Abstract
Objective
We assessed cross-sectional and longitudinal associations of 3 non-traditional cardiovascular disease (CVD) risk factors - human immunodeficiency virus (HIV), cocaine use, and chronic hepatitis C virus (HCV) infection - with 3 validated markers of subclinical CVD: carotid artery plaque, albuminuria, and aortic pulse wave velocity (PWV) in a well-characterized cohort.
Approach and Results
We measured carotid plaque at baseline and after 24 months, urine albumin-creatinine ratio (ACR) every 6 months, and PWV annually for up to 36 months in a predominantly African-American cohort of 292 participants (100 HIV-negative and 192 HIV-positive). Thirty-nine percent had chronic HCV infection, and 20%, 28%, and 52% were never, past, and current cocaine users, respectively. Sixteen percent, 47%, and 64% of those with none, 1 or 2, or all 3 non-traditional risk factors had ≥2 abnormal CVD risk markers (P=0.001). In fully adjusted models that included all 3 non-traditional risk factors, HIV infection was independently associated with carotid plaque progression (increase in the number of anatomic segments with plaque), albuminuria (ACR >30mg/g), albuminuria progression (doubling of ACR from baseline to a value >30mg/g), and PWV. Cocaine use was associated with an approximately 3-fold higher odds of carotid plaque at baseline and HCV infection was significantly associated with a higher risk of carotid plaque progression.
Conclusions
These results suggest that HIV infection, cocaine use, and HCV infection are important non-traditional risk factors for CVD and highlight the need to understand the distinct and overlapping mechanisms of the associations.
Introduction
Human immunodeficiency virus (HIV), cocaine use, and hepatitis C virus (HCV) are overlapping epidemics in many US cities. Individually, each of these “non-traditional” risk factors has been implicated in cardiovascular disease (CVD),1–13 although with reports of null associations as well.14–16 As combination antiretroviral therapy has substantially prolonged the life expectancy of HIV-infected persons, it is important to understand the traditional and non-traditional determinants of age-related comorbidities such as CVD. Few studies have rigorously assessed all three factors in a single population.
Using data from a longitudinal cohort, we sought to explore the independent associations of HIV, cocaine use (biologically validated), and active HCV (detectable HCV RNA) with cross-sectional and longitudinal trajectories of well-validated markers of subclinical CVD that predict CVD risk. We used ultrasonography to assess intima-media thickness (IMT) of the right common and internal carotid arteries and carotid plaque in all extracranial carotid artery segments. These carotid arterial measures are associated with the risk of future CVD events17–19 and with CVD risk reduction in response to interventions such as use of lipid and blood pressure lowering medications.20 Our second measure, albuminuria (urine albumin-creatinine ratio >30 mg/g), a component of chronic kidney disease,21 is believed to reflect systemic endothelial dysfunction22, 23 and is a strong predictor of CVD events.24 Finally, we measured aortic pulse wave velocity (PWV), a measure of arterial stiffness that is an independent predictor of CVD events in a variety of populations.25
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
A total of 292 participants completed at least one study visit and were included in the analysis (100 HIV-negative and 192 HIV-positive). The median (25th percentile [P25], 75th percentile [P75]) follow-up was 37 months (36, 37). The median (P25, P75) enrollment and last follow-up months were July 2011 (February 2011, February 2012) and August 2014 (March 2014, March 2015), respectively. Compared with HIV-negative participants, HIV-positive participants were more likely to be women, have a diagnosis of hypertension, be taking antihypertensive and anti-lipidemic medication, have a history of cocaine use, and have HCV infection (Table 1). Age, race, smoking status, body mass index, total to high-density lipoprotein cholesterol ratios, estimated glomerular filtration rate (eGFR), and American College of Cardiology/American Heart Association (ACC/AHA) CVD risk scores26 were similar in the two groups.
Table 1.
Baseline characteristics of 292 study participants according HIV status, Baltimore, Maryland
| Characterisitic | HIV-negative (n=100) |
HIV-positive (n=192) |
P value |
|---|---|---|---|
| Female, n (%) | 19 (19) | 67 (35) | 0.005 |
| Age, years, median (P25, P75) | 49 (45, 54) | 49 (45, 53) | 0.59 |
| African American, n (%) | 92 (92) | 181 (94) | 0.46 |
| Smoking status, n (%) | 0.80 | ||
| Never | 27 (27) | 46 (24) | |
| Past | 12 (12) | 22 (11) | |
| Current | 61 (61) | 124 (65) | |
| Hypertension, n (%) | 21 (21) | 67 (35) | 0.016 |
| Taking antihypertensive medication, n (%) | 19 (19) | 63 (33) | 0.014 |
| Systolic blood pressure, mm Hg, median (P25, P75) | 126 (113, 135) | 119 (108, 131) | 0.007 |
| Body mass index, kg/m2, median (P25, P75) | 27 (23, 33) | 25 (23, 31) | 0.15 |
| eGFR*, mL/min/1.73m2, median (P25, P75) | 103 (91, 114) | 103 (84, 118) | 0.74 |
| ≥90 mL/min/1.73m2, n (%) | 76 (76) | 131 (68) | 0.10 |
| 60–89 mL/min/1.73m2, n (%) | 24 (24) | 54 (28) | |
| <60 mL/min/1.73m2, n (%) | 0 | 7 (4) | |
| Total to HDL cholesterol ratio, median (P25, P75) | 3.1 (2.4, 4.1) | 3.1 (2.5, 4.2) | 0.87 |
| Using HMG-CoA reductase inhibitor, n (%) | 4 (4) | 27 (14) | 0.008 |
| Self-reported history of CVD†, n (%) | 4 (4) | 21 (11) | 0.05 |
| ACC/AHA CVD risk score†, median (P25, P75) | 6.1 (3.6, 9.8) | 5.2 (2.2, 9.0) | 0.095 |
| Cocaine use§, n (%) | 0.002 | ||
| Never | 31 (31) | 26 (14) | |
| Past | 24 (24) | 58 (30) | |
| Current | 45 (45) | 108 (56) | |
| Active hepatitis C virus, n (%) | 23 (23) | 92 (48) | <0.001 |
| Time since enrollment in HIV clinic, years, median (P25, P75) | - | 8.1 (3.5, 12.2) | - |
| History of AIDS-defining condition, n (%) | - | 48 (25) | - |
| Nadir CD4 cell count, cells/mm3, median (P25, P75) | - | 146 (43, 302) | - |
| Taking antiretroviral therapy, n (%) | - | 175 (91) | - |
| Ritonavir-boosted protease inhibitor, n (%) | - | 127 (66) | - |
| Non-nucleoside reverse transcriptase inhibitor, n (%) | - | 47 (24) | - |
| Integrase strand transfer inhibitor, n (%) | - | 40 (21) | - |
| Tenofovir, n (%) | - | 130 (68) | - |
| Abacavir, n (%) | - | 30 (16) | - |
| Current CD4 cell count, cells/mm3, median (P25, P75) | - | 467 (248, 627) | - |
| HIV RNA < 400 copies/mL, N (%) | - | 152 (79) | - |
| Number of study visits completed, median (P25, P75) | 6 (5, 7) | 6 (6, 7) | 0.005 |
HDL, high density lipoprotein; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A
Glomerular filtration rate estimated with serum creatinine using the CKD-EPI equation.
25 participants reported one or more prior CVD events at baseline including 1) “heart attack” (n=5), “open heart surgery for blocked blood vessels” (n=2), “balloon treatment or stent placed in heart” (n=1), or “stroke or mini-stroke” (n=21).
ACC/AHA CVD risk score25 is the predicted 10-year risk of cardiovascular disease derived from an equation that includes age, sex, race, total cholesterol, HDL cholesterol, diabetes, systolic blood pressure, smoking status, and use of antihypertensive medication. Point estimates are expressed per 5 percentage point increase in the risk score.
Cocaine use categorized for each participant on the basis of self-report and urine drug tests obtained over the course of study observation (see text for details).
We categorized cocaine use status for each participant on the basis all self-report and urine drug test data collected during follow-up: 1) never users denied historical and recent (prior 6 months) cocaine use at all visits and had all negative urine cocaine tests; 2) past users reported prior cocaine use at the baseline survey, but denied recent use in all surveys and had all negative urine cocaine tests; 3) current users either reported recent cocaine use or had cocaine detected by urine drug test at one or more visits. Urine drug test data led to substantial reclassification of cocaine use compared with self-report alone; of 85 participants categorized as never users and 136 categorized as past users by self-reported data, 28 (33%) and 54 (40%), respectively, were re-categorized as current users on the basis of cocaine detection in at least one drug test. Consequentially, 57 participants were categorized as never cocaine users, 82 as past users, and 153 as current users (Table 1). Among participants categorized as current cocaine users during the study period, recent cocaine use (either by self-report [past 6 months] or urine drug test) was detected at median (P25, P75) of 57% (33%, 100%) of study visits. Baseline participant characteristics stratified by cocaine use status are shown in Table I in the online-only Data Supplement.
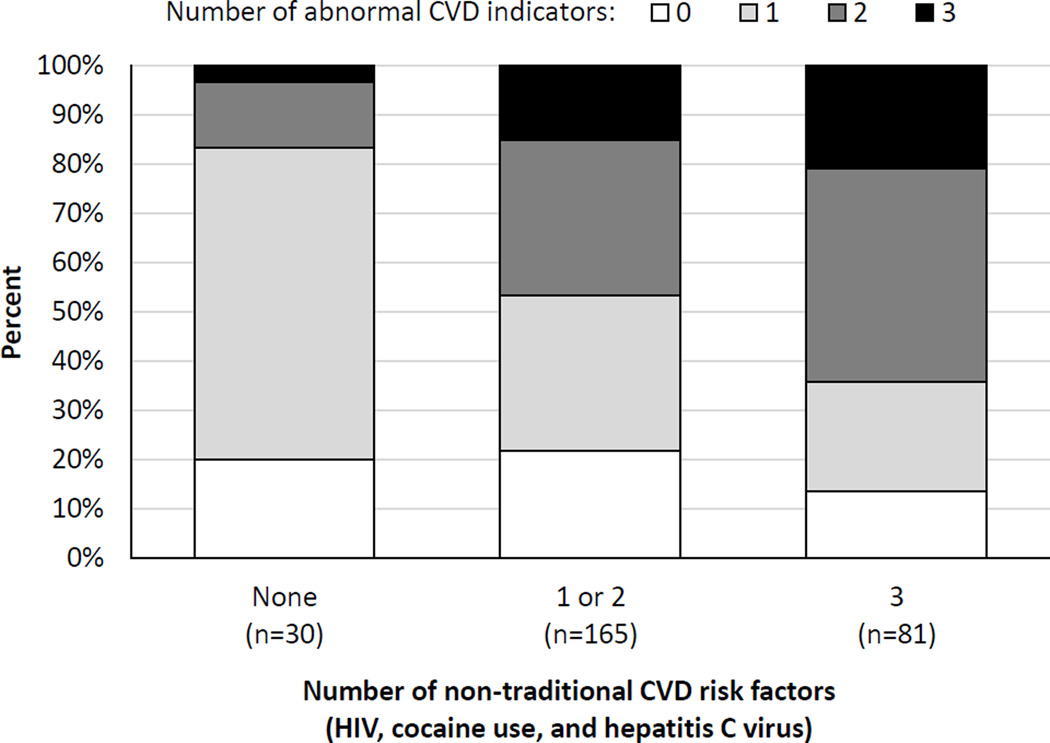
Correlations among subclinical CVD markers were modest, with Spearman’s rho values of 0.13, 0.17, and 0.19 for albumin-creatinine ratio vs. carotid IMT, PWV vs. IMT, and albumin-creatinine ratio vs. PWV, respectively (P<0.05 for all comparisons). Considering the sum of non-traditional risk factors at the individual level, we found evidence that having more risk factors (HIV, cocaine use [past or current], and HCV) was associated with having a larger number of abnormal subclinical CVD markers (carotid plaque, albuminuria, and PWV > 9.6 m/s at any visit) (Figure, P=0.001 for overall difference).
Figure.
Bar chart showing percentages of participants with 0, 1, 2, or 3 CVD indicators (carotid plaque, albuminuria, and pulse wave velocity > 9.6 m/s measured at any visit) according to the number of non-traditional risk factors (HIV, cocaine use [past or current], and HCV). The figure includes 276 participants (95%) with at least one valid measure of each CVD indicator). The percentages with 0, 1, 2, and 3 cardiovascular indicators were 20%, 63%, 13%, and 3% in those with no non-traditional risk factors; 22%, 32%, 32%, and 15% in those with 1 or 2 non-traditional risk factors; and 14%, 22%, 43%, and 21% in those with all 3 non-traditional risk factors (P=0.001 for overall difference).
A total of 279 participants (96%) had technically adequate carotid assessments at baseline and of these, 221 completed technically adequate assessments at 24 months. One hundred forty-nine (53%) participants had carotid plaque detected at baseline. Plaque progression was defined as the presence of plaque in a larger number of the 12 anatomic segments at the 24-month assessment compared with the baseline assessment. Of participants with repeat imaging at 24 months, plaque progression was detected in 47 (40%) and 20 (20%) of those with and without plaque at baseline, respectively. (P=0.002 for difference). Two participants had clinically-significant carotid stenosis, on the basis of visual stenosis > 50% with peak blood flow velocity >160 cm/s. Both past and current cocaine use had strong independent associations with the presence of carotid plaque at baseline in a model that included all 3 non-traditional risk factors and ACC/AHA CVD risk scores (Table 2). Past cocaine use, HIV-positive status and HCV-positive status had significant unadjusted associations with plaque progression at 24 months, although only HIV and HCV status remained significantly associated with plaque progression in the fully adjusted model. In a supplemental modeling approach using individual components of the ACC/AHA CVD risk score rather than the risk score itself, results were similar, however HCV status no longer was significantly associated with plaque progression (OR 1.8 [0.9, 3.5, Table II in the online-only Data Supplement). In contrast, neither HIV, cocaine use, nor HCV had significant associations with baseline carotid IMT or with subsequent change in IMT (Table 2).
Table 2.
Associations of HIV, cocaine use, and hepatitis C with carotid artery plaque and intima-media thickness among 279 participants, Baltimore, Maryland
| Risk factor | Presence of carotid plaque at baseline |
Carotid plaque progression |
||||
|---|---|---|---|---|---|---|
| Frequency (%) |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
Frequency (%) |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
|
| Cocaine use | ||||||
| Never (n=55) | 16 (29) | ref. | ref. | 9 (20) | ref. | ref. |
| Past (n=78) | 49 (63) | 4.1 (2.0, 8.6)† | 3.3 (1.5, 7.3)‡ | 27 (42) | 2.9 (1.2, 7.1)‡ | 1.9 (0.8, 4.9) |
| Current (n=146) | 84 (58) | 3.3 (1.7, 6.4)† | 2.7 (1.3, 5.5)‡ | 31 (28) | 1.5 (0.7, 3.5) | 1.0 (0.4, 2.4) |
| HIV status | ||||||
| Negative (n=96) | 79 (43) | ref. | ref. | 14 (20) | ref. | ref. |
| Positive (n=183) | 107 (57) | 1.5 (.9, 2.5) | 1.4 (0.8, 2.5) | 53 (35) | 2.2 (1.1, 4.4)‡ | 2.1 (1.0, 4.3)‡ |
| Hepatitis C status | ||||||
| Negative (n=169) | 83 (49) | ref. | ref. | 32 (24) | ref. | ref. |
| Positive (n=110) | 66 (60) | 1.6 (1.0, 2.5) | 1.0 (0.6, 1.8) | 35 (41) | 2.3 (1.3, 4.1)‡ | 1.9 (1.0, 3.6)‡ |
| CVD risk score§ | - | - | 1.8 (1.4, 2.4)† | - | - | 1.3 (1.0, 1.8)‡ |
|
Carotid IMT at baseline (µm) |
Annualized change in carotid IMT (µm/year) |
|||||
| Mean ± sd | Unadjusted difference (95% CI) |
Adjusted* difference (95% CI) |
Mean ± sd | Unadjusted difference (95% CI) |
Adjusted* difference (95% CI) |
|
| Cocaine use | ||||||
| Never (n=55) | 788 ± 259 | ref. | ref. | 16 ± 58 | ref. | ref. |
| Past (n=78) | 852 ± 243 | 64 (−26, 154) | 30 (−60, 121) | 16 ± 52 | −1 (−23, 22) | −10 (−33, 13) |
| Current (n=146) | 852 ± 268 | 64 (−17, 145) | 32 (−51, 11) | 14 ± 63 | −2 (−23, 18) | −12 (−33, 10) |
| HIV status | ||||||
| Negative (n=96) | 841 ± 224 | ref. | ref. | 13 ± 43 | ref. | ref. |
| Positive (n=183) | 839 ± 277 | −2 (−67, 62) | 3 (−62, 68) | 16 ± 65 | 4 (−13, 20) | 5 (−12, 22) |
| Hepatitis C status | ||||||
| Negative (n=169) | 825 ± 268 | ref. | ref. | 9 ± 61 | ref. | ref. |
| Positive (n=110) | 862 ± 246 | 38 (−25, 99) | 15 (−49, 79) | 24 ± 55 | 15 (−1,31) | 14 (−2, 31) |
| CVD risk score§ | - | - | 76 (47, 105)† | - | - | 2 (1, 4)‡ |
OR, odds ratio; CI, confidence interval; ref., reference group; IMT, intima-media thickness; sd, standard deviation
Adjusted models include all variables shown
P < 0.001
P < 0.05 and P ≥ 0.001
ACC/AHA CVD risk score25 is the predicted 10-year risk of cardiovascular disease derived from an equation that includes age, sex, race, total cholesterol, HDL cholesterol, diabetes, systolic blood pressure, smoking status, and use of antihypertensive medication. Point estimates are expressed per 5 percentage point increase in the risk score.
Forty-three participants (15%) had albuminuria at baseline, and albuminuria progression - defined as a urine albumin-creatinine ratio during follow-up that was >30/mg/g and at least 2-fold higher than the baseline value - was detected in 14 (33%) and 57 (23%) of those with and without albuminuria at baseline, respectively (P=0.18 for difference). HIV-positive status was independently associated with an increased risk of both baseline albuminuria and with albuminuria progression, while cocaine use categories and HCV had no significant associations with albuminuria or albuminuria progression in models that included all 3 non-traditional risk factors and ACC/AHA CVD risk scores (Table 3). In a supplemental modeling approach using individual components of the ACC/AHA CVD risk score rather than the score itself, results were similar, however HIV status no longer was significantly associated with baseline albuminuria (OR 1.8 [0.7, 4.4], Table III in the online-only Data Supplement). HIV did remain significantly associated with albuminuria progression in the supplemental model.
Table 3.
Associations of cocaine use, HIV, and hepatitis C with baseline albuminuiria and albuminuria progression among 292 participants, Baltimore, Maryland
| Presence of albuminuria at baseline |
Albuminuria progression |
|||||
|---|---|---|---|---|---|---|
| Risk factor | Frequency (%) |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
Frequency (%) |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
| Cocaine use | ||||||
| Never (n=57) | 5 (9) | ref. | ref. | 11 (19) | ref. | ref. |
| Past (n=82) | 15 (18) | 2.3 (0.8, 6.8) | 1.7 (0.6, 5.2) | 16 (20) | 1.0 (0.4, 2.4) | 0.7 (0.3, 1.7) |
| Current (n=153) | 23 (15) | 1.8 (0.7, 5.1) | 1.3 (0.5, 3.9) | 44 (29) | 1.7 (0.8, 3.6) | 1.2 (0.5, 2.6) |
| HIV status | ||||||
| Negative (n=100) | 8 (8) | ref. | ref. | 14 (14) | ref. | ref. |
| Positive (n=192) | 35 (18) | 2.6 (1.1, 5.8)† | 2.4 (1.0, 5.6)† | 57 (30) | 2.6 (1.4, 4.9)† | 2.5 (1.3, 4.8)† |
| Hepatitis C status | ||||||
| Negative (n=177) | 22 (12) | ref. | ref. | 36 (20) | ref. | ref. |
| Positive (n=115) | 21 (18) | 1.6 (0.8, 3.0) | 1.2 (0.6, 2.4) | 35 (30) | 1.7 (1.0, 2.9) | 1.4 (0.8, 2.5) |
| CVD risk score‡ | - | - | 1.2 (0.9, 1.6) | - | - | 1.1 (0.8, 1.4) |
OR, odds ratio; CI, confidence interval; ref., reference group
Adjusted models include all variables shown.
P < 0.05
ACC/AHA CVD risk score25 is the predicted 10-year risk of cardiovascular disease derived from an equation that includes age, sex, race, total cholesterol, HDL cholesterol, diabetes, systolic blood pressure, smoking status, and use of antihypertensive medication. Point estimates are expressed per 5 percentage point increase in the risk score.
Over the course of follow-up, 288 participants (99%) underwent 975 technically adequate PWV measurements in 1038 study visits (94%), with a median (P25, P75) of 4 (3, 4) measures per participant. The median (P25, P75) PWV at baseline was 8.2 m/s (7.1, 9.6). In a model accounting for correlations in repeated measures from individual participants and adjusted for all 3 non-traditional risk factors and ACC/AHA CVD risk scores, we found that HIV-positive status was significantly associated with higher PWV, while HCV was not associated with PWV (Table 4). Unexpectedly, we found that cocaine use (both past and current) was associated with lower PWV values, and for current cocaine users this association was statistically significant in the fully adjusted model (−57 cm/s versus never users, 95% CI, −107, −7). In a supplemental modeling approach using individual components of the ACC/AHA CVD risk score rather than the score itself, current cocaine use was not significantly associated with PWV (Table IV in the online-only Data Supplement). A total of 137 participants (48%) with one or more valid PWV measures had at least one value > 9.6 m/s. Analysis of PWV as a binary variable at this cutoff produced similar results to the analysis of PWV as a continuous variable (data not shown).
Table 4.
Associations of HIV, cocaine use, and hepatitis C with aortic pulse wave velocity among 288 participants, Baltimore, Maryland
| Risk factor | Unadjusted difference, cm/s (95% CI) |
Adjusted* difference, cm/s (95% CI) |
|---|---|---|
| Cocaine use | ||
| Never | ref. | ref. |
| Past | 0 (−55, −55) | −37 (−92, 18) |
| Current | −21 (−70, 28) | −57 (−107, −7)† |
| HIV status | ||
| Negative | ref. | ref. |
| Positive | 31 (−8, 70) | 45 (6, 84)† |
| Hepatitis C status | ||
| Negative | ref. | ref. |
| Positive | 26 (−12, 64) | 20 (−17, 57) |
| CVD risk score‡ | - | 46 (28, 64)§ |
ref., reference group
Adjusted model includes all variables shown.
P < 0.05 and P ≥ 0.001
ACC/AHA CVD risk score25 is the predicted 10-year risk of cardiovascular disease derived from an equation that includes age, sex, race, total cholesterol, HDL cholesterol, diabetes, systolic blood pressure, smoking status, and use of antihypertensive medication. Point estimates are expressed per 5 percentage point increase in the risk score.
P < 0.001
To further explore these findings we assessed systolic blood pressure at the time of PWV measurements. As anticipated, blood pressure was positively correlated with PWV, with each 10 mm Hg increase in mean arterial blood pressure associated with a 55 cm/s (95% CI, 44, 65, P<0.001) increase in PWV. However, there were no associations of cocaine use or HCV status with mean arterial blood pressure (data not shown). HIV-positive participants had significantly lower adjusted mean arterial blood pressure than HIV-negative participants (adjusted difference −4 mm Hg, 95% CI, −6, −1). There was no evidence of effect modification among HIV infection, cocaine use, and HCV infection with any of the subclinical CVD markers assessed. Average outcome measurement data by study visit are shown in Table V of the online-only Data Supplement.
Discussion
In this unique longitudinal study, we found evidence that HIV infection, cocaine use, and HCV infection were independently associated with subclinical CVD in a sample of middle-aged, predominantly African-American individuals. In adjusted models, HIV disease was independently associated with carotid plaque progression, albuminuria, albuminuria progression, and PWV. Cocaine use was associated with an approximately 3-fold higher odds of carotid plaque at baseline, and HCV infection was significantly associated with a higher risk of carotid plaque progression. In analyses of longitudinal changes in subclinical CVD markers, we did not find evidence that current cocaine use affected indicator trajectories differently than past cocaine use.
Our finding should be interpreted in light of the study’s strengths and weaknesses. Strengths include a longitudinal design, high follow-up rates, and three well-validated measures of subclinical CVD. The markers of interest - carotid plaque/IMT, albuminuria, and PWV - were modestly correlated with one another, although each was strongly associated with ACC/AHA CVD risk score, suggesting that these different markers may capture different aspects of CVD. Additional study strengths include characterization of HCV status on the basis of detectable viremia, and use of urine toxicology to validate self-reported cocaine use. Urine drug testing led to reclassification of 33% and 40% of self-reported never users and past users, respectively, to current user status. Study weaknesses include a relatively small sample size and only 3 years of longitudinal follow-up. Our study participants were predominantly African American, reflecting local demographics, and our findings may not extrapolate to other racial groups.
Among the non-traditional risk factors assessed, we found the most consistent evidence for an association between HIV status and subclinical CVD. In our study, HIV status had independent associations with all three subclinical CVD markers. This finding accords with studies linking HIV infection to an increased risk for CVD events8, 11, 12 and to a higher prevalence of subclinical CVD markers.1, 2, 4, 9 Although the mechanism underlying the association of HIV infection with CVD risk is incompletely elucidated, a high prevalence of traditional CVD risk factors in the population, adverse effects of antiretroviral drugs,27 inflammation, and immune activation have all been posited as contributing factors.28, 29
We found that cocaine use (both past and current) was strongly associated with the presence of carotid plaque at baseline and with plaque progression in the unadjusted model, although not in the fully adjusted model. This finding supports data from Lai and colleagues, who found strong associations between chronic cocaine use, coronary calcium, and coronary plaque30, 31 among African American participants. The acute sympathomimetic effects of cocaine use, including myocardial infarction, are well characterized,32 but evidence also suggests chronic irreversible vascular damage from cocaine use.33, 34
A unique aspect of our study was the ability to distinguish past and current cocaine users with biological validation. We did not, however, find evidence of more rapid progression of CVD surrogates in current versus past users. In fact, point estimates for these two groups, relative to never users, were similar in most analyses. Similarly, one small study that used contingency management to reduce cocaine use in chronic users found that cocaine abstinence was associated with significantly reduced serum endothelin-1 concentrations, but not with the incidence of coronary plaque progression.35 These data imply that cocaine use may initiate a trajectory of CVD that, at some point, progresses independently of continued cocaine use. It should also be noted that our ability to distinguish small longitudinal differences in past and current cocaine users was limited by relatively short follow-up.
Unexpectedly, we found a protective association between current cocaine use and PWV. This finding may be spurious, as the association was non-significant in univariate analysis, but became statistically significant in the adjusted model. Moreover, the association of current cocaine use with PVW was not statistically significant in a supplemental modeling approach where we adjusted models for individual components of the ACC/AHA CVD risk, rather than the risk score itself. One small Australian study found cocaine use to be associated with increased aortic PWV.36 That study measured PWV with cardiac and aortic magnetic resonance imaging and we used applanation tonometry for PWV measurement, limiting the ability to make direct comparisons.
The evidence of an independent association between HCV and subclinical CVD was relatively weaker than the associations of HIV and cocaine use with CVD. Although, HCV had moderate associations with several CVD indicators in univariate analyses, HCV retained statistical significance only with carotid plaque progression in multivariate analysis. This association did not retain statistical significance in a supplemental modeling approach, although the point estimate was similar to that in the primary analysis. An important caveat is that our study was not sufficiently powered to assess modest associations between HCV and subclinical CVD, consistent with the effect sizes reported in large observational studies.3, 7, 37 However, our study highlights the importance of considering drug use and other behavioral factors as confounders of the observed relationship between HCV and CVD, factors which may be difficult to characterize in administrative databases.
A final consideration is the incongruity between associations assessed with carotid plaque and those assessed with carotid IMT. HIV infection, cocaine use, and HCV infection all had significant independent associations with carotid plaque, but none had significant associations with carotid IMT or change in IMT, and most point estimates approximated the null. Of note, our plaque assessments included the common, bifurcation, and internal carotid artery segments on both the right and left, whereas IMT measurements were acquired only in the right carotid artery. However, there has also been substantial variability in the findings across studies focused on HIV and HCV associations with carotid IMT.28, 38 Different imaging protocols may account for some inconsistencies, and one study suggested that HIV- and inflammation-related progression of carotid disease may be more marked at the carotid bifurcation than in the common or internal carotid arteries, due to lower shear stress at the bifurcation.39 Indeed, carotid plaque is more prevalent at the bifurcation and internal carotid than in the common carotid39, as we observed. Carotid plaque consistently has been shown to be a better predictor of future CVD events than carotid IMT.19, 40
This study assessed independent associations of HIV infection, cocaine use, and HCV infection with validated surrogate CVD markers. We found HIV infection to be independently associated with carotid plaque progression, albuminuria, albuminuria progression, and PWV, whereas cocaine use was associated with carotid plaque at baseline, and HCV was associated with carotid plaque progression. These results suggest that HIV, cocaine use and HCV are important non-traditional risk factors for CVD in some populations and highlight the need to understand the distinct and overlapping mechanisms of the associations.
Supplementary Material
Highlights.
Human immunodeficiency virus (HIV) infection, cocaine use, and hepatitis C virus (HCV) infection are overlapping epidemics in many US cities.
This study prospectively assessed the independent associations of these “non-traditional” risk factors with well-validated cross-sectional and longitudinal markers of subclinical cardiovascular disease (CVD).
We found strong evidence that HIV, cocaine use, and HCV infection were independently associated with subclinical CVD. There was no evidence of effect modification among these factors.
Compared with never use, past and current cocaine use had similar associations with longitudinal CVD markers. This suggests that adverse effects from cocaine use were relatively irreversible in this middle-aged cohort.
Acknowledgments
We want to thank the study participants.
Sources of funding
This project was supported by the National Institute on Drug Abuse (R01 DA026770, K24 DA035684). Other support was provided by the National Institute of Diabetes and Digestive and Kidney Disease (P01DK056492), Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by grant number UL1-TR000424 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH, and by the Johns Hopkins Center for AIDS Research (P30 AI094189). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviation
- HIV
human immunodeficiency virus
- HCV
hepatitis C virus
- CVD
cardiovascular disease
- IMT
intima-media thickness
- PWV
pulse wave velocity
- eGFR
estimated glomerular filtration rate
- ACC/AHA
American College of Cardiology/American Heart Association
- ACR
albumin-creatinine ratio
Footnotes
Disclosures
Dr. Stein received royalties from the Wisconsin Alumni Research Foundation for a patent related to carotid ultrasound and arterial age.
References
- 1.Post WS, Budoff M, Kingsley L, Palella FJ, Jr, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between hiv infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, Williams KC, Lo J, Grinspoon SK. Arterial inflammation in patients with hiv. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang CC, Skanderson M, et al. The risk of incident coronary heart disease among veterans with and without hiv and hepatitis c. Circ Cardiovasc Qual Outcomes. 2011;4:425–432. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, Waters DD. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with hiv infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 5.Lai S, Lima JA, Lai H, Vlahov D, Celentano D, Tong W, Bartlett JG, Margolick J, Fishman EK. Human immunodeficiency virus 1 infection, cocaine, and coronary calcification. Arch. Intern Med. 2005;165:690–695. doi: 10.1001/archinte.165.6.690. [DOI] [PubMed] [Google Scholar]
- 6.Ishizaka N, Ishizaka Y, Takahashi E, Tooda E, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis c virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002;359:133–135. doi: 10.1016/s0140-6736(02)07339-7. [DOI] [PubMed] [Google Scholar]
- 7.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis c virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–232. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freiberg MS, Chang CC, Kuller LH, et al. Hiv infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, Tien PC, Shlipak MG, Sidney S, Polak JF, O'Leary D, Bacchetti P, Kronmal RA. Preclinical atherosclerosis due to hiv infection: Carotid intima-medial thickness measurements from the fram study. AIDS. 2009;23:1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKibben RA, Haberlen SA, Post WS, Brown TT, Budoff M, Witt MD, Kingsley LA, Palella FJ, Jr, Thio CL, Seaberg EC. A cross-sectional study of the association between chronic hepatitis c virus infection and subclinical coronary atherosclerosis among participants in the multicenter aids cohort study. J Infect Dis. 2016;213:257–265. doi: 10.1093/infdis/jiv396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, Gerstoft J. Ischemic heart disease in hiv-infected and hiv-uninfected individuals: A population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 12.Klein D, Hurley LB, Quesenberry CP, Jr, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with hiv-1 infection? J Acquir Immune Defic Syndr. 2002;30:471–477. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Maia-Leite LH, Catez E, Boyd A, Haddour N, Curjol A, Lang S, Nuernberg M, Duvivier C, Desvarieux M, Kirstetter M, Girard P-M, Cohen A, Boccara F. Aortic stiffness aging is influenced by past profound immunodeficiency in hiv-infected individuals: Results from the evas-hiv (evaluation of aortic stiffness in hiv-infected individuals) Journal of Hypertension. 2016;34:1338–1346. doi: 10.1097/HJH.0000000000000957. [DOI] [PubMed] [Google Scholar]
- 14.Tien PC, Schneider MF, Cole SR, Cohen MH, Glesby MJ, Lazar J, Young M, Mack W, Hodis HN, Kaplan RC. Association of hepatitis c virus and hiv infection with subclinical atherosclerosis in the women's interagency hiv study. AIDS. 2009;23:1781–1784. doi: 10.1097/QAD.0b013e32832d7aa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Data Collection on Adverse Events of Anti HIVDSG. Weber R, Sabin C, et al. Hbv or hcv coinfections and risk of myocardial infarction in hiv-infected individuals: The d:A:D cohort study. Antivir Ther. 2010;15:1077–1086. doi: 10.3851/IMP1681. [DOI] [PubMed] [Google Scholar]
- 16.Currier JS, Kendall MA, Henry WK, Alston-Smith B, Torriani FJ, Tebas P, Li Y, Hodis HN. Progression of carotid artery intima-media thickening in hiv-infected and uninfected adults. AIDS. 2007;21:1137–1145. doi: 10.1097/QAD.0b013e32811ebf79. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 18.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the american society of echocardiography carotid intima-media thickness task force. Endorsed by the society for vascular medicine. J. Am. Soc. Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR, Liu K, Kaufman J, Stein JH. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espeland MA, O'Leary DH, Terry JG, Morgan T, Evans G, Mudra H. Carotid intimal-media thickness as a surrogate for cardiovascular disease events in trials of hmg-coa reductase inhibitors. Curr Control Trials Cardiovasc Med. 2005;6:3. doi: 10.1186/1468-6708-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidney disease: Improving global outcomes (kdigo) ckd work group. Kdigo 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 22.Ritz E. Albuminuria and vascular damage--the vicious twins. N. Engl. J Med. 2003;348:2349–2352. doi: 10.1056/NEJMe030066. [DOI] [PubMed] [Google Scholar]
- 23.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 24.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, Appleyard M, Jensen JS. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 25.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 26.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 acc/aha guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with hiv infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: The data collection on adverse events of anti-hiv drugs (d:A:D) study. J Infect Dis. 2010;201:318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 28.Stein JH, Currier JS, Hsue PY. Arterial disease in patients with human immunodeficiency virus infection: What has imaging taught us? JACC Cardiovasc Imaging. 2014;7:515–525. doi: 10.1016/j.jcmg.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in hiv-infected adults. J Infect Dis. 2012;205(Suppl 3):S375–S382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai H, Moore R, Celentano DD, Gerstenblith G, Treisman G, Keruly JC, Kickler T, Li J, Chen S, Lai S, Fishman EK. Hiv infection itself may not be associated with subclinical coronary artery disease among african americans without cardiovascular symptoms. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai SH, Fishman EK, Lai H, Moore R, Cofrancesco J, Pannu H, Tong WJ, Du JF, Bartlett J. Long-term cocaine use and antiretroviral therapy are associated with silent coronary artery disease in african americans with hiv infection who have no cardiovascular symptoms. Clin. Infect. Dis. 2008;46:600–610. doi: 10.1086/526782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351–358. doi: 10.1056/NEJM200108023450507. [DOI] [PubMed] [Google Scholar]
- 33.Fine DM, Garg N, Haas M, Rahman MH, Lucas GM, Scheel PJ, Atta MG. Cocaine use and hypertensive renal changes in hiv-infected individuals. Clin J Am Soc Nephrol. 2007;2:1125–1130. doi: 10.2215/CJN.02450607. [DOI] [PubMed] [Google Scholar]
- 34.Saez CG, Olivares P, Pallavicini J, Panes O, Moreno N, Massardo T, Mezzano D, Pereira J. Increased number of circulating endothelial cells and plasma markers of endothelial damage in chronic cocaine users. Thromb Res. 2011;128:e18–e23. doi: 10.1016/j.thromres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Lai H, Stitzer M, Treisman G, Moore R, Brinker J, Gerstenblith G, Kickler TS, Li J, Chen S, Fishman E, Lai S. Cocaine abstinence and reduced use associated with lowered marker of endothelial dysfunction in african americans: A preliminary study. J Addict Med. 2015;9:331–339. doi: 10.1097/ADM.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozor R, Grieve SM, Buchholz S, Kaye S, Darke S, Bhindi R, Figtree GA. Regular cocaine use is associated with increased systolic blood pressure, aortic stiffness and left ventricular mass in young otherwise healthy individuals. PLoS One. 2014;9:e89710. doi: 10.1371/journal.pone.0089710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis c virus coinfection and the risk of cardiovascular disease among hiv-infected patients. HIV Med. 2010;11:462–468. doi: 10.1111/j.1468-1293.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 38.Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. Hiv positivity, protease inhibitor exposure and subclinical atherosclerosis: A systematic review and meta-analysis of observational studies. Heart. 2009;95:1826–1835. doi: 10.1136/hrt.2009.177774. [DOI] [PubMed] [Google Scholar]
- 39.Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC, Maka K, Martin JN, Ganz P, Deeks SG. Carotid intima-media thickness progression in hiv-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc. 2012;1 doi: 10.1161/JAHA.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.