Abstract
Background and Aim
Hepatic fibrosis is marked by activation of hepatic stellate cells (HSCs). Cholestatic injury precedes liver fibrosis and cholangiocytes interact with HSCs promoting fibrosis. Mast cells (MCs) infiltrate following liver injury and release histamine increasing biliary proliferation. We evaluated if inhibition of MC-derived histamine decreases biliary proliferation and fibrosis.
Methods
WT and Mdr2−/− mice (9-11 weeks) were treated with cromolyn sodium for 1 week to block MC-derived histamine. Biliary mass and proliferation were evaluated by immunohistochemistry for CK-19 and Ki-67. Bile flow, bicarbonate excretion and total bile acids were measured in all mice. Fibrosis was evaluated by Sirius Red/Fast Green staining and by qPCR for α-SMA, fibronectin, collagen type 1a and TGF-β1. HSC activation was evaluated by qPCR in total liver and immunofluorescent staining in tissues for synaptophysin 9. Histamine serum secretion was measured by EIA. Mouse liver and human liver samples from control or PSC patients were evaluated for MC markers by qPCR and immunohistochemistry. In vitro, cultured MCs were transfected with HDC shRNA to decrease histamine secretion and subsequently co-cultured with cholangiocytes or HSCs prior to measuring fibrosis markers, proliferation and TGF-β1 secretion.
Results
Treatment with cromolyn sodium decreased biliary proliferation, fibrosis, histamine secretion, and bile flow in Mdr2−/− mice. PSC mice and patients have increased MCs. Knockdown of MC HDC decreased cholangiocyte and HSC proliferation/activation.
Conclusion
MCs are recruited to proliferating cholangiocytes and promote fibrosis. Inhibition of MC-derived histamine decreases fibrosis and regulation of MC mediators may be a therapeutic for PSC.
Keywords: mast cells, fibrosis, histamine, Primary Sclerosing Cholangitis
Introduction
Primary sclerosing cholangitis (PSC) is a progressive disease of the liver characterized by chronic cholestasis leading to inflammation and fibrosis of the biliary epithelium. PSC often occurs in the 3rd – 4th decade of life and can affect intrahepatic and/or extrahepatic ducts leading to diffuse stricturing and thickening(1). Though the etiology of PSC is not fully known, it has autoimmune features which may explain the presence of serum markers such as antinuclear antibodies and perinuclear antineutrophil cytoplasmic antibodies which are found in up to 50% and 80% of cases, respectively(1, 2). No effective treatment has been found to slow or alter the progression of PSC, and it remains a leading cause of liver transplantation.
The multidrug resistance gene 2 is an ABC transporter, which encodes a P-type glycoprotein responsible for the excretion of biliary phospholipids(1, 3). Mdr2−/− mice, which mimic human PSC progression(3), lack this gene causing bile acids to accumulate in the intrahepatic biliary system and disrupt portal tract function. The proper function of the bicarbonate umbrella has been demonstrated to be important in the regulation of cholangiopathies including PSC, and Mdr2−/− mice have been described to be vulnerable to toxic bile formation, which can be induced by decreased bicarbonate excretion, abnormal bile composition, increased biliary pressure and biliary casts (4, 5). While hepatic stellate cells (HSCs) are the primary producers of collagen leading to fibrosis in most chronic liver diseases (3), cholangiocytes have also been found to play a role in promoting hepatic fibrosis by interacting with HSCs via hedgehog signaling (6).
Mast cells are developed from pluripotent stem cells found in the bone marrow and the spleen. They secrete histamine, as well as a variety of other cytokines and play an important role in both the innate immune system and the allergic response(7). Mast cell-derived histamine can stimulate biliary proliferation and fibrosis via autocrine and paracrine mechanisms(8). Furthermore, the number of hepatic mast cells increases as liver disease progresses(9). Following bile duct ligation, mast cells are found in close proximity to bile ducts and portal tracts(8). Mast cell infiltration correlates with increased bile duct mass and proliferation and treatment with the mast cell stabilizer, cromolyn sodium, decreases BDL-induced bile duct mass and biliary proliferation(8).
Cromolyn sodium has also been used to treat the autoimmune disorder, irritable bowel disease(10, 11). Furthermore, it has been demonstrated that cromolyn sodium acts directly on mast cells but has no direct effect on cholangiocytes, suggesting that this compound may be useful to modulate mast cell degranulation during cholangiopathies(8). Such evidence is important for studies aimed at treatments for cholangiopathies because it further details the paracrine role served by mast cells in the setting of liver diseases such as PSC.
Materials and Methods
Reagents and other materials
Chemical grade reagents were purchased from Sigma Aldrich Co (St. Louis, MO, USA) unless stated otherwise. All mouse primers, shRNA and real-time PCR materials were obtained from Qiagen (Fredrick, MD, USA). Antibodies for immunoblotting, immunohistochemistry and immunofluorescence were purchased from Santa Cruz Biotechnology (Dallas, TX). Histamine EIA kits were purchased from Cayman Chemical (An Arbor, MI, USA).
In vivo models
We utilized the genetically modified mouse model of Primary Sclerosing Cholangitis (PSC), Mdr2−/− mice. Both FVB/NJ (WT) and Mdr2−/− male mice (9 - 11 weeks of age) obtained from Jackson Laboratory (Sacramento, CA) were implanted with osmotic minipumps to deliver cromolyn sodium (24 mg/kg/BW) for 1 week(8). All mice were housed in the Baylor Scott and White Health Animal Facility and given free access to drinking water and standard chow. All animals were kept in a temperature-controlled environment with a 12:12 light/dark cycle, and all protocols strictly adhered to regulations set forth by the local IACUC committee.
From all animals we collected serum and liver blocks (frozen and paraffin-embedded). We performed hematoxylin and eosin (H&E) staining in livers to evaluate lobular damage, hepatic necrosis and inflammation.
Detection of mast cells, mast cell markers and histamine secretion in PSC mice
In all groups hepatic mast cell presence was detected by immunohistochemistry for mouse mast cell protease 1 (mMCPT-1). Mast cell marker expression (c-kit, chymase and tryptase) was measured in total liver by real-time PCR(8, 12). Histamine secretion was evaluated by EIA in serum from all groups of mice(13).
Evaluation of mast cells and mast cell markers in human PSC
To determine if mast cells are present in human PSC, we measured the expression of mast cell markers by toluidine blue staining, immunohistochemistry and real-time PCR for c-Kit, FCεR1, chymase and tryptase in human liver sections from control (no disease) and PSC patients (84% late stage PSC and 16% advanced PSC). The classification of late and advanced stage PSC is described as the following: Stage I, inflammation and connective tissue proliferation around the small bile ducts; Stage II, inflammation passes into the liver with the formation of scar tissue; Stage III, more severe scar tissue forms and Stage IV represents biliary liver cirrhosis. Stages I and II represent early stage PSC and Stages III and IV represent late to advanced stage that have similar characteristics. Liver sections (4-5 μm thick) obtained by needle biopsies from three control patient and three PSC patients were provided by Dr. Pietro Invernizzi (Humanitas Research Hospital, Rozzano, Italy) under a protocol approved by the Ethics Committee of the Humanitas Research Hospital; the protocol was also reviewed by the local Veterans’ Administration IRB and R&D Committee. The use of human tissue was also approved by the Baylor Scott & White Health Institutional Review Board.
Evaluation of biliary mass and proliferation
Since we have previously demonstrated that mast cells infiltrate the liver following BDL and participate in sustaining biliary proliferation(8), we measured the effects of cromolyn sodium on intrahepatic bile duct mass (IBDM) and proliferation in Mdr2−/− mice. In liver sections from all groups we performed immunohistochemistry for the biliary marker, CK-19(8, 13) to measure IBDM and the number of cholangiocytes positive for Ki-67 to evaluate biliary proliferation.
Cromolyn sodium effects on bile flow, bicarbonate excretion, and total bile acids
Bile was collected from all animal groups according to previous work(14). Incannulation of the gallbladder was performed using a 30 gauge needed attached to PE 10 tubing immediately following ligation of the common bile duct. Bile was collected for up to 20 minutes and stored for bicarbonate and total bile acid analysis. Body temperature was monitored and maintained at 37°C throughout the collection and fluids were introduced into the peritoneal cavity. Bicarbonate levels were assessed in all groups by National Mouse Metabolic Phenotyping Centers (MMPC, Yale University School of Medicine), and total bile acids were measured in bile, serum and total liver homogenate from all groups using a commercially available Mouse Total Bile Acid Assay Kit (Crystal Chemical Co, Wakefield, MA).
Effects of mast cells and mast cell-derived histamine on hepatic fibrosis
To evaluate the effects of mast cells on hepatic fibrosis we measured collagen content in liver sections from all experimental groups by Fast Green/Sirius Red (along with semi-quantification) and Masson's Trichrome(13). In total liver we measured the following fibrotic markers by real-time PCR: α-smooth muscle actin (α-SMA), collagen type-1a and fibronectin(13). TGF-β1 is a known regulator of HSC-driven fibrosis(1) and we evaluated the expression of TGF-β1 in total liver by real-time PCR(8, 13).
Alterations to vascular endothelial cells following mast cell-derived histamine inhibition
We measured the effects of cromolyn sodium on the vascular bed by evaluating the expression of Von Wilenbrand (Factor VIII) by immunofluorescence in liver sections and the expression of vascular endothelial growth factor A (VEGF-A) in total liver RNA by real-time PCR as previously described (8, 13).
Evaluation of HSC activation
We evaluated the effects of mast cells on hepatic stellate cell (HSC) activation. In tissue sections from all groups we measured HSC activation by immunofluorescence for synaptophysin-9 (SYP-9). Livers were co-stained with CK-19 to visualize bile ducts. In total liver we evaluated SYP-9 gene expression by real-time PCR.
In vitro evaluation of mast cell/cholangiocyte/HSC interactions
Our hypothesis is that, following cholangiocyte injury (i.e. BDL or in Mdr2−/−), mast cells infiltrate the liver and release factors (e.g. histamine) to increase cholangiocyte proliferation and activate HSCs thereby contributing to fibrosis progression. To evaluate if mast cells alter both cholangiocytes and HSCs in vitro, we utilized immortalized murine biliary cell lines that we have previously used in numerous studies and are comprised of all-sized mouse cholangiocytes (13, 15), human HSCs (hHSCs) and cultured mouse mast cells (MCs). To determine if MC-derived histamine alters either cholangiocyte or HSC proliferation/activation, MCs were first transfected with empty vector or shRNA targeting histidine decarboxylase (HDC) to inhibit the production and release of MC histamine(13, 16).
Cholangiocytes were plated and allowed to come to confluency before the addition of non-adherent MC cultures (transfected with empty vector (MCneg) or HDC shRNA (MCshHDC) to manipulate mast cell histamine levels)(15, 16). After up to 6 days of co-culture, proliferation/activation and fibrosis were measured by real-time PCR for PCNA, α-SMA, fibronectin and TGF-β1(8).
Human HSCs (hHSCs) were stimulated with mast cell supernatants collected from transfected mast cells, MCneg (stable levels of histamine), or MCshHDC (depleted levels of histamine) and we measured hHSC expression of PCNA, α-SMA, fibronectin and TGF-β1.
Statistical Methods
All data is expressed as mean ± SEM. Groups were analyzed by the Student unpaired t test when two groups are analyzed or a two-way ANOVA when more than 2 groups are analyzed, followed by an appropriate post hoc test. p<0.05 was considered significant.
Results
Detection of mast cells, mast cell markers and histamine secretion
Since mast cells have been found in close proximity to proliferating bile ducts and infiltrate the liver following BDL in rats(8) and are found in human PSC(17), we aimed to demonstrate that mast cells are present in Mdr2−/− mice. Immunohistochemistry for mMCPT-1 demonstrated that there is an increase in the number of mast cells in Mdr2−/− mice compared to WT and mast cell numbers were reduced in Mdr2−/− mice treated with cromolyn sodium (Figure 1A). Further, mast cells were found in close proximity to large bile ducts (marked with black arrows, Figure 1A). In serum from Mdr2−/− mice, histamine secretion increased compared to WT, whereas in Mdr2−/− mice treated with cromolyn sodium, histamine secretion was significantly downregulated (Figure 1B). The expression of c-Kit, chymase and tryptase increased in Mdr2−/− mice compared to WT and treatment with cromolyn sodium decreased these genes (Figure 1C).
Figure 1.
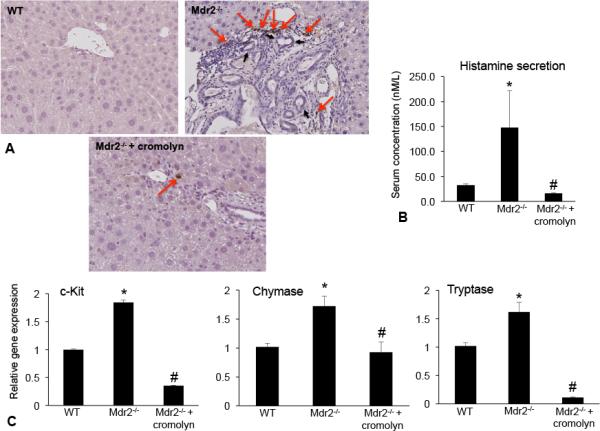
Mast cells were found in close proximity to bile ducts in Mdr2−/− mice. (A) The number of mMCPT-1-positive mast cells (found close to large bile ducts) increased in Mdr2−/− mice compared to WT and treatment with cromolyn sodium decreased the number of infiltrating mast cells. (A) Mast cells are indicated with red arrows and large bile ducts are marked with black arrows. Images are 20× magnification. Histamine secretion was measured by EIA in serum from WT, Mdr2−/− and Mdr2−/− + cromolyn sodium. (B) Histamine levels increased in Mdr2−/− mice, but decreased in Mdr2−/− mice treated with cromolyn sodium compared to WT. (C) c-Kit, chymase and tryptase gene expression are increased in Mdr2−/− mice compared to WT, whereas treatment with cromolyn sodium decreased the gene expression in Mdr2−/− mice. Data are expressed as mean ± SEM of at least 6 experiments for real-time PCR and 12 experiments for EIA. *p<0.05 versus WT mice; #p<0.05 versus Mdr2−/− mice.
Using samples collected from patients diagnosed with late or advanced stage PSC and corresponding control tissues, we confirmed the presence of mast cells and mast cell markers (Figure 2). Similar to previous reports, livers from human PSC patients display robust mast cell marker expression including c-Kit, FCεR1, chymase and tryptase as shown in Figure 2 by real-time PCR (A), immunostaining for toluidine blue and immunohistochemistry for chymase and tryptase (B) when compared to control tissue that had little or no mast cells. These data support the concept that mast cells are an important player in the regulation and progression of human PSC.
Figure 2.
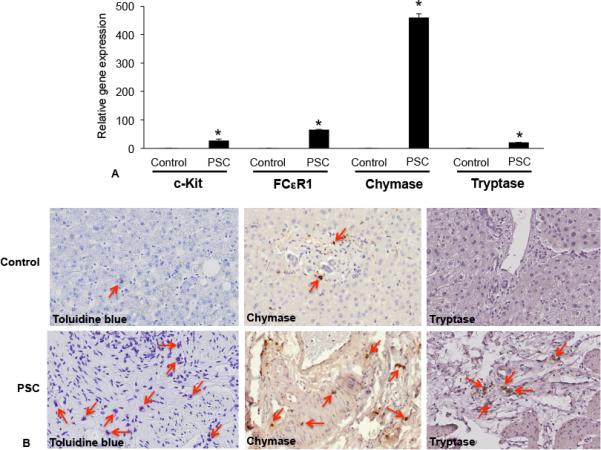
Mast cell presence was assessed in human liver biopsy samples from control (no disease) and PSC patients (late and advanced PSC) by real-time PCR, toluidine blue staining and immunohistochemistry for mast cell markers (chymase and tryptase). (A) The gene expression of c-Kit, FCεR1, chymase and tryptase increased in samples from advanced and late stage PSC when compared to normal, non-diseased tissues. (B) By immunostaining (toluidine blue) and immunohistochemistry (chymase and tryptase), there is an infiltration of mast cells found surrounding damaged bile ducts in PSC patients compared to normal tissue (red arrows depict mast cells). Data are expressed as mean ± SEM of at least 6 experiments for real-time PCR. *p<0.05 versus control. Images are 20× magnification
Cromolyn sodium treatment ameliorates liver damage and decreases biliary mass and proliferation in Mdr2−/− mice
In Mdr2−/− mice necrosis, lobular damage and portal inflammation increased compared to WT, whereas treatment with cromolyn sodium ameliorated these pathological features (Figure 3A). The liver/BW ratio increased in Mdr2−/− mice compared to WT, whereas treatment with cromolyn sodium decreased the liver/BW ratio compared to Mdr2−/− mice (Figure 3B).
Figure 3.
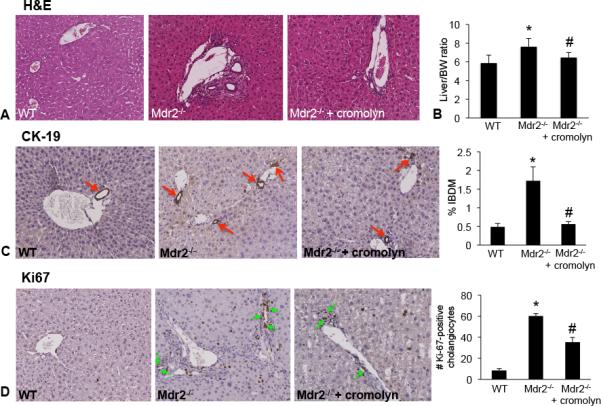
(A) Liver damage was assessed by H&E staining in liver sections from all groups. Inflammation, necrosis and lobular damage increased in Mdr2−/− mice compared to WT mice and these features were reduced in Mdr2−/− mice treated with cromolyn sodium. Images are 20× magnification. (B) Liver weight and body weights were recorded for each animal and the ratio was calculated. Mdr2−/− mice have increased liver/BW ratio compared to WT and treatment with cromolyn sodium significantly decreases liver/BW ratio compared to Mdr2−/−. (C) Bile duct mass and (D) Ki-67 were evaluated in liver sections from WT, Mdr2−/− mice and Mdr2−/− mice treated with cromolyn sodium. We found that bile duct mass (CK-19 staining, red arrows) and Ki-67 increased in Mdr2−/− mice compared to WT mice, whereas treatment with cromolyn sodium decreased both bile duct mass (red arrows) and the number of proliferating cholangiocytes (green arrows). Data has been semi-quantitated. Data are expressed as mean ± SEM of at least 10 experiments. *p<0.05 versus WT mice; #p<0.05 versus Mdr2−/− mice.
We have demonstrated that cromolyn sodium treatment decreases IBDM and proliferation in rats following BDL(8). In our current study we found that IBDM and cholangiocyte proliferation were both increased in Mdr2−/− mice compared to WT mice (Figure 3C and 3D). In Mdr2−/− mice treated with cromolyn sodium, IBDM and proliferation decreased compared to Mdr2−/− mice (Figure 3C and3).
Blocking mast cell-derived histamine inhibits bile flow, decreases bicarbonate levels and reduces the total bile acid pool
In Mdr2−/− mice, there was increased bile flow; however, treatment with cromolyn sodium reduced bile flow (Figure 4A). Bicarbonate excretion was unchanged between WT and Mdr2−/− mice, but was reduced in Mdr2−/− mice treated with cromolyn sodium compared to Mdr2−/− mice (Figure 4B). Total bile acid composition in bile was reduced in Mdr2−/− mice treated with or without cromolyn sodium compared to WT (Figure 4C), whereas serum bile acids (Figure 4D) and total liver homogenate bile acids (Figure 4E) were increased in Mdr2−/− mice compared to WT and treatment with cromolyn sodium decreased both serum and total liver homogenate bile acid levels.
Figure 4.
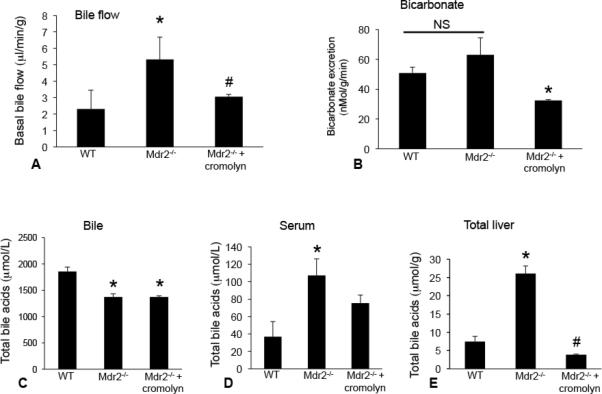
In vivo, we measured the effects of cromolyn sodium treatment on bile flow, bicarbonate excretion and total bile acid (TBA) concentration. In Mdr2−/− mice there was increased bile flow; however, treatment with cromolyn sodium significantly reduced bile flow (A). Bicarbonate excretion was unchanged between WT and Mdr2−/− mice (NS = not significant), but was reduced in Mdr2−/− mice treated with cromolyn sodium compared to Mdr2−/− mice (B). Total bile acid composition in bile was reduced in Mdr2−/− mice treated with or without cromolyn sodium compared to WT (C), whereas serum bile acids (D) and total liver homogenate bile acids (E) were increased in Mdr2−/− mice compared to WT and treatment with cromolyn sodium decreased both serum and total liver homogenate bile acid levels. Data are expressed as mean ± SEM of at least 6 experiments from each animal for bile flow; 6 experiments from each animal for bicarbonate excretion, 4 experiments for bile TBA, 10 experiments for serum TBA and 4 experiments for total liver TBA. *p<0.05 versus WT; #p<0.05 versus Mdr2−/− mice.
Inhibition of mast cell-derived histamine decreases hepatic fibrosis in Mdr2−/− mice
In liver sections we evaluated collagen content by Fast Green/Sirius Red and Masson's Trichrome staining (Figure 5A). Mdr2−/− mice have increased collagen deposition compared to WT, whereas in Mdr2−/− mice treated with cromolyn sodium collagen deposition was reduced (Figure 5A). Fast Green/Sirius Red staining was semi-quantified and demonstrates that there is a significant upregulation of collagen content in Mdr2−/− mice compared to WT and treatment with cromolyn sodium decreases collagen content in Mdr2−/− mice (Figure 5B). By real-time PCR, we found that α-SMA, collagen type-1a and fibronectin increased in Mdr2−/− mice compared to WT. When Mdr2−/− mice were treated with cromolyn sodium, the expression of these fibrotic genes decreased (Figure 5C).
Figure 5.
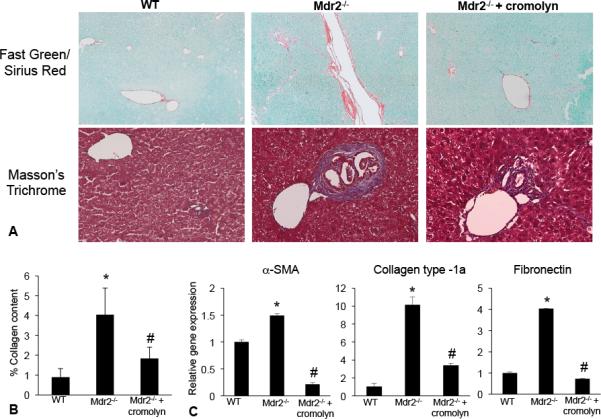
Fibrosis and collagen content was evaluated by immunostaining and real-time PCR in WT, Mdr2−/− mice and Mdr2−/− mice treated with cromolyn sodium. (A) Staining for Fast Green/Sirius Red and Masson's Trichrome demonstrate an increase in collagen content in Mdr2−/− mice compared to WT. (B) Treatment with cromolyn sodium decreased collagen content and the fibrotic reaction in Mdr2−/− mice as shown by semi-quantification of Fast Green/Sirius Red staining. (C) The expression of α-SMA, collagen-type 1a and fibronectin were increased in total liver mRNA from Mdr2−/− mice compared to WT and treatment with cromolyn sodium decreased these fibrotic genes. Data are expressed as mean ± SEM of at least 9 experiments. *p<0.05 versus WT mice; #p<0.05 versus Mdr2−/− mice. Images are 20× magnification.
Blocking mast cell-derived histamine alters the vascular cell proliferation in Mdr2−/− mice
Immunofluorescence for Factor VIII reveals that there is an upregulation of vascular cell positivity in Mdr2−/− mice compared to WT and treatment with cromolyn decreases the intensity/postivity of Factor VIII (Supplemental Figure 1). Further, VEGF-A gene expression was increased in Mdr2−/− mice compared to WT and decreased in Mdr2−/− mice treated with cromolyn sodium (Supplemental Figure 1).
HSC activation and TGF-β1 signaling are decreased in Mdr2−/− mice treated with cromolyn sodium
SYP-9 expression was significantly upregulated in Mdr2−/− mice compared to WT (Figure 6A and 6B). As expected, we found that SYP-9 protein and gene expression significantly decreased in Mdr2−/− treated with cromolyn sodium (Figure 6A and 6B). To evaluate the downstream targets of HSC-driven fibrosis, we measured TGF-β1 levels in total liver from WT, Mdr2−/− and Mdr2−/− + cromolyn sodium. In Mdr2−/− mice TGF-β1 expression increased compared to WT, whereas in Mdr2−/− mice treated with cromolyn sodium, expression decreased (Figure 6C).
Figure 6.
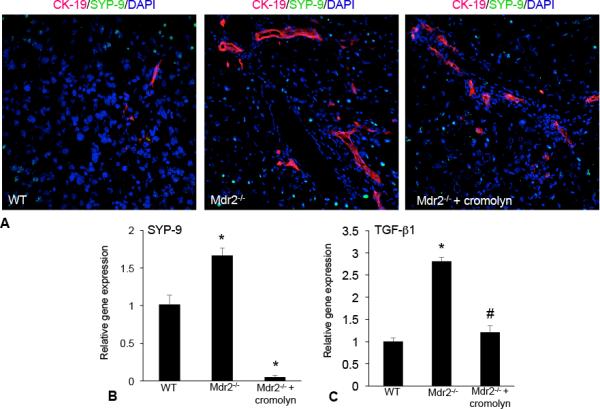
HSC activation was evaluated by immunofluorescence and real-time PCR for the expression of SYP-9 in WT, Mdr2−/− mice and Mdr2−/− mice treated with cromolyn sodium. (A) SYP-9 expression (green staining) was increased in Mdr2−/− mice compared to WT; whereas, treatment with cromolyn sodium decreased SYP-9 expression in Mdr2−/− mice (bile ducts are depicted by red staining). (B) Further, the gene expression of SYP-9 increased in total liver mRNA from Mdr2−/− mice compared to WT and treatment with cromolyn sodium decreased SYP-9 expression. The expression of TGF-β1 was measured by real-time PCR in total liver from WT, Mdr2−/− mice and Mdr2−/− mice treated with cromolyn sodium. (C) TGF-β1 expression significantly increased in total liver mRNA from Mdr2−/− mice compared to WT and decreased after treatment with cromolyn sodium. Data are expressed as mean ± SEM of at least 6 experiments for real-time PCR. *p<0.05 versus WT mice; #p<0.05 versus Mdr2−/− mice.
Mast cell-derived histamine regulates biliary proliferation, fibrosis and HSC activation, in vitro
We have demonstrated that cholangiocytes express HDC and the histamine receptors (H1- H4 HR)(18) and prior to performing in vitro experiments, we found that hHSCs express HDC and H1 – H4 HRs (results not shown). Next we focused on the effects that mast cells (MCs) have on biliary proliferation and fibrotic reaction. MCs were transfected with HDCshRNA (MCshHDC) to inhibit histamine production or empty vector (MCneg) prior to co-culture with cultured cholangiocytes (Figure 7A). When cholangiocytes were co-cultured with MCneg (stable levels of HDC), there was an increase in proliferation, fibrosis markers and TGF-β1 as shown by PCR (Figure 7B). In contrast, in cholangiocytes co-cultured with MCshHDC, there was a significant decrease in these parameters suggesting that MC-derived histamine regulates biliary proliferation and fibrotic reaction, in vitro (Figure 7B).
Figure 7.
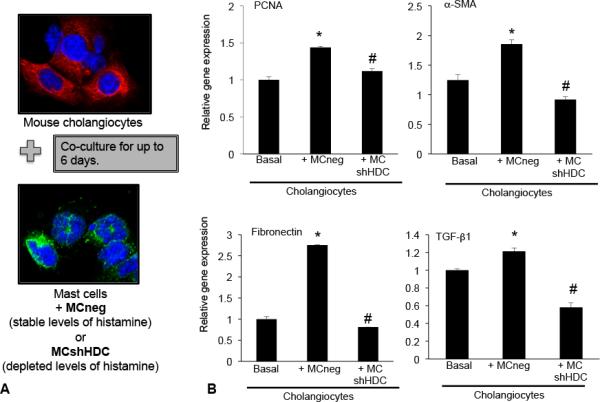
(A) In vitro, we measured the effects of mast cells transfected with empty vector (MCneg) or HDC shRNA (MCshHDC) on cholangiocytes in culture. (B) Following co-culture with mast cells containing stable levels of histamine (MCneg), cholangiocyte PCNA, fibronectin and α-SMA expression are increased; whereas, in cholangiocytes co-cultured with mast cells containing depleted levels of histamine (MCshHDC), these parameters were decreased. (B) TGF-β1 gene expression was increased in cholangiocytes co-cultured with MCneg compared to basal and was decreased when cholangiocytes were co-cultured with MCshHDC. Data are expressed as mean ± SEM of at least 6 experiments for EIA and real-time PCR. *p<0.05 versus basal cholangiocytes; #p<0.05 versus MCneg.
Next, humans HSCs (hHSCs) were stimulated with supernatants collected from MCneg and MCshHDC transfected cells for up to 48 hours (Figure 8A). In hHSCs stimulated with MCneg (stable levels of MC histamine), there was a significant increase in proliferation (Figure 8B) and fibrotic markers (Figure 8C) compared to basal treatment, however in hHSCs stimulated with supernatants from MCshHDC (inhibited levels of MC histamine), these parameters decreased. These data strongly indicate that hHSCs are influenced by mast cell-derived histamine that ultimately alters HSC activation driving fibrosis.
Figure 8.
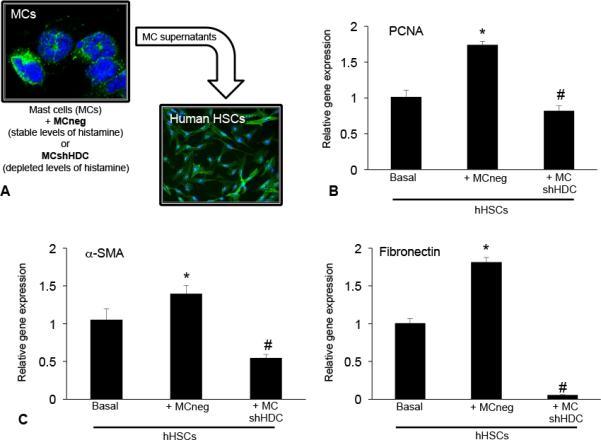
(A) In vitro, we measured the effects of mast cells transfected with empty vector (MCneg) or HDC shRNA (MCshHDC) on human HSCs (hHSCs) in culture. Following stimulation with supernatants from mast cells containing stable levels of histamine (MCneg), (B) hHSC PCNA, (C) α-SMA and fibronectin expression are increased; whereas, in hHSCs stimulated with supernatants from mast cells containing depleted levels of histamine (MCshHDC), these parameters were decreased. Data are expressed as mean ± SEM of at least 4 experiments for EIA and real-time PCR. *p<0.05 versus basal hHSCs; #p<0.05 versus MCneg.
Discussion
We have described the effects of the infiltration of hepatic mast cells in the PSC mouse model, Mdr2−/− and found that inhibition of mast cell-derived histamine (via cromolyn sodium treatment) decreases proliferation and hepatic fibrosis associated with PSC. In support of our findings, it has been demonstrated that mast cells are present in human PSC(19, 20) and contribute to the progression of fibrosis. c-Kit positive mast cells have been found in the portal tract(19) and increased mast cell infiltration has been shown within the sclerosing areas of PSC patients(20). Our results demonstrate that mast cells are found in both advanced and late stage human PSC and may contribute to the fibrotic phenotype. Mast cells have also been found in primary biliary cirrhosis and alcoholic liver injury(17, 21) implicating these immune cells in liver damage.
Our previous work demonstrates that in models of damage or cellular proliferation, histamine levels are increased(15, 18). We have shown that cholangiocytes secrete histamine and during cholangiocarcinoma progression there is an autocrine regulation of cholangiocyte histamine release that increases tumor growth(16). Following BDL, mast cells infiltrate the liver and increase proliferation (8). In addition, patients with PSC have increased serum histamine concentrations and the presence of mast cells is also increased(22). These studies indicate a link between increased histamine levels, that is derived primarily from infiltrated hepatic mast cells and hepatic damage, which is marked by increased biliary proliferation and periportal or portal fibrosis. Further, our previous work has also shown that inhibition of histamine decreases biliary proliferation and damage(8), therefore modulation of mast cell-derived histamine induces an alteration in biliary response and hepatic fibrosis.
Mdr2−/− mice undergo a transformation from birth to maturity that involves biliary proliferation associated with liver damage peaking anywhere from 6 – 12 weeks of age(3). The male mice we used were 9-11 weeks of age and, as shown by H&E, there is marked inflammation along with increased bile duct mass and biliary proliferation that are alleviated by cromolyn sodium treatment. Cromolyn sodium has been found to inhibit cellular proliferation in a number of models and diseases. In various cancer cell lines cromolyn sodium decreased proliferation including adenocarcinoma, hepatocellular carcinoma, mammary gland carcinoma and epidermoid larynx carcinoma demonstrating that cromolyn sodium is anti-proliferative(23).
It has been reported that Mdr2−/− mice are susceptible to toxic bile formation, which can be caused by increased bicarbonate excretion, abnormal bile composition, increased biliary pressure and biliary casts (4, 5). In our study we found an increase in bile flow in Mdr2−/− mice that was ameliorated by cromolyn sodium treatment. Bicarbonate levels were elevated compared to WT and significantly reduced by cromolyn sodium treatment. In support of our findings, an early study found that histamine treatment increased choleresis in sheep, and while treatment with an H1HR antagonist did not alter histamine-induced bile flow the H2HR antagonists, cimetidine and ranitidine, both inhibited histamine-induced choleresis(24). Further, in rats, ischemic reperfusion has been shown to decrease hepatic bile flow and treatment with ranitidine reversed these effects (25), again demonstrating that histamine (via histamine receptor activation) has a relevant effect on choleresis and hepatic function. Interestingly, a study investigating mast cell effects on ischemic reperfusion and bile flow found that there was no difference in bile flow excretion between wild-type rats or mast cell deficient, Ws/Ws rats (26); which, in contrast to our study, might be explained by the difference in injury and rodent model. Our data demonstrates that hepatic bile flow is increased in PSC mice (that have more mast cells compared to WT) and our studies are the first to demonstrate that inhibition of mast cell-derived histamine may regulate the bicarbonate umbrella and have a protective role against toxic bile in Mdr2−/− mice. Total bile acids were increased in the Mdr2−/− mice, which were lowered in serum and significantly reduced in liver homogenates in mice treated with cromolyn sodium. To our knowledge, our study is the first to demonstrate that inhibition of mast cell-derived histamine alters and decreases the total bile acid pool in a PSC model and further studies are planned to better understand this important event.
The pattern of PSC progression in Mdr2−/− mice has been described to be similar to that that is seen in humans. Popov, et al. have reported that Mdr2−/− mice display a robust fibrotic reaction beginning at 2 weeks after birth and by 8 weeks of age there is an increase in collagen deposition and fibrosis-related genes are elevated including α-SMA that is 20 fold greater than wild-type mice(27). Our studies reveal that Mdr2−/− mice exhibit large portal and periportal fibrosis, but not parenchymal fibrosis and examinations of Mdr2−/− mice at later ages also confirm the presence of bile duct disease and hepatic fibrosis(28), which is concurrent with our findings in the present manuscript. Mdr2−/− mice were found to have large amounts of collagen content and an increase in α-SMA and fibronectin. When we treated mice with cromolyn sodium, there was a marked decrease in fibrosis progression. Since our model induces portal and periportal fibrosis, we can presume that these changes are dependent on ductal fibrosis and not just cholestatic liver injury. Studies attest to the ability of cromolyn sodium to alter the remodeling of tissues, a process that is critical in fibrotic scarring. In pulmonary vascular remodeling, it has been shown that mast cell stabilization via cromolyn sodium treatment ameliorated the vascular remodeling in rats subjected to pulmonary arterial hypertension and resulted in more favorable hemodynamics(29). Further, mast cells can stimulate collagen synthesis in the skin by dermal fibroblasts and thereby may alter scar formation. A study by Chen, et al. found that cromolyn sodium treatment reduced the formation of cutaneous scars and inflammation, but did not weaken the healing wound suggesting that cromolyn sodium plays an important role in tissue alteration(30).
Since hepatic fibrosis is also considered an inflammatory event(31), cromolyn sodium may be reducing the degree of inflammation and inflammatory mediators and thereby attenuating fibrosis progression. To understand this further we examined a primary inflammatory mediator of fibrosis, TGF-β1(32, 33). Once activated, mast cells release numerous factors into the tissue environment including inflammatory cytokines like TGF-β1, histamine and other growth factors(12, 34, 35). Our present study also includes an examination of hepatic stellate cells (HSCs) that, once activated, promote fibrosis via numerous mechanisms including TGF-β1, NF-κB and hedgehog signaling(36-38). We found that HSC activation and TGF-β1 expression was increased in Mdr2−/− mice compared to wild-type, but when mice were treated with cromolyn sodium there was a decrease in activated HSCs(39). These results suggest that mast cells interact with HSCs and that mast cell-derived histamine may alter HSC activation. To support these findings, a recent study has demonstrated that mast cell tryptase induces the activation of protease-activated receptor 2 (PAR-2) and increases HSC activation and proliferation, thus promoting hepatic fibrosis and implying that mast cells interact directly with HSCs to drive fibrosis(40). To back our findings, it has been demonstrated that, in rats with myocardial fibrosis, mast cells infiltrate correlating with increased fibrosis and TGF-β1 levels. When these rats were treated with cromolyn sodium, mast cell infiltration was reduced along with myocardial fibrosis and TGF-β1 expression(41). Taken together, inhibition of mast cell-derived histamine may be beneficial to preventing the fibrotic reaction found in PSC.
There is a dynamic regulation of cellular proliferation and activation during PSC induced fibrosis(42, 43). HSCs are a key player in this event and recent studies have demonstrated that cholangiocytes are also involved in the progression of hepatic fibrosis(44, 45). Our past studies have shown that mast cells and cholangiocytes interact and that mast cell-derived histamine alters cholangiocyte proliferation(8). To understand if mast cells alter cholangiocyte and hHSC fibrotic events, we performed in vitro studies using established cultured cell lines. To this end we found that when either cholangiocytes or HSCs were co-cultured or treated with mast cells containing stable levels of histamine, there was an increase in proliferation, activation and fibrotic gene expression. In contrast, when these cells were co-cultured with mast cells containing diminished levels of histamine, the fibrotic reaction and cellular activation was reduced. Our data imply that mast cells may interact with both cholangiocytes and HSCs to drive fibrosis and that mast cell-derived histamine alters both the cholangiocyte and hHSC fibrotic reaction. It has been demonstrated by Omenetti, et al. that HSCs and cholangiocytes interact to promote epithelial mesenchymal transformation, cell migration and the progression of fibrosis(46). And, in support of the concept that mast cells may also influence HSCs, it has been shown that PAR-2 (activated by mast cell tryptase) stimulates the activation of HSCs and increases profibrogenic cytokines and collagen and in PAR-2 knockout mice, these phenomena are decreased, again supporting the concept that mast cells may interact with HSCs during fibrosis(47).
Portal fibroblasts are also key regulators of hepatic fibrosis and, in Mdr2−/− mice portal myofibroblasts are activated and contribute to fibrosis(28, 48). An early study by Akiyoshi, et al. reported that mast cells, portal myofibroblasts and cholinergic nerve terminals work synergistically to promote liver fibrosis(49) demonstrating that the paracrine influence from mast cells is not limited to altered HSCs and cholangiocyte function. In human fibrosis, mast cell chymase expression is increased which is coupled with increased expression of myofibroblast Angiotensin II receptor, AT1 suggesting that fibrosis can be mediated by mast cells and myofibroblasts(50). Our data demonstrates that mast cells influence cholangiocytes and HSCs; however, we cannot rule out the important role that activated myofibroblasts may play in our model and further studies are warranted to examine this further (Supplemental Figure 2, working model).
In summary, we have demonstrated that mast cells are present in the Mdr2−/− PSC mouse model and human PSC and mast cells contribute to HSC activation and fibrosis. Inhibition of mast cell-derived histamine decreases proliferation/fibrosis and may act on the bicarbonate umbrella. Targeting mast cells and mast cell-derived components may offer new therapeutic benefits for patients with PSC.
Supplementary Material
Acknowledgments
Financial support: Portions of this work were supported by (i) a VA Merit Award (1I01BX003031) from the United States Department of Veteran's affairs, Biomedical Laboratory Research and Development Service and an RO1 from NIH NIDDK (1R01DK108959) (HF) and a VA Research Career Scientist Award and VA Merit Award 5101BX000574 from the United States Department of Veteran's affairs, Biomedical Laboratory Research and Development Service (GA); (ii) funds from the PSC Partners Seeking a Cure (HF & SD); (iii) a Scott and White Research Mentor Award (HF), (iv) the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White Health (GA) and (v) a core grant (U24 DK05963, Yale University School of Medicine). This research was supported in part by the Veterans Health Administration. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Abbreviations
- PSC
Primary Sclerosing Cholangitis
- Mdr2−/−
multidrug resistance knockout mouse
- HSC
hepatic stellate cells
- TGF-β1
transforming growth factor-beta 1
- BDL
bile duct ligation
- EIA
enzymatic immunoassay
- H&E
hematoxylin & eosin
- PCR
polymerase chain reaction
- WT
wild-type
- IBDM
intrahepatic bile duct mass
- CK-19
cytokeratin 19
- PCNA
proliferating cellular nuclear antigen
- TBA
total bile acids
- α-SMA
alpha-smooth muscle actin
- SYP-9
synaptophysin 9
- MCs
cultured mast cells
- shRNA
short hairpin ribonucleic acid
- HDC
histidine decarboxylase
- hHSCs
human hepatic stellate cells
Footnotes
Conflict of interest: The authors have no financial conflict of interest to report.
References
- 1.Penz-Osterreicher M, Osterreicher CH, Trauner M. Fibrosis in autoimmune and cholestatic liver disease. Best Pract Res Clin Gastroenterol. 2011;25:245–258. doi: 10.1016/j.bpg.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo P, Peter JB, Gershwin ME, DeSotel CK, Shoenfeld Y, Ahmed AE, Lindor KD. Serum autoantibodies in patients with primary sclerosing cholangitis. J Hepatol. 2000;32:182–187. doi: 10.1016/s0168-8278(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 3.Osterreicher CH, Trauner M. Animal models of biliary tract injury. Curr Opin Gastroenterol. 2012;28:239–243. doi: 10.1097/MOG.0b013e32835264d9. [DOI] [PubMed] [Google Scholar]
- 4.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77–98. doi: 10.1055/s-2006-960172. [DOI] [PubMed] [Google Scholar]
- 6.Omenetti A, Bass LM, Anders RA, Clemente MG, Francis H, Guy CD, McCall S, et al. Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology. 2011;53:1246–1258. doi: 10.1002/hep.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003;111:24–32. doi: 10.1067/mai.2003.60. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy LL, Hargrove LA, Graf AB, Francis TC, Hodges KM, Nguyen QP, Ueno Y, et al. Inhibition of mast cell-derived histamine secretion by cromolyn sodium treatment decreases biliary hyperplasia in cholestatic rodents. Lab Invest. 2014;94:1406–1418. doi: 10.1038/labinvest.2014.129. [DOI] [PubMed] [Google Scholar]
- 9.Francis H, Meininger CJ. A review of mast cells and liver disease: What have we learned? Dig Liver Dis. 2010;42:529–536. doi: 10.1016/j.dld.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One. 2012;7:e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefanini GF, Prati E, Albini MC, Piccinini G, Capelli S, Castelli E, Mazzetti M, et al. Oral disodium cromoglycate treatment on irritable bowel syndrome: an open study on 101 subjects with diarrheic type. Am J Gastroenterol. 1992;87:55–57. [PubMed] [Google Scholar]
- 12.Halova I, Draberova L, Draber P. Mast cell chemotaxis - chemoattractants and signaling pathways. Front Immunol. 2012;3:119. doi: 10.3389/fimmu.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graf A, Meng F, Hargrove L, Kennedy L, Han Y, Francis T, Hodges K, et al. Knockout of histidine decarboxylase decreases bile duct ligation-induced biliary hyperplasia via downregulation of the histidine decarboxylase/VEGF axis through PKA-ERK1/2 signaling. Am J Physiol Gastrointest Liver Physiol. 2014;307:G813–823. doi: 10.1152/ajpgi.00188.2014. [DOI] [PubMed] [Google Scholar]
- 14.Yeh TH, Krauland L, Singh V, Zou B, Devaraj P, Stolz DB, Franks J, et al. Liver-specific beta-catenin knockout mice have bile canalicular abnormalities, bile secretory defect, and intrahepatic cholestasis. Hepatology. 2010;52:1410–1419. doi: 10.1002/hep.23801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng F, Onori P, Hargrove L, Han Y, Kennedy L, Graf A, Hodges K, et al. Regulation of the histamine/VEGF axis by miR-125b during cholestatic liver injury in mice. Am J Pathol. 2014;184:662–673. doi: 10.1016/j.ajpath.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Francis H, DeMorrow S, Venter J, Onori P, White M, Gaudio E, Francis T, et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut. 2012;61:753–764. doi: 10.1136/gutjnl-2011-300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koda W, Harada K, Tsuneyama K, Kono N, Sasaki M, Matsui O, Nakanuma Y. Evidence of the participation of peribiliary mast cells in regulation of the peribiliary vascular plexus along the intrahepatic biliary tree. Lab Invest. 2000;80:1007–1017. doi: 10.1038/labinvest.3780106. [DOI] [PubMed] [Google Scholar]
- 18.Francis HL, Demorrow S, Franchitto A, Venter JK, Mancinelli RA, White MA, Meng F, et al. Histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP3/Ca2+ and cAMP-dependent signaling mechanisms. Lab Invest. 2012;92:282–294. doi: 10.1038/labinvest.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii M, Iwai M, Harada Y, Morikawa T, Okanoue T, Kishikawa T, Tsuchihashi Y, et al. A role of mast cells for hepatic fibrosis in primary sclerosing cholangitis. Hepatol Res. 2005;31:127–131. doi: 10.1016/j.hepres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Tsuneyama K, Saito K, Ruebner BH, Konishi I, Nakanuma Y, Gershwin ME. Immunological similarities between primary sclerosing cholangitis and chronic sclerosing sialadenitis: report of the overlapping of these two autoimmune diseases. Dig Dis Sci. 2000;45:366–372. doi: 10.1023/a:1005429130150. [DOI] [PubMed] [Google Scholar]
- 21.Ferrier L, Berard F, Debrauwer L, Chabo C, Langella P, Bueno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148–1154. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gittlen SD, Schulman ES, Maddrey WC. Raised histamine concentrations in chronic cholestatic liver disease. Gut. 1990;31:96–99. doi: 10.1136/gut.31.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motawi TM, Bustanji Y, El-Maraghy S, Taha MO, Al-Ghussein MA. Evaluation of naproxen and cromolyn activities against cancer cells viability, proliferation, apoptosis, p53 and gene expression of survivin and caspase-3. J Enzyme Inhib Med Chem. 2014;29:153–161. doi: 10.3109/14756366.2012.762645. [DOI] [PubMed] [Google Scholar]
- 24.Lee SP. Effects of histamine and histamine antagonists on hepatic bile flow in the conscious sheep. Clin Exp Pharmacol Physiol. 1984;11:61–69. doi: 10.1111/j.1440-1681.1984.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 25.Okajima K, Harada N, Uchiba M. Ranitidine reduces ischemia/reperfusion-induced liver injury in rats by inhibiting neutrophil activation. J Pharmacol Exp Ther. 2002;301:1157–1165. doi: 10.1124/jpet.301.3.1157. [DOI] [PubMed] [Google Scholar]
- 26.Shibamoto T, Tsutsumi M, Kuda Y, Ohmukai C, Zhang W, Kurata Y. Mast cells are not involved in the ischemia-reperfusion injury in perfused rat liver. J Surg Res. 2012;174:114–119. doi: 10.1016/j.jss.2010.11.900. [DOI] [PubMed] [Google Scholar]
- 27.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Baghdasaryan A, Claudel T, Kosters A, Gumhold J, Silbert D, Thuringer A, Leski K, et al. Curcumin improves sclerosing cholangitis in Mdr2−/− mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut. 2010;59:521–530. doi: 10.1136/gut.2009.186528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartelds B, van Loon RL, Mohaupt S, Wijnberg H, Dickinson MG, Boersma B, Takens J, et al. Mast cell inhibition improves pulmonary vascular remodeling in pulmonary hypertension. Chest. 2012;141:651–660. doi: 10.1378/chest.11-0663. [DOI] [PubMed] [Google Scholar]
- 30.Chen L, Schrementi ME, Ranzer MJ, Wilgus TA, DiPietro LA. Blockade of mast cell activation reduces cutaneous scar formation. PLoS One. 2014;9:e85226. doi: 10.1371/journal.pone.0085226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karsdal MA, Manon-Jensen T, Genovese F, Kristensen JH, Nielsen MJ, Sand JM, Hansen NU, et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2015;308:G807–830. doi: 10.1152/ajpgi.00447.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Yi J, Ye R, Liu J, Duan Q, Xiao J, Liu F. miR-144 regulates transforming growth factor-beta1 iduced hepatic stellate cell activation in human fibrotic liver. Int J Clin Exp Pathol. 2015;8:3994–4000. [PMC free article] [PubMed] [Google Scholar]
- 33.Woods LT, Camden JM, El-Sayed FG, Khalafalla MG, Petris MJ, Erb L, Weisman GA. Increased Expression of TGF-beta Signaling Components in a Mouse Model of Fibrosis Induced by Submandibular Gland Duct Ligation. PLoS One. 2015;10:e0123641. doi: 10.1371/journal.pone.0123641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachelet I, Levi-Schaffer F, Mekori YA. Mast cells: not only in allergy. Immunol Allergy Clin North Am. 2006;26:407–425. doi: 10.1016/j.iac.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balta C, Herman H, Boldura OM, Gasca I, Rosu M, Ardelean A, Hermenean A. Chrysin attenuates liver fibrosis and hepatic stellate cell activation through TGF-beta/Smad signaling pathway. Chem Biol Interact. 2015;240:94–101. doi: 10.1016/j.cbi.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Lan T, Kisseleva T, Brenner DA. Deficiency of NOX1 or NOX4 Prevents Liver Inflammation and Fibrosis in Mice through Inhibition of Hepatic Stellate Cell Activation. PLoS One. 2015;10:e0129743. doi: 10.1371/journal.pone.0129743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Yuan X, Tao L, Cheng Z, Dai X, Sheng X, Xue D. Xia-yu-xue decoction (XYXD) reduces carbon tetrachloride (CCl4)-induced liver fibrosis through inhibition hepatic stellate cell activation by targeting NF-kappaB and TGF-beta1 signaling pathways. BMC Complement Altern Med. 2015;15:201. doi: 10.1186/s12906-015-0733-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.van de Bovenkamp M, Groothuis GM, Meijer DK, Slooff MJ, Olinga P. Human liver slices as an in vitro model to study toxicity-induced hepatic stellate cell activation in a multicellular milieu. Chem Biol Interact. 2006;162:62–69. doi: 10.1016/j.cbi.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Chen B, Li S, Sun Q. Tryptase inhibitor APC 366 prevents hepatic fibrosis by inhibiting collagen synthesis induced by tryptase/protease-activated receptor 2 interactions in hepatic stellate cells. Int Immunopharmacol. 2014;20:352–357. doi: 10.1016/j.intimp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Palaniyandi Selvaraj S, Watanabe K, Ma M, Tachikawa H, Kodama M, Aizawa Y. Involvement of mast cells in the development of fibrosis in rats with postmyocarditis dilated cardiomyopathy. Biol Pharm Bull. 2005;28:2128–2132. doi: 10.1248/bpb.28.2128. [DOI] [PubMed] [Google Scholar]
- 42.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–841. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594. e571. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 44.Kaur S, Siddiqui H, Bhat MH. Hepatic Progenitor Cells in Action: Liver Regeneration or Fibrosis? Am J Pathol. 2015;185:2342–2350. doi: 10.1016/j.ajpath.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Spirli C, Villani A, Mariotti V, Fabris L, Fiorotto R, Strazzabosco M. Posttranslational regulation of polycystin-2 protein expression as a novel mechanism of cholangiocyte reaction and repair from biliary damage. Hepatology. 2015;62:1828–1839. doi: 10.1002/hep.28138. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knight V, Tchongue J, Lourensz D, Tipping P, Sievert W. Protease-activated receptor 2 promotes experimental liver fibrosis in mice and activates human hepatic stellate cells. Hepatology. 2012;55:879–887. doi: 10.1002/hep.24784. [DOI] [PubMed] [Google Scholar]
- 48.Latasa MU, Gil-Puig C, Fernandez-Barrena MG, Rodriguez-Ortigosa CM, Banales JM, Urtasun R, Goni S, et al. Oral methylthioadenosine administration attenuates fibrosis and chronic liver disease progression in Mdr2−/− mice. PLoS One. 2010;5:e15690. doi: 10.1371/journal.pone.0015690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akiyoshi H, Terada T. Mast cell, myofibroblast and nerve terminal complexes in carbon tetrachloride-induced cirrhotic rat livers. J Hepatol. 1998;29:112–119. doi: 10.1016/s0168-8278(98)80185-2. [DOI] [PubMed] [Google Scholar]
- 50.Ikura Y, Ohsawa M, Shirai N, Sugama Y, Fukushima H, Suekane T, Hirayama M, et al. Expression of angiotensin II type 1 receptor in human cirrhotic livers: Its relation to fibrosis and portal hypertension. Hepatol Res. 2005;32:107–116. doi: 10.1016/j.hepres.2005.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


