Abstract
Background
Pteris vittata L. is rich in flavonoids which exhibit different bioactivities. In order to investigate the flavonoids components of P. vittata L., extracts of the plant were isolated by column chromatography on silica gel and Sephadex LH-20.
Results
Four flavonoids compounds were obtained, the structures were identified as kaempferol (1), quercetin (2), kaempferol-3-O-d-glucopyranoside (3) and rutin (4), respectively, on the basis of NMR spectroscopic analyses.
Conclusions
Compound 3 was reported for the first time from P. vittata L.
Electronic supplementary material
The online version of this article (doi:10.1186/s40064-016-3308-9) contains supplementary material, which is available to authorized users.
Keywords: Pteris vittata L., Flavonoids, Kaempferol-3-O-d-glucopyranoside
Background
Pteris vittata L., a common fern known as ‘Chinese Brake Fern’, is native from China and widespread all over the world (Ma et al. 2001). It received much attentions in recent years because it was known to be a hyperaccumulator plant of arsenic used in phytoremediation (Cesaro et al. 2015; Tisarum et al. 2015; de Oliveira et al. 2016; Tiwari et al. 2016). It is also widely used in traditional Chinese medicine for diverse therapeutic applications, such as the treatment of influenza, dysentery, rheumatism, injury and scabies (Xie 1996). Previous qualitative phytochemical screening studies on P. vittata L. have showed a substantial amount of flavonoids (Ding et al. 2009; Zhou et al. 2010). Compounds like leucocyanidin, leucodelphinidin, flavone ester apigenin 7-O-p-hydroxybenzoate, as well as a number of glycosides of apigenin, leutolin, isocutellarein-8-O-methyl-ether, kaempherol and quercetin were isolated from this plant in the past years (Salatino and Prado 1998; Imperato 2006). But, it attracts little attention on its chemical constituents or bioactivities recently. Numerous reports showed that flavonoids exhibited different bioactivities, such as anti-inflammatory, anti-oxidative, hypolipidemic, or antitumor effects (Bao et al. 2016; Feng et al. 2014; Matias et al. 2014; Raman et al. 2016). Plants are one of the important sources for screening active compounds. Hence, we attempt to obtain flavonoids components from P. vittata L. to provide more information about its chemical constituents in this experiment.
Methods
Plant material
The whole plant of P. vittata L. was collected from Guangzhou, China, in October 2015 and identified by Prof. P. T. Li (College of Forestry, South China Agricultural University, Guangzhou, P. R. China). The voucher specimen (No. PGZ090239) has been deposited at the CANT Herbarium, South China Agricultural University, Guangzhou, P. R. China.
Experimental material
Silica gel (100–200 and 200–300 mesh) for column chromatography (CC) and GF254 silica gel for thin layer chromatography (TLC) were purchased from Qingdao Marine Chemical Ltd., Qingdao, China. Sephadex LH-20 obtained from Amersham Biosciences, Sweden was used for CC. Other reagents were of analytical grade purchased from Guangzhou reagent Co. Ltd. NMR spectra (1H, 13C-NMR) were determined on a Bruker AV-600 instrument using TMS as an internal reference.
Extraction and isolation
The whole plant (7.0 kg) of P. vittata L. was extracted with methanol for three times at 25 °C. After the solvent was removed under vacuum, the concentrated extract was further extracted successively with petroleum ether, ethyl acetate and n-butanol. The ethyl acetate extract (80 g) was subjected to silica gel column chromatography using gradient hexane–EtOAc (50:1–1:1) to obtain 6 fractions. Fraction 2 (12 g) was subjected to silica gel column chromatography eluted with gradient hexane–acetone (100:1–10:1) to afford compound 1 (52 mg). Fraction 3 (8 g) was subjected repeatedly to silica gel column chromatography eluted with hexane–acetone (100:5) and was finally purified by column chromatography on Sephadex LH-20 eluted with CHCl3–MeOH (1:1) to give compound 2 (53 mg). Fraction 5 (2 g) was subjected to silica gel column chromatography eluted with CHCl3–MeOH (95:5) to give compound 3 (36 mg). Fraction 6 (5 g) was subjected to silica gel column chromatography eluted with CHCl3–MeOH (10:1) led to the isolation of compound 4 (280 mg).
Results
Four compounds were isolated and their structures were identified as kaempferol (1), quercetin (2), kaempferol-3-O-d-glucopyranoside (3) and rutin (4), respectively, by spectral analysis and comparison with the spectroscopic data reported in previous literatures (Fig. 1). NMR spectra data of the compounds were listed as follows (Additional file 1).
Fig. 1.
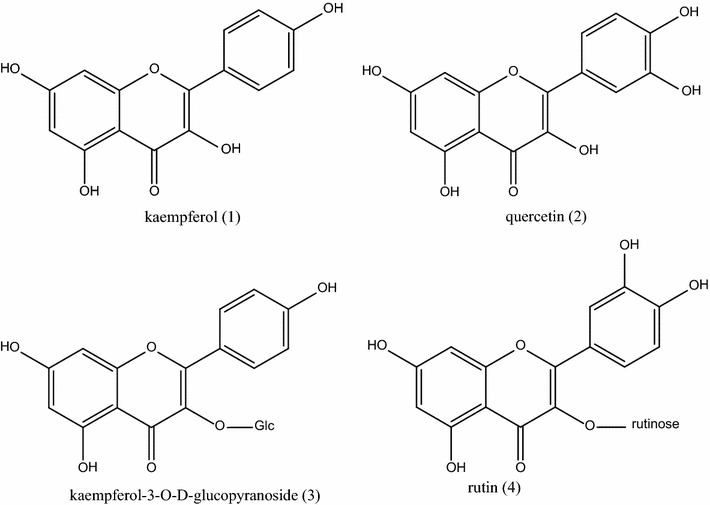
Structures of flavonoids from P. vittata L
Compound 1 was obtained as yellow amorphous powder. 1H-NMR (600 MHz, DMSO-d6) δH: 6.19 (1H, d, J = 1.8 Hz), 6.44 (1H, d, J = 1.8 Hz), 6.92 (2H, d, J = 9.0 Hz), 8.04 (2H, d, J = 9.0 Hz). 13C-NMR (150 MHz, DMSO-d6) δC: 93.5 (C-8), 98.2 (C-6), 103.0 (C-10), 115.4 (C-3′, C-5′), 121.7 (C-1′), 129.5 (C-2′), 130.5 (C-6′), 135.6 (C-3), 146.8 (C-2), 156.2 (C-5), 159.2 (C-4′), 160.7 (C-9), 163.9 (C-7), 175.9 (C-4). It was determined as kaempferol by comparison with the spectroscopic data reported in the literature (Liu et al. 2009).
Compound 2 was obtained as yellow amorphous powder. 1H-NMR (600 MHz, DMSO-d6) δH: 6.19 (1H, d, J = 1.8 Hz), 6.40 (1H, d, J = 2.4 Hz), 6.88 (1H, d, J = 8.4 Hz), 7.54 (1H, dd, J = 2.4, 8.4 Hz), 7.67 (1H, d, J = 2.4 Hz). 13C-NMR (150 MHz, DMSO-d6) δC: 93.3 (C-8), 98.1 (C-6), 102.9 (C-10), 114.9 (C-2′), 115.5 (C-5′), 119.9 (C-6′), 121.8 (C-1′), 135.6 (C-3), 144.9 (C-1′), 146.7 (C-1), 147.6 (C-4′), 156.1 (C-5), 160.7 (C-9), 163.8 (C-7), 175.8 (C-4). It was identified as quercetin by comparison with the spectroscopic data reported in the literature (Ma et al. 2007).
Compound 3 was obtained as yellow amorphous powder. 1H-NMR (600 MHz, DMSO-d6) δH: 3.09 (2H, d, J = 4.2 Hz), 3.21 (1H, d, J = 7.8 Hz), 3.32 (1H, d, J = 11.4 Hz), 3.55 (1H, d, J = 11.4 Hz), 4.29 (1H, s), 4.96 (1H, s), 5.07 (1H, s), 5.36 (1H, s), 5.46(1H, d, J = 7.8), 6.21 (1H, d, J = 1.8 Hz), 6.43 (1H, d, J = 1.8 Hz), 6.88 (2H, d, J = 9.0 Hz), 8.04 (2H, d, J = 9.0 Hz). 13C-NMR (150 MHz, DMSO-d6) δC: 61.1 (C-6″), 70.2 (C-4″), 74.5 (C-2″), 76.8 (C-5″), 77.8 (C-3″), 94.0 (C-8), 99.1 (C-6), 101.2 (C-1″), 104.3 (C-10), 115.4 (C-3′, C-5′), 121.2 (C-1′), 131.2 (C-2′, C-6′), 133.5 (C-3), 156.6 (C-2), 156.7 (C-9), 160.3 (C-4′), 161.5 (C-5), 164.5 (C-7), 177.8 (C-4). It was determined as kaempferol-3-O-d-glucopyranoside by comparison with the spectroscopic data reported in the literature (Long et al. 2011).
Compounds 4 was obtained as yellow amorphous powder. 1H-NMR (600 MHz, DMSO-d6) δH: 1.00 (3H, d, J = 6.0 Hz), 3.08 (3H, m), 3.70 (1H, d, J = 12.0 Hz), 4.39 (1H, d, J = 2.4 Hz), 5.10 (2H, d, J = 18.0 Hz), 5.28 (1H, s), 5.35 (1H, d, J = 6.0 Hz), 6.20 (1H, d, J = 2.4 Hz), 6.39 (1H, d, J = 2.4 Hz), 7.54 (1H, d, J = 2.4 Hz), 7.56 (1H, dd, J = 2.4, 12.0 Hz) 13C-NMR (150 MHz, DMSO-d6): δC: 17.6 (C-6′″), 66.9 (C-6″), 68.1 (C-5′″), 69.9 (C-4″), 70.2 (C-2′″), 70.4 (C-3′″), 71.7 (C-4′″), 73.9 (C-2″), 75.8 (C-5″), 76.3 (C-3″), 93.5 (C-8), 98.5 (C-6), 100.6 (C-1′″), 101.0 (C-1″), 103.8 (C-10), 115.1 (C-2′), 116.1 (C-5′), 121.0 (C-6′), 121.5 (C-1′), 133.2 (C-3), 144.6 (C-3′), 148.3 (C-4′), 156.3 (C-9), 156.5 (C-2), 161.1 (C-5), 164.0 (C-7), 177.2 (C-4). It was identified as rutin compared with the spectroscopic data reported in the literature (Zhang et al. 2010).
Conclusion
Phytochemical screening of P. vittata L. was carried out. Four flavonoids were obtained and identified as kaempferol (1), quercetin (2), kaempferol-3-O-d-glucopyranoside (3) and rutin (4), respectively. Compound 1, 2 and 4 were known components which were mentioned in the previous results (Imperato and Telesca 2000; Imperato 2006). Compound 3 was a derivative of kaempferol with a glucopyranoside. It is reported for the first time from P. vittata L. The current results may provide more information about flavonoids profiles of this plant.
Authors’ contributions
LZC designed the experiment and prepared the manuscript. HXB isolated and purified the flavonoid components. LLJ identified structures of the compounds. All authors read and approved the final manuscript.
Acknowledgements
This work was financially supported by Foundation for Distinguished Young Talents in Higher Education of Guangdong (2014KQNCX214) and Technology Research and Development Program of Huizhou (2014B040008001).
Competing interests
The authors declare that they have no competing interests.
Additional file
10.1186/s40064-016-3308-9 NMR spectra data of the compounds can be found online as Additional file for the present article.
Contributor Information
Li-jing Lin, Email: 49031788@qq.com.
Xiao-bing Huang, Email: 351772657@qq.com.
Zhen-cheng Lv, Phone: +86-752-2529555, Email: szsky@126.com.
References
- Bao L, Hu L, Zhang Y, Wang YI. Hypolipidemic effects of flavonoids extracted from Pteris vittata. Exp Ther Med. 2016;11(4):1417–1424. doi: 10.3892/etm.2016.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaro P, Cattaneo C, Bona E, Berta G, Cavaletto M. The arsenic hyperaccumulating Pteris vittata expresses two arsenate reductases. Sci Rep. 2015;5:14525. doi: 10.1038/srep14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira LM, Gress J, De J, Rathinasabapathi B, Marchi G, Chen Y, Ma LQ. Sulfate and chromate increased each other’s uptake and translocation in As-hyperaccumulator Pteris vittata. Chemosphere. 2016;147:36–43. doi: 10.1016/j.chemosphere.2015.12.088. [DOI] [PubMed] [Google Scholar]
- Ding LJ, Su GL. Extraction of flavonoids from ladder brake with microwave and its antioxidative activity. J Food Sci Bio. 2009;28(5):623–626. [Google Scholar]
- Feng Z, Hao W, Lin X, Fan D, Zhou J. Antitumor activity of total flavonoids from Tetrastigma hemsleyanum Diels et Gilg is associated with the inhibition of regulatory T cells in mice. Onco Targets Ther. 2014;7:947–956. doi: 10.2147/OTT.S61794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato F. The new flavones ester Apigenin-7-O-oxy-p-hydroxybenzoate and 3-Di-C-glycosyl flavones from Pteris vittata. Am Fern J. 2006;96(2):62–65. doi: 10.1640/0002-8444(2006)96[62:SN]2.0.CO;2. [DOI] [Google Scholar]
- Imperato F, Telesca A. 6-C-cellobiosylisoscutellarein-8-methyl ether, a new flavonoid from Pteris vittata. Am Fern J. 2000;96(1):42–47. doi: 10.2307/1547261. [DOI] [Google Scholar]
- Liu CD, Chen J, Wang JH. Confirmation of the structure of tiliroside, an acylated kaempferol glycoside, by 13C-Nuclear Magnetic Resonance. Chem Nat Comp. 2009;45(6):808–810. doi: 10.1007/s10600-010-9500-1. [DOI] [Google Scholar]
- Long F, Deng L, Chen Y. Study on the chemical constituents in the flowers of Ligustrum lucium. West China J Pharm Sci. 2011;26(2):97–100. [Google Scholar]
- Ma LQ, Komar KMN, Tu C, Zhang W, Cai Y, Kennelley E. A fern that hyperaccumulates arsenic. Nature. 2001;409:579. doi: 10.1038/35054664. [DOI] [PubMed] [Google Scholar]
- Ma XM, Liu Y, Shi YP. Phenolic derivatives with free-radical-scavenging activities from Ixeridium gracile (DC.) Shih. Chem Biodivers. 2007;4(9):2172–2181. doi: 10.1002/cbdv.200790174. [DOI] [PubMed] [Google Scholar]
- Matias A, Nunes SL, Poejo J, Mecha E, Serra AT, Madeira PJ, Bronze MR, Duarte CM. Antioxidant and anti-inflammatory activity of a flavonoid-rich concentrate recovered from Opuntia ficus-indica juice. Food Funct. 2014;5(12):3269–3280. doi: 10.1039/C4FO00071D. [DOI] [PubMed] [Google Scholar]
- Raman ST, Ganeshan AK, Chen C, Jin C, Li SH, Chen HJ, Gui Z. In vitro and in vivo antioxidant activity of flavonoid extracted from mulberry fruit (Morus alba L.) Pharmacogn Mag. 2016;12(46):128–133. doi: 10.4103/0973-1296.177910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salatino MLF, Prado J. Flavonoid glycosides of Pteridaceae from Brazil. Biochem Syst Ecol. 1998;26:761–769. doi: 10.1016/S0305-1978(98)00032-5. [DOI] [Google Scholar]
- Tisarum R, Chen Y, Dong X, Lessl JT, Ma LQ. Uptake of antimonite and antimonate by arsenic hyperaccumulator Pteris vittata: effects of chemical analogs and transporter inhibitor. Environ Pollut. 2015;206:49–55. doi: 10.1016/j.envpol.2015.06.029. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Sarangi BK, Thul ST. Identification of arsenic resistant endophytic bacteria from Pteris vittata roots and characterization for arsenic remediation application. J Environ Manage. 2016;180:359–365. doi: 10.1016/j.jenvman.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Xie WZ. Compilation of national Chinese herbal medicine. Beijing: People Health Press; 1996. pp. 646–647. [Google Scholar]
- Zhang S, Tao ZM, Zhang Y. Chemical constituents from the stems and leaves of Elaeocarpus glabripetalus. Chin J Nat Med. 2010;8(1):21–24. doi: 10.3724/SP.J.1009.2010.00021. [DOI] [Google Scholar]
- Zhou XJ, Yang ZQ, Jing HY, Gao YX. Study on optimization of total flavonoids extraction technology in Pteris Vittata L. Res Dev Mark. 2010;26(3):210–211. [Google Scholar]


