Abstract
Study Objectives:
Low flow supplemental oxygen is commonly prescribed to patients with obesity hypoventilation syndrome (OHS). However, there is a paucity of data regarding its efficacy and safety. The objective of this study was to assess the medium-term treatment efficacy of adding supplemental oxygen therapy to commonly prescribed treatment modalities in OHS.
Methods:
In this post hoc analysis of a previous randomized controlled trial, we studied 302 sequentially screened OHS patients who were randomly assigned to noninvasive ventilation, continuous positive airway pressure, or lifestyle modification. Outcomes at 2 mo included arterial blood gases, symptoms, quality of life, blood pressure, polysomnography, spirometry, 6-min walk distance, and hospital resource utilization. Statistical analysis comparing patients with and without oxygen therapy in the three treatment groups was performed using an intention-to-treat analysis.
Results:
In the noninvasive ventilation group, supplemental oxygen reduced systolic blood pressure although this could be also explained by a reduction in body weight experienced in this group. In the continuous positive airway pressure group, supplemental oxygen increased the frequency of morning confusion. In the lifestyle modification group, supplemental oxygen increased compensatory metabolic alkalosis and decreased the apnea-hypopnea index during sleep. Oxygen therapy was not associated with an increase in hospital resource utilization in any of the groups.
Conclusions:
After 2 mo of follow-up, chronic oxygen therapy produced marginal changes that were insufficient to consider it, globally, as beneficial or deleterious. Because supplemental oxygen therapy did not increase hospital resource utilization, we recommend prescribing oxygen therapy to patients with OHS who meet criteria with close monitoring. Long-term studies examining outcomes such as incident cardiovascular morbidity and mortality are necessary.
Clinical Trials Registration:
Clinicaltrial.gov, ID: NCT01405976
Citation:
Masa JF, Corral J, Romero A, Caballero C, Terán-Santos J, Alonso-Álvarez ML, Gomez-Garcia T, González M, López-Martínez S, De Lucas P, Marin JM, Marti S, Díaz-Cambriles T, Chiner E, Merchan M, Egea C, Obeso A, Mokhlesi B, Spanish Sleep Network. The effect of supplemental oxygen in obesity hypoventilation syndrome. J Clin Sleep Med 2016;12(10):1379–1388.
Keywords: noninvasive ventilation, obesity hypoventilation syndrome, oxygen therapy, sleep apnea
INTRODUCTION
Obesity hypoventilation syndrome (OHS) is defined by obesity and chronic hypercapnic respiratory failure not related to neuromuscular, metabolic, lung, or chest wall diseases.1
In addition to weight reduction surgery or lifestyle modification, other treatments widely used to treat OHS include positive airway pressure therapy in the form of noninvasive ventilation (NIV), normally with bilevel positive airway pressure2 or continuous positive airway pressure (CPAP).3
BRIEF SUMMARY
Current Knowledge/Study Rationale: Supplemental oxygen is commonly prescribed to patients with obesity hypoventilation syndrome (OHS) treated with noninvasive ventilation (NIV) and continuous positive airway pressure (CPAP), in order to improve residual hypoxemia during daytime and/or sleep and as a palliative treatment in OHS patients who reject NIV or CPAP therapies. However, there is paucity of data regarding its efficacy and safety.
Study Impact: Oxygen therapy added to NIV, CPAP, or lifestyle modification during 2 mo produced mixed results that were insufficient to consider it, globally, as beneficial or deleterious. Because supplemental oxygen therapy did not increase hospital resource utilization, we recommend prescribing oxygen therapy to patients with OHS who meet criteria with close monitoring.
In clinical practice supplemental oxygen therapy is frequently added to NIV or CPAP in order to improve residual hypoxemia during daytime and sleep periods.4 Oxygen therapy has also been added to lifestyle modification treatment in OHS patients who reject NIV or CPAP. An observational study reported that in patients with OHS supplementary oxygen therapy was an independent risk factor for mortality,5 although another study did not find the same association.6
Two experimental studies reported an increase in daytime partial pressure of carbon dioxide (PCO2) with exposure to 20 min of intermediate or high concentrations of supplemental oxygen in a proportion of patients with stable OHS.7,8 In another study an increase in nocturnal PCO2 was observed in 11 obese patients with nocturnal hypoventilation treated with low oxygen concentrations during 15 days, although no changes in daytime PCO2 or clinical symptoms were observed.9 However, there is no information about medium or long-term treatment efficacy of adding oxygen to treatment strategies typically used in patients with OHS, such as NIV, CPAP, and lifestyle modification.
The objective of this post hoc analysis of a randomized controlled trial was to assess the medium-term efficacy of adding supplemental oxygen therapy to patients with OHS randomized to NIV, CPAP, or lifestyle modification.10
METHODS
Patients
From May 2009 to March 2013, we screened consecutive patients between age 15 and 80 y who were referred for pulmonary consultation for suspected OHS or obstructive sleep apnea (OSA) at 16 tertiary care hospitals in Spain with the main objective to evaluate, in a randomized controlled trial, the comparative efficacy of NIV, CPAP, and lifestyle modification (control group). For the initial medium-term outcomes of this study (see supplemental material) we enrolled subjects who had OHS with10 or without severe OSA. OHS was defined as obesity with body mass index (BMI) ≥ 30 kg/m2 plus stable hypercapnic respiratory failure during wakefulness (PaCO2 ≥ 45 mmHg, pH ≥ 7.35, and no clinical worsening during the previous 2 mo) and no evidence of relevant chronic obstructive pulmonary disease [forced expiratory volume in the first second (FEV1) > 70% of predicted when FEV1/(forced vital capacity (FVC) > 70], neuromuscular, chest wall, or metabolic disease. Other inclusion criteria were an absence of narcolepsy or restless legs syndrome, and a correctly executed 30-min CPAP/NIV treatment test (see supplemental material). The exclusion criteria were: (1) a psychophysical inability to complete questionnaires, (2) severe chronic debilitating illness, (3) severe chronic nasal obstruction, and (4) lack of informed consent. The study was approved by the ethics committees of the 16 centers and written informed consent was obtained from all patients.
In the current post hoc analysis we used data obtained from 302 patients with OHS, with10 and without OSA, enrolled in the randomized controlled trial.
Interventions
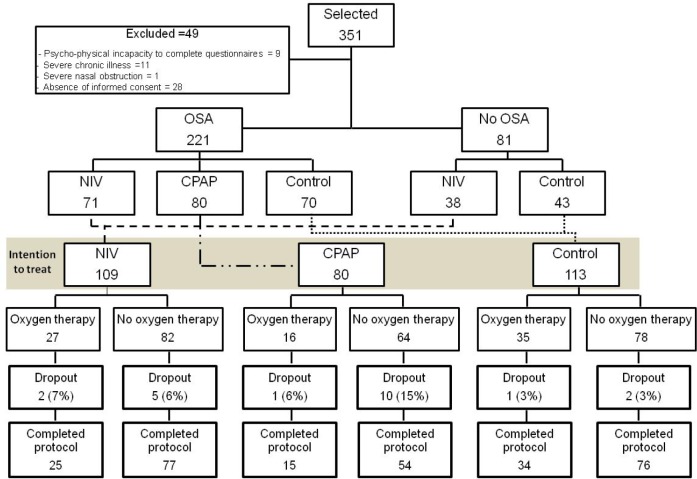
Patients were randomized by an electronic database (simple randomization) to NIV, CPAP, or lifestyle modification (control group) arms (Figure 1).
Figure 1. Flow chart of the study protocol.
Of the 351 selected patients, 49 were excluded, 221 had OSA (apnea-hypopnea index ≥ 30) and they were randomized into NIV, CPAP, or control groups and 81 without OSA were randomized to NIV and control groups. Patients with and without OSA randomized to NIV and control were considered together. The resulting groups (NIV, CPAP, and control) were divided in patients treated with and without supplemental oxygen therapy to be compared. CPAP, continuous positive airway pressure; NIV, noninvasive ventilation; OSA, obstructive sleep apnea.
Control Group: Lifestyle Modification
The lifestyle modification consisted of a 1,000-calorie diet and the maintenance of correct sleep hygiene and habits (avoiding supine position during sleep; maintaining regular sleep habits and exercise; not consuming sedatives, stimulants, or alcohol; not smoking tobacco; and avoiding heavy meals within 4 h before bedtime).
Continuous Positive Airway Pressure
In addition to lifestyle modification and oxygen (if required), patients were instructed to use at-home fixed CPAP during the entire sleep period prior to conventional CPAP titration (see supplemental material).
Noninvasive Ventilation
In addition to lifestyle modification and oxygen (if required), patients were instructed to use NIV treatment during the entire sleep period. The ventilator mode was set at bilevel pressure with assured volume. More information about the setting, daytime and polysomnographic adjustments, ventilators, and masks used are available in the supplemental material.
Criteria for Oxygen Supplementation
LIFESTYLE (CONTROL) ARM: There were two pathways to qualify for supplemental oxygen therapy in patients randomized to this group: (1) Daytime assessment of oxygenation based on baseline arterial blood gases (ABG): If on the ABG the PaO2 was ≤ 55 mmHg on room air during wakefulness, supplemental oxygen was prescribed.11 Of the 35 patients on supplemental oxygen in the lifestyle group (Figure 1), 22 (63%) qualified for supplemental oxygen based on hypoxemia during wakefulness on the baseline ABG. (2) Sleep assessment during baseline polysomnography (PSG): The PSG was performed on room air. The decision to add oxygen was made in the morning by the expert sleep clinicians based on the review and analysis of the PSG data. If the mean peripheral oxygen saturation (SpO2) during sleep from the pulse oximetry signal during the PSG was ≤ 88%, then supplemental oxygen was prescribed. Of the 35 patients on supplemental oxygen in the lifestyle group, 13 (37%) qualified for supplemental oxygen based on hypoxemia during sleep.
CPAP AND NIV ARMS: In patients randomized to CPAP or NIV there were two pathways by which patients could get prescribed supplemental oxygen. (1) Sleep assessment during CPAP/NIV titration PSG: For the sleep assessment, all PSGs were performed on room air. The decision to add oxygen was made in the morning by the expert sleep clinicians based on the review and analysis of the titration PSG data. For those who underwent CPAP or NIV titration, the decision to add oxygen to positive airway pressure (PAP) therapy was made if the mean SpO2 from the pulse oximetry signal during the titration PSG was ≤ 88% on adequate PAP levels. Of the 16 patients in the CPAP group who required oxygen (Figure 1), 14 (88%) qualified for oxygen based on hypoxemia on the CPAP titration PSG. Of the 27 patients in the NIV group who required oxygen, 26 (96%) qualified for oxygen based on the NIV titration PSG. (2) Daytime assessment of oxygenation based on ABG: In patients randomized to CPAP or NIV, oxygen could also be prescribed if the PaO2 was ≤ 55 mmHg on an ABG obtained on room after 1 mo of NIV or CPAP therapy. The reason for this initial approach in the protocol was to assess whether baseline hypoxemia improves after 1 mo of nocturnal NIV/CPAP therapies. However, of the 16 patients in the CPAP group that required oxygen, only 2 qualified based on the ABG after 1 mo of CPAP therapy. The other 14 required oxygen based on sleep hypoxemia as described previously. Of the 27 patients in the NIV group that required supplemental oxygen, only 1 patient qualified based on the ABG after 1 mo of therapy. All the other patients had already been started on oxygen because of hypoxemia during the NIV titration as described previously.
Instructions to Patients on How to Use Supplemental Oxygen
In our protocol, all patients requiring supplemental oxygen therapy regardless of the arm they were randomized to (i.e. lifestyle, CPAP, or NIV) were recommended to use it for at least 17 h per day, including during the sleep period. In other words, if a patient had only oxygen during sleep criterion, they were also instructed to use supplemental oxygen for at least 17 h a day. The reason we recommended oxygen therapy even during the daytime in these individuals was to create uniformity in the three groups as it pertains to the duration of exposure to supplemental oxygen.
Oxygen therapy was only titrated in patients who demonstrated hypoxemia during daytime assessment on ABG with PaO2 ≤ 55 mmHg (i.e. 22 in the lifestyle group, 2 in the CPAP group, and 1 patient in the NIV group). In these individuals, oxygen therapy was titrated during wakefulness to ascertain the necessary flow to maintain waking SpO2 between 88% and 92%. The level of oxygen required to keep the SpO2 above 88% during wakefulness was the same level used during sleep (either added to the PAP system or via nasal cannula in the lifestyle group). For the patients who had only criterion for oxygen supplementation during sleep (i.e., 13 in the lifestyle group, 14 in the CPAP group, and 26 patients in the NIV group), oxygen was prescribed empirically at 2 L/min flow during sleep and wakefulness. As stated previously, all PSGs (baseline and follow-up PSGs) were performed without supplemental oxygen. Therefore, oxygen titration during sleep was not possible. In those randomized to CPAP or NIV, oxygen was bled-in into their home PAP system using a T-tube connector. In those randomized to lifestyle modification, oxygen was used via a nasal cannula. Despite these differences in approach, oxygen flow (i.e. L/min during sleep and wakefulness) and duration of use was not significantly different between the three groups.
Follow-up and Outcomes
Patients were evaluated at baseline and after 2 mo. We assessed ABG parameters while breathing room air (see supplemental material), anthropometric data, clinical symptoms, habitual dyspnea (defined as a score of ≥ 2 points in the Medical Research Council scale),12 subjective sleepiness based on the Epworth Sleepiness Scale (ESS), health-related quality of life (HRQL) using the Functional Outcomes of Sleep Questionnaire, the Medical Outcomes Study Short Form-36 (SF-36), and the visual analog well-being scale,13,14 PSG, spirometry,15 the 6-min walk distance test,16 adherence to NIV, CPAP, or oxygen therapy using an hourly counter, hospitalizations (number of times hospitalized and duration of hospital stay), emergency room visits and side effects of treatments during the 2 mo of follow-up. Dropout definition is described in the supplemental material. An evaluation of side effects and an ABG was also performed after the first month of treatments.
Polysomnography
We performed PSG at baseline, for titration (only for the NIV and CPAP groups), and after 2 mo of treatment (with NIV and CPAP in place and without treatment for the control group). Standard protocols were used to perform and analyze the PSG (see supplemental material).
Statistical Analysis
Intention-to-treat analysis was performed. Missing values were imputed following a multiple imputation method with iterative multivariable regression because the missing data had characteristics compatible with a missing-at-random pattern. Baseline variables between patients with and without supplementary oxygen in each of interventions groups (NIV, CPAP, and control) were compared using unpaired Mann-Whitney U tests for continuous and an x2 test for categorical variables. Changes in the continuous variables from baseline to 2 mo were compared between patients with and without supplementary oxygen in each of interventions groups (NIV, CPAP, and control) using also unpaired Mann-Whitney U tests. When the overall comparison demonstrated a statistical significance (p < 0.05), an analysis of covariance (ANCOVA) was carried out, taking into account the baseline values of the variable analyzed, age, sex, BMI, FEV1 and apnea-hypopnea index (AHI) (henceforth “baseline adjustment”). In order to ascertain whether the results were dependent or independent of weight change or CPAP/NIV compliance, we carried out an additional ANCOVA with the baseline adjustment plus introducing variables such as change in BMI and adherence to CPAP or NIV as a categorical variable (below or ≥ 4 h/day use). In the control group the additional ANCOVA was only adjusted for BMI change.
To evaluate the change in clinical symptoms presented as categorical variables, we compared the percentage at the end of the follow-up between patients with and without supplementary oxygen therapy in each of interventions groups. When there was statistical significance (p < 0.05), two similar ANCOVA analysis as previously described were carried out.
Data management, the imputation and statistical analyses were performed using SPSS software (IBM SPSS Statistics, Version 22.0. Armonk, NY, IBM Corporation).
RESULTS
Of the 351 patients who met the inclusion criteria, 49 were excluded (Figure 1). Patients with AHI ≥ 30 (n = 221) were randomized to NIV, CPAP or lifestyle modification. Patients with AHI < 30 (n = 81) were randomized to NIV or lifestyle modification. For the current analysis patients with or without AHI ≥ 30 who were randomized to NIV were analyzed as one group (NIV group). Similarly, patients with or without AHI ≥ 30 who were randomized to lifestyle modification were analyzed as one group (controls) (Figure 1). These newly constructed groups had similar percentages of patients receiving supplemental oxygen. Dropout frequency was similar between patients receiving or not receiving supplemental oxygen.
Table 1 describes the baseline values with and without supplemental oxygen in the three intervention groups (NIV, CPAP, and control). In general terms, patients needing supplemental oxygen therapy had worse daytime and nocturnal hypoxemia and exercise tolerance. Table 2 summarizes the changes in the respiratory functional test and blood pressure with and without supplemental oxygen therapy in the three intervention groups. In the NIV group, there was a reduction in systolic blood pressure with supplemental oxygen therapy (p = 0.015), but the statistical significance disappeared when we adjusted for the baseline values (baseline adjustment) plus change in BMI and NIV compliance (p = 0.098). In the control group, oxygen therapy increased the pH (p = 0.032) and the statistical significance persisted despite baseline adjustment as well as adjustment for change in BMI. Bicarbonate in NIV group (p = 0.003), PaO2 in CPAP group (p = 0.031) and 6-min walk distance test in the control group (p = 0.028) improved more with supplemental oxygen therapy but the statistical significance disappeared after baseline adjustment. Therefore, these improvements were likely explained by the different baseline characteristics between groups with and without oxygen therapy and not necessarily due to a beneficial effect of oxygen supplementation.
Table 1.
Baseline variables.
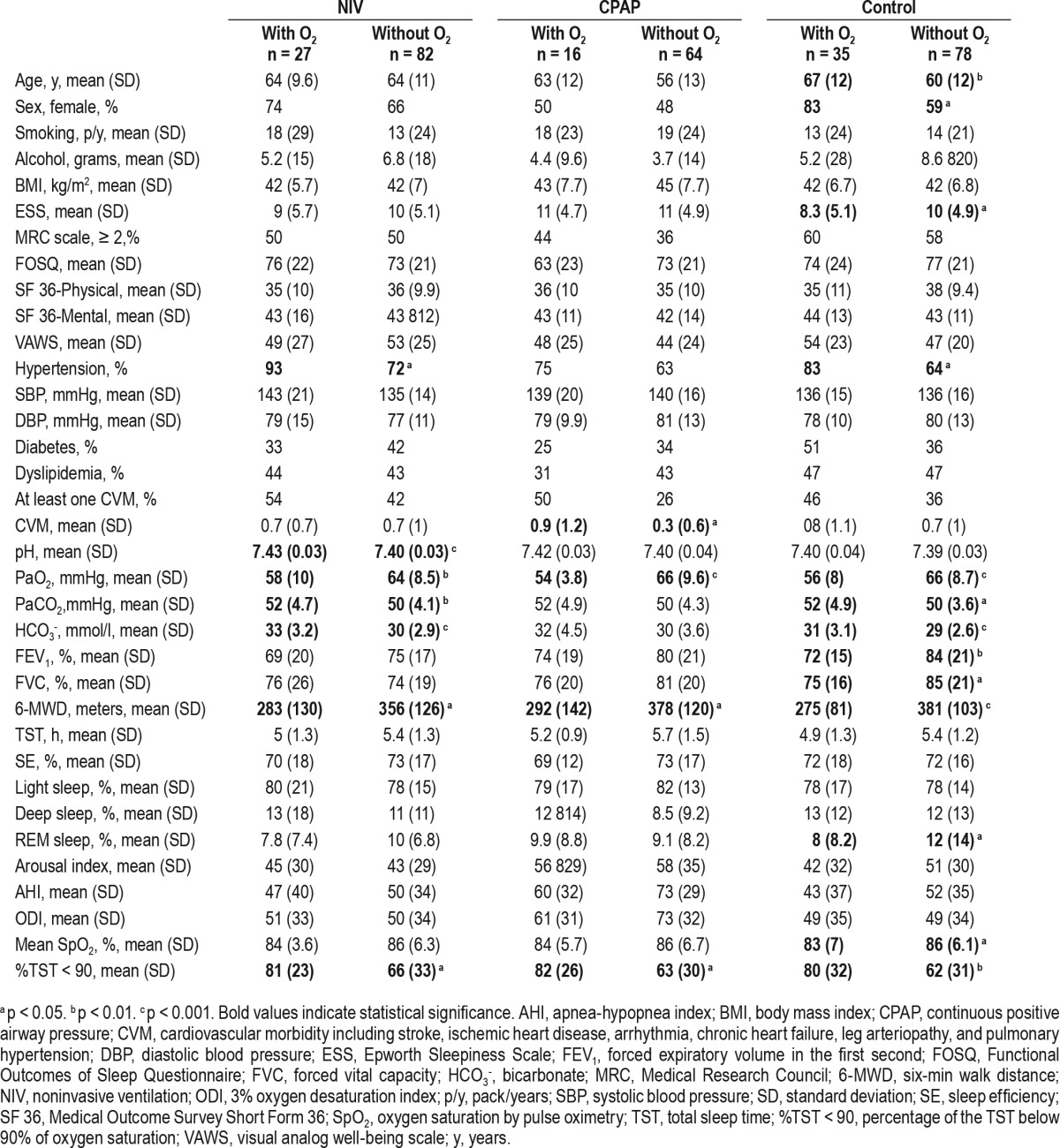
Table 2.
Changes in pulmonary function parameters and blood pressure based on assigned treatment.
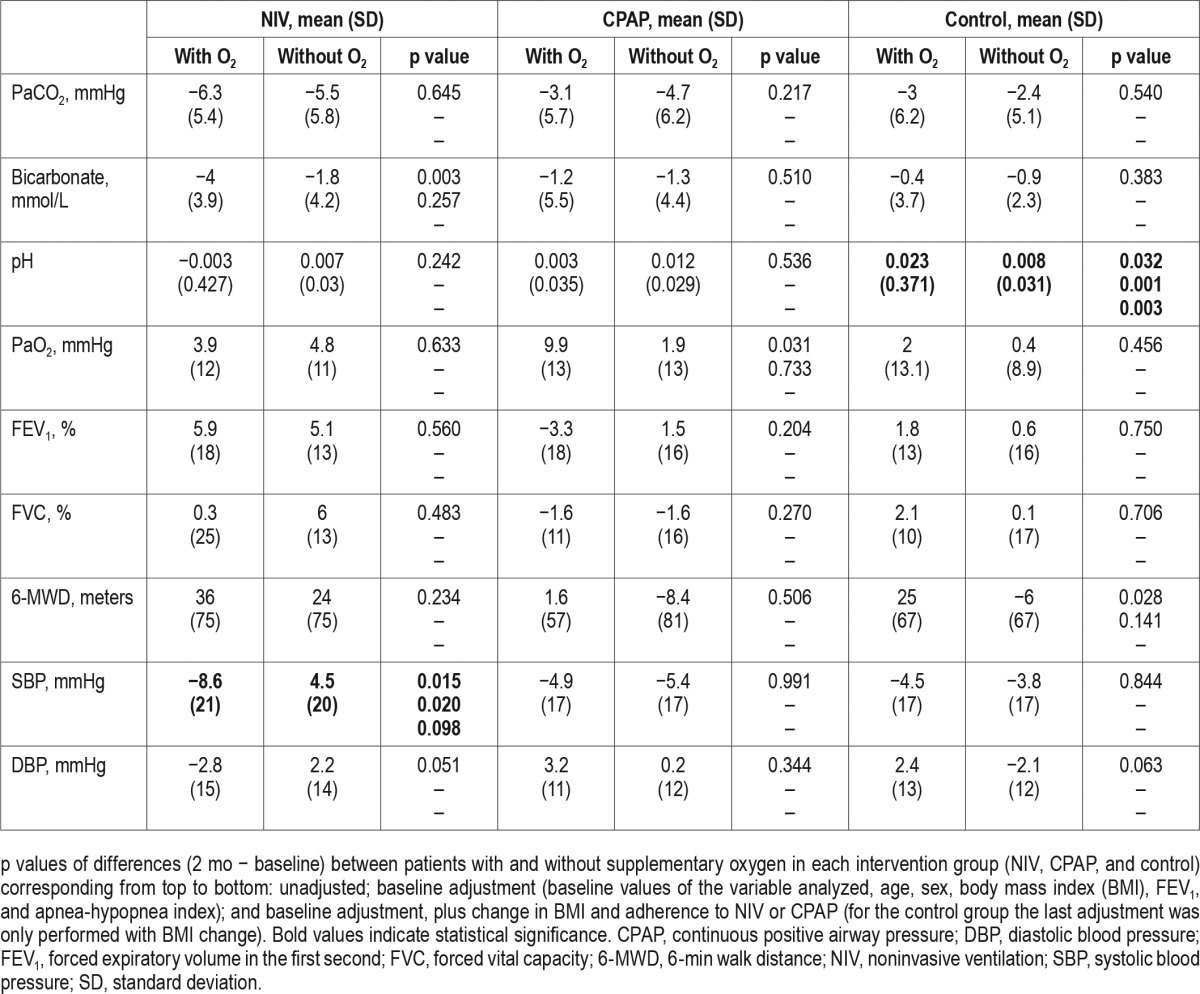
Table 3 presents the changes in the ESS score and HRQL tests with and without oxygen therapy in the three intervention groups. In the CPAP group, the physical component of the SF-36 had a tendency (p = 0.056) to improve more with oxygen therapy.
Table 3.
Changes in the Epworth Sleepiness Scale score and health-related quality-of-life tests based on assigned treatment.
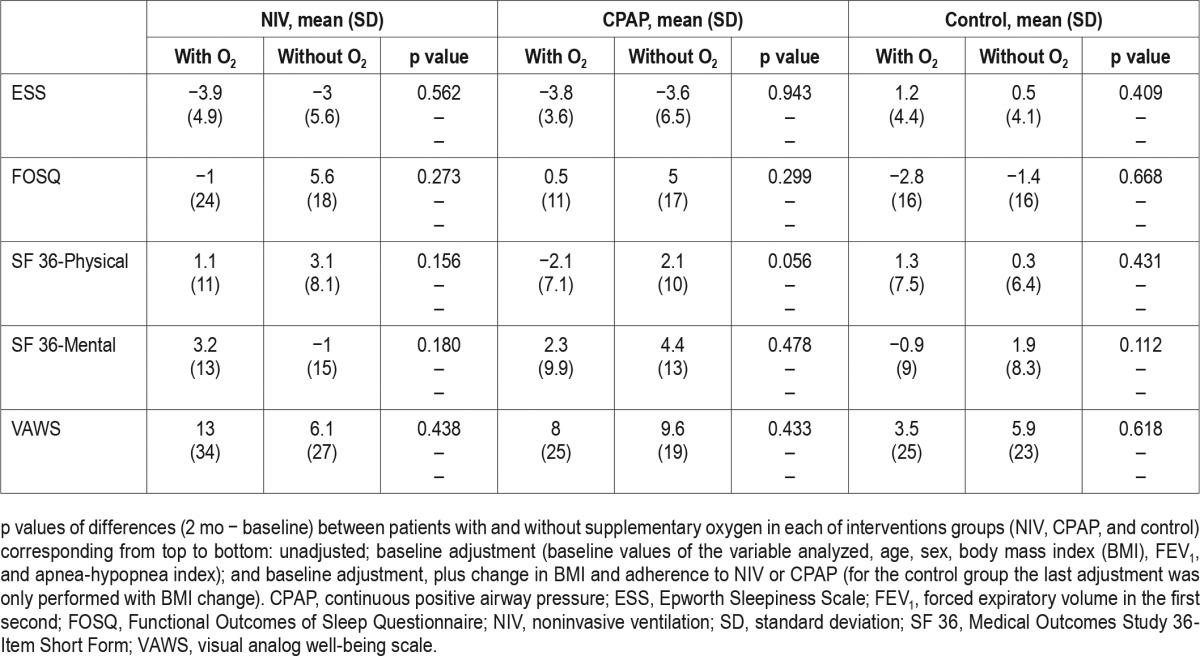
Figure 2 illustrates changes in other clinical symptoms. Patients treated with CPAP plus supplemental oxygen had a tendency for reporting morning headaches (p = 0.056) and a significantly higher frequency of morning confusion (p = 0.006). This finding persisted despite adjustments. There were no statistically significant differences in symptoms in NIV and control groups treated with or without supplemental oxygen.
Figure 2. Frequency of clinical symptoms in the three intervention groups (NIV, CPAP, and control) distributed in baseline with and without supplemental oxygen therapy and after 2 mo with and without supplemental oxygen therapy.
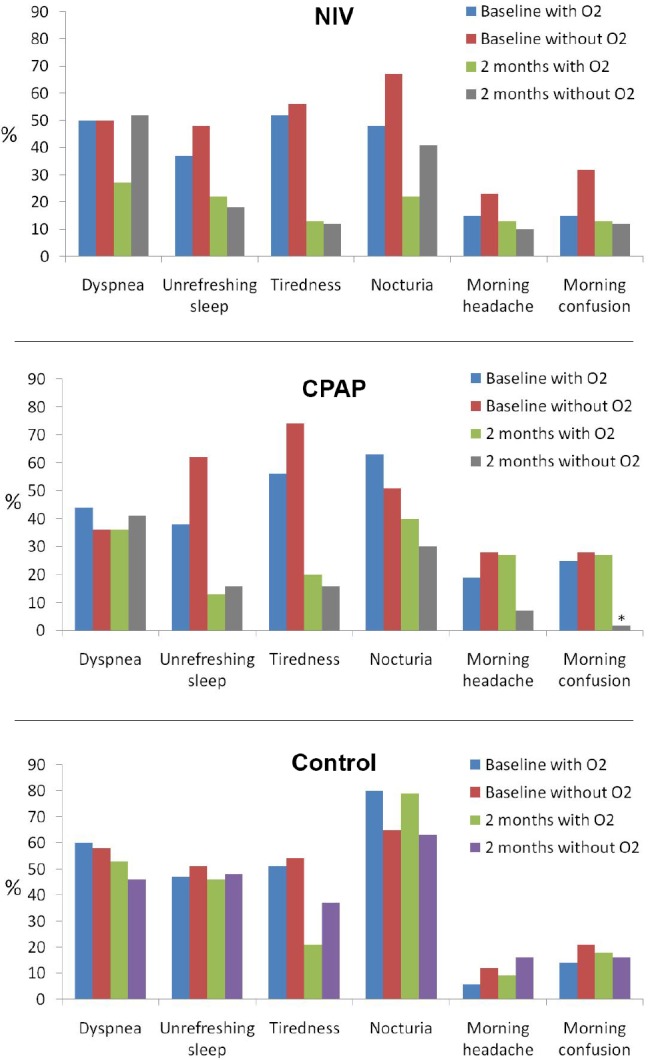
Statistically we compared the percentage at the end of the follow-up between patients with and without supplementary oxygen therapy in each of interventions groups by unadjusted analysis and the following adjusted models: (1) baseline values of the variable analyzed, age, sex, body mass index (BMI), the forced expiratory volume in 1 sec, and apnea-hypopnea index (baseline adjustment); and (2) baseline adjustment, BMI change, and CPAP or NIV compliance (hour/day); in the control group the last adjustment was only performed with BMI change. *Unadjusted p < 0.01; baseline adjusted p < 0.01; and all adjusted p < 0.01. CPAP, continuous positive airway pressure; NIV, noninvasive ventilation.
Table 4 presents the changes in polysomnographic parameters with and without chronic oxygen therapy in the three intervention groups. In the control group, there was a significant reduction in the AHI in patients treated with oxygen therapy (p = 0.033) which was maintained after both adjustments (for baseline values as well as for change in BMI). The improvement in mean oxygen saturation during sleep, however, did not remain significant after adjusting for baseline characteristics (p = 0.195).
Table 4.
Changes in polysomnographic parameters based on assigned treatment.
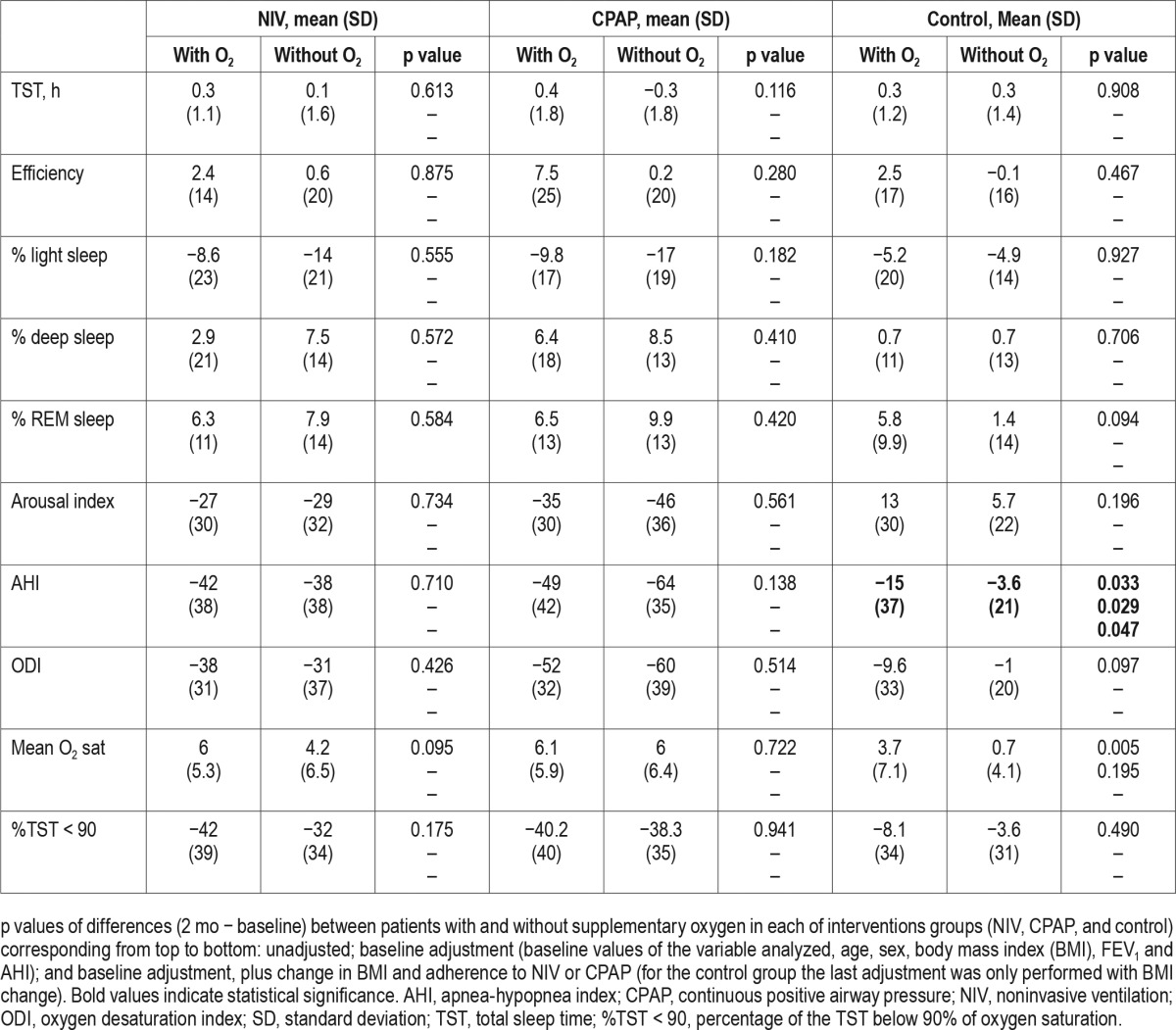
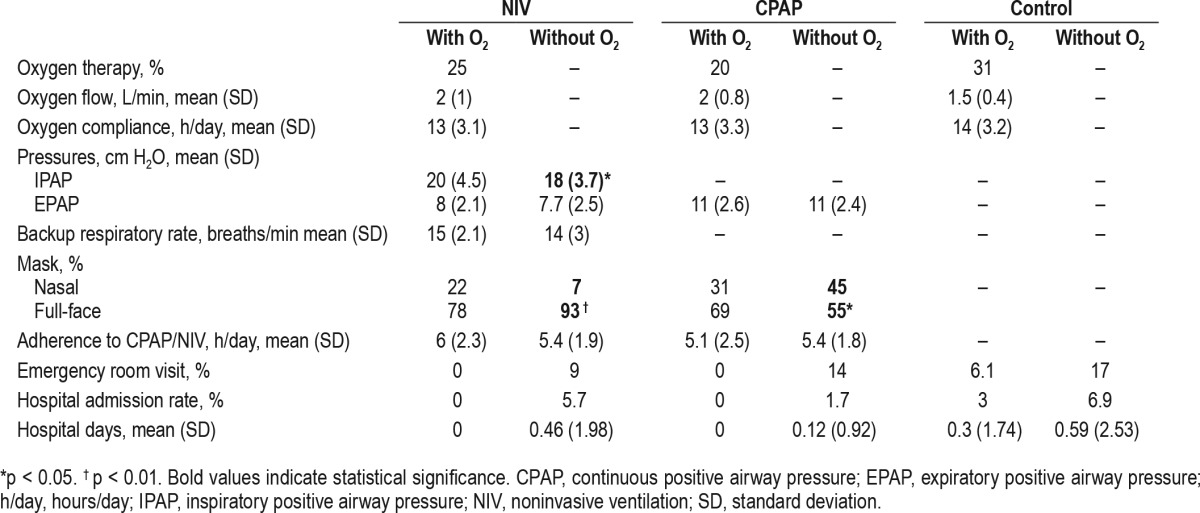
Table 5 presents the NIV, CPAP, oxygen settings, hospital admission rates, duration of hospital stay (hospital days), and emergency room visits during the follow-up period. In the NIV group, patients treated with oxygen needed more inspira-tory PAP than patients treated without oxygen. The hospital resource utilization was higher in patients treated with supplemental oxygen therapy in all three groups. However, these differences were not statistically significant.
Table 5.
Therapy settings, compliance and hospital admissions and days.
DISCUSSION
To the best of our knowledge, this is the first study comparing the medium-term efficacy and safety of adding chronic supplemental oxygen therapy to NIV, CPAP or lifestyle modification therapies in patients with OHS. The main results can be summarized as follows: (1) chronic oxygen therapy led to marginal changes in daytime respiratory functional tests, polysomnographic parameters, HRQL tests, and clinical symptoms; (2) the observed changes were dissimilar among the three commonly prescribed therapies for OHS; and (3) there was no evidence of an increase in hospital resource utilizations after 2 mo of supplemental oxygen therapy.
In clinical practice supplemental oxygen therapy is frequently prescribed for patients with OHS, either with or without PAP therapy.4 The expected benefit is to decrease nocturnal and daytime hypoxemia. However, there is a concern that oxygen therapy may result in worsening of chronic respiratory acidosis or compensatory chronic metabolic alkalosis. In the current study, oxygen therapy led to an increase in pH in the control group only, suggesting that adding supplemental oxygen therapy to lifestyle modification treatment can worsen respiratory acidosis, which leads to compensatory chronic metabolic alkalosis.17 This may have not occurred in the CPAP and NIV group because these interventions reduced PaCO2 more efficiently than lifestyle modification.10 Two laboratory-based randomized crossover studies examined the effect of supplemental oxygen therapy in ambulatory patients with stable OHS. In one study, 28 subjects were exposed to 28% and 50% oxygen concentrations for 20 min8 and in another study, 24 subjects were exposed to 100% oxygen for 20 min.7 The arterialized-venous PCO28 and transcutaneous PCO27 increased with oxygen therapy in a subgroup of patients with OHS. Respiratory acidosis occurred with a fraction of inspired oxygen (FiO2) of 0.50 but not with a FiO2 0.28. The pH was not measured in the study using 100% oxygen.7 In our study, there was not a significant change in PaCO2 although there was a greater likelihood of alkalosis in the control group when treated with supplemental oxygen for 2 mo. In addition to different methods of measuring PCO2, compared to the prior studies our study has several differences that could explain the divergent results: (1) our longer period of treatment with oxygen can compensate the initial respiratory acidosis; (2) our FiO2 was lower than in the afore-mentioned studies; and (3) ABG was obtained while the patients were off supplemental oxygen and breathing room air for at least 20 min.
Some clinical series have evaluated predictors of mortality in OHS patients treated with NIV.5,6,18,19 In two of these studies supplementary oxygen therapy was also assessed as a risk factor associated with mortality.5,6 Only in one study the addition of oxygen to NIV was independently associated with increased mortality.5 In another study,19 alkalosis (pH > 7.44) was independently associated with increased mortality in patients with OHS treated with NIV. However, in that study the investigators did not adjust for oxygen therapy as a confounder. Although our study cannot provide further information on long-term outcomes such as mortality, we only noted a trend to mild alkalosis when supplemental oxygen was added to lifestyle changes without PAP treatment.
Patients treated with oxygen reported poorer changes in the clinical symptoms compared with patients treated with habitual treatments alone without oxygen (i.e. NIV, CPAP, or lifestyle changes). The only change with oxygen was the significant increased frequency of morning confusion and a tendency (p < 0.1) toward increased morning headache when supplemental oxygen therapy was added to CPAP treatment. This finding suggests that, in contrast to NIV treatment, supplemental oxygen added to CPAP treatment may lead to a worsening of the hypoventilation during sleep. These results may be related to the tendency toward lower improvement in quality of life in patients treated with CPAP plus supplemental oxygen in comparison with patients with CPAP alone (SF-36 physical component in Table 3). Thus, we speculate that when supplemental oxygen is added to PAP therapy, NIV may be better than CPAP in counterbalancing the tendency toward worsening of nocturnal hypoventilation.
There was a significant decrease in systolic blood pressure in patients treated with supplemental oxygen in the NIV group after adjustment for confounders as well as a trend toward reduction in diastolic blood pressure (Table 2). This may be relevant as intermittent hypoxemia during sleep has been recognized as a risk factor for hypertension in patients with OSA.20,21 Although oxygen therapy can decrease the severity of OSA,22 blood pressure is not appreciably impacted with supplemental oxygen.22,23 In fact, when we adjusted the change in systolic blood pressure for the change in BMI and NIV compliance the statistical significance disappeared (Table 2), suggesting that adherence to NIV and weight loss may be more important contributors to improvement in blood pressure than supplemental oxygen added to NIV. Although patients with supplemental oxygen therapy added to NIV had higher NIV compliance than patients with NIV alone (6 h/day vs. 5.4 h/day on average, respectively), those who received oxygen and NIV experienced a greater degree of weight loss (−5.5 kg vs. −1.8 kg on average, respectively). Therefore, weight loss may have been an even more relevant factor than compliance to NIV.
All PSGs, as the rest of tests (i.e. ABG, spirometry and 6-min walk distance), were carried out without supplemental oxygen in order to better assess the efficacy of the different treatments (NIV, CPAP, and lifestyle modification). In the follow-up PSGs from control the group that were performed on room air, we found a significant reduction in the AHI when these patients were treated with supplemental oxygen therapy at home. The statistical significance persisted despite adjustment for baseline values and change in BMI, perhaps because the mean change in weight was very similar between lifestyle groups with and without supplemental oxygen subgroups (mean weight loss of −1.6 kg in both subgroups of lifestyle). We know that oxygen therapy reduces the AHI in OSA22 but we do not know if a certain positive effect remains at night without oxygen in place if it has been used at home for 2 mo. Change in oxygenation level during sleep was also better in the supplemental oxygen group (Table 4), although it could be explained based on the differences in baseline characteristics. Although variability in AHI could potentially explain these differences, we think it is less likely because it would affect both subgroups (with and without supplemental oxygen) equally. Therefore, we speculate that 2 mo of chronic oxygen therapy at home may have a “residual” carryover effect after 1 night of oxygen withdrawal, therefore reducing the AHI. However, further research in this area is necessary.
Although patients without supplemental oxygen therapy in the three groups (NIV, CPAP, and control) had greater hospital resource utilization than patients requiring supplemental oxygen therapy, the differences did not reach statistical significance. It is likely, however, that 2 mo of follow-up is insufficient to assess this outcome.
Our study has several limitations. First, it is a post hoc analysis of a randomized controlled trial and as such it lacked a priori sample size calculation based on a main outcome variable. However, our sample size is the largest of any randomized controlled trial in patients with OHS. Moreover, the current study represents the largest sample of OHS patients on supplemental oxygen followed carefully after 2 mo of therapy. Second, although we enrolled consecutive patients across several participating centers, our inclusion and exclusion criteria may have led to some selection bias and may, therefore, not be reflective of “real life” patients with OHS seen in clinical practice. Third, patients treated with supplemental oxygen had differences in some anthropometric and clinical characteristics in comparison with OHS patients without oxygen therapy. The influence of these differences was minimized by adjusting for baseline confounders. Fourth, we did not use transcutaneous CO2 measurement during the PSG test due to limited resources for incorporating it in all participating centers.
In summary, our findings suggest that 2 mo of supplemental oxygen in patients with OHS did not produce clear deleterious effects or an increase in hospital resource utilization. However, when oxygen was added to NIV it was associated with a reduction in blood pressure; when added to CPAP it was associated with increased frequency of morning confusion; and when added to lifestyle modification it was associated with increased alkalosis and lower AHI. Long-term studies are necessary to ensure that supplemental oxygen therapy does not increase the risk of cardiovascular morbidity and mortality in patients with OHS. While we await the results of these studies, we believe low-flow supplemental oxygen is safe therapy in patients with OHS as long as they are closely monitored.
DISCLOSURE STATEMENT
Funding for this study was provided by the Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo) PI050402, Spanish Respiratory Foundation 2005 (FEPAR) and Air Liquide Spain. Juan F. Masa has full access to all data from the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are indebted to Verónica Rodríguez for her assistance in the translation of the manuscript and to Vanessa Iglesias for her technical assistance.
Author contributions: Substantial contributions to study conception and design, acquisition of data, or analysis and interpretation of data: Juan F. Masa, MD, PhD; Jaime Corral, MD, Maria L. Alonso, MD, PhD; Estrella Ordax, MD; Maria F. Troncoso, MD, PhD; Monica Gonzalez, MD, PhD; Soledad Lopez-Martín, MD; Jose M. Marin, MD, PhD; Sergi Marti, MD, PhD; Trinidad Díaz-Cambriles, MD; Eusebi Chiner, MD, PhD; Carlos Egea, MD, PhD; Joaquin Teran, MD, PhD; Nicolas Gonzalez-Mangado, MD, PhD; Angeles Martínez, MD; Pilar De Lucas, MD, PhD; Santiago J. Carrizo, MD, PhD; Odile Romero, MD; Josefa Diaz, MD, PhD; Cristina Senent, MD; Miguel Merchan, MStat; Francisco Rivas, MD; Auxiliadora Romero MD; Candela Caballero, MD; Jose Maria Benítez, MD; Rafael Golpe, MD, PhD; Ana Santiago-Recuerda, MD, PhD; Silvia Gomez, MD; Monica Bengoa, MD; Estefanía García-Ledesma, MD; and M-Angeles Sánchez-Quiroga, MD. Drafting the article or revising the article critically for important intellectual content: Juan F Masa, MD, PhD; Babak Mokhlesi MD, MSc, Jaime Corral, MD; Jose M Marin, MD, PhD; Sergi Marti, MD, PhD; Eusebi Chiner, MD, PhD; Miguel Merchan, MStat, PhD; Joaquin Teran, MD, PhD; Nicolas Gonzalez-Mangado, MD, PhD; and Pilar De Lucas, MD, PhD. Final approval of the version to be published: Juan F Masa, MD, PhD; Babak Mokhlesi MD, MSc; and Miguel Merchan, MStat.
ABBREVIATIONS
- ABG
arterial blood gases
- ANCOVA
analysis of covariance
- AHI
apnea-hypopnea index
- BMI
body mass index
- CO2
carbon dioxide
- CPAP
continuous positive airway pressure
- EES
Epworth sleepiness scale
- FEV1
forced expiatory volume in the first second
- FiO2
fraction of inspired oxygen
- FVC
forced vital capacity
- HRQL
health-related quality of life
- NIV
noninvasive ventilation
- OHS
obesity hypoventilation syndrome
- OSA
obstructive sleep apnea
- PaO2
arterial partial pressure of oxygen
- PAP
positive airway pressure
- PCO2
partial pressure of carbon dioxide
- PSG
polysomnography
- SpO2
peripheral oxygen saturation
REFERENCES
- 1.Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc. 2008;5:218–25. doi: 10.1513/pats.200708-122MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borel JC, Tamisier R, Gonzalez-Bermejo J, et al. Noninvasive ventilation in mild obesity hypoventilation syndrome: a randomized controlled trial. Chest. 2012;141:692–702. doi: 10.1378/chest.10-2531. [DOI] [PubMed] [Google Scholar]
- 3.Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008;63:395–401. doi: 10.1136/thx.2007.081315. [DOI] [PubMed] [Google Scholar]
- 4.Mokhlesi B, Tulaimat A, Parthasarathy S. Oxygen for obesity hypoventilation syndrome: a double-edged sword? Chest. 2011;139:975–7. doi: 10.1378/chest.10-2858. [DOI] [PubMed] [Google Scholar]
- 5.Priou P, Hamel JF, Person C, et al. Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome. Chest. 2010;138:84–90. doi: 10.1378/chest.09-2472. [DOI] [PubMed] [Google Scholar]
- 6.Borel JC, Burel B, Tamisier R, et al. Comorbidities and mortality in hypercapnic obese under domiciliary noninvasive ventilation. PLoS One. 2013;8:e52006. doi: 10.1371/journal.pone.0052006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijesinghe M, Williams M, Perrin K, Weatherall M, Beasley R. The effect of supplemental oxygen on hypercapnia in subjects with obesity-associated hypoventilation: a randomized, crossover, clinical study. Chest. 2011;139:1018–24. doi: 10.1378/chest.10-1280. [DOI] [PubMed] [Google Scholar]
- 8.Hollier CA, Harmer AR, Maxwell LJ, et al. Moderate concentrations of supplemental oxygen worsen hypercapnia in obesity hypoventilation syndrome: a randomized crossover study. Thorax. 2014;69:346–53. doi: 10.1136/thoraxjnl-2013-204389. [DOI] [PubMed] [Google Scholar]
- 9.Masa JF, Celli BR, Riesco JA, Sánchez de Cos J, Disdier C, Sojo A. Noninvasive positive pressure ventilation and not oxygen may prevent overt ventilatory failure in patients with chest wall diseases. Chest. 1997;112:207–13. doi: 10.1378/chest.112.1.207. [DOI] [PubMed] [Google Scholar]
- 10.Masa JF, Corral J, Alonso ML, et al. Spanish Sleep Network. Efficacy of different rreatment alternatives for obesity hypoventilation syndrome. Pickwick Study. Am J Respir Crit Care Med. 2015;192:86–95. doi: 10.1164/rccm.201410-1900OC. [DOI] [PubMed] [Google Scholar]
- 11.Ortega Ruiz F, Díaz Lobato S, Galdiz Iturri JB, et al. SEPAR. Continuous home oxygen therapy. Arch Bronconeumol. 2014;50:185–200. doi: 10.1016/j.arbres.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–8. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 13.Masa JF, Jiménez A, Durán J, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004;170:1218–24. doi: 10.1164/rccm.200312-1787OC. [DOI] [PubMed] [Google Scholar]
- 14.Spanish Group of Breathing Sleep Disorders. Masa JF, Jiménez A, Durán J, et al. Visual analogical well-being scale for sleep apnea patients: validity and responsiveness: a test for clinical practice. Sleep Breath. 2011;15:549–59. doi: 10.1007/s11325-010-0399-3. [DOI] [PubMed] [Google Scholar]
- 15.García-Río F, Calle M, Burgos F, et al. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Spirometry. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Arch Bronconeumol. 2013;49:388–401. doi: 10.1016/j.arbres.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 16.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 17.Berger KI, Norman RG, Ayappa I, Oppenheimer BW, Rapoport DM, Goldring RM. Potential mechanism for transition between acute hypercapnia during sleep to chronic hypercapnia during wakefulness in obstructive sleep apnea. Adv Exp Med Biol. 2008;605:431–6. doi: 10.1007/978-0-387-73693-8_75. [DOI] [PubMed] [Google Scholar]
- 18.Ojeda Castillejo E, de Lucas Ramos P, López Martin S, et al. Noninvasive mechanical ventilation in patients with obesity hypoventilation syndrome. Long-term outcome and prognostic factors. Arch Bronconeumol. 2015;51:61–8. doi: 10.1016/j.arbres.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Budweiser S, Riedl SG, Jörres RA, Heinemann F, Pfeifer M. Mortality and prognostic factors in patients with obesity-hypoventilation syndrome undergoing noninvasive ventilation. J Intern Med. 2007;261:37–83. doi: 10.1111/j.1365-2796.2007.01765.x. [DOI] [PubMed] [Google Scholar]
- 20.Barbé F, Durán-Cantolla J, Capote F, et al. Spanish Sleep and Breathing Group. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–26. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 21.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–76. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta V, Vasu TS, Phillips B, Chung F. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2013;9:271–9. doi: 10.5664/jcsm.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–85. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.