Abstract
Fingolimod (FTY720, Gilenya), a sphingosine-1-phosphate receptor (S1PR) modulator, is one of the first-line immunomodulatory therapies for treatment of relapsing-remitting multiple sclerosis (MS). Human S1PR1 variants have been reported to have functional heterogeneity in vitro, suggesting that S1PR1 function may influence FTY720 efficacy. In this study, we examined the influence of S1PR1 phosphorylation on response to FTY720 in neuroinflammation. We found that mice carrying a phosphorylation-defective S1pr1 gene [S1PR1(S5A) mice] were refractory to FTY720 treatment in MOG35-55-immunized and Th17-mediated experimental autoimmune encephalomyelitis (EAE) models. Long-term treatment with FTY720 induced significant lymphopenia and suppressed Th17 response in the peripheral immune system via downregulating STAT3 phosphorylation in both WT and S1PR1(S5A) mice. However, FTY720 did not effectively prevent neuroinflammation in the S1PR1(S5A) EAE mice as a result of encephalitogenic cells expressing C-C chemokine receptor 6 (CCR6). Combined treatment with FTY720 and anti-CCR6 delayed disease progression in S1PR1(S5A) EAE mice, suggesting that CCR6-mediated cell trafficking can overcome the effects of FTY720. This work may have translational relevance regarding FTY720 efficacy in MS patients and suggests that cell type–specific therapies may enhance therapeutic efficacy in MS.
Defective sphingosine-1-phosphate receptor 1 phosphorylation compromises the efficacy of FTY720 in a murine experimental autoimmune encephalomyelitis model via a CCR6-mediated CNS homing mechanism.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disorder of the CNS that affects over 2 million individuals worldwide (1, 2). Timely treatment with immune modulatory therapies, such as IFN-β or glatiramer acetate, decreases relapse rates and prevents neural tissue damage (3). Thirteen FDA-approved MS therapies are currently available; however, approximately 50% of MS patients develop varying degrees of neurologic disability despite immune modulation (4, 5). Understanding mechanisms dictating proinflammatory responses in MS and the effects of immune therapies on these pathways is essential to maximize therapeutic efficacy and achieve long-term favorable outcome.
The bioactive lipid second messenger sphingosine-1-phosphate (S1P) pathway is a major immune regulatory pathway in MS pathogenesis (6–8). Targeting the S1P pathway with fingolimod (FTY720, Gilenya), a functional antagonist of S1P receptors (S1PRs) 1 and 3–5, is an exciting advance in MS therapy because FTY720 treatment effectively decreases annualized relapse rates and prevents progressive neurologic disability (9–12). Until recently, insights into S1P signaling in MS have primarily been derived from studies on lymphocyte trafficking from secondary lymphoid organs (SLOs) (13–15). The S1P-S1PR1 interaction is essential for lymphocyte entry into the systemic circulation (16–18). FTY720 retains lymphocytes within SLOs by promoting phosphorylation, internalization, and degradation of S1PR1 (19–22). However, whether FTY720’s mode of action in MS is solely by regulating lymphocyte trafficking or if alternative mechanisms of immune regulation exist remains to be elucidated. Current studies suggest that FTY720 also regulates Th17 and Th1 development (6, 23) and Th1 and Treg balance (23) and has direct effects on the CNS by modulating astrocytes (24) and oligodendrocytes (25, 26).
We recently reported that failure to phosphorylate S1PR1 in the C-terminal peptide (a region crucial for receptor internalization) led to Th17-mediated autoimmune CNS demyelination by activating the IL-6/Jak/STAT3 pathway (6). An unbiased phosphoproteomic analysis of MS brain lesions also demonstrated that S1PR1 is phosphorylated on S351, suggesting that a parallel mechanism might occur in the human disease. Due to the presence of S1PR1 gene variations among the general population (27) and the observation of breakthrough clinical disease and proinflammatory peripheral blood immune cell profiles in a subset of MS patients treated with FTY720 (28–31), we questioned how S1PR1 gene mutation that leads to impaired receptor phosphorylation might determine the response to FTY720 therapy.
Here, we show that mice carrying a phosphorylation-defective S1pr1 gene [S1PR1(S5A)] are significantly less responsive to treatment with FTY720, especially in the Th17 adoptive transfer experimental autoimmune encephalomyelitis (EAE) model. In vivo and in vitro experiments suggest that FTY720 treatment decreased IL-17 expression by downregulating STAT3 phosphorylation. Interestingly, FTY720 treatment did not arrest a subset of lymphocytes from trafficking to the CNS despite significant lymphopenia. Further analysis suggests that these cells expressed the CNS homing receptor C-C chemokine receptor 6 (CCR6) and that treatment with a blocking antibody against CCR6 ameliorated EAE and delayed disease progression in S1PR1(S5A) mice. In summary, S1PR1 internalization is critical for responsiveness to FTY720, and the CCR6-dependent CNS homing mechanism appears to overcome FTY720-induced blockade of lymphocyte egress when receptor phosphorylation is defective. These findings may illuminate the pathogenesis of MS and may have translational relevance to the response to immune modulatory therapies in CNS autoimmunity.
Results
S1PR1(S5A) EAE mice were less responsive to treatment with FTY720.
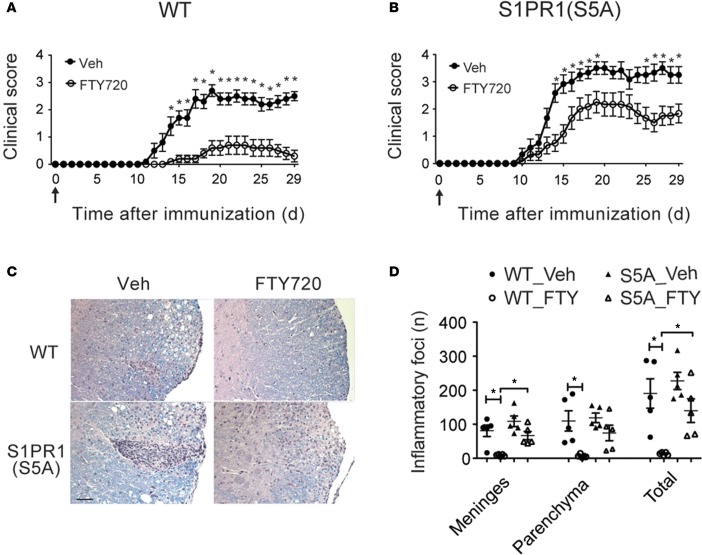
Our previous work showed that mice carrying a phosphorylation-defective S1pr1 gene (in which 5 serines in the C-terminal fragment of the protein were mutated into alanines) developed more severe Th17-mediated EAE (6). Existing studies suggest that S1PR1 and S1PR4 are predominantly expressed in CD4+ T cells (32). FTY720 has been shown to signal via all S1PRs (S1PR1–5) except S1PR2 (33). Therefore, we first examined the expression of S1PR1–5 in splenic CD4+ T cells isolated from C57BL/6J wild-type (WT) and S1PR1(S5A) mice. There was no significant difference in the expression of S1PRs in CD4+ T cells from WT and S1PR1(S5A) mice (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/jci.insight.86462DS1). Thus, we focused our study mainly on the effects of FTY720 on S1PR1 function. We treated myelin oligodendrocyte glycoprotein (MOG) peptide35–55-immunized (MOG35-55-immunized) C57BL/6J WT and S1PR1(S5A) mice with FTY720 from the day of immunization throughout the disease course in the preventative EAE model. We observed that treatment with FTY720 resulted in significantly less severe paralysis in WT EAE mice (Figure 1A and Supplemental Table 1). FTY720 treatment also decreased EAE severity in S1PR1(S5A) EAE mice (Figure 1B and Supplemental Table 2). However, FTY720 was less effective in preventing EAE in the S1PR1(S5A) mice (cumulative disease index [CDI = 30.17]) compared with their WT counterparts (CDI = 7.4, P = 0.0081). In support of these findings, we also observed higher disease incidence, earlier onset of EAE (Supplemental Figure 2), and more severe CNS inflammation (Figure 1, C and D) in S1PR1(S5A) EAE mice. These findings suggest that S1PR1 phosphorylation may influence not only EAE severity, as we have observed previously (6), but also response to FTY720 therapy.
Figure 1. S1PR1(S5A) EAE mice were less responsive to treatment with FTY720.
Mean clinical score ± SEM of MOG35-55-immunized C57BL/6J (WT) mice (A) and mice carrying phosphorylation-defective S1PR1 [S1PR1(S5A) mice] (B) (females, 8–12 weeks) treated with either vehicle (1% cyclodextrin in PBS ) or FTY720 (0.5 mg/kg) by daily i.p. injections. Arrows indicate time of initiation of therapy. *P < 0.05, Mann-Whitney U test. This experiment was performed 3 times with n = 10–12 mice/arm. (C) Histopathology of a representative section from formalin-fixed, paraffin-embedded CNS tissue from WT and S1PR1(S5A) EAE mice. Scale bar: 50 μm. (D) Quantification of inflammatory foci in the CNS of WT and S1PR1(S5A) (S5A) EAE mice from A and B. mean ± SEM, *P < 0.05, ANOVA with Tukey’s multiple comparison test, n = 5 mice/arm.
S1PR1(S5A) Th17 adoptive transfer EAE mice were refractory to FTY720.
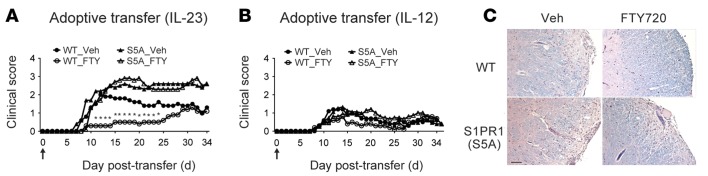
We then investigated the cellular mechanism of unresponsiveness to FTY720 treatment in the S1PR1(S5A) EAE mice by performing Th-specific adoptive transfer EAE experiments. Donor encephalitogenic cells from the splenocytes of MOG35-55-immunized C57BL/6J WT and S1PR1(S5A) EAE mice (day 8 after immunization) activated in vitro with either recombinant IL-12 (rIL-12) (10 ng/ml for Th1 polarization) or rIL-23 (10 ng/ml for Th17 polarization) in the presence of MOG35-55 peptide were adoptively transferred into naive WT recipients and treated with vehicle or FTY720 by daily i.p. injections up to day 34. We observed that the recipients of donor WT Th17 cells showed improvement of paralysis upon treatment with FTY720 (Figure 2A and Supplemental Table 3). In contrary, the recipients of donor S1PR1(S5A) Th17 cells developed more severe clinical EAE and were refractory to FTY720 treatment (Figure 2A and Supplemental Table 4). In support of these findings, histopathological analysis also showed more severe CNS inflammation in the S1PR1(S5A) Th17 recipients with or without FTY720 therapy, whereas their WT counterparts showed markedly less severe CNS inflammation following FTY720 treatment (Figure 2C). We also observed that the recipients of encephalitogenic Th1 cells from either WT or S1PR1(S5A) mice developed milder EAE (Figure 2B and Supplemental Tables 5 and 6) and showed minimal response to FTY720 treatment. These findings suggest that S1PR1(S5A) Th17 cells are likely responsible for the poor responsiveness to FTY720 therapy.
Figure 2. Th17 adoptive transfer recipient EAE mice were refractory to treatment with FTY720.
Mean clinical scores of C57BL/6J (WT) recipients of donor encephalitogenic cells from MOG35-55-immunized C57BL/6J (WT) or S1PR1(S5A) mice. Splenocytes were cultured and treated with rIL-23 (10 ng/ml) to generate Th17-polarized cells (A) or with rIL-12 (10 ng/ml) to generate Th1-polarized cells (B) for 72 hours in the presence of MOG35-55 peptide before transfer. Recipient mice were treated with either vehicle (1% cyclodextrin in PBS) or FTY720 (0.5 mg/kg) by daily i.p. injections from day 0 to 34 after transfer. Arrows indicate time of initiation of therapy. This experiment was performed 3 times with n = 10–15 mice/arm. (C) Histopathology of a representative section from formalin-fixed, paraffin-embedded CNS tissue from mice recipient of Th17-polarized cells from WT or S1PR1(S5A) mice following treatment with vehicle or FTY720. Scale bar: 50 μm. Donors: females, 8–12 weeks; recipients: females, 5–6 weeks. *P < 0.05, Mann-Whitney U test.
FTY720 downregulated Th17 differentiation in vivo and in vitro.
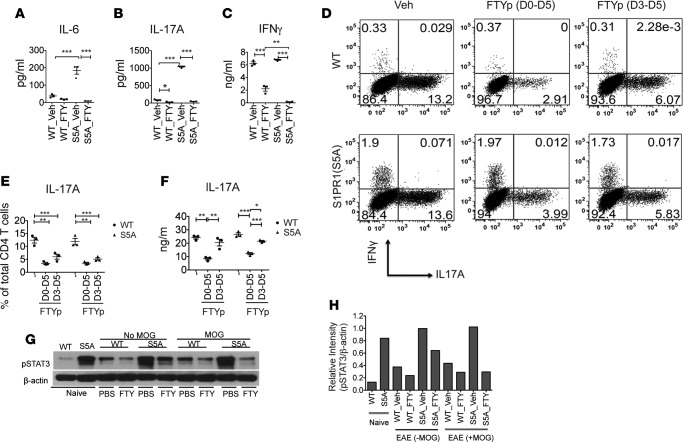
Findings from our adoptive transfer EAE experiments suggested that Th17 cells were responsible for the impaired response to FTY720 treatment in S1PR1(S5A) EAE mice. To investigate whether FTY720 treatment interferes with Th17 differentiation, we examined the peripheral immune response of WT and S1PR1(S5A) mice treated with FTY720. We first performed an ex vivo recall assay with MOG35-55 peptide stimulation in splenocyte cultures (day 8 after immunization) from C57BL/6J WT and S1PR1(S5A) EAE mice. Mice were treated in vivo with FTY720 or vehicle starting from day 0 by daily i.p. injections. Analysis of cytokine expression in culture supernatants by ELISA revealed that in vivo FTY720 treatment downregulated MOG35-55-specific IL-6 and IL-17 expression in both WT and S1PR1(S5A) EAE splenocytes (Figure 3, A and B). As previously reported (6), S1PR1(S5A) EAE mice expressed higher levels of IL-6 and IL-17A compared with WT EAE mice (Figure 3, A and B). We also observed the downregulation of IFN-γ (Th1 cytokine) by FTY720 treatment in both WT and S1PR1(S5A) mice (Figure 3C). We next investigated the effects of FTY720 on in vitro Th17 differentiation by using FTYp (phosphorylated form of FTY720), an active form utilized in in vitro studies. We isolated CD4+ T cells from splenocytes of naive WT and S1PR1(S5A) mice and cultured them in a Th17-polarizing condition in the presence of FTYp (1 μM) for 5 days. Similar to our findings in the ex vivo recall assay, we observed that in vitro FTYp treatment also suppressed Th17 differentiation (Figure 3, D and E) and IL-17A expression (Figure 3F). We have previously shown that Th17-mediated CNS inflammation in S1PR1(S5A) EAE mice was due to enhanced STAT3 phosphorylation (6). Based on this, we hypothesized that FTY720 may downregulate STAT3 activation. Indeed, immunoblot analysis of WT and S1PR1(S5A) EAE splenocytes following in vivo FTY720 treatment revealed downregulation of pSTAT3 expression with FTY720 treatment (Figure 3, G and H). Taken together, these findings suggest that FTY720 treatment suppresses Th17 development in both WT and S1PR1(S5A) mice via targeting STAT3 activation.
Figure 3. FTY720 treatment downregulated Th17 cell development via targeting STAT3 activation.
Splenocytes from MOG35-55 -immunized C57BL/6J (WT) and S1PR1(S5A) presymptomatic (day 8) EAE mice, treated with FTY720 (0.5 mg/kg, daily i.p injections) or vehicle (1% cyclodextrin in PBS) in vivo, were cultured in the presence of MOG35-55 peptide (10 μg/ml) for 3 days. Culture supernatant was then collected at respective time points (0–72 hours) and analyzed for cytokine expression by ELISA. IL-6 (A) was examined at 24 hours; IL-17A (B) and IFN-γ (C) were examined at 72 hours. CD4+ T cells were isolated from spleens of naive C57BL/6J (WT) and S1PR1(S5A) mice and activated in vitro with anti-CD3 (5 μg/ml) and anti-CD28 (2 μg/ml) for 5 days in Th17 media supplemented with IL-6 (20 ng/ml), IL-23 (20 ng/ml), and TGF-β (1 ng/ml) in the presence of phosphorylated FTY720 (FTYp, 1 μM). Cells were then restimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 4 hours, labeled with antibodies against IFN-γ and IL-17A, and analyzed by flow cytometry (D and E). Culture supernatants were examined by ELISA (F). Splenocytes from MOG35-55-immunized WT and S1PR1(S5A) presymptomatic EAE mice treated in vivo with either FTY720 (0.5 mg/kg) or vehicle by daily i.p. injections were reactivated in vitro with MOG35-55 peptide for 30 minutes. These cells were harvested and lysate was prepared and analyzed by immunoblot analysis utilizing an antibody against pSTAT3 and β-actin (G). Relative protein expression (H) was quantified by densitometric normalization against β-actin expression in the respective samples. Data represent 3 independent experiments (n = 3–5 mice/arm, mean ± SEM, *P < 0.05; **P < 0.01; ***P < 0.001, ANOVA with Tukey’s multiple comparison test).
FTY720 treatment suppressed Th17 differentiation in the peripheral immune system.
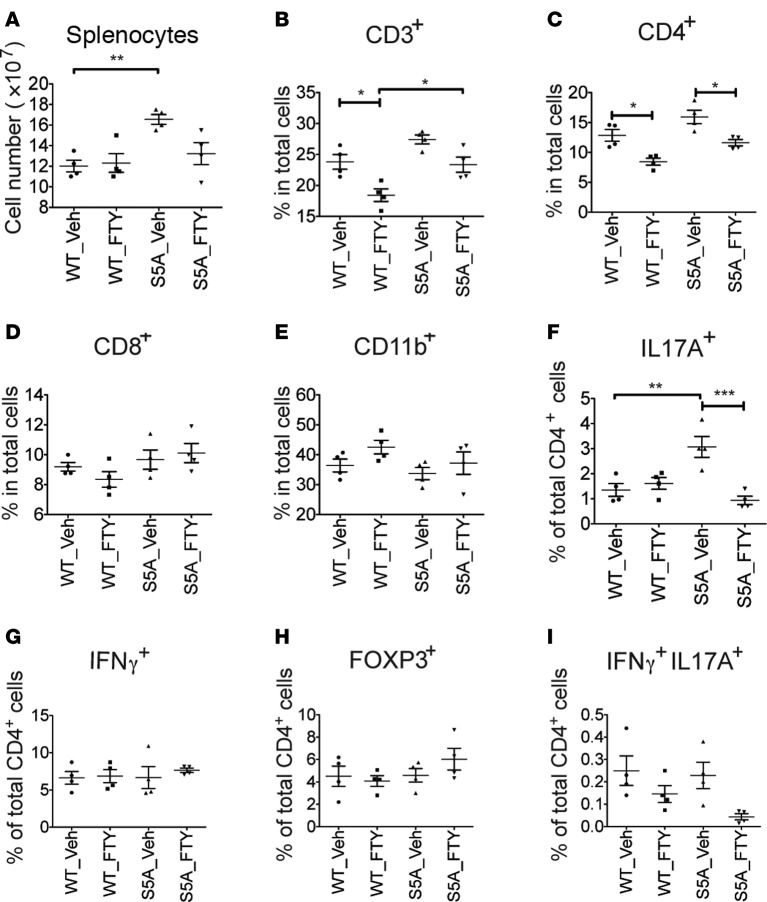
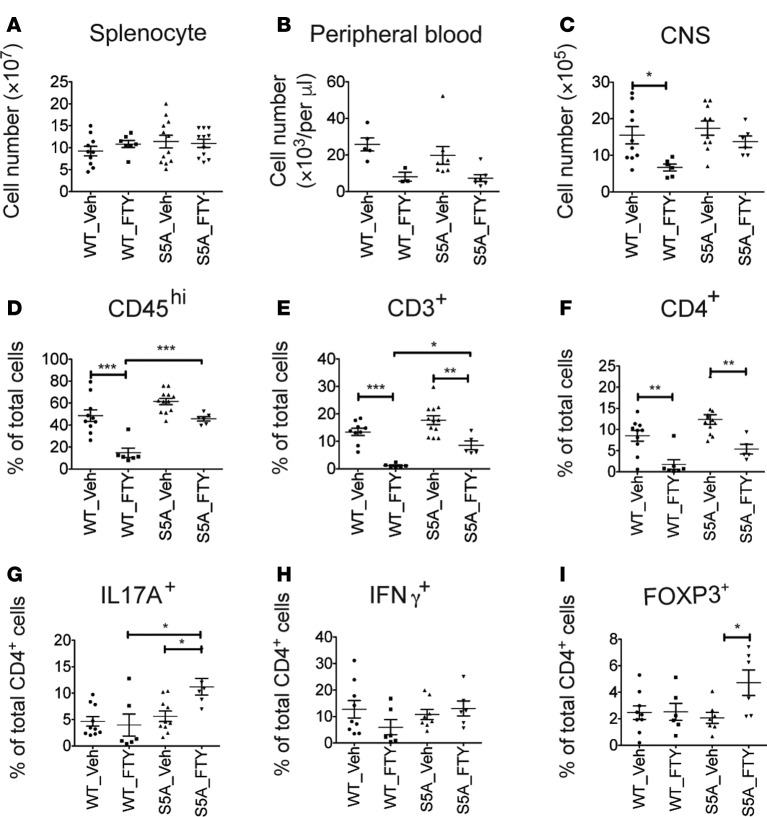
We next investigated FTY720’s effects on the splenocyte population in FTY720-treated, MOG35-55-immunized WT and S1PR1(S5A) EAE mice (day 8 after immunization). We found that in vivo FTY720 treatment decreased proliferation of splenocytes from both WT and S1PR1(S5A) EAE mice in response to ex vivo reactivation with MOG35-55 peptide (Supplemental Figure 3A). We also observed a decrease in percentage and total cell number of CD4+ T cells in the spleens of S1PR1(S5A) mice treated with FTY720, in agreement with the immune cell proliferation studies (Figure 4, A–D, and Supplemental Figure 3, B–D). No significant changes were observed in CD11b+ myeloid cells in WT and S1PR1(S5A) EAE mice following FTY720 treatment (Figure 4E and Supplemental Figure 3E).
Figure 4. FTY720 treatment in vivo downregulated Th17 expression in the spleen of S1PR1(S5A) EAE mice.
MOG35-55-immunized C57BL/6J (WT) and S1PR1(S5A) EAE mice were treated with vehicle (1% cyclodextrin in PBS) or FTY720 (0.5 mg/kg) by daily i.p. injections up to day 8 after immunization. Splenocytes were harvested, and the total splenocyte counts were quantified by hemocytometer (A). Percentages of CD3+ (B), CD4+ (C), CD8+ (D), and CD11b+ (E) cells were quantified by flow cytometry. Splenocytes were also stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 4 hours, and intracellular staining was performed to measure the expression of IL-17A, IFN-γ, and FOXP3 among CD4+ cells (F–I). Data represent 2 independent experiments (n = 4, mean ± SEM, *P < 0.05; **P < 0.01, ANOVA with Tukey’s multiple comparison test).
Further investigation of splenic T cell function showed that the total number and percentage of Th17 cells were higher in the splenocytes of vehicle-treated S1PR1(S5A) EAE mice than WT EAE mice. However, the percentage and total number of IL-17–positive CD4+ T cells were significantly decreased by FTY720 treatment in S1PR1(S5A) EAE mice (Figure 4F and Supplemental Figure 3F) but not the number of IFN-γ–positive and FOXP3-positive CD4+ T cells in both WT and S1PR1(S5A) EAE mice (Figure 4, G and H, and Supplemental Figure 3,G and H). The prevalence of IL-17/IFN-γ double-positive CD4+ cells was also decreased in S1PR1(S5A) mice treated with FTY720 (Figure 4I and Supplemental Figure 3I). Similarly, we observed decreased levels of IL-17A transcripts in CD4+ cells from the spleens of S1PR1(S5A) EAE mice following FTY720 treatment, as analyzed by quantitative PCR (Supplemental Figure 3J). These data further support the finding that FTY720 treatment downregulates the peripheral Th17 response in S1PR1(S5A) EAE mice.
Severe CNS inflammation in FTY720-treated S1PR1(S5A) EAE mice despite significant lymphopenia.
FTY720 arrests immune cell trafficking from SLOs into the systemic circulation (17). Our findings also showed that FTY720 treatment downregulated Th17 differentiation in the peripheral immune system (spleen) in the presymptomatic (day 8) S1PR1(S5A) EAE mice. However, we observed a high number of infiltrating immune cells in the CNS of S1PR1(S5A) EAE mice despite treatment with FTY720 (Figure 1D). In order to characterize which immune cell subsets are resistant to FTY720 treatment, we performed multidimensional flow cytometry analysis on the splenocytes, peripheral blood mononuclear cells, and CNS-infiltrating cells of MOG35-55-immunized WT and S1PR1(S5A) EAE mice with disease scores of 2 to 3 (day 13–16 after immunization) with daily FTY720 treatment. The gating scheme and strategy for CNS-infiltrating immune cell profiles are depicted in Supplemental Figure 4. There was no difference in the splenocyte counts in the WT mice and S1PR1(S5A) mice with established EAE (disease score 2–3) (Figure 5A). FTY720 treatment resulted in lymphopenia in both WT and S1PR1(S5A) EAE mice (Figure 5B). Although FTY720 treatment decreased the percentage and total number of CD4+ cells in the CNS of both WT and S1PR1(S5A) EAE mice, S1PR1(S5A) EAE mice still had higher numbers of CD45hi, CD3+, and CD4+ cells in the CNS compared with WT EAE mice upon FTY720 treatment (Figure 5, C–F, and Supplemental Figure 5, A–C). Interestingly, FTY720 treatment also did not arrest entry of CD8+ cells into the CNS of S1PR1(S5A) EAE mice compared with their WT counterparts (Supplemental Figure 5, D and E). Furthermore, cells of myeloid lineage, especially dendritic cells (CD11b+CD11c+), were also present in the CNS of S1PR1(S5A) EAE mice following FTY720 treatment (Supplemental Figure 6).
Figure 5. FTY720 treatment failed to arrest immune cell trafficking to the CNS of S1PR1(S5A) EAE mice.
MOG35-55-immunized C57BL/6J (WT) and S1PR1(S5A) EAE mice were treated with vehicle (1% cyclodextrin in PBS) or FTY720 (0.5 mg/kg) by daily i.p. injections. Immune cells from the brains and spinal cords were collected from EAE mice with a score of 2 to 3. Total cell counts from splenocytes (A), peripheral blood mononuclear cells (B), and CNS immune cells (C) were quantified using a hemocytometer. Percentages of CD45hi (D), CD3+ (E), and CD4+ (F) cells among CNS immune cells were quantified by flow cytometry. CNS immune cells were restimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 4 hours, and intracellular staining was performed to measure the expression of IL-17A (G), IFN-γ (H), and FOXP3 (I) among CD4+ cells. This experiment was performed 3 times with n ≥ 5 mice/arm, mean ± SEM, *P < 0.05; **P < 0.01; ***P < 0.001, ANOVA with Tukey’s multiple comparison test.
We hypothesized that the paradoxical effect of FTY720 on the peripheral immune system and the CNS may be due to the FTY720’s action on immune activation and trafficking in S1PR1(S5A) mice. Thus, we treated MOG35-55-immunized WT mice and S1PR1(S5A) mice with established EAE (clinical score 2) with daily FTY720 i.p. injections. FTY720 treatment successfully prevented disease progression in WT EAE mice compared with S1PR1(S5A) EAE mice (Supplemental Figure 7A). These findings suggest that FTY720 treatment failed to reverse paralysis and prevent further progression in S1PR1(S5A) mice once EAE is established. Furthermore, there was no difference in the immune cell profiles in the SLOs (spleen) of the vehicle- and FTY720-treated S1PR1(S5A) mice when disease score reached 2–3 (Supplemental Figure 7, B–E). Therefore, FTY720 likely suppresses Th17 differentiation in the earlier stages of EAE and not when the disease activity is established.
Taken together, these findings suggest that FTY720 treatment arrests overall lymphocyte trafficking from SLOs in both WT and S1PR1(S5A) EAE mice. However, a subset of encephalitogenic cells still appear to be able to enter into the CNS of S1PR1(S5A) EAE mice despite FTY720 treatment.
CCR6-positive lymphocytes evaded FTY720 blockade and trafficked to the CNS.
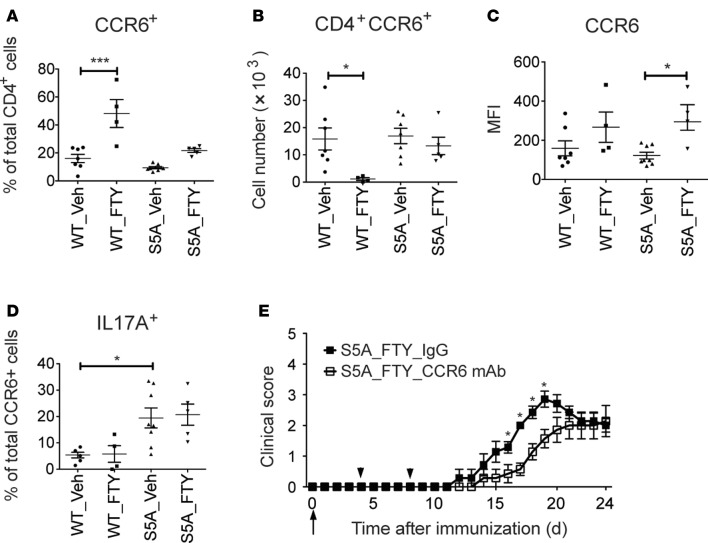
We then characterized the phenotype and function of CNS-infiltrating CD4+ T cells that escaped FTY720 treatment in WT and S1PR1(S5A) EAE mice. Multidimensional flow cytometry and intracellular cytokine staining revealed that the total number of IL-17A+CD4+ lymphocytes was decreased (without change in the percentage of IL-17A–positive cells among CD4+ lymphocytes) in the CNS of WT EAE mice. Contrary to this finding, the CNS of S1PR1(S5A) EAE mice showed significant enrichment in the percentage of IL-17A–positive CD4+ cells, without change in the total cell numbers (Figure 5G and Supplemental Figure 8A). FTY720 treatment also prevented entry of IFN-γ–positive CD4+ cells into the CNS of WT EAE mice but did so less effectively in S1PR1(S5A) EAE mice (Figure 5H and Supplemental Figure 8B). Furthermore, the number of FOXP3-positive cells (Figure 5I and Supplemental Figure 8C) was enriched in the CNS of S1PR1(S5A) EAE mice. We then investigated the contribution of CCR6, the CNS homing Th17 chemokine receptor, in this process. We observed high percentages of CCR6-positive CD4+ cells in the CNS of both WT and S1PR1(S5A) EAE mice following FTY720 treatment compared with their vehicle-treated counterparts (Figure 6A). FTY720 treatment decreased the total number of CCR6-positive cells in the CNS of WT EAE mice but not S1PR1(S5A) mice (Figure 6B). The mean expression (MFI) of CCR6 on CD4 T cells in the CNS was also increased in S1PR1(S5A) mice treated with FTY720 (Figure 6C). Finally, we observed a higher percentage of CCR6-positive CD4+ T cells that expressed IL-17A in the CNS of S1PR1(S5A) mice (~20% CCR6 cells) compared with that of WT mice (Figure 6D). A lesser percentage of CCR6+ cells expressed FOXP3 (3%–10%) and IFN-γ (1%–5%, Supplemental Figure 9). Taken together, our studies suggest that FTY720 treatment prevents overall immune cell infiltration into the CNS; however, CCR6+ immune cells appear to escape the action of FTY720, and this phenomenon was more pronounced in S1PR1(S5A) EAE mice.
Figure 6. CCR6-mediated encephalitogenic cell homing overrode FTY720-induced lymphocyte retention in S1PR1(S5A) EAE mice.
MOG35-55-immunized C57BL/6J (WT) and S1PR1(S5A) EAE mice were treated with vehicle (1% cyclodextrin in PBS) or FTY720 (0.5 mg/kg) by daily i.p. injections. Immune cells from the brains and spinal cords were collected when clinical EAE scores reached 2–3. CNS immune cells were restimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 4 hours, and then cells were labeled with antibodies against CD4, CCR6, and IL-17A. Percentage (A), total number (B), and MFI (C) of CCR6+ in CD4+ T cells and the percentage of IL-17A+ in CD4+CCR6+ cells (D) were quantified by flow cytometry. This experiment was performed 3 times with n ≥ 4 mice/arm, mean ± SEM, *P < 0.05; ***P < 0.001, ANOVA with Tukey’s multiple comparison test. Mean clinical score ± SEM of MOG35-55-immunized S1PR1(S5A) mice (E) (females, 8–12 weeks) treated with FTY720 (0.5 mg/kg) by daily i.p. injections. The arrow indicates time of initiation of therapy. Additionally, mice were also i.p. injected with either IgG control or anti-CCR6 mAb (100 μg/per mouse) at day 4 and day 8. Arrowheads indicate the time of antibody injection (n = 7/arm, *P < 0.05, Mann-Whitney U test).
To further investigate whether S1PR1 regulates CCR6 expression, we examined CCR6 expression in splenic T cells in both MOG35-55-immunized mice at day 8 and in vitro Th17 differentiation. We observed enhanced CCR6 expression together with IL-17A in the S1PR1(S5A) EAE mice. We also found that FTY720 treatment downregulated both IL-17A and CCR6 (Figure 4F and Supplemental Figure 10, A–C). Similarly, FTYp treatment of in vitro Th17 differentiation cultures significantly decreased the percentage of CCR6+ cells (Supplemental Figure 10D). Collectively, these findings suggest that FTY720 treatment in the early stages of Th differentiation downregulates both IL-17A and CCR6; however, once cells are committed, FTY720 treatment is unable to prevent CCR6+ cells from entry into the CNS.
CCR6 is responsible for the impaired response to FTY720 therapy in S1PR1(S5A) EAE mice.
CCR6 is a known chemokine receptor responsible for homing of encephalitogenic Th17 cells to the CNS in autoimmune neuroinflammation (34). To investigate whether CCR6 is primarily responsible for the poor response to FTY720 in S1PR1(S5A) mice, we treated MOG35-55-immunized S1PR1(S5A) EAE mice with blocking mAb against CCR6 (CCR6 mAb) (days 4 and 8 after immunization) along with FTY720 daily injections (days 0–24 after immunization). The CCR6 mAb utilized in the study is not a cell-depleting antibody. The binding affinity of this mAb to CCR6 on B cells in vivo lasted at least 8 days (35). Treatment with CCR6 mAb delayed the disease onset in S1PR1(S5A) EAE mice compared with the isotype control IgG treatment group (Figure 6E and Supplemental Table 7). The isotype control IgG antibody had no significant effect on EAE disease activity (Supplemental Figure 11). These findings support the hypothesis that CCR6-mediated lymphocyte migration to CNS overrides FTY720’s action in the SLOs and contributes to neuroinflammation in S1PR1(S5A) EAE mice.
Discussion
In this study, we investigated how S1PR1 phosphorylation and internalization affects the response to FTY720 therapy during autoimmune neuroinflammation. Utilizing mutant mice carrying a global phosphorylation–defective S1pr1 gene (S5A) in the EAE models, we elucidated the mechanisms that govern Th17 cell development and trafficking from SLOs to the CNS. Our findings suggest that surface residence of S1PR1 is not only responsible for the pro-Th17 phenotype, but for the responsiveness to treatment with FTY720. We also observed that FTY720 attenuated Th17 differentiation by downregulating STAT3 phosphorylation if FTY720 was exposed before establishment of Th17 phenotype. In agreement with previous reports (16, 17), our data suggest that FTY720 suppresses the trafficking of T lymphocytes from SLOs. However, in the case of impaired receptor internalization, a subset of encephalitogenic cells expressing CCR6 evade blockade by FTY720, resulting in severe and intractable CNS inflammation.
Our findings illuminate mechanistic insights on S1P signaling and may have clinical relevance on the utility of FTY720 as an immune modulatory therapy in MS. Case studies reporting breakthrough relapses and/or the development of tumefactive MS in association with FTY720 therapy suggest potential proinflammatory properties of FTY720 (28–31). The link between S1PR1 gene variations, MS pathogenesis, and FTY720 efficiency should be investigated in light of the presence of S1PR1 variants in the general population, including those involving S351 (27). Functional analysis also revealed that mutations I45T and G305C in S1PR1 resulted in impaired receptor endocytosis and degradation in response to FTY720 treatment. In addition, S1PR1 variants were also identified as MS susceptibility genes in GWAS (36). However, the frequency and the effects of S1PR1 gene mutation in MS patients have yet to be investigated. Ongoing translational studies are focusing on the relationship between MS immune phenotype and response to FTY720 therapy by using next-generation gene sequencing and flow cytometry-based immune phenotyping to correlate patient’s genotype with clinical outcome.
The critical question of what connects S1PR1 and CCR6 expression and the mechanism by which CCR6-expressing encephalitogenic cells overcome FTY720 blockade arises from our findings. A possible explanation is that, in the case of impaired S1PR1 internalization, CCR6-mediated immune cells are generated and egress into the systemic circulation before FTY720 can retain these cells within the SLOs. Existing studies have shown that FTY720 preferentially depletes naive and central memory T cells, but not effector memory T cells, from the circulation (37–39), suggesting that alternative mechanisms may exist to allow CCR6 cell egress from SLOs. In addition, CCR6 expression on Th17 cells (35, 40) and CCL-20 expression in choroid plexus are responsible for the initiation of autoimmune neuroinflammation (34). In a subset of patients treated with natalizumab (Tysabri), blockade of VLA-4 with a mAb promotes encephalitogenic Th17 cell homing by way of choroid plexus via a P-selectin glycoprotein ligand-1– (PSGL-1–) and myeloma cell adhesion molecule– (MCAM-mediated) mechanism (41). Whether FTY720 also promotes other CNS homing pathways in S1PR1(S5A) EAE mice remains to be elucidated as do the mechanisms by which Th17 cells traffic to the CNS under the influence of FTY720.
We also observed that FTY720 treatment does not prevent CD11b+CD11c+ dendritic cells from entering into the CNS in S1PR1(S5A) EAE mice, suggesting that these cells may contribute to the worsening CNS inflammation. Mixed lymphocyte culture experiments also suggested that antigen-presenting cells (APCs) from S1PR1(S5A) mice showed more IL-17A expression in T cells compared with their WT counterparts (6). Studies by others have also shown that S1P signaling modulates macrophage and dendritic cell functions (18). These observations imply that dysregulation of S1PR1 signaling may promote the activation of APCs during neuroinflammation. The exact mechanism of APCs in neuroinflammation and in response to FTY720 remains to be addressed.
In summary, impaired S1PR1 internalization not only worsens Th17-mediated autoimmune CNS inflammation, but also compromises the response to FTY720 therapy. Notably, CCR6+ cells appear to be responsible for the impaired response to FTY720. How this mechanism translates to the MS pathogenesis remains to be investigated. Translation from this work will likely further elucidate the molecular underpinnings of MS pathogenesis and tools for patient stratification and therapeutic optimization, leading to better clinical outcome in MS.
Methods
Materials.
The generation of S1PR1(S5A) mice (C57BL/6J background) was previously described (42). FTY720 was a gift from Novartis Pharma AG. S1P and FTY720 (S)-Phosphate (CAS 402616-26-6) were purchased from Cayman Chemical. Anti-CD3 (clone 145-2C11) and anti-CD28 (clone 37.51) antibodies were from Biolegend. Mouse recombinant IL-6 (406-ML), IL-12 (419-ML), IL-23 (1887-ML), and TGF-β (7666-MB) were from R&D Systems. Liberase enzyme cocktail was from Roche Biosciences. For flow cytometry, mAbs against CD45 (clone 30-F11), CD3 (clone 145-2c11), CD8 (clone 53-6.7), SiglecF (clone E50-2440), and IFN-γ (clone XMG-1.2) were from BD Biosciences; anti-CD11b (clone M1/70), CD11c (clone N418), CD4 (clone GK1.5), Gr1 (clone RB6-8C5), and CCR6 (clone 29-2L17) antibodies were from Biolegend, and anti–IL-17 (clone ebio17B7) and FOXP3 (clone FJK-16s) antibodies were from eBioscience. Antibodies used for immunoblotting pSTAT3 (catalog 9131) and STAT3 (catalog 9132) were from Cell Signaling Technology, and β-actin (clone AC-74) was from Sigma-Aldrich. For CCR6-blocking experiments, anti-CCR6 (clone 140706) and isotype control IgG (clone 54447) antibodies were from eBioscience.
EAE experiments and treatment with FTY720.
S1PR1(S5A) mice and WT C57BL/6J mice were housed in the Research Animal Facility at Stanford University. EAE was induced in 8- to 12-week-old female WT or S1PR1(S5A) mice immunized with CFA, MOG35–55, and Bordetella pertussis toxin (List Biological Laboratories) at 400 ng per mouse on days 0 and 2 (43). Mice were treated with FTY720 (a gift from Novartis, 0.5 mg per kg body weight) at indicated time points by daily i.p. injections. EAE mice were scored as follows: 0, normal; 1, tail paralysis; 2, hind limb weakness; 3, complete hind limb paralysis; 4, hind limb paralysis with forelimb weakness; and 5, moribund or death (43). EAE clinical score assessment was not blinded.
Histology and quantification of CNS inflammation.
To assess the degree of CNS inflammation, brains and spinal cords from WT C57BL/6J and S1PR1(S5A) mice were harvested and fixed in 10% formaldehyde. Paraffin-embedded sections were prepared as previously described (43). Tissue sections were stained with hematoxylin, eosin, and luxol fast blue. An investigator blinded to the experimental groups quantified the numbers of CNS inflammatory foci.
Th1 and Th17 adoptive transfer experiments.
Eight- to twelve-week-old C57BL/6J (WT) or S1PR1(S5A) female mice were immunized with CFA and MOG35–55 as described above. Eight days following immunization, splenocytes were harvested, pooled, and cultured in RPMI 1640 media supplemented with 10% FBS with 10 ng/ml of IL-12 (a Th1-promoting cytokine) or IL-23 (a Th17-promoting cytokine) (R&D Systems) for 72 hours in the presence of 10 μg/ml MOG35-55 peptide. Cells were then harvested and washed once with PBS. 1.5 × 107 cells per mouse were then injected i.p. into 5- to 6-week-old WT naive recipient mice. Pertussis toxin (400 ng) was injected i.p. on days 0 and 2. From day 0, mice were treated with vehicle (1% cyclodextrin in PBS) for the control group or FTY720 (0.5 mg/kg, dissolved in 1% cyclodextrin in PBS) for the experimental group daily by i.p. injections. Mice were then followed clinically for up to 30 days.
Ex vivo recall and quantification of cytokine expression.
For in vivo treatment, 8- to 12-week-old female MOG35-55-immunized WT or S1PR1(S5A) mice (n= 3 per group) were treated with FTY720 (0.5 mg/kg) administered i.p. daily from the time of immunization up to day 8. The control group was injected i.p. with vehicle (1% cyclodextrin in PBS). Splenocytes were harvested and cultured with MOG35–55 peptide (0, 5, 10, and 20 μg/ml) for 24, 48, 72, and 96 hours. Cytokine expression in the supernatant was measured using anti-mouse ELISA kits (IL-6, IL-12p40, and IFN-γ, OptEIA; IL-17, R&D Systems).
Immunoblot analysis.
Splenocytes from MOG35-55-immunized WT and S1PR1(S5A) mice treated with FTY720 (0.5 mg/kg, daily i.p. injections from day 0 to 8) were activated in vitro with MOG35-55 (10 μg/ml) at 37 °C for 30 minutes. Controls were incubated in media alone. Cells were then washed once with PBS and harvested in lysis buffer supplemented with protease and phosphatase inhibitors (Roche Applied Sciences). The protein homogenate was quantified by BCA assay (Thermo Scientific). A total of 15 μg protein was loaded on a 10% Novex NuPAGE precast Bis-Tris Gel (Life Technologies), resolved at 80 V for 2 hours, transferred onto a cellulose membrane, blocked with 5% BSA in TBST at room temperature overnight, and probed with primary antibodies against pSTAT3 (D3A7, Cell Signaling), STAT3 (79D7, Cell Signaling), and β-actin (4C2, Sigma-Aldrich) at 4°C overnight. Horseradish peroxidase–linked secondary antibodies (Cell Signaling) were used to incubate the blot at room temperature for 1 hour and developed utilizing ECL Chemiluminescence kit (GE Healthcare). For quantification of protein expression, bands on the X-ray film corresponding to the respective proteins were scanned using a HP laser scanner. The normalization step was done by dividing the densitometric units of protein of interest by their corresponding β-actin from the same sample and plotted on a bar graph. This experiment was repeated at least 3 times for each analysis with n = 3–5 mice/arm.
In vitro Th17 polarization.
For in vitro Th17 polarization experiments, CD4+ T cells were isolated from spleens of 8- to 12-week-old naive WT and S1PR1(S5A) mice utilizing magnetic CD4 microbeads (Miltenyi Biotec). Cell were then cultured in RPMI 1640 media supplemented with 10% FBS for 5 days in 24-well plates precoated with anti-mouse CD3 (5 μg/ml) and CD28 (2 μg/ml) in a Th17 polarization condition (IL-6 [20 ng/ml], IL-23 [20 ng/ml], TGF-β [1 ng/ml], anti–IFN-γ [5 μg/ml], and anti–IL-4 [5 μg/ml] antibodies). FTYp (1 μM) or vehicle (DMSO) was added every 24 hours as indicated. Cells were then harvested at day 5 and restimulated with 50 ng/ml phorbol 12-myristate, 13-acetate (PMA) and 500 ng/ml ionomycin in the presence of Golgi-stop (BD Biosciences) for 4 hours. Stimulated cells were labeled with anti–IFN-γ, IL-17A, and CCR6 antibodies by using the Cytofix/Cytoperm kit (BD Biosciences) and were analyzed by BD LSRII flow cytometry. The culture supernatants were also analyzed by ELISA for the expression of IL-17A cytokines.
RNA isolation and quantitative RT-PCR.
Total RNA was extracted from cells with TRIzol according to the manufacturer’s instructions (Ambion, Thermo Fisher Scientific). SYBR Green quantitative real-time PCR (Roche) was carried out to quantify the levels of gene expression after normalization with the housekeeping gene, β-actin. Primers were designed by Primer 3 (NCBI) and are listed in Supplemental Table 8.
Characterization of CNS immune cells.
Brains and spinal cords from MOG35-55-immunized C57BL/6J (WT) and S1PR1(S5A) EAE mice were harvested on days 13 to 16 (at disease scores of 2–3) after immunization. Immune cells were isolated from the CNS tissue according to methods outlined in Arac et al. (44). Briefly, mice were perfused with 30 ml of cold saline. Brains and spinal cords were homogenized through a 70-μm filter and treated with liberase enzyme cocktail for 1 hour at 37 °C. Immune cells were harvested from a 30% Percoll gradient by centrifugation, and residual red blood cells were lysed by hypotonic solution. Cells were washed once with PBS and labeled with mAbs against CD45, CD3, CD4, CD8, CD11b, CD11c, Gr1, SiglecF, and CCR6. Immune cells harvested from inflamed CNS tissue were also stimulated in vitro with 50 ng/ml PMA and 500 ng/ml ionomycin in the presence of Golgi-stop (BD Biosciences) for 4 hours. Cells were then labeled with anti–IL-17, anti–IFN-γ, and anti-FOXP3 antibodies by using the Biolegend FOXP3 Staining Kit (Biolegend). Cells (0.5 to 1 × 106) labeled with respective antibodies for 30 minutes on ice were washed twice with PBS, resuspended in staining buffer (3% FBS in PBS), and then analyzed by a LSRII Flow Cytometer (BD) at the Stanford Shared Flow Facility. BD FACSDiva 6.0 software was used to acquire data, and FlowJo 7.6.2 was used for data analysis.
CCR6-blocking experiment.
MOG35-55-immunized 8- to 12-week-old female WT or S1PR1(S5A) mice were treated with either FTY720 (0.5 mg per kg body weight) or vehicle (1% cyclodextrin in PBS) by daily i.p. injections from day 0 to day 21, except day 4 and day 8. For CCR6-blocking experiments, WT and S1PR1(S5A) EAE mice were administered i.p. with either blocking antibody against CCR6 or isotype control IgG (100 μg per mouse) on day 4 and day 8 following immunization. Clinical scores of these EAE mice were followed up to day 21.
Statistics.
Data represent the mean ± SEM. The Mann-Whitney U test was performed for comparison of EAE clinical scores. Sample size of n = 7–15 was selected for Mann-Whitney analysis. One-way ANOVA followed by Tukey’s multiple comparison test to compare all groups was used to determine significance between 3 or more test groups. The 2-tailed Student’s t test was used for direct comparison of 2 groups. A P value of less than 0.05 was considered statistically significant.
Study approval.
Animal experiments were approved by the Institutional Animal Care and Use Committee at Stanford University and performed in compliance with the US National Institutes of Health guidelines provided by the committee.
Author contributions
HCT and MHH formulated the hypothesis, designed the research, and wrote the manuscript. HCT performed most of the experiments and data analysis in this research. YH performed the ex vivo experiments and adoptive transfer EAE models. CSG performed the active EAE models. MAM helped to collect histological images. CWG helped to perform animal experiments.
Supplementary Material
Acknowledgments
We thank Raymond A. Sobel for quantification of CNS inflammation in EAE experiments; Jeanette Baker for the quantification of splenocyte proliferations; and Volker Brinkmann and Paul Smith (Novartis Pharma AG) for FTY720. This study is supported by Fondation Leducq (Sphingosine 1-Phosphate in Neurovascular Biology and Disease [SphingoNet]) and internal funding from the Department of Neurology and Neurological Sciences at Stanford University School of Medicine.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:JCI Insight. 2016;1(9):e86462. doi:10.1172/jci.insight.86462.
Contributor Information
Hsing-Chuan Tsai, Email: jhctsai@stanford.edu.
Yingxiang Huang, Email: Yih108@eng.ucsd.edu.
References
- 1.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354(9):942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 2.Casserly C, Ebers GC. Relapses do not matter in relation to long-term disability: yes. Mult Scler. 2011;17(12):1412–1414. doi: 10.1177/1352458511427514. [DOI] [PubMed] [Google Scholar]
- 3.Haghikia A, Hohlfeld R, Gold R, Fugger L. Therapies for multiple sclerosis: translational achievements and outstanding needs. Trends Mol Med. 2013;19(5):309–319. doi: 10.1016/j.molmed.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Farina C, Wagenpfeil S, Hohlfeld R. Immunological assay for assessing the efficacy of glatiramer acetate (Copaxone) in multiple sclerosis. A pilot study. J Neurol. 2002;249(11):1587–1592. doi: 10.1007/s00415-002-0904-0. [DOI] [PubMed] [Google Scholar]
- 5.Vosslamber S, van Baarsen LG, Verweij CL. Pharmacogenomics of IFN-beta in multiple sclerosis: towards a personalized medicine approach. Pharmacogenomics. 2009;10(1):97–108. doi: 10.2217/14622416.10.1.97. [DOI] [PubMed] [Google Scholar]
- 6.Garris CS, et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat Immunol. 2013;14(11):1166–1172. doi: 10.1038/ni.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin R, Sospedra M. Sphingosine-1 phosphate and central nervous system. Curr Top Microbiol Immunol. 2014;378:149–170. doi: 10.1007/978-3-319-05879-5_7. [DOI] [PubMed] [Google Scholar]
- 8.Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest. 2015;125(4):1379–1387. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumruker T, Billich A, Brinkmann V. FTY720, an immunomodulatory sphingolipid mimetic: translation of a novel mechanism into clinical benefit in multiple sclerosis. Expert Opin Investig Drugs. 2007;16(3):283–289. doi: 10.1517/13543784.16.3.283. [DOI] [PubMed] [Google Scholar]
- 10.Kappos L, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JA, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 12.Devonshire V, et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol. 2012;11(5):420–428. doi: 10.1016/S1474-4422(12)70056-X. [DOI] [PubMed] [Google Scholar]
- 13.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 14.Chi H, Flavell RA. Cutting edge: regulation of T cell trafficking and primary immune responses by sphingosine 1-phosphate receptor 1. J Immunol. 2005;174(5):2485–2488. doi: 10.4049/jimmunol.174.5.2485. [DOI] [PubMed] [Google Scholar]
- 15.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5(7):560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 16.Zhi L, et al. FTY720 blocks egress of T cells in part by abrogation of their adhesion on the lymph node sinus. J Immunol. 2011;187(5):2244–2251. doi: 10.4049/jimmunol.1100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 18.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8(10):753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oo ML, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282(12):9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 20.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33(2):91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingwersen J, Aktas O, Kuery P, Kieseier B, Boyko A, Hartung HP. Fingolimod in multiple sclerosis: mechanisms of action and clinical efficacy. Clin Immunol. 2012;142(1):15–24. doi: 10.1016/j.clim.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Garris CS, Blaho VA, Hla T, Han MH. Sphingosine-1-phosphate receptor 1 signalling in T cells: trafficking and beyond. Immunology. 2014;142(3):347–353. doi: 10.1111/imm.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11(11):1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JW, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108(2):751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui QL, Fang J, Kennedy TE, Almazan G, Antel JP. Role of p38MAPK in S1P receptor-mediated differentiation of human oligodendrocyte progenitors. Glia. 2014;62(8):1361–1375. doi: 10.1002/glia.22688. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, et al. Fingolimod treatment promotes proliferation and differentiation of oligodendrocyte progenitor cells in mice with experimental autoimmune encephalomyelitis. Neurobiol Dis. 2015;76:57–66. doi: 10.1016/j.nbd.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Obinata H, et al. Individual variation of human S1P1 coding sequence leads to heterogeneity in receptor function and drug interactions. J Lipid Res. 2014;55(12):2665–2675. doi: 10.1194/jlr.P054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourdette D, Gilden D. Fingolimod and multiple sclerosis: four cautionary tales. Neurology. 2012;79(19):1942–1943. doi: 10.1212/WNL.0b013e3182735edf. [DOI] [PubMed] [Google Scholar]
- 29.Visser F, Wattjes MP, Pouwels PJ, Linssen WH, van Oosten BW. Tumefactive multiple sclerosis lesions under fingolimod treatment. Neurology. 2012;79(19):2000–2003. doi: 10.1212/WNL.0b013e3182735cb3. [DOI] [PubMed] [Google Scholar]
- 30.Mehling M, Johnson TA, Antel J, Kappos L, Bar-Or A. Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurology. 2011;76(8 Suppl 3):S20–S27. doi: 10.1212/WNL.0b013e31820db341. [DOI] [PubMed] [Google Scholar]
- 31.Sato DK, et al. Changes in Th17 and regulatory T cells after fingolimod initiation to treat multiple sclerosis. J Neuroimmunol. 2014;268(1-2):95–98. doi: 10.1016/j.jneuroim.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res. 2014;55(8):1596–1608. doi: 10.1194/jlr.R046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 34.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10(5):514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 35.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.International Multiple Sclerosis Genetics Consortium. Network-based multiple sclerosis pathway analysis with GWAS data from 15,000 cases and 30,000 controls. Am J Hum Genet. 2013;92(6):854–865. doi: 10.1016/j.ajhg.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofmann M, Brinkmann V, Zerwes HG. FTY720 preferentially depletes naive T cells from peripheral and lymphoid organs. Int Immunopharmacol. 2006;6(13-14):1902–1910. doi: 10.1016/j.intimp.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 38.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28(1):122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song ZY, et al. Peripheral blood T cell dynamics predict relapse in multiple sclerosis patients on fingolimod. PLoS One. 2014;10(4):e86462. doi: 10.1371/journal.pone.0124923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamazaki T, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181(12):8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider-Hohendorf T, et al. VLA-4 blockade promotes differential routes into human CNS involving PSGL-1 rolling of T cells and MCAM-adhesion of TH17 cells. J Exp Med. 2014;211(9):1833–1846. doi: 10.1084/jem.20140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thangada S, et al. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207(7):1475–1483. doi: 10.1084/jem.20091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han MH, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451(7182):1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 44.Arac A, et al. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci USA. 2011;108(32):13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.