Abstract
The present study aims to assess the toxic effect of latex and ethanolic leaf extract of Calotropis procera (C. procera), in comparison to abamectin, on serum biomarkers of function and histological integrity of heart and testis in male albino rats. To achieve this aim, the albino rats were separately administered 1/20 and 1/10 of LD50 of C. procera latex, ethanolic C. procera leaf extract and abamectin respectively by oral gavage for 4 and 8 weeks. C. procera latex and leaf extract as well as abamectin markedly elevated the activities of serum CK-MB, AST and LDH at the two tested periods in a dose dependent manner. Lipid peroxidation was significantly increased while GSH level and GPx, GST and SOD activities were significantly depleted in heart and testis of all treated rats. All treatments also induced a marked increase in serum TNF-α and decrease in serum IL-4, testosterone, FSH and LH levels in a dose dependent manner. The latex seemed to be more effective in deteriorating the testicular function and sex hormones’ levels while the ethanolic leaf extract produced more deleterious effects on oxidative stress and antioxidant defense system in both heart and testis. The normal histological architecture and integrity of the heart and testis were perturbed after treatments and the severity of lesions, which include odema, inflammatory cell infiltration, necrosis and degeneration, is dose and time dependent. In conclusion, the findings of this study indicated that C. procera latex and ethanolic extract of leaves could induce marked toxicity in heart and testis and these toxic effects may be more or less similar to those of abamectin. The cardiotoxicity and testicular toxicity may be mediated via stimulation of inflammation, increased oxidative stress and suppression of antioxidant defense system.
Keywords: Calotropis procera, Abamectin, Heart, Testis, Toxicity
Background
Development of new rodent control methodologies and strategies continues to be an exciting subject for researchers. In the last two decades, there has been a shift in rodenticide use, with researchers and pest control practitioners taking a renewed interest in alternatives to anticoagulants; this stems from increased resistance of pests to 1st generation anticoagulants (Quy et al. 1995), as well as concerns over the secondary-poisoning risks and wildlife contamination associated with field use of 2nd generation anticoagulants (Eason and Turck 2002; Thomas et al. 2011). It was found by Carvalho et al. (2003) that many natural compounds have been suggested as alternatives to conventional chemicals used for pest control. It was reported that plant extracts have been used as pesticides by humans (Abou-Hashem 2013). The use of toxic plants is especially prevalent in the developing and underdeveloped countries, where plants grown locally are cheaper than the synthetic chemical pesticides (EL-Gengaihi et al. 1997; Paul and Kumar 2009; Million et al. 2010).
Calotropis is a small genus having six species of shrubs or small trees, distributed in tropical and subtropical Africa, Asia and America. Two species namely Calotropis procera (C. procera) and Calotropis gigantea (C. gigantea) are found in India which closely resembled to each other in structure and in functional uses (Bhatnagar 1950). It was revealed that C. procera includes various chemicals which are useful for various activities (Sheth 2011; Begum et al. 2013). The entire plant has been reported to contain alkaloids, sterols, flavonoids, cardiac glycosides, triterpenoids and usharin (Suresh Kumar et al. 2013). In an earlier study, various medicinal properties such as a laxative, anthelmintic, purgative, anti-inflammatory and diuretic have been documented (Iqbal et al. 2005). Different parts of C. procera and its latex have shown analgesic, antibacterial and wound healing properties in traditional medicine (Laitiff et al. 2010; Lima-Filho et al. 2010). The previous pharmacological studies on C. procera include reports of its anticancer, antifungal and insecticidal activity (Ahmed et al. 2006; Hassan et al. 2006).
Despite these uses, C. procera poses varying toxic effects in animals through air borne allergies, touch and consumption in livestock. Vadlapudi and Naidu (2010) revealed that the plant is also known for its toxic properties that include iridocyclites, dermatitis and acts like a poison and produces lethal effects. Toxicity of C. procera is reported in sheep in the form of anorexia and diarrhea. Consumption of this plant leads to severe poisoning to livestock as well as man. Incidental ingestion of fresh C. procera leaves has been suggested as toxic to many ruminants by several farmers from the Brazilian semi-arid region. These observations are supported by some studies that have reported toxic effects promoted by C. procera latex and leaves (Mahmoud et al. 1979a, b; Singhal and Kumar 2009). The latex of C. procera contains several cardenolides such as calotropin, catotoxin, calcilin and gigantin which are caustic and considered poisonous in nature (Kuriachen and Dave 1989).
Biocides are widely used in agriculture and can contaminate rivers and other water bodies due to transport from cultivated areas (Cerejeira et al. 2003; Maloschik et al. 2007). Abamectin, the non-proprietary name assigned to avermectin B1, is a mixture of two components, with the major component avermectin B1a 80 % of the mixture, and the minor component avermectin B1b, 20 % of the mixture, differing by a single methylene group (Agarwal 1998). The two components, B1a and B1b, have similar biological and toxicological properties (Lankas and Gordon 1989; Gallo and Lawryk 1991). As indicated by Kolar et al. (2008), abamectin has been used in several countries as a pest control agent in livestock and as an active substance of nematicides and insecticides for agricultural use. ABM may be valuable in agriculture; it may be highly toxic to mammals (Moline et al. 2000).
Therefore, this study aims to verify the toxic effect of latex and ethanolic extract of leaves of C. procera on heart and testis compared with the biocide abamectin.
Methods
Plant materials
The leaves and latex of C. procera were obtained from East desert of Beni-Suef Governorate. The plant was authenticated by Dr. Walaa Azmy Hasan, lecturer of plant taxonomy, Department of Botany, Faculty of Science, Beni Suef University.
Collection of leaves and extract preparation
Only mature leaves without sign of lesion were used. The leaves of C. procera were extracted by ethanol according to Freedman et al. (1979). Leaves were washed with distilled water, air dried at room temperature and ground into fine powder using electrical mixer. Five hindered grams of the powder were suspended in 1 l of ethanol 95 % for 72 h then filtered and the filtrate was evaporated by rotary evaporator at high pressure and temperature 40–50 °C at Faculty of pharmacy, Beni-Suef University, Egypt. The extract was kept in a refrigerator at −30 °C until use.
Latex collection
Fresh latex was obtained by breaking the leaf stock and allowing the latex to flow into a glass beaker. It was freshly prepared before injection.
Pesticide and chemicals
Abamectin (1.8 % EC) is a mixture of 80 % avermectin B1a and maximum of avermectin B1b used as an acaricide. It was obtained from Synganta Agro. Co. (Switzerland).
Reagent kits used for determination of creatinine kinase-MB (CK-MB) activity was purchased from Spinreact Company (Spain). Aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) reagent kits were purchased from Biosystems Company (Spain). Testosterone reagent kits were purchased from BioSource Company (Belgium). Follicle stimulating hormone (FSH) and luteinizing hormone (LH) reagent kits were purchased from Monobind, INC. (USA). Tumor necrosis factor-alpha (TNF-α) and Interleukin-4 (IL-4) kits were purchased from R&D Systems, Inc. (USA). All other used chemicals are of analytical grade and were obtained from Sigma-Aldrich Chemical Company (USA).
Experimental animals
Male albino rats weighing 120–150 g (8–10 weeks of age) were used as experimental animals in this investigation. They were obtained from the Animal House of Research Institute of Ophthalmology, Giza, Egypt. Animals were supplied daily standard pellet diet and were given water ad libitum. The animals were housed in polypropylene cages with good aerated stainless steel in Animal House of Zoology Department, Faculty of Science, Beni-Suef University, Egypt at temperature 20–25 °C and 12-h daily light dark cycles. The animals were kept for 2 weeks under observation before the onset of the experiment to exclude any intercurrent infection. All animal procedures are in accordance with the recommendation of the Experimental Animals Ethics Committee of Faculty of Science, Beni-Suef University. All efforts were done to decrease the suffering of animals to a minimum.
Experimental design
Experimental animals were divided into seven groups as follow:
Group 1: Rats of this group are regarded as control group and were administered 1 % carboxy methyl cellulose (CMC) by oral gavage for 4 and 8 weeks.
Group 2: Rats of this group were orally administered 1/20 of LD50 (50 % lethal dose) of C. procera latex (66 µl/kg b. wt), dissolved in 1 % CMC, for 4 and 8 weeks. LD50 of C. procera latex is 1.316 ml/kg b. wt as detected by Fahim et al. (2016).
Group 3: Rats of this group were orally administered 1/10 of LD50 of C. procera latex (132 µl/kg b. wt), dissolved in 1 % CMC, for 4 and 8 weeks.
Group 4: Rats of this group were orally administered 1/20 of LD50 of ethanolic extract of C. procera leaves (4.78 mg/kg b. wt), dissolved in 1 % CMC, for 4 and 8 weeks. LD50 of ethanolic extract of C. procera leaves is 95.52 mg/kg b. wt (El-Shafey et al. 2011).
Group 5: Rats of this group were orally administered 1/10 of LD50 of ethanolic extract of C. procera leaves (9.56 mg/kg b. wt), dissolved in 1 % CMC, for 4 and 8 weeks.
Group 6: Rats of this group were orally administered 1/20 of LD50 of abamectin (vertimec 1.8 % EC) (0.44 mg/kg b. wt), dissolved in 1 % CMC, for 4 and 8 weeks. LD50 of abamectin vertimec (1.8 % EC) is 8.7 mg/kg body weight (Lankas and Gordon 1989; El-Shafey et al. 2011).
Group 7: Rats of this group were orally administered 1/10 of LD50 of abamectin (vertimec 1.8 % EC) (0.87 mg/kg b. wt), dissolved in 1 % CMC, for 4 and 8 weeks.
Samples preparation
At the end of the 4th and 10th weeks, six animals of each group were sacrificed under diethyl ether anesthesia. Blood samples were obtained from cervical vein, left to coagulate at room temperature and then they were centrifuged at 3000 rpm for 30 min. The clear non-haemolysed supernatant sera were quickly removed and were divided into 3 portions. The obtained samples were kept in deep freezer at −30 °C till used. Half of heart and testis were excised and removed quickly, homogenized by using in isotonic solution (0.9 % NaCl) and kept in deep freezer at −30 °C till used. The other half of heart and other testis were immediately excised and fixed in 10 % neutral buffered formalin for histopathological processing.
Biochemical investigations
Detection of serum parameters related to heart function
Serum CK-MB activity was determined according to the method of Gerhart and Waldenström (1979). Serum AST and LDH activities were detected according to the methods of Gella et al. (1985) and Young (2000) respectively.
Assay of male sex hormones levels
Concentrations of FSH and LH in serum were detected according to the method of Odell et al. (1968) and Braunstein et al. (1976). Serum testosterone concentration was determined according to the method of Andreyko et al. (1986).
Assays TNF-α and IL-4 levels
Serum TNF-α and IL-4 levels were determined by the quantitative sandwich enzyme immunoassay technique according to the methods of Howard and Harada (1994) and Croft et al. (2012) respectively.
Assay of oxidative stress and antioxidant defense markers
Heart and testis glutathione content and lipid peroxidation were determined according to the methods of Beutler et al. (1963) and Preuss et al. (1998) respectively. Glutathione peroxidase (GPx), glutathione-S-transferase (GST) and superoxide dismutase (SOD) activities in heart and testis were assayed according to the methods of Matkovics et al. (1997), Mannervik and Gutenberg (1981) and Marklund and Marklund (1974) respectively.
Statistical analysis
The data obtained from the experiment were analyzed using the one-way analysis of variance (ANOVA) (Roa et al. 1985) followed by LSD test to compare various groups with each other. Results were expressed as mean ± standard error (SE) and values of P > 0.05 were considered non-significantly different while those of P < 0.05 and P < 0.01 were considered significant and highly significant respectively. F-probability expresses the general effect between groups. Multi-factor analysis of variance (MANOVA) was also performed to evaluate the effect of time, dose and time–dose interaction.
Results
Effect on levels of serum parameters related to heart function
Data showing the effects of latex and ethanolic extract and ABM pesticide on serum markers of heart function are represented in Tables 1 and 2. The treatments of normal rats with latex and ethanolic extract as well as ABM for 4 weeks induced a highly significant increase (P < 0.01; LSD) in the activities of CK-MB and LDH. With the exception of the effect of 1/20 LD50 of ABM, serum AST and LDH activities were significantly increased (P < 0.01; LSD) after administration of the three tested materials for 8 weeks. One-way ANOVA (Table 1) depicted that the general effect between groups on serum CK-MB, AST and LDH activities was very highly significant (P < 0.001; F-probability). On the other hand, two-way ANOVA of normal–latex effect showed that the effect of dose was very highly significant (P < 0.001; F-probability) on CK-MB, AST and LDH activities while the time of administration had a significant effect (P < 0.05; F-probability) on CK-MB and insignificant effect (P > 0.05; F-probability) on AST and LDH activities. The dose–time interaction had insignificant effect (P > 0.05; F-probability) on CK-MB, AST and LDH activities. Regarding normal–extract effect, the dose, time and dose–time interaction had very highly significant effect (P < 0.001; F-probability) on CK-MB and LDH activities while the effect of dose and time on AST activity were very highly significant and significant respectively. Similar to latex and ethanolic extract effect, the dose effect of ABM was very highly significant (P < 0.001; F-probability). The time had insignificant effect (P > 0.05; F-probability) on CK-MB and AST activities and very highly significant effect (P < 0.001; F-probability) on LDH activity with ABM treatment. The effect of dose–time interaction was very highly significant on LDH activity due to ABM administration.
Table 1.
Effect of latex and ethanolic leaf extract and abamectin on serum CK-MB, AST and LDH activities of normal rats
| Treatments | Parameter | |||||
|---|---|---|---|---|---|---|
| CK-MB (U/L) | AST (U/L) | LDH (U/L) | ||||
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | |
| Vehicle (CMC 1 %) control | 93.26 ± 5.49f | 94.99 ± 4.70f | 96.57 ± 2.55fg | 86.65 ± 6.01g | 749.58 ± 80.83g | 738.89 ± 80.25g |
| 1/20 LD50 latex | 178.16 ± 21.79bcde | 103.37 ± 18.49f | 124.68 ± 5.48abcd | 116.64 ± 2.27bcde | 2897.51 ± 310.79bc | 2775.36 ± 146.39c |
| 1/10 LD50 latex | 217.63 ± 25.89b | 185.50 ± 18.35bcde | 138.67 ± 5.95a | 126.66 ± 10.24ab | 3488.61 ± 182.80a | 3471.67 ± 25.63a |
| 1/20 LD50 ethanolic extract | 177.62 ± 16.82bcde | 135.00 ± 3.09ef | 109.43 ± 1.59cdef | 105.98 ± 3.26ef | 3341.78 ± 296.19ab | 1533.90 ± 23.68f |
| 1/10 LD50 ethanolic extract | 366.10 ± 31.39a | 140.34 ± 3.69def | 140.50 ± 6.32a | 125.98 ± 6.36ab | 3457.25 ± 325.48a | 2085.50 ± 223.34de |
| 1/20 LD50 ABM | 194.03 ± 20.98bcd | 142.40 ± 16.58cdef | 108.87 ± 1.32def | 99.45 ± 8.72fg | 2195.30 ± 141.03d | 1283 ± 10.57fg |
| 1/10 LD50 ABM | 195.09 ± 21.01bc | 201.76 ± 23.23b | 111.10 ± 4.70bcdef | 125.34 ± 3.99abc | 3194.32 ± 256.22abc | 1581 ± 81.17ef |
| F-probability | P < 0.001 | P < 0.001 | P < 0.001 | |||
| LSD at 5 % level | 53.828 | 16.0947 | 549.503 | |||
| LSD at 1 % level | 72.491 | 21.675 | 735.985 | |||
Data are expressed as mean ± SE
Number of animals in each group is six
For each parameter, means, which do not share the same superscript symbol(s), are significantly different at P < 0.05
Table 2.
Analysis of variance for CK-MB, AST and LDH activities in serum of normal and treated rats
| Source of variation | F-probability | ||
|---|---|---|---|
| CK-MB (U/L) | AST (U/L) | LDH (U/L) | |
| A—Normal–latex effect | |||
| Dose | P < 0.001 | P < 0.001 | P < 0.001 |
| Time | P < 0.05 | P > 0.05 | P > 0.05 |
| Dose–time | P > 0.05 | P > 0.05 | P > 0.05 |
| B—Normal–extract effect | |||
| Dose | P < 0.001 | P < 0.001 | P < 0.001 |
| Time | P < 0.001 | P < 0.05 | P < 0.001 |
| Dose–time | P < 0.001 | P > 0.05 | P < 0.001 |
| C—Normal–Abamectin Effect | |||
| Dose | P < 0.001 | P < 0.001 | P < 0.001 |
| Time | P > 0.05 | P > 0.05 | P < 0.001 |
| Dose–time | P > 0.05 | P < 0.05 | P < 0.001 |
Effect on male sex hormones levels
Serum testosterone level was highly significantly reduced (P < 0.01; LSD) in male rats ingested latex, ethanolic extract and ABM for 4 and 8 weeks; the effect seemed to dose dependent. Treatments with 1/10 LD50 of the tested materials for 4 weeks induced a highly significant decrease (P < 0.01) in LH level while 1/20 extract only produced a significant effect at the same experimental period. With regard to FSH, the administration of the high dose of latex and extract significantly (P < 0.01; LSD) decreased serum FSH level after 4 weeks whereas the high dose of the three tested treatments induced a significant depletion (P < 0.01; LSD) of FSH level at the 8th week (Table 3). Concerning one way ANOVA, it was found that the general effect between groups on serum testosterone, FSH and LH concentrations was very highly significant (P < 0.001; F-probability) throughout the experiment (Table 3). Two-way ANOVA (Table 4) stated that the dose effect of latex was very highly significant (P < 0.001; LSD) on testosterone, FSH and LH while the time had very highly significant effect (P < 0.001; F-probability) on FSH level, highly significant effect (P < 0.01; F-probability) on LH level and insignificant effect (P > 0.05; F-probability) on testosterone level. The dose–time interaction had insignificant effect (P > 0.05; F-probability) on the three tested hormones. Concerning extract effect, the dose had very highly significant effect (P < 0.001; F-probability) on testosterone and FSH levels and highly significant effect (P < 0.01; F-probability) on LH level. Time had significant effect (P < 0.05; F-probability) on FSH level and insignificant effect significant effect (P > 0.05; F-probability) on testosterone and LH levels. The interaction between dose and time had insignificant effect (P > 0.05; F-probability) on the three tested hormones. Regarding ABM effect, dose had very highly significant effect (P < 0.001; F-probability) on testosterone and LH levels and highly significant effect (P < 0.01; F-probability) on FSH level. Time had significant effect (P < 0.05; F-probability) on testosterone and LH levels and very highly significant effect (P < 0.001; F-probability) on FSH level. The dose–time interaction had insignificant effect (P > 0.05; F-probability) on the three tested hormones.
Table 3.
Effect of latex and ethanolic leaf extract and abamectin on serum testosterone, FSH and LH levels in normal rats
| Treatments | Parameter | |||||
|---|---|---|---|---|---|---|
| Testosterone (ng/ml) | FSH (mlU/ml) | LH (mlU/ml) | ||||
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | |
| Vehicle (CMC1 %) control | 2.71 ± 0.28a | 2.22 ± 0.35a | 0.26 ± 0.01a | 0.23 ± 0.01abc | 0.19 ± 0.01a | 0.17 ± 0.01abcd |
| 1/20 LD50 latex | 1.21 ± 0.11bcd | 1.1 ± 0.16bcd | 0.25 ± 0.01a | 0.20 ± 0.01defg | 0.18 ± 0.01ab | 0.14 ± 0.01fg |
| 1/10 LD50 latex | 0.75 ± 0.13d | 0.84 ± 0.04cd | 0.19 ± 0.01efg | 0.18 ± 0.01fg | 0.15 ± 0.01efg | 0.14 ± 0.01g |
| 1/20 LD50 ethanolic extract | 1.63 ± 0.21b | 1.38 ± 0.36bc | 0.23 ± 0.01abcd | 0.21 ± 0.01cdef | 0.16 ± 0.01cdef | 0.16 ± 0.01bcde |
| 1/10 LD50 ethanolic extract | 1.19 ± 0.06bcd | 0.88 ± 0.16cd | 0.20 ± 0.01efg | 0.19 ± 0.02efg | 0.16 ± 0.01def | 0.14 ± 0.01efg |
| 1/20 LD50 ABM | 1.02 ± 0.15cd | 0.81 ± 0.11d | 0.25 ± 0.01abc | 0.22 ± 0.01bcde | 0.18 ± 0.01ab | 0.18 ± 0.01abc |
| 1/10 LD50 ABM | 0.88 ± 0.07cd | 0.68 ± 0.06d | 0.24 ± 0.02ab | 0.18 ± 0.02g | 0.16 ± 0.01cdef | 0.15 ± 0.01efg |
| F-probability | P < 0.001 | P < 0.001 | P < 0.001 | |||
| LSD at 5 % level | 0.5479 | 0.0311 | 0.0199 | |||
| LSD at 1 % level | 0.7379 | 0.0419 | 0.0269 | |||
Data are expressed as mean ± SE
Number of animals in each group is six
For each parameter, means, which do not share the same superscript symbol(s), are significantly different at P < 0.05
Table 4.
Analysis of variance for testosterone, FSH and LH concentrations in serum of normal and treated rats
| Source of variation | F-probability | ||
|---|---|---|---|
| Testosterone (ng/ml) | FSH (mlU/ml) | LH (mlU/ml) | |
| A—Normal–latex effect | |||
| Dose | P < 0.001 | P < 0.001 | P < 0.001 |
| Time | P > 0.05 | P < 0.001 | P < 0.01 |
| Dose–time | P > 0.05 | P > 0.05 | P > 0.05 |
| B—Normal–extract effect | |||
| Dose | P < 0.001 | P < 0.001 | P < 0.01 |
| Time | P > 0.05 | P < 0.05 | P > 0.05 |
| Dose–time | P > 0.05 | P > 0.05 | P > 0.05 |
| C—Normal–abamectin effect | |||
| Dose | P < 0.001 | P < 0.01 | P < 0.001 |
| Time | P < 0.05 | P < 0.001 | P < 0.05 |
| Dose–time | P > 0.05 | P > 0.05 | P > 0.05 |
Effect on serum TNF-α and IL-4 levels
Data represented in Tables 5 and 6 depicted that all treatments induced strong adverse effects on the normal levels of serum TNF-α and IL-4 of normal rats. Administrations of latex, ethanolic extract of C. procera and ABM for 4 and 8 weeks induced a highly significant increases (P < 0.01; LSD) in serum levels of TNF-α. On the other hand, while the higher doses of the ethanolic extract and ABM induced a highly significant effect on serum level of IL-4 after 4 weeks, the latex produced a significant effect as a result of the two tested doses after 4 and 8 weeks. ABM seemed to be the most effective in increasing serum TNF-α while latex is the most potent in lowering serum IL-4 level. One way ANOVA (Table 5) indicated that the general effect on serum TNF-α and IL-4 levels between groups was very highly significantly (P < 0.001, F-probability) throughout the experiment. Two-way ANOVA (Table 6) revealed that the dose effect of latex and extract was very highly significant (P < 0.001; F-probability). The dose effect of ABM was highly significant (P < 0.01; F-probability) on IL-4 level while it was very highly significant (P < 0.001; F-probability) on TNF-α level. The time and dose–time interaction of extract and ABM had a highly significant effect (P < 0.01; F-probability) effect on IL-4 level. However, while time of administration of latex had significant effect (P < 0.05; F-probability) on IL-4 level, its interaction with dose had insignificant effect (P > 0.05; F-probability). The effect of time of ABM on TNF-α level was significant (P < 0.05; F-probability) while its interaction with dose was insignificant (P > 0.05; F-probability).
Table 5.
Effect of latex and ethanolic leaf extract and abamectin on TNF-α and IL-4 levels in serum of normal rats
| Treatment | Parameter | |||
|---|---|---|---|---|
| TNF-α (pg/ml) | IL-4 (ng/ml) | |||
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | |
| Vehicle (CMC 1 %) control | 36.72 ± 1.02h | 38.72 ± 0.62h | 202 ± 4.37a | 197 ± 1.64a |
| 1/20 LD50 latex | 78.55 ± 6.28fg | 72.55 ± 7.10g | 130.7 ± 13.63c | 170.5 ± 15.17b |
| 1/10 LD50 latex | 91.43 ± 6.12def | 80.30 ± 5.74efg | 101.53 ± 3.99d | 112.9 ± 5.34cd |
| 1/20 LD50 ethanolic extract | 102.42 ± 6.53cd | 92.40 ± 3.67de | 179.3 ± 7.96ab | 186.97 ± 4.27ab |
| 1/10 LD50 ethanolic extract | 109.85 ± 4.29bc | 100.98 ± 3.52cd | 138 ± 11.67c | 181.4 ± 3.96ab |
| 1/20 LD50 abamectin | 100.67 ± 5.61cd | 86.62 ± 3.83ef | 184.9 ± 8.89ab | 199.27 ± 2.80a |
| 1/10 LD50 abamectin | 124 ± 3.08a | 117.95 ± 1.08ab | 134.97 ± 19.22c | 197.23 ± 1.95a |
| F-probability | P < 0.001 | P < 0.001 | ||
| LSD at 5 % level | 13.4927 | 26.4573 | ||
| LSD at 1 % level | 18.1709 | 35.6306 | ||
Data are expressed as mean ± SE
Number of animals in each group is six
For each parameter, means, which do not share the same superscript symbol(s), are significantly different at P < 0.05
Table 6.
Analysis of variance for concentrations in serum on TNF-α and IL-4 levels of normal and treated rats
| Source of variation | F-probability | |
|---|---|---|
| TNF-α (pg/ml) | IL-4 (ng/ml) | |
| A—Normal–latex effect | ||
| Dose | P < 0.001 | P < 0.001 |
| Time | P > 0.05 | P < 0.05 |
| Dose–time | P > 0.05 | P > 0.05 |
| B—Normal–extract effect | ||
| Dose | P < 0.001 | P < 0.001 |
| Time | P > 0.05 | P < 0.01 |
| Dose–time | P > 0.05 | P < 0.01 |
| C—Normal–abamectin effect | ||
| Dose | P < 0.001 | P < 0.01 |
| Time | P < 0.05 | P < 0.01 |
| Dose–time | P > 0.05 | P < 0.01 |
Effect on heart and testis oxidative stress and antioxidant markers levels
The effects of latex, ethanolic extract of leaves of C. procera and ABM on GSH, LPO concentrations and GPx, GST and SOD activities are expressed in Tables 7, 8, 9 and 10. GSH content in heart and testis was highly significantly decreased (P < 0.01; LSD) after administration of the plant latex and extract as well as ABM at 4th and 8th week. With the exception of 1/20 LD50 of latex on testis LPO, the two tested doses of latex, ethanolic extract and ABM induced a significant elevation of LPO in heart and testis at the 2 tested periods. Heart GPx, GST and SOD activities were detectably decreased after administration of the tested materials at the two tested periods. With the exception of the effect of 1/20 ABM on GPx activity, the GPx, GST and SOD activities were significantly decreased (P < 0.01; LSD) after all treatments at the 4th and 8th week as a result of both tested doses. Regarding one way ANOVA (Tables 8, 9), it was found that the general effect on heart and testis GSH content, LPO and the activities of GPx, GST and SOD between groups was very highly significantly (P < 0.001, F-probability) throughout the experiment.
Table 7.
Effect of latex and ethanolic leaf extract and abamectin on cardiac GSH content, LPO and GPx, GST and SOD activities in normal rats
| Treatments | Parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GSH (nmol/100 mg tissue) | LPO (nmol/100 mg tissue) | GPx (U/g tissue) | GST (U/g tissue) | SOD (U/g tissue) | ||||||
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 week | 8 weeks | |
| Vehicle (CMC 1 %) control | 163.34 ± 1.44a | 143.42 ± 0.93b | 46.31 ± 0.71f | 47.85 ± 2.11f | 84.85 ± 0.99a | 85.50 ± 2.25a | 489.93 ± 14.33ab | 506.25 ± 3.74a | 14.35 ± 0.18bc | 15.31 ± 0.71ab |
| 1/20 LD50 latex | 109.87 ± 7.52cd | 119.65 ± 8.74c | 64.50 ± 3.68de | 61.84 ± 1.13e | 77.39 ± 2.04bc | 69.00 ± 1.24defg | 447.92 ± 2.69ef | 481.80 ± 2.60bc | 9.07 ± 0.23e | 11.95 ± 0.29d |
| 1/10 LD50 latex | 69.69 ± 6.29e | 97.71 ± 6.10d | 72.82 ± 2.17cd | 67.16 ± 2.65cde | 69.93 ± 2.70defg | 63.96 ± 0.43g | 429.86 ± 12.39f | 460.07 ± 6.63cde | 1.93 ± 0.23h | 4.62 ± 0.30g |
| 1/20 LD50 ethanolic extract | 68.39 ± 6.23e | 108.40 ± 1.00cd | 65.19 ± 3.75de | 59.78 ± 4.33e | 68.06 ± 1.69efg | 79.63 ± 1.3ab | 480.49 ± 13.70bc | 454.51 ± 8.65de | 15.71 ± 0.14a | 6.84 ± 0.78f |
| 1/10 LD50 ethanolic extract | 45.35 ± 5.28f | 102.50 ± 8.26cd | 84.53 ± 4.66a | 72.71 ± 2.56cd | 63.96 ± 2.96g | 77.11 ± 0.74bc | 472.31 ± 5.63bcd | 386.06 ± 8.17h | 2.11 ± 0.25h | 4.04 ± 0.06g |
| 1/20 LD50 ABM | 118.73 ± 11.35c | 116.51 ± 4.20c | 76.55 ± 6.46abc | 72.56 ± 2.02cd | 74.22 ± 2.59bcd | 72.35 ± 3.33cdef | 470.49 ± 3.39bcde | 487.85 ± 0.77ab | 13.39 ± 0.11c | 7.12 ± 0.15f |
| 1/10 LD50 ABM | 77.61 ± 0.70e | 77.43 ± 1.31e | 84.30 ± 5.44ab | 74.19 ± 3.12bcd | 72.54 ± 2.35cde | 84.85 ± 0.99a | 447.92 ± 7.12ef | 395.83 ± 3.36g | 12.26 ± 0.22d | 4.84 ± 0.38g |
| F-probability | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| LSD at 5 % level | 17.2065 | 10.2926 | 6.0102 | 22.709 | 1.013 | |||||
| LSD at 1 % level | 23.1723 | 13.8613 | 8.0941 | 30.5827 | 1.3642 | |||||
Data are expressed as mean ± SE
Number of animals in each group is six
For each parameter, means, which do not share the same superscript symbol(s), are significantly different at P < 0.05
Table 8.
Effect of latex and ethanolic leaf extract and abamectin on testicular GSH content, LPO and GPx, GST and SOD activities in normal rats
| Treatments | Parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GSH (nmol/100 mg tissue) | LPO (nmol/100 mg tissue) | GPx (U/g tissue) | GST (U/g tissue) | SOD (U/g tissue) | ||||||
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 weeks | 8 weeks | 4 week | 8 weeks | |
| Vehicle (CMC 1 %) control | 86.78 ± 9.11a | 69.60 ± 6.12b | 13.68 ± 1.72e | 14.46 ± 2.40e | 71.89 ± 3.25a | 73.20 ± 6.68a | 976.18 ± 23.75a | 1011.18 ± 27.05a | 10.24 ± 1.77a | 9.33 ± 0.97ab |
| 1/20 LD50 latex | 68.97 ± 3.94b | 33.41 ± 5.68e | 16.30 ± 0.56de | 23.33 ± 1.97bc | 50.85 ± 6.52cd | 44.53 ± 3.42cde | 683.16 ± 37.74bcd | 787.50 ± 52.34b | 6.45 ± 0.38cde | 5.12 ± 0.44defg |
| 1/10 LD50 latex | 54.24 ± 1.85cd | 28.32 ± 3.02ef | 29.76 ± 1.98a | 30.06 ± 3.02a | 48.50 ± 4.38cde | 46.99 ± 4.29cde | 637.50 ± 42.81cde | 769.38 ± 29.28b | 5.53 ± 0.54def | 4.40 ± 0.24efg |
| 1/20 LD50 ethanolic extract | 64.06 ± 2.18bc | 19.03 ± 0.55fg | 25.37 ± 2.67abc | 21.35 ± 1.73cd | 55.13 ± 3.69bc | 49.06 ± 1.37cd | 753.96 ± 33.91bc | 673.09 ± 76.22bcd | 7.93 ± 0.98bc | 6.36 ± 0.56cde |
| 1/10 LD50 ethanolic extract | 48.05 ± 1.77d | 14.87 ± 0.59g | 28.10 ± 2.42ab | 22.56 ± 1.65bc | 49.44 ± 2.15cd | 37.27 ± 3.28e | 489.58 ± 6.99f | 584.21 ± 47.88def | 7.13 ± 0.46cd | 3.07 ± 0.76g |
| 1/20 LD50 ABM | 53.45 ± 4.58cd | 31.06 ± 3.74e | 21.72 ± 1.61cd | 21.80 ± 1.50cd | 64.25 ± 3.05ab | 51.47 ± 2.67cd | 672.29 ± 21.79bcd | 596.88 ± 66.45def | 6.99 ± 0.80cd | 4.05 ± 0.37fg |
| 1/10 LD50 ABM | 48.87 ± 3.79d | 18.42 ± 0.96fg | 23.33 ± 0.87bc | 27.92 ± 1.99ab | 52.10 ± 3.18cd | 41.90 ± 3.22de | 569.17 ± 29.95def | 546.87 ± 51.57ef | 5.06 ± 0.48defg | 3.59 ± 0.40fg |
| F-probability | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| LSD at 5 % level | 11.946 | 5.6821 | 11.3121 | 124.2059 | 2.1784 | |||||
| LSD at 1 % level | 16.0879 | 7.6522 | 15.2342 | 167.2704 | 2.9337 | |||||
Data are expressed as mean ± SE
Number of animals in each group is six
For each parameter, means, which do not share the same superscript symbol(s), are significantly different at P < 0.05
Table 9.
Analysis of variance for oxidative stress and antioxidant enzymes in heart of normal and treated rats
| Source of variation | F-probability | ||||
|---|---|---|---|---|---|
| GSH | LPO | GPx | GST | SOD | |
| A—Normal–latex effect | |||||
| Dose | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| Time | P > 0.05 | P > 0.05 | P < 0.01 | P < 0.001 | P < 0.001 |
| Dose–time | P < 0.01 | P > 0.05 | P < 0.05 | P > 0.05 | P < 0.05 |
| B—Normal–extract effect | |||||
| Dose | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| Time | P < 0.001 | P > 0.05 | P < 0.001 | P < 0.001 | P < 0.001 |
| Dose–time | P < 0.001 | P > 0.05 | P < 0.01 | P < 0.001 | P < 0.001 |
| C—Normal–abamectin effect | |||||
| Dose | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| Time | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P < 0.001 |
| Dose–time | P > 0.05 | P > 0.05 | P > 0.05 | P < 0.001 | P < 0.001 |
Table 10.
Analysis of variance for oxidative stress and antioxidant markers in testis of normal and treated rats
| Source of variation | F-probability | ||||
|---|---|---|---|---|---|
| GSH | LPO | GPx | GST | SOD | |
| A—Normal–latex effect | |||||
| Dose | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| Time | P < 0.001 | P > 0.05 | P > 0.05 | P < 0.01 | P > 0.05 |
| Dose–time | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 |
| B—Normal–extract effect | |||||
| Dose | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| Time | P < 0.001 | P > 0.05 | P > 0.05 | P > 0.05 | P < 0.05 |
| Dose–time | P < 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 |
| C—Normal–abamectin effect | |||||
| Dose | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
| Time | P < 0.001 | P > 0.05 | P < 0.05 | P > 0.05 | P < 0.05 |
| Dose–time | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 |
Concerning two-way ANOVA (Tables 9, 10), the dose effect of latex, ethanolic extract and ABM on GSH content and GPx, GST and SOD activities in heart and testis was very highly significant (P < 0.001, F-probability) throughout the experiment. Regarding latex effect, the time had insignificant effect (P > 0.05, F-probability) on heart GSH content and LPO, testis LPO, testis GPx and SOD activities and highly significantly effect (P < 0.01, F-probability) on heart GPx and testis GST activities and very highly significantly effect (P < 0.001, F-probability) on testis GSH level and activities of heart SOD and GST. The effect of interaction between dose and time was highly significant (P < 0.01, F-probability) on heart GSH content and only significant (P < 0.05, F-probability) on heart GPx and SOD activities. Concerning extract effect, the time had very highly significant effect (P < 0.001, F-probability) on cardiac GPx, GST and SOD activities and cardiac and testicular GSH levels and only significant effect on testicular SOD activity. Dose–time interaction had a very highly significant effect (P < 0.001, F-probability) on cardiac GSH content and GST and SOD activities and a highly significant effect on GPx activity. Concerning to ABM, the time had a significant effect (P < 0.05, F-probability) on testicular GPx and SOD activities and a very highly significant effect (P < 0.001, F-probability) on cardiac SOD activity and testicular GSH level. Dose–time interaction had a very highly significant effect (P < 0.001, F-probability) on cardiac GST and SOD activities.
Histopathological effects
Normal myocytes architecture of normal rat heart in control animals were observed (Fig. 1a, b). Treatments of normal rats with 1/20 of LD50 of C. procera latex caused intermuscular odema (Fig. 1c–e) associated with inflammatory cell infiltration (Fig. 1c). Administration of 1/10 of LD50 of latex caused marked alterations of normal structure of heart by affecting on cardiac myocytes causing odema and inflammatory cell infiltration (Figs. 1f, 2a) in short time (4 weeks) while after 8 weeks, it caused necrosis associated with inflammatory cells infiltration and congestion of blood vessels (Fig. 2b–d). The treatment of rats with 1/20 of LD50 of ethanolic extract caused inflammatory cells infiltration at the end of the 4th week (Fig. 2e) and it caused intermuscular odema, necrosis of cardiac myocytes associated with inflammatory cells infiltration and congestion of blood vessels at the end of the 8th week (Fig. 2f). Increasing the concentration of the ethanolic extract (1/10 of LD50) caused odema associated with inflammatory cell infiltration (Fig. 3a, b) after 4 week. Prolongation of period of administration of this dose caused congestion of blood vessels, necrosis of cardiac myocytes associated with inflammatory cell infiltration (Fig. 3c–e). Regarding ABM administration, low dose caused congestion of blood vessels and inflammatory cells infiltration (Fig. 4a, b) and high dose caused necrosis of cardiac myocytes associated with inflammatory cell infiltration (Fig. 4c, d).
Fig. 1.
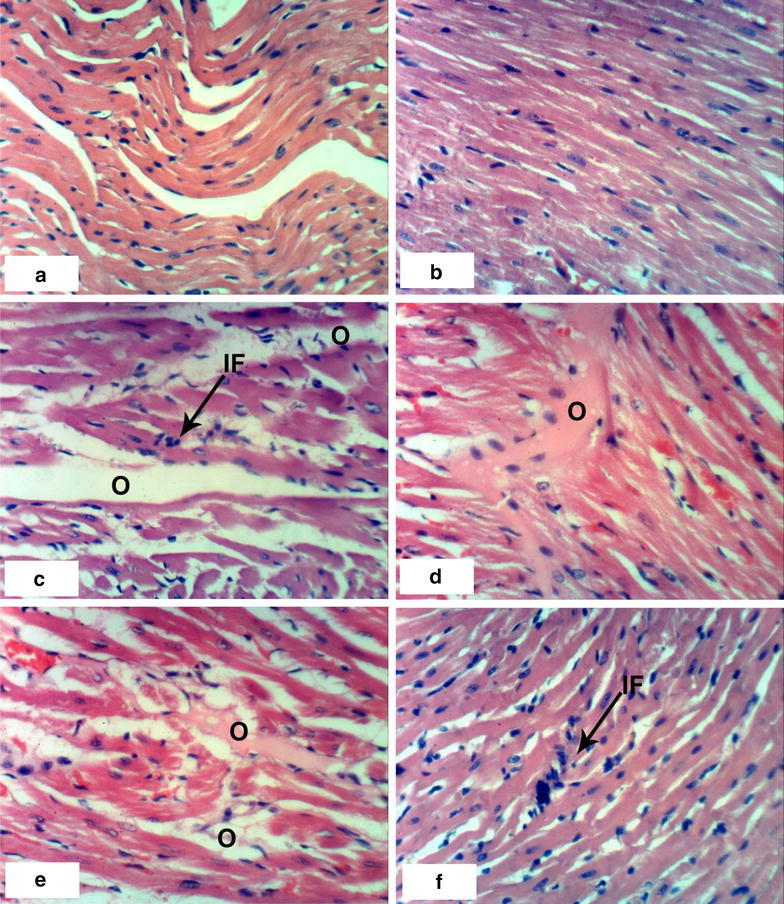
Photomicrographs of H and E stained heart sections of normal and latex treated rats. Sections of control rats administered 1 % CMC for 4 weeks (a) and 8 weeks (b) showing normal myocytes of heart. c Section of rat treated with 1/20 LD50 of latex for 4 weeks showing intermuscular odema (O) associated with inflammatory cell infiltration (IF), d, e sections of rats treated with 1/20 LD50 of latex for 8 weeks showing intermuscular odema (O), f section of rat treated with 1/10 LD50 of latex for 4 weeks showing inflammatory cell infiltration (IF) (×400)
Fig. 2.
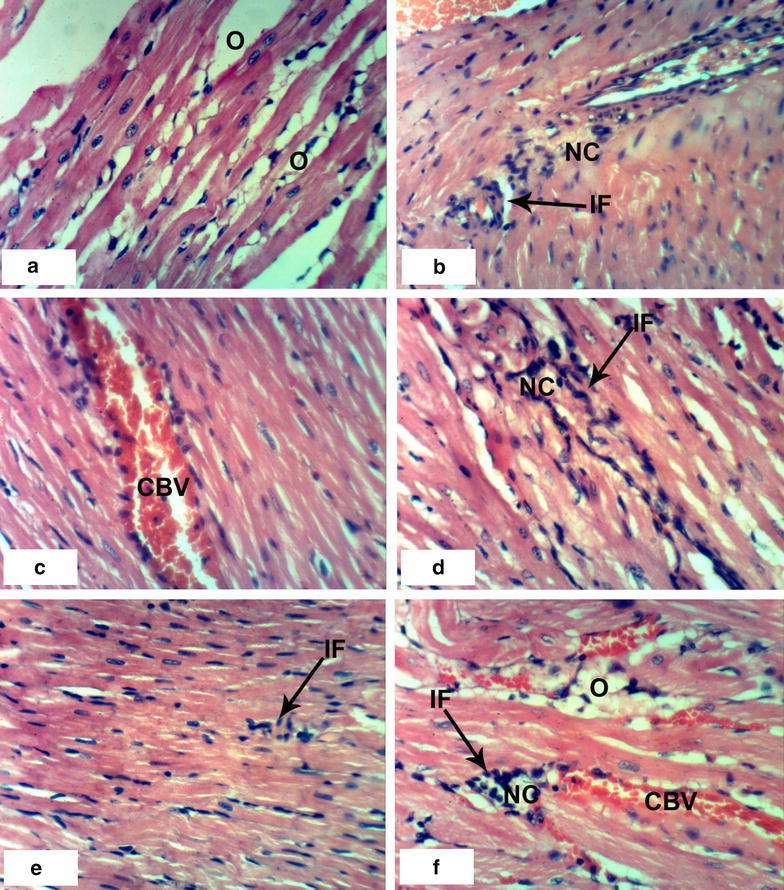
Photomicrographs of H and E stained heart sections of latex and extract treated rats. a Section of rat treated with 1/10 LD50 of latex for 4 weeks showing intermascular odema (O), b–d sections of rats treated with 1/10 LD50 of latex for 8 weeks showing congestion of blood vessels (CBV) and necrosis (NC) associated with inflammatory cell infiltration (IF), e SECTION of rat treated with 1/20 LD50 of extract for 4 weeks showing inflammatory cell infiltration (IF), f section of rat treated with 1/20 LD50 of extract for 8 weeks showing intermuscular odema (O), necrosis (NC) and congestion of blood vessels (CBV) (×400)
Fig. 3.
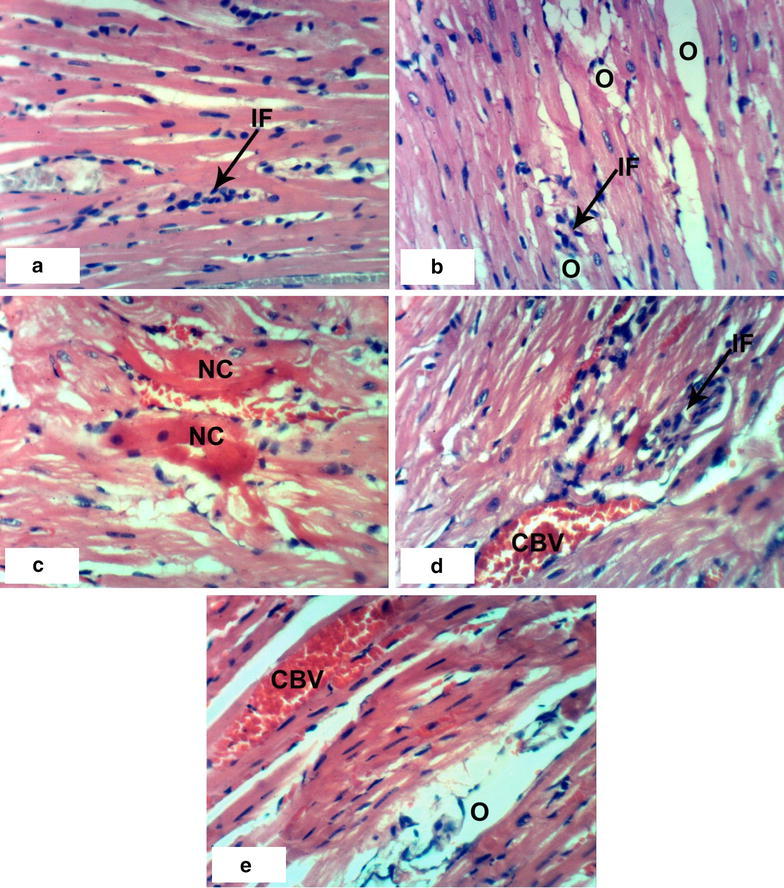
Photomicrographs of H and E stained heart sections of rats treated with extract. a, b Sections of rats treated with 1/10 LD50 extract for 4 weeks showing odema and inflammatory cell infiltration (IF), c–e sections of rats treated with 1/10 LD50 of extract for 8 weeks showing necrosis (NC), congestion of blood vessels (CBV), inflammatory cell infiltration (IF) and odema (O) (×400)
Fig. 4.
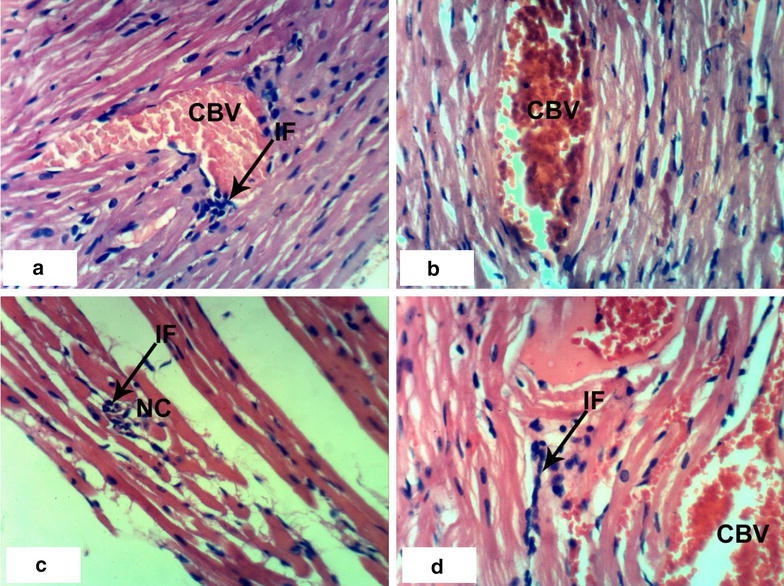
Photomicrographs of H and E stained heart sections of ABM treated rats. a Section rat treated with 1/20 LD50 ABM for 4 weeks showing inflammatory cell infiltration (IF) and congestion of blood vessels (CBV), b section of rat treated with 1/20 LD50 of ABM for 8 weeks showing congestion of blood vessels (CBV), c section of rat treated with 1/10 LD50 ABM for 4 weeks showing necrosis (NC) associated with inflammatory cell infiltration (IF), d section of rat treated with 1/10 LD50 ABM for 8 weeks showing congestion of blood vessels (CBV) and inflammatory cell infiltration (IF) (×400)
Control groups demonstrated normal testicular histology with all successive stages of spermatogenesis (Fig. 5a, b). Administration of 1/20 LD50 latex for 4 weeks altered the normal testis structure by causing degeneration of spermatogonial cells lining seminiferous tubules (Fig. 5c). Interstitial oedema associated with inflammatory cells infiltration (Fig. 5e) occurred after administration of the high dose of latex after 4 weeks and degeneration observed after 8 weeks (Fig. 5f). Administration of 1/10 LD50 ethanolic extract of C. procera for 8 weeks caused atrophy of the seminiferous tubules, necrosis, degeneration and desquamation of spermatogonial cells lining seminiferous tubules (Fig. 6d–f). Treatment with ABM at the low dose caused degeneration and atrophy of the seminal vesicles and desquamation of spermatogonial cells (Fig. 7b–d). Treatment with the high dose of ABM for 4 weeks caused degeneration and interstitial odema and necrosis (Fig. 7e, f). After 8 weeks administration, appearance of degeneration, interstitial odema and atrophy of seminal vesicles (Fig. 8a–c) were observed.
Fig. 5.
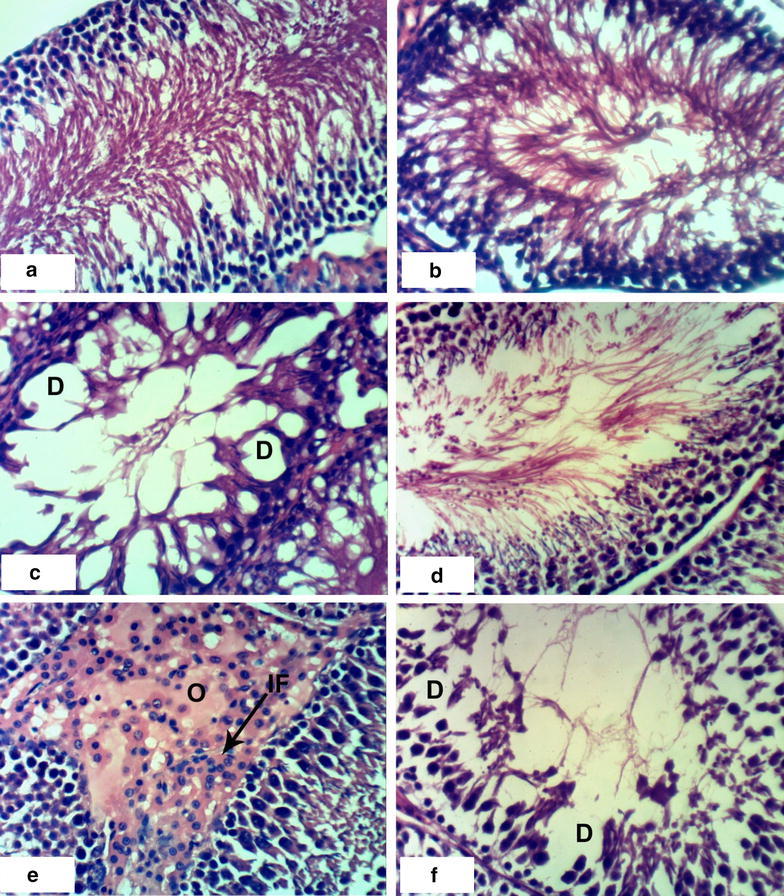
Photomicrographs of H and E stained testis sections of control and latex treated rats. Sections of control rats administered 1 % CMC for 4 weeks (a) and for 8 weeks (b) showing normal histologic structure of testis and successive stages of spermatogenesis, c section of rat treated with 1/20 LD50 latex for 4 weeks showing degeneration of spermatogonial cells (D), d section of rat treated with 1/20 LD50 latex for 8 weeks showing mild degeneration of spermatids (D), e section of rat treated with 1/10 LD50 latex for 4 weeks showing odema (O) associated with inflammatory cell infiltration (IF), f section of rat treated with 1/10 LD50 latex for 8 weeks showing degeneration of spermatogonial cells (D) (×400)
Fig. 6.
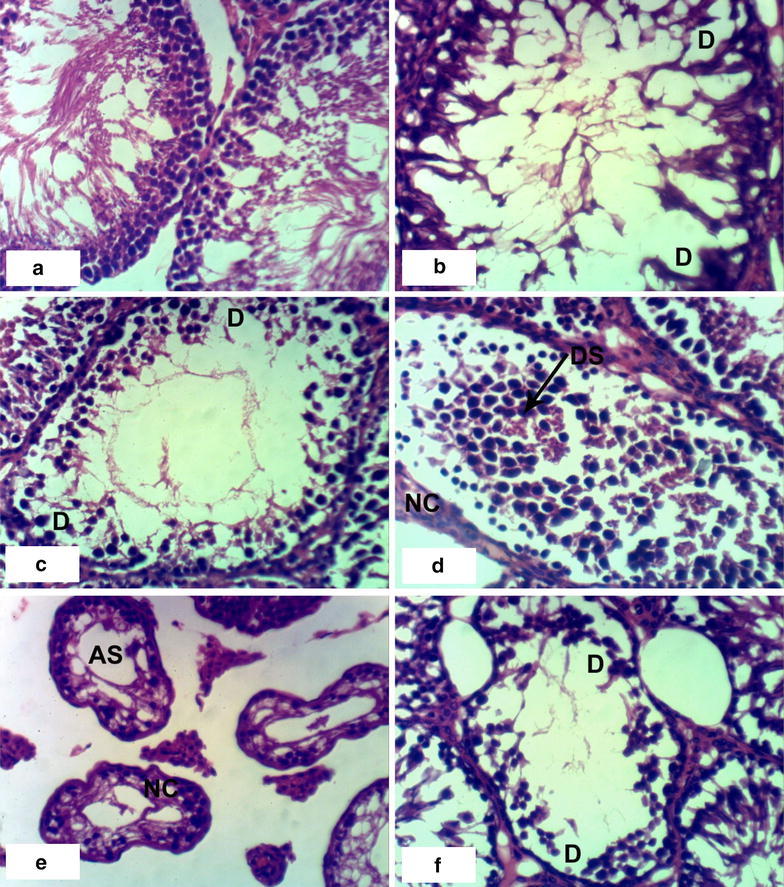
Photomicrographs of H and E stained testis sections of extract treated rats. a Section of rat treated with 1/20 LD50 extract for 4 weeks showing normal structure of testis, b section of rat treated with 1/20 LD50 extract for 8 weeks showing degeneration of spermatogonial cells (D), c section of rat treated with 1/10 LD50 extract for 4 weeks showing degeneration of spermatogonial cells (D), d–f sections of rats treated with 1/10 LD50 extract for 8 weeks showing desquamation of cells (DS), necrosis (NC), atrophy of seminiferous tubules (AS) and degeneration of spermatogonial cells (D) (×400)
Fig. 7.
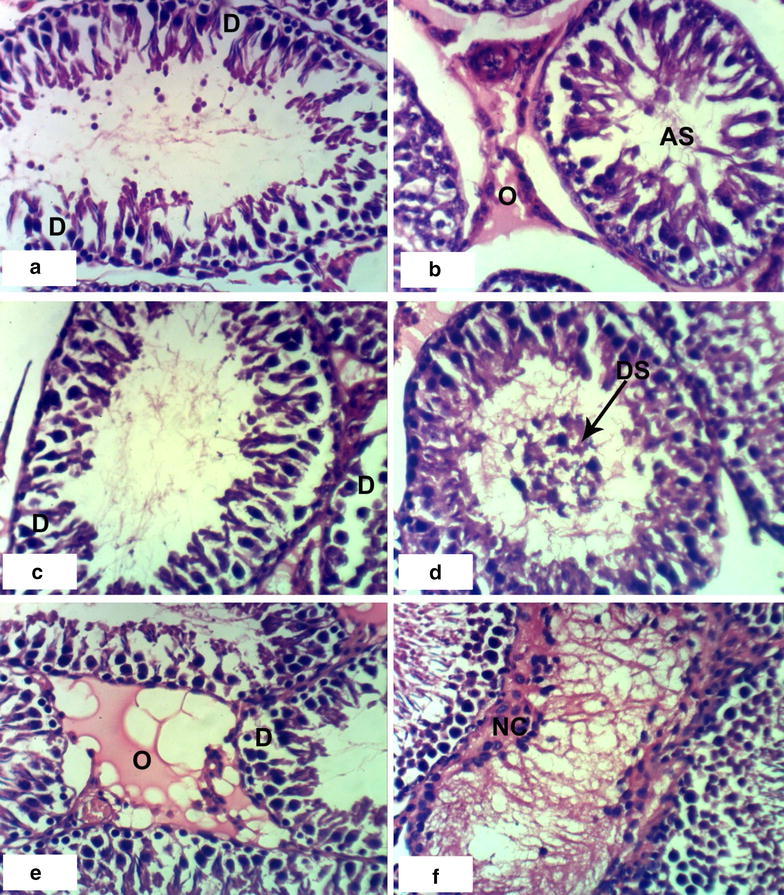
Photomicrographs of H and E stained testis sections of ABM treated rats. a Section of rat treated with 1/20 LD50 ABM for 4 weeks showing degeneration of spermatogonial cells (D), b–d sections of rats treated with 1/20 LD50 ABM for 8 weeks showing atrophy of seminiferous tubules (AS), odema (O) degeneration of spermatogonial cells (D) and desquamation of cells (DS), e, f sections of rats treated with 1/10 LD50 ABM for 4 weeks showing degeneration of spermatogonial cells (D), odema (O) and necrosis of cells (NC) (×400)
Fig. 8.
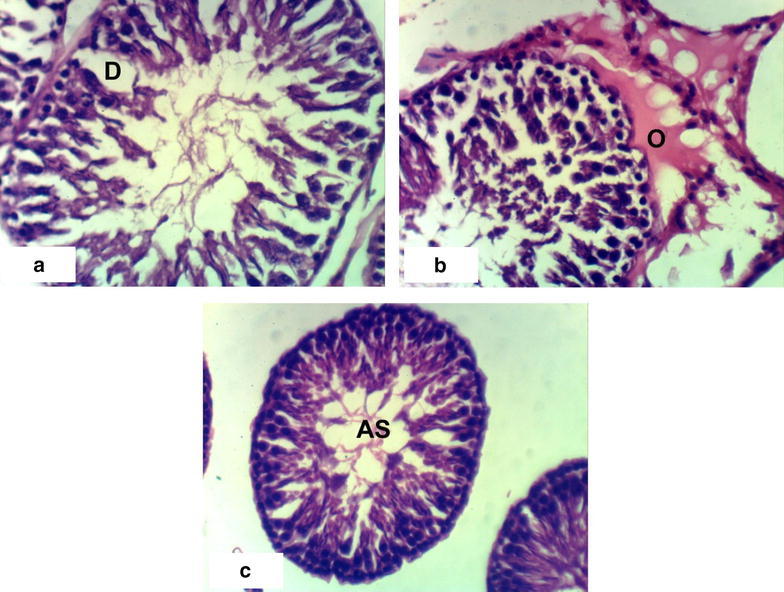
Photomicrographs of H and E stained testis sections of ABM treated rats for 8 weeks showing degeneration of spermatogonial cells (D) (photomicrograph a), odema (O) (photomicrograph b) and atrophy of seminiferous tubules (AS) (photomicrograph c) (×400)
Discussion
Plant extracts may provide an alternative method to currently applied pesticides, as they constitute a rich source of bioactive chemicals (Kim et al. 2005).
Calotropis procera plant commonly called Sodom apple or Giant milkweed belong to the family of Asclepiadaceae. It is a major grazing plant found in Asian temperate region, Asia-tropical and Africa (Agaie et al. 2007). It was reported that ingestion of fresh C. procera leaves and latex has been suggested as toxic to many ruminants by several farmers (Mahmoud et al. 1979a; Singhal and Kumar 2009). It was reported by Thankamma (2003) and Basak et al. (2009) that C. procera latex administered to rats revealed toxic, wound healing, and pain-killing effects. Chemical compounds in the latex are calotropagenin glycosides/derivatives, cardenolides, flavonoids, and saponins (Kanojiya and Madhusudanan 2012). Cardenolides in the C. procera latex are associated with the toxic effects in mammals (Elgamal et al. 1999). Phytochemical screening of the extracts of C. procera leaves indicated the presence of alkaloids, carbohydrates, cardiac glycosides, saponins, phenols, tannins, terpenoids and flavanoids which are known to possess medicinal and pesticidal properties (Verma et al. 2013). It was reported by De Lima et al. (2011) that the plant as hepatotoxic and cardiotoxic. Other researchers have documented the renal toxicity in addition to hepatic toxicity of the plant (Basak et al. 2009; Lin and Will 2012). The chemical poisons from plants such as Argel (Solenostemma argel) and Usher (C. procera) are mostly alkaloids which are nitrogenous heterocyclic compounds having strong effects on the nervous system of animals and may result in death (Badshah et al. 2004).
In view of this study, administration of C. procera latex and ethanolic extract of leaves for 4 and 8 weeks induced significant elevations in the activities of CK-MB, AST and LDH. These results are in accordance with those of El Badwi and Bakhiet (2010) and El-Badwi et al. (2010).
In the current study, histological examination of rats treated with C. procera latex and ethanolic leaf extract showed impairment of the normal structure of heart. Early histopathological changes at the 4th week of latex and extract administration include intermuscular odema, inflammatory cell infiltration. As the period of latex and extract administration extended to 8 weeks, alterations are more pronounced and include necrosis of cardiac myocytes associated with inflammatory cells infiltration and congestion of blood vessels. These results go parallel with these of El Badwi and Bakhiet (2010) who reported that the heart muscle fibers were focally vacuolated or necrotic with lymphatic infiltration. It was reported by De lima et al. (2011) that the toxic effects, established by intraperitoneal injection of C. procera latex to rats and oral administration of chopped leaves in a with the lowest amount of water to sheep appeared as cardiac muscle fibers separated by edematous fluid, and the rats exhibited subendocardic hemorrhages, infiltration of mononuclear inflammatory cells, multi-focal coagulation necrosis of the muscular fibers evidenced by granular appearance of the sarcoplasm, distinct eosinophilic cytoplasm lacking transverse striations, basophilic granulation and prominent vacuolization of the sarcoplasm of some fibres and presenting pyknotic or absent nuclei.
Phytochemical studies have revealed that C. procera contains a mixture of cardenolides, including proceragenin and 2″-oxovoruscharin (Van Quaquebeke et al. 2005). Cardenolides are cardiac-active compounds that inhibit the cellular membrane Na+/K+ ATPase, resulting in an electrolytic disturbance that affects the electrical conductivity of the heart (Poindexter et al. 2007). Thus, the heart dysfunction and the elevations in heart enzymes (CK-MB, AST and LDH) in serum and cardiac toxicity induced by the plant, in this study, may attributed to its constituting cardenolides. The milky latex contains a powerful bacteriolytic enzyme, toxic glycoside calactin, calotropin D1, calotropin D2 calotropin F11, and a nontoxic powerful proteolytic enzyme and it exhibited local anesthetic activity (Samar et al. 2009). In this study, administration of ABM showed marked elevations in LDH, AST and CK-MB. These results reflected the toxic effect of ABM and impairment of heart function. The elevated of serum enzymes related to heart function was associated with cardiac histopathological lesions which include oedema, inflammatory cells infiltration and necrosis observed in the present investigation.
In view of this study, treatments with C. procera latex and ethanolic extract of leaves induced marked decrease in the levels of male sex hormones testosterone, FSH and LH. In conduction with the present study, Sharma and Jacob (2001) found that intermuscular administration of aqueous and ethanolic extracts of flowers of C. procera has been shown to induce functional sterility and has a potent antispermatogenic activity in the albino mouse, but at the doses and experimental regimen employed, had no apparent effect on sexual behavior or libido. In the same way, Akinloye et al. (2002) reported that fresh leaves extract has depicted potential deleterious effects on the rat testes and accessory sex organs represented by degeneration of seminiferous epithelium of varying degrees as well as presence of large-sized multimate cells in the tubules and empty interstitial spaces. Calotropis is extensively used in both male and female rats for understanding its role in fertility (Akinloye et al. 2002; Circosta et al. 2001; Ahirwar et al. 2007). It was indicated previously that, an active principle of flower extract of C. procera showed spermicidal effect on testicular functions in Indian desert male Gerbil (Garg 1979).
Histologic profile of testes in the present study revealed extensive deleterious changes in the germinal tubules which contained mainly necrotic and degenerating germ cells. Further, the epididymal lumina appeared devoid of spermatozoa and exhibited mainly cell debris. Also, the seminiferous tubules were atrophied and necrosis and desquamation of spermatogonial cells after administration of the high dose of the extract. The interstitium was observed to be devoid of leydig cells in this study. This change may be due to decreased production of testosterone known to be responsible for normal testicular architecture. Histological changes observed in the testes of treated rats in this study may be due to the cardiac glycosides found in the plant which was incriminated to be responsible also for pathological and ultra-structural changes in the kidney tubules of Wistar rats. These changes are in concordance with Akinloye et al. (2002). In the current study, the plant showed toxic effect on the testis through effect on the germ cell and this is conducted by Akinloye et al. (2002) who said C. procera extract has destructive effect on the germ cells which are actively dividing. In addition, it was reported that testosterone maintained the viability of spermatozoa (Bhargava 1989). The toxic effect of the plant in this study to decrease the serum level of testosterone may be mediated via affecting on leydig cells and impairing their functions and structures.
Previous reports have indicated a strong link between male infertility and exposure to more than 50 pesticides (Victor-Costa et al. 2010; Manfo et al. 2010; Tiwari et al. 2011). The adverse effects of ABM on the fertility of adult male rats have been demonstrated in the present study. The serum levels of testosterone, FSH and LH was significantly reduced in rats treated with 1/20 and 1/10 LD50 of ABM for 4 and 8 weeks. These results are in agreement with those of Elbetieha and Daas (2003) and Abd-Elhady and Abou-Elghar (2013) who found reduction in testosterone level at dose of 2.13 mg ABM/animal/day. Elbetieha and Daas (2003) indicated that ingestion of ABM for 6 weeks induced adverse effects on male rat fertility and reproduction. The decrease in male fertility via decrease in male sex hormones in rats treated with ABM in the present study is explained by Abd-Elhady and Abou-Elghar (2013) who suggested that ABM may have acted directly on the testes and affected the androgen biosynthesis pathway. These are strongly supported by the wide array of abnormalities seen when histopathological sections of the testes were examined. These abnormalities include necrotic changes in the tissues, interstitial odema, and degeneration and atrophy of seminal vesicles as well as desquamation of spermatogonial cells. In our opinion, it can be suggested that ABM may act directly on the testes and affected the androgen biosynthesis pathway or may directly act on the brain, hypothalamus or anterior pituitary gland which will indirectly affect the testes and possibly affect sexual activity. Both attributions are supported by the present study which revealed direct histological effect of ABM on testis and direct effects on pituitary hormones FSH and LH that respectively control spermatogenesis and testosterone secretion from leydig cells.
The shift in balance between oxidant/antioxidant in favor of oxidants is termed oxidative stress. Oxidative stress contributes to many pathological conditions. When oxidative stress occurs, cells attempt to counteract the oxidant effects and restore the redox balance by activation or silencing of genes encoding defensive enzymes, transcription factors, and structural proteins Scandalios (2004). Glutathione (GSH) is highly abundant in all cell compartments and is the major soluble antioxidant. It detoxifies hydrogen peroxide and lipid peroxides via action of glutathione peroxidase. GSH donates its electron to H2O2 to reduce it into H2O and O2. GSH protects cells against apoptosis by interacting with proapoptotic and antiapoptotic signaling pathways (Masella et al. 2005). In this study, it is observed that administration of latex and ethanolic extract induced marked decrease in heart and testis GSH levels and GPx, GST and SOD activities and on the other hand, it induced significant increase in lipid peroxidation. This increase in lipid peroxidation and suppression of antioxidant defense system as a result of latex and ethanolic extract administration lead to excess production and less scavenging of reactive oxygen species which in turn result in cardiac and testicular oxidative damage. In the current study, the deterioration of non-enzymatic and enzymatic antioxidants and exacerbated production of lipid peroxidation was associated with the elevation in the levels of various serum biochemical markers of cardiac and testicular damage including CK-MB, AST and LDH.
Administration of different doses of ABM caused depletion in GSH content, GPx, GST and SOD activities in this study. These results are in line with those of El-Shenawy (2010) who studied the toxic effect of ABM on isolated rat hepatocytes and found ABM decreased GSH concentration and GPx and SOD activities. Since superoxide is the primary ROS produced from the toxic substances, its dismutation by SOD is the primary importance for each cell. So, the depletion of the activity of SOD in this study caused accumulations of ROS in tissues causing disturbance of cell membrane and damage of cells.
Lipid peroxidation is the oxidative deterioration of polyunsaturated lipids to form radical intermediates that bring about cellular damage. Malondialdehyde (MDA), a major end product of this reaction, is an index of lipid peroxidation and has been estimated as thiobarbituric acid (TBARS) (Kohen and Nyska 2002). The increase in MDA level after the latex and ethanolic extract administration reflects that the plant induced increase in ROS and lipid peroxides. The elevation of lipid peroxides caused disturbance in cell membrane structure, damage of cell and cell death. Degree of toxicity induced by the plant is dose dependent. Significant increases in lipid peroxidation in heart and testis after ingestion of ABM in this study were resulted. Increasing dose progressively increased the toxic effect on the normal oxidant/antioxidant state in tissues. These results are in line with those of El-Shenawy (2010) who studied the toxic effect of ABM on isolated rat hepatocytes and found that ABM increased LPO.
Another substantiation of the impairment of the oxidant/antioxidant status in cells occurred with the plant and ABM administration is the depletion of the GPx, GST and SOD activities in tissues in a dose dependent manner. These results are in line with those of El-Shafey et al. (2011) and in contrast with those of El-Shenawy (2010). Based on the findings of the present study, it can be concluded that the cardiotoxic and testicular toxicity of C. procera leaf extract and latex as well as ABM is due to the elevation of lipid peroxidation and ROS and depletion of antioxidant levels.
The milky sap is a mixture of various chemicals including calotropis glycosides such as calotropin, calotoxin, calactin, uscharidin, voruscharin which are caustic in nature and are considered poisonous. The irritant and pro-inflammatory property of latex of C. procera has been well established (Alencar et al. 2006). Accidental exposure to the latex has been reported to cause inflammation of the skin and eyes (Shivkar and Kumar 2003; Al-Mezaine et al. 2005). In the present study, it was resulted that the latex and ethanolic extract as well as ABM induced marked inflammations as observed in photomicrographs of heart and testis histological sections. This heart and testis inflammatory status was concomitant with the marked elevation of serum levels of pro-inflammatory cytokine, TNF-α, and depletion of anti-inflammatory cytokine, IL-4, in a dose and time dependent manner.
Overall, C. procera latex and ethanolic extract of leaves as well as ABM induced cardiotoxic and testicular toxic effects which was evidenced by increases in the activities of heart enzymes in serum and decreases in male sex hormones in serum in addition to heart and testis histological perturbances. However the ethanolic leaf extract seemed to be more effective in deteriorating oxidative stress and antioxidant defense system in both heart and testis, the latex produced more deleterious effects on the testicular function and sex hormones’ levels. So, the plant latex and ethanolic extract are considered toxic and they may be suggested as rodenticides at 1/10 and 1/20 of LD50 which are more or less similar to the reference pesticide, ABM.
Authors’ contributions
OMA, HIA and MWB planned and designed the experiment. HYA measured the detected parameters. OMA and HYA performed statistical analysis. All authors drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to express their sincere appreciation to Dr. Walaa Azmy Hasan for her assistance in recognition and classification of the plant. The authors also acknowledged Prof. Dr. Kawkab Abd El Aziz Ahmed, Professor of Pathology, Pathology Department, Faculty of Veterinary Medicine, Cairo University for her great help in the examination of liver sections and description of histopathological changes.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Osama M. Ahmed, Phone: 00201001084893, Email: osamamoha@yahoo.com, Email: osama.ahmed@science.bsu.edu.eg
Hanaa I. Fahim, Email: hanaa_fahim@yahoo.com
Magdy W. Boules, Email: magdy129@yahoo.com
Heba Y. Ahmed, Email: heba_younes_1@yahoo.com
References
- Abd-Elhady HK, Abou-Elghar GE. Abamectin induced biochemical and histopathological changes in the albino rat, Rattus norvegicus. Plant Prot Res. 2013;53(3):263–270. [Google Scholar]
- Abou-Hashem AAM. Rodenticidal effect of argel (Gomphocarpus sinaicus Boiss) leaves on the Norway rat (Albino), Rattus norvegicus, Berkenhout under laboratory conditions. J Appl Sci Res. 2013;9(3):690–1695. [Google Scholar]
- Agaie BM, Salisu A, Ebobo AA. A survey of common toxic plants of livestock in Sokoto State, Nigeria. Sci Res Essay. 2007;2(2):40–42. [Google Scholar]
- Agarwal AK. Avermectin. In: Wexler P, editor. Encyclopedia of toxicology. 1. San Diego: Academic Press; 1998. pp. 89–90. [Google Scholar]
- Ahirwar D, Ahirwar B, Khary MD. Influence of Calotropis procera roots on biochemistry of reproductive organs of ovariectomized rats. Indian J Pharm Sci. 2007;69:459–461. doi: 10.4103/0250-474X.34565. [DOI] [Google Scholar]
- Ahmed UAM, Zuhua S, Bashier NHH, Muafi H, Zhongping H, Yuling G. Evaluation of insecticidal potentialities of aqueous extracts from Calotropis procera (Ait) against Henosepilachna elaterii rossi. J Appl Sci. 2006;6:2466–2470. doi: 10.3923/jas.2006.2466.2470. [DOI] [Google Scholar]
- Akinloye AK, Abatan MA, Akaka OO, Oke BA. Histomorphometric and histopathological studies on the effects of Calotropis procera (Giant milkwood) on the male reproductive organs of Wistar rats. Afr J Biomed Res. 2002;5:57–61. [Google Scholar]
- Alencar NM, Oliveira JS, Mesquita RO, Lima MW, Vale MR, Etchells JP, et al. Pro- and anti-inflammatory activities of the latex from Calotropis procera (Ait.) R.Br. are triggered by compounds fractionated by dialysis. Inflamm Res. 2006;55(12):559–564. doi: 10.1007/s00011-006-6025-y. [DOI] [PubMed] [Google Scholar]
- Al-Mezaine HS, Al-Rajhi AA, Al-Assiri A, Wagoner MD. Calotropis procera (ushaar) keratitis. Am J Ophthalmol. 2005;139(1):199–202. doi: 10.1016/j.ajo.2004.07.062. [DOI] [PubMed] [Google Scholar]
- Andreyko JL, Bhavnani BR, Nisker JA, Walker WH, Woolever CA. Role of serum androgens and sex hormones binding globulin capacity in the evaluation of hirsutism in women. Clin Biochem. 1986;19(1):58–61. doi: 10.1016/S0009-9120(86)80074-1. [DOI] [PubMed] [Google Scholar]
- Badshah H, Farmanullah Salihah Z, Saljoqi A, Shakur M. Toxic effects of AK (Calotropis procera) plant extracts against termites (Heterotermes indicola and Coptotermes heimi) Isoptera: Rhinotermitidae. Pak J Biol Sci. 2004;7(9):1603–1606. doi: 10.3923/pjbs.2004.1603.1606. [DOI] [Google Scholar]
- Basak SK, Bhaumik A, Mohanta A, Singhal P. Ocular toxicity by latex of Calotropis procera (Sodom apple) Indian J Opthalmol. 2009;57(3):232–234. doi: 10.4103/0301-4738.49402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum N, Sharma B, Pandey RS. Calotropis procera and Annona squamosa: potential alternatives to chemical pesticides. Br J Appl Sci Technol. 2013;3(2):254–267. doi: 10.9734/BJAST/2014/2205. [DOI] [Google Scholar]
- Beutler E, Duron O, Kelly BM. Improved method for determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Bhargava SK. Antiandrogenic effects of a flavonoid rich fraction of Vitex negundo seeds: a histological and biochemical study in dogs. J Ethnopharmacol. 1989;27:327–339. doi: 10.1016/0378-8741(89)90007-X. [DOI] [PubMed] [Google Scholar]
- Bhatnagar SS. The wealth of India. New Delhi: CSIR; 1950. p. 23. [Google Scholar]
- Braunstein GD, Rasor J, Alder D, Danzer H, Wade ME. Serum human luteinizing hormone levels through normal pregnancy. Am J Obstet Gynecol. 1976;126:678–681. doi: 10.1016/0002-9378(76)90518-4. [DOI] [PubMed] [Google Scholar]
- Carvalho AFU, Melo VMM, Craveiro AA, Machado MIL, Bantim MB, Rabelo EF. Larvicidal activity of the essential oil from Lippia sidoides Cham. against Aedes aegypti Linn. Mem Inst Oswaldo Cruz. 2003;98:569–571. doi: 10.1590/S0074-02762003000400027. [DOI] [PubMed] [Google Scholar]
- Cerejeira MJ, Viana P, Batista S, Pereira T, Silva E, Valério MJ, et al. Pesticides in Portuguese surface and ground waters. Water Res. 2003;37(5):1055–1063. doi: 10.1016/S0043-1354(01)00462-6. [DOI] [PubMed] [Google Scholar]
- Circosta C, Sanogo R, Occhiuto F. Effects of Calotropis procera on oestrous cycle and oestrogentic functionality in rats. IL Farm. 2001;56:373–378. doi: 10.1016/S0014-827X(01)01089-8. [DOI] [PubMed] [Google Scholar]
- Croft M, Duan M, Choi H, Eun S, Madireddi S, Mehta A. TNF superfamily in inflammatory disease: translating basic insights. Trends Immunol. 2012;33:144–152. doi: 10.1016/j.it.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lima JM, De Freitas FJ, Amorim RN, Camara AC, Batista JS, Soto-Blanco B. Clinical and pathological effects of Calotropis procera exposure in sheep and rats. Toxicon. 2011;57(1):183–185. doi: 10.1016/j.toxicon.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Eason CT, Turck P. A 90-day toxicological evaluation of compound 1080 (sodium monofluoroacetate) in Sprague Dawley rats. Toxicol Sci. 2002;69:439–447. doi: 10.1093/toxsci/69.2.439. [DOI] [PubMed] [Google Scholar]
- El Badwi SMA, Bakhiet AO. Toxicity of Calotropis procera latex in pregnant and non-pregnant goats. Sci Res Essays. 2010;5(17):2404–2408. [Google Scholar]
- El-Badwi SMA, Bakhiet AO, Medani AB, Shamseldin ZY. Influence of phenobarbital pretreatment on toxicity of Calotropis procera latex in Nubian goats. Res J Vet Sci. 2010;5(1):25–31. doi: 10.3923/rjvs.2012.25.31. [DOI] [Google Scholar]
- Elbetieha A, Daas SI. Assessment of antifertility activities of ABM pesticide in male rats. Ecotoxicol Environ Saf. 2003;55(3):307–313. doi: 10.1016/S0147-6513(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Elgamal MHA, Hanna AG, Morsy NAM, Duddeck H, Simon A, Toth G. Complete 1H and 13C signal assignments of 5α cardenolides isolated from Calotropis procera R.Br. J Mol Struct. 1999;477:201–208. doi: 10.1016/S0022-2860(98)00615-2. [DOI] [Google Scholar]
- EL-Gengaihi SE, Dimetry NZ, Mohamed SM. Chemical and biological investigation of harmal plant. 2-Alkaloidal investigation. J Appl Entomol. 1997;12(3):165–167. doi: 10.1111/j.1439-0418.1997.tb01387.x. [DOI] [Google Scholar]
- El-Shafey AAM, Seliem MME, El-Mahrouky F, Gabr WM, Kandil RA. Some physiological and biochemical effects of oshar extract and abamectin biocide on male albino rats. J Am Sci. 2011;7(12):254–261. [Google Scholar]
- El-Shenawy NS. Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytes. Toxicol In Vitro. 2010;24:1148–1157. doi: 10.1016/j.tiv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Fahim HE, Ahmed OM, Boules MW, Ahmed HY. Nephrotoxic effects of abamectin and Calotropis procera latex and leaf extract in male albino rats. Am J Med Med Sci. 2016;6(3):73–86. [Google Scholar]
- Freedman B, Nowak J, Kwolek WF. Abiossay for plant derived pest control agent using the European comborer. Econ Entomol. 1979;72:45–54. doi: 10.1093/jee/72.4.541. [DOI] [PubMed] [Google Scholar]
- Gallo MA, Lawryk NJ. Organic phosphorus pesticides. In: Hayes WJ Jr, Laws ER Jr, editors. Handbook of Pesticide Toxicology. San Diego: Academic Press; 1991. pp. 917–1123. [Google Scholar]
- Garg A. Effect of Aak Calotropis procera (Ait.) R. Br. flower extract on testicular function of the Indian desert male gerbil Meriones hurrianae Jerdon: a biochemical & histological study. Indian J Exp Biol. 1979;17(9):859–862. [PubMed] [Google Scholar]
- Gella FJ, Olivella T, Cruz Pastor M, Arenas J, Moreno R, Durban R, et al. A simple procedure for routine determination of aspartate aminotransferase and alanine aminotransferase with pyridoxal phosphate. Clin Chim Acta. 1985;153:241–247. doi: 10.1016/0009-8981(85)90358-4. [DOI] [PubMed] [Google Scholar]
- Gerhart W, Waldenström J. Creatine kinase B-Subunit activity in serum after immunoinhibition of M-Subunit activity. Clin Chem. 1979;25(7):1274–1280. [PubMed] [Google Scholar]
- Hassan SW, Bilbis FL, Ladan MJ, Umar RA, Dangoggo SM, Saidu Y, Abubakar MK, Faruk UZ. Evaluation of antifungal activity and phytochemical analysis of leaves, roots and stem bark extracts of Calotropis procera (Asclepiadaceae) Pak J Biol Sci. 2006;9(14):2624–2629. doi: 10.3923/pjbs.2006.2624.2629. [DOI] [Google Scholar]
- Howard M, Harada N (1994) Guidebook to cytokines and their receptors. In: Nicola NA (ed) Oxford University Press, New York, p 44
- Iqbal Z, Lateef M, Jabbar A, Muhammad G, Khan MN. Anthelmintic activity of Calotropis procera (Ait.) Ait F. flowers in sheep. J Ethnopharmacol. 2005;102(2):256–261. doi: 10.1016/j.jep.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Kanojiya S, Madhusudanan KP. Rapid identification of calotropagenin glycosides using high-performance liquid chromatography electrospray ionisation tandem mass spectrometry. Phytochem Anal. 2012;23(2):117–125. doi: 10.1002/pca.1332. [DOI] [PubMed] [Google Scholar]
- Kim HG, Jeon JH, Kim MK, Lee HS. Pharmacological effects of asaronaldehyde isolated from Acorus gramineus rhizome. Food Sci Biotechnol. 2005;14:685–688. [Google Scholar]
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30(6):620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Kolar L, Erzen NK, Hogerwerf L, Van Gestel CAM. Toxicity of abamectin and doramectin to soil invertebrates. Environ Pollut. 2008;151(1):182–189. doi: 10.1016/j.envpol.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Kuriachen PM, Dave Y. Structural, developmental and histochemical studies in the collectors of Calotropis procera (Asclepiadaceae) J Phytologic Res. 1989;2:7–14. [Google Scholar]
- Laitiff AA, Teoh SL, Das S. Wound healing in diabetes mellitus: traditional treatment modalities. Clin Ter. 2010;161(4):359–364. [PubMed] [Google Scholar]
- Lankas GR, Gordon LR. Toxicology. In: Campbell WC, editor. “Ivermectin and abamectin. New York: Springer; 1989. p. 363. [Google Scholar]
- Lima-Filho JV, Patriota JM, Silva AF, Filho NT, Oliveira RS, Alencar NM, et al. Proteins from latex of Calotropis procera prevent septic shock due to lethal infection by Salmonella enterica serovar Typhimurium. J Ethnopharmacol. 2010;129:327–334. doi: 10.1016/j.jep.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Lin Z, Will Y. Evaluation of drugs with specific organ toxicities in organ-specific cell lines. Toxicol Sci. 2012;126(1):114–127. doi: 10.1093/toxsci/kfr339. [DOI] [PubMed] [Google Scholar]
- Mahmoud OM, Adam SEI, Tartour G. The effects of Calotropis procera on small ruminants. I. Effects of feeding sheep with the plant. J Comp Pathol. 1979;89:241–250. doi: 10.1016/0021-9975(79)90063-X. [DOI] [PubMed] [Google Scholar]
- Mahmoud OM, Adam SEI, Tartour G. The effects of Calotropis procera on small ruminants. II. Effects of administration of the latex to sheep and goats. J Comp Pathol. 1979;89:251–263. doi: 10.1016/0021-9975(79)90064-1. [DOI] [PubMed] [Google Scholar]
- Maloschik E, Ernst A, Hegedus G, Darvas B, Székács A. Monitoring water-polluting pesticides in Hungary. Microchem J. 2007;85(1):88–97. doi: 10.1016/j.microc.2006.05.002. [DOI] [Google Scholar]
- Manfo FP, Moundipa PF, Déchaud H, Tchana AL, Nantia EA, Zabot MT, et al. Effect of agropesticides use on male reproductive function: a study on farmers in Djutitsa (Cameroon) Environ Toxicol. 2010;27(7):423–432. doi: 10.1002/tox.20656. [DOI] [PubMed] [Google Scholar]
- Mannervik B, Gutenberg C. Glutathione transferase (human placenta) Methods Enzymol. 1981;77:231–235. doi: 10.1016/S0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Matkovics B, Kotorman M, Varga IS, Hai DQ, Varga C. Oxidative stress in experimental diabetes induced by streptozotocin. Acta Physiol Hung. 1997;85(1):29–38. [PubMed] [Google Scholar]
- Million T, Kassa H, Charles K. The toxicity of plant material, Drimia altissima (Urginea altissima), against the field rat, Arvicanthis abyssinicus: a potential non synthetic rodenticide. Ethiop J Health Dev. 2010;24(3):175–179. [Google Scholar]
- Moline JM, Golden AL, Bar-Chama N, Smith E, Rauch ME, Chapin RE, et al. Exposure to hazardous substances and male reproductive health: a research framework. Environ Health Perspect. 2000;108:803–813. doi: 10.1289/ehp.00108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell WD, Parlow AF, Cargille CM, Ross GI. Radioimmunioassay for human FSH: physiological studies. J Clin Invest. 1968;47:2551–2562. doi: 10.1172/JCI105937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul TK, Kumar A. Dendrocnide sinuata (Blume) Chew (Urticaceae)—a plant that can be grown to repulse the wild elephants. ENVIS Newsl. 2009;14(2):5–6. [Google Scholar]
- Poindexter B, Feng W, Dasgupta A, Bick R. Oleandrin produces changes in intracellular calcium levels in isolated cardiomyocytes, a real-time fluorescence imaging study comparing adult to neonatal cardiomyocytes. J Toxicol Environ Health. 2007;70:568–574. doi: 10.1080/15287390600882408. [DOI] [PubMed] [Google Scholar]
- Preuss HG, Jarrel ST, Scheckenbach R, Lieberman S, Anderson RA. Comparative effects of chromium, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr. 1998;17(2):116–123. doi: 10.1080/07315724.1998.10718736. [DOI] [PubMed] [Google Scholar]
- Quy RJ, Cowan DP, Prescott CV, Gill JE, Kerins GM, Dunsford G, et al. Control of a population of Norway rats resistant to anticoagulant rodenticides. Pest Sci. 1995;45:247–256. doi: 10.1002/ps.2780450308. [DOI] [Google Scholar]
- Roa M, Blane K, Zonneberg M (1985) PC-STAT one-way analysis of variance. Version IA (C) copyright. The University of Georgia, University of Georgia, USA
- Samar KB, Arup B, Ayan M, Prashant S. Ocular toxicity by latex of Calotropis procera. Indian J Ophthalmol. 2009;57:232–234. doi: 10.4103/0301-4738.49402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. Genomic responses to oxidative stress. In: Meyers RA, editor. Encyclopedia of molecular cell biology and molecular medicine. 2. Weinheim: Wiley-VCH; 2004. pp. 489–512. [Google Scholar]
- Sharma N, Jacob D. Inhibition of fertility and functional alteration in the genital organs of male Swiss albino mouse after administration of Calotropis procera flower extract. Pharma Biol. 2001;39(6):403–407. doi: 10.1076/phbi.39.6.403.5882. [DOI] [Google Scholar]
- Sheth F. Range of seasonal phytochemical variations in Calotropis procera (Ait.) R. Br. Int J Med Arom Plants. 2011;1(2):180–183. [Google Scholar]
- Shivkar YM, Kumar VL. Histamine mediates the pro-inflammatory effect of latex of Calotropis procera in rats. Mediat Inflamm. 2003;12(5):299–302. doi: 10.1080/096293503310001619708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal A, Kumar VL. Effect of aqueous suspension of dried latex of Calotropis procera on hepatorenal functions in rat. J Ethnopharmacol. 2009;122(1):172–174. doi: 10.1016/j.jep.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Suresh Kumar P, Suresh E, Kalavathy S. Review on a potential herb Calotropis gigantea (L.) R. Br. Sch Acad J Pharm. 2013;2(2):135–143. [Google Scholar]
- Thankamma L. Hevea latex as a wound healer and pain killer. Curr Sci. 2003;84(8):971–972. [Google Scholar]
- Thomas PJ, Mineau P, Shore RF, Champoux L, Martin PA, Wilson LK, Fitzgerald G, Elliot JE. Second generation anticoagulant rodenticides in predatory birds: probabilistic characterization of toxic liver concentrations and implications for predatory bird populations in Canada. Environ Int. 2011;37:914–920. doi: 10.1016/j.envint.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Pragya P, Ram KR, Chowdhuri DK. Environmental chemical mediated male reproductive toxicity: Drosophila melanogaster as an alternate animal model. Theriogenology. 2011;76(2):197–216. doi: 10.1016/j.theriogenology.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Vadlapudi V, Naidu CK. In vitro bioactivity of indian medicinal plant Calotropis procera (Ait) J Glob Pharm Technol. 2010;2:43–45. [Google Scholar]
- Van Quaquebeke E, Simon G, Andre A, Dewelle J, El Yazidi M, Bruyneel F, et al. Identification of a novel cardenolide (200-oxovoruscharin) from Calotropis procera and the hemisynthesis of a novel derivative displaying potent in vitro antitumor activities and high in vivo tolerance: structure–activity relationship analyses. J Med Chem. 2005;48:849–856. doi: 10.1021/jm049405a. [DOI] [PubMed] [Google Scholar]
- Verma R, Satsangi GP, Shrivastava JN. Analysis of phytochemical constituents of the ethanolic and chloroform extracts of Calotropis procera using gas chromatography-mass spectroscopy (GC-MS) technique. J Med Plants Res. 2013;7(40):2986–2991. [Google Scholar]
- Victor-Costa AB, Bandeira SM, Oliveira AG, Mahecha GA, Oliveira CA. Changes in testicular morphology and steroidogenesis in adult rats exposed to Atrazine. Reprod Toxicol. 2010;29:323–331. doi: 10.1016/j.reprotox.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Young DS (2000) Effects of drugs on clinical laboratory tests, 5th edn. American Association for Clinical Chemistry (AACC) Press, Washington, DC, USA. https://www.aacc.org/store/books/6600/effects-of-drugs-on-clinical-laboratory-tests-5th-edition.aspx


