Summary
Muscarinic receptors represent a promising therapeutic target for schizophrenia, but the mechanisms underlying the antipsychotic efficacy of muscarinic modulators are not well understood. Here we report that activation of M4 receptors on striatal spiny projection neurons results in a novel form of dopaminergic regulation resulting in a sustained depression of striatal dopamine release that is observed more than 30 minutes after removal of the muscarinic receptor agonist. Furthermore, both the M4-mediated sustained inhibition of dopamine release and the antipsychotic-like efficacy of M4 activators were found to require intact signaling through CB2 cannabinoid receptors. These findings highlight a novel mechanism by which striatal cholinergic and cannabinoid signaling leads to sustained reductions in dopaminergic transmission and concurrent behavioral effects predictive of antipsychotic efficacy.
Introduction
Currently available treatments for schizophrenia are often effective in ameliorating psychotic symptoms but also induce metabolic, cognitive, and motor side-effects, limiting their therapeutic utility. The seminal finding that the M1/M4-preferring muscarinic acetylcholine receptor (mAChR) agonist xanomeline demonstrated robust antipsychotic efficacy in schizophrenia patients (Shekhar et al., 2008) generated a major interest in developing selective M1 and M4 agonists and understanding the roles of these receptors in forebrain circuits (Foster et al., 2014a). Unfortunately, all known mAChR agonists are non-selective and activate peripheral M2 and M3 receptors, leading to adverse effects (Bymaster et al., 2003). By targeting allosteric sights on mAChRs, we and others have succeeded in identifying highly subtype-selective positive allosteric modulators (PAMs) of individual mAChR subtypes that avoid activation of peripheral mAChRs. Interestingly, highly selective M4 positive allosteric modulators (PAMs) have robust antipsychotic-like effects in multiple rodent models. While the mechanisms by which M4 PAMs exert their behavioral effects are not entirely clear, these compounds reverse multiple in vivo effects of psychomotor stimulants that induce increases in extracellular dopamine (DA; Bubser et al., 2014; Byun et al., 2014; Chan et al., 2008; Leach et al., 2010). These studies raise the possibility that M4 PAMs may act by inhibiting DA release from midbrain DA neurons. However, the hypothesis that selective M4 PAMs inhibit DA release has not been directly tested. Furthermore, while M4 can act as a presynaptic heteroreceptor to inhibit glutamate release in the striatum (Pancani et al., 2014), this receptor is not expressed in DA neurons and is not likely to inhibit DA release by direct actions on DA terminals. We now report that activation of M4 induces a sustained inhibition of electrically- or optically-induced DA release in midbrain slices that is potentiated by M4 PAMs and persists for more than 30 minutes after receptor activation. This sustained inhibition of DA release is independent of nicotinic receptor (nAChR) signaling, suggesting that it is not mediated by autoreceptors on cholinergic interneurons. Instead, use of genetically-modified mice revealed that this sustained inhibition of striatal DA release is mediated by M4 expressed on a subpopulation of striatal spiny projection neurons (SPNs) that express D1 DA receptors (D1-SPNs). Surprisingly, both the sustained inhibition of DA release and antipsychotic-like behavioral effects induced by an M4 PAM were found to require intact endocannabinoid (eCB)-mediated CB2 cannabinoid receptor signaling. Taken together, these studies identify a novel signaling pathway through which activation of M4 receptors on SPNs dampens dopaminergic signaling and highlights the importance of this pathway to the antipsychotic-like efficacy observed with M4 PAMs.
Results
mAChR activation induces a sustained inhibition of DA release that is independent of nAChR signaling
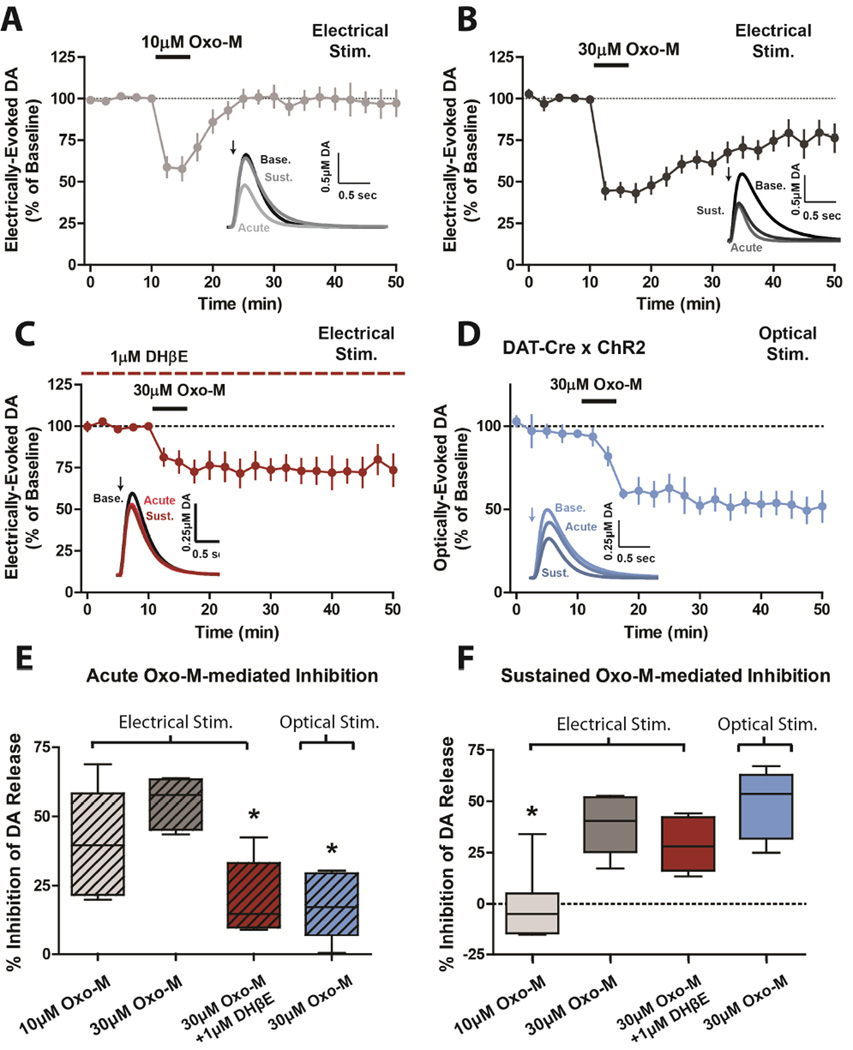
We determined the ability of mAChR activation to modulate striatal DA release by bath applying the non-selective mAChR agonist oxotremorine-M (Oxo-M) while monitoring electrically-evoked DA release via fast scan voltammetry (Figure S1A). Consistent with previous studies (Threlfell et al., 2010; Zhang et al., 2002), addition of 10µM Oxo-M caused a transient inhibition of DA release that returned to baseline within 10 minutes of removing Oxo-M from the bath (Figure 1A). However, at higher concentrations (30µM), Oxo-M induced a sustained inhibition of DA release that persisted long after (>30 minutes) drug washout (Figure 1B).
Figure 1.
mAChR activation induces a sustained reduction in DA release that is independent of nAChR signaling. Time-courses of Oxo-M-induced inhibition of DA release in the presence or absence of the nAChR antagonist DHβE using electrically-evoked (A–C) or optically-evoked (D) DA release paradigms. (E,F) Box plot summaries depicting the % inhibition of DA release observed under different conditions at acute (15 min) or sustained (30 min) time points (n=5–7; * Significant difference from 30µM Oxo-M, p<0.05; one-way ANOVA with a post hoc Dunnett’s test).
Previous studies have demonstrated that electrical stimulation of cholinergic afferents in the striatum can increase tonic DA release by activation of β2-containing nicotinic ACh receptors (nAChRs) on DA neuron terminals (Champtiaux et al., 2003). Furthermore, mAChR inhibition of DA release induced by electrical stimulation in striatal slices has been ascribed in large part to activation of mAChR autoreceptors on cholinergic terminals that inhibit acetylcholine (ACh) release and thereby inhibit nAChR-mediated increases in DA release (Threlfell et al., 2010). To assess the effects of mAChR activation on nAChR-independent DA release we pretreated the slices with the β2-selective nAChR antagonist dihydro-β-erythroidine hydrobromide (DHβE, 1µM). Pretreatment with DHβE completely blocked DA release induced by optical stimulation of cholinergic interneurons (Figure S1B) while partially blocking electrically-induced DA transients (Figure S1C). This suggests that this nAChR antagonist can completely inhibit nAChR-mediated increases in DA release as previously observed (Threlfell et al., 2012). Pretreatment with DHβE significantly reduced the Oxo-M induced inhibition of electrically-evoked DA release at early time points (Figure 1C,E). However, the Oxo-M-induced sustained inhibition was not significantly altered by DHβE pretreatment (Figure 1C,F), suggesting that this sustained inhibition of DA release is not mediated by inhibition of nAChR effects. To remove effects of electrical stimulation on cholinergic terminals and selectively activate DA terminals, we utilized mice that selectively express ChR2 in dopamine transporter (DAT)-expressing neurons (DAT-Cre × ChR2 mice). Optical stimulation in slices from DAT-Cre × ChR2 mice induced robust DA transients (Figure S1D). In these mice, application of 30µM Oxo-M had a blunted effect at early time points (Figure 1D,E), whereas the sustained inhibition at later time points was unchanged (Figure 1D,F). Importantly, inclusion of DHβE had no effect on optically-evoked DA transients or Oxo-M mediated regulation of optically-evoked DA release from DAT-Cre × ChR2 mice (Figure S1E). Collectively, these findings suggest that while the acute inhibition is largely dependent on nAChR activation, the sustained inhibition of DA release is independent of nAChR signaling.
The sustained inhibition of DA release induced by Oxo-M is mediated by M4 receptors expressed on D1-SPNs
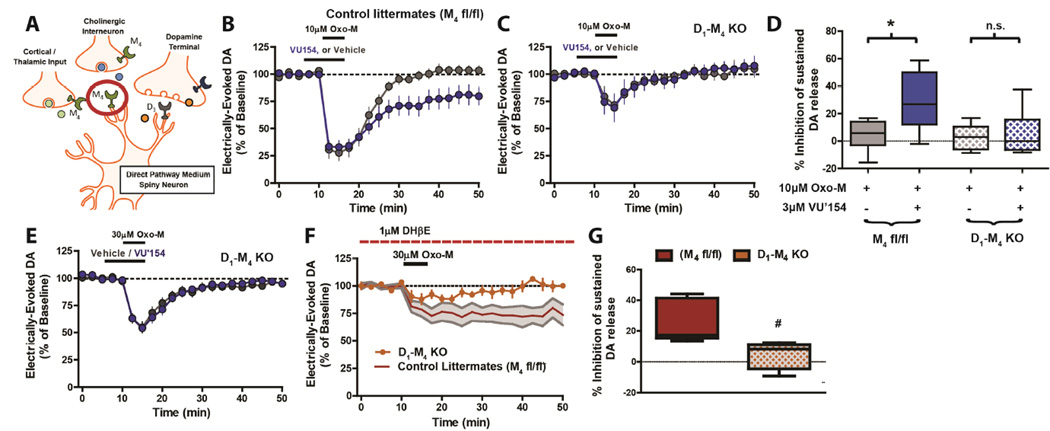
The M4 receptor is expressed in numerous neuronal populations in the striatum (Figure 2A). In order to determine the role of M4 in mediating the sustained inhibition of DA release observed with Oxo-M we utilized tissue specific knock-out mice in combination with the novel M4-selective PAM VU0467154. Consistent with its activity as a PAM, application of VU0467154 (3µM) had no effect on DA release when applied alone (Figure S1F); however, VU0467154 significantly potentiated the inhibition of DA release induced by 10µM Oxo-M at sustained time points (Figure 2B,D) suggesting a key role for M4 in mediating the sustained inhibition of DA release. However, M4 is not expressed in DA neurons (Weiner et al., 1990), suggesting that M4 is not likely to serve as a presynaptic heteroreceptor that directly inhibits DA release in DA terminals. Since this sustained effect of Oxo-M on DA release is independent of nAChR activation, it is also not likely to be mediated by activation of M4 autoreceptors on cholinergic terminals. The large majority of M4 in striatum is present on D1-SPNs (Ince et al., 1997), raising the possibility that this subpopulation of M4 could in some way modulate DA release. To assess this, we used mice in which M4 was selectively deleted from D1-expressing neurons (D1-M4 knockout (KO) mice). Electrically-evoked DA release was similar in magnitude in both D1-M4 KOs and control littermates (floxed M4 receptor mice; M4 fl/fl; data not shown). However, the acute inhibition observed upon application of 10µM Oxo-M was significantly reduced in the D1-M4 KO mice (28.2 ± 9.1% acute inhibition of DA release, n=6) compared to littermate controls (72.3 ± 7.1% % acute inhibition of DA release, n=6; p<0.05, two-tailed Mann-Whitney test), suggesting that M4 expressed on D1 neurons may, in part, contribute to the acute inhibition seen with Oxo-M. In the D1-M4 KO mice, the acute inhibition was further enhanced by increasing the Oxo-M concentration to 30µM (Figure 2E), suggesting that M4 expressed on D1-SPNs is not the only subpopulation of mAChRs contributing to this acute response. Importantly, VU0467154 had no effect on Oxo-M modulation of DA release in D1-M4 KO slices at any of the Oxo-M concentrations examined (Figure 2C–E). Furthermore, the sustained inhibition of DA release observed in littermate control mice after application of Oxo-M (30µM) in the presence of DHβE was completely absent in D1-M4 KO mice (Figure 2F,G). Collectively, these findings suggest that M4 on D1-SPNs contributes to the acute inhibition observed with application of mAChR agonist but is solely responsible for the sustained inhibition of DA signaling observed after mAChR activation.
Figure 2.
M4 expressed on spiny projection neurons mediate the muscarinic-induced sustained suppression of DA release. (A) Cartoon depicting the anatomical location of M4 in the striatum. (B,C) Time-courses and (D) box plot summaries of DA release following bath application of 10µM Oxo-M +/− 3µM VU0467154 (VU’154) in D1-M4 KO mice and control littermates (M4 fl/fl; n=5–6; * Significant difference from 10µM Oxo-M, p<0.05; one-way ANOVA with a post hoc Bonferroni test). (E) Time-course showing the effect of 30µM Oxo-M +/− 3µM VU0467154 in D1-M4 KO mice. Time-course (F) and box plot summaries (G) depicting the effects of 30µM Oxo-M following pretreatment with DHβE (n=5, # Significant difference from control littermate; p<0.05; two-tailed Mann-Whitney test).
M4-mediated sustained inhibition of DA release requires intact diacylglycerol (DAG) lipase and CB2 cannabinoid receptor signaling
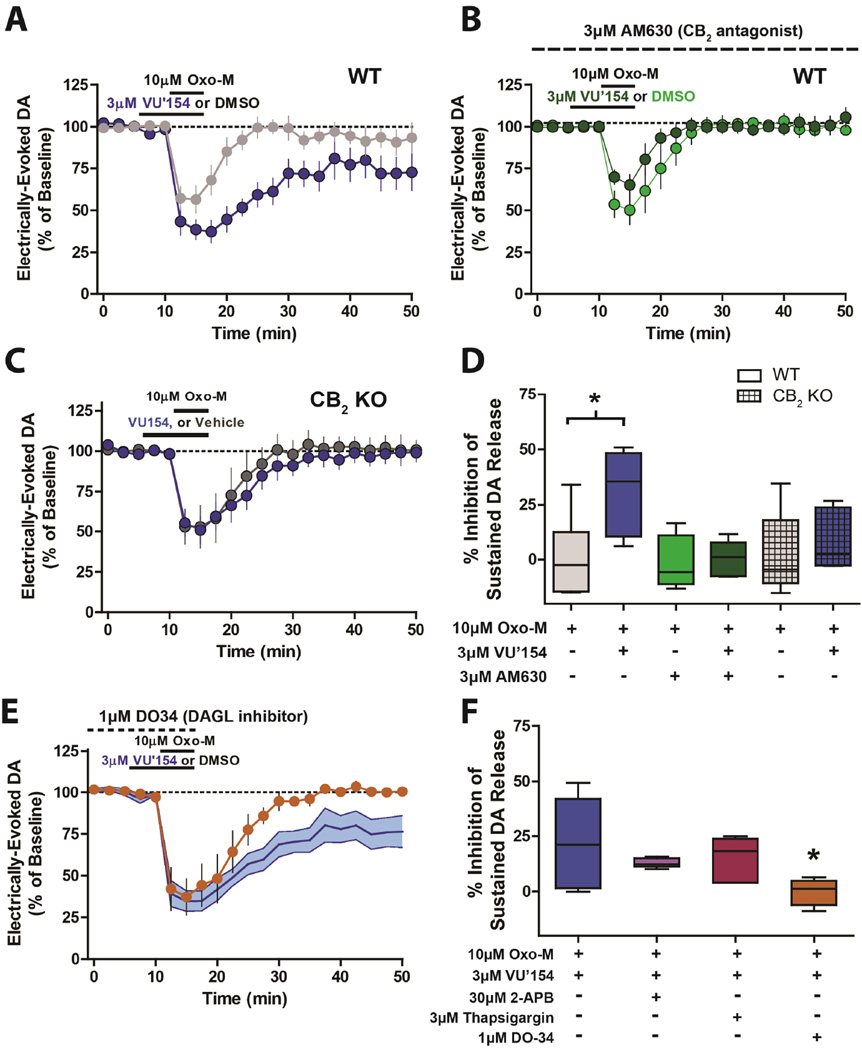
SPNs do not project to DA terminals, suggesting that in these acute slices M4 must alter DA release via a form of non-synaptic transmission. One of the best studied forms of non-synaptic communication in the striatum involves eCB signaling and activation of CB1 receptors and this pathway has been demonstrated to play a key role in M4–mediated modulation of striatal synaptic plasticity (Shen et al., 2015). However, the selective CB1 receptor antagonist AM251 (1µM) had no effect on M4 modulation of DA release, suggesting that CB1 is not involved (data not shown). Interestingly, while the CB2 cannabinoid receptor is not thought to play a major role in regulation of neurotransmitter release, a recent study suggests that DA neurons express the CB2 receptor (Zhang et al., 2014). Thus, we used a combination of genetic and pharmacological tools to determine whether CB2 receptors play a role in M4 effects on DA release. Similar to results in M4 fl/fl mice, VU0467154 significantly potentiated the sustained inhibition of DA release observed after bath application of 10µM Oxo-M in wild type (WT) mice (Figure 3A,D). Strikingly, the CB2 antagonist AM630 (3µM) had no effect on DA release alone (data not shown), but blocked VU0467154 potentiation of Oxo-M effects (Figure 3B,D). Furthermore, the effects of VU0467154 were absent in CB2 KO mice (Figure 3C,D) confirming a critical role for the CB2 receptor in mediating this response.
Figure 3.
VU0467154-mediated effects on DA release are CB2-dependent. (A,B,C) Time-courses and (D) box plot summaries showing that the effects of VU0467154 (VU’154) on DA release are blocked by pretreatment with the CB2 antagonist AM630 (3µM) and absent in CB2 KO mice (n=5–6; * Significant difference from 10µM Oxo-M, p<0.05; one-way ANOVA with a post hoc Bonferroni test). (E) Time-course and (F) box plot summaries showing that VU’154-mediated effects on sustained dopamine release are blocked by pretreating with DAG-Lipase inhibitor DO34 (1µM) but unaffected by treatment with 2-APB or thapsigargin (n=5–6; * Significant difference from VU’154 + Oxo-M, p<0.05; one-way ANOVA with a post hoc Dunnett’s test).
The CB2-dependence of the sustained reductions in DA release suggests that activation of M4 on SPNs could facilitate the formation and/or release of an eCB. Production and concurrent physiological effects of eCBs can be stimulated via a multitude of mechanisms at various synapses with some of these mechanisms being Ca2+-dependent and others being Ca2+-independent (Gladding et al., 2009). While M4 canonically signals through the G-protein Gαi, there are many examples of G-protein-coupled receptors (GPCRs) that, in addition to coupling with Gαi, can activate multiple G-proteins and signaling pathways including intracellular Ca2+ mobilization. To evaluate if M4 in SPNs could promote Ca2+ release, single-cell Ca2+ mobilization experiments were performed in M1 KO mice that expressed tdTomato in D1-expressing neurons. These mice allowed us to visualize and patch D1-SPNs as well as isolate M4 as the only known mAChR subtype in these cells. Either current injection or activation of Group I metabotropic glutamate (mGlu) receptors produced robust Ca2+ transients (Figure S2). In contrast, mAChR activation did not induce Ca2+ transients in D1-SPNs (Figure S2A). To further evaluate a potential role for intracellular Ca2+ signaling in the mAChR-induced inhibition of dopamine release, slices were pretreated with either 3µM thapsigargin to deplete intracellular Ca2+ stores or 30µM 2-Aminoethoxydiphenyl borate (2-APB) to antagonize the inositol 1,4,5-trisphosphate (IP3) receptor. Neither of these treatments had any effect on M4-mediated sustained inhibitions of DA release (Figure 3F). However, bath application of thapsigargin blocked mGlu-mediated Ca2+ transients in D1-SPNs suggesting that this treatment was effective at depleting intracellular Ca2+ stores (Figure S2C,D). These data suggest that the observed M4-mediated effects on sustained DA release are not likely to be dependent on M4-induced Ca2+ mobilization. Previous examples of Ca2+-independent eCB-mediated effects arising from SPNs as well as other brain regions have been demonstrated to occur through DAG lipase which plays a key role in the synthesis of the endocannabinoid 2-arachinonoylglycerol (2-AG; Chevaleyre and Castillo, 2003; Lerner and Kreitzer, 2012). Interestingly, we found that mAChR-induced sustained reductions in DA release were blocked by pretreatment with the DAG lipase inhibitor DO-34 (Figure 3E,F). Collectively, these studies suggest that M4 expressed on SPNs can lead to CB2 activation through activation of DAG-lipase and formation of the eCB 2-AG.
Efficacy of VU0467154 in rodent models of antipsychotic efficacy are mediated by M4 in D1 expressing neurons and are blocked by a CB2 antagonist
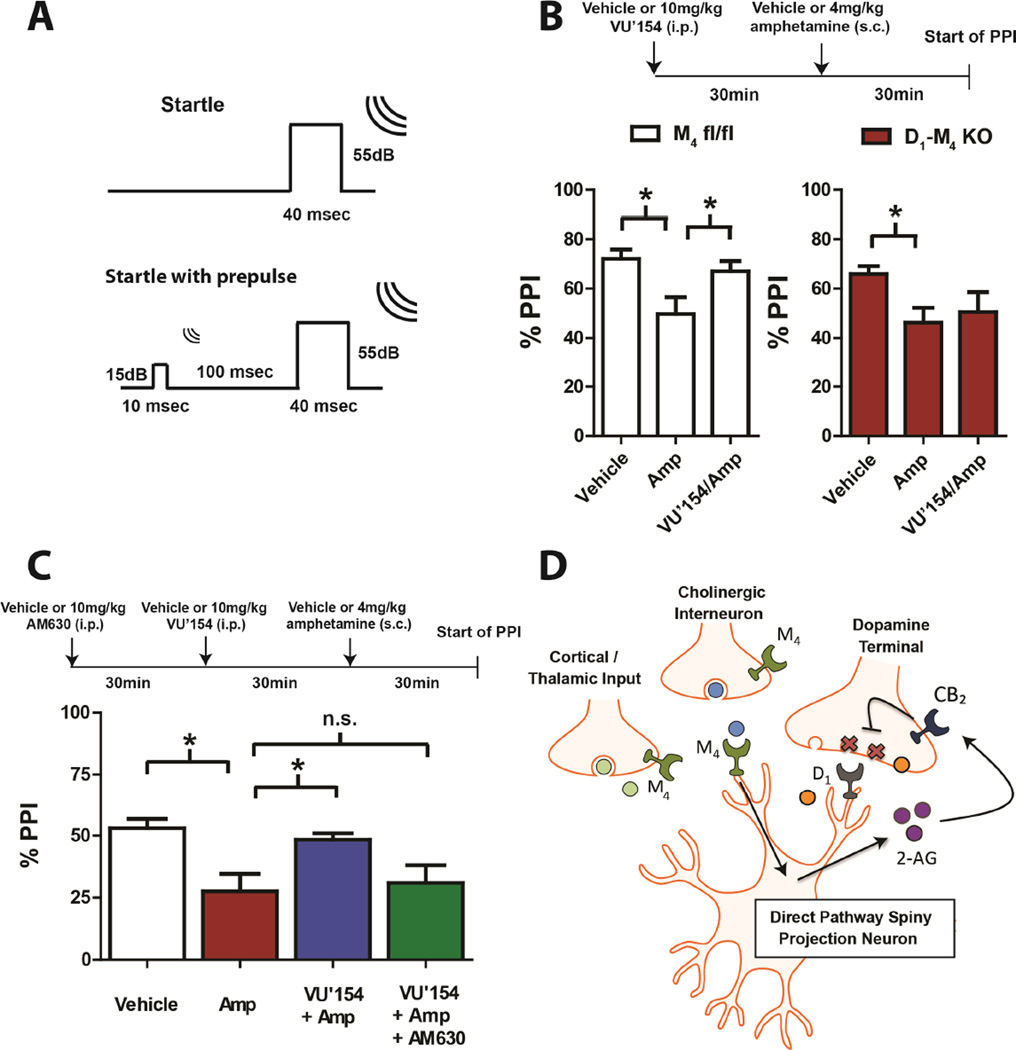
We next evaluated the effects of VU0467154 on amphetamine-induced disruption of prepulse inhibition (PPI; Figure 4A) of the acoustic startle response, a preclinical model of sensory motor gating deficits in schizophrenia patients that is reversed by M4 PAMs (Byun et al., 2014). Both D1-M4 KO mice and control littermates (M4 fl/fl) had similar baseline acoustic startle responses that were not altered by dosing with amphetamine or VU0467154 alone or in combination (Figure S3). VU0467154 (10mg/kg; intraperitoneal (i.p.)) alone had no effect on PPI compared to vehicle (74.2±3.7% and 72.1±3.8% PPI respectively). Amphetamine (4 mg/kg; subcutaneous (s.c.)) induced a robust decrease in PPI in both control and D1-M4 KO mice (Figure 4B). In control littermates, this amphetamine disruption was significantly reversed by pretreatment with VU0467154 (Figure 4B left). However, VU0467154 had no effect on amphetamine-disrupted PPI in D1-M4 KO mice (Figure 4B right), highlighting the importance of this M4 subpopulation in mediating VU0467154 effects on antipsychotic-like behaviors.
Figure 4.
VU0467154 reverses disrupted prepulse inhibition (PPI) via a mechanism requiring activation of M4 on D1-expressing neurons and subsequent CB2 receptor activation. (A) Experimental paradigm for monitoring PPI (B) Averaged data depicting % PPI observed in the absence or presence of VU0467154 (VU’154; 10mg/kg), amphetamine (Amp; 4mg/kg) or vehicle in D1-M4 KO mice and control littermates (M4 fl/fl; n=11–21; * Significant difference from Amp, p<0.05). (C) Averaged data depicting the % PPI observed in WT mice dosed with VU’154, AMP, and/or 10mg/kg AM630 (n=11–21; * Significant difference from Amp, p<0.05; one-way ANOVA with a post hoc Dunnett’s test). (D) Cartoon depicting mechanistic model for how striatal M4 receptors mediate sustained reductions in DA release and antipsychotic-like efficacy.
In addition, the CB2 receptor antagonist AM630 (10mg/kg; i.p.) inhibited the ability of VU0467154 (10mg/kg; i.p.) to reverse amphetamine-induced disruption of PPI (Figure 4C) but had no effect in the absence of VU0467154 (27.6±7% and 26.3±4.9% PPI in the absence and presence of AM630). We also examined MK801-induced hyperlocomotion, another behavioral paradigm that is used to predict antipsychotic-like efficacy of novel compounds. Consistent with our recent report (Bubser et al., 2014), VU0467154 significantly reversed MK-801-induced ambulation. This effect of VU0467154 was partially blocked by AM630 (Figure S4). Taken together, these findings support the novel concept that activation of M4 in D1-SPNs induces eCB release, resulting in a CB2-mediated sustained inhibition of DA release and that these actions contribute to the antipsychotic-like effects of the M4 PAM (Figure 4D).
Discussion
Clinical findings suggest that modulation of mAChR signaling can be efficacious in the cognitive, negative, and psychotic symptoms in schizophrenic and Alzheimer’s disease patients (Bodick et al., 1997; Shekhar et al., 2008). Preclinical studies suggest that selective activation of the M4 receptor may provide a strategy to obtain therapeutic effects while minimizing the side-effects observed with broad spectrum cholinergic/muscarinic compounds (Foster et al., 2014a). However, the mechanism by which M4 activation exerts antipsychotic efficacy has not been established. Here we make two key findings that further elucidate the mechanism whereby M4 activation leads to antipsychotic-like effects in preclinical models. First, we report that activation of M4 in the striatum causes a sustained reduction in striatal DA release. This M4-mediated reduction in DA release is novel in that it is sustained at time points well after washout of mAChR agonists which is reminiscent of long-term depression (LTD) observed at glutamatergic synapses (Gladding et al., 2009). Furthermore, the inhibition of DA release is mediated by M4 receptors expressed on D1-SPNs, which are striatal projection neurons that are postsynaptic targets of dopaminergic projections to the striatum. Interestingly, M4-mediated sustained reductions in DA release are blocked by inhibiting DAG lipase, suggesting that M4 on SPNs modulates DA terminals via the eCB signaling molecule 2-AG. Secondly, we demonstrate that both the VU0467154-mediated effects on DA release and the antipsychotic-like behavioral effects require CB2 receptor signaling. Collectively, these studies highlight a novel interaction between muscarinic and eCB signaling pathways in the striatum leading to reduced dopaminergic signaling and highlight the importance of these pathways in mediating the antipsychotic-like behavioral effects observed with M4 PAMs.
Previous studies have suggested that mAChR agonist-induced inhibition of striatal DA release is mediated primarily by autoreceptors on cholinergic interneurons (Threlfell et al., 2010; Zhang et al., 2002), which may include both M2 and M4 mAChR subtypes. Here, we find that Oxo-M induced an acute inhibition of DA release that was significantly reduced either in the presence of nAChR antagonist or with direct optical stimulation of DA terminals. While the acute inhibition was largely nAChR-dependent, and accordingly likely to be mediated by autoreceptors, it was not completely abolished under conditions that removed nAChR signaling. Furthermore, the acute inhibition seen with Oxo-M was reduced in D1-M4 KO mice compared to littermate controls. These findings suggest that the acute inhibition observed after mAChR agonist application involves multiple receptor subtypes and populations likely including: M2, M4 (located both pre-synaptically and post-synaptically), and M5 (Foster et al., 2014b, Shin et al., 2015). Importantly, we also report a novel sustained inhibition of DA release that was observed at time points well after removal of Oxo-M. Unlike the acute inhibition, this sustained inhibition of DA release is independent of nAChR-signaling. Application of the M4-selective PAM VU0467154 in acute brain slices had no effect on its own, likely owing to the reduced activity of striatal cholinergic interneurons, and corresponding low levels of ACh tone, in ex vivo brain slices compared to in vivo (Shen et al., 2015). However, when VU0467154 was included with a submaximal concentration of agonist, it potentiated the sustained inhibition of DA release. In the striatum the majority of M4 is expressed on D1-expressing SPNs and it was found that the VU0467154-mediated effects on DA release and reversal of specific behavioral effects of psychomotor stimulants were dependent on expression of M4 expressed in D1-SPNs. These data are consistent with a previous report suggesting that some behavioral effects of the non-selective orthosteric mAChR agonist xanomeline also require activation of M4 receptors on D1-expressing neurons (Dencker et al., 2011). While these data suggest that M4 on D1-SPNs are critically important for mediating antipyschotic-like efficacy, we cannot rule out a role for M4 expressed on other D1-expressing populations. Collectively, these data provide exciting new insights into the mechanisms by which activation of M4 receptors in these neurons can regulate DA-dependent behaviors.
Because SPNs do not make synaptic connections with DA terminals, we hypothesized that M4 receptors on SPNs must alter DA release via a form of non-synaptic transmission. A recent study demonstrated that M4 activation with M4 PAMs could modulate multiple forms of spike-timing dependent plasticity at corticostriatal synapses via an eCB-dependent mechanism. These effects on plasticity were absent when M4 was deleted from D1-expressing neurons and required intact CB1 receptor signaling (Shen et al., 2015). Most previously described eCB actions in the CNS are primarily mediated by CB1. However, we found that a selective antagonist of CB1 receptors did not inhibit the effect of M4 activation on DA release. In contrast, selective blockade or genetic deletion of CB2 receptors completely blocked the ability of M4 PAMs to inhibit DA release. Furthermore, the M4-mediated sustained reductions in DA release were completely blocked by a DAG-lipase inhibitor suggesting a key role for 2-AG in mediating these effects. Collectively, these findings suggest that the subpopulation of M4 on direct pathway SPNs can efficiently promote eCB signaling in the striatum with important physiological implications. In addition to changes in synaptic plasticity induced by CB1 activation, reduced DA release via CB2 activation could impact forms of synaptic plasticity that are modulated by D1 and D2 receptor activation. In the future it will be important to fully parse out the effects of M4 activation on overall striatal function that are mediated by M4 on SPNs (regulation of DA release through CB2 activation, activation of CB1 receptors on glutamatergic and GABAergic terminals) and activation of M4 in other neuronal populations.
The finding that CB2 was required for M4-mediated modulation of DA release was surprising in the light of previous studies indicating that CB1 and CB2 were thought to be primarily expressed in the CNS and immune system, respectively. However, this original dichotomy has been challenged by recent studies that have found CB2 expressed in numerous brain areas (Mechoulam and Parker, 2013), including data suggesting that DA neurons express CB2 receptors (Zhang et al., 2014). Furthermore, CB2 activation has been demonstrated to modulate cocaine-induced locomotion (Xi et al., 2011) and CB2 function has been linked to schizophrenia in patients and to schizophrenia-related behaviors in rodent models (Ishiguro et al., 2010; Ortega-Alvaro et al., 2011). Furthermore, we demonstrate that CB2 blockade occluded M4-mediated behavioral effects in two preclinical behavioral models predictive of antipsychotic efficacy. While activation of M4 leading to CB2 activation on DA terminals is the most parsimonious explanation of our results, it is not clear what population(s) of CB2 mediate these effects. Further studies with tissue-specific CB2 KO mice will be needed to determine the role of CB2 receptors expressed on various neuronal and glial populations in modulating DA release and DA-dependent behaviors. In addition to providing a novel mechanism whereby M4 activation can lead to antipsychotic-like efficacy, these studies suggest that CB2–selective agonists could provide an exciting new therapeutic strategy for the treatment of schizophrenia.
The present study is especially important in the context of currently available antipsychotic agents that act by blocking striatal DA receptors. While exerting antipsychotic activity through blockade of midbrain DA receptors, DA receptor antagonists also block DA receptors in the cortex and other brain regions where they induce adverse effects, including impaired cognitive function. In contrast, M4 PAMs improve cognitive function in animal models (Bubser et al., 2014). These studies provide a mechanism by which M4 may act locally to reduce DA signaling without blockade of DA receptors throughout the CNS. The combined cellular and behavioral studies reported suggest that this mechanism may be responsible, at least in part, for the antipsychotic-like effects of M4 PAMs.
Experimental Procedures
Voltammetry data plotted with box and whisker plots was analyzed using one-way analysis of variance (ANOVA) with a Dunnett’s or Bonferroni post hoc test where appropriate. Time courses of DA release are depicted as the mean ± SEM. PPI data are expressed as mean ± SEM and analyzed using one-way ANOVA with a Dunnett’s post hoc test comparing all dosing groups to amphetamine-treated controls. Experimental procedures are described in detail in the Supplemental Experimental Procedures. All animals were handled in accordance with federal guidelines and protocols approved by Vanderbilt University.
Supplementary Material
Acknowledgments
We would like to thank Weimin Peng for her invaluable assistance. This work was supported by the Olga V. Tedeschi Young Investigator Award from the Brain & Behavior Research Foundation to DJF, as well as grants from the NIH (NIMH and NINDS) to DJF, PJC, CKJ and ZX, and the Tourette Association of America to ZX.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
D.J.F. and P.J.C. conceived the study and wrote the manuscript. D.J.F., P.J.C, J.M.R., C.K.J., and J.M.W designed the experiments. D.J.F., J.M.R., D.H.R, M.S.M, and J.M.W. conducted the experiments and analyzed the data. C.W.L, J.W., S.P., L.J.M, and M.J.U provided genetic and pharmacological tools utilized in this study. C.M.N., S.P., L.J.M., J.W., C.K.J., Z.X., C.W.L., and J.M.R. reviewed and edited the manuscript.
References
- Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, Lamsal A, Niswender CM, Daniels JS, Poslusney MS, et al. Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem Neurosci. 2014;5:920–942. doi: 10.1021/cn500128b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003;17:1403–1410. doi: 10.1046/j.1460-9568.2003.02588.x. [DOI] [PubMed] [Google Scholar]
- Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishankar R, Kelm ND, Damon S, Bridges TM, et al. Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology. 2014;39:1578–1593. doi: 10.1038/npp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;3:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, Christopoulos A, Lazareno S, Birdsall NJ, Bymaster FP, Felder CC. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci USA. 2008;105:10978–10983. doi: 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker D, Wortwein G, Weikop P, Jeon J, Thomsen M, Sager TN, Mork A, Woldbye DP, Wess J, Fink-Jensen A. Involvement of a subpopulation of neuronal M4 muscarinic acetylcholine receptors in the antipsychotic-like effects of the M1/M4 preferring muscarinic receptor agonist xanomeline. J Neurosci. 2011;31:5905–5908. doi: 10.1523/JNEUROSCI.0370-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Choi DL, Conn PJ, Rook JM. Activation of M1 and M4 muscarinic receptors as potential treatments for Alzheimer's disease and schizophrenia. Neuropsychiatr Dis Treat. 2014a;10:183–191. doi: 10.2147/NDT.S55104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Gentry PR, Lizardi-Ortiz JE, Bridges TM, Wood MR, Niswender CM, Sulzer D, Lindsley CW, Xiang Z, Conn PJ. M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor's location. J Neurosci. 2014b;34:3253–3262. doi: 10.1523/JNEUROSCI.4896-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladding CM, Fitzjohn SM, Molnar E. Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms. Pharmacol Rev. 2009;61:395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Horiuchi Y, Ishikawa M, Koga M, Imai K, Suzuki Y, Morikawa M, Inada T, Watanabe Y, Takahashi M, et al. Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry. 2010;67:974–982. doi: 10.1016/j.biopsych.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Leach K, Loiacono RE, Felder CC, McKinzie DL, Mogg A, Shaw DB, Sexton PM, Christopoulos A. Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacology. 2010;35:855–869. doi: 10.1038/npp.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Kreitzer AC. RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits. Neuron. 2012;2:347–359. doi: 10.1016/j.neuron.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, Navarrete F, Manzanares J. Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology. 2011;36:1489–1504. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancani T, Bolarinwa C, Smith Y, Lindsley CW, Conn PJ, Xiang Z. M4 mAChR-mediated modulation of glutamatergic transmission at corticostriatal synapses. ACS Chem Neurosci. 2014;5:318–324. doi: 10.1021/cn500003z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mallinckrodt C, Bymaster FP, McKinzie DL, Felder CC. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- Shen W, Plotkin JL, Francardo V, Ko WK, Xie Z, Li Q, Fieblinger T, Wess J, Neubig RR, Lindsley CW, et al. M4 Muscarinic Receptor Signaling Ameliorates Striatal Plasticity Deficits in Models of L-DOPA-Induced Dyskinesia. Neuron. 2015;88:762–773. doi: 10.1016/j.neuron.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Adrover MF, Wess J, Alvarez VA. Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens. Proc Natl Acad Sci USA. 2015;112:8124–8129. doi: 10.1073/pnas.1508846112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB(2) receptors modulate cocaine's actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, Gardner EL, Wu J, Xi ZX. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci USA. 2014;111:E5007–E5015. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci. 2002;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.