Abstract
Study Objectives:
We hypothesized that patient reported outcomes (PROs) improve with positive airway pressure (PAP) in patients with sleep-disordered breathing (SDB) and hypertension (HTN).
Methods:
Questionnaire-based PROs (sleepiness [Epworth Sleepiness Scale, (ESS)], depression [Patient Health Questionnaire-9 (PHQ-9)], and fatigue [Fatigue Severity Scale (FSS)]) were retrospectively examined in patients with SDB and HTN at baseline and within a year following PAP initiation. PRO changes were estimated using multivariable linear mixed-effect models adjusted for baseline age, sex, race, body mass index, resistant hypertension (RHTN) status, cardiac and diabetes history, and correlation between repeated measurements. Age and race by PAP interaction terms (mean change, 95% CI) were examined.
Results:
894 patients with HTN and SDB were examined. 130 (15%) had baseline RHTN (age 58 ± 12 y, 52.9 % male, BMI 36.2 ± 9.1 kg/m2). In multivariable models, a significant improvement in sleepiness ESS (−2.09, 95% CI: −2.37, −1.82), PHQ-9 (−1.91, 95% CI: −2.25, −1.56), and FSS scores (−4.06 95% CI: −4.89, −3.22) was observed. A significant race by PAP effect interaction was observed (p < 0.0001 for all PROs); Caucasians had greater improvements than non-Caucasians. The interaction term of effect of PAP and age was significant for ESS (p = 0.04) and PHQ-9 (p = 0.0003), indicating greater improvement in younger patients.
Conclusions:
Consistent improvement of broad PRO domains in response to PAP in SDB was observed in this clinic-based hypertensive cohort; Caucasians and younger patients derived greater benefit.
Citation:
Walia HK, Griffith SD, Thompson NR, Moul DE, Foldvary-Schaefer N, Mehra R. Impact of sleep-disordered breathing treatment on patient reported outcomes in a clinic-based cohort of hypertensive patients. J Clin Sleep Med 2016;12(10):1357–1364.
Keywords: patient reported outcomes, hypertension, positive airway pressure
INTRODUCTION
Sleep-disordered breathing (SDB) is a major public health problem with a progressive increase in prevalence over the last two decades1 accompanied by a myriad of negative health consequences and associated with decrements in quality of life, the latter a focus of a recent American Academy of Sleep Medicine statement.2 SDB is characterized by repetitive upper airway collapse, and pressure stenting with continuous positive airway pressure (CPAP)3 is the usual first-line treatment. Intermittent bouts of hypoxemia and sympathetic surges accompany SDB, thereby resulting in sustained elevations in blood pressure even during wakefulness.4 Hypertension (HTN) is also a highly prevalent disorder affecting one in three adults and represents a leading cause of death in United States.5 Over one-third of the hypertensive population has RHTN, making it a prevalent condition managed by both primary care clinicians and specialists.6 Additionally, the prevalence of SDB in patients with HTN7 and RHTN8 is very high, with estimates ranging from 50% to 85%.
BRIEF SUMMARY
Current Knowledge/Study Rationale: There exists a strong association between sleep-disordered breathing and hypertension, particularly resistant hypertension, and these are associated with impaired quality of life. There is a need for a greater understanding of patient-reported outcomes responsiveness to positive airway pressure therapy in individuals suffering from both conditions
Study Impact: This study provides a novel longitudinal measurement on improvement in daytime sleepiness, depressive symptoms, and fatigue in patients with sleep-disordered breathing and hypertension/ resistant hypertension. Caucasians and younger patients derived greater benefit, thus informing clinicians of expected patient reported outcomes response to positive airway pressure therapy.
In many patients, SDB is associated with worsening of a variety of patient reported outcomes (PROs), e.g., excessive daytime sleepiness (EDS),9 depressive symptoms,10–12 and fatigue.9 These symptoms have been observed to demonstrate salient yet complex inter-relationships in SDB and hypertension (HTN). For instance, increased risk of HTN observed in OSA may be modulated by daytime somnolence.13 Moreover, sleepiness is a not an uncommon symptomatic manifestation of depression, the latter representing the second leading cause of common psychiatric problems in adults.14 While it is fairly well established that SDB serves as a risk for HTN development, depression has also been identified as an independent risk factor for HTN.15 Data also support an improvement of sleepiness symptoms in SDB with CPAP16,17 and depressive symptoms also improve with CPAP in SDB in the general population.11,18–20 However, there are few studies which examine improvement of sleepiness and depressive symptoms in SDB in those with HTN. Furthermore, there is scant literature showing improvement of fatigue in patients with CPAP therapy, particularly those with HTN.21
Recognizing the strong association between SDB and HTN, particularly RHTN, there is a need for a greater understanding of PROs in individuals suffering from both conditions. Moreover, these two groups could potentially demonstrate varying subjective changes in response to SDB treatment. Patients with HTN have decreased quality of life (QOL) compared to those without HTN.22 Given that the effect of BP reduction may be dependent on the somnolence status,23 examination of PROs in this population is of interest. In addition, as data have demonstrated that African Americans have elevated ESS scores compared to Caucasians,24 and a high prevalence of depression and fatigue in African Americans,25,26 the effect modification of race on PAP response relative to PROs was examined. We also investigated the modification of age on the PAP response and PRO relationships given literature suggesting that EDS in SDB is associated with younger age group27 and some data suggesting increased depressive symptoms and fatigue symptoms in older adults.28,29 We therefore postulated that the treatment of SDB with PAP will result in improvement of PRO measures ranging from sleepiness, depressive symptoms and fatigue and hypothesize that race and age will serve as effect modifiers of these relationships.
METHODS
Study Sample
Electronic medical record data (EMR) (Epic Systems) were extracted for patients presenting for outpatient clinic visits in the Cleveland Clinic Sleep Disorders Center between 2/18/2008 and 7/16/2013 who had physician-confirmed HTN diagnosis (based on the ICD-9 billing code of 401-405) and self-reported use of PAP therapy. Inclusion criteria included age (≥ 18 years), diagnosis of SDB determined by PAP usage, history of HTN and PRO data at baseline (before PAP initiation) and at least one visit with PRO data after PAP initiation in a year follow-up period. Patient confirmation of HTN and SDB was based on physician diagnosis and self-reported use of PAP therapy for SDB collected through the Cleveland Clinic's Knowledge Program (KP), an electronic system for systematically collecting PROs at outpatient clinic visits. Patients with secondary hypertension due to chronic renal disease, primary hyperaldosteronism, Cushing syndrome, and renal artery stenosis were excluded.
Date of PAP initiation during the one-year follow-up period was based on a change to a self-report question regarding PAP therapy use at the baseline clinic visit to the affirmative compared to that for the baseline visit. The baseline visit was defined by the last Sleep Center visit in which the patient answered “no” to PAP usage. Baseline demographic and clinical comorbid data from the EMR regarding the patient's age, sex, race, median income by zip code, history of diabetes, and history of cardiac comorbidities (peripheral vascular disease, coronary heart disease, heart failure, or stroke) were obtained by the usage of ICD-9 codes. Socioeconomic status was measured using median income for each patient's zip code using 2010 United States Census data. Baseline body mass index (BMI) values were calculated based upon available weight and height data at baseline. Information on antihypertensive medications, BP, and self-reported PAP adherence were obtained from EMR and self-report data sources for the baseline visit and all subsequent visits to the Sleep Center in the following year. Medications were only included if indicated in the EMR as an active prescription on the date in question. RHTN at baseline was defined based upon the American Heart Association criteria where the blood pressure was above goal despite the use of ≥ 3 antihypertensive medications or controlled blood pressure on ≥ 4 medications.30 Patients not meeting these criteria were classified into the non-RHTN group. This study was approved by Institutional Review Board of the Cleveland Clinic.
The Electronic Outcome Data Collection System
The Knowledge Program (KP) at the Cleveland Clinic is an electronic data collection system of disease based PROs that are entered into the patient's EMR at the point of care making these measures clinically available to providers.31 PROs in the Sleep Center include the Epworth sleepiness scale (ESS), Patient Health Questionnaire-9 (PHQ-9), Fatigue Severity Scale (FSS) and self-reported number of days per week and hours per night of PAP usage. PAP adherence was defined based on the Centers for Medicare and Medicaid criteria which is PAP usage ≥ 5 days per week and ≥ 4 hours per day.32
The ESS, is a commonly used 8-item questionnaire to assess subjective daytime time sleepiness.33 The patient answers each question, rating their chance of dozing on a scale of 0 (never) to 3 (high chance of dozing) for each item. The final ESS score can range from 0–24, with a score ≥ 10 indicative of excessive daytime sleepiness.34 The PHQ-9 is a 9-item depression scale that provides psychometric measurement of depression,35 with each item scored on a scale of 0 (not at all) to 3 (nearly every day). A score of ≥ 10 is indicative of moderate to severe depression. The FSS is a 9-item tool validated for assessing fatigue36. Each item is graded on a scale of 1 (disagree) to 7 (agree) with a score ≥ 36 considered to be abnormal.
Polysomnogram
Attended overnight polysomnography (PSG) was performed on patients who underwent sleep studies at Cleveland Clinic using Nihon Kohden (Japan, 1951) system following standard guidelines.37 The recording montage included (F3- M2, C3-M2, O1-M2, F4-M1, C4-M1, O2-M1) bilateral electrooculography, submental and bilateral anterior tibial electromyography, thoracic and abdominal respiratory inductance plethysmography, and finger pulse oximetry. Nasal airflow and nasal pressure was measured using oronasal thermistor and nasal cannula, respectively. Hypopnea was defined as airflow ≥ 50% in the nasal pressure channel for ≥ 10 s resulting in an arousal or ≥ 3% oxygen desaturation.
Statistical Analyses
Descriptive statistics were stratified by HTN group (RHTN vs. non-RHTN). Group differences in baseline variables were examined using Wilcoxon and Fisher exact tests, as applicable, using R version 3.1.1 software,38 where p values of < 0.05 were considered to be statistically significant.
Primary Analyses
To examine change in PROs, separate linear mixed-effects models were estimated in which the dependent variable was either the ESS, PHQ-9, or FSS. Predictors included an indicator for pre/post PAP initiation, HTN group, and other clinically relevant baseline variables including age (years), sex, race, BMI, and cardiac (peripheral vascular disease, coronary heart disease, heart failure, or stroke), median income by zip code (per $10,000), and diabetes history. Normally distributed subject-specific random effects were included to account for multiple patient visits. Fixed effects were included for all other variables. To test whether the difference in PRO score from baseline averaged over the follow-up period differed between the RHTN and non-RHTN groups, an interaction term between pre/post PAP and HTN group was tested to address the primary hypothesis.
Secondary Analyses
A sensitivity analysis assessed the robustness of the primary analysis by including the data from follow-up visits during which patients' self-reported PAP adherence (adherence defined per the Centers of Medicare and Medicaid services (CMS) criteria,32 as self-reported PAP usage of ≥ 5 days per week and ≥ 4 h per day) as well as data from corresponding baseline visits. We also ascertained objective adherence data by contacting the durable medical equipment company and obtained valid data in 245 patients and conducted secondary subgroup analyses on these patients.
To explore the potentially non-additive effects of race and PAP as well as age and PAP on PROs fixed-effect interaction terms were used in each of the models with age as a continuous variable and race (Caucasians versus non-Caucasians) in relation to the pre/post PAP indicator, adjusted for factors in the mixed models described above. Additionally, the differences in PAP adherence in the race and age subgroups were further explored by excluding PAP non-adherent patients.
RESULTS
Baseline Characteristics
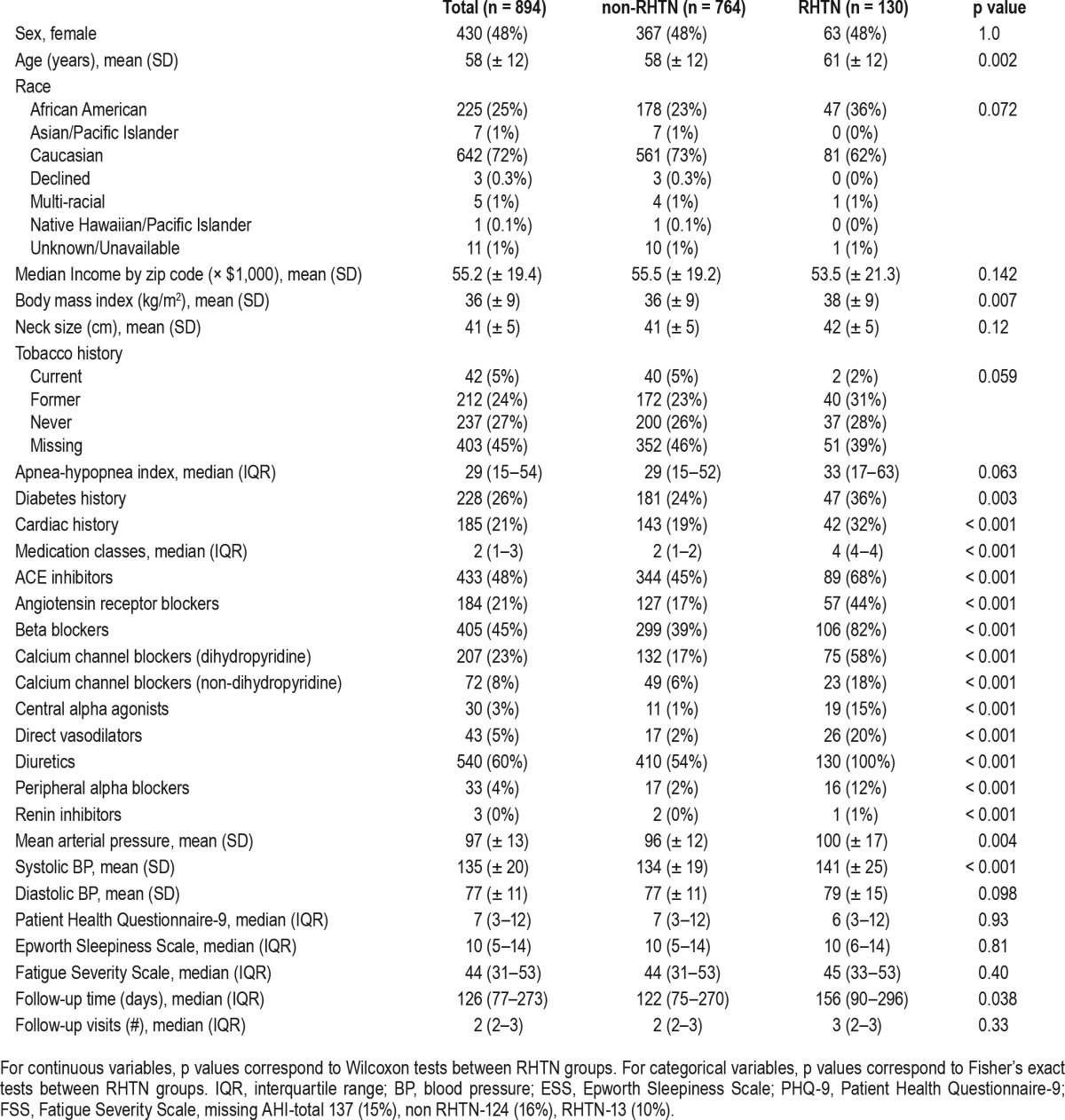
Of a total of 2,500 patients with pre and post PAP PRO data, there were 1,000 patients with HTN and SDB reporting use of PAP, 894 (89%) had complete Cleveland Clinic visit data over the course of the study from 2/18/2008 and 7/16/2013 (Table 1). Overall, the median AHI was 29 (interquartile range (IQR), 15–54 events/h). The median follow-up time was 126 (IQR, 77–273) days. Of the total sample, 130 (15%) had RHTN. The mean age (61 ± 12 vs. 58 ± 12 years, p = 0.002) and mean BMI (38 ± 9 kg/m2 vs. 36 ± 9 kg/m2, p = 0.007) was significantly higher in the RHTN than non-RHTN, respectively. As anticipated, those with RHTN had a higher percentage of diabetes and cardiac disease (p < 0.01). There was no difference in PRO between HTN and RHTN group.
Table 1.
Descriptive statistics at baseline by resistant hypertension status.
Primary Analyses
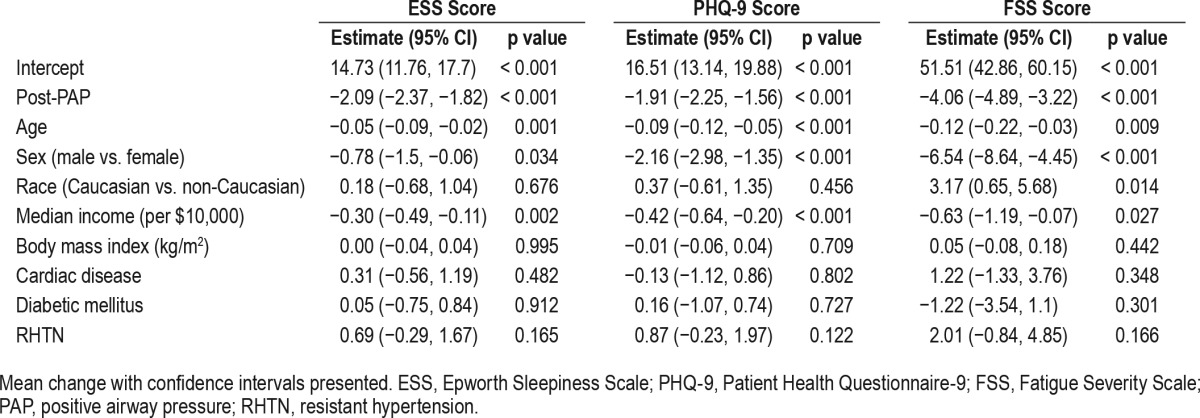
In models fully adjusted for age, sex, race, BMI, median income by zip code (per $10,000), cardiac and diabetes history in the year following PAP initiation, there was a significant decrease in ESS (−2.09, 95% CI: −2.37, −1.82), PHQ-9 (−1.91, 95% CI: −2.25, −1.56), and FSS scores (−4.06 95% CI: −4.89, −3.22). This improvement was observed in the sample overall and was not dependent on RHTN status (Table 2).
Table 2.
Linear mixed effects multivariable regression model examining effect of positive airway pressure on patient reported outcomes.
Secondary Analyses
Of the full dataset, 728 (81.4%) were adherent with PAP, according to self-report. In sensitivity analyses including only adherent patients, the impact of PAP appeared to be slightly more pronounced with improvement of ESS (−2.29, 95% CI: −2.58, −2.01), PHQ-9 (−2.20, 95% CI: −2.55, −1.84), and FSS scores (−4.53, 95% CI: −5.42, −3.64); findings which were once again independent of RHTN status (Table 3). Of the 245 subjects with valid objective adherence data, 147 (60.0%) were objectively adherent. In the sub-analyses of the 147 objectively-adherent patients, we found the results to be consistently more pronounced improvement across the measures, i.e., ESS (−2.55, 95% CI: −3.24 to −1.87), PHQ-9 (−2.84, 95% CI: −3.54 to −2.13) and FSS (5.82, 95% CI: −7.79 to −3.84).
Table 3.
Linear mixed effects multivariable regression model examining effect of positive airway pressure on patient reported outcomes restricted to positive airway pressure adherent patients.

The difference in model-adjusted average baseline scores was not significantly different for PHQ-9 (7.1, 95% CI: 0.2, 14.0 vs. 6.3, 95 % CI: −0.6, 13.2, p = 0.12), but was significantly higher in Caucasians vs. African Americans/others for ESS (9.5, 95% CI: 3.8, 15.2 vs. 8.6, 95% CI: 2.8, 14.4, p = 0.04) and for FSS (39.7, 95% CI: 22.3, 57.1, vs. 34.7, 95% CI: 17.2, 52.2 p = 0.0002, respectively). The statistical interaction of race and PAP effect on PRO changes demonstrated the association between PAP treatment and PRO outcome to be dependent on race (p < 0.05 for all). Greater improvements after PAP therapy were observed in Caucasians compared to other races for all PROs, i.e., ESS reduced by 2.4 in Caucasians and 0.5 in African Americans/others; PHQ-9 reduced by 2.1 in Caucasians and 1.3 in African Americans/others; and FSS reduced by 5.0 in Caucasians and by 2.0 in African Americans/others (Figure 1). Given that PAP adherence was higher in Caucasians (83.8%) versus African Americans/others (75.1%), sensitivity analyses excluding those non-adherent with PAP was performed which demonstrated persistence of a greater reduction of PROs in Caucasians versus other races.
Figure 1. Changes in outcomes of ESS, PHQ-9 and FSS after institution of CPAP by race category (p < 0.05 for all).
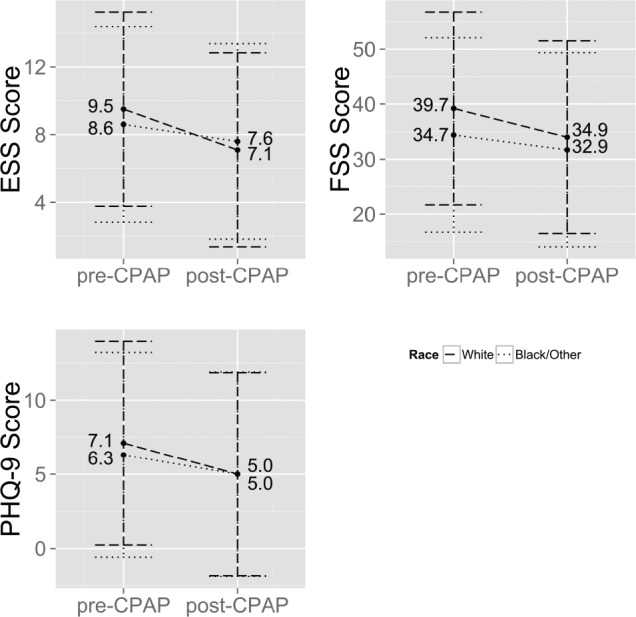
ESS, Epworth Sleepiness Scale; PHQ-9, Patient Health Questionnaire-9; FSS, Fatigue Severity Scale; CPAP, continuous positive airway pressure.
In terms of age, younger patients had worse scores than older patients on all 3 PROs at baseline (p < 0.01). The statistical interaction of effect of PAP and age relative to PROs was statistically significant for ESS (p = 0.04) and PHQ-9 (p = 0.0003), demonstrating greater improvement in younger versus older patients. However, the age-PAP use interaction was not statistically significant for FSS (p = 0.42; Figure 2). As the average baseline age of those who were PAP adherent versus non-adherent was 58.7 years (SD = 11.6) versus 56.3 years (SD = 12.1), respectively, we conducted sensitivity analyses excluding those who were non-adherent with PAP, which demonstrated similar findings. For instance, for a 35-year-old, ESS improved by 3.4 compared to a 70-year-old whose ESS improved by 2.3 with PAP. Similarly, in a 35-year-old, the PHQ-9 improved, on average, by 4.1 and in a 70-year-old improved by 1.7.
Figure 2. Changes in outcomes of ESS, PHQ-9 and FSS after institution of CPAP by age.

ESS (p = 0.04), PHQ-9 (p = 0.0003), FSS (p = 0.42). ESS, Epworth Sleepiness Scale; PHQ-9, Patient Health Questionnaire-9; FSS, Fatigue Severity Scale; CPAP, continuous positive airway pressure.
DISCUSSION
This single-center observational study of a sample of patients treated with PAP indicated that regardless of hypertension status, PAP therapy for SDB was associated with improvement in daytime sleepiness, depressive symptoms, and fatigue. To our knowledge, no previous study has examined changes in sleep-related functional outcomes with PAP therapy in a strictly hypertensive cohort enriched with resistant hypertension. This is especially interesting as there was no difference in the outcomes between the two groups studied. Our findings suggest a positive impact of PAP therapy in hypertensive patients in a real-world setting of patients with health care insurance with a follow up period up to a year. As anticipated, these effects were slightly more robust in patients with better PAP adherence and the relationships were modified by race and age such that also in Caucasians, and younger patients appeared to derive greater PAP treatment benefit.
Our work has important implications for population health, and is also aligned with the American Academy of Sleep Medicine imperatives for tracking outcomes in SDB care paths.2 Moreover, untreated SDB provides a potential etiology for the impairment of quality of life reported in patients with HTN.22 Specifically, it has been established that EDS is a significant public health problem in patients with SDB39 and is useful to identify individuals with high risk of HTN or RHTN.13 The observation that patients with controlled BP had less dozing propensity further corroborates this point.40 Moreover, there is minimal data available examining change in ESS in those with HTN. One randomized controlled trial demonstrated improvements in ESS with CPAP usage in RHTN patients and that the CPAP effect on BP levels was independent of daytime sleepiness.41 Similarly, another prospective study that evaluated the effect of CPAP on HTN in coronary artery disease patients with SDB showed significant improvement in ESS.42 Meta-analyses of randomized controlled trials in non-hypertensive population have suggested the mean difference of ESS of −2.7 points which is similar to the magnitude of improvement noted in our real-world hypertensive cohort.43 Furthermore, there is a paucity of data in terms of change in important mood and fatigue symptom-based outcomes (depression, fatigue) in response to CPAP usage in SDB and HTN in clinic-based cohorts, particularly those enriched with RHTN.
Evidence shows varying degrees of improvement of the depressive symptoms with the use of CPAP in SDB.18,44,45 For example, data from a smaller study demonstrated sustained improvement in depression measures utilizing a different questionnaire than used in the current study (Beck Depression Inventory) over a prolonged follow up period in response to CPAP in SDB.46 A recent prospective study demonstrated a larger magnitude in change in PHQ-9 from 11.3 ± 6.1 to 3.7 ± 2.9 with 3 months of treatment of SDB with CPAP.47 Albeit similar to our study improvement in PHQ-9 was noted, the difference in magnitude of decrease from our study can be attributed to the differences in the study design, duration of treatment and type of population studied. Moreover, the results from the current study extend these findings by corroborating these improvements in depression scores with PAP treatment in SDB in a group of hypertensive patients. These data therefore support another pathway, i.e., via impact on depression symptoms, by which SDB treatment may improve HTN outcomes particularly in light of data identifying depression as a risk factor for HTN development.15
Limited data exist regarding the improvement of fatigue with CPAP usage in SDB. In a randomized controlled trial involving 59 subjects, significant improvement in fatigue was noted. However the study period was limited to 3 weeks and the scale for fatigue measurement was profile of mood states.21
Another small scale study (n = 14), showed improvement with profile of mood states fatigue subscale.48 The results of the current larger study support a more extended improvement of fatigue symptoms, with a follow-up period of 1 year, in response to SDB treatment with PAP. Findings of improvement in fatigue symptoms appear to be consistent across all of these studies irrespective of fatigue scale considered. Moreover, it was recently reported that improvement of fatigue (by FSS) was related to better CPAP adherence.49
In our study, Caucasians were noted to have greater improvement than non-Caucasians (the majority of whom were African Americans) in PROs in response to PAP therapy in SDB despite taking into account PAP adherence and socioeconomic status—findings which were counter to anticipated results. This occurred despite the fact that African Americans have more symptoms of sleepiness and depression compared to Caucasians. A recent large randomized controlled trial of African American individuals indicated EDS to be a significant predictor of non-adherence to antihypertensive medications, suggesting that EDS should be targeted for patients with HTN.50 However, mitigated responsiveness in non-Caucasians of PROs to CPAP therapy compared to Caucasians was observed independent of consistency of PAP usage. Potential reasons for these findings include genetic susceptibilities or possibly residual confounding by features of SES. There are minimal data exploring the relationship between race, CPAP adherence, and functional outcomes of SDB. For instance, a study of approximately 200 veterans suggested that race is a modifier of the relationship between CPAP adherence and some functional outcomes of sleep; in particular measures of the Functional Outcomes of Sleep Questionnaire (FOSQ).51
The differing population-specific factors studied and varying study designs involving differing QOL measures (FOSQ) may explain some similarities and differences.
Younger patients were noted to have more improvement in the depression and ESS scores compared to older patients. It has been consistently demonstrated that older patients may not perceive sleepiness to be a major symptom of sleep apnea52; this therefore may explain the less responsiveness to ESS compared to younger patients. Similarly, some studies report that older patients may appear to have less depressive symptoms as they tend to underreport it53 and that overall SDB has less impact on QOL in older patients.54 This may explain reduced responsiveness of PAP in older patients in depressive symptoms; to our knowledge, no studies have compared the age effects in regards to PAP responsiveness in depression and fatigue symptoms.
Strengths of our study include a large sample size and assessment of the real world effectiveness of PAP to the change in PRO in a group of hypertensive, i.e., individuals at risk for not only SDB, but also adverse cardiovascular outcomes. Moreover, we utilized existing PRO data with a comprehensive electronic data capture system.31 In terms of limitations, we relied on self-report for PAP compliance; however, we have previously reported a significant correlation of objective and subjective PAP adherence in our center and correlated well with those with available objective data.55 The socioeconomic status was an assessment based on median income by zip code and therefore may not have effectively incorporated important aspects of socioeconomic status. The retrospective approach to analysis is a limitation; therefore, future prospective data are required to corroborate these findings.
Our study provides a novel longitudinal measurement on improvement in daytime sleepiness, depressive symptoms and fatigue in patients with HTN and RHTN. Future investigations should build upon these findings and involve a focus on the prospective examination of PROs in response to PAP in SDB and HTN and implementation of randomized controlled trials to gain a systematic sense of the magnitude of treatment effect. Also, another area of future investigation would be to have a comparative group of non-hypertensive individuals. Furthermore, prospective investigation of groups in terms of HTN and RHTN and subgroups including different ethnic backgrounds and age confirming the current findings of similar PRO responsiveness in RHTN and HTN and enhanced responsiveness in the younger age group and Caucasians would be valuable and inform SDB treatment guidelines.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by the National Heart, Lung, and Blood Institute (NHLBI) [R21HL108226 and R01HL109493] and Cleveland Clinic Neurological Institute-Center for Outcomes Research and Evaluation Scholar Award. Dr. Mehra reports that she has received NIH funding for which she has served as Principal Investigator (NHLBI RO1 1 R01 HL 109493, R21 HL108226). Her institution has received positive airway pressure machines and equipment from Philips Respironics for use in NIH-funded research. She has received honorarium from the American Academy of Sleep Medicine for speaking. She serves as a Associate Editor for the journal Chest. She has received royalties from Up to Date. Dr. Foldvary-Schaefer receives grant support from UCB, Inc., Jazz Pharmaceuticals plc, and ResMed and serves on the Speaker's Bureau for Jazz Pharmaceuticals plc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the Cleveland Clinic Knowledge Program and Optimizing Healthcare Decisions teams for the electronic health record data and Brian Wells, MD, PhD, for assistance in identifying medications for data queries. The authors thank Cleveland Clinic Home Care including Mary Mertens for assisting us obtaining the objective PAP compliance data. The authors also thank Srividya Ramachandran, PhD, for medical writing and editorial services for the manuscript from Neurological Institute. Dr. Walia had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Griffith, Mr. Thompson, Dr. Moul, Dr. Foldvary-Schaefer, and Dr. Mehra contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
ABBREVIATIONS
- ESS
Epworth Sleepiness Scale
- FSS
Fatigue Severity Scale
- HTN
hypertension
- PAP
positive airway pressure
- PHQ-9
Patient Health Questionnaire-9
- PROs
patient reported outcomes
- RHTN
resistant hypertension
- SDB
sleep-disordered breathing
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aurora RN, Collop NA, Jacobowitz O, Thomas SM, Quan SF, Aronsky AJ. Quality measures for the care of adult patients with obstructive sleep apnea. J Clin Sleep Med. 2015;11:357–83. doi: 10.5664/jcsm.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 4.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Bruen BK, Lantz PM, Mendez D. Impact of Health Insurance Expansions on Nonelderly Adults With Hypertension. Prev Chron Dis. 2015;12:E105. doi: 10.5888/pcd12.150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57:1076–80. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 7.Torres G, Sanchez-de-la-Torre M, Barbe F. The relationship between obstructive sleep apnea and hypertension. Chest. 2015;148:824–32. doi: 10.1378/chest.15-0136. [DOI] [PubMed] [Google Scholar]
- 8.Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–7. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 9.Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118:372–9. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- 10.Wheaton AG, Perry GS, Chapman DP, Croft JB. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005-2008. Sleep. 2012;35:461–7. doi: 10.5665/sleep.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13:437–44. doi: 10.1016/j.smrv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Chen YH, Keller JK, Kang JH, Hsieh HJ, Lin HC. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med. 2013;9:417–23. doi: 10.5664/jcsm.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng J, He QY, Zhang XL, Chen BY. Epworth Sleepiness Scale may be an indicator for blood pressure profile and prevalence of coronary artery disease and cerebrovascular disease in patients with obstructive sleep apnea. Sleep Breath. 2012;16:31–40. doi: 10.1007/s11325-011-0481-5. [DOI] [PubMed] [Google Scholar]
- 14.Le Port A, Gueguen A, Kesse-Guyot E, et al. Association between dietary patterns and depressive symptoms over time: a 10-year follow-up study of the GAZEL cohort. PLoS One. 2012;7:e51593. doi: 10.1371/journal.pone.0051593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng L, Chen D, Yang Y, Zheng Y, Hui R. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens. 2012;30:842–51. doi: 10.1097/HJH.0b013e32835080b7. [DOI] [PubMed] [Google Scholar]
- 16.McDaid C, Duree KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009;13:427–36. doi: 10.1016/j.smrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–71. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 18.Millman RP, Fogel BS, McNamara ME, Carlisle CC. Depression as a manifestation of obstructive sleep apnea: reversal with nasal continuous positive airway pressure. J Clin Psychiatry. 1989;50:348–51. [PubMed] [Google Scholar]
- 19.Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142–51. doi: 10.1183/09031936.00064214. [DOI] [PubMed] [Google Scholar]
- 20.Povitz M, Bolo CE, Heitman SJ, Tsai WH, Wang J, James MT. Effect of treatment of obstructive sleep apnea on depressive symptoms: systematic review and meta-analysis. PLoS Med. 2014;11:e1001762. doi: 10.1371/journal.pmed.1001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomfohr LM, Ancoli-Israel S, Loredo JS, Dimsdale JE. Effects of continuous positive airway pressure on fatigue and sleepiness in patients with obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2011;34:121–6. doi: 10.1093/sleep/34.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trevisol DJ, Moreira LB, Kerkhoff A, Fuchs SC, Fuchs FD. Health-related quality of life and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2011;29:179–88. doi: 10.1097/HJH.0b013e328340d76f. [DOI] [PubMed] [Google Scholar]
- 23.Bratton DJ, Stradling JR, Barbe F, Kohler M. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax. 2014;69:1128–35. doi: 10.1136/thoraxjnl-2013-204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes AL, Spilsbury JC, Patel SR. The Epworth score in African American populations. J Clin Sleep Med. 2009;5:344–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Sohail Z, Bailey RK, Richie WD. Misconceptions of depression in African Americans. Front Psychiatry. 2014;5:65. doi: 10.3389/fpsyt.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steele L, Dobbins JG, Fukuda K, et al. The epidemiology of chronic fatigue in San Francisco. Am J Med. 1998;105:83S–90S. doi: 10.1016/s0002-9343(98)00158-2. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Zhang C, Jia P, et al. The association between the phenotype of excessive daytime sleepiness and blood pressure in patients with obstructive sleep apnea-hypopnea syndrome. Int J Med Sci. 2014;11:713–20. doi: 10.7150/ijms.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011;129:126–42. doi: 10.1016/j.jad.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci. 2009;64:76–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–26. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 31.Katzan I, Speck M, Dopler C, et al. The Knowledge Program: an innovative, comprehensive electronic data capture system and warehouse. AMIA Annu Symp Proc. 2011;2011:683–92. [PMC free article] [PubMed] [Google Scholar]
- 32.Billings ME, Kapur VK. Medicare long-term CPAP coverage policy: a cost-utility analysis. J Clin Sleep Med. 2013;9:1023–9. doi: 10.5664/jcsm.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 34.Benbadis SR, Mascha E, Perry MC, Wolgamuth BR, Smolley LA, Dinner DS. Association between the Epworth sleepiness scale and the multiple sleep latency test in a clinical population. Ann Intern Med. 1999;130:289–92. doi: 10.7326/0003-4819-130-4-199902160-00014. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 37.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 38.R Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2014. R: A language and environment for statistical computing. http://www.R-project.org/ [Google Scholar]
- 39.Ronksley PE, Hemmelgarn BR, Heitman SJ, et al. Excessive daytime sleepiness is associated with increased health care utilization among patients referred for assessment of OSA. Sleep. 2011;34:363–70. doi: 10.1093/sleep/34.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deleanu OC, Malaut AE, Nebunoiu AM, Micheu MM, Mihaltan FD. Obstructive sleep apnea syndrome and arterial hypertension--a complicated relationship? The role of controlling blood pressure values in patients with OSAS. Pneumologia. 2014;63:36–43. [PubMed] [Google Scholar]
- 41.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–15. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 42.Huang Z, Liu Z, Luo Q, et al. Long-term effects of continuous positive airway pressure on blood pressure and prognosis in hypertensive patients with coronary heart disease and obstructive sleep apnea: a randomized controlled trial. Am J Hypertens. 2015;28:300–6. doi: 10.1093/ajh/hpu147. [DOI] [PubMed] [Google Scholar]
- 43.McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 2009;13:43–274. doi: 10.3310/hta13040. iii-iv, xi-xiv, 1-119. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz DJ, Kohler WC, Karatinos G. Symptoms of depression in individuals with obstructive sleep apnea may be amenable to treatment with continuous positive airway pressure. Chest. 2005;128:1304–9. doi: 10.1378/chest.128.3.1304. [DOI] [PubMed] [Google Scholar]
- 45.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz DJ, Karatinos G. For individuals with obstructive sleep apnea, institution of CPAP therapy is associated with an amelioration of symptoms of depression which is sustained long term. J Clin Sleep Med. 2007;3:631–5. [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards C, Mukherjee S, Simpson L, Palmer LJ, Almeida OP, Hillman DR. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med. 2015;11:1029–38. doi: 10.5664/jcsm.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derderian SS, Bridenbaugh RH, Rajagopal KR. Neuropsychologic symptoms in obstructive sleep apnea improve after treatment with nasal continuous positive airway pressure. Chest. 1988;94:1023–7. doi: 10.1378/chest.94.5.1023. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Geater AF, Chai Y, et al. Pre- and in-therapy predictive score models of adult OSAS patients with poor adherence pattern on nCPAP therapy. Patient Prefer Adherence. 2015;9:715–23. doi: 10.2147/PPA.S83105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams NJ, Jean-Louis G, Pandey A, Ravenell J, Boutin-Foster C, Ogedegbe G. Excessive daytime sleepiness and adherence to antihypertensive medications among Blacks: analysis of the counseling African Americans to control hypertension (CAATCH) trial. Patient Prefer Adherence. 2014;8:283–7. doi: 10.2147/PPA.S53617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallace DM, Wohlgemuth WK. Does race-ethnicity moderate the relationship between CPAP adherence and functional outcomes of sleep in US veterans with obstructive sleep apnea syndrome? J Clin Sleep Med. 2014;10:1083–91. doi: 10.5664/jcsm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaz Fragoso CA, Van Ness PH, Araujo KL, Iannone LP, Klar Yaggi H. Age-related differences in sleep-wake symptoms of adults undergoing polysomnography. J Am Geriatr Soc. 2015;63:1845–51. doi: 10.1111/jgs.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Addison-Brown KJ, Letter AJ, Yaggi K, et al. Age differences in the association of obstructive sleep apnea risk with cognition and quality of life. J Sleep Res. 2014;23:69–76. doi: 10.1111/jsr.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez-Garcia MA, Soler-Cataluna JJ, Roman-Sanchez P, Gonzalez V, Amoros C, Montserrat JM. Obstructive sleep apnea has little impact on quality of life in the elderly. Sleep Med. 2009;10:104–11. doi: 10.1016/j.sleep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Su CS, Liu KT, Panjapornpon K, Andrews N, Foldvary-Schaefer N. Functional outcomes in patients with REM-related obstructive sleep apnea treated with positive airway pressure therapy. J Clin Sleep Med. 2012;8:243–7. doi: 10.5664/jcsm.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]


