Abstract
We previously described a novel germ cell-specific X-linked reproductive homeobox gene (Rhox13) that is upregulated at the level of translation in response to retinoic acid (RA) in differentiating spermatogonia and preleptotene spermatocytes. We hypothesize that RHOX13 plays an essential role in male germ cell differentiation, and have tested this by creating a Rhox13 gene knockout (KO) mouse. Rhox13 KO mice are born in expected Mendelian ratios, and adults have slightly reduced testis weights, yet a full complement of spermatogenic cell types. Young KO mice (at ~7–8 weeks of age) have a ≈50% reduction in epididymal sperm counts, but numbers increased to WT levels as the mice reach ~ 17 weeks of age. Histological analysis of testes from juvenile KO mice reveal a number of defects during the first wave of spermatogenesis. These include increased apoptosis, delayed appearance of round spermatids, and disruption of the precise stage-specific association of germ cells within the seminiferous tubules. Breeding studies reveal that both young and aged KO males produce normal-sized litters. Taken together, our results indicate that RHOX13 is not essential for mouse fertility in a controlled laboratory setting, but that it is required for optimal development of differentiating germ cells and progression of the first wave of spermatogenesis.
Keywords: Differentiating spermatogonia, testis, fertility, homeobox, Rhox genes, apoptosis
Introduction
Spermatogenesis begins in the neonatal mouse testis with the transition of quiescent prospermatogonia into stem, progenitor, and differentiating type A spermatogonia (Busada and Geyer 2015, Drumond, et al. 2011, Niedenberger, et al. 2015, Yang and Oatley 2014, Yoshida, et al. 2006). These three types of spermatogonia provide the foundation for the ‘first wave of spermatogenesis’ that occurs during the first ~6 weeks of postnatal life, and after that the spermatogonial stem cells (SSCs) supply subsequent waves during steady-state spermatogenesis. SSCs divide asymmetrically to either produce another SSC (self-renewal) or an undifferentiated progenitor spermatogonium that will proliferate before eventually differentiating in response to retinoic acid (RA) (Busada and Geyer 2015, Yang and Oatley 2014). Differentiating type A spermatogonia will then proceed through 6 divisions (A1, A2, A3, A4, In, B) before entering prophase I of meiosis as preleptotene spermatocytes. Following meiosis, haploid round spermatids undergo a dramatic morphological transformation during the process of spermiogenesis that culminates in the release of testicular sperm into the lumina of the seminiferous tubules.
The first cohort of differentiating spermatogonia proceed through these above steps with remarkable precision during the first 5 weeks of life in mice such that specific advanced germ cell types appear in the testis in consistent numbers on particular days of postnatal development (Bellve, et al. 1977), with minor strain variation. For example, in testes of C57BL/6 mice these germ cell types first appear on the following postnatal (P) days: preleptotene spermatocytes = P8, leptotene spermatocytes = P10, zygotene spermatocytes = P12, pachytene spermatocytes = P14, round spermatids = P20, and testicular sperm ≈P35. The first wave of spermatogenesis results in the appearance of functional sperm in the epididymis by 6 weeks of age in mice, which ensures their reproductive competence at an early age. It also provides researchers with an excellent model system for studying spermatogenesis, as relatively pure populations of specific germ cell types can be isolated for study, and perturbations in germ cell development are readily identified.
The ‘reproductive homeobox X-linked’, or Rhox, genes are located on the X chromosome in mammals and encode homeodomain-containing transcription factors. The Rhox genes are expressed primarily in reproductive tissues including the testis, ovary, epididymis, and placenta (Geserick, et al. 2002, Maclean, et al. 2005, MacLean and Wilkinson 2010, Song, et al. 2013, Wayne, et al. 2002). Their expression is regulated, at least in part, at the level of transcription by DNA methylation (Jackson-Grusby, et al. 2001, Maclean, et al. 2011, Oda, et al. 2006, Richardson, et al. 2014). The genomes of multiple mammals contain Rhox genes that are located on a syntenic region on the X chromosome and are likely expressed in reproductive tissues (Maclean, et al. 2005, Song, et al. 2013, Wang and Zhang 2006, Wilming, et al. 2015). There are only three genes in humans (RHOXF1, RHOXF2, and RHOXF2B), and there is evidence that these genes are rapidly evolving in primates (Niu, et al. 2011). In mice, an evolutionary expansion generated a collinear cluster of 33 Rhox genes discovered in 2005 by the Wilkinson laboratory (Maclean, et al. 2005). The cluster contains 13 unique Rhox genes (named Rhox1-13), as well as multiple duplicated copies of Rhox2-4 (MacLean, et al. 2006). A few of the Rhox genes have been targeted in mice by knockout (KO) or RNAi (KD) approaches to determine their roles in fertility. Inactivation of the Sertoli-expressed Rhox5 (by gene KO) and Rhox8 (by siRNA KD) resulted in male subfertility, with increased germ cell apoptosis, reduced cauda epididymal sperm counts, and impaired sperm motility (Maclean, et al. 2005, Welborn, et al. 2015).
After the initial reports of the Rhox gene cluster (in which 12 unique genes were described), we discovered another gene just downstream of Rhox12 that we designated Rhox13 that is only expressed in male and female germ cells (Geyer and Eddy 2008). In the female, Rhox13 mRNA is present in the fetal ovary, with RHOX13 protein detectable in oogonia and preleptotene oocytes (Geyer and Eddy 2008). Based on the coincidence of mRNA and protein solely in the fetal ovary, we concluded that Rhox13 expression is under transcriptional control in female germ cells. In contrast, Rhox13 mRNA is present in all stages of testis development from embryonic day (E) 12.5 through adulthood, including in pachytene spermatocytes and round spermatids (our unpublished data). However, RHOX13 protein is only readily detectable in spermatogonia and preleptotene spermatocytes. We found that Rhox13 mRNAs are repressed in prospermatogonia through a direct interaction with the RNA binding protein NANOS2, and this repression is apparently alleviated in response to RA (Chappell, et al. 2013, Geyer, et al. 2012). This translational control in the testis results in a protein expression pattern for RHOX13 that is remarkably similar to that in the female (oogonia/spermatogonia and preleptotene oocytes/spermatocytes), although they seem to be determined by distinct mechanisms. Therefore, we hypothesize that RHOX13 plays an essential role in germ cell differentiation in both sexes prior to meiotic initiation.
In this report, we examine the requirement for RHOX13 in male germ cell development by generating Rhox13 KO mice and analyzing their reproductive phenotype. Testes of Rhox13−/y males exhibit delayed appearance of germ cell types and a significant increase in germ cell apoptosis during the first wave of spermatogenesis, which results in reduced numbers of epididymal sperm in young adult males. These defects are not seen in subsequent waves of spermatogenesis as Rhox13−/y males age, and their fertility is not significantly altered, at least in a controlled laboratory setting. Altogether, our results indicate that RHOX13 is required for a quantitatively normal first wave of spermatogenesis in the mouse.
Materials and Methods
Animal Care
All animal procedures were performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committees of East Carolina University (AUP #A181) and the National Institute of Environmental Health Sciences. C57BL/6NCrl mice were obtained from Charles River Laboratories, Inc. (Raleigh, NC, USA). Euthanasia of neonatal and juvenile mice up to P12 was performed by decapitation, and euthanasia of mice older than P12 was done by CO2 asphyxiation followed by cervical dislocation.
Generation of Rhox13 KO mice
The mouse Rhox13 gene (MGI:1920864) was targeted in E14Tg2a.4 (129/Ola) mouse embryonic stem (ES) cells derived from male embryos (BayGenomics, MMRRC-015890) using homologous recombination. The Rhox13tm1a(KOMP)Wtsi vector, (CDS79660), a pre-conditional ‘knockout first’ vector containing loxP and FRT sites, was obtained from the KOMP repository (www.komp.org/geneinfo.php?geneid=3451). The vector was linearized and electroporated into ES cells grown and selected as described previously (Cho, et al. 2001). Recombinant clones that correctly incorporated the Rhox13 targeting construct into the X chromosome were identified by PCR and Southern blot analysis. One correctly targeted ES cell clone was electroporated with the circular pCAGGS-FLPe-IRES Spuro construct (Gene Bridges SSRF001) to excise the sequence flanked by FRT sequences, and then re-cloned. The resulting clones were re-screened to verify excision of the FRT-flanked regions and selected sub-clones were injected into C57BL/6N blastocysts to create chimeras. Male chimeras were bred to female C57Bl/6N mice, and pups were screened by coat color and genotyping to confirm germline transmission. Because only female pups carrying the mutant allele were produced (since Rhox13 is X-linked), some were used to establish a colony of mice carrying the deleted Rhox13 allele and others were mated to mice dizygous for the FLP deleter allele (B6.Cg-Tg (ACTFLPe) 9205 Dym/J, JAX Stock No: 005703). Resulting offspring with the FRT flanked region deleted were mated with Hspa2-Cre mice (Inselman, et al. 2010) to produce mice with exon 2 of Rhox13 deleted (Rhox13−/y males and Rhox13−/x females). The colony was maintained by mating Rhox13−/y males with C57Bl/6N females.
PCR Genotyping
The genotypes of Rhox13 WT and KO mice were determined using PCR to amplify genomic DNA isolated from tail tip tissue. The WT or deleted alleles were amplified using forward (5’-AGGGTTCTCCTAGGGTTGAGACC) and reverse (5’-TCCCTGGAAATGTCCAGAGCAG) primers. Amplification of DNA from wild type and KO mice should yield single 915 and 267 nt bands when run on a 1.5% agarose gel.
Breeding studies
We performed both long-term and short-term breeding studies. For the long-term breeding study, WT and KO male mice aged ≥P60 were paired with a single WT C57BL/6N female. Mice were paired for 92 days, and then males were euthanized. Female mice were monitored for another 22 days for the appearance of any final litters. For the short-term breeding study, P35 WT males and KO littermates were paired separately with a single female WT C57BL/6N female for 15 days. Male mice were euthanized at P50, and reproductive parameters analyzed. Female breeders continued to be monitored for 22 more days for the appearance of any final litters.
Cell quantitation from testis sections
Identification of preleptotene spermatocytes, zygotene spermatocytes, and round spermatids was performed during the first wave of spermatogenesis based on characteristic differences in position within the seminiferous cords and tubules, nuclear diameter, and chromatin appearance (Bellve, et al. 1977). Paraffin sections from Bouin’s-fixed testes isolated from P8, P12, and P20 mice were stained with hematoxylin and eosin using standard methods. Male germ cells were manually counted from photomicrographs captured with an Axio Observer A1 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) equipped with an XL16C digital camera and Exponent version 1.3 software (Dage-MTI, Michigan City, IN, USA). Photomicrographs were analyzed using Zeiss Axiovision software. Seminiferous cords were outlined manually, and the total germ cell numbers were divided by total seminiferous cord surface area and multiplied by 1,000 to obtain cells/mm2. Cell counting was performed on ~30 round seminiferous cords or tubules in duplicate on testes from at least 4 different animals.
Cauda epididymal sperm counts
Sperm obtained from one cauda epididymis were counted in duplicate from each mouse. The cauda epididymides were removed from each mouse immediately after euthanasia and placed into 1 ml of 1× PBS at room temperature. Each epididymis was cut into small pieces and gently mixed by repeated passage through a P1000 pipet tip. The solution was diluted with distilled water to immobilize the sperm, and counts were obtained using a hemocytometer.
Daily sperm production counts
Whole testes were weighed and snap-frozen in liquid nitrogen. A portion of the frozen testis was cut off, weighed, detunicated, and homogenized in 1 ml of lysis solution (0.9% NaCl and 0.05% Triton X-100). The solution was further homogenized by multiple passages through a 26-gauge needle. The solution was then diluted and the numbers of step 14–16 spermatid heads were counted. The total number of spermatid heads was multiplied by the dilution and then by 2,500 and then divided by the weight of the testis fragment, multiplied by the weight of the whole testis, and divided by 4.84 to calculate daily sperm production per testis (Ashby, et al. 1999, Joyce, et al. 1993, Robb, et al. 1978).
Immunohistochemistry (IHC) and Indirect Immunofluorescence (IIF)
IHC was performed as previously described (Geyer, et al. 2012). Briefly, Bouin’s-fixed testis sections were deparaffinized and then RHOX13 antiserum (generated previously, (Geyer and Eddy 2008)) was applied at 1:500 for 1 h at room temperature without antigen retrieval. Primary antibody was omitted as a negative control. Detection was performed using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA), and sections were counterstained with hematoxylin. Images were captured as described above. IIF was performed as before (Busada, et al. 2015a, Busada, et al. 2014, Busada, et al. 2015b, Niedenberger, et al. 2015). Briefly, 4% PFA-fixed and O.C.T.-embedded 5 µm frozen sections were incubated in blocking buffer for 30 min at room temperature and then incubated in primary antibody for 1 h at room temperature. Primary antibodies were against RHOX13 (1:500, (Geyer and Eddy 2008)), cleaved poly(ADP-ribose) polymerase 1 (PARP1, 1:100, #9544 Cell Signaling Technology, Danvers, MA, USA), BrdU (1:50, #B35130 ThermoFisher Scientific, Waltham, MA, USA), and STRA8 (1:3,000, #ab49602 Abcam, Cambridge, MA, USA). Following stringency washes, sections were incubated in secondary antibody (1:1,000 Alexa Fluor-488 donkey anti-rabbit or donkey anti-goat IgG, Invitrogen, Carlsbad, CA, USA) plus phalloidin (1:500, Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. Blocking and antibody incubations were done in 1× PBS containing 3% BSA + 0.1% Triton X-100, and stringency washes were done with 1× PBS + 0.1% Triton X-100. Primary antibodies were omitted as negative controls. Coverslips were mounted with Vectastain containing DAPI (Vector Laboratories, Burlingame, CA, USA), and images obtained using a Fluoview FV1000 confocal laser-scanning microscope (Olympus America, Center Valley, PA, USA).
Statistics
Statistical analyses were performed using Student’s t-test, and the level of significance was set at P<0.05.
Results
Generation of the disrupted Rhox13 allele
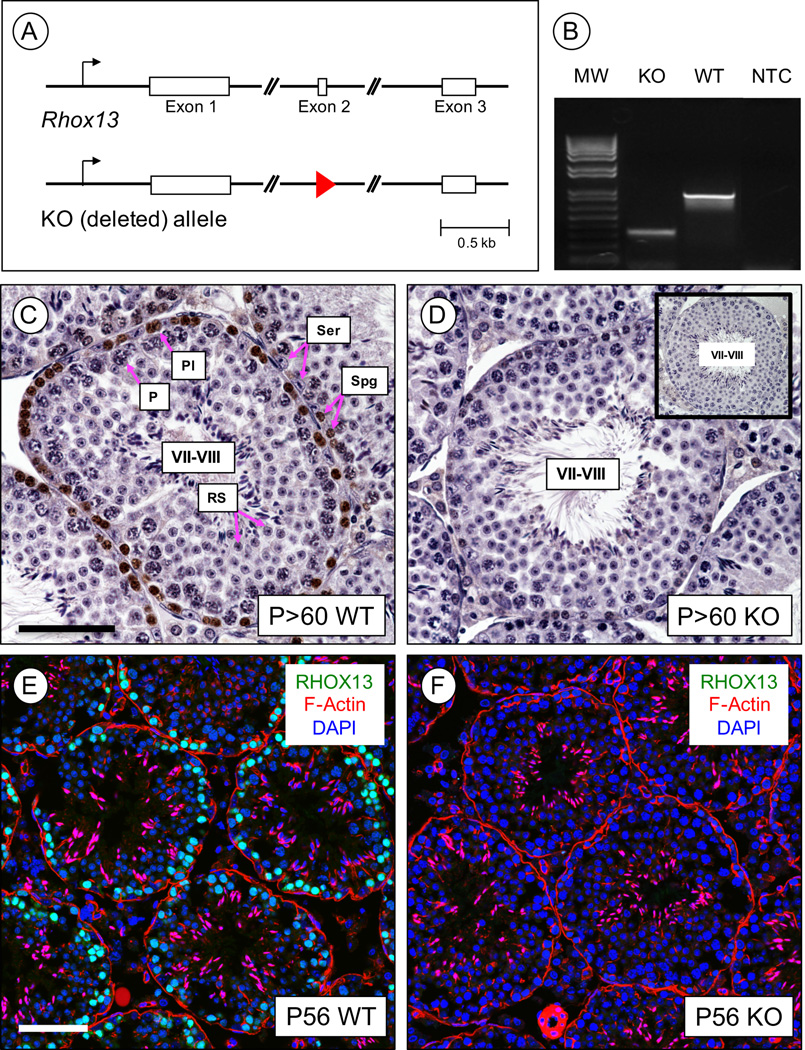
The germ cell expressed X-linked Rhox13 gene has a rather unique expression pattern. In the male, it is transcribed solely in germ cells throughout testis development, and mRNAs are detectable from E12.5 through adulthood (Geyer and Eddy 2008, Geyer, et al. 2012). However, RHOX13 protein is only readily detectable in spermatogonia and preleptotene spermatocytes, is decreased dramatically in leptotene spermatocytes, and is undetectable in pachytene spermatocytes and spermatids (despite both cell types containing abundant Rhox13 mRNA) (Geyer and Eddy 2008, Geyer, et al. 2012). To test the hypothesis that RHOX13 plays an essential role in male germ cell development, we took a reverse genetics approach to disrupt the Rhox13 gene. We introduced loxP sites flanking the second exon of the Rhox13 gene, which encodes a portion of the homeodomain. Since Rhox13 is only expressed in germ cells, we decided to convert the floxed allele into a deleted allele. This was accomplished by breeding Rhox13fl/fl female and Rhox13+/y;Hspa2-Cre male mice, which express Cre recombinase in spermatocytes (Inselman, et al. 2010). We successfully deleted the floxed allele, and therefore one half of all resultant male pups are Rhox13−/y (KO) and the other half are Rhox13+/y (WT), and we distinguished between them by PCR genotyping (Fig. 1A–B). Deletion of Rhox13 was confirmed at the protein level by immunostaining, as no RHOX13 was detectable in male germ cells of any of the Rhox13 KO mice (Fig. 1C–F).
Figure 1. Generation of Rhox13 KO mice.
(A) The Rhox13 gene is comprised of three exons (shown in boxes), as is typical of most homeobox genes. We created a KO allele by deleting exon 2, and a single loxP site remains (red triangle). (B) PCR amplification using primers on either side of exon 2 distinguishes KO from WT alleles. MW = molecular weight ladder, and NTC = no template control. (C–D) IHC was done to verify loss of RHOX13 protein (brown staining) in P>60 adult testes. Shown are stage VII-VIII seminiferous tubule sections from WT (C) and KO (D) mice. Sections were counterstained with hematoxylin, and inset (in D) is a no primary antibody control. Spg = spermatogonia, Pl = preleptotene spermatocytes, P = pachytene spermatocytes, RS = round spermatids, Ser = Sertoli cells. (E–F) IIF was done to localize RHOX13 (in green) in WT (E) and KO (F) P56 testes. DNA is stained with DAPI (blue), and F-actin is stained with fluorescently-conjugated phalloidin (red). Scale bar = 50 µm.
Analysis of phenotypic traits of Rhox13 KO mice
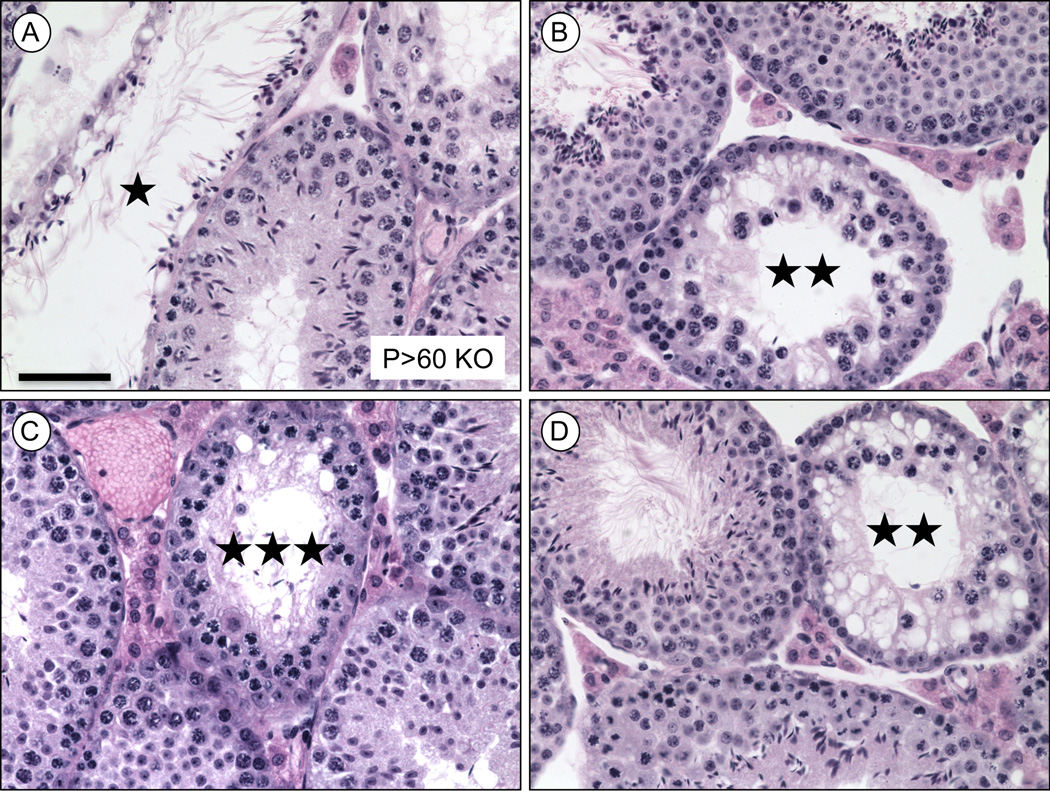
Rhox13 WT and KO mice were generated in equivalent ratios throughout this study. We analyzed males from litters from the first generation after constitutively deleting the Rhox13 allele and found a relatively high degree of phenotypic heterogeneity both within and between Rhox13 KO males. Some had extremely low cauda sperm counts and abnormal testis histology, while others had apparently normal testis histology, sperm counts, and motility. Most KO mice had a mixture of normal-appearing tubules adjacent to those missing layers of spermatogenic cells, including basal spermatogonia or adluminal spermatocytes and spermatids (Fig. 2A–D). In addition, there were many vacuolated spaces within the seminiferous epithelia of these KO tubules, suggesting germ cell loss by apoptosis.
Figure 2. Histological abnormalities of adult (P>60) testes from Rhox13 KO on a mixed genetic background.
(A–D) Bouin’s-fixed KO sections are stained with H&E and abnormal tubules are indicated by asterisks (★ = only containing condensed spermatids and Sertoli cells; ★★ = lacking spermatids; ★★★ = lacking spermatids and almost all spermatogonia). Scale bar = 50 µm.
To determine whether these histological abnormalities caused differences in reproductive performance, we entered adult (P>60) male WT and KO mice (n=4 for each genotype) into a 5-month breeding study. Males of both genotypes exhibited apparently normal breeding behavior, and we found there were no statistically significant differences in either the average numbers of pups per litter or in the time taken for birth of the first litter. However, there was a statistically significant increase in the time between litters sired by Rhox13 KO males when paired with WT females (Fig. 3, P=0.008).
Figure 3. Fecundity results from long-term breeding trial of Rhox13 WT and KO mice on mixed genetic background.
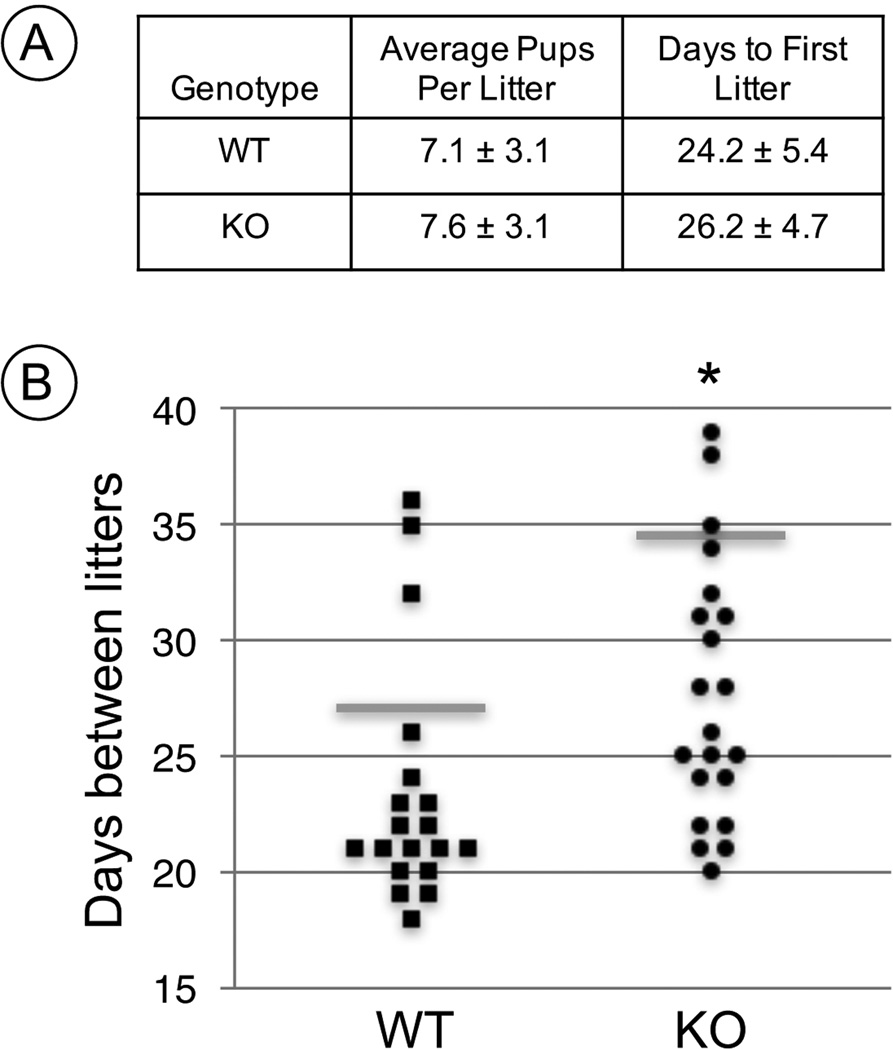
(A) The average litter size and days to first litter were not statistically different between Rhox13 WT and KO males. (B) In a graphical representation of time between litters, a horizontal gray line represents the average. Statistical significance is indicated by an asterisk (P=0.008).
The Rhox13 KO mice were produced by injecting 129/Ola ES cells into C57BL/6 blastocysts. Based on the high variability in testis histology and sperm counts, we chose to backcross the Rhox13 KO line onto the C57BL/6N genetic background in order to remove any confounding genetic modifiers and obtain a stable phenotype. Rhox13−/+ females were bred with WT males for 7 generations, and the resulting ‘incipient congenic’ pups were predicted to have a >99% C57BL/6-derived genome. All subsequent analyses were performed using 7th or later generation mice. Males were euthanized at P56 (unpaired, or virgin males) and at P50 and P120 (after short-term and longer-term breeding studies) for reproductive phenotypic assessment, and the results are summarized in Table 1. As expected, there were no differences in body weight at any of the ages examined. Testis weights were ~10–15% lower in KO mice at P56 and P120, but this difference did not reach the level of statistical significance. The size and weight of paired seminal vesicles are under androgen control, and therefore differences would suggest differences in testosterone levels (reviewed in (Dohle, et al. 2003)). Although seminal vesicle weights vary considerably between even WT mice, there were no significant differences between genotypes in the 3 groups of mice. We counted cauda epididymal sperm from WT and KO males at P50, P56, and P120 to measure the output of spermatogenesis. These ages are significant because sperm in P50 and P56 epididymides should be products of the first wave of spermatogenesis, while those in P120 epididymides arise from subsequent rounds of adult SSC-derived steady-state spermatogenesis. There were no statistically significant differences in cauda sperm quantity between P120 WT and KO males. In contrast, young KO males had reduced numbers of cauda epididymal sperm as compared to WT control littermates at both P56 (~1.6-fold decrease, P<0.001) and P50 (~1.5-fold decrease, not statistically significant). Importantly, P50 males were actively breeding until the day of euthanasia, while P56 males were never paired with females.
Table 1.
Reproductive phenotype of backcrossed Rhox13 KO males.
| Genotype (Age) | Body weight (g) |
Testis weight (mg) |
Paired seminal vesicle weight (mg) |
Cauda epididymal sperm (× 106/ml) |
|---|---|---|---|---|
| Rhox13+/y (WT, P50) | 22.4 ± 0.9 | 86.4 ± 2.5 | 115.4 ± 12.0 | 2.5 ± 1.3 |
| Rhox13−/y (KO, P50) | 22.0 ± 1.3 | 82.5 ± 2.9 | 104.3 ± 35.2 | 1.7 ± 0.9 |
| Rhox13+/y (WT, P56) | 22.4 ± 0.2 | 87.5 ± 0.7 | ND | 9.41 ± 0.7* |
| Rhox13−/y (KO, P56) | 22.4 ± 1.3 | 78.7 ± 6.3 | ND | 5.71 ± 0.6* |
| Rhox13+/y (WT, P120) | 32.9 ± 4.0 | 100.9 ± 18.1 | 296.3 ± 30.7 | 12.5 ± 4.0 |
| Rhox13−/y (KO, P120) | 32.4 ± 1.3 | 92 ± 5.3 | 252.8 ± 151.8 | 13.9 ± 1.9 |
All values are presented as averages with standard deviation.
ND = not determined.
In comparisons of cauda epididymal sperm counts, asterisks indicate statistical differences, at P<0.001.
N = 3 WT, 4 KO (P50); N = 2 WT, 4 KO (P56); N = 4 WT, 4 KO (P120) WT, 4 KO (P50); N = 2 WT, 4 KO (P56); N = 4 WT, 4 KO (P120).
Histological abnormalities during the first wave of spermatogenesis
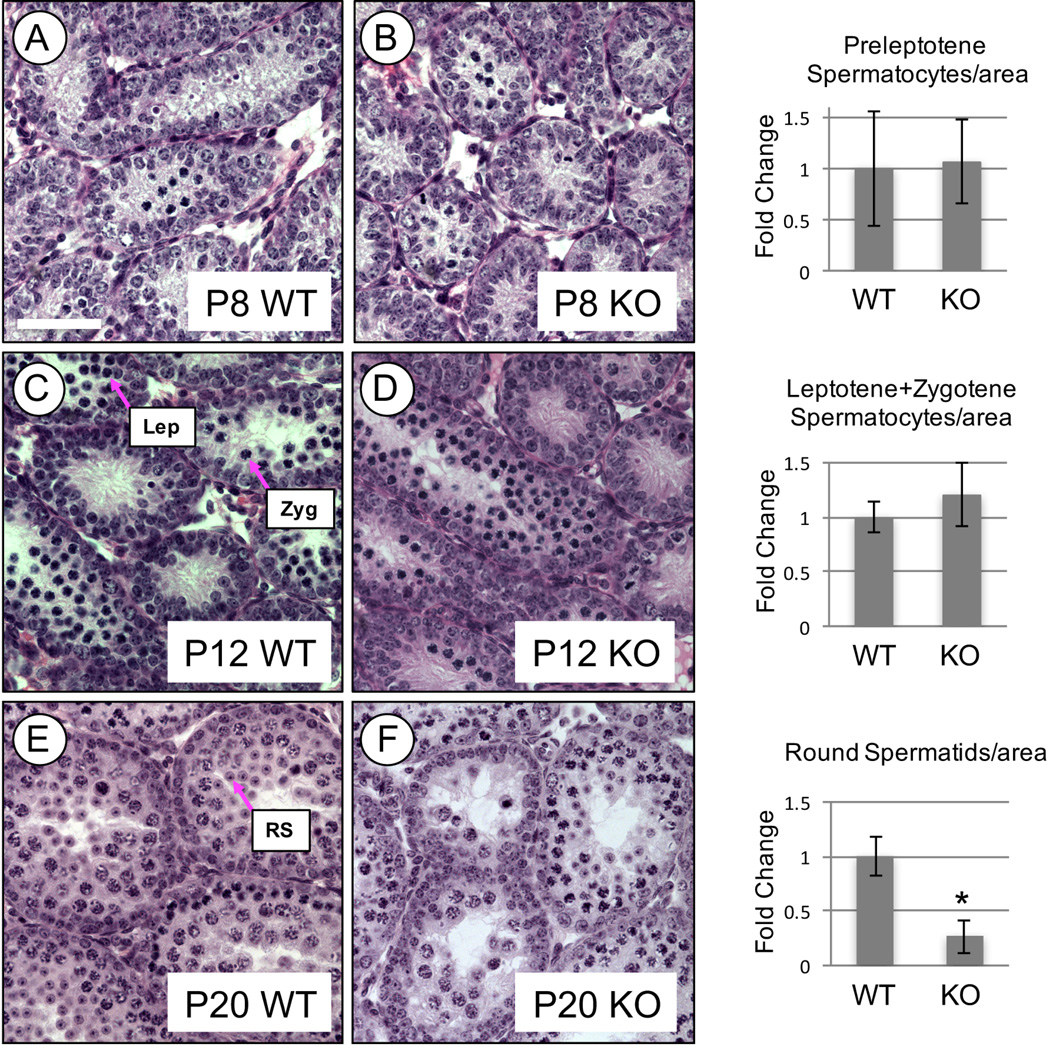
The reduced sperm counts in younger KO males (P50 and P56) suggested that the absence of RHOX13 had an effect during the first wave of spermatogenesis. We examined the histology of testes from WT and KO mice at P8, P12, and P20 because these ages coincide with the appearance of preleptotene spermatocytes, pachytene spermatocytes and round spermatids, respectively (Bellve, et al. 1977). We found that at P8 there were similar numbers of emergent preleptotene spermatocytes in WT and KO testes (Fig. 4A–B). At P12, there was no difference in the number of leptotene or zygotene spermatocytes (Fig. 4C–D). Round spermatids are the most advanced germ cell type present in the P20 testis, and in WT mice are found adjacent to the lumina of the seminiferous tubules (Bellve, et al. 1977). In KO testes, there was a 3.8-fold reduction in the number of round spermatids per area of seminiferous tubules (Fig. 4E–F), and an apparent difference in the number of tubules containing round spermatids. By P22, both WT and KO males had similar numbers of round spermatids (data not shown), indicating that this developmental delay was temporary.
Figure 4. Developmental delays and histological abnormalities during the first wave of spermatogenesis in backcrossed Rhox13 KO mice.
There were no significant differences in the numbers of preleptotene spermatocytes at P8 (A–B) or leptotene (Lep) and zygotene (Zyg) spermatocytes at P12 (C–D). In contrast, P20 KO testes had significantly reduced numbers of round spermatids (RS) (E–F). Asterisk indicates significance at P=0.0002. Scale bar = 50 µm.
Germ cell proliferation is reduced in Rhox13 KO mice during the first wave of spermatogenesis
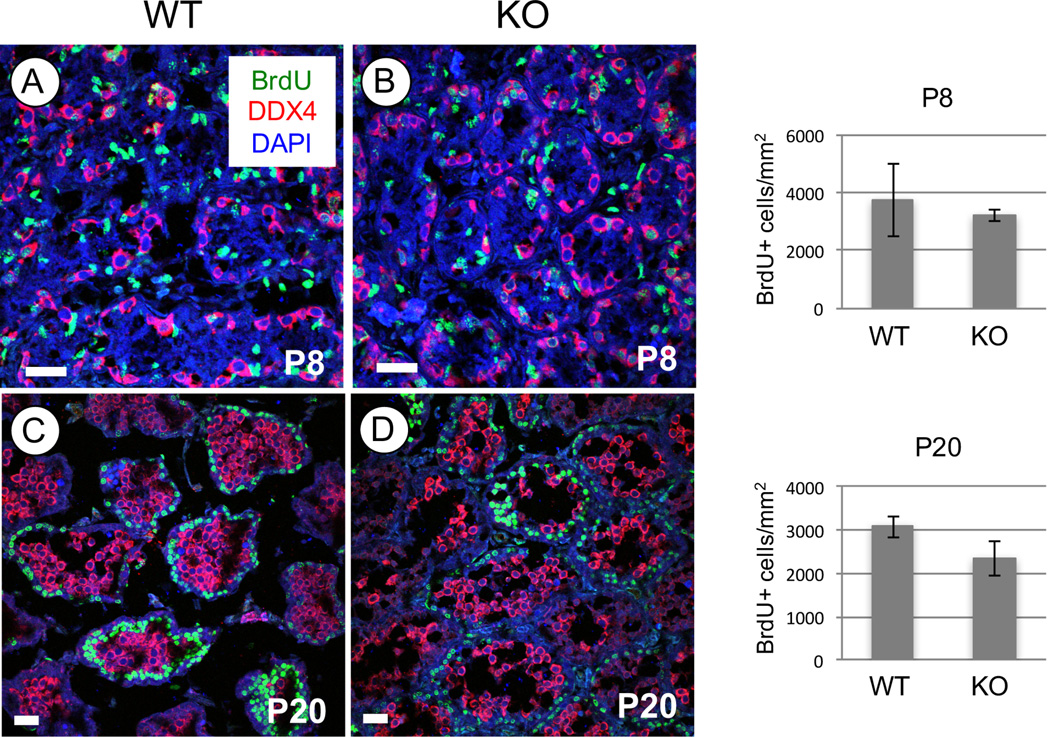
The preceding observations suggested an approximate 1-day delay in the appearance of specific germ cell types in Rhox13 KO testes during the first wave of spermatogenesis. We assessed whether this might be explained by a change in the extent of germ cell proliferation. We injected juvenile mice with the nucleotide analog 5-bromo-2-deoxyuridine (BrdU), which is only incorporated into DNA of cells that are mitotically active and have passed through S phase. Mice were euthanized 20 h after injection, and testis sections were stained with an antibody recognizing BrdU. We counted BrdU+ proliferating germ cells, and found there were no significant differences in the numbers of BrdU+ germ cells in Rhox13 KO mice at P8 and P20 (Fig. 5).
Figure 5. Germ cell proliferation decreases in backcrossed KO testes during the first wave of spermatogenesis.
(A–D) Immunostaining was done to detect BrdU (green) and DDX4 (red), and nuclei were labeled with DAPI (blue) in testes from P8 (A–B) and P20 (C–D) WT (A, C) and KO (B, D) mice. Ages are indicated on each panel. Scale bars = 30 µm.
Rhox13 testes have higher levels of germ cell apoptosis
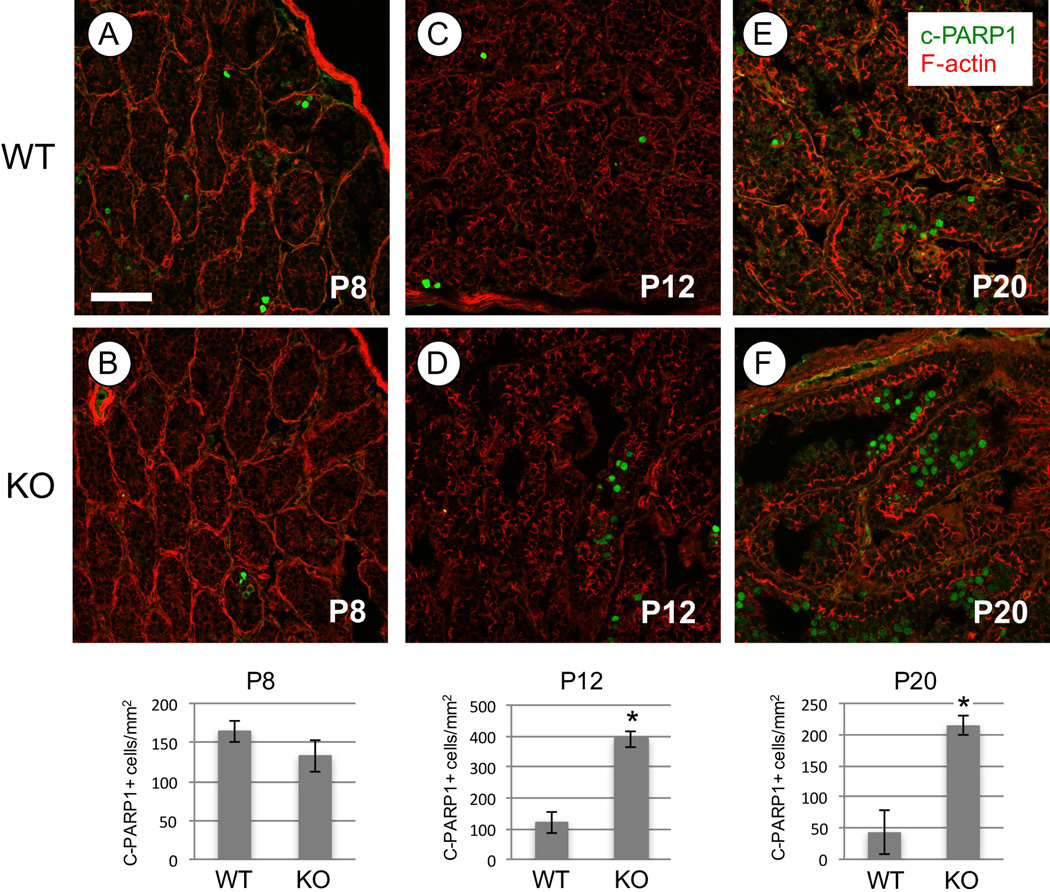
We noted an apparent increase in the number of apoptotic-appearing germ cells with pyknotic nuclei and brightly eosinophilic cytoplasm in H&E-stained Rhox13 KO testis sections during the first wave of spermatogenesis (Fig. 4). To quantify the extent of apoptosis, we stained frozen testis sections with an antibody recognizing cleaved PARP1 (c-PARP1). At P8, there were similar numbers of c-PARP1+ germ cells in WT and KO testes (Fig. 6A–B). At both P12 and P20, there was a significant increase of approximately 3-fold more c-PARP1+ germ cells in KO versus WT testes (Fig. 6C–F, P<0.05). We did not observe significant differences in any of the reproductive parameters measured from P120 adult mice undergoing SSC-derived steady-state spermatogenesis (data not shown).
Figure 6. Increased germ cell apoptosis in backcrossed KO testes during the first wave of spermatogenesis.
(A–F) Immunostaining was done to detect c-PARP1 (green), and phalloidin labeled F-actin (red). The c-PARP1+ cells were counted at each age and in each genotype, and the results are presented for each age below their respective panels as c-PARP1+ cells per mm2 of seminiferous cord (P8, P12) or tubule (P20). Asterisk indicates statistical significance (P<0.05). Scale bar = 30 µm.
Breeding studies assess the fertilization ability and efficiency of first wave- and adult SSC-derived sperm
We could not rule out the possibility that RHOX13 performs an essential role in a process required for fertility that we could not discern from the analyses described above. To examine this, we performed a 5-month breeding trial with 4 adult males of each genotype (age = P60 at the beginning of the trial). There were no significant differences in the average time to first litter (WT = 22 days, KO = 22 days), average time between subsequent litters (WT = 25 days, KO = 20 days), or average numbers of pups per litter (WT = 6.75, KO = 7.5).
During the first wave of spermatogenesis, KO testes exhibited a variety of histological abnormalities, decreased proliferation, increased apoptosis, and 50% fewer cauda epididymal sperm. Therefore, we performed a short-term breeding study to assess the fertility of these young male KO mice. KO and WT males at P35 were housed with mature females (P>60) for 15 days, and were then euthanized at P50 (n=4 KO, n=3 WT). The female mice were monitored for the next 22 days to record any subsequent litters. Two out of the three WT males produced a litter, while two out of four KO males produced a litter. There were no statistically significant differences in average numbers of pups per litter or days to first litter (Table 1). Thus, we conclude that deletion of Rhox13 does not significantly impair mouse fertility, at least in a controlled laboratory setting.
Discussion
These studies examined the reproductive phenotype of male Rhox13 KO mice during the first wave of spermatogenesis and during steady-state spermatogenesis in older adult mice. We found that, although RHOX13 is not essential for fertility, its loss led to a number of histological abnormalities and developmental delays that were particularly evident during the first wave of spermatogenesis. Compared to WT littermates, KO testes had delayed appearance of specific germ cell types, histological abnormalities, and an approximate 3-fold increase in germ cell apoptosis, culminating in a ~50% reduction in cauda epididymal sperm content in young adult males. These defects were not seen in older adult male mice, indicating a reduced role for RHOX13 in steady-state spermatogenesis.
The effect of genetic background on the reproductive phenotype
The first Rhox13 KO mice that we analyzed were on a mixed 129/Ola x C57BL/6N genetic background. Adult males that we analyzed exhibited a remarkable variety of reproductive phenotypes; some had apparently normal histology and sperm counts, while others had disrupted spermatogenesis. There is precedence in the literature for genetic background effects on fertility phenotypes, and this is likely due to unlinked genetic modifiers. One example is provided from studies of Stra8 KO mice, which exhibited an inconsistent array of spermatogenic defects on a mixed genetic background; the phenotype became consistent once the mutation was backcrossed onto a C57BL/6N background (Anderson, et al. 2008, Baltus, et al. 2006). We bred 129/Ola x C57BL/6N KO mice with WT inbred C57BL/6N mice for 7 generations to progressively dilute the 129/Ola-derived genome. Based on classical genetics, these ‘incipient congenic’ mice should have a genetic background that is >99% derived from the C57BL/6 strain (Berry and Linder 2007). These C57BL/6N Rhox13 males had less of a phenotype than those on the mixed background, suggesting that defects might have been more severe had we chosen to backcross onto the 129/Ola background.
Altered timing and increased apoptosis in Rhox13 KO males during the first wave of spermatogenesis
We observed a consistent delay in the appearance of round spermatids accompanied by a significant increase in apoptosis beginning at P12. Spermatogenesis in rodents is a tightly coordinated and precisely timed process, and as a consequence the adult seminiferous tubule segments contain predictable combinations of germ cells in various stages of development. The stage is set for this during the first wave of spermatogenesis, as specific germ cell types appear on defined days of development accompanied by a series of coordinated events such as Sertoli cell terminal differentiation and formation of the blood-testis barrier (BTB). Therefore, it is logical to presume that if the progression of germ cell development were delayed, then the coordination between developing germ cells and their environment would be disrupted. This might contribute to the significant increase in apoptosis during the first wave of spermatogenesis. There are other examples of altered timing of spermatogenesis during the first wave of spermatogenesis. The first is provided by studies of the ‘follicle stimulating hormone (FSH) receptor’ KO mouse, which exhibited decreased testicular weight, reduced seminiferous tubule diameters, and a delay in the appearance of spermatids (Krishnamurthy, et al. 2001). Another is provided by studies of mice deficient for ‘mediator complex subunit 1’ (Med1). Med1 KO spermatocytes appeared precociously, approximately 3 days earlier than in WT littermate controls (Huszar, et al. 2015). This interesting phenotype included a significant increase in the abundance of pachytene spermatocytes that persisted through at least P42, but did not result in reduced fertility.
The cauda epididymis is the main site for sperm storage in most mammals (Bedford 2015, Hinton, et al. 1996, Jones 1999, Moore 1998), and therefore quantifying sperm content provides a reasonable measure of the functional output of spermatogenesis. In young adult males (~P50), we observed a ~50% reduction in cauda epididymal sperm, the final product of the first wave of spermatogenesis. The fact that virgin males had not expelled any sperm likely explains why there were higher sperm numbers in their cauda epididymides.
A potential role of Rhox gene cluster in fertility
Rhox genes are present in all mammalian species examined to-date (MacLean and Wilkinson 2010). There are 3 Rhox genes in humans (RHOXF1, RHOXF2, and RHOXF2B) (Geserick, et al. 2002, Richardson, et al. 2014, Song, et al. 2013, Wayne, et al. 2002), and there are at least 8 polymorphic sites (Niu, et al. 2011). It will be important to determine whether any polymorphisms are associated with human male infertility. The 33 Rhox genes in mice exhibit unique expression patterns throughout the male and female reproductive systems (Maclean, et al. 2005, MacLean, et al. 2006, MacLean and Wilkinson 2010). It is apparent that the individual rodent Rhox genes exhibit a much more restricted expression pattern than the human RHOX genes, which suggests that their encoded proteins may play more refined or specialized roles within various reproductive tissues. In addition, the expansion of the Rhox gene cluster on the X chromosome may contribute to, among other things, regulation of the first wave of spermatogenesis. Indeed, the defects reported here in Rhox13 KO mice are most evident during the first wave, and decrease or disappear during steady-state spermatogenesis in the adult testis.
Three other Rhox genes have been disrupted in mice. Rhox9 (previously termed Gpbox) is expressed in the placenta and in fetal germ cells of both sexes, and targeted disruption did not lead to observable defects in spermatogenesis (Takasaki, et al. 2000, Takasaki, et al. 2001). However, it has been proposed that the closely related Rhox6 gene product could provide functional compensation, as it shares sequence similarity and expression pattern (MacLean and Wilkinson 2010). Rhox5 and Rhox8 are both expressed selectively in Sertoli cells, and their ablation resulted in male subfertility (Maclean, et al. 2005, Welborn, et al. 2015). It is likely that RHOX proteins may have overlapping functional roles, which would explain why the reproductive defects reported to-date are relatively minor. Future studies in our laboratory will examine the transcriptome-wide changes in gene expression in Rhox13 KO mice in order to identify RHOX13-regulated genes, and it is possible that other Rhox genes are upregulated in the absence of RHOX13 to provide functional compensation in the adult testis.
The first wave of spermatogenesis occurs in rodents, and results in the production of functional sperm capable of fertilization as early as 7–8 weeks of age in mice. Most rodents become independent by 4–5 weeks of age and the vast majority are polygamous (Curtis 2010), relying on opportunistic breeding encounters while leading an existence fraught with the danger of being consumed by a wide variety of different predators. Therefore, the ability to successfully reproduce a week or more earlier because of first wave-derived sperm (rather than waiting on germ cell production from SSCs) would increase a male’s chances of passing genes to the next generation before a potential early demise. Therefore, the role of the germ cell-expressed Rhox13 gene may be to support early fertility and increased fecundity by providing additional support for the first wave of spermatogenesis, thus favoring the evolutionary expansion of the Rhox gene cluster.
Acknowledgments
Funding
This work was supported by a grant from the NIH, National Institute of Child Health and Human Development (HD072552 to C.B.G.) and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES070076 to E.M.E.).
The authors thank Joani Zary-Oswald (ECU) and Linwood Koonce (NIEHS) for technical assistance.
Footnotes
Declaration of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby J, Tinwell H, Haseman J. Lack of effects for low dose levels of bisphenol A and diethylstilbestrol on the prostate gland of CF1 mice exposed in utero. Regul Toxicol Pharmacol. 1999;30:156–166. doi: 10.1006/rtph.1999.1317. [DOI] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–1434. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- Bedford JM. The epididymis re-visited: a personal view. Asian J Androl. 2015;17:693–698. doi: 10.4103/1008-682X.153297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry ML, Linder CC. Breeding systems: considerations, genetic fundamentals, genetic background, and strain types. Second. Boston, MA: Elsevier; 2007. [Google Scholar]
- Busada JT, Chappell VA, Niedenberger BA, Kaye EP, Keiper BD, Hogarth CA, Geyer CB. Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse. Dev Biol. 2015a;397:140–149. doi: 10.1016/j.ydbio.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busada JT, Geyer CB. The Role of Retinoic Acid (RA) in Spermatogonial Differentiation. Biol Reprod. 2015 doi: 10.1095/biolreprod.115.135145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busada JT, Kaye EP, Renegar RH, Geyer CB. Retinoic acid induces multiple hallmarks of the prospermatogonia-to-spermatogonia transition in the neonatal mouse. Biol Reprod. 2014;90:64. doi: 10.1095/biolreprod.113.114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busada JT, Niedenberger BA, Velte EK, Keiper BD, Geyer CB. Mammalian target of rapamycin complex 1 (mTORC1) Is required for mouse spermatogonial differentiation in vivo. Dev Biol. 2015b doi: 10.1016/j.ydbio.2015.08.004. Dbio15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell VA, Busada JT, Keiper BD, Geyer CB. Translational activation of developmental messenger RNAs during neonatal mouse testis development. Biol Reprod. 2013;89:61. doi: 10.1095/biolreprod.113.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C, Willis WD, Goulding EH, Jung-Ha H, Choi YC, Hecht NB, Eddy EM. Haploinsufficiency of protamine-1 or-2 causes infertility in mice. Nat Genet. 2001;28:82–86. doi: 10.1038/ng0501-82. [DOI] [PubMed] [Google Scholar]
- Curtis JT. Does fertility trump monogamy? Anim Behav. 2010;80:319–328. doi: 10.1016/j.anbehav.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohle GR, Smit M, Weber RF. Androgens and male fertility. World J Urol. 2003;21:341–345. doi: 10.1007/s00345-003-0365-9. [DOI] [PubMed] [Google Scholar]
- Drumond AL, Meistrich ML, Chiarini-Garcia H. Spermatogonial morphology and kinetics during testis development in mice: a high-resolution light microscopy approach. Reproduction. 2011;142:145–155. doi: 10.1530/REP-10-0431. [DOI] [PubMed] [Google Scholar]
- Geserick C, Weiss B, Schleuning WD, Haendler B. OTEX, an androgen-regulated human member of the paired-like class of homeobox genes. Biochem J. 2002;366:367–375. doi: 10.1042/BJ20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer CB, Eddy EM. Identification and characterization of Rhox13, a novel X-linked mouse homeobox gene. Gene. 2008;423:194–200. doi: 10.1016/j.gene.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer CB, Saba R, Kato Y, Anderson AJ, Chappell VK, Saga Y, Eddy EM. Rhox13 is translated in premeiotic germ cells in male and female mice and is regulated by NANOS2 in the male. Biol Reprod. 2012;86:127. doi: 10.1095/biolreprod.111.094938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton BT, Palladino MA, Rudolph D, Lan ZJ, Labus JC. The role of the epididymis in the protection of spermatozoa. Curr Top Dev Biol. 1996;33:61–102. doi: 10.1016/s0070-2153(08)60337-3. [DOI] [PubMed] [Google Scholar]
- Huszar JM, Jia Y, Reddy JK, Payne CJ. Med1 regulates meiotic progression during spermatogenesis in mice. Reproduction. 2015;149:597–604. doi: 10.1530/REP-14-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselman AL, Nakamura N, Brown PR, Willis WD, Goulding EH, Eddy EM. Heat shock protein 2 promoter drives Cre expression in spermatocytes of transgenic mice. Genesis. 2010;48:114–120. doi: 10.1002/dvg.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, Dausman J, Lee P, Wilson C, Lander E, Jaenisch R. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet. 2001;27:31–39. doi: 10.1038/83730. [DOI] [PubMed] [Google Scholar]
- Jones RC. To store or mature spermatozoa? The primary role of the epididymis. Int J Androl. 1999;22:57–67. doi: 10.1046/j.1365-2605.1999.00151.x. [DOI] [PubMed] [Google Scholar]
- Joyce KL, Porcelli J, Cooke PS. Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J Androl. 1993;14:448–455. [PubMed] [Google Scholar]
- Krishnamurthy H, Babu PS, Morales CR, Sairam MR. Delay in sexual maturity of the follicle-stimulating hormone receptor knockout male mouse. Biol Reprod. 2001;65:522–531. doi: 10.1095/biolreprod65.2.522. [DOI] [PubMed] [Google Scholar]
- Maclean JA, 2nd, Chen MA, Wayne CM, Bruce SR, Rao M, Meistrich ML, Macleod C, Wilkinson MF. Rhox: a new homeobox gene cluster. Cell. 2005;120:369–382. doi: 10.1016/j.cell.2004.12.022. [DOI] [PubMed] [Google Scholar]
- MacLean JA, 2nd, Lorenzetti D, Hu Z, Salerno WJ, Miller J, Wilkinson MF. Rhox homeobox gene cluster: recent duplication of three family members. Genesis. 2006;44:122–129. doi: 10.1002/gene.20193. [DOI] [PubMed] [Google Scholar]
- MacLean JA, 2nd, Wilkinson MF. The Rhox genes. Reproduction. 2010;140:195–213. doi: 10.1530/REP-10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean JA, Bettegowda A, Kim BJ, Lou CH, Yang SM, Bhardwaj A, Shanker S, Hu Z, Fan Y, Eckardt S, McLaughlin KJ, Skoultchi AI, Wilkinson MF. The rhox homeobox gene cluster is imprinted and selectively targeted for regulation by histone h1 and DNA methylation. Mol Cell Biol. 2011;31:1275–1287. doi: 10.1128/MCB.00734-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HD. Contribution of epididymal factors to sperm maturation and storage. Andrologia. 1998;30:233–239. doi: 10.1111/j.1439-0272.1998.tb01165.x. [DOI] [PubMed] [Google Scholar]
- Niedenberger BA, Busada JT, Geyer CB. Marker expression reveals heterogeneity of spermatogonia in the neonatal mouse testis. Reproduction. 2015;149:329–338. doi: 10.1530/REP-14-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu AL, Wang YQ, Zhang H, Liao CH, Wang JK, Zhang R, Che J, Su B. Rapid evolution and copy number variation of primate RHOXF2, an X-linked homeobox gene involved in male reproduction and possibly brain function. BMC Evol Biol. 2011;11:298. doi: 10.1186/1471-2148-11-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M, Yamagiwa A, Yamamoto S, Nakayama T, Tsumura A, Sasaki H, Nakao K, Li E, Okano M. DNA methylation regulates long-range gene silencing of an X-linked homeobox gene cluster in a lineage-specific manner. Genes Dev. 2006;20:3382–3394. doi: 10.1101/gad.1470906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson ME, Bleiziffer A, Tuttelmann F, Gromoll J, Wilkinson MF. Epigenetic regulation of the RHOX homeobox gene cluster and its association with human male infertility. Hum Mol Genet. 2014;23:12–23. doi: 10.1093/hmg/ddt392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb GW, Amann RP, Killian GJ. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil. 1978;54:103–107. doi: 10.1530/jrf.0.0540103. [DOI] [PubMed] [Google Scholar]
- Song HW, Anderson RA, Bayne RA, Gromoll J, Shimasaki S, Chang RJ, Parast MM, Laurent LC, de Rooij DG, Hsieh TC, Wilkinson MF. The RHOX homeobox gene cluster is selectively expressed in human oocytes and male germ cells. Hum Reprod. 2013;28:1635–1646. doi: 10.1093/humrep/det043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki N, McIsaac R, Dean J. Gpbox (Psx2), a homeobox gene preferentially expressed in female germ cells at the onset of sexual dimorphism in mice. Dev Biol. 2000;223:181–193. doi: 10.1006/dbio.2000.9741. [DOI] [PubMed] [Google Scholar]
- Takasaki N, Rankin T, Dean J. Normal gonadal development in mice lacking GPBOX, a homeobox protein expressed in germ cells at the onset of sexual dimorphism. Mol Cell Biol. 2001;21:8197–8202. doi: 10.1128/MCB.21.23.8197-8202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang J. Remarkable expansions of an X-linked reproductive homeobox gene cluster in rodent evolution. Genomics. 2006;88:34–43. doi: 10.1016/j.ygeno.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Wayne CM, MacLean JA, Cornwall G, Wilkinson MF. Two novel human X-linked homeobox genes, hPEPP1 and hPEPP2, selectively expressed in the testis. Gene. 2002;301:1–11. doi: 10.1016/s0378-1119(02)01087-9. [DOI] [PubMed] [Google Scholar]
- Welborn JP, Davis MG, Ebers SD, Stodden GR, Hayashi K, Cheatwood JL, Rao MK, MacLean JA., 2nd Rhox8 Ablation in the Sertoli Cells Using a Tissue-Specific RNAi Approach Results in Impaired Male Fertility in Mice. Biol Reprod. 2015;93:8. doi: 10.1095/biolreprod.114.124834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilming LG, Boychenko V, Harrow JL. Comprehensive comparative homeobox gene annotation in human and mouse. Database (Oxford) 2015;2015 doi: 10.1093/database/bav091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QE, Oatley JM. Spermatogonial stem cell functions in physiological and pathological conditions. Curr Top Dev Biol. 2014;107:235–267. doi: 10.1016/B978-0-12-416022-4.00009-3. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]