Abstract
Introduction / Objectives
Malignancy is a major cause of death in patients with inflammatory disease. The risk of individual malignancies is altered in some inflammatory diseases, such as Rheumatoid Arthritis and Psoriasis. This study aimed to examine malignancy incidence in patients with psoriatic arthritis (PsA), a related inflammatory disease.
Method
Institutional cancer registry and medical record linkage systems were retrospectively reviewed in a population-based incidence cohort of 217 patients with PsA and 434 age/sex matched comparators. Malignancy rates were compared using adjusted Cox models.
Results
Incidence of overall malignancy (excluding NMSC; hazard ratio [HR]:1.64; 95% confidence interval [CI]:1.03-2.61) and breast cancer (HR: 3.59; 95% CI: 1.22-10.61), but not NMSC (HR: 1.23; 95% CI: 0.72-2.09), were significantly elevated in the PsA cohort. Age and female sex were similar predisposing risk factors in both cohorts.
Conclusions
The overall incidence of malignancy, as well as the risk of breast cancer, was higher in patients with PsA than in the general population.
Keywords: Psoriatic arthritis, malignancy, epidemiology, spondylarthropathies
INTRODUCTION
The overall risk of malignancy and specific malignancy bias is altered in some chronic inflammatory diseases. Patients with rheumatoid arthritis (RA) have an increased risk of lymphoma and lung cancer [1,2]. Similarly, patients with psoriasis have an increased risk of non-melanoma skin cancers (NMSC) and T cell lymphomas [3,4]. The risk of malignancy in the related psoriatic arthritis (PsA) is understudied and knowledge of this risk is important because malignancy is a prominent comorbidity and cause of death in PsA [5].
This study aimed to determine the overall and site-specific malignancy risk in a population-based cohort of patients with PsA in comparison to a sex and age matched non-PsA cohort from the same geographic area. Hematologic malignancies and NMSC were specifically investigated as cancers that are of particular interest in this population.
MATERIALS AND METHODS
Study Design
This retrospective, population-based cohort study of cancer incidence in PsA and comparator cohorts was approved by the Mayo Clinic (#14-009917) and Olmsted Medical Center (013-OMC-15) Institutional Review Boards and included data from the Rochester Epidemiology Project (REP) resources and Mayo Clinic Cancer Registry [6].
A previously assembled incidence cohort of 217 patients with PsA [7,8] was used. Briefly, this included all adult (≥18 years of age) residents of Olmsted County, Minnesota first diagnosed with PsA between January 1, 1970 and December 31st, 2008. All patients with PsA were previously verified [7] to fulfill the Classification of Psoriatic Arthritis (CASPAR) criteria [9]. Data on family history of psoriasis, inflammatory joint pain at diagnosis, enthesitis, spine involvement, psoriatic nail dystrophy, rheumatoid factor status, and dactylitis were also collected previously by manual record review. A comparison cohort of 434 subjects included 2 randomly chosen age and sex matched subjects for each patient with PsA from Olmsted County residents without PsA. Malignancy information was collected in relation to the PsA patient’s date of PsA diagnosis and followed until death, migration from Olmsted County or December 31st, 2014.
Cancer Diagnosis
Cancer diagnoses were retrieved from medical charts (NMSC) and the Mayo Clinic Cancer Registry (all other malignancies) using a standardized abstraction form. Cancer categories included: head/neck, gastric, pancreatic, liver, colon/rectal, other digestive, lung, other thorax, bone, soft tissue, melanoma, NMSC, breast (female-only), ductal carcinoma in situ, ovarian, other gynecologic, prostate, kidney, bladder, other genitourinary, ophthalmic, central nervous system, lymphoma, leukemia, multiple myeloma, myeloproliferative syndrome, myelodysplastic syndrome and other.
Statistical Analysis
Descriptive statistics were used to summarize demographic data. The proportion of patients with malignancies prior to PSA incidence/index date was compared using Fisher’s exact tests. The cumulative incidence of malignancy adjusted for the competing risk of death was estimated [10]. Cumulative incidence rates were not reported for malignancy sites with fewer than 5 events per cohort. Patients with malignancy at a particular site prior to PsA incidence/index date were excluded from the analyses of malignancy development of that site. Cox proportional hazards models were used to compare the rate of malignancy development, both overall and by site, between patients with PsA and the non-PsA comparison cohort. Among patients with PsA, disease characteristics were assessed individually as potential risk factors for malignancy using Cox models adjusted for age, sex and calendar year of PsA incidence. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Clinical Characteristics
The mean age at PsA diagnosis (217 subjects diagnosed by CASPAR criteria) was 43.9 years (standard deviation [SD] 14.2). The comparator cohort (434 age / sex matched comparison subjects) had a mean age of 44.0 years at index date (SD 14.2, p=0.89). Both cohorts were 60% male with similar follow-up time (mean 15.3 years in PsA and 15.4 years in non-PsA).
Observed Cancer Incidence Prior to PsA Diagnosis
. A total of 11 (5%) and 25 (6%) patients with at least 1 malignancy diagnosis in the PsA and comparator cohort, respectively (p=0.86) including cases of NMSC (3 [1%] in PsA vs 11 [3%] in non-PsA; p=0.41) and breast cancer in females (2 [1%] in PsA and 6 [1%] in non-PsA; p=0.72). No individual cancer site was significantly different between the cohorts prior to PsA incidence/ index date.
Observed Cancer Incidence Following PsA Diagnosis
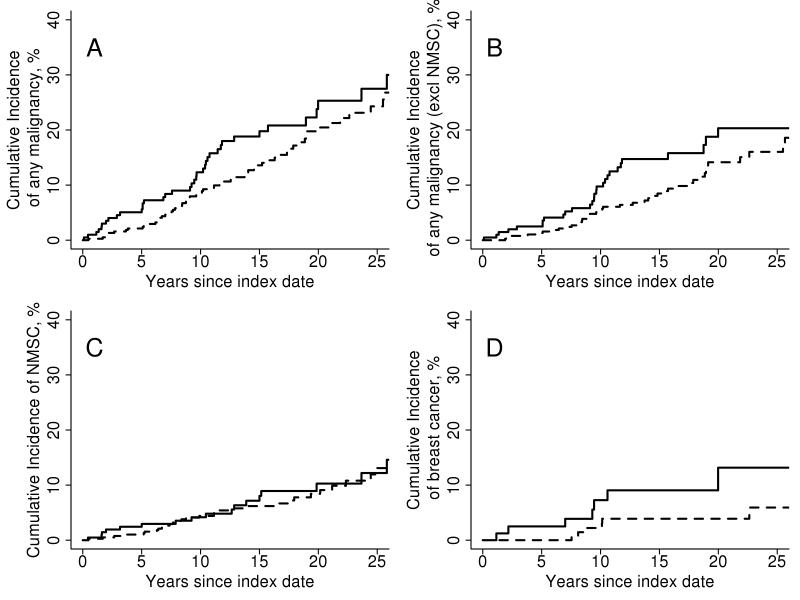
There were 43 patients in the PsA cohort who were diagnosed with at least 1 malignancy during follow-up compared to 70 patients in the comparator cohort. The cumulative incidence of malignancy at 10 years was 12.3% (±2.5%) in the PsA cohort and 8.6 (±1.5) in the non-PsA cohort (Figure 1A), representing a marginally increased risk of malignancy in the PsA cohort (hazard ratio [HR]: 1.41; 95% confidence interval [CI]: 0.96, 2.07 adjusted for age, sex and index calendar year; Table 1). When NMSC was excluded, the risk of malignancy was significantly increased in the PsA cohort (HR: 1.64; 95% CI: 1.03, 2.61; Figure 1B). Considered separately, there was no significant difference in risk of hematologic or solid cancers (HR: 1.48; 95% CI: 0.89, 2.48 and HR: 2.48; 95% CI: 0.75, 8.13, respectively).
Figure 1. Cumulative incidence of malignancy among psoriatic arthritis patients and non-psoriatic arthritis comparators subjects.
Rates of malignancy: A. Overall including non-melanoma skin cancers, B. Overall excluding non-melanoma skin cancers. C. Non-melanoma skin cancers alone. D. Breast cancers in female patients in the psoriatic arthritis cohort (solid line) and comparator cohort (dashed line).
Table 1.
Cumulative incidence rate of malignancy within the first 10 years after diagnosis in 217 patients with psoriatic arthritis (PsA) compared to 434 subjects without PsA
| Malignancy Site* | Number of events after incidence in PsA/index in non-PsA |
Cumulative incidence at 10 years for PsA patients (± SE) |
Cumulative incidence at 10 years for non-PsA subjects (± SE) |
Hazard ratio (95% confidence interval) |
|---|---|---|---|---|
| Any malignancy (including NMSC) |
43/70 | 12.3 ± 2.5 | 8.6 ± 1.5 | 1.41 (0.96, 2.07) |
| Any malignancy (excluding NMSC) |
30/45 | 9.7 ± 2.2 | 5.1 ± 1.2 | 1.64 (1.03, 2.61) |
| Solid | 24/39 | 8.0 ± 2.1 | 4.2 ± 1.1 | 1.48 (0.89, 2.48) |
| Hematologic | 5/6 | 1.6 ± 0.9 | 0.8 ± 0.5 | 2.48 (0.75, 8.13) |
| NMSC | 22/36 | 4.2 ± 1.5 | 4.4 ± 1.1 | 1.23 (0.72, 2.09) |
| Breast (female only) |
8/6 | 7.3 ± 3.2 | 2.2 ± 1.3 | 3.59 (1.22, 10.61) |
| Prostate (male only) |
9/11 | 5.5 ± 2.2 | 1.8 ± 0.9 | 1.83 (0.75, 4.46) |
No malignancies were observed in these sites: liver, other thorax, bone, ovary, other genitourinary, ophthalmologic, multiple myeloma, myeloproliferative syndrome, myelodysplastic syndrome. Comparisons were not performed for the following sites with fewer than 5 malignancies per cohort (number of events after incidence in PsA/ index in non-PsA): head/neck (2/3), gastric (1/1), pancreatic (1/1), colon/rectal (1/3), other digestive (0/4), lung (2/9), soft tissue (0/1), melanoma (1/1), DCIS (1/1), Other gynecological (1/2), kidney (0/1), bladder (0/1), central nervous system (2/1), lymphoma (2/4), leukemia (4/1) and other (1/1).
Abbreviations: NMSC=non-melanoma skin cancer; DCIS=ductal carcinoma in situ; SE=standard error
There was also no evidence of a difference in incidence between the two cohorts in most individual sites of cancers. Of all malignancy sites analyzed, only breast cancer was statistically more frequent in the PsA cohort. In 86 females with PsA, there were eight cases of breast cancer during follow-up, compared to only six cases in the corresponding 172 comparator females (Figure 1D and Table 2, HR: 3.59, 95% CI: 1.22, 10.61). The incidence of NMSC was not different between the two cohorts (HR: 1.23; 95% CI: 0.72-2.09; Figure 1C).
Table 2.
Multivariate risk factor analysis for cancer incidence in 217 patients with psoriatic arthritis (PsA)
| N (%) or | Hazard ratio | ||
|---|---|---|---|
| Characteristic | mean (SD) | (95% CI) | P value |
| Age, per 1 year increase | 44.0 (±14,2) | 1.08 (1.05, 1.11) | <0.001 |
| Female sex | 86 (40%) | 2.17 (1.05, 4.48) | 0.037 |
| Calendar year of PsA incidence | 1994 (± 9) | 0.98 (0.94, 1.02) | 0.320 |
| Inflammatory joint pain present | 190 (88%) | 0.85 (0.19, 3.77) | 0.829 |
| Enthesitis PsA | 74 (34%) | 1.10 (0.46, 2.60) | 0.830 |
| Spine PsA | 17 (8%) | 1.35 (0.31, 5.80) | 0.687 |
| Family history of psoriasis | 52/123 (42%) | 0.92 (0.25, 3.40) | 0.901 |
| Psoriatic nail dystrophy | 88 (41%) | 0.52 (0.22, 1.24) | 0.142 |
| Negative rheumatoid factor | 178/182 (98%) | -- | 0.991 |
| Dactylitis | 102 (47%) | 0.66 (0.31, 1.39) | 0.270 |
Demographic characteristics were assessed as possible predisposing factors for cancer development in patients with PsA, but most were not associated with cancer development (Table 2). As expected, cancer risk increased with age (HR 1.08 per 1 year increase; 95% CI: 1.05-1.11, p<0.001), but was not different from age-related risk in the non-PsA cohort (interaction p=0.39). There was no effect of calendar year of diagnosis (HR 0.98 per 1 year increase; 95% CI: 0.94-1.02) on the incidence of cancer, indicating no statistically significant change in cancer incidence in PsA over time. This effect of calendar time was consistent between the two cohorts (interaction p=0.53) as was the time since diagnosis (interaction p=0.29). Females had an increased risk of overall incidence of cancer in the PsA (HR 2.17, 95% CI: 1.05-4.48, p=0.037), and non-PsA (interaction p=0.17) cohorts.
DISCUSSION
In this population-based age and sex matched cohort study, there was a 64% and 41% increased incidence of overall malignancy risk following diagnosis of PsA excluding and including NMSC, respectively, as well an increased risk of breast cancer.
A similar study from the University of Toronto Psoriatic Arthritis Clinic compared cancer incidence in 665 patients with PsA to the historical Toronto malignancy incidence [11], reporting a standard incidence ratio (SIR) of 0.98 (95% CI 0.77-1.24) for all malignancy. Both studies had at least a trending increase in female breast cancer risk (Toronto study SIR 1.55 95% CI 0.92-2.62). However, the incidence of NMSC was not examined in the Toronto study, so the current report represents new findings about NMSC.
Interestingly, these findings do not parallel those seen in RA, such as the increases in lymphoma and lung cancer risk [11,12], or those seen in psoriasis, including an increased risk of T cell lymphoma and NMSC [3,4]. This could reflect different root pathologies or treatment practices between these three inflammatory conditions. For example, ultra-violet light therapy might be more frequently used in localized psoriasis, leading to an increased risk of NMSC, whereas systemic treatments are often employed in PsA, obviating this risk.
This is the first comparison of malignancy incidence in patients with PsA to that of a specific geographically-defined age and sex matched cohort of patients without PsA. Previous studies have reported cancer prevalence in patients with PsA, but mostly within therapeutic clinical trials without comparison to the general population. This study benefitted from a comprehensive medical record system which allowed selection of a geographically similar age and sex matched cohort which controls for additional risk factors not accounted for in historical comparisons.
This is a retrospective study with inherent reporting biases and fewer patients than some previous studies. The Olmsted County population is predominantly Caucasian, so results may also not extend to other populations. Increased clinical surveillance and confounding by therapeutic intervention may have affected the results.
Although this study is strictly epidemiologic and does not establish a causal relationship, knowledge about potential increased malignancy risk is clinically useful. Numerous studies have reported on malignancy risk associated with immunologically active medications [1,12-14]. Properly defining cancer incidence in patients with PsA provides a reference point for examination of additional risk secondary to therapeutic regimens. Although the majority of the data includes subject cases with traditional treatments, there were no significant differences in cancer rates by decade of PsA diagnosis (HR 0.98; 95% CI: 0.94-1.02), indicating that the malignancy incidence conclusions likely represent risk under current treatment paradigms. The increased overall and breast malignancy incidence in this PsA cohort reflects an altered malignancy risk profile in patients with PsA.
Acknowledgments
This publication was made possible by the Rochester Epidemiology Project (R01 AG 034676 from the National Institutes of Health) and the CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). KMW was supported by the National Institutes of General Medical Sciences (grant T32-GM-65841) and the Mayo Clinic College of Medicine’s Medical Scientist Training Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors have no conflicts of interest.
REFERENCES
- 1.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50(6):1740–1751. doi: 10.1002/art.20311. doi:10.1002/art.20311. [DOI] [PubMed] [Google Scholar]
- 2.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212. doi: 10.1186/s13075-015-0728-9. doi:10.1186/s13075-015-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006;126(10):2194–2201. doi: 10.1038/sj.jid.5700410. doi:10.1038/sj.jid.5700410. [DOI] [PubMed] [Google Scholar]
- 4.Frentz G, Olsen JH. Malignant tumours and psoriasis: a follow-up study. Br J Dermatol. 1999;140(2):237–242. doi: 10.1046/j.1365-2133.1999.02655.x. [DOI] [PubMed] [Google Scholar]
- 5.Gladman DD. Mortality in psoriatic arthritis. Clin Exp Rheumatol. 2008;26(5 Suppl 51):S62–65. [PubMed] [Google Scholar]
- 6.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. doi:10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernste FC, Sanchez-Menendez M, Wilton KM, Crowson CS, Matteson EL, Maradit Kremers H. Cardiovascular risk profile at the onset of psoriatic arthritis: a population-based cohort study. Arthritis Care Res (Hoboken) 2015;67(7):1015–1021. doi: 10.1002/acr.22536. doi:10.1002/acr.22536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population-based study. J Rheumatol. 2009;36(2):361–367. doi: 10.3899/jrheum.080691. doi:10.3899/jrheum.080691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673. doi: 10.1002/art.21972. doi:10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 10.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Rohekar S, Tom BD, Hassa A, Schentag CT, Farewell VT, Gladman DD. Prevalence of malignancy in psoriatic arthritis. Arthritis Rheum. 2008;58(1):82–87. doi: 10.1002/art.23185. doi:10.1002/art.23185. [DOI] [PubMed] [Google Scholar]
- 12.Hellgren K, Smedby KE, Backlin C, Sundstrom C, Feltelius N, Eriksson JK, Baecklund E, Askling J. Ankylosing spondylitis, psoriatic arthritis, and risk of malignant lymphoma: a cohort study based on nationwide prospectively recorded data from Sweden. Arthritis Rheumatol. 2014;66(5):1282–1290. doi: 10.1002/art.38339. doi:10.1002/art.38339. [DOI] [PubMed] [Google Scholar]
- 13.Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64(6):1035–1050. doi: 10.1016/j.jaad.2010.09.734. doi:10.1016/j.jaad.2010.09.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanto M, Peragallo MS, Pietrosanti M, Di Rosa R, Picchianti Diamanti A, Salemi S, D'Amelio R. Risk of malignancy in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis under immunosuppressive therapy: a single-center experience. Intern Emerg Med. 2016;11(1):31–40. doi: 10.1007/s11739-015-1270-0. doi:10.1007/s11739-015-1270-0. [DOI] [PubMed] [Google Scholar]