Abstract
Study Objectives:
Patients with central disorders of hypersomnolence sometimes do not achieve satisfactory symptom control with currently available wake-promoting medications. Based on the finding that the cerebrospinal fluid from some patients with hypersomnolence demonstrates potentiation of gamma-aminobutyric acid (GABA)-A receptors in excess of that of controls, a finding that reverses with flumazenil, we initiated prescribing compounded flumazenil to carefully selected, treatment-refractory hypersomnolent patients.
Methods:
This retrospective chart review evaluated the first 153 consecutive patients treated with transdermal and/or sublingual flumazenil by physicians at our center from 2013 through January 2015.
Results:
Patients were 35.5 y old (± 14.4) and 92 (60.1%) were women. Mean Epworth Sleepiness Scale scores prior to flumazenil were 15.1 (± 4.5) despite prior or current treatment with traditional wake-promoting therapies. Symptomatic benefit was noted by 96 patients (62.8%), with a mean reduction in Epworth Sleepiness Scale score of 4.7 points (± 4.7) among responders. Of these, 59 remained on flumazenil chronically, for a mean of 7.8 mo (± 6.9 mo). Female sex and presence of reported sleep inertia differentiated flumazenil responders from nonresponders. Adverse events were common, but often did not result in treatment discontinuation. Serious adverse events included a transient ischemic attack and a lupus vasculopathy, although whether these events occurred because of flumazenil administration is unknown.
Conclusions:
This chart review demonstrates that sublingual and transdermal flumazenil provided sustained clinical benefit to 39% of patients with treatment-refractory hypersomnolence. Prospective, controlled studies of this GABA-A receptor antagonist for the treatment of hypersomnolence are needed.
Commentary:
A commentary on this article appears in this issue on page 1321.
Citation:
Trotti LM, Saini P, Koola C, LaBarbera V, Bliwise DL, Rye DB. Flumazenil for the treatment of refractory hypersomnolence: clinical experience with 153 patients. J Clin Sleep Med 2016;12(10):1389–1394.
Keywords: flumazenil, GABA-A receptor, GABA-related hypersomnia, hypersomnolence, idiopathic hypersomnia, narcolepsy
INTRODUCTION
The central disorders of hypersomnolence are generally treated with wake-promoting medications, such as modafinil or armodafinil, and traditional psychostimulants. In the case of narcolepsy, a robust evidence base supports treatment decisions,1 whereas treatment of idiopathic hypersomnia calls for off-label use of medications that are FDA-approved for narcolepsy. Despite available medication options, satisfactory control of symptoms cannot always be achieved and there is a subgroup of approximately 15% to 20% of patients who are treatment refractory.2–4 Evaluation of cerebrospinal fluid from patients whose hypersomnolence persists despite extensive trials of different wake-promoting agents has shown that affected patient cerebrospinal fluid can potentiate gamma-aminobutyric acid (GABA) currents at GABA-A receptors in vitro in excess of that of nonhypersomnolent controls.5 This increased activity can be reversed experimentally using flumazenil, which is a GABA-A receptor antagonist. Intravenous injection of flumazenil improved objective measures of vigilance and reduced subjective sleepiness in seven hypersomnolent patients with this cerebrospinal fluid finding.5 Because flumazenil is formulated and marketed for intravenous use, exhibits high first-pass metabolism, and has a short plasma half-life, translation of these findings into clinical trials and clinical practice is challenging. However, two cases of successful, long-term treatment of particularly intransigent hypersomnolence with flumazenil, one using transdermal and sublingual preparations5 and one using subcutaneous preparations,6 offer proof-of-concept that the long-term use of flumazenil in otherwise treatment-refractory hypersomnolence is feasible.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Hypersomnolence can be treated with a variety of wake-promoting medications. However, a subgroup of patients have persistent, severe sleepiness despite use of standard therapies.
Study Impact: This large case series suggests that sublingual and transdermal flumazenil may be beneficial for some patients with treatment-refractory sleepiness. A prospective, controlled study of flumazenil is needed to confirm these observations.
Based on the aforementioned findings, we began prescribing compounded flumazenil to carefully selected patients in 2013. Our criteria for use of flumazenil included the presence of a central hypersomnolence disorder that was: (1) not due to an identifiable, reversible cause (such as iron or carnitine deficiencies, hypothyroidism, insufficient sleep time, or an untreated primary disorder of sleep); (2) refractory to multiple conventional wake-promoting medications because of lack of effect, intolerable side effects, or both; and (3) severely affecting quality of life, often resulting in disability. Patients were extensively counselled about the off-label nature of this medication, the relative lack of long-term safety data, and the novel preparation forms, and some patients elected to pursue this avenue of treatment. Here, we present our clinical experience in the first 153 patients treated with compounded sublingual or transdermal flumazenil, to provide a preliminary assessment of the effectiveness and safety of flumazenil in the patients with treatment-refractory hypersomnolence.
METHODS
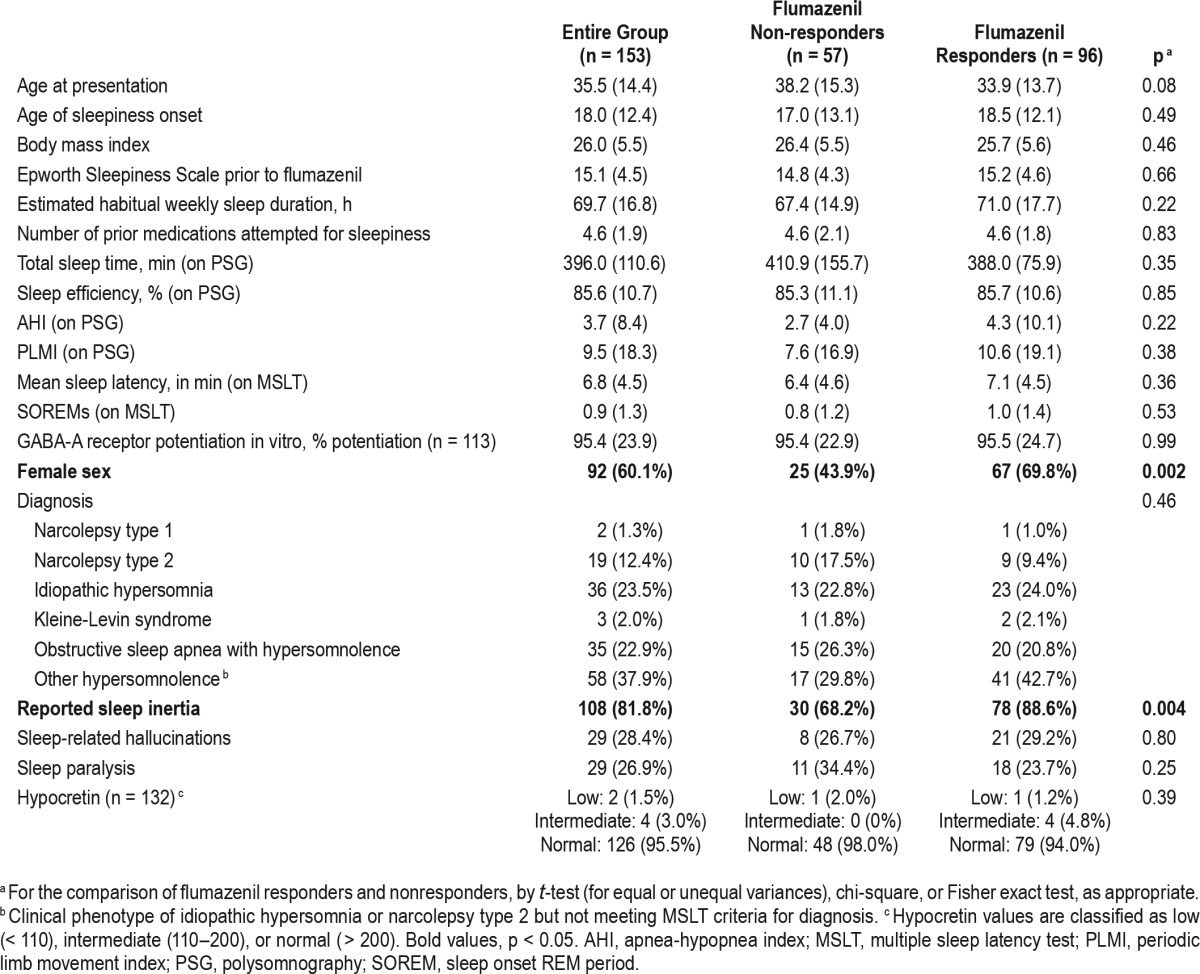
All prescriptions written for flumazenil by the authors (LMT and DBR) from 2013 through January 2015 were sent to a single, local compounding pharmacy (Pavilion Compounding, Atlanta, GA). We examined records of all sleep center patients who filled flumazenil prescriptions during this time. Clinical charts were reviewed to determine clinical characteristics and course of the patients through late January 2015. Lumbar punctures were performed in the majority of patients (see Table 1). Clinical diagnosis was assigned based on clinical phenotype and most recent multiple sleep latency testing results.
Table 1.
Clinical characteristics of flumazenil responders and nonresponders.
Based on our previously published case of successful use of compounded flumazenil for idiopathic hypersomnia,5 flumazenil lozenges were prescribed as 6-mg lozenges for sublingual delivery, with a typical starting dose of 6 mg four times a day and titration to effective dose or 12 mg four times per day, whichever was lower. Rarely, individual patients were prescribed a divided daily dose up to 60 mg/day. Flumazenil cream was prescribed as 1 mL of 12 mg/mL cream applied to forearms at bedtime, as a possible strategy to combat sleep inertia the following morning. Over time, patients reported that daytime use of cream was also beneficial for sleepiness, and some patients were then titrated up to 12 mg transdermally four times daily. The decision of which formulation of flumazenil to use was individualized by patient, but generally fit one of three patterns: (1) beginning treatment with both lozenges and cream together (in the presence of reported sleep inertia and daytime sleepiness); (2) beginning treatment with lozenges alone (in patients for whom sleep inertia was a less prominent or absent symptom); and (3) beginning treatment with cream alone (for patients who were evaluated later in the observation period, after patients had begun to report benefit with cream alone). In patients begun on one formulation, the second was sometimes added based on clinical response. For patients reporting partial symptomatic benefit from a traditional wake-promoting agent, this medication was generally continued during flumazenil titration and chronic treatment.
Patient response to flumazenil was determined using a combination of clinical records, review of electronic correspondence from patients, and pharmacy refill records. Exploratory comparisons between flumazenil responders and nonresponders were made on demographic and clinical features, using t-test, chi-square, or Fisher exact test as appropriate. This study was approved by the Emory Institutional Review Board.
RESULTS
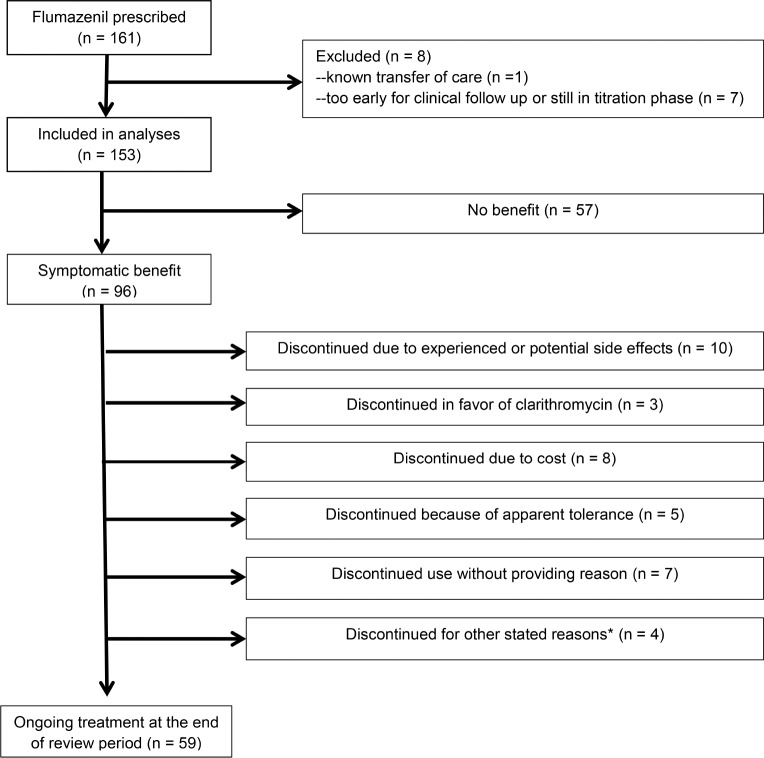
Flumazenil prescriptions were filled by 161 patients. Eight patients were excluded from analyses because flumazenil had been prescribed too close to the end of the observation period for clinical and refill data to be available (n = 7) or because the patient transferred care elsewhere such that no follow- up data were anticipated (n = 1). Analyses were performed on the remaining 153 patients, in whom the mean age was 35.5 y (standard deviation 14.4). Ninety-two patients (60.1%) were women. Patients had treatment-refractory sleepiness, with mean Epworth Sleepiness Scale (ESS) scores of 15.1 (± 4.5) despite having tried an average of 4.6 (± 1.9) medications for sleepiness prior to flumazenil. Most patients (89%) had taken modafinil (n = 40), armodafinil (n = 32), or both (n = 60; prior use unknown in 4 cases). Traditional psychostimulants (at least one, 86%) and clarithromycin (85%) had commonly been used. Wake-promoting antidepressants (i.e., bupropion or protripty-line) and sodium oxybate had been attempted in smaller proportions of patients, 50% and 25% respectively. In the case of patients with sleep apnea (n = 35), sleepiness was persistent despite treatment of sleep apnea (n = 29), known to predate the development of sleep apnea based on multiple polysomno-grams (n = 2), or was judged to be disproportionately severe relative to the mild degree of apnea such that sleep-disordered breathing was thought unlikely to account for sleepiness (n = 4). That is, these 35 patients were considered to have a central disorder of hypersomnolence that could not be fully accounted for by the presence of sleep apnea. One hundred two patients were initially prescribed lozenges alone, 12 were initially prescribed cream alone, and 39 were prescribed both lozenges and cream together as initial treatment. Eighty-eight patients (57.5 %) tried both cream and lozenges during the course of treatment.
Symptomatic benefit from flumazenil was noted by 96 patients (62.8%). ESS scores were significantly better during flumazenil treatment (baseline ESS of 15.0 ± 4.7, flumazenil ESS of 10.3 ± 5.1, p < 0.0001) among responders who provided ESS scores at both time points (n = 40). Nineteen patients reported worsening sleepiness on flumazenil, although this was most often a transient feeling lasting minutes after each dose, and nine patients who noted initial worsening of sleepiness continued flumazenil because this was transient or resolved with change in dosing. Of the 96 patients who reported symptomatic improvement, 59 patients were continuing to use flumazenil at the end of the review period (38.6% of the total group). Patients remaining on flumazenil at the end of the review period had been using it chronically for an average of 7.8 mo (± 6.9 mo). This excludes a single patient continuing treatment with flumazenil for idiopathic hypersomnia begun in 2008; her chronic experience with flumazenil has been previously reported.5 Reasons for discontinuation in the remaining 37 patients are detailed in Figure 1. Comparing those who noted symptomatic benefit from flumazenil and those who did not notice benefit (Table 1), the only clinical characteristics that differed were an increased proportion of women and an increased proportion with reported sleep inertia among those who responded. Among women, the response rate was 73% versus 48% in men (p = 0.002). Among those with sleep inertia, the response rate was 72% versus 42% in those without sleep inertia (p = 0.004). Nearly all patients (94%) were prescribed lozenges at some point and there was no difference in response rate between those who had or had not tried the lozenge form of flumazenil. In contrast, patients who were prescribed cream were more likely to report a benefit from flumazenil than those never prescribed cream (71% response rate among the 97 prescribed cream and only 48% response rate in the 56 not prescribed cream, p = 0.005). However, as patients were not randomly assigned to cream or lozenge, this may reflect confounding by indication.
Figure 1. Flowchart shows clinical course of patients treated with flumazenil for refractory hypersomnolence.
*Other reasons for discontinuation included one each of: difficulty with dosing while at work, short duration of benefit, desire to become pregnant, and apparent spontaneous remission.
Adverse events while on flumazenil were noted by 79 patients (52%), although only 17 of these cited experiencing adverse events as the reason for medication discontinuation. In one of these patients, mild, asymptomatic elevation in liver function tests was the adverse event requiring medication discontinuation. Thirty-four of the patients who reported adverse events chose to continue flumazenil, because side effects were mild, transient, resolved with change of dosing or formulation, or were outweighed by benefits. The remaining 28 patients endorsing side effects reported other reasons for discontinuation or did not provide a reason. Two serious adverse events occurred: one patient with a history of atrial fibrillation and branch retinal artery occlusion experienced a transient ischemic attack; and one patient with systemic lupus erythematosus had asymptomatic, radiographic evidence of a central nervous system lupus vasculopathy. Dizziness was the most commonly reported adverse event, occurring in 20 patients (13%). Anxiety was reported by 10 patients (7%) and other mood disturbances by 9 patients (6%). Adverse events reported by at least 2 patients included: headache (n = 10), insomnia (n = 6), cognitive dysfunction (n = 6), variability in medication effectiveness or worsening symptoms as the medication wore off (n = 5), paresthesias (n = 4), weight gain or increase in appetite (n = 4), nausea (n = 4), bad taste (n = 3), dry mouth (n = 2), weakness (n = 2), abdominal pain (n = 2), change in dream content (n = 2), and muscle twitches (n = 2).
DISCUSSION
This retrospective chart review demonstrates that sublingual and/or transdermal flumazenil were associated with sustained and clinically meaningful improvement in symptoms in 39% of patients with treatment-refractory hypersomnolence. Patients were selected for flumazenil treatment on the basis of severe, often profound and disabling, symptoms that persisted despite standard wake-promoting medications, and therefore are not representative of all hypersomnolent patients. Although the results of this retrospective chart review should be confirmed in a controlled, prospective study, this percentage of responders appears clinically meaningful, especially in a population who in whom a mean of 4.6 other medications for the treatment of hypersomnolence had failed. The reasons for the incomplete response rate might reflect variable absorption, incomplete penetration into the central nervous system, or disease heterogeneity. The percentage of sustained improvement seen in our series is similar to the clinical response rate reported for clarithromycin (38%)7 and pitolisant (37%)8 in patients with similar treatment-refractory hypersomnolence. Yet, the incomplete response to all of these therapies, as well as to more traditional treatments such as modafinil (continuation rate of 50% in patients with idiopathic hypersomnia)9 or amphetamines (continuation rate of 29% to 66% in patients with idiopathic hypersomnia),9 suggests that as-yet-unidentified clinical or biological factors could help predict which patients will respond optimally to which therapies. In the current series, only female sex and reported sleep inertia were associated with positive response to flumazenil. The presence of sleep inertia is supportive for the diagnosis of idiopathic hypersomnia.10 Intravenous flumazenil has been approved by the United States Food and Drug Administration for the reversal of benzodiazepine sedation since the early 1990s, and there is a long history of consideration of off-label use of flumazenil for neurologic and psychiatric diseases. In addition to potential utility as a treatment for benzodiazepine dependence,11 intravenous flumazenil has been preliminarily evaluated for the treatment of Parkinson disease,12 hepatic encephalopathy,13 and schizophrenia.14 However, because the half-life of flumazenil is short15 and the benefit of intravenous flumazenil on sleepiness and vigilance is correspondingly short lived,5 intravenous flumazenil is not a viable clinical solution for refractory hypersomnolence. Oral flumazenil has been safely administered at single doses as high as 100–600 mg.15–18 However, due to large hepatic first-pass metabolism, the oral bioavailability is only 16% of the intravenous bioavailability.15 To bypass first-pass metabolism while using a delivery system compatible with long-term use, we employ compounded sublingual and/or transdermal flumazenil. For other clinical applications, especially the treatment of benzodiazepine addiction and hepatic encephalopathy, sublingual spray, subcutaneous pump, and implantable wafer preparations of flumazenil have been tested or are in development.19–21 The latter two preparations have been reported to be beneficial in a single patient with idiopathic hypersomnia.6
The safety profile of intravenous flumazenil is well established. Serious adverse effects include seizures, arrhythmias, and re-sedation as the medication is cleared, but the risk of seizures is thought to be most prominent with chronic use of benzodiazepines or in cases of combined benzodiazepine and tricyclic antidepressant overdose.22 In contrast, for patients with refractory epilepsy not on benzodiazepines, there is some evidence of an antiepileptic effect of flumazenil.18,23 No seizures were reported in our patients. Anxiety was relatively common among patients in our series, consistent with previously reported effects of flumazenil on anxiety and mood.16,17,23,24 Because flumazenil is most often used as an acute, rather than chronic, treatment, data regarding the effects of medium- or long-term use of flumazenil are more limited. Subcutaneous or intravenous flumazenil infusions for a duration of 4 to 8 days have been well tolerated and have shown benefit in management of benzodiazepine withdrawal symptoms, although these interventions await further testing in large controlled trials.19 Oral flumazenil at divided doses up to 90 mg per day was used successfully in 27 patients with epilepsy for an average of 22 mo (range, 1–42 mo) with no serious adverse events and side effects in 30%.23 Oral flumazenil taken for days to months has also been used in individual cases of hepatic encephalopathy, with mixed results.25,26 Here, we contribute to existing data on chronic use of flumazenil by demonstrating preliminary evidence for effectiveness and few serious adverse events, with treatment-limiting adverse events in 11% of patients. The relationship between adverse events and flumazenil use in this uncontrolled chart review cannot be determined. In particular, we were unable to locate literature demonstrating a relationship between flumazenil and vascular disease, given the two serious adverse events noted in our patients. However, in light of the relatively limited available literature, we have conservatively opted to extensively detail all adverse events experienced, regardless of relationship, or lack thereof, with flumazenil use.
In summary, our clinical experience in a large group of patients with treatment-refractory hypersomnolence demonstrates meaningful and sustained clinical response in a substantial fraction of patients. Important questions remain about optimal formulation, dosing, long-term safety, and effectiveness. Prospective, controlled studies, ideally with measurement of plasma or cerebrospinal fluid flumazenil levels, are clearly needed. However, our experience suggests the possibility of clinical use of flumazenil in carefully selected, severely affected patients lacking other treatment options.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by K23 NS083748 (LMT), R01 NS089719 and the Mind Science Foundation (DBR), and UL1 TR000424 from the National Institutes of Health. Dr. Trotti reports fees to her institution from Jazz Pharmaceutical and Balance Therapeutics, outside the submitted work. Dr. Bliwise reports personal fees from New England Research Institute, Ferring Pharmaceuticals, Morehouse School of Medicine, Vantia Therapeutics, Merck, and Georgia Institute of Technology, outside the submitted work. Dr. Rye has a US patent application (U.S. 20110028418A1) pending for the use of GABA-A receptor antagonists for the treatment of hypersomnia and other disorders of excessive sleepiness. Dr. Rye reports personal fees from Flamel Technologies on topics related to the current work, however the current work was in no way supported by or funded by Flamel Technologies. Dr. Rye reports personal fees from Jazz Pharmaceuticals, UCB Pharma, and Xenoport Inc., outside the submitted work. The other authors have indicated no financial conflicts of interest. Off-label and investigational use: This manuscript presents new data on off-label use of flumazenil and discusses published work regarding off-label use of flumazenil, modafinil, armodafinil, psychostimulants, clarithromycin, and investigational use of pitolisant.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Brad Cherson, RPh, for providing information on flumazenil prescriptions compounded by Pavilion Pharmacy; Amanda Freeman, PhD and Andrew Jenkins, PhD, for performing patch-clamp assays of GABA-A receptor function in the presence of patient cerebrospinal fluid; and Drs. Emmanuel Mignot (Stanford Center for Sleep Sciences and Medicine), Joan Santamaria and Alex Iranzo (Neurology Service, Hospital Clinic of Barcelona), and Glenda Keating (Emory University), for performing hypocretin measurements.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ESS
Epworth Sleepiness Score
- GABA
gamma-aminobutyric acid
- MSLT
multiple sleep latency test
- PLMI
periodic limb movement index
- PSG
polysomnography
- SD
standard deviation
- SOREM
sleep onset REM period
REFERENCES
- 1.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–11. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KN, Pilsworth S, Sharples LD, Smith IE, Shneerson JM. Idiopathic hypersomnia: a study of 77 cases. Sleep. 2007;30:1274–81. doi: 10.1093/sleep/30.10.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise MS, Arand DL, Auger RR, Brooks SN, Watson NF. Treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1712–27. doi: 10.1093/sleep/30.12.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics. 2012;9:739–52. doi: 10.1007/s13311-012-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rye DB, Bliwise DL, Parker K, et al. Modulation of Vigilance in the Primary Hypersomnias by Endogenous Enhancement of GABAA Receptors. Sci Transl Med. 2012;4:161ra51. doi: 10.1126/scitranslmed.3004685. [DOI] [PubMed] [Google Scholar]
- 6.Kelty E, Martyn V, O'Neil G, Hulse G. Use of subcutaneous flumazenil preparations for the treatment of idiopathic hypersomnia: a case report. J Psychopharmacol. 2014;28:703–6. doi: 10.1177/0269881114523865. [DOI] [PubMed] [Google Scholar]
- 7.Trotti LM, Saini P, Freeman AA, et al. Improvement in daytime sleepiness with clarithromycin in patients with GABA-related hypersomnia: clinical experience. J Psychopharmacol. 2013;28:697–702. doi: 10.1177/0269881113515062. [DOI] [PubMed] [Google Scholar]
- 8.Leu-Semenescu S, Nittur N, Golmard J-L, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med. 2014;15:681–7. doi: 10.1016/j.sleep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Ali M, Auger RR, Slocumb NL, Morgenthaler T. Idiopathic hypersomnia: clinical features and response to treatment. J Clin Sleep Med. 2009;5:562–8. [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 11.Hulse G, Kelty E, Hood S, Norman A, Basso MR, Reece AS. Novel indications for benzodiazepine antagonist flumazenil in GABA mediated pathological conditions of the central nervous system. Curr Pharm Des. 2015;21:3325–42. doi: 10.2174/1381612821666150619092720. [DOI] [PubMed] [Google Scholar]
- 12.Ondo WG, Silay YS. Intravenous flumazenil for Parkinson's disease: a single dose, double blind, placebo controlled, cross-over trial. Mov Disord. 2006;21:1614–7. doi: 10.1002/mds.21022. [DOI] [PubMed] [Google Scholar]
- 13.Als-Nielsen B, Gluud LL, Gluud C. Benzodiazepine receptor antagonists for hepatic encephalopathy. Cochrane Database Syst Rev. 2004:CD002798. doi: 10.1002/14651858.CD002798.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Menzies L, Ooi C, Kamath S, et al. Effects of gamma-aminobutyric acid-modulating drugs on working memory and brain function in patients with schizophrenia. Arch Gen Psychiatry. 2007;64:156–67. doi: 10.1001/archpsyc.64.2.156. [DOI] [PubMed] [Google Scholar]
- 15.Roncari G, Ziegler WH, Guentert TW. Pharmacokinetics of the new benzodiazepine antagonist Ro 15-1788 in man following intravenous and oral administration. Br J Clin Pharmacol. 1986;22:421–8. doi: 10.1111/j.1365-2125.1986.tb02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgitt A, Lader M, Fonagy P. The effects of the benzodiazepine antagonist Ro 15-1788 on psychophysiological performance and subjective measures in normal subjects. Psychopharmacology. 1986;89:395–403. doi: 10.1007/BF02412110. [DOI] [PubMed] [Google Scholar]
- 17.Darragh A, Lambe R, O'Boyle C, Kenny M, Brick I. Absence of central effects in man of the benzodiazepine antagonist Ro 15-1788. Psychopharmacology. 1983;80:192–5. doi: 10.1007/BF00427969. [DOI] [PubMed] [Google Scholar]
- 18.Sharief MK, Sander JW, Shorvon SD. The effect of oral flumazenil on interictal epileptic activity: results of a double-blind, placebo-controlled study. Epilepsy Res. 1993;15:53–60. doi: 10.1016/0920-1211(93)90009-v. [DOI] [PubMed] [Google Scholar]
- 19.Hulse G, O'Neil G, Morris N, Bennett K, Norman A, Hood S. Withdrawal and psychological sequelae, and patient satisfaction associated with subcutaneous flumazenil infusion for the management of benzodiazepine withdrawal: a case series. J Psychopharmacol. 2013;27:222–7. doi: 10.1177/0269881112446532. [DOI] [PubMed] [Google Scholar]
- 20.Hood SD, Norman A, Hince DA, Melichar JK, Hulse GK. Benzodiazepine dependence and its treatment with low dose flumazenil. Br J Clin Pharmacol. 2014;77:285–94. doi: 10.1111/bcp.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saadi T, Kramskay R, Zilberman Peled B, Katz N, Peled N, Baruch Y. Pharmacokinetics and safety of sublingual flumazenil (CRLS035) in healthy adults (potential therapy for hepatic encephalopathy) J Pharmacogenom Pharmacoproteom. 2014;5:140. [Google Scholar]
- 22.Spivey WH. Flumazenil and seizures: analysis of 43 cases. Clin Ther. 1992;14:292–305. [PubMed] [Google Scholar]
- 23.Scollo-Lavizzari G. The clinical anti-convulsant effects of flumazenil, a benzodiazepine antagonist. Eur J Anaesthesiol Suppl. 1988;2:129–38. [PubMed] [Google Scholar]
- 24.Girdler NM, Lyne JP, Wallace R, et al. A randomised, controlled trial of cognitive and psychomotor recovery from midazolam sedation following reversal with oral flumazenil. Anaesthesia. 2002;57:868–76. doi: 10.1046/j.1365-2044.2002.02785.x. [DOI] [PubMed] [Google Scholar]
- 25.Meier R, Gyr K. Treatment of hepatic encephalopathy (HE) with the benzodiazepine antagonist flumazenil: a pilot study. Eur J Anaesthesiol Suppl. 1988;2:139–46. [PubMed] [Google Scholar]
- 26.Ferenci P, Grimm G, Meryn S, Gangl A. Successful long-term treatment of portal-systemic encephalopathy by the benzodiazepine antagonist flumazenil. Gastroenterology. 1989;96:240–3. doi: 10.1016/0016-5085(89)90787-7. [DOI] [PubMed] [Google Scholar]