Summary
Diamond Blackfan anemia (DBA) is an inherited syndrome usually presenting with severe macrocytic anemia in infancy, paucity of erythroid precursors in the bone marrow, and congenital anomalies. We describe a child with mild, transfusion independent normocytic anemia whose diagnosis of DBA was established by identification of a novel de novo mutation disrupting normal splicing of the ribosomal protein RPL5. The diagnosis of DBA was confirmed by elevated erythrocyte adenosine deaminase levels and an abnormal ribosomal RNA profile. This case demonstrates the usefulness of genomic analysis in establishing the diagnosis of DBA in patients with a nonclassical presentation of the disease.
Keywords: Diamond Blackfan anemia, DBA, next-generation sequencing, targeted sequencing, high throughput sequencing
Diamond Blackfan anemia (DBA) is a disorder of ribosomal biogenesis, classically presenting with macrocytic anemia at or shortly after birth, reticulocytopenia, and a normocellular marrow with reduced erythroid precursors. Erythrocyte adenosine deaminase (ADA) and hemoglobin F levels are usually elevated.1,2 Extrahematopoietic manifestations include growth retardation, thumb abnormalities, facial dysmorphism, and congenital heart defects. Genetic analysis of DBA has raised awareness that patients can also present in “nonclassical” ways.1 We describe a toddler with genetically diagnosed DBA who presented with mild normocytic anemia, neutropenia, and a triphalengeal thumb. This case emphasizes the need to consider DBA in the diagnosis of patients with nonclassical presentations and the contribution of genomic analysis to diagnosis of inherited marrow failure.
METHODS
Blood and bone marrow samples were collected after obtaining informed consent. Bone marrow aspiration smears were stained with Hematek (Siemens, Germany).
Genomic DNA, extracted from peripheral blood, was evaluated by multiplexed massively parallel sequencing following hybridization to MarrowSeq, a targeted-capture panel of 85 genes involved in bone marrow failure and myelodysplastic syndrome.3 (Details of the MarrowSeq panel are available at: http://web.labmed.washington.edu/tests/genetics/MRW).
cDNA was synthesized from RNA obtained from peripheral blood mononuclear cells. Amplified polymerase chain reaction (PCR) products were sequenced on an ABI Prism 3130xl Genetic Analyzer. For expression analysis, cDNAs were amplified using the ABsolute Blue SYBR Green master mix (ThermoFisher Scientific). Amplification products were detected by real-time PCR using the ECO system (Illumina). Gene expression was normalized to the expression of the housekeeping genes RPLP0 and GAPDH. Primers for RPL5 exons 1 to 2: F-ggtctctgttccgcaggatg, R-cagttttaccctctcgtcgtct. Primers for exons 6 to 8: F-agaacagcgtaactccagaca, R-ttgggacggttccacctctt.
Erythrocyte ADA levels were measured as previously described.4 Ribosomal RNA species (rRNA 18S, 28S, 32S) were evaluated as reported by Farrar et al.5
CASE REPORT
A 15-month-old girl was referred to our hematology clinic for evaluation of anemia. She was born at term, with birth weight of 2200 g, and was found to have a single umbilical artery and a right triphalangeal thumb, which was surgically corrected at age 8 months. Her parents are not related and family history was unremarkable. Echocardiography at age 4 months demonstrated a small patent foramen ovale.
At age 6 months the patient’s hemoglobin level was 8.6 g/dL with a mean corpuscular volume (MCV) of 86.4 fL. Ferritin, serum iron, vitamin B12 levels, thyroid function tests, and osmotic fragility test were normal. Hemoglobin electrophoresis at age 15 months showed hemoglobin F of 2.4% (normal <1%) and hemoglobin A2 of 2.7% (normal < 3.5%). Molecular testing excluded the presence of mutations in the alpha globin genes.
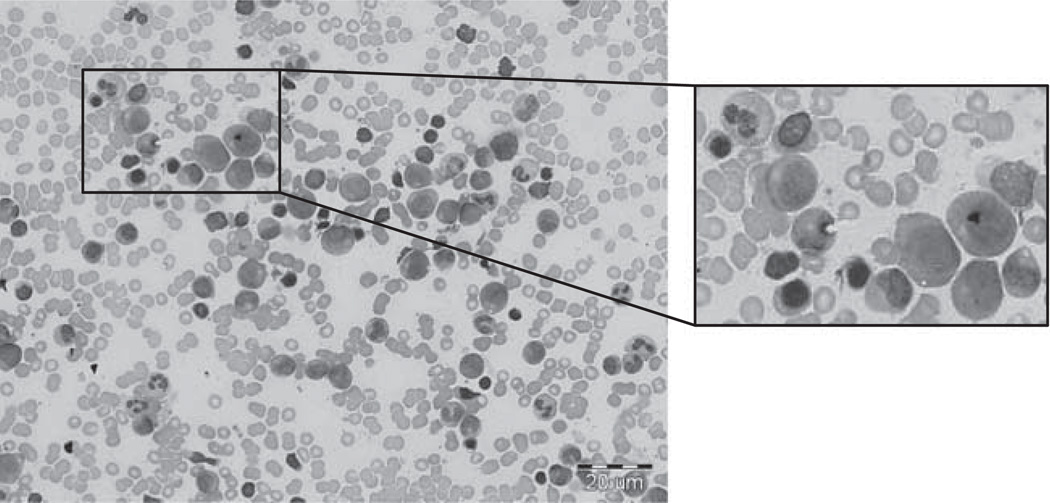
At presentation, physical examination was notable for a mild systolic murmur and a surgical scar on the right thumb. Laboratory studies revealed an underproduction, normocytic anemia (hemoglobin, 10.9 g/dL; MCV, 81 fL; corrected reticulocyte count, 1%), absolute neutropenia (900/µL), and normal platelet count. Peripheral blood smear was unremarkable. Bone marrow aspirate and biopsy revealed a normocellular marrow for age with a relative decrease in erythroid precursors (myeloid:erythroid ratio 5:1) and no morphologic dysplasia (Fig. 1). The bone marrow karyotype was 46,XX. Telomere lengths and chromosome fragility testing of the peripheral blood to evaluate for Fanconi anemia and dyskeratosis congenita were normal. Given her mild neutropenia, we obtained serial neutrophil counts to evaluate for cyclic neutropenia, and obtained sequences of ELANE and SBDS; all results were normal.
FIGURE 1.
Bone marrow aspiration of the patient. Left, a representative field. A scale bar is located in the right lower corner. Right, a higher magnification of the marked field. Photographs were taken with a light microscope (BX51; Olympus, × 50 magnification).
Over 3 years of follow-up, the patient manifested failure to thrive, mild hypotonia, and an intermittent mild normocytic anemia (hemoglobin, ~9.2 to 11.4 g/dL; MCV, ~80 to 84 fL). Neutrophil counts normalized and she remained transfusion independent.
Given both the substantial phenotypic overlap among inherited bone marrow failure syndromes and phenotypic heterogeneity within these syndromes, we undertook a genetic approach to diagnosis. Specifically, we evaluated genomic DNA by targeted-capture next-generation sequencing. The proband, her unaffected brother, and parents were sampled. Median coverage across the targeted genomic region was 542-fold, with 97% of targeted bases having > 50-fold coverage and 98.5% of targeted bases having > 10-fold coverage.
Sequence analysis revealed the proband to be heterozygous for RPL5 c.527(+ 1)G > A (IVS5 + 1G > A) at chr1:93,301,950 (hg19). The mutation disrupts a splice donor site of RPL5 and is predicted to lead to out-of-frame skipping of exon 5 (203 bp), leading to a premature stop. RPL5 encodes a component of the 60S ribosome. Mutations in RPL5 are diagnostic for DBA.6 The mutation was absent from the sequences of the parents and unaffected brother, so de novo in the patient. Sanger sequencing confirmed the presence of the mutation in the child, and its absence in her parents and brother. This mutation has not been previously reported and is not found on any of the public databases, which in total include exome sequences of approximately 80,000 persons.
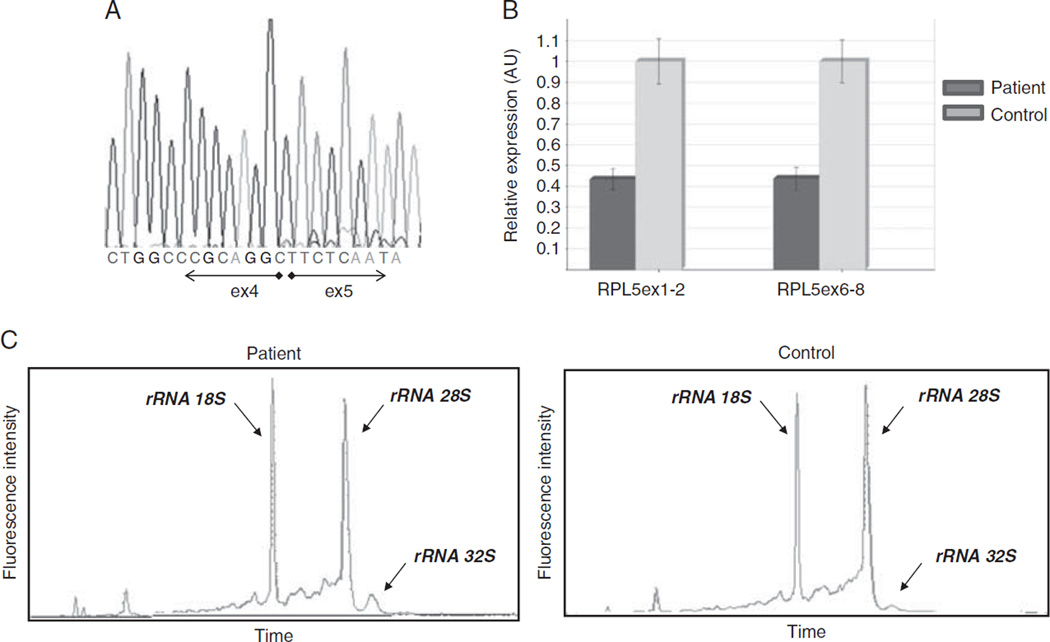
Evaluation of RNA from peripheral blood of the patient suggested that the mutant RNA is unstable. Sanger sequencing of cDNA revealed only a normal sequence of RPL5 (Fig. 2A). Also, the patient’s RNA expression levels of RPL5 exons 1 to 2 and 6 to 8 were reduced by about 50% below control (Fig. 2B).
FIGURE 2.
Genetic and molecular analysis confirms the diagnosis of DBA. A, A normal cDNA sequence of RPL5. B, Relative expression levels of RPL5 of the patient (dark gray bars) compared with a normal control (light gray bars), demonstrating a reduced expression of RPL5 in the patient. RPL5 expression was analyzed using 2 independent reactions. PCR was performed in triplicates; error bars represent the standard error. C, Defect in pre-rRNA processing evidenced by accumulation of 32S rRNA, confirmed by an electropherogram of ribosomal rRNA. Total RNA of peripheral blood mononuclear cells of the patient (left panel) compared with a healthy control (right panel) is presented. 18S, 28S, and 32S rRNA species are indicated. A reduction of the 28S peak and increase of the 32S rRNA peak are evident in the patient’s sample compared with the control. ex indicates exons.
Evaluation of pre-rRNA processing in mononuclear blood cells of the patient yielded results consistent with her RPL5 genotype. An increase of 32S rRNA species (a precursor of the 28S rRNA) was evident (Fig. 2C). Accumulation of this rRNA precursor is characteristic of DBA patients with defects in the maturation of the 60S ribosomal subunit.5 The patient’s erythrocyte ADA levels were elevated (4.5 U/g hemoglobin; normal, 0.8 to 1.2), consistent with a diagnosis of DBA.
DISCUSSION
The classical presentation of DBA includes early onset severe macrocytic anemia, no other significant cytopenias, paucity of the erythroid precursors in the bone marrow, and congenital anomalies. We describe a patient with mild normocytic anemia and transient neutropenia who had a few congenital anomalies: a single umbilical artery, patent foramen ovale, and a triphalengeal thumb. The primary evaluation, therefore, did not raise suspicion of DBA. Despite the atypical presentation, genomic sequencing revealed a de novo mutation in the RPL5 gene, consistent with a diagnosis of DBA. Identification of an abnormal ribosomal RNA profile and elevated levels of erythrocyte ADA established the diagnosis of DBA.
The case emphasizes the growing evidence of nonclassical presentation of DBA. In this case, the main clue for the diagnosis of DBA was the thumb abnormality. Triphalangeal thumb may occur as an isolated congenital anomaly, in association with other hand abnormalities, or as a part of a syndrome.7 Thumb abnormalities are found in 9% to 19% of patients with DBA.1 However, the very mild anemia and the lack of macrocytosis reduced suspicion of DBA and led to a delay in the diagnosis of this patient.
DBA patients with a late onset anemia or a relatively mild course have been reported. A patient with an RPL5 mutation was anemic from infancy but became transfusion-dependent at age 12 years.8 Adult onset of anemia was described in a patient with congenital thumb abnormalities and a genetic diagnosis of DBA.9 In reported series of patients with DBA, a few patients were diagnosed older than age 12 months or with a mild anemia that required no therapy.6,10–12
The present case, along with those previously reported, emphasize the phenotypic heterogeneity of DBA and the need to consider this disorder even in patients with mild anemia and only a moderate reduction of red cell precursors in the marrow. Incorporation of genomic analysis will assist an accurate diagnosis of patients with DBA, especially in cases with nonclassical presentation, and may also broaden our understanding of the clinical heterogeneity of the disease.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Vlachos A, Ball S, Dahl N, et al. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol. 2008;142:859–876. doi: 10.1111/j.1365-2141.2008.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fargo JH, Kratz CP, Giri N, et al. Erythrocyte adenosine deaminase; diagnostic value for Diamond Blackfan Anaemia. Br J Haematol. 2013;160:547–554. doi: 10.1111/bjh.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang MY, Keel SB, Walsh T, et al. Genomic analysis of bone marrow failure and myelodysplastic syndromes reveals phenotypic and diagnostic complexity. Haematologica. 2015;100:42–48. doi: 10.3324/haematol.2014.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glader BE, Backer K. Elevated red cell adenosine deaminase activity: a marker of disordered erythropoiesis in Diamond-Blackfan anaemia and other haematologic diseases. Br J Haematol. 1988;68:165–168. doi: 10.1111/j.1365-2141.1988.tb06184.x. [DOI] [PubMed] [Google Scholar]
- 5.Farrar JE, Quarello P, Fisher R, et al. Exploiting pre-rRNA processing in Diamond Blackfan anemia gene discovery and diagnosis. Am J Hematol. 2014;89:985–991. doi: 10.1002/ajh.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazda HT, Sheen MR, Vlachos A, et al. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am J Hum Genet. 2008;83:769–780. doi: 10.1016/j.ajhg.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qazi Q, Kassner EG. Triphalangeal thumb. J Med Genet. 1988;25:505–520. doi: 10.1136/jmg.25.8.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farruggia P, Quarello P, Garelli E, et al. The spectrum of nonclassical Diamond-Blackfan anemia: a case of late beginning transfusion dependency associated to a new RPL5 mutation. Pediatr Rep. 2012;4:91–93. doi: 10.4081/pr.2012.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballester EF, Gil-Fernandez JJ, Vazquez-Blanco M, et al. Adult-onset Diamond Blackfan anemia with a novel mutation in the exon 5 of RPL11: too late and too rare. Clin Case Rep. 2015;3:392–395. doi: 10.1002/ccr3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konno Y, Toki T, Tandai S, et al. Mutations in the ribosomal protein genes in Japanese patients with Diamond-Blackfan anemia. Haematologica. 2010;95:1293–1299. doi: 10.3324/haematol.2009.020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerrard G, Valgañón M, Foong HE, et al. Targeted enrichment and high throughput sequencing of 80 ribosomal protein genes to identify mutations associated with Diamond-Blackfan anaemia. Br J Haematol. 2013;162:530–536. doi: 10.1111/bjh.12397. [DOI] [PubMed] [Google Scholar]
- 12.Willig TN, Draptchinskaia N, Dianzani I, et al. Mutations in ribosomal protein S19 gene and Diamond Blackfan anemia: wide variations in phenotypic expression. Blood. 1999;94:4294–4306. [PubMed] [Google Scholar]