Abstract
Study Objectives:
REM sleep behavior disorder (RBD) is a common manifestation of Parkinson disease (PD). In this study, we assessed the effects of rotigotine transdermal patch on RBD features in patients with PD.
Methods:
In this prospective open-label study, eleven PD patients with untreated RBD were administered rotigotine patches for up to seven months to ameliorate their parkinsonism. The severities of their RBD symptoms before and after rotigotine therapy were evaluated through patient and bed partner interviews, a validated evaluation scale (REM sleep behavior disorder questionnaire-Hong Kong, RBDQ-HK), and blinded assessments based on video-polysomnographic (VPSG) measure.
Results:
Rotigotine improved parkinsonism and subjective sleep quality in PD patients with RBD. The RBDQ-HK total score, especially the Factor 2 score, was decreased, which demonstrated that the subjective severity of RBD symptoms was improved after rotigotine treatment, especially the frequency and severity of abnormal RBD-related motor behaviors. The VPSG analyses showed that the total sleep time (TST) and stage 1% were increased and that the PLMS index was decreased. However, no differences in the RBD-related sleep measures were observed.
Conclusions:
The improved RBD symptoms and VPSG measures of PD patients in this study (TST, stage 1%, and PLMS index) suggest that, in PD, rotigotine may partially improve RBD-related symptoms. Rotigotine should be considered to be an optional drug for the treatment of RBD symptoms in PD.
Citation:
Wang Y, Yang Y, Wu H, Lan D, Chen Y, Zhao Z. Effects of rotigotine on REM sleep behavior disorder in Parkinson disease. J Clin Sleep Med 2016;12(10):1403–1409.
Keywords: Parkinson disease, rotigotine transdermal patch, dopamine agonist, REM sleep behavior disorder, video-polysomnography
INTRODUCTION
REM sleep behavior disorder (RBD) is characterized by dream-enacting behaviors, unpleasant dreams, and the intermittent loss of normal muscle atonia during REM sleep (RWA) based on video-polysomnography.1 Approximately 15% to 50% of Parkinson disease (PD) patients suffer from RBD.2 These patients manifest shouting, talking, laughing and motor movements that are potentially harmful to themselves or to their bed partners and that cause severe sleep disruption.3 No randomized, double-blind, placebo-controlled study has been reported regarding treatment of RBD. However, a few drugs have been reported as effective, such as clonazepam,4 melatonin,5 and pramipexole,6,7 a dopamine agonist. However, little is known regarding the relationship between RBD and dopaminergic deficiency.
The non-ergolinic dopamine agonist rotigotine is formulated in a silicone-based transdermal patch for administration once per day.8 The efficacy of rotigotine has been demonstrated as a monotherapy in early PD8 and an adjuvant to levodopa in advanced PD.9 With continuous drug release that allows for a constant plasma concentration over 24 h, rotigotine provides an attractive option for the management of PD patients with end-of-dose symptom deterioration, such as nocturnal sleep disorders.10 Rotigotine induced a greater improvement in sleep quality, as recorded by the Parkinson's Disease Sleep Scale-2 (PDSS-2) compared with placebo.10,11 However, no published trails have investigated the efficacy of rotigotine on RBD symptoms.
BRIEF SUMMARY
Current Knowledge/Study Rationale: REM sleep behavior disorder (RBD) is a common manifestation of Parkinson disease (PD). No published trails have investigated the efficacy of nonergolinic dopamine agonist rotigotine on RBD symptoms.
Study Impact: In PD, rotigotine can improve the frequency and severity of abnormal motor behaviors, improve the quality of sleep of patients. Rotigotine could be used as an alternative to clonazepam for RBD patients with PD.
The present exploratory open-label study was designed to observe clinical and video-polysomnographic changes to determine the efficacy of rotigotine on PD patients with RBD.
METHODS
Subjects
This study was approved by the local ethics committee of Shanghai Chang Zheng Hospital. All patients gave written informed consent prior to participation. Rotigotine is an adjunct dopaminergic treatment to further improve the symptoms of PD patients. Among them, patients with RBD symptoms (sleep disruption, unpleasant dreams, dream-enacting behaviors, or sleep-related injury) performed VPSG tests. Patients who underwent VPSG confirmed the presence of RBD and thus were eligible for enrollment in the study. PD was diagnosed according to the UK Brain Bank Criteria.12 Diagnosis of RBD was based on the International Classification of Sleep Disorders, 2nd Edition (ICSD-2) criteria.1 The PSG criteria of RBD was according to a published method.13,14 Patients were allowed to be prescribed levodopa (except controlled-release levodopa or > 5 daily doses of immediate-release levodopa), anticholinergic agents, entacapone, monoamine oxidase-B inhibitors or amantadine, provided that doses were stable for ≥ 28 days prior to baseline assessment and stable during the trial. Exclusion criteria were dementia, hallucinations, psychosis, current or previous treatment with other dopamine agonists, or antidopaminergic agents. None of the patients had received drug-treatment for RBD, such as clonazepam. As severe obstructive sleep apnea-hypopnea (OSAH) could mimic the symptoms of RBD, patients with sleep-disordered breathing, as evidenced by clinical interview and PSG recording, were excluded. None of the patients showed an apnea index > 10 or an index of respiratory events > 15.
Study Design and Clinical Assessment
The study included a pretreatment period (for eligibility assessment, review of patch application procedure), a 3-day baseline period (including 2 overnight inpatient stays at a medical center for the VPSG measurement and other clinical assessments), up to 8 weeks' titration and a 12–20 weeks' dose-maintenance period. Over at least 12 weeks, 2 overnight VPSG measurements and other clinical assessments were performed before the end of the maintenance period with the patch still attached. Rotigotine was initiated at 2 mg/24 h and gradual increased by 2 mg/24 h according to parkinsonism response and tolerance until a maximum dose of 16 mg/24 h was reached. The VPSG results and other clinical assessments before and after rotigotine treatment were compared.
Parkinsonism and subjective sleep quality were assessed using the Unified Parkinson's Disease Rating Scale (UPDRS)-II (for daytime functioning), the Unified Parkinson's Disease Rating Scale (UPDRS)-III (for motor performance), the PD Sleep Scale-2 (PDSS-2), and the Epworth Sleepiness Scale (ESS). The RBD symptoms frequency and severity were measured by the REM sleep behavior disorder questionnaire-Hong Kong (RBDQ-HK).13 These questionnaires were completed shortly after the patients took their regular morning dose of oral anti-parkinsonian medications and were in the “on” state. As patients in this study only had up to 7 months of follow-up, RBDQ-HK scores were based on the patients' average symptoms during the last 3 months instead of the last one year.15
Nocturnal Video-Polysomnography
Participants underwent 2 overnight nocturnal VPSG reviews. The first night was regarded as a night for adaptation, and the measures taken during the second night were used for analysis. VPSG recordings were collected and stored digitally using a U.S. Polysmith SW-SM2000C polysomnography recorder. VPSG recording contained the following montages: bilateral electro-oculogram (EOG) derivations, standard electroencephalographic (EEG) derivations (C3-A2, C4- A1, O1-A2, O2-A1), electrocardiogram chin and 2 lower limb surface EMG derivations (right and left extensor digitorum communis), oronasal airflow based on a thermocouple and nasal pressure measurements, sonogram, oxyhemoglobin saturation, and chest and abdomen inductance plethysmography. All PSG data were recorded in synchrony with continuous video monitoring.
The data were scored by a board certified sleep specialist blinded to the clinical information. VPSG staging and scoring followed the American Academy of Sleep Medicine (AASM) guidelines,16 with the exception that REM sleep stage was scored based only on electroencephalogram and electoroculograms14 to account for the frequent of occurrence of REM sleep without atonia (RWA) in PD. During REM sleep, we calculated the REM density, the phasic and tonic electromyographic activity in the submentalis muscle,17 and the percentage of REM sleep with abnormal behaviors,7 as previously reported. To evaluate movements and vocalization during REM sleep in the VPSG recordings, we employed the REM sleep behavior disorder severity scale (RBDSS).18 RBDSS categorizes sleep on an event-to-event basis. The final score was determined by the highest score obtained in each VPSG recording.4
Statistical Analysis
Descriptive statistical analyses were performed using the SPSS19.0 program. Changes in questionnaire scores and polysomnographic variables after rotigotine treatment were assessed using the Wilcoxon signed rank test. Missing data were not imputed. P < 0.05 was considered statistically significant.
RESULTS
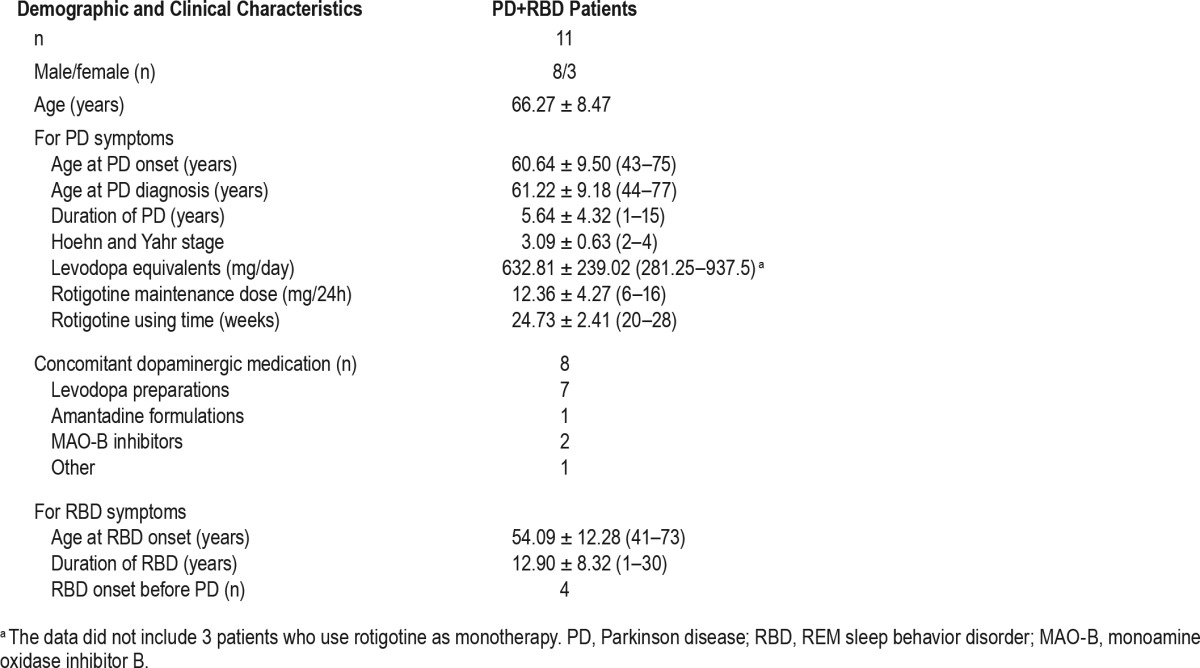
Eleven PD patients (8 men and 3 women) who were clinical diagnosed with RBD and confirmed by VPSG were enrolled. All patients at baseline had at least submental tonic EMG activity over 30% and/or submental phasic EMG activity over 15% of REM sleep time. The mean parkinsonism duration was 5.64 ± 4.32 years, and the H&Y stage was 3.09 ± 0.63. The mean RBD duration was 12.90 ± 8.32 years. RBD developed after parkinsonism in 4 patients, occurred simultaneously in 3 patients, and preceded from the onset of parkinsonism in the remaining 4 patients. Among the enrolled patients, 8 patients were taking other parkinsonism drugs: 4 were taking levodopa, 1 was taking monoamine oxidase inhibitor (MAO)-B, 1 was taking levodopa and amantadine, 1 was taking levodopa and MAO-B, and 1 was taking levodopa and entacapone. At study entrance, the mean levodopa daily dose was 632.81 ± 239.02 mg, and the mean levodopa treatment duration was 5.44 ± 4.41 years. At the end of the study, the rotigotine daily dose was 12.36 ± 4.27 mg, and the mean duration of rotigotine therapy was 24.73 ± 2.41weeks in all patients (shown in Table 1).
Table 1.
Baseline demographic and clinical characteristics.
The comparison of scales and VPSG parameters obtained before and after rotigotine therapy are shown in Table 2. The mean UPDRS-III and UPDRS-II scores decreased (from 37.18 ± 10.43 to 33.18 ± 10.02, p = 0.012, and from 17.18 ± 6.74 to 14.82 ± 5.23, p = 0.023, respectively), indicating that parkinsonian motor symptoms improved after the introduction of rotigotine. The PDSS-2 scores decreased (from 20.27 ± 7.72 to 17.36 ± 5.71, p = 0.046), indicating an improvement in nocturnal sleep. A worsening of daytime sleepiness as measured by the ESS was not statistically significant. The mean score for the overall RBDQ-HK scale decreased (from 44.18 ± 17.71 to 36.42 ± 17.96, p = 0.011), and the RBDQ-HK factor 2 score (Q6-Q12, behavioral factor) decreased (from 30.36 ± 12.26 to 25.27 ± 12.34, p = 0.040). There was no statistically significant difference in the mean score for factor 1 (Q1-Q5 and Q13, dream-related factor). These results demonstrated that the subjective severity of RBD symptoms was improved after rotigotine therapy (RBDQ-HK total score), especially for the frequency and severity of abnormal RBD-related motor behaviors (RBDQ-HK Factor 2). Two individual RBDQ-HK items (Q8 and Q9) decreased with rotigotine treatment, indicating a more significant improvement in some individual symptomatology of RBD, such as “dream-related movements” or “falling out of bed” (shown in Figure 1). In total, 63.64% patients (n = 7) demonstrated reductions in RBD symptoms after initiating rotigotine therapy.
Table 2.
Variables (questionnaire scores and VPSG parameters) before and after stable rotigotine treatment of RBD patients.
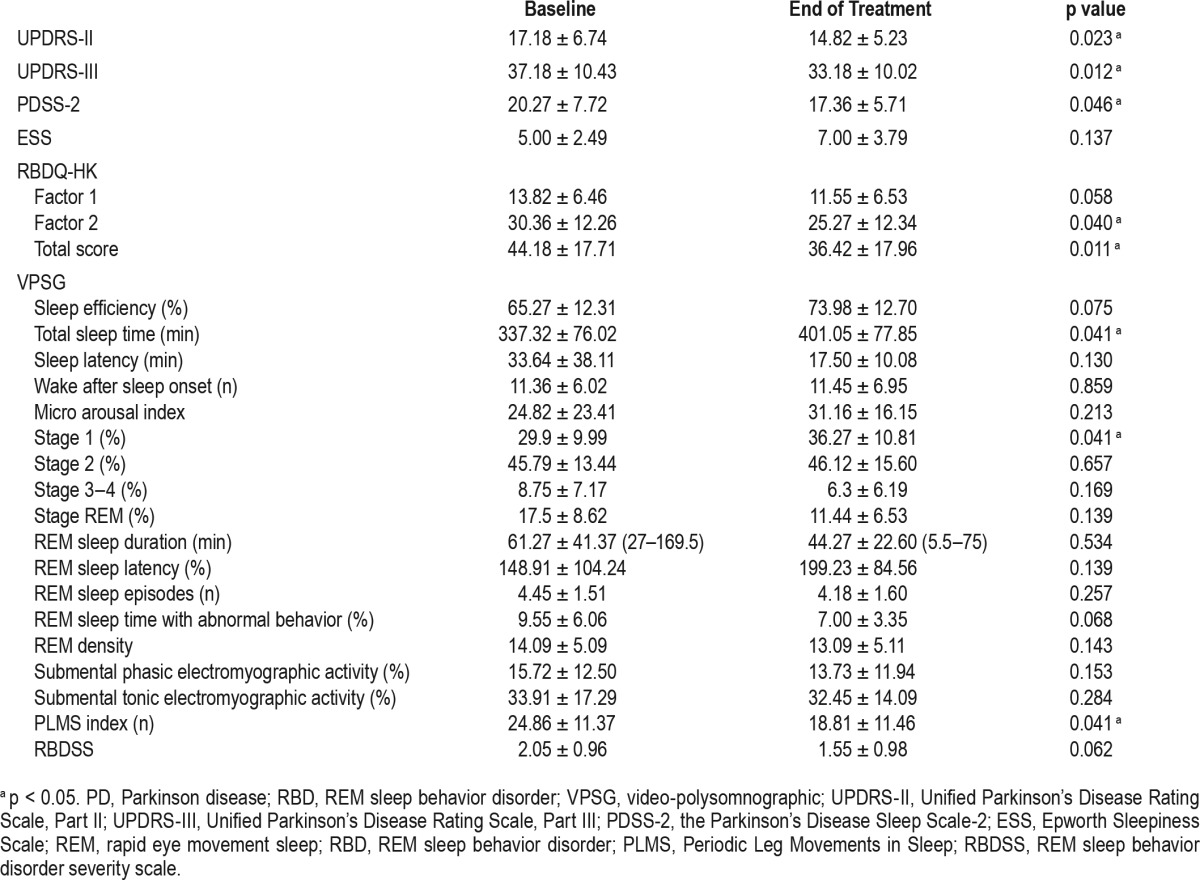
Figure 1. Changes of RBDQ-HK individual items before and after stable rotigotine treatment.
*p < 0.05 for mean changes of RBDQ-HK individual items from baseline to end of maintenance of rotigotine treatment.
After rotigotine treatment, TST and stage 1% were increased (from 337.32 ± 76.02 to 401.05 ± 77.85, p = 0.041 and from 29.9 ± 9.99 to 36.27 ± 10.81, p = 0.041, respectively), and the PLMS index was significantly decreased (from 24.86 ± 11.37 to 18.81 ± 11.46, p = 0.041). However, there were no significant differences in sleep efficiency, sleep latency, wake after sleep onset, micro arousal index, stage 2 %, stage 3–4 %, stage REM %, REM sleep latency, or REM sleep episodes. No differences were found in RBD specific sleep measures, including tonic submental electromyographic activity, phasic submental electromyographic activity, and percentage of REM sleep time spent with abnormal behaviors. There was no significant difference in the mean RBDSS score, which represents movements and vocalization during REM sleep in VPSG recordings. In these patients, 54.55% of patients (6 patients) exhibited a decrease in the RBDSS score, indicating a reduction in the RBD symptoms based on the VPSG video.
The common adverse events were application site reactions (n = 2), nausea (n = 1) and somnolence (n = 1) and were mild in intensity.
DISCUSSION
After the introduction of rotigotine, the mean UPDRS-III and UPDRS-II scores were decreased. The PDSS-2 score decreased, indicating an improvement in motor symptoms and nocturnal sleep in PD comorbidity with RBD patients, which is consistent with previous reports in PD patients.8–10 The subjective severity of RBD symptoms was improved after rotigotine therapy (RBDQ-HK total score), especially for the frequency and severity of abnormal RBD related motor behaviors (RBDQHK Factor 2). Two individual RBDQ-HK items significantly decreased (“dream-related movements” and “falling out of bed”). The VPSG analyses showed that TST and stage 1% significantly increased; the PLMS index decreased, and 54.55% of patients (6 patients) exhibited reductions in RBD symptoms based on the VPSG video (RBDSS score).
RBD has been observed in up to half of all PD patients.2 Until now, systematic controlled studies of drugs for treatment of RBD in PD have been lacking. Only few reports have studied the effect of dopaminergic agents on RBD and the data are conflicting. Some PD patients have experienced subjective improvement of RBD symptoms after the administration of levodopa,19 but others have reported that RBD onset was temporarily associated with the initiation of levodopa, selegiline, and dopamine agonists.20 Dopamine agonists, such as pramipexole,6 have been reported as sufficient effective drugs for treatment of RBD. Three small case studies reported that approximately 61.7% ∼ 89% of idiopathic RBD (iRBD) patients experienced a reduction in RBD symptoms after pramipexole treatment,6,21,22 whereas Kumru7 reported that pramipexole did not modify RBD related symptoms or VPSG abnormalities in PD patients (symptomatic RBD, sRBD). However, these conclusions were unconvincing due to the differing entrance criteria (iRBD patients, sRBD patients, or both of them); without a validated evaluation scale for the severity of RBD symptoms6,7,21; without VPSG data in some studies.6 Recently, Taeko Sasai23 found that RWA/REM was a predictive factor of the responsiveness to pramipexole treatment in 98 patients. Pramipexole is applicable, especially for mild iRBD cases with a lower rate of RWA.23 However, another possibility is that dopamine agonists could treat iRBD patients, but not sRBD patients, as reported by Kumru, which indicates that the pathogenesis of sRBD may differ from that of iRBD and that the dopamine mechanism may not play a central role in the pathogenesis of sRBD.7
However, our results showed that the subjective severity of RBD symptoms was significantly improved after rotigotine therapy in PD patients, which differs from the results of pramipexole on PD patients reported by Kumru for various reasons: (1) We employed a validated scale (RBDQ-HK) evaluation for RBD symptoms instead of subjective reports by the patients, and therefore, our results may be more reliable. (2) Rotigotine may have a different mechanism of action than pramipexole on RBD. Rotigotine, which provides continuous drug delivery over 24 h, favors maintenance of the drug concentration in the blood and is effective at night. Activation of D1 receptors is unique to rotigotine among the non-ergot-derived dopamine receptor agonists, and the dopamine D1 receptor is involved in the regulation of REM sleep.24,25
Previous VPSG studies on the efficacy of dopamine agonists in RBD patients are rare, and all of which have focused on pramipexole. In these studies, no differences were observed in sleep architecture or general sleep measure variables after treatment.7,21,22 However, Fantini et al. reported that pramipexole increased tonic, but not phasic, electromyographic activity during REM sleep,21 and a reduction in the sleep motor behaviors was confirmed by video recording. Kumru et al.7 did not find any difference in sleep measures detected on video recordings. Sasai et al.22 reported reduced REM density, and the rate of change in RBD symptoms was positively correlated with the rate of REM density reduction. Our study reports for the first time that rotigotine increases TST and the stage 1 % of patients, which was consistent with the result that the PDSS-2 scores decreased after rotigotine treatment. The result that rotigotine decreased the PLMS index is consistent with previous studies.26
In this study, the results that there were no changes in RBD-specific sleep measures assessed via VPSG were not confirmed with the reduction in the sleep motor behaviors assessed via the RBDQ-HK test, which may be due to the following reasons: (1) The clinical and VPSG measures of RBD vary every night in the individual PD patients. VPSG analysis was based on measures from only one night in this study. However, the RBDQ-HK was designed to evaluate RBD symptoms over a period of time. (2) How VPSG parameters represent the severity of RBD symptoms remains a problem worth studying. In RBD patients with multiple system atrophy, the REM sleep tonic, but not phasic, electromyographic activity was inversely correlated to monoaminergic binding in the striatum.27 Some researchers believe that excessive phasic, but not tonic, electromyographic activity during REM sleep reflects the clinical behavioral manifestations of RBD.28 (3) In this study, we employed RBDSS, a polysomnographic video-based scale, for rating the severity of abnormal motor behavior and vocalizations of RBD.18 Although, the RBDSS score tended to decrease, the difference was not significant. On one hand, this result might represent type 2 error due to the small number of patients in this study. On the other hand, RBDSS also has some limitations, such as defining RBD severity according to the most severe episode and neglecting the frequency and duration of the movement18; movements could be hidden or not recorded on the video based on VPSG records from only one night. Therefore, other VPSG parameters that more reliably reflect the severity of RBD symptoms are needed to further test the effect of rotigotine on RBD patients.
The role of dopamine mechanisms in RBD pathogenesis not fully understood. The lack of effects of pramipexole on RBD7 suggests that, in PD, dopamine mecha nisms do not play a central role in the pathogenesis of RBD. However, iRBD is a prodrome of PD, and the fact that RBD frequently appears secondary to PD indicates a possible connection between dopamine and RBD.29 Furthermore, patients with iRBD show nigrostriatal dopaminergic dysfunction manifested in SPECT imaging as reduced striatal dopamine transporter levels,30,31 reduced striatal dopamine transmission,32 and decreased putamen dopamine uptake.33 In addition, dopamine depletion engenders increased muscle tone during REM sleep, and RBD onset was affected by an imbalance of DA levels in an MPTP model of PD.34 Structures such as the substantia nigra pars compacta (SNpc) have also been purportedly linked to REM and NREM sleep circuits.35 A more recent study revealed that dopamine inhibits neurons from the rat dorsal subcoeruleus nucleus, which is a critical structure for the generation and maintenance of REM sleep through the activation of α2-adrenergic receptors.36 In agreement with the above results, the result that rotigotine improved RBD symptoms in PD patients in this study also suggests a relationship between dopamine and RBD pathogenesis. Dopaminergic impairment might be one of the major substrates for RBD pathogenesis, although RBD pathogenesis could be heterogeneous.37,38
This study has some limitations. First, given its open nature and lack of a control group as well as the small number of patients, the reliability of the results may be questioned. Second, the results of our study were assessed using a validated rating scale of RBD related to clinical symptomatology (RBDQ-HK), which indicated that the frequency and severity of dream-enacting behaviors were reduced after rotigotine therapy. However, the RBDQ-HK was originally designed to evaluate RBD symptoms during the last one year.13 Although previous studies have used it to evaluate short-term therapeutic effects,15 a verification system regarding its usefulness for short-term evaluation has not been published thus far. Third, the mean UPDRS-III score and the mean UPDRS-II score decreased after rotigotine in this study. It cannot be excluded that the improvement in RBD symptoms may be associated with improved patient PD motor symptoms, mobility at bedtime, and attenuation of dystonia or pain in specific patients, which have been demonstrated as therapeutic effects of rotigotine in other previous studies.10,39 Although not significant, the decrease in REM duration may have contributed to the reduced behavioral manifestations of RBD after rotigotine treatment. Therefore, this study is only a preliminary exploration. Further research using a large sample size and considering various additional influencing factors should be conducted to confirm the results reported herein.
In conclusion, rotigotine can improve the frequency and severity of abnormal motor behaviors, especially for dream-related movements and falling out of bed; improve the quality of sleep of patients; increase TST and the stage 1%; and decrease the PLMS index. Rotigotine could be used as an alternative to clonazepam for RBD patients with PD, especially for elderly patients or patients with obstructive sleep apnea syndrome. A double-blinded, placebo-controlled trial of rotigotine treatment in RBD patients should be conducted to confirm the results reported here.
DISCLOSURE STATEMENT
This study was supported by National Natural Science Foundation of China (81100990, 81171252); The Ministry of Science and Technology Plan Fund Major Projects (2011ZXJ09202-015). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All authors have read this version of the article, approve the submission. The present work is not currently under consideration for publication elsewhere. No conflict of interest, off-label or investigational use exists in the submission of this manuscript. The corresponding author Zhong-xin Zhao takes full responsibility for the data, the analyses and interpretation, and the conduct of the research; has full access to all of the data; and has the right to publish any and all data separate and apart from any sponsor. The authors have indicated no financial conflicts of interest. This work was performed in the department of neurology, Changzheng Hospital, Second Military Medical University, Shanghai, China.
ACKNOWLEDGMENTS
The authors thank all patients for their participation.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- ESS
Epworth Sleepiness Scale
- ICSD
International Classification of Sleep Disorders
- PD
Parkinson disease
- PDSS
the Parkinson's Disease Sleep Scale
- PLMS
periodic leg movements in sleep
- RBD
REM sleep behavior disorder
- RBDQ-HK
REM sleep behavior disorder questionnaire-Hong Kong
- RBDSS
REM sleep behavior disorder severity scale
- REM
rapid eye movement sleep
- RWA
REM sleep without atonia
- UPDRS
Unified Parkinson's Disease Rating Scale
- TST
total sleep time
- VPSG
video-polysomnographic
REFERENCES
- 1.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders 2nd ed: diagnostic and coding manual. [Google Scholar]
- 2.Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatry. 2008;79:387–91. doi: 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- 3.Iranzo A, Santamaria J, Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev. 2009;13:385–401. doi: 10.1016/j.smrv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Ferri R, Marelli S, Ferini-Strambi L, et al. An observational clinical and video-polysomnographic study of the effects of clonazepam in REM sleep behavior disorder. Sleep Med. 2013;14:24–29. doi: 10.1016/j.sleep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 5.McCarter SJ, Boswell CL, St Louis EK, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013;14:237–42. doi: 10.1016/j.sleep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt MH, Koshal VB, Schmidt HS. Use of pramipexole in REM sleep behavior disorder: results from a case series. Sleep Med. 2006;7:418–23. doi: 10.1016/j.sleep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Kumru H, Iranzo A, Carrasco E, et al. Lack of effects of pramipexole on REM sleep behavior disorder in Parkinson disease. Sleep. 2008;31:1418–21. [PMC free article] [PubMed] [Google Scholar]
- 8.Watts RL, Jankovic J, Waters C, Rajput A, Boroojerdi B, Rao J. Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology. 2007;68:272–6. doi: 10.1212/01.wnl.0000252355.79284.22. [DOI] [PubMed] [Google Scholar]
- 9.Poewe WH, Rascol O, Quinn N, et al. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson's disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol. 2007;6:513–20. doi: 10.1016/S1474-4422(07)70108-4. [DOI] [PubMed] [Google Scholar]
- 10.Trenkwalder C, Kies B, Rudzinska M, et al. Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER) Mov Disord. 2011;26:90–9. doi: 10.1002/mds.23441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray Chaudhuri K, Martinez-Martin P, Antonini A, et al. Rotigotine and specific non-motor symptoms of Parkinson's disease: post hoc analysis of RECOVER. Parkinsonism Relat Disord. 2011;19:660–5. doi: 10.1016/j.parkreldis.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen SS, Shen Y, Xiong KP, et al. Validation study of REM sleep behavior disorder questionnaire-Hong Kong (RBDQ-HK) in east China. Sleep Med. 2014;15:952–8. doi: 10.1016/j.sleep.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Montplaisir J, Gagnon JF, Fantini ML, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010;25:2044–51. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 15.Howell MJ, Arneson PA, Schenck CH. A novel therapy for REM sleep behavior disorder (RBD) J Clin Sleep Med. 2011;7:639–644. doi: 10.5664/jcsm.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. [Google Scholar]
- 17.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42:1371–4. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 18.Sixel-Doring F, Schweitzer M, Mollenhauer B, Trenkwalder C. Intraindividual variability of REM sleep behavior disorder in Parkinson's disease: a comparative assessment using a new REM sleep behavior disorder severity scale (RBDSS) for clinical routine. J Clin Sleep Med. 2011;7:75–80. [PMC free article] [PubMed] [Google Scholar]
- 19.Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25:140–2. doi: 10.1016/s0163-8343(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 20.Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Age, drugs, or disease: what alters the macrostructure of sleep in Parkinson's disease? Sleep Med. 2012;13:1178–83. doi: 10.1016/j.sleep.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Fantini ML, Gagnon JF, Filipini D, Montplaisir J. The effects of pramipexole in REM sleep behavior disorder. Neurology. 2003;61:1418–20. doi: 10.1212/wnl.61.10.1418. [DOI] [PubMed] [Google Scholar]
- 22.Sasai T, Inoue Y, Matsuura M. Effectiveness of pramipexole, a dopamine agonist, on rapid eye movement sleep behavior disorder. Tohoku J Exp Med. 2012;226:177–81. doi: 10.1620/tjem.226.177. [DOI] [PubMed] [Google Scholar]
- 23.Sasai T, Matsuura M, Inoue Y. Factors associated with the effect of pramipexole on symptoms of idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2013;19:153–7. doi: 10.1016/j.parkreldis.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Wen XS, Chen XM, Rong F, Jing T, Chen S, Ma WL. The regulation of SKF38393 on the dopamine and D1 receptor expression in hippocampus during chronic REM sleep restriction. CNS Neurosci Ther. 2013;19:730–3. doi: 10.1111/cns.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trampus M, Ferri N, Adami M, Ongini E. The dopamine D1 receptor agonists, A68930 and SKF 38393, induce arousal and suppress REM sleep in the rat. Eur J Pharmacol. 1993;235:83–7. doi: 10.1016/0014-2999(93)90823-z. [DOI] [PubMed] [Google Scholar]
- 26.Trenkwalder C, Beneš H, Poewe W, et al. Efficacy of rotigotine for treatment of moderate-to-severe restless legs syndrome: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2008;7:595–604. doi: 10.1016/S1474-4422(08)70112-1. [DOI] [PubMed] [Google Scholar]
- 27.Gilman S, Koeppe RA, Chervin RD, et al. REM sleep behavior disorder is related to striatal monoaminergic deficit in MSA. Neurology. 2003;61:29–34. doi: 10.1212/01.wnl.0000073745.68744.94. [DOI] [PubMed] [Google Scholar]
- 28.Iranzo A, Frauscher B, Santos H, et al. Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med. 2011;12:284–8. doi: 10.1016/j.sleep.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 30.Mossa EP, Niccoli Asabella A, Iuele F, Stabile Ianora AA, Giganti M, Rubini G. Striatal dopamine transporter levels in patients with REM sleep behavior disorder: assessment with 123I-FP-CIT SPECT. Recent iProg Med. 2012;103:500–4. doi: 10.1701/1166.12896. [DOI] [PubMed] [Google Scholar]
- 31.Eisensehr I, Linke R, Tatsch K, et al. Increased muscle activity during rapid eye movement sleep correlates with decrease of striatal presynaptic dopamine transporters. IPT and IBZM SPECT imaging in subclinical and clinically manifest idiopathic REM sleep behavior disorder, Parkinson's disease, and controls. Sleep. 2003;26:507–12. doi: 10.1093/sleep/26.5.507. [DOI] [PubMed] [Google Scholar]
- 32.Wing YK, Lam SP, Zhang J, et al. Reduced striatal dopamine transmission in REM sleep behavior disorder comorbid with depression. Neurology. 2015;84:516–22. doi: 10.1212/WNL.0000000000001215. [DOI] [PubMed] [Google Scholar]
- 33.Arnulf I. REM sleep behavior disorder: motor manifestations and pathophysiology. Mov Disord. 2012;27:677–89. doi: 10.1002/mds.24957. [DOI] [PubMed] [Google Scholar]
- 34.Verhave PS, Jongsma MJ, Van den Berg RM, et al. REM sleep behavior disorder in the marmoset MPTP model of early Parkinson disease. Sleep. 2011;34:1119–25. doi: 10.5665/SLEEP.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lima MM. Sleep disturbances in Parkinson's disease: the contribution of dopamine in REM sleep regulation. Sleep Med Rev. 2013;17:367–75. doi: 10.1016/j.smrv.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Yang N, Zhang KY, Wang FF, Hu ZA, Zhang J. Dopamine inhibits neurons from the rat dorsal subcoeruleus nucleus through the activation of alpha2-adrenergic receptors. Neurosci Lett. 2014;559:61–6. doi: 10.1016/j.neulet.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 37.Kotagal V, Albin RL, Muller ML, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol. 2012;71:560–8. doi: 10.1002/ana.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YE, Jeon BS, Paek SH, et al. Rapid eye movement sleep behavior disorder after bilateral subthalamic stimulation in Parkinson's disease. J Clin Neurosci. 2015;22:315–9. doi: 10.1016/j.jocn.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Swick TJ, Friedman JH, Chaudhuri KR, et al. Associations between severity of motor function and nonmotor symptoms in Parkinson's disease: a post hoc analysis of the RECOVER Study. Eur Neurol. 2014;71:140–7. doi: 10.1159/000355019. [DOI] [PubMed] [Google Scholar]


