition, and all subjects were used only once. All procedures were approved by Nathan Kline Institute’s and NYU School of Medicine’s Institutional Animal Care and Use Committee and followed the guidelines from the National Institutes of Health.
Treatment and testing procedure
All experiments were run in an experimental room with a fume hood to control duration and specificity of experimental odors. Before each experiment, animals were weighed and marked for individual identification.
Behavioral testing
Two behavioral tests were used: a Y-maze test to verify odor preference and a nipple attachment test to verify maternal odor’s ability to control nipple attachment.
Y-maze
To investigate preference/avoidance behavior, we used a Y-maze test consisting of a start box (10 × 8.5 × 8 cm for PN7 and 14; 19 × 10 × 10 cm for PN23) and two arms (24 × 8.5 × 8 cm for PN7 and 14; 29 × 10 × 9.5 cm for PN23) separated via two sliding doors with a stainless steel mesh floor. Pups had to choose between an alley containing maternal odor (air stream: 2 L/min flow rate, 1:10 maternal odor:air) versus familiar clean bedding (20 mL clean shavings in a petri dish), or were tested using familiar clean bedding versus acetophenone odor (20 mL of acetophenone diluted to 10 parts per million (PPM) with mineral oil on a KimWipe, Kimthech Science, Dallas, Texas). Acetophenone was chosen due to its relative attractiveness to rat pups (Perry, Al Aïn, McSky, Wilson, & Sullivan, 2014). The odor stimuli were placed at the end of the alleys. For maternal odor presentations, two anesthetized mothers were placed in an airtight glass enclosure (20 × 21 cm) connected to a flow dilution olfactometer. A pup was introduced in the start box for 5 s and the test itself began when the two sliding doors were opened and the pup was free to choose an arm for a 1-min period. An animal was considered to have made a choice toward the odor stimulus when the pup’s entire body crossed the alley entrance. Each pup was tested for five trials and was kept in a holding chamber for the 30 s inter-trial period while the Y-maze mesh floor was thoroughly washed with distilled water, and then dried. The Y-maze was cleaned with 95% ethyl alcohol and washed with water between animals. All testing/analyses was conducted by an experimenter blind to the experimental procedures.
Nipple attachment test
Before testing, a lactating mother was anesthetized (urethane—1.5 g/kg, to prevent milk letdown) and washed with acetone, alcohol, and water (5-min wash with each) to remove the natural maternal odor. Removing maternal odor disrupts nipple attachment, although replacing the maternal odor in the air near the mother is sufficient to reinstate nipple attachment (Hofer, Shair, & Singh, 1976; Raineki, Moriceau, & Sullivan, 2010; Teicher & Blass, 1977). The washed and dried mother was positioned on her side in a testing chamber (25 × 40 × 20 cm) that enabled pups to approach the ventrum and nipple attach. The experimental odor stimuli (maternal odor or 10 PPM acetophenone diluted in mineral oil) was presented via a flow dilution olfactometer (2 L/min, 1:10 odor:air) via tubing located 2 cm from the center of the mother’s ventrum. For maternal odor, two anesthetized mothers were placed in an airtight glass enclosure connected to the olfactometer. A control condition consisted of diffusing an air stream without any additional odor stimuli. Testing began by introducing a pup into the opposite side of the testing chamber. Pups were observed for the 3-min test, and the time to attach to the nipple was recorded. The tests were also videotaped.
14C 2-DG autoradiography
Pups were injected subcutaneously with 14C 2-DG (20 μCi/100 g) 5 min prior to the odor presentation. Pups were individually acclimated for 10 min in a beaker (600 mL beakers for PN7, 2000 mL beakers for PN14 and 23). After the first 5 min, the pups were injected and placed back in the beaker. Afterward, an odor tube, taped to the beaker and linked to a flow dilution olfactometer, diffused either air stream only, maternal odor (two anesthetized mothers placed in an airtight chamber connected to olfactometer; 2 L/min, 1:10 maternal odor:air) or acetophenone embedded in the air stream (10 PPM acetophenone diluted in mineral oil; 2 L/min; 1:10 acetophenone vapor). Each odor stimulus was delivered using an odor-specific olfactometer 11 times every 4 min for 30 s. Olfactometers were controlled by Ethovision software (Noldus, Leesburg, VA). Following odor presentations (45 min after 2-DG injection), animals were decapitated and their brains were removed, frozen in 2-methylbutane (−45°C), and stored at −80°C. Brains were sectioned in a cryostat (20 μm) at −20°C. Every third section was collected on a cover slip and exposed for 5 days along with standards (14C standards 10 × 0.02 mCi, American Radiolabeled Chemicals Inc., St Louis, MO) and exposed to X-ray film in a cassette (Kodak, Rochester, NY) (Boulanger Bertolus et al., 2014; Debiec & Sullivan, 2014; Plakke, Freeman, & Poremba, 2009; Sullivan & Wilson, 1995).
Regions of interest analysis
Regions of interest (ROI) were analyzed by an experimenter blind to the experimental conditions using ImageJ image analysis software (National Institutes of Health, USA) that allows pseudocolor imaging and quantitative optical densitometry. An increase in autoradiographic density corresponds to increased neural activation. Data were analyzed as the average 2-DG uptake within the PFC, including the subareas of the ACC and mPFC (consists of prelimbic and infralimbic cortices), as well as within the Basolateral Amygdala (BLA; consists of lateral and BLA nuclei). All measures were relative to the uptake in the corpus callosum, which did not vary between groups, to control for differences in section thickness and exposure (Coopersmith & Leon, 1986; Sullivan et al., 2000). Specifically, relative uptake measurements were computed as a ratio of average 2-DG uptake in each ROI to that of the corpus callosum. For autoradiography analysis of the BLA, given that anatomical landmarks are not visible with 2-DG, specific amygdala nuclei were identified by counterstaining sections with cresyl violet, which were used to make a template of that brain area for use with the autoradiographs. Cresyl violet staining was not used for analysis of the ACC and mPFC because anatomical landmarks are visible with 2-DG. Rather, brain areas were outlined with the aid of a stereotaxic atlas (Paxinos & Watson, 1986). Four sections were analyzed for each ROI per subject.
Functional connectivity analysis
An estimate of functional connectivity between individual ROI’s was calculated as the Pearson correlation across all animals (n = 15/odor/age) for each pairwise brain region comparison for each odor condition, at each age. Functional connectivity does not imply direct anatomical connectivity, but rather is indicative of a significant relationship in activity between two regions across animals. Thus, for example, if a given odor was associated with consistently elevated activity in region X across animals at a given age and consistently decreased activity in area Y, this would emerge as a negative correlation and imply relatively strong functional connectivity. If the activity in the two areas was unrelated and varied randomly across animals experiencing the same odor, the correlation would be near 0, suggesting relatively little functional connectivity. ROI’s for this analysis included the subareas of the PFC and amygdala listed above. After calculating all bivariate correlations across these ROI, a mean functional connectivity measure was determined for ROI within the PFC module, within the amygdala module and within the cross PFC–amygdala module (termed “joint” module here). The mean difference in correlation between each odor and the no-odor condition was used for display and for statistical analyses. This difference highlights the odor effect on functional connectivity beyond basal (no odor) conditions.
Statistical analysis
For the behavioral and 2-DG ROI data, comparisons were made between groups using two-factor (odor × age) analysis of variance (ANOVA) followed by post hoc Fisher’s tests. For functional connectivity analysis, bivariate correlation matrices were created by calculating ratios of mean 2-DG uptake for all pair-wise combinations of brain regions analyzed for maternal, acetophenone, and no odor conditions at each age. To allow for quantitative analysis of functional connectivity, the difference between each odor’s correlation matrix and the no odor correlation matrix was computed at each age. Group differences were analyzed by two-way ANOVA followed by post hoc Fisher’s tests. In all cases, the level of significance was set at p < 0.05.
Results
Behavioral responses to odors across ontogeny
Y-maze test
As presented in Figure 1(A), for the Y-maze maternal odor test, there was a significant difference as indicated by a two-way ANOVA (main effect (odor): F(1,23) = 18.032; p < 0.05; interaction effect (odor × age): F(2,23) = 2.117, p < 0.147, n = 7–8/group), with post hoc tests indicating that pups chose the maternal odor significantly more than the familiar odor (i.e., clean bedding) at each age (p < 0.05). The statistical analysis for acetophenone (Figure 1(A)), adjacent to the maternal odor graphs for each age) also showed statistical significance as indicated by a two-way ANOVA (main effect (odor): F(1,23) = 214.422; p < 0.05; interaction effect (odor × age): F(2,23) = 1.063; p = 0.364, n = 7–8/group) with post hoc tests showing that pups chose the acetophenone odor significantly more than the familiar odor at PN7 and PN14 (p < 0.05).
Figure 1.
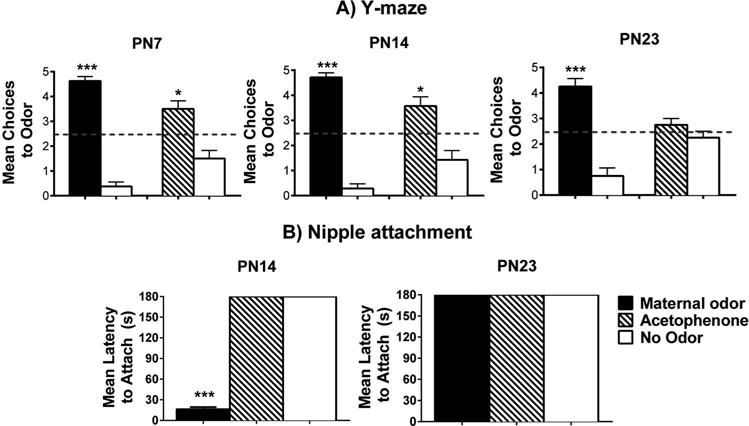
Behavioral responses toward maternal odor and acetophenone odor in developing rat pups. Y-maze tests show the number of choices toward odors during five trials (±S.E.M.) for PN7, PN14, and PN23 pups (A). Odor choices were determined in separate tests consisting of maternal odor versus familiar odor (clean bedding) or acetophenone versus. familiar odor. (B) The nipple attachment test shows latency to attach to the nipple (±S.E.M.) by PN14 and PN23 pups exposed to a lactating female with maternal odor removed (“no odor”), or with presentation of maternal odor or acetophenone in an airstream above the mother’s ventrum. *p < 0.050 and ***p < 0.001. A total of 94 pups were used, with N = 7–8/group.
Nipple attachment test
Maternal odor or the novel acetophenone was assessed for its ability to reinstate nipple attachment following removal of the natural maternal odor (Figure 1(B)). Statistical significance was found for PN14 (F(2,23) = 41.170, p < 0.001, n = 8/group) but not PN23 (all S.E.M. = 0), which replicates previous data from the lab (Raineki et al., 2010). Fisher’s post hoc tests revealed that when maternal odor was infused into the testing environment, pups showed significantly shorter latencies to nipple attach relative to groups when acetophenone or no odor was presented (p < 0.05). In fact, when acetophenone or no odor was presented, pups failed to nipple attach, demonstrating the powerful control that maternal odor has over young pup behavior, and the loss of some of the maternal odor’s influence by the weaning age as pups prepare for independence (PN23).
Neural responses to odors across ontogeny
To assess maternal odor’s impact on the amygdala and PFC across early development, pups were presented with maternal odor, a novel artificial preferred odor (acetophenone), or no odor. Following odor presentations at PN7, PN14, and PN23, brains were removed and neural activity was measured in the PFC (ACC, mPFC) and amygdala (BLA) through the use of 2-DG ROI analysis and functional connectivity analysis. Results are illustrated in Figure 2 (ROI analysis) and Figure 3 (functional connectivity analysis).
Figure 2.
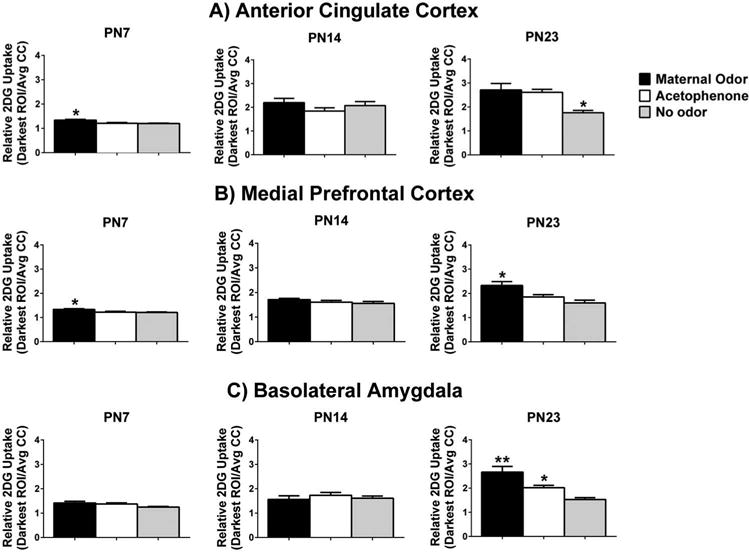
Regions of Interest (ROI) analysis of relative 2-DG uptake in response to maternal odor, acetophenone, and no odor presentations in prefrontal cortex (PFC) areas of the anterior cingulate cortex (ACC; A), medial prefrontal cortex (mPFC; B), and basolateral amygdala (BLA; C) of pups at postnatal day (PN)7, PN14, and PN23. Bars represent the mean level of 2-DG uptake (±S.E.M.) in the three ROI. Significance indicated by *, with ** used to indicate the two odors are significantly different from one another. A total of 73 pups were used, with N = 7–9/group.
Figure 3.
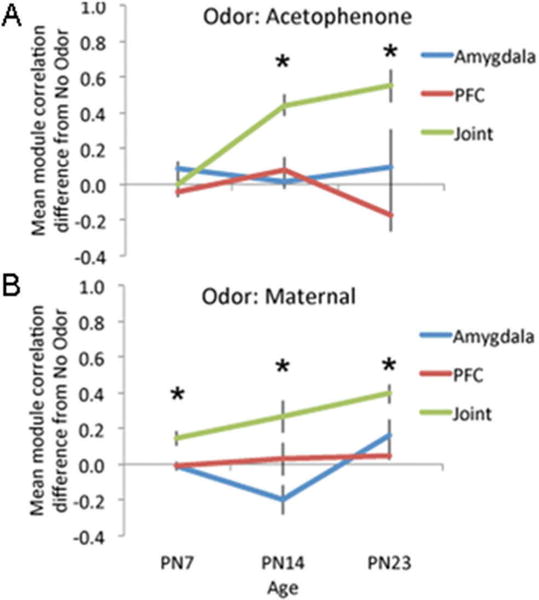
Functional connectivity for acetophenone (A) and maternal odor (B) between ROI’s within the amygdala, the PFC, and between the amygdala and PFC (joint) at PN7, PN14, and PN23. Maternal odor modified functional connectivity between the amygdala and prefrontal cortex (PFC) relative to a no odor control condition at all ages. Data is derived from bivariate correlation matrices representing the difference in mean 2-DG uptake for maternal odor and acetophenone relative to no odor control (see Methods). Error bars represent S.E.M. (N = 15/group). Asterisks denote significant difference from 0 for the Joint module, p < 0.05.
ROI analysis
ACC subregion of PFC
Significant differences in ACC activation (Figure 2(A)) were seen at PN7 (F(2,20) = 7.731, p = 0.003, n = 7–8/group), with post hoc tests indicating higher activation of the ACC in response to maternal odor relative to each of the two other groups (no odor and acetophenone) (p < 0.05). However, no significant differences were found for the ACC at PN14 (F(2,20) = 1.310, p = 0.292, n = 7–8/group). By PN23, significant differences in ACC activation re-emerged (F(2,21) = 8.399, p = 0.002, n = 8/group). Post hoc tests revealed that both maternal and acetophenone odors showed significantly higher 2-DG uptake within the ACC, relative to no odor in these oldest pups (p < 0.05).
mPFC subregion of PFC
Similarly to the ACC, the mPFC showed significant differences in 2-DG uptake (Figure 2(B)) at PN7 (F(2,20) = 5.445, p = 0.013, n = 7–8/group) and at PN23 (F(2,21) = 8.388, p = 0.002, n = 8/group), but not at PN14 (F(2,20) = 1.345, p = 0.282, n = 8/group). Post hoc tests revealed that only maternal odor caused significant activation in mPFC at PN7 and PN23 relative to acetophenone and no odor (p < 0.05).
BLA
As seen in Figure 2(C), significant group differences in 2-DG uptake within the BLA were not found at PN7 (F(2,21) = 2.863, p = 0.079, n = 8) or PN14 (F(2,21) = 0.534, p = 0.594, n = 8/group). However, at PN23, the BLA showed significant group differences in 2-DG uptake (F(2,22) = 14.260, p < 0.001, n = 8–9/group). Specifically, post hoc tests revealed that both maternal and acetophenone odor activated the BLA significantly more than the no odor condition at PN23 (p < 0.05), and maternal odor was also associated with significantly higher 2-DG uptake compared to the activation seen in response to acetophenone (p < 0.05).
Functional connectivity analysis
The functional connectivity within each module (amygdala, PFC, and joint between amygdala and PFC) varied with age and odor. As shown in Figure 3, the strongest effects were on the joint amygdala-PFC functional connectivity module. Thus, while functional connectivity within the amygdala and within the PFC did not differ significantly from the no-odor condition at any age, the mean functional connectivity between the amygdala and PFC was significantly modified by odor. Maternal odor, which induces behavioral attraction and nipple attachment behaviors at PN7 and PN14, significantly enhanced amygdala-PFC functional connectivity (compared to no odor) at all ages (module × age ANOVA, main effect of module, F(2,40) = 10.692, p < 0.001). Post hoc tests revealed a significant difference of the joint functional connectivity from 0 at all three ages. This maternal odor-induced functional connectivity increased with age (ANOVA, main effect of age, F(2,40) = 4.231, p < 0.05). Acetophenone, which induces attraction at PN7 and PN14, but does not control nipple attachment, also enhanced amygdala-PFC functional connectivity compared to the no odor condition. However, this effect was not observed until PN14 (module × age ANOVA, module × age interaction effect, F(4,40) = 5.194, p < 0.01, post hoc tests revealed a significant difference from 0 at PN14 and PN23), suggesting a unique effect of maternal odor in the youngest animals.
Discussion
Our results are summarized in Table 1 and show that the neural response to an infant’s primary social buffering agent, the maternal odor, has major transitions during early life. While maternal odor remains preferred across early life, maternal odor’s behavioral control of pup behavior diminishes with maturation. Specifically, maternal odor has strong control over pup nipple attachment at PN14 and younger (Raineki et al., 2010), but this is diminished by weaning age as pups are preparing to transition to independence. Comparing our maternal odor results with a novel, but naturally preferred odor (acetophenone) that did not support nipple attachment, highlights unique brain areas activated by maternal odor that go beyond an odor preference (odor approached). Our neural assessment results show that maternal odor produces dramatic changes in the pattern of brain activation across development. Specifically, the youngest pups (PN7) that are still in the SHRP with no adult-like social buffering show maternal odor activation of the mPFC and ACC, albeit at very low levels. At PN14, as adult-like social buffering emerges and maternal odor still strongly controls pup behavior, maternal social buffering completely blocks CORT release, and mPFC and ACC activations wanes (not significantly different from no odor). At PN23, maternal odor activates the mPFC, ACC, and BLA. At this age maternal odor’s robust behavioral control over pup behavior has diminished, but maternal odor retains its ability to socially buffer pups (decreasing the robust stress hormone release by about half) (Figure 4) (Shionoya et al., 2007; Stanton & Levine, 1990; Suchecki et al., 1995).
Table 1.
Summary of neurobehavioral changes of pups in response to maternal odor versus acetophenone across development. Social buffering of the stress response occurs at both PN14 and PN23 (Hennessy et al., 2009; Sullivan & Perry, 2015).
| Assessments
|
PN7
|
PN14
|
PN23
|
|
|---|---|---|---|---|
| Social buffering | No* | Yes | Yes | |
| Behaviors | Y-maze test | Preference maternal & acetophenone | Preference maternal | |
| Nipple attachment test | Only maternal odor | Neither | ||
| Brain area activation | Anterior cingulate cortex (ACC) | Maternal odor | None | Maternal odor = acetophenone > no odor |
| Medial prefrontal cortex (mPFC) | Maternal odor | None | Maternal odor | |
| Basolateral amygdala (BLA) | None | None | Maternal odor > Acetophenone > no odor | |
| Functional connectivity | PFC–amygdala | Maternal odor | Maternal odor acetophenone | Maternal odor acetophenone |
Adult-like social buffering does not occur at PN7. However, maternal cues produce prolonged social buffering by preventing corticosterone (CORT) release unless pups are deprived of maternal cues for 1–2 h (Lehmann & Feldon, 2000). The results of the two behavioral tests are summarized, indicating which odors were approached (preferred), and which odors support nipple attachment, at each test age. The ROI and functional connectivity results for each brain areas are also summarized, indicating which regions showed significant activity or connectivity levels, at each test age.
Figure 4.
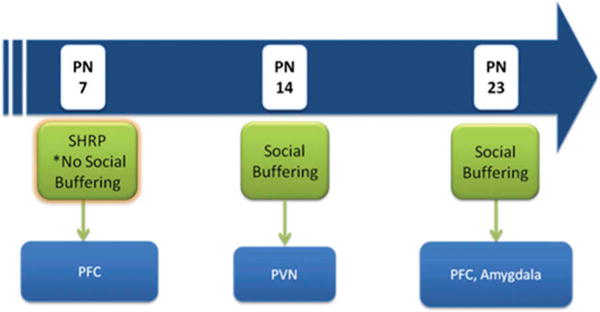
Schematic of potential circuits underlying social buffering of pups by the mother across development. The first period in PN7 pups is characterized by the stress hyporesponsive period (SHRP), when adult-like social buffering does not occur, although maternal cues maintain the SHRP. The ability of maternal odor to activate the prefrontal cortex (PFC) suggests that PFC–paraventricular nucleus of the hypothalamus (PVN) connections may be involved in the mother’s maintenance of pups’ low corticosterone levels. By PN14, maternal cues socially buffers pups, with data suggesting that this primarily occurs by directly blocking the hypothalamic–pituitary axis (HPA) at the level of the PVN. Maternal odor did not activate the PFC or amygdala, suggesting that these routes for social buffering are not active at this age. Finally, in the PN23 pups, the circuitry underlying the processing of maternal cues seems to expand to include areas of the PFC and the amygdala, suggesting that both direct (PVN) and indirect (PFC, amygdala) routes might underlie social buffering.
The complex role of maternal odor
The maternal odor socially buffers the stress response of infant pups, although it functions via a complex system that undergoes developmental transitions and is best viewed within the animal’s ecological niche. Pups depend upon their chemosensory and somatosensory systems for survival in early life, since the auditory and visual systems only begin to emerge around PN14–15 (Ehret, 1976; Weber & Olsson, 2008). The maternal odor is used by pups to locate the mother but once located, pups use a combination of maternal olfactory and somatosensory cues to socially interact with the mother and nipple attach (Blass, 1990; Blass & Teicher, 1980; Hofer et al., 1976; Pedersen & Blass, 1981, 1982; Polan & Hofer, 1999; Raineki et al., 2010; Singh & Tobach, 1975; Stern, 1997; Teicher & Blass, 1976, 1977). However, the presence of maternal odor alone facilitates and normalizes social interactions with the mother and is required for pups to respond to the nipple with the stereotypical, rapid lateral head movements that typically precede grasping the nipple (Bruno, Teicher, & Blass, 1980; Hofer et al., 1976; Teicher & Blass, 1976; Teicher & Blass, 1977).
In humans, infant–mother interactions are similarly initiated by sensory stimuli broadcast by the mother, including visual, auditory and olfactory stimuli, which elicit specific behaviors in the newborn during the first days/weeks of life (DeCasper & Fifer, 1980; Fleming, O’Day, & Kraemer, 1999; Marlier, Schaal, & Soussignan, 1998; Sullivan & Toubas, 1998). The mother’s features, such as her voice and odors, have been learned in utero by the fetus (DeCasper & Fifer, 1980; Mennella, Jagnow, & Beauchamp, 2001; Sullivan et al., 1991; Varendi & Porter, 2001). Specifically, maternal odor elicits head turns toward the source of the odor, attenuation of crying, and an optimization of state and mouthing in the newborn (Bingham, Churchill, & Ashikaga, 2007; Doucet, Soussignan, Sagot, & Schaal, 2009; Marlier et al., 1998; Raimbault, Saliba, & Porter, 2007; Rattaz, Goubet, & Bullinger, 2005; Schaal et al., 2009; Schleidt & Genzel, 1990; Sullivan et al., 1991; Sullivan & Toubas, 1998).
Despite the stereotyped response of infants to maternal odor, there is little evidence that maternal odor is a pheromone and there is no indication of a “specialized labeled line” within the brain (Logan et al., 2012; Moriceau & Sullivan, 2006; Raineki et al., 2010, 2010; Roth & Sullivan, 2005; Sullivan et al., 1990). Rather, human and animal research suggests that the maternal odor is learned repeatedly, beginning in utero and throughout early life, presumably to support learning of the amniotic fluid odors and the mother’s diet-dependent odor (Al Aïn, Belin, Schaal, & Patris, 2013; Blass, 1990; Brake, 1981; Cheslock, Varlinskaya, Petrov, & Spear, 2000; Fillion & Blass, 1986; Hofer et al., 1976; Johanson & Teicher, 1980; Mainardi, Marsan, & Pasquali, 1965; Pedersen & Blass, 1982; Raineki et al., 2010, 2010; Ronca & Alberts, 1994; Sullivan & Leon, 1986; Sullivan, Perry, Sloan, Kleinhaus, & Burtchen, 2011; Sullivan et al., 1990; Teicher & Blass, 1977). The infant is also able to quickly learn new olfactory signature of the mother, which acquires the power of eliciting/controlling the baby–mother interactions (Marlier et al., 1998; Schleidt & Genzel, 1990; Sullivan et al., 1991; Sullivan & Toubas, 1998).
Not surprisingly, maternal deprivation triggers increased HPA activity, high cortisol levels, and distress-like behavior in infant, whereas reunion with the mother results in a strong attenuation of the cortisol response in human as well as in non-human primates (Gunnar & Donzella, 2002; Dettling, Feldon, & Pryce, 2002; Dettling, Pryce, Martin, & Döbeli, 1998; Sanchez, 2006; Taylor, Mustoe, Hochfelder, & French, 2015). Moreover, olfactory and auditory cues from the mother dampen cortisol reactivity in isolated young squirrel monkeys (Levine, Wiener, & Coe, 1993). Cortisol is also highly released in response to stressful/fearful situations, and the mother, who acts as a safety signal, socially buffers the stress response of the child by reducing cortisol activity, even if the infant exhibits fear or pain-related behaviors (Gunnar, & Donzella, 2002; Hostinar et al., 2014; Nachmias et al., 1996). Interestingly, in children with autism, social buffering via maternal odor may subsequently facilitate motor planning abilities in the context of an automatic imitation task (Parma, Bulgheroni, Tirindelli, & Castiello, 2014).
Neurobiology of maternal odor and social buffering: PN7 pups
During the early days of life, pups rarely leave the nest and spend approximately 80% of their time attached to the mother’s nipples (Galef, 1981; Leon, Coopersmith, Beasley, & Sullivan, 1990; Weber & Olsson, 2008). Similarly to other altricial species, young pups have limited capability of self-regulation and rely on the caregiver to regulate physiology and neural functioning (Hofer, 1973, 1984, 1994; Sarro, Sullivan, & Wilson, 2014; Schaal et al., 2009). In these deaf and blind pups, olfaction, somatosensory, and feeding-related stimuli are important for regulation, and this is most obvious during the first week of life (Sullivan & Perry, 2015). For example, tactile stimulation from the mother maintains high levels of growth hormone in pups, and maternal odor and warmth control behavioral activity levels (Hofer, 1984; Kuhn, Butler, & Schanberg, 1978).
Unique features of stress regulation: persistent neonatal social buffering
During the early days of life, one regulatory role of maternal stimuli is to maintain the HPA axis in semi-quiescence during the SHRP (van Oers et al., 1998). The importance of the mother’s regulatory effect on the pup’s HPA axis and stress hormone release is best seen in the maternal deprivation/separation procedure. Removal of pups from the mother causes pups’ stress hormones to begin to increase after a couple of hours of separation, but is decreased by reuniting the infant with the caregiver or presenting stimuli associated with the caregiver (Levine, 2005; Plotsky et al., 2005). Thus, while adult-like social buffering is not present in early life, some form of stress hormone reduction exists in pups.
The neural control of maternal regulation of pups’ stress hormone levels at this age is not well understood. Maternal regulation of CORT occurs, at least in part, via suppression of the HPA axis though maternal cues and behavior (Levine, 2000). For these very young pups, the neural response of maternal cues is rarely assessed, although previous work has also shown that the basic olfactory system is activated by maternal odor, including the olfactory bulb and piriform (olfactory) cortex indicating at least one pathway within the brain to support this early life maternal control of stress hormones (Moriceau & Sullivan, 2004; Raineki et al., 2010; Sullivan et al., 1990). To our knowledge the present work is the first attempt to look beyond the olfactory system and areas of the HPA axis and assess higher order brain areas.
At PN7, our ROI analysis indicated that maternal odor induced activity in two subareas of the medially located PFC, the ACC, and mPFC. No activation by acetophenone was detected in these areas. In the rat, the PFC is a late developing brain area with protracted development continuing through adolescence (Andersen, Lyss, Dumont, & Teicher, 1999; Andersen, LeBlanc, & Lyss, 2001; Bertolino et al., 1997; Cunningham, Bhattacharyya, & Benes, 2002; Seminowicz et al., 2004; Sripanidkulchai, Sripanidkulchai, & Wyss, 2004; Sturrock, 1978; Zhang, 2004). In fact, the PFC is not distinguishable from surrounding areas until around PN8 (Bouwmeester, Smits, & Van Ree, 2002; Bouwmeester, Wolterink, & Van Ree, 2002; Kalsbeek et al., 2006; Schonheit, 1980; Schönheit, 1982; Van Eden & Uylings, 2004a, 2004b). Other indices of brain maturation suggest that peak neurogenesis within the PFC does not occur until the second week of life (Bouwmeester, Smits, et al., 2002; Bouwmeester, Wolterink, et al., 2002; Kolb, Petrie, & Cioe, 1996), with the majority of spine maturation occurring from PN11–20 (Cunningham et al., 2002; Schonheit, 1980). There is a paucity of experiments assessing the role of the infant PFC; thus, it is unclear if this brain area is functionally developed and contributing to behavior at this young age.
Additionally, at PN7, our functional connectivity analysis suggests that while connectivity (mean bivariate correlations) within the amygdala and within the PFC is not modified by odor stimulation when compared to the no odor group, it is noteworthy that maternal odor enhances functional connectivity between the amygdala and PFC at this age. The novel, but behaviorally attractive odor acetophenone does not enhance amygdala-PFC functional connectivity at this age, suggesting a potentially unique effect of maternal odor in very young pups on network function.
In summary, maternal odor has unique behavioral control over PN7 pups, including control of nipple attachment. Additionally, maternal odor produces a unique neural response in PN7 pups, including activation of mPFC and ACC based on ROI analysis and enhanced functional connectivity between the amygdala and PFC.
Neurobiology of maternal odor and emergence of adult-like social buffering: PN14 pups
By PN14, pups are more mature and vision and audition will emerge in a day or two (Bolles & Woods, 1964; Brunjes & Alberts, 1981; Fagiolini, Pizzorusso, Berardi, Domenici, & Maffei, 1994). The transition from crawling to walking occurred at PN10 and pups venture from the nest and begin to eat small amounts of solid food (Galef, 1981). Self-regulation has also significantly matured, although similarly to other altricial species, full independence from the caregiver is a protracted process and has not yet occurred at this age. Specifically, the mother continues to have significant influence over the infant’s physiology and stress response, including via social buffering.
Emergence of adult-like social buffering and neurobiology
By PN14, the SHRP has ended, although the increase in stress hormone levels produced by stressors (i.e., shock) is relatively small compared to older weaning-aged pups and adults (Sevelinges et al., 2007; Upton & Sullivan, 2010). Social buffering by the mother is functional and leads to the complete reduction of CORT in the presence of stressors (Moriceau & Sullivan, 2006; Moriceau et al., 2006; Stanton & Levine, 1990; Suchecki et al., 1995; Van Oers et al., 1998).
Our ROI analysis revealed that the maternal odor did not activate either the BLA or PFC at this age. For the BLA, ample evidence indicates that it is mature enough to function at this age, at least in response to threat (Berdel & Moryś, 2000; Berdel, Moryś, & Maciejewska, 1997; Chareyron, Lavenex, & Lavenex, 2012; Sullivan et al., 2000). However, the amygdala is suppressed by maternal presence and maternal odor, although this is mediated by the decrease in CORT produced by social buffering at the level of the PVN (Moriceau et al., 2006; Shionoya et al., 2007). This conclusion is further supported by independent manipulation of CORT at the level of the amygdala and manipulation of NE at the level of the PVN, which indicates that the presence of CORT is causal to amygdala function (Shionoya et al., 2007). This HPA NE pathway, which originates from the medullary A1/A2, is a major pathway to activation and attenuation of the HPA axis (Herman, Prewitt, & Cullinan, 1996; Nicolaides, Kyratzi, Lamprokostopoulou, Chrousos, & Charmandari, 2015; Tsigos & Chrousos, 2002). Thus, these data further suggest that at this age maternal odor alters behavior through changes in HPA activation and not the amygdala.
Our functional connectivity analyses suggest that both maternal odor and acetophenone elevate functional connectivity between the amygdala and PFC compared to no odor controls at this age. Interestingly, maternal odor also induced a trend for decreased functional connectivity within the amygdala at PN14, though this did not reach significance (p = 0.111). Acetophenone did not induce a change in intra-amygdala functional connectivity.
Previous research has provided some understanding of the neural circuitry supporting social buffering at this age, identifying the importance of NE in the PVN (Hennessy et al., 2009, 2015; Moriceau & Sullivan, 2006; Shionoya et al., 2007). Together, this research suggests that the PVN is a major focus for HPA activation but also attenuation and further suggests that alternate pathways from the PFC and amygdala may not be a source of social buffering in pups at this age. Thus, our findings support previous results that maternal buffering at this age works primarily by decreasing CORT release from the HPA axis (Hennessy et al., 2015; Shionoya et al., 2007). This seems to occur by interfacing directly with the PVN of the HPA axis, rather than involving indirect afferents to the HPA axis, such as via the amygdala and PFC (Hennessy et al., 2015; Shionoya et al., 2007). Interestingly, maternal odor exerts a particularly powerful control over pup behavior at this age range (i.e., PN10–15) and can block threat/fear learning by attenuating the PVN, and thus CORT release into the amygdala. Indeed, this highlights the critical role of social buffering in these young pups.
In summary, maternal odor continues to show unique behavioral control of PN14 pups, including control of nipple attachment and strong social buffering of the stress response via attenuation of PVN activity. However, our ROI analysis suggests maternal odor no longer activates the PFC, and functional connectivity analyses do not differ between maternal odor and another preferred odor (acetophenone). Considering that maternal odor has unique and specific abilities to modify the PVN, this may suggest that this age pup has a minimal neural circuit for social buffering.
Neurobiology of maternal odor and social buffering: PN23 pups
By PN23, an age typical for weaning, pups are transitioning to independence. By this age, maternal control over the infant’s behavior and brain is diminished (Sarro et al., 2014). Specifically, maternal odor no longer controls nipple attachment, and the mother, while still providing social buffering, can no longer completely block CORT release in her young. Stress-induced CORT release is now close to adult levels and social buffering by the mother robustly decreases the CORT release by about half (Stanton & Levine, 1990). Additionally, there are major changes in how the brain responds to CORT. Social buffering by the mother can no longer block fear learning since amygdala-dependent fear learning no longer requires CORT (Corodimas, LeDoux, Gold, & Schulkin, 1994; Hui et al., 2004; Moriceau & Sullivan, 2006; Thompson, Erickson, Schulkin, & Rosen, 2004; Upton & Sullivan, 2010).
Expansion of brain areas activated by maternal odor
By weaning, our ROI analysis revealed an expansion of brain areas activated by maternal odor. Specifically, maternal odor caused odor-specific increased activity in the mPFC and BLA. These data suggest that neural processing of maternal cues at this age likely involves an expanded network to influence HPA function, including brain areas known to modulate the HPA axis (Eisenberger et al., 2011; Ferguson, Latchford, & Samson, 2008; Gee et al., 2014; Herman et al., 2003; Swanson & Sawchenko, 1980). By weaning, functional connectivity analysis revealed a continued strengthening of PFC–amygdala functional connectivity in response to maternal odor, relative to no odor controls. In the rat, there are strong anatomical connections between the PFC and the amygdaloid complex, which are reciprocal. Specifically, the mPFC projects heavily to the BLA and central nucleus of the amygdala (Hurley, Herbert, Moga, & Saper, 1991; McDonald, 1998; McDonald, Mascagni, & Guo, 1996; Vertes, 2004). The ACC projects only to restricted regions of the BLA (Heidbreder & Groenewegen, 2003; McDonald, 1998; McDonald et al., 1996; Ottersen, 1982; Sesack, Deutch, Roth, & Bunney, 1989). Overall, little attention has been given to PFC functional development in rodents, although PFC activity, including within the ACC, begins to show complex behavioral control and is involved in extinction training around PN17 (Li, Kim, & Richardson, 2012; Lilliquist, Nair, Gonzalez-Lima, & Amsel, 1999; Nair, Berndt, Barrett, & Gonzalez-Lima, 2001a, 2001b). The specific role of each of these connections within social buffering has not been explored.
Consistent with the present results, evidence in humans suggests that social buffering induced by an attachment figure goes beyond simple attenuation of the PVN and CORT release by involving brain activation of a complex network, including the mPFC and the amygdala (Eisenberger, Lieberman, & Williams, 2003; Eisenberger et al., 2011; Evanson & Herman, 2015; Gee et al., 2014; Hennessy et al., 2015; Hostinar et al., 2014; Parma et al., 2014; Radley & Sawchenko, 2015; Sullivan & Perry, 2015). For example, it has been reported that maternal stimuli (image or physical presence) can modulate amygdala–mPFC circuitry and affect behavior in children but not in adolescents, assuming that mothers exert a potent influence in emotional regulation through social buffering in early life (Gee et al., 2014). Similarly, in infant humans, increased PFC–amygdala functional connectivity has been shown in the mother–infant attachment context (Berridge & Kringelbach, 2008; Riem et al., 2012). This convergence of data across species strongly suggests that the caregiver has a profound ability to modulate neurobehavioral measures of social buffering (Gunnar & Hostinar, 2015).
In adults, the amygdala has indirect connections to the PVN, with the central amygdala and basolateral complex projecting to the PVN via the PFC, solitary nucleus of the brainstem, and the bed nucleus of the stria terminalis (Kiyokawa, Wakabayashi, Takeuchi, & Mori, 2012; Schwaber, Kapp, Higgins, & Rapp, 1982; Ulrich-Lai & Herman, 2009). The PFC also has afferents to the HPA axis, via connections to the PVN (Sandi & Haller, 2015; Smith & Vale, 2006; Ziegler & Herman, 2002). Furthermore, our results converge with findings in adult humans that safety cues produce heightened activity in the mPFC during a social buffering task (Eisenberger et al., 2011; Schiller, Levy, Niv, LeDoux, & Phelps, 2008), suggesting that a shift to a more adult-like social buffering circuit may occur by this age. These findings provide mechanistic explanations on maternal social buffering that may provide insight into how social buffering works in humans. In rodents and humans, the emergence of the PFC and PFC–amygdala connections might result in a developmental shift in the potency of maternal buffering. Before then, maternal odor seems to be a critical safety signal allowing to reduce/prevent the behavioral and physiological distress responses and to inhibit the odor fear learning.
Interestingly, research in rodents and humans suggests that with continued maturation the mother will continue to lose her ability to socially buffer her progeny, whereas conspecifics, such as a friend or a potential mating partner, become the more effective socially buffering partners in adolescence and adulthood (Gee et al., 2014; Hennessy, 1984, 1986; Hostinar et al., 2015; Kiyokawa, Kikusui, Takeuchi, & Mori, 2004). This developmental transition appears to be related to the functional emergence of the PFC around the age of weaning. However, maternal odor retains value and can influence behavior into adulthood (Raineki et al., 2015; Sevelinges et al., 2007, 2011; Sevelinges, Sullivan, Messaoudi, & Mouly, 2008), albeit its value is transiently reduced after weaning (Perry & Sullivan, 2015).
Conclusion
Assessment of the neurobehavioral response to maternal odor, a major cue for social buffering in infants, suggests that there is a dynamic neural system supporting social buffering during the first few weeks of life. Specifically, the infant’s neural response to maternal odor undergoes dramatic transitions in brain area activation, indicating that mechanisms of social buffering transition from a simplistic circuit in infancy to a more complex circuitry at weaning. Furthermore, integration of the present findings with the literature on the development of the HPA system, social buffering, and the impact of maternal cues on neural activity, suggests that maternal buffering of infants involves a complex system. Specifically, it seems that how maternal odor interfaces with the HPA axis to lower CORT release is complex and changes with development. However, there may be greater reliance on the PVN as a major site of integration for social buffering information during infancy. This simplistic pathway may provide insight into how the mother has such strong control over the pups’ CORT levels, as indicated by complete blockade of CORT during social buffering until at least PN14.
By weaning, the present data suggest that a more complex pathway of HPA control through social buffering (maternal buffering) appears to be emerging. While it is clear that social buffering interfaces with the HPA axis at the level of the PVN at all ages, the present results likely highlight two additional control pathways for social buffering by weaning age, via the amygdala and PFC. However, additional multiple pathways likely exist that show developmental changes (Hostinar et al., 2014). In adults, the HPA axis is controlled through multiple levels within the brain and we speculate that social buffering potentially attenuates CORT release through many of these levels of control (Herman et al., 2003; Herman, Ostrander, Mueller, & Figueiredo, 2005; Herman et al., 1996; Herman, Tasker, Ziegler, & Cullinan, 2002; Laryea, Arnett, & Muglia, 2015; Panagiotakopoulos & Neigh, 2014). Our assessment of the changing neural network underlying maternal odor processing across development provides novel insight into potential mechanisms underlying social buffering throughout development.
How this system compares with development of the human social buffering systems remains unclear. However, elements of social buffering show great convergence across species, and undergo similar developmental transitions with robust caregiver social buffering occurring in early life (Gee et al., 2014; Hostinar et al., 2015, 2014; Sanchez, 2006).
Acknowledgments
Funding
This work was supported by the NIH DC009910, MH091451, HD083217 (RMS, DAW), REP was supported by T32MH096331. The conference that occasioned this special issue was supported by NSF grant BCS-1439258 to PI Megan Gunnar, and co-PIs Nim Tottenham, Mar Sanchez and Regina Sullivan.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Al Aïn S, Belin L, Schaal B, Patris B. How does a newly born mouse get to the nipple? Odor substrates eliciting first nipple grasping and sucking responses. Developmental Psychobiology. 2013;55(8):888–901. doi: 10.1002/dev.21082. [DOI] [PubMed] [Google Scholar]
- Andersen S, Lyss P, Dumont N, Teicher M. Enduring neurochemical effects of early maternal separation on limbic structures. Annals of the New York Academy of Sciences. 1999;877(1):756–759. doi: 10.1111/j.1749-6632.1999.tb09317.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41(4):345–350. doi: 10.1002/(ISSN)1098-2396. [DOI] [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S. Transitions in infant learning are modulated by dopamine in the amygdala. Nature Neuroscience. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdel B, Moryś J. Expression of calbindin-D28k and parvalbumin during development of rat’s basolateral amygdaloid complex. International Journal of Developmental Neuroscience. 2000;18(6):501–513. doi: 10.1016/S0736-5748(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Berdel B, Moryś J, Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. International Journal of Developmental Neuroscience. 1997;15(6):755–765. doi: 10.1016/S0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology (Berl) 2008;199(3):457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Saunders R, Mattay V, Bachevalier J, Frank J, Weinberger D. Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporolimbic lesions: A proton magnetic resonance spectroscopic imaging study. Cerebral Cortex. 1997;7(8):740–748. doi: 10.1093/cercor/7.8.740. [DOI] [PubMed] [Google Scholar]
- Bingham PM, Churchill D, Ashikaga T. Breast milk odor via olfactometer for tube-fed, premature infants. Behavior Research Methods. 2007;39(3):630–634. doi: 10.3758/BF03193035. [DOI] [PubMed] [Google Scholar]
- Blass EM. Suckling: Determinants, changes, mechanisms, and lasting impressions. Developmental Psychology. 1990;26(4):520–533. doi: 10.1037/0012-1649.26.4.520. [DOI] [Google Scholar]
- Blass EM, Teicher MH. Suckling. Science. 1980;210(4465):15–22. doi: 10.1126/science.6997992. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Woods PJ. The ontogeny of behaviour in the albino rat. Animal Behaviour. 1964;12(4):427–441. doi: 10.1016/0003-3472(64)90062-4. [DOI] [Google Scholar]
- Boulanger Bertolus J, Hegoburu C, Ahers JL, Londen E, Rousselot J, Szyba K. Infant rats can learn time intervals before the maturation of the striatum: Evidence from odor fear conditioning. Frontiers in Behavioral Neuroscience. 2014;8:176. doi: 10.3389/fnbeh.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester H, Smits K, Van Ree JM. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. The Journal of Comparative Neurology. 2002;450(3):241–255. doi: 10.1002/(ISSN)1096-9861. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Wolterink G, Van Ree JM. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. The Journal of Comparative Neurology. 2002;442(3):239–249. doi: 10.1002/(ISSN)1096-9861. [DOI] [PubMed] [Google Scholar]
- Brake SC. Suckling infant rats learn a preference for a novel olfactory stimulus paired with milk delivery. Science. 1981;211(4481):506–508. doi: 10.1126/science.7192882. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Alberts JR. Early auditory and visual function in normal and hyperthyroid rats. Behavioral and Neural Biology. 1981;31(4):393–412. doi: 10.1016/S0163-1047(81)91468-0. [DOI] [PubMed] [Google Scholar]
- Bruno JP, Teicher MH, Blass EM. Sensory determinants of suckling behavior in weanling rats. Journal of Comparative and Physiological Psychology. 1980;94(1):115–127. doi: 10.1037/h0077646. [DOI] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Lavenex P. Postnatal development of the amygdala: A stereological study in rats. The Journal of Comparative Neurology. 2012;520(16):3745–3763. doi: 10.1002/cne.23132. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Spear NE. Rapid and robust olfactory conditioning with milk before suckling experience: Promotion of nipple attachment in the newborn rat. Behavioral Neuroscience. 2000;114(3):484–495. doi: 10.1037/0735-7044.114.3.484. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response by adult rats to odors experienced early in life. Brain Research. 1986;371(2):400–403. doi: 10.1016/0006-8993(86)90384-7. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, LeDoux JE, Gold PW, Schulkin J. Corticosterone potentiation of conditioned fear in rats. Annals of the New York Academy of Science. 1994;746:392–393. doi: 10.1111/j.1749-6632.1994.tb39264.x. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. The Journal of Comparative Neurology. 2002;453(2):116–130. doi: 10.1002/(ISSN)1096-9861. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Moments in time–the neonatal rat hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141(5):1590–1592. doi: 10.1210/endo.141.5.7527. [DOI] [PubMed] [Google Scholar]
- Debiec J, Sullivan RM. Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proceedings of the National Academy of Sciences. 2014;111(33):12222–12227. doi: 10.1073/pnas.1316740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers’ voices. Science. 1980;208(4448):1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacology Biochemistry and Behavior. 2002;73(1):259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Pryce CR, Martin RD, Döbeli M. Physiological responses to parental separation and a strange situation are related to parental care received in juvenile Goeldi’s monkeys (Callimico goeldii) Developmental Psychobiology. 1998;33(1):21–31. doi: 10.1002/(sici)1098-2302(199807)33:1<21::aid-dev3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Heinrichs M. Psychobiology of social support: The social dimension of stress buffering. Restorative Neurology and Neuroscience. 2014;32(1):149–162. doi: 10.3233/rnn-139008. [DOI] [PubMed] [Google Scholar]
- Doucet S, Soussignan R, Sagot P, Schaal B. The secretion of areolar (montgomery’s) glands from lactating women elicits selective, unconditional responses in neonates. PLoS One. 2009;4(10):e7579. doi: 10.1371/journal.pone.0007579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C. Sparse encoding of natural scents. Neuron. 2006;50(6):816–818. doi: 10.1016/j.neuron.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) Journal of American Audiology Society. 1976;1(5):179–184. [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Master SL, Inagaki TK, Taylor SE, Shirinyan D, Lieberman MD. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Science U S A. 2011;108(28):11721–11726. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson NK, Herman JP. Metabotropic glutamate receptor-mediated signaling dampens the HPA axis response to restraint stress. Physiology & Behavior. 2015;150:2–7. doi: 10.1016/j.physbeh.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: Dark rearing and monocular deprivation. Vision Research. 1994;34(6):709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus – a potential target for integrative treatment of autonomic dysfunction. Expert Opinion on Therapeutic Targets. 2008;12(6):717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillion TJ, Blass EM. Infantile experience with suckling odors determines adult sexual behavior in male rats. Science. 1986;231(4739):729–731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- Fleming AS, O’Day DH, Kraemer GW. Neurobiology of mother-infant interactions: Experience and central nervous system plasticity across development and generations. Neuroscience & Biobehavioral Reviews. 1999;23(5):673–685. doi: 10.1016/S0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Galef BG. The ecology of weaning: Parasitism and the achievement of independence by altricial mammals. In: Gubernick DJ, Klopfer PH, editors. Parental Care in Mammals. New York: Plenum Publishing Corporation; 1981. pp. 211–241. [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Sciences. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume V, Conte-Devolx B, Szafarczyk A, Malaval F, Pares-Herbute N, Grino M. The corticotropin-releasing factor release in rat hypophysial portal blood is mediated by brain catecholamines. Neuroendocrinology. 1987;46(2):143–146. doi: 10.1159/000124811. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Hostinar CE. The social buffering of the hypothalamic–pituitary–adrenocortical axis in humans: Developmental and experiential determinants. Social Neuroscience. 2015;10(5):479–488. doi: 10.1080/17470919.2015.1070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: Evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience & Biobehavioral Reviews. 2003;27(6):555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Presence of companion moderates arousal of monkeys with restricted social experience. Physiology & Behavior. 1984;33(5):693–698. doi: 10.1016/0031-9384(84)90033-7. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Effects of social partners on pituitary-adrenal activity during novelty exposure in adult female squirrel monkeys. Physiology & Behavior. 1986;38(6):803–807. doi: 10.1016/0031-9384(86)90046-6. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Schiml PA, Willen R, Watanasriyakul W, Johnson J, Garrett T. Selective social buffering of behavioral and endocrine responses and Fos induction in the prelimbic cortex of infants exposed to a novel environment. Developmental Psychobiology. 2015;57(1):50–62. doi: 10.1002/dev.21256. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Critical Reviews™ in Neurobiology. 1996;10(3–4):371–394. doi: 10.1615/CritRevNeurobiol.v10.i3-4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: Glutamate-GABA connections. Pharmacology Biochemistry and Behavior. 2002;71(3):457–468. doi: 10.1016/S0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hofer MA. The effects of brief maternal separations on behavior and heart rate of two week old rat pups. Physiology & Behavior. 1973;10(3):423–427. doi: 10.1016/0031-9384(73)90200-X. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Relationships as regulators: A psychobiologic perspective on bereavement. Psychosomatic Medicine. 1984;46(3):183–197. doi: 10.1097/00006842-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatrica. 1994;83:9–18. doi: 10.1111/apa.1994.83.issue-s397. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Shair H, Singh P. Evidence that maternal ventral skin substances promote suckling in infant rats. Physiology & Behavior. 1976;17(1):131–136. doi: 10.1016/0031-9384(76)90279-1. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Gunnar MR. Future directions in the study of social relationships as regulators of the HPA axis across development. Journal of Clinical Child & Adolescent Psychology. 2013;42(4):564–575. doi: 10.1080/15374416.2013.804387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Science. 2015;18(2):281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiology of Learning and Memory. 2004;81(1):67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. The Journal of Comparative Neurology. 1991;308(2):249–276. doi: 10.1002/(ISSN)1096-9861. [DOI] [PubMed] [Google Scholar]
- Iacobucci P, Colonnello V, Fuchs T, D’Antuono L, Panksepp J. Differential ultrasonic indices of separation distress in the presence and absence of maternal cues in infant rats bred for high and low positive social affect. Acta Neuropsychiatrica. 2013;25(5):289–296. doi: 10.1017/neu.2013.6. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Teicher MH. Classical conditioning of an odor preference in 3-day-old rats. Behavioral and Neural Biology. 1980;29(1):132–136. doi: 10.1016/S0163-1047(80)92596-0. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Ruiter M, La Fleur SE, Cailotto C, Kreier F, Buijs R. The hypothalamic clock and its control of glucose homeostasis. Progress in Brain Research. 2006;153:283–307. doi: 10.1016/S0079-6123(06)53017-1. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361(1476):2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner’s stress status influences social buffering effects in rats. Behavioral Neuroscience. 2004;118(4):798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Wakabayashi Y, Takeuchi Y, Mori Y. The neural pathway underlying social buffering of conditioned fear responses in male rats. European Journal of Neuroscience. 2012;36(10):3429–3437. doi: 10.1111/j.1460-9568.2012.08257.x. [DOI] [PubMed] [Google Scholar]
- Kolb B, Petrie B, Cioe J. Recovery from early cortical damage in rats, VII. Comparison of the behavioural and anatomical effects of medial prefrontal lesions at different ages of neural maturation. Behavioural Brain Research. 1996;79(1–2):1–13. doi: 10.1016/0166-4328(95)00254-5. [DOI] [PubMed] [Google Scholar]
- Kuhn CM, Butler SR, Schanberg SM. Selective depression of serum growth hormone during maternal deprivation in rat pups. Science. 1978;201(4360):1034–1036. doi: 10.1126/science.684424. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Developmental Neuroscience. 2012;34(2–3):101–114. doi: 10.1159/000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laryea G, Arnett M, Muglia LJ. Ontogeny of hypothalamic glucocorticoid receptor-mediated inhibition of the hypothalamic–pituitary–adrenal axis in mice. Stress. 2015;18(4):400–407. doi: 10.3109/10253890.2015.1046832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: Consistent or confusing? Reviews in the Neurosciences. 2000;11(4):383–408. doi: 10.1515/REVNEURO.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Russig H, Feldon J, Pryce CR. Effect of a single maternal separation at different pup ages on the corticosterone stress response in adult and aged rats. Pharmacology Biochemistry and Behavior. 2002;73(1):141–145. doi: 10.1016/S0091-3057(02)00788-8. [DOI] [PubMed] [Google Scholar]
- Leon M, Coopersmith R, Beasley LJ, Sullivan RM. Thermal aspects of parenting. In: Krasnegor A, Bridges RS, editors. Mammalian Parenting: Biolchemical, Neurobiological, and Behavioral Determinants. New York Oxford: Oxford University Press; 1990. pp. 400–415. [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic–pituitary–adrenal axis. European Journal of Pharmacology. 2000;405(1–3):149–160. doi: 10.1016/S0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiology & Behavior. 2001;73(3):255–260. doi: 10.1016/S0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30(10):939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Levine S, Johnson DF, Gonzalez CA. Behavioral and hormonal responses to separation in infant rhesus monkeys and mothers. Behavioral Neuroscience. 1985;99(3):399–410. doi: 10.1037/0735-7044.99.3.399. [DOI] [PubMed] [Google Scholar]
- Levine S, Stanton ME, Gutierrez YR. Maternal modulation of pituitary-adrenal activity during ontogeny. Advances in Experimental Medicine and Biology. 1988;245:295–310. doi: 10.1007/978-1-4899-2064-5_24. [DOI] [PubMed] [Google Scholar]
- Levine S, Wiener SG, Coe CL. Temporal and social factors influencing behavioral and hormonal responses to separation in mother and infant squirrel monkeys. Psychoneuroendocrinology. 1993;18(4):297–306. doi: 10.1016/0306-4530(93)90026-h. [DOI] [PubMed] [Google Scholar]
- Li S, Kim JH, Richardson R. Differential involvement of the medial prefrontal cortex in the expression of learned fear across development. Behavioral Neuroscience. 2012;126(2):217–225. doi: 10.1037/a0027151. [DOI] [PubMed] [Google Scholar]
- Lilliquist MW, Nair HP, Gonzalez-Lima F, Amsel A. Extinction after regular and irregular reward schedules in the infant rat: Influence of age and training duration. Developmental Psychobiology. 1999;34(1):57–70. doi: 10.1002/(ISSN)1098-2302. [DOI] [PubMed] [Google Scholar]
- Logan DW, Brunet LJ, Webb WR, Cutforth T, Ngai J, Stowers L. Learned recognition of maternal signature odors mediates the first suckling episode in mice. Current Biology. 2012;22(21):1998–2007. doi: 10.1016/j.cub.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainardi D, Marsan M, Pasquali A. Causation of sexual preferences of the house mouse. The behaviour of mice reared by parents whose odour was artificially altered. Atti Della Societa Italiana Di Scienze Nationali E Del Museo Civico Di Storia Naturale Di Milano. 1965;104:325–338. [Google Scholar]
- Maken DS, Weinberg J, Cool DR, Hennessy MB. An investigation of the effects of maternal separation and novelty on central mechanisms mediating pituitary-adrenal activity in infant guinea pigs (Cavia porcellus) Behavioral Neuroscience. 2010;124(6):800–809. doi: 10.1037/a0021465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier L, Schaal B, Soussignan R. Neonatal responsiveness to the odor of amniotic and lacteal fluids: A test of perinatal chemosensory continuity. Child Development. 1998;69(3):611–623. doi: 10.1111/j.1467-8624.1998.tb06232.x. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior. 2009;55(4):538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: A Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55(3):257–332. doi: 10.1016/S0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and Postnatal Flavor Learning by Human Infants. Pediatrics. 2001;107(6):E88. doi: 10.1542/peds.107.6.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. International Journal of Developmental Neuroscience. 2004;22(5–6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. The Journal of Neuroscience. 2004;24(5):1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. The Journal of Neuroscience. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development. 1996;67(2):508–522. doi: 10.2307/1131829. [DOI] [PubMed] [Google Scholar]
- Nair HP, Berndt JD, Barrett D, Gonzalez-Lima F. Maturation of extinction behavior in infant rats: Large-scale regional interactions with medial prefrontal cortex, orbitofrontal cortex, and anterior cingulate cortex. The Journal of Neuroscience. 2001a;21(12):4400–4407. doi: 10.1523/JNEUROSCI.21-12-04400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair HP, Berndt JD, Barrett D, Gonzalez-Lima F. Metabolic mapping of brain regions associated with behavioral extinction in preweanling rats. Brain Research. 2001b;903(1–2):141–153. doi: 10.1016/S0006-8993(01)02469-6. [DOI] [PubMed] [Google Scholar]
- Nicolaides NC, Kyratzi E, Lamprokostopoulou A, Chrousos GP, Charmandari E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation. 2015;22(1– 2):6–19. doi: 10.1159/000362736. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. The Journal of Comparative Neurology. 1982;205(1):30–48. doi: 10.1002/(ISSN)1096-9861. [DOI] [PubMed] [Google Scholar]
- Pacak K, Armando I, Fukuhara K, Kvetnansky R, Palkovits M, Kopin IJ. Noradrenergic activation in the paraventricular nucleus during acute and chronic immobilization stress in rats: An in vivo microdialysis study. Brain Research. 1992;589(1):91–96. doi: 10.1016/0006-8993(92)91165-B. [DOI] [PubMed] [Google Scholar]
- Panagiotakopoulos L, Neigh GN. Development of the HPA axis: Where and when do sex differences manifest? Frontiers in Neuroendocrinology. 2014;35(3):285–302. doi: 10.1016/j.yfrne.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Parma V, Bulgheroni M, Tirindelli R, Castiello U. Facilitation of action planning in children with autism: The contribution of the maternal body odor. Brain and Cognition. 2014;88:73–82. doi: 10.1016/j.bandc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Pedersen PE, Blass EM. Olfactory control over suckling in albino rats. In: Aslin RN, Alberts JR, Pedersen MR, editors. The Development of Perception: Psychobiological Perspectives. New York: Academic Press; 1981. pp. 359–381. [Google Scholar]
- Pedersen PE, Blass EM. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Developmental Psychobiology. 1982;15(4):349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- Perry RE, Al Aïn S, McSky K, Wilson DA, Sullivan RM. Development of hedonics: Ontogeny of olfactory and limbic system circuits supporting maternal odor and predator odor responses in rats. 44th meeting of the Society for Neuroscience (SfN); Washington, DC. 2014. Nov, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Sullivan RM. Rescue of neurobehavioral deficits following infant abuse: The role of maternal odor. 45th meeting of the Society for Neuroscience (SfN); Chicago, IL. 2015. Oct, [Google Scholar]
- Plakke B, Freeman JH, Poremba A. Metabolic mapping of rat forebrain and midbrain during delay and trace eyeblink conditioning. Neurobiology of Learning and Memory. 2009;92(3):335–344. doi: 10.1016/j.nlm.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30(12):2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Polan HJ, Hofer MA. Maternally directed orienting behaviors of newborn rats. Developmental Psychobiology. 1999;34(4):269–279. doi: 10.1002/(ISSN)1098-2302. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE. Evidence for involvement of a limbic paraventricular hypothalamic inhibitory network in hypothalamic–pituitary–adrenal axis adaptations to repeated stress. Journal of Comparative Neurology. 2015;523(18):2769–2787. doi: 10.1002/cne.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimbault C, Saliba E, Porter RH. The effect of the odour of mother’s milk on breastfeeding behaviour of premature neonates. Acta Paediatrica. 2007;96(3):368–371. doi: 10.1111/j.1651-2227.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry. 2010;67(12):1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Pickenhagen A, Roth TL, Babstock DM, McLean JH, Harley CW. The neurobiology of infant maternal odor learning. Brazilian Journal of Medical and Biological Research. 2010;43(10):914–919. doi: 10.1590/S0100-879X2010007500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Sarro E, Rincon-Cortes M, Perry R, Boggs J, Holman CJ. Paradoxical neurobehavioral rescue by memories of early-life abuse: The safety signal value of odors learned during abusive attachment. Neuropsychopharmacology. 2015;40(4):906–914. doi: 10.1038/npp.2014.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattaz C, Goubet N, Bullinger A. The calming effect of a familiar odor on full-term newborns. Journal of Developmental & Behavioral Pediatrics. 2005;26(2):86–92. doi: 10.1097/00004703-200504000-00003. [DOI] [PubMed] [Google Scholar]
- Riem MM, Van Ijzendoorn MH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ. No laughing matter: Intranasal oxytocin administration changes functional brain connectivity during exposre to infant laughter. Neuropsychopharmacology. 2012;37(5):1257–1266. doi: 10.1038/npp.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronca A, Alberts J. Sensory stimuli associated with gestation and parturition evoke cardiac and behavioral responses in fetal rats. Psychobiology. 1994;22(4):270–282. doi: 10.3758/BF03327110. [DOI] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack KM, Howell BR. Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Social Neuroscience. 2015;10(5):512–526. doi: 10.1080/17470919.2015.1087426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Haller J. Stress and the social brain: Behavioural effects and neurobiological mechanisms. Nature Reviews Neuroscience. 2015;16(5):290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Sarro EC, Sullivan RM, Wilson DA. Maternal regulation of infant brain state. Current Biology. 2014;24:1664–1669. doi: 10.1016/j.cub.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal B, Coureaud G, Doucet S, Delaunay-El Allam M, Moncomble A-S, Montigny D. Mammary olfactory signalisation in females and odor processing in neonates: Ways evolved by rabbits and humans. Behavioural Brain Research. 2009;200(2):346–358. doi: 10.1016/j.bbr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: Reversal of fear in the human brain. The Journal of Neuroscience. 2008;28(45):11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleidt M, Genzel C. The significance of mother’s perfume for infants in the first weeks of their life. Ethology & Sociobiology. 1990;11(3):145–154. doi: 10.1016/0162-3095(90)90007-S. [DOI] [Google Scholar]
- Schonheit B. The development of neurons in the cingulate cortex in postnatally undernourished rats. Folia Morphology (Praha) 1980;28(4):337–340. [PubMed] [Google Scholar]
- Schönheit B. The influence of early postnatal undernourishment on the development of cortical neurons in the rat] Journal Für Hirnforschung. 1982;23(6):681. [PubMed] [Google Scholar]
- Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. The Journal of Neuroscience. 1982;2(10):1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z. Limbic-frontal circuitry in major depression: A path modeling metanalysis. Neuroimage. 2004;22(1):409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. The Journal of Comparative Neurology. 1989;290(2):213–242. doi: 10.1002/(ISSN)1096-9861. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R. Enduring effects of infant memories: Infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biological Psychiatry. 2007;62(10):1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Mouly A-M, Raineki C, Moriceau S, Forest C, Sullivan RM. Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infant’s sensitive period attachment learning. Developmental Cognitive Neuroscience. 2011;1(1):77–87. doi: 10.1016/j.dcn.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Sullivan RM, Messaoudi B, Mouly A-M. Neonatal odor-shock conditioning alters the neural network involved in odor fear learning at adulthood. Learning & Memory. 2008;15(9):649–656. doi: 10.1101/lm.998508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Hormones and Behavior. 2007;52(3):391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PJ, Tobach E. Olfactory bulbectomy and nursing behavior in rat pups (Wistar DAB) Developmental Psychobiology. 1975;8(2):151–164. doi: 10.1002/dev.420080207. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypotha-lamic–pituitary–adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripanidkulchai K, Sripanidkulchai B, Wyss J. The cortical projection of the basolateral amygdaloid nucleus in the rat: A retrograde fluorescent dye study. The Journal of Comparative Neurology. 2004;229(3):419–431. doi: 10.1002/cne.902290310. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Levine S. Brief separation elevates cortisol in mother and infant squirrel monkeys. Physiology & Behavior. 1985;34(6):1007–1008. doi: 10.1016/0031-9384(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: Specific role of maternal cues. Developmental Psychobiology. 1990;23(5):411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stern JM. Offspring-induced nurturance; Animal-human parallels. Developmental Psychobiology. 1997;31(1):19–37. doi: 10.1002/(ISSN)1098-2302. [DOI] [PubMed] [Google Scholar]
- Sturrock R. Development of the indusium griseum. I. A Quantitative Light Microscopic Study of Neurons and Glia. Journal of Anatomy. 1978;125(Pt 2):293. [PMC free article] [PubMed] [Google Scholar]
- Suchecki D, Nelson DY, Van Oers H, Levine S. Activation and inhibition of the hypothalamic–pituitary–adrenal axis of the neonatal rat: Effects of maternal deprivation. Psychoneuroendocrinology. 1995;20(2):169–182. doi: 10.1016/0306-4530(94)00051-B. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic–pituitary–adrenal axis in the infant rat: The roles of feeding and stroking. Developmental Brain Research. 1993;75(2):185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neuroscience & Biobehavioral Reviews. 2010;34(6):835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407(6800):38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Developmental Brain Research. 1986;27(1):278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Perry RE. Mechanisms and functional implications of social buffering in infants: Lessons from animal models. Social Neuroscience. 2015;10(5):500–511. doi: 10.1080/17470919.2015.1087425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Perry RE, Sloan A, Kleinhaus K, Burtchen N. Infant bonding and attachment to the caregiver: Insights from basic and clinical science. Clinics in Perinatology. 2011;38(4):643–655. doi: 10.1016/j.clp.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Taborsky-Barba S, Mendoza R, Mendoza R, Itano A, Leon M. Olfactory classical conditioning in neonates. Pediatrics. 1991;87(4):511–518. [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Toubas P. Clinical usefulness of maternal odor in newborns: Soothing and feeding preparatory responses. Biology of the Neonate. 1998;74(6):402–408. doi: 10.1159/000014061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Dissociation of behavioral and neural correlates of early associative learning. Developmental Psychobiology. 1995;28(4):213–219. doi: 10.1002/dev.420280403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Developmental Brain Research. 1990;53(2):243–247. doi: 10.1016/0165-3806(90)90013-O. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: A site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31(6):410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kiyokawa Y, Kodama Y, Arata S, Takeuchi Y, Mori Y. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behavioural Brain Research. 2013;240:46–51. doi: 10.1016/j.bbr.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Taylor JH, Mustoe AC, Hochfelder B, French JA. Reunion behavior after social separation is associated with enhanced HPA recovery in young marmoset monkeys. Psychoneuroendocrinology. 2015;57:93–101. doi: 10.1016/j.psyneuen.2015.03.019.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Blass EM. Suckling in newborn rats: Eliminated by nipple lavage, reinstated by pup saliva. Science. 1976;193(4251):422–425. doi: 10.1126/science.935878. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Blass EM. First suckling response of the newborn albino rat: The roles of olfaction and amniotic fluid. Science. 1977;198(4317):635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behavioral Brain Research. 2004;149(2):209–215. doi: 10.1016/S0166-4328(03)00216-X. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53(4):865–871. doi: 10.1016/S0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119(3):488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton KJ, Sullivan RM. Defining age limits of the sensitive period for attachment learning in rat pups. Developmental Psychobiology. 2010;52(5):453–464. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden C, Uylings H. Cytoarchitectonic development of the prefrontal cortex in the rat. The Journal of Comparative Neurology. 2004a;241(3):253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Van Eden C, Uylings H. Postnatal volumetric development of the prefrontal cortex in the rat. The Journal of Comparative Neurology. 2004b;241(3):268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- Van Oers HJ, De Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. The Journal of Neuroscience. 1998;18(23):10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varendi H, Porter RH. Breast odour as the only maternal stimulus elicits crawling towards the odour source. Acta Paediatrica. 2001;90(4):372–375. doi: 10.1080/080352501750126131. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Walker CD, Deschamps S, Proulx K, Tu M, Salzman C, Woodside B. Mother to infant or infant to mother? Reciprocal regulation of responsiveness to stress in rodents and the implications for humans. Journal of Psychiatry Neuroscience. 2004;29(5):364–382. [PMC free article] [PubMed] [Google Scholar]
- Weber EM, Olsson IAS. Maternal behaviour in Mus musculus sp.: An ethological review. Applied Animal Behaviour Science. 2008;114:1–2. 1–22. doi: 10.1016/j.applanim.2008.06.006. [DOI] [Google Scholar]
- Zhang ZW. Maturation of layer V pyramidal neurons in the rat prefrontal cortex: Intrinsic properties and synaptic function. Journal of Neurophysiology. 2004;91(3):1171–1182. doi: 10.1152/jn.00855.2003. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP. Neurocircuitry of stress integration: Anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integrational Compative Biology. 2002;42(3):541–551. doi: 10.1093/icb/42.3.541. [DOI] [PubMed] [Google Scholar]


