Abstract
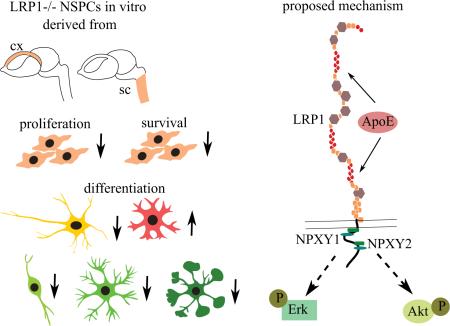
The LDL family of receptors and its member LRP1 have classically been associated with a modulation of lipoprotein metabolism. Current studies, however, indicate diverse functions for this receptor in various aspects of cellular activities, including cell proliferation, migration, differentiation and survival. LRP1 is essential for normal neuronal function in the adult CNS, whereas the role of LRP1 in development remained unclear. Previously we have observed an upregulation of LewisX (LeX) glycosylated LRP1 in the stem cells of the developing cortex and demonstrated its importance for oligodendrocyte differentiation. In the current study we show that LeX-glycosylated LRP1 is also expressed in the stem cell compartment of the developing spinal cord and has broader functions in the developing CNS. We have investigated the basic properties of LRP1 conditional knockout on the neural stem/progenitor cells (NSPCs) from the cortex and the spinal cord, created by means of Cre-loxp mediated recombination in vitro. The functional status of LRP1-deficient cells has been studied using proliferation, differentiation and apoptosis assays. LRP1 deficient NSPCs from both CNS regions demonstrated altered differentiation profiles. Their differentiation capacity towards oligodendrocyte progenitor cells (OPCs), mature oligodendrocytes and neurons was reduced. In contrast, astrocyte differentiation was promoted. Moreover, LRP1 deletion had a negative effect on NSPCs proliferation and survival. Our observations suggest that LRP1 facilitates NSPCs differentiation via interaction with ApoE. Upon ApoE4 stimulation wild type NSPCs generated more oligodendrocytes, but LRP1 knockout cells showed no response. The effect of ApoE seems to be independent of cholesterol uptake, but is rather mediated by downstream MAPK and Akt activation.
Keywords: neural stem and progenitor cells, LRP1, LewisX carbohydrate, neural stem cell differentiation, LRP1-dependent signaling, membrane permeant Cre-recombinase
Introduction
Radial glia and neural stem precursor cells (NSPCs) are multipotent cells with a capacity to self-renew and differentiate into neurons, oligodendrocytes and astrocytes (Kriegstein and Alvarez-Buylla 2009; Malatesta and Gotz 2013; Taverna et al. 2014). After completion of development, NSPCs are restricted to the neurogenic niches of the adult brain (Götz and Huttner 2005; Ming and Song 2011). Radial glia display regional differences and may comprise a complex set of subpopulations (Malatesta et al. 2003; Pinto and Götz 2007). In order to enrich for and study the properties of distinct neural stem cell populations, reliable cell-surface markers would represent useful tools for the isolation of cellular subsets. In fact, glycoconjugates that are glycan-epitopes exposed by proteoglycans, glycoproteins and glycolipids expressed on the cell surface can successfully be used for the live cell sorting (Faissner and Reinhard 2015; von Holst et al. 2006; Yanagisawa et al. 2005; Yu et al. 2010). For example, LewisX (LeX) glycan, also known as stage-specific embryonic antigen 1 (SSEA-1), or CD15 (leucocyte cluster of differentiation 15) was originally characterized as a surface antigen of pluripotent stem cells expressed at an early developmental stage of the embryo (Solter and Knowles 1978). Interestingly, LeX remains strongly upregulated in the radial glia compartments of the developing telencephalon, spinal cord and in the neurogenic zones of the adult brain (Capela and Temple 2006; Dodd and Jessell 1986; Hennen et al. 2011; Karus et al. 2013; Theocharidis et al. 2014; Yamamoto et al. 1985). LeX can be successfully used as a target for live sorting of neural stem cells (Capela and Temple 2006; Hennen et al. 2011; Pruszak et al. 2007) Distinct monoclonal antibodies distinguish LeX variants within the glycan moieties and recognize different neural stem cell subpopulations. For instance, mAb 487LeX binds to the terminal LeX motif and marks already committed progenitors, whereas mAb 5750LeX targets an internal LeX motif, typical for multipotent undifferentiated NSPCs (Hennen and Faissner 2012). In order to understand the differential expression profile of Lex-determinants, we identified the corresponding carrier proteins in the developing CNS. We confirmed already known LeX presenting proteins such as Phosphacan, Tenascin-C and L1-CAM (Garwood et al. 1999; Hennen et al. 2013; Streit et al. 1990; Yaji et al. 2015).
Furthermore, we identified the Low-Density Lipoprotein Receptor-related Protein 1 (LRP1) as a novel LeX carrier. LRP1 is highly enriched in the developing cortex and LeX-glycosylated in the radial glia compartment (Hennen et al. 2013). LRP1 is a large ubiquitously expressed cell membrane receptor which functions as a major cholesterol transporter, and interacts with a variety of ligands involved in cell signaling (Lillis et al. 2008). Thereby, LRP1 modulates numerous cellular activities, including cell proliferation, migration and survival in vascular smooth muscle cells, fibroblasts, osteoblasts, keratinocytes, hepatic stellate cells or Schwann cells (Boucher and Herz 2011; Campana et al. 2006; Grey et al. 2006; Llorente-Cortes et al. 2012; Mantuano et al. 2010; Tang et al. 2010). Also, the expression of LRP1 in radial glia of the adult spinal cord and the involvement of LRP1 in neuroblast migration along the rostral-migratory stream has recently been reported (Petit et al. 2011; Rabiej et al. 2016). Furthermore, LRP1 is responsible for amyloid precursor protein (APP)-transport and post-synaptic receptor trafficking, which underlines its essential function in the adult brain (Jaeger and Pietrzik 2008; Maier et al. 2013).
By comparison, its role in the developing nervous system was largely unknown. Recently, we reported that LRP1-deficient NSPCs generate less O4-positive cells compared to control cells (Hennen et al. 2013). In the present study, we demonstrate that LRP1 expression and glycosylation in the developing spinal cord is similar to the pattern in the cortex. Our observations suggest that LRP1 modulates proliferation, survival and differentiation of NSPCs derived from both explored CNS regions. We provide further evidence that ApoE promotes oligodendroglial differentiation via LRP1. This effect seems to depend on downstream activation of ERK/MAPK (extracellular signal-regulated kinases/mitogen-activated protein kinases) and PI3K/Akt (phosphatidylinositol 3-kinase/protein kinase B) signaling, rather than on alterations of the cholesterol content of NSPCs.
Material and methods
Animals
The animals were housed under the 12h light/dark cycle with free access to food and water. LRP1flox/flox animals (B6;129S7-LRP1tm2Her/J); (Rohlmann et al. 1996) were obtained from Jackson Laboratories and kept on C57Bl6 background. LRP1flox/wt were mated with LRP1flox/wt animals in order to obtain LRP1flox/flox and LRP1wt/wt littermates. The day of the vaginal plug was considered embryonic day 0.5 (E0.5). The genotyping of the animals was performed according to Jackson Laboratories protocol.
Neurosphere culture
Radial glia stem cells can be isolated from the developing or adult brain and cultivated as neurospheres in the presence of Epithelial Growth Factor (EGF) and Fibroblast Growth Factor 2 (FGF2) (Alvarez-Buylla and García-Verdugo 2002; Reynolds and Weiss 1996). Neurospheres contain NSPCs, which can be expanded and differentiated into neurons, oligodendrocytes and astrocytes in vitro (Reynolds and Rietze 2005).
NSPCs were obtained by acute dissociation of E14.5 cortex and spinal cord, as described previously (Hennen et al. 2011; Karus et al. 2011). The cells were plated at the density of 100,000 cells/ml in T25 flasks (Nunc, Wiesbaden, Germany) in the NSPC medium containing 1:1 DMEM/F12, 0.2 mg/ml L-Glutamine (all Sigma-Aldrich, Munich, Germany), 100 U/ml Penicillin, 100U/ml Streptomycin, 2% (v/v) B27 (all Life Technologies, Breda, Germany), supplemented with 20 ng/ml EGF, 20 ng/ml FGF (all Preprotech, Rocky Hill, NJ, USAHiH) and 0.5 U/ml Heparin (Sigma-Aldrich) and cultivated for 5-7 days.
Cre-recombinase transduction
Cre-recombinase transduction was performed as described previously (Hennen et al. 2013) with minor changes. 5 days in vitro (DIV 5) cortical neurospheres and DIV 7 spinal cord neurospheres were dissociated with the help of 0.05% (v/v) trypsin-EDTA (Invitrogen, Karlsruhe, Germany), and 50,000 cortical or 40,000 spinal cord derived NSPCs were plated on 16-mm dishes (Nunc) pre-coated with 15 μg/ml polyornithine (Sigma-Aldrich) and 2 μg/ml laminin (BD Bioscience, Franklin Lakes, NJ, USA). The cells were cultivated in NSPC medium supplemented with 10 ng/ml EGF and FGF2 overnight (ON). On the next day the medium was replaced by NSPC medium containing 0.5 μM His-TAT-NLS-Cre-recombinase (Nolden et al. 2006) and 20 ng/ml of EGF and FGF2 and incubated for 8-15 h. After incubation, the Cre-containing NSPC medium was carefully exchanged with the standard NSPC medium supplemented with 20 ng/ml of EGF and FGF2. 24 h later the cells were removed from the surface by trypsinization and cultivated as free-floating neurospheres for additional 3-5 days prior to further experiments. The effectiveness of LRP1 deletion was confirmed by Western-blot, using 10 μg of protein isolated from the Cre-treated neurospheres. The Cre-treated LRP1flox/flox cells became LRP1 knockout (LRP1−/−) cells and Cre-treated LRP1wt/wt remained LRP1 wild type (LRP1+/+). These NSPCs were compared to each other in the following experiments.
Proliferation and apoptosis assay
The Cre-treated neurospheres were trypsinized and plated on polyornithine and 2 μg/ml laminin/entactin (Corning, Corning, NY, USA) coated dishes at a density of 20,000 cells/cm2 in NSPC medium supplemented with 10 ng/ml EGF and FGF2 overnight. Proliferation and apoptosis assays were performed in separate dishes. On the next day 10 μM BrdU (Roche, Grenzach-Wyhlen, Germany) was added to the medium for the proliferation assay and incubated for 4 h. Afterwards, the cells were fixed with Ethanol fixative (50 mM glycine, pH 2.0 in 70% (v/v) EtOH) for 10 min. For the apoptosis assay the cells were mildly stressed by growth factor withdrawal for 6 h followed by 4% (w/v) paraformaldehyde (PFA) in PBS fixation for 10 min.
Differentiation assay
The Cre-treated neurospheres were trypsinized and plated on polyornithine (Sigma-Aldrich) and 5 μg/ml laminin (Corning) coated dishes at a density of 30,000 cells/cm2 in NSPC medium supplemented with 1% (v/v) fetal calf serum (FCS) (Invitrogen) for 5 days. For some experiments cells were treated with 7 μg/ml of the lipidated apolipoprotein E4 (ApoE4) rHDL/apoE4, or 7 μg/ml of the lipid-free ApoE4 or 110 μM of methyl-β-cyclodextrin MβCD (Sigma-Aldrich) according to Swaroop et al., (2012). Supplement containing medium was exchanged every second day. After 5 days the cells were live stained, fixed with 4% (w/v) PFA in PBS for 10 min or lysed for the Western-blot.
Preparation of reconstituted HDL (rHDL) bearing apoE4
Recombinant apoE4(1-299) with a hexa-His tag at the N-terminal end was isolated from a bacterial over expression system and purified as described previously (Choy et al. 2003). Protein concentration was determined using a NanoDrop 2000 spectrometer applying a molar extinction coefficient of 44,460 M-1 cm-1 for apoE4 at 280 nm. Since no detectable lipid has been identified in bacterially expressed recombinant apoE (Narita et al, 2002) we refer to the added protein as lipid-free apoE4. rHDL was prepared by the cholate dialysis method (Nichols et al. 1987) with slight modifications to the lipid components. The reconstitution was initiated with 1-palmitoyl-2-oleoyl-sn-glycerophosphocholine (POPC) (Avanti Polar Lipids, Inc. (Alabaster, AL), cholesterol (Avanti Polar Lipids, Alabaster, AL) and apoE4 in the ratio 9:1:2.5 (w/w/w), respectively, in the presence of sodium deoxycholate. In cases where cellular uptake of rHDL was visually monitored, TopFluor® cholesterol (Avanti Polar Lipids, Alabaster, AL, USA) was included at 1/10th the total amount of cholesterol in the above mixture. Following reconstitution, the fractions containing rHDL were isolated by KBr density gradient ultracentrifugation and dialyzed against 20 mM sodium phosphate buffer, pH 7.4, containing 150 mM NaCl, for 48 hours with 3 changes. Throughout the manuscript, the reconstituted particles bearing POPC/cholesterol/apoE4 and POPC/cholesterol/TopFluor/apoE4 are simply referred to as rHDL/apoE4 and rHDL/TopFluor/apoE4, respectively.
Cholesterol uptake assay
The NSPCs were plated on 35 mm FluoroDishes (World Precision Instruments, Sarasota, FL, USA) in NSPCs-medium at a density of 30,000 cells/cm2. On the next day the cells were loaded with 7 μg/ml of “rHDL//TopFluor/apoE4” for 15 min at 37°, washed twice with NSPCs medium and imaged live with a confocal laser-scanning microscope LSM 510 Meta (Zeiss, Göttingen, Germany). To test the effectiveness of cholesterol extraction by Methyl-β-Cyclodextrin (MβCD), the NSPCs pre-loaded with 2.5 μM TopFluor Cholesterol (Avanti) for 15 min at 37° were treated with 110 μM of MβCD (Sigma-Aldrich) for 24 h and live imaged to estimate the loss of fluorescence due to cholesterol extraction.
Cholesterol concentration measurement
The lipids were extracted from 1*106 NSPCs per condition dissociated from the free-floating neurospheres. The cell pellets were homogenized in 200 μl of the extracting solution (38.5 % (v/v) Chloroform, 61 % (v/v) Isopropanol, 0.5 % (v/v) nonidet-P40 (NP-40)) with the help of a TissueLyser (Qiagen, Hilden, Germany), the supernatant was dried ON at 50°C. Cholesterol concentration determination was performed with an Amplex Red Cholesterol Assay Kit (Invitrogen), according to the manufacturer's protocol. The cholesterol concentration was normalized to total protein concentration, as measured by the Pierce BCA Protein Assay Kit (Life Technologies).
NSPCs Transfection
NSPCs transfection was performed as described previously (Bertram et al. 2012) with minor changes. Briefly, the 2nd-passage neurospheres were dissociated. Cell pellets of 500,000 cells were mixed with 20 μl of P3 Primary Cell 4D-Nucleofector™ Kit solution (Lonza, Basel, Switzerland) containing plasmid DNA. For the LRP1 mini-receptor constructs the last 2307 nucleotides of LRP1 (Roebroek et al. 2006) have been subcloned into a plBCX backbone with the 5′BamH1 and 3′Xba1 restriction sites by using the following forward primer: 5′- GAGCTCGGATCCGATTGCAGCATCGACCCC and the reverse primer 3′- GGGCCCTCTAGACTATGCCAAGGGATCTCC and were fused to a myc tag at the 5′end. The LRP1 minireceptor constructs contain the c-terminus, the transmembrane domain and a short part of the ectodomain of LRP1. The NPxY1 motif was replaced from NPTY to AATA and the NPxY2 motif was changed from NPVYATL to AAVAATL in the LRP1 NPxY 1+2 minireceptor construct (Roebroek et al., 2006). The following conditions were used: LRP1 wild type plasmid 0,5 μg + 0,25 μg GFP, LRP1 LRP1 NPxY 1+2 0,5 μg + 0,25 μg GFP. Cells were transferred into the 16 well nucleofector cuvette and pulsed according to the manufacturer's protocol (Lonza). Directly after pulsing, 180 μl pre-warmed neurosphere medium was added to the electroporated NSPCs. The cells were gently re-suspended and plated as free-floating neurospheres in the NSPCs medium containing 20 ng/ml of EGF and FGF2. 24 h later the cells were subjected to the differentiation assay.
ERK/MAPK or Akt activation test
The cells were plated in the same way as for differentiation assay overnight. On the next day the cells were washed with minimal essential medium (MEM) (Sigma-Aldrich) for 1 h, then stimulated for additional 90 min with 50 nM of ApoE4 and lysed for the Western blot. ERK and Akt activation were measured as a relation of the amount of phosphorylated ERK and Akt protein to total ERK and Akt protein.
Immunoprecipitation
For immunoprecipitation, the spinal cord tissue was isolated from E12.5 C57Bl6 embryos and lysed in the RIPA-buffer (50 mM Tris/HCl pH 7.4, 150 mM NaCl, Triton X-100 1 % (v/v), Na-Deoxycholat 0.5 % (w/v), all Sigma-Aldrich) with protease inhibitors: 1 mM phenylmethanesulfonylfluoride (PMSF) and 1 μg/ml Aprotinin, all Roche. 30 μl Protein A/G agarose slurry (Santa Cruz Biotechnology, Dallas, TX) was incubated with 2.5 μg goat anti-mouse IgG conjugated with Cy3 (Dianova, Hamburg, Germany) for 1 h on a rotating wheel in PBS, followed by incubation with 4 μg of mAb 11E2 mouse anti-LRP1 α-chain (Storck et al. 2016) or 4 μg of isotype control mAb actin (BD) for 2 h in 1 ml PBS/A. 1000 μg of total protein was incubated with pre-labeled agarose slurry in 500 μl of RIPA buffer overnight, washed gently 3 times. Before SDS-polyacrylamide gel electrophorese, samples were boiled for 5 min in loading buffer (60 mM Tris-HCl pH 6.8, 2.5% (w/v) SDS, 10% (v/v) glycerol, 5% (v/v) β-mercaptoethanol, 0.01 % bromophenol blue). 5750LeX, 487LeX and LRP1 α-chain epitopes were subsequently detected by Western blot analysis.
Western blot
The cells were lysed in the SDS-buffer with protease inhibitors (same as for immunoprecipitation). The protein concentration was defined by the Pierce BCA Protein Assay Kit (Life Technologies). The Western blot analysis was performed according to a standard protocol as outlined in Hennen et al. (2011), the images were acquired using a MicroChemie Chemiluminescence-Reader (Biostep, Burkhardtsdorf, Germany). The following antibodies were used: rabbit anti-LRP1 1:10,000 (Abcam, Cambridge, UK), mouse anti-MAP2 (clone AP20, Millipore, Schwalbach, Germany) 1:2000, rabbit anti-PDGFRα (Santa Cruz Biotechnology) 1:3000, mouse anti-GFAP 1:3000 (Sigma-Aldrich), mouse anti-α-tubulin (clone DMA1, Sigma-Aldrich) 1:10,000, rabbit-anti pERK1/2 Thr202/Tyr204 (Cell signaling, Cambridge, UK 1:1000, rabbit-anti ERK1/2 (Santa Cruz Biotechnology) 1:1000, rabbit-anti pAkt Ser473 (Cell signaling) 1:1000, rabbit-anti Akt (Santa Cruz Biotechnology) 1:1000, mouse anti-actin (BD Bioscience, Erembodegem, Belgium) 1:5000, mouse anti-βIII-tubulin (Sigma-Aldrich) 1:500.
Immunohistochemistry
For the immunohistochemical staining the E12.5 embryos were fixed in 4% (w/v) PFA in PBS ON followed by subsequent incubation in 10%, 20% and 30% (w/v) sucrose (each 12 h), and frozen in Tissue Freezing Medium (Leica, Solms, Germany). 40 μm-thick free-floating frontal sections were cut, blocked with 10% (w/v) normal goat serum (NGS), 0.1% (v/v) Triton in PBS for 1 h. The sections were incubated with primary antibodies: rabbit anti-LRP1 1:500 (Abcam), rat anti-LeX 5750 mAb 5750LeX 1:50 (Hennen et al., 2011) ON in the blocking solution, washed 3×30 min with PBS and incubated with appropriate secondary antibodies: Alexa Flour 488 (Life Technologies) 1:500, Cy3 (Dianova) 1:500 and Topro3 (BioTechniques, New York, NY, USA) 1:500 for 2 h, washed again 3×30 min with PBS and mounted with Immumount (ThermoScientific).
Immunocytochemistry
For O4 staining, the primary antibodies mouse anti-O4 (Sommer and Schachner 1981) (1:30) were applied on the living cells in KRHA (Krebs–Ringer-Hepes buffer with 0.1% (w/v) BSA) for 30 min, then the cells were washed with PBS and fixed with 4% (w/v) PFA in PBS for 10 min at RT.
The staining against other epitopes was performed on fixed cells. The cells were blocked with 10% (w/v) NGS, 0.1% (v/v) Triton in PBS for 30 min and incubated for additional 30 min with primary antibodies in the blocking solution. The following primary antibodies have been used: rabbit anti-platelet-derived growth factor receptor α (PDGFRα) (Santa Cruz Biotechnology) 1:200, mouse anti-myelin basic protein (MBP) (Millipore) 1:50, mouse anti-βIII-tubulin (Sigma-Aldrich) 1:300, rabbit anti-glial fibrillary acidic protein (GFAP) (Dako, Hamburg, Germany) 1:400, mouse anti-Nestin (Millipore) 1:300, mouse anti-bromodeoxyuridine BrdU (Roche) 1:50. Further on, the cells were washed 3×10 min with PBS, incubated with secondary antibodies (see immunohistochemistry) and 4′,6-diamidino-2-phenylindole DAPI 1:50,000 for 30 min, washed again 3×10 min with PBS and mounted in PBS/glycerin 1:1.
Image acquisition and data processing
Immunostainings were imaged with the help of a confocal laser-scanning microscope LSM 510 Meta (Zeiss) or were examined using an Axioplan 2 microscope with UV epifluorescence (Zeiss). For the proliferation, apoptosis and differentiation assays at least 1500 cells per experimental condition were quantified, at least 5 independent experiments were performed, the percentage of positive cells for each investigated marker was compared. Neurite length of βIII-tubulin-positive neurons differentiated from LRP1+/+ and LRP1−/− NSPCs was assessed using the NeuronJ ImageJ plugin. At least 100 neurons in total per condition were analyzed in 4 independent experiments. The longest neurite was considered as an axon, the shorter neurites were designated as dendrites. Cholesterol uptake was quantified by the assessment of fluorescence intensity of accumulated rHDL/TopFluor/apoE4. The fluorescence intensity was quantified per image and divided by the number of cells. Western blots were analyzed by comparing the band intensity of the marker protein normalized to α-tubulin or actin band intensity in case of differentiation marker gene expression analysis and total Akt or total ERK in case of signaling activation analysis. Cell counting, fluorescence intensity measurements and densitometry were performed in ImageJ software. All data analysis required pairwise comparison, the quantified parameters were compared between knockout and control conditions or between treatment and control conditions in each experiment. The data were first subjected to the Kolmogorov-Smirnov normality test. As the observed distributions deviated significantly from normality in most of the cases, the conditions were compared with the aid of the non-parametric Mann-Whitney U test. Statistical tests and diagrams were made in R v. 3.0.2 (https://www.r-project.org/).
Results
LeX-glycosylated LRP1 is expressed in the developing spinal cord
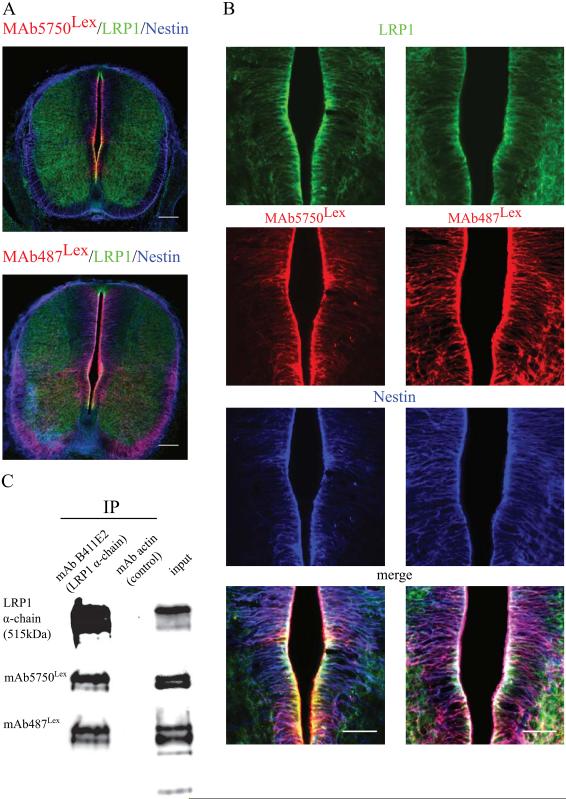
Our previous studies showed that LewisX glycosylated LRP1 is highly upregulated in the cortical plate (Hennen et al. 2013). To investigate whether this is a unique phenomenon for the developing cortex or a more common feature of the developing CNS, we tested LRP1 and LeX expression in the spinal cord. Immunohistochemical staining of the E12.5 spinal cord with mAb5750LeX or mAb487Lex together with the antibody against LRP1 and radial glia specific intermediate filament, Nestin, showed the co-localization of LeX with LRP1 and Nestin in the radial glia cell compartment along the central canal of the spinal cord (Fig 1 A, B). To test if LRP1 is a LeX-carrier in the spinal cord, E14 spinal cord protein lysates were immunoprecipitated with an antibody against the α-chain of LRP1. The two different isoforms of the LeX-carbohydrate recognized by the MAbs 5750LeX and 487Lex could be detected on the precipitated LRP1 α-chain, thereby confirming LeX-glycosylation of LRP1 (Fig 1 C).
Fig. 1. LRP1 is LewisX glycosylated in the developing spinal cord.
A. An overview showing frontal spinal cord sections of E12.5 C57Bl6 mouse embryos immunostained against the glycan LewisX epitope mAb5750LeX and mAb487LeX (red) co-localizing with LRP1 (green) and Nestin (blue) in the radial glia cells of the central canal, scale bar 100 μm. B. High magnification images of the central canal region, immunostained for the same markers as in A, confirming co-localization, scale bar 50 μm. C. Western blot detecting LRP1α-chain, 5750LeX and 487LeX epitopes in the E14.5 spinal cord protein lysate, immunoprecipitated with mAb B411E2, illustrating the LeX glycosylation of mature LRP1 in the developing spinal cord. IP = immunoprecipitation, AB = antibody.
High expression and the specific glycosylation of LRP1 in the NSPCs compartment of at least two developing CNS regions may indicate its essential role in CNS development. In the current study, we addressed the question of LRP1 function in neural stem cells by studying the basic properties of LRP1 negative NSPCs, such as proliferation, survival and differentiation. Due to the fact that constitutive LRP1 knockout is lethal (Herz et al., 1992), we employed the in vitro conditional knockout approach, by applying cell-permeable Cre-recombinase to the NSPCs generated from LRP1flox/flox embryos.
Proliferation and survival are negatively affected in LRP1-deficient NSPCs
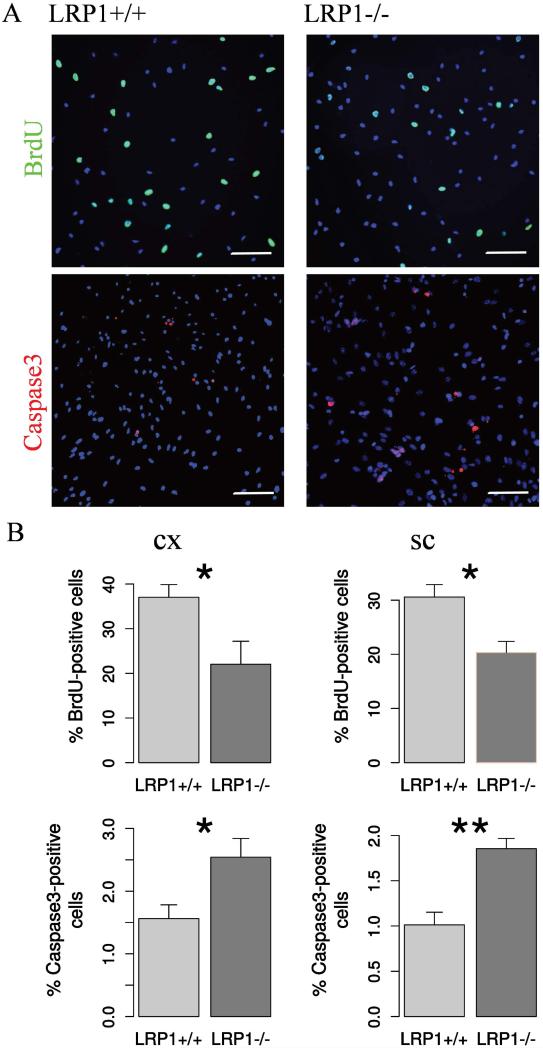
In a series of proliferation assays, LRP1-knockout cells from the cortex and spinal cord demonstrated less BrdU incorporation compared to the control cells, indicating a reduction in proliferation (Figure 2 A, B).
Fig. 2. LRP1 deletion interferes with NSPC proliferation and survival.
A. Representative photomicrographs of LRP1+/+ and LRP1−/− NSPCs immunostained against BrdU (N = 7, cx; N = 10, sc) and apoptosis marker active Caspase3 (N = 9, cx; N = 11, sc). Scale bar 50 μm. B. Quantification results indicate 1.5 fold reduction in proliferation and 2 fold increase in the apoptosis rate of LRP1 NSPCs derived from cortex and spinal cord. Data are expressed as mean ± SE (* indicates P < 0.05, ** — P < 0.01). Cx = cortex, sc = spinal cord.
As a result of the apoptosis assay, more LRP1 knockout cells derived from both regions were positive for the active form of Caspase3 compared to the control NSPCs, which reveals a higher apoptosis rate (Figure 2 A, B). Overall, LRP1 seems to have a protective function in the NSPCs, promoting their proliferation and survival.
LRP1 modulates NSPC differentiation
NSPCs are capable of spontaneous in vitro differentiation into neurons, astrocytes and oligodendroglia upon growth factor withdrawal and in the presence of FCS (Louis and Reynolds 2005).
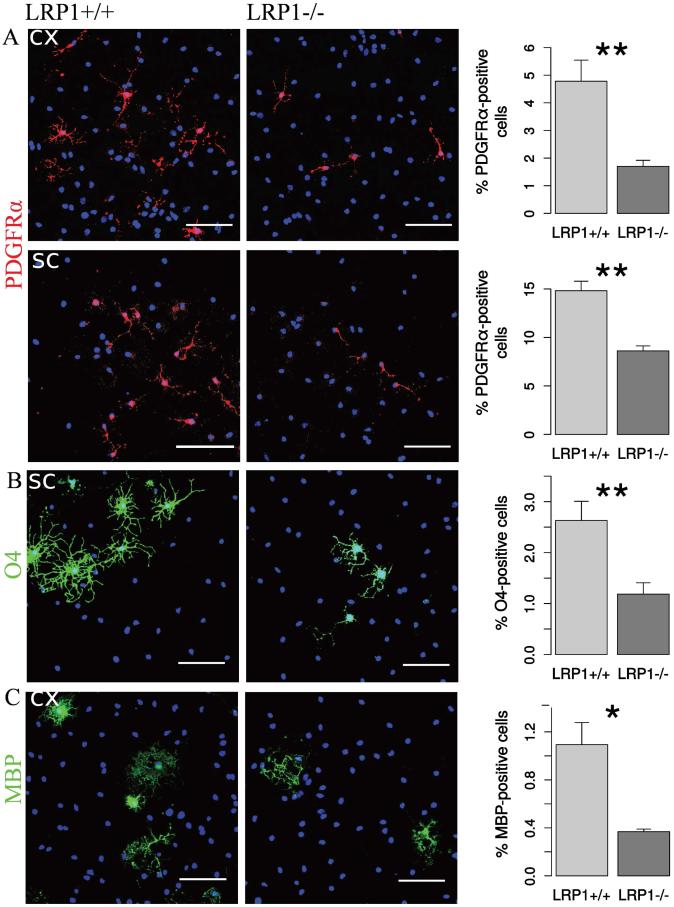
We previously demonstrated that cortical LRP1-deficient cells generate fewer O4-positive oligodendrocytes (Hennen et al. 2013). Here we asked whether other stages of oligodendroglial development, including early PDGFRα-positive progenitors and mature MBP-positive oligodendrocytes, were also affected by the in vitro knockout.
LRP1 deletion seemed to have a negative effect on different stages of oligodendroglial development in both examined tissues. We observed a reduction in PDGFRα-positive cells (Figure 3 A). In agreement with the results obtained earlier for the cortical NSPCs (Hennen et al. 2013), there was also a similarly strong reduction in the numbers of O4-positive immature oligodendroglia derived from the spinal cord NSPCs (Figure 3 B). MBP-positive cells derived from cortical NSPCs were also found in proportionally lower numbers in LRP1−/− condition (Figure 3 C).
Fig. 3. LRP1 deletion impairs lineage progression of oligodendrocytes.
Representative photomicrographs of LRP1+/+ and LRP1 differentiated NSPCs derived from cortex and spinal cord immunostained against: A. PDGFRα — a marker of OPCs (N = 5, cx; N = 13, sc). LRP1−/− cells generate 2 times less OPCs. B. Sulfatide O4 — a marker of differentiated immature oligodendrocytes (N = 5, cx; N = 10, sc). LRP1−/− cells generate 2 times less immature oligodendrocytes. C. MBP — a marker of mature oligodendrocytes (N = 4, cx). LRP1−/− cells generate 3 times less mature oligodendrocytes. Scale bar 50 μm. Data are expressed as mean ± SE (* indicates P < 0.05, ** — P < 0.01). Cx = cortex, sc = spinal cord
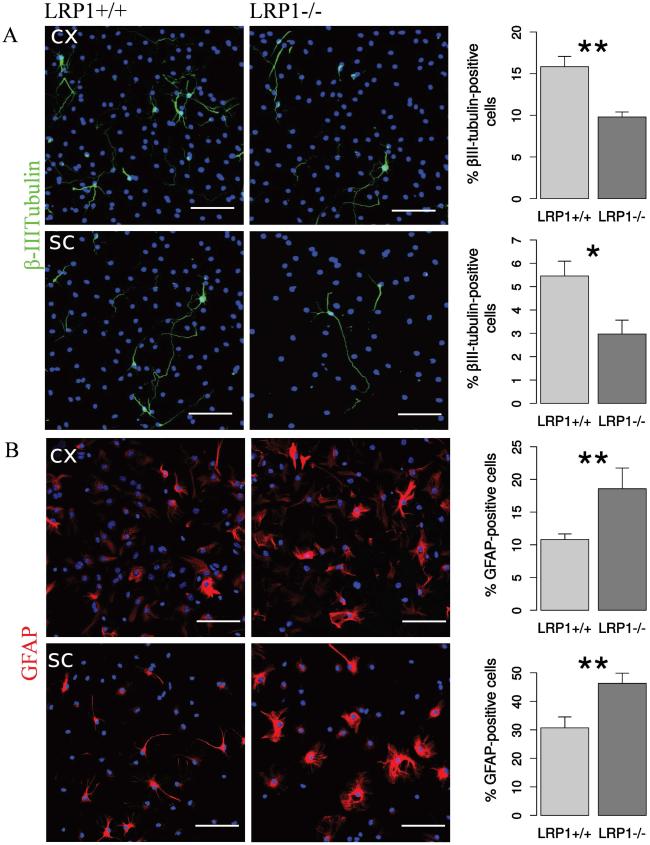
LRP1 deletion influenced neuronal differentiation in a similar way. The number of the βIII-tubulin-positive neurons derived from both cortical and spinal cord NSPCs was also decreased (Figure 4 A). However, LRP1-deficient cells generated significantly more GFAP-positive astrocytes in the case of both cortex and spinal cord derived cells (Figure 4 B).
Fig. 4. LRP1 knockout NSPCs generated less neurons, but more astrocytes.
Representative photomicrographs of LRP1+/+ and LRP1−/−differentiated NSPCs derived from cortex and spinal cord immunostained against: A. βIII-tubulin — a marker of young neurons (N = 10, cx, N = 10, sc). LRP1−/− NSPCs derived from both tissues generate nearly twice less neurons. B. GFAP — an astrocytic marker (N = 7, cx; N = 14, sc). LRP1 NSPCs derived from both tissues generate around 1.5 times more astrocytes. Scale bar 50 μm. Data are expressed as mean ± SE (* indicates P < 0.05, ** — P < 0.01). Cx = cortex, sc = spinal cord.
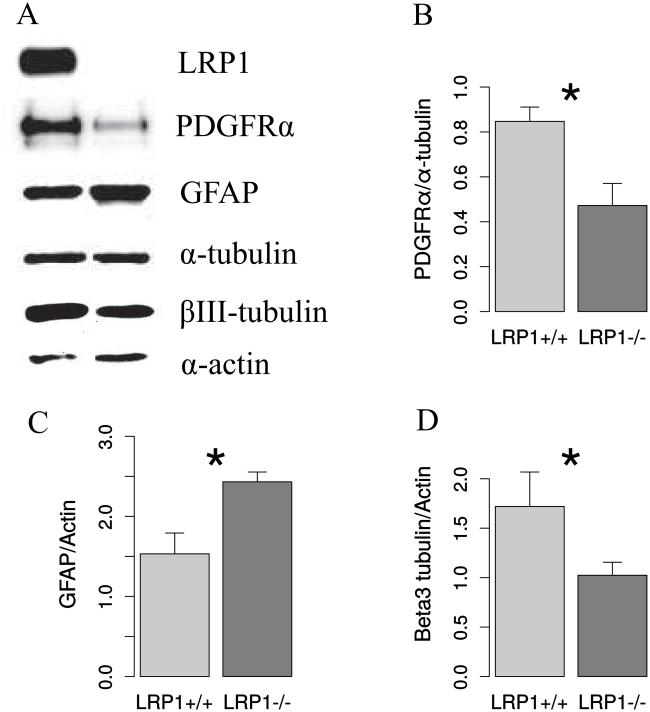
To validate the results of the immunocytochemical analysis, we performed western blot analysis, which confirmed the reduction of expression of both, the neuronal marker, βIII-tubulin and the OPCs marker, PDGFRα, and an increase of GFAP expression in LRP1−/− cells (Figure 5).
Fig. 5. Western blot confirms the modulation of NSPC differentiation by LRP1 deletion.
A. Representative Western blot analysis of protein lysates from differentiated NSPCs detecting LRP1, two house keeping genes: α-tubulin and actin and cell specific markers: PDGFRα, GFAP and βIII-tubulin. Quantification of Western blot results reveals a reduction of OPCs marker PDGFRα (B), neuronal marker βIII-tubulin (C), and an increase of GFAP expression in LRP1−/− cells. N=5, data are expressed as mean ± SE (* indicates P < 0.05, ** — P < 0.01).
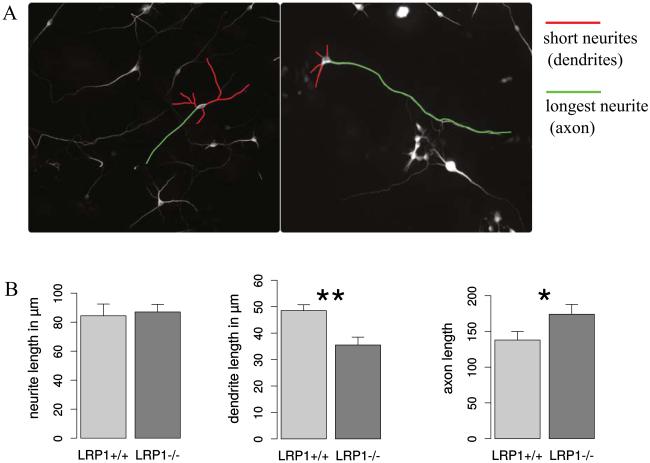
We have observed that LRP1 deletion interferes with neuronal differentiation. Other studies demonstrated that neuronal LRP1 is also important for normal synaptic and motor function (Martin et al. 2008; May et al. 2004). The functional neuronal deficits could be partially related to the morphological changes. Blocking LDL-receptors with RAP results in severe deficits in neurite outgrowth (Qiu et al. 2004). LRP1 deletion leads to a milder but noticeable reduction in neurite length (Nakajima et al. 2013). Here we investigated the neurite outgrowth in neurons differentiated from LRP1−/− and control NSPCs. There were no changes observed in the total neurite length. However, the length of the shorter neurites, which could correspond to dendrites, were reduced, while the length of the longer neurites, or possibly axons, was increased in the LRP1 knockout neurons (Fig 6). These results hint at changes in the neuronal polarity caused by LRP1 deletion.
Fig. 6. LRP1 deletion modifies neuronal polarity.
A. Representative photomicrographs of LRP1+/+ and LRP1−/− differentiated NSPCs derived from cortex immunostained against βIII-tubulin. The longer neurites (presumably axons) and the shorter processes (presumably dendrites) are indicated with green and red false color traces, respectively. B. The comparison of total neurite, dendrite and axon length between LRP1+/+ and LRP1−/− neurons. LRP1 knockout neurons exhibit significantly shorter dendrites and longer axons compared to wild type neurons, indicating alterations in neuronal polarity upon LRP1 deletion.
The mechanism of LRP1 action in NSPCs differentiation
As shown above, LRP1 deletion in NSPCs had a negative effect on their proliferation, survival and differentiation towards oligodendrocytes and neurons. The function of LRP1 in proliferation and survival has previously been described for other cell types (Boucher and Herz 2011; Campana et al. 2006; Grey et al. 2006; Llorente-Cortes et al. 2012; Mantuano et al. 2010; Tang et al. 2010). Therefore we decided to focus on the role of LRP1 in NSPCs' differentiation, especially towards oligodendrocyte, as this lineage was most severely affected. Since LRP1 seemed to exert similar functions in NSPCs derived from both CNS regions investigated, namely cortex and spinal cord, we have used only cortical NSPCs in our experiments, because these can be obtained in higher numbers. According to our previous results, blocking of the extracellular ligand binding domains with RAP had a negative impact on oligodendroglial differentiation, which was similar to complete LRP1 deletion (Hennen et al. 2013). As the ligand binding domains are clearly involved in the differentiation process, our next step here was to test which ligand triggers the LRP1 mediated effect.
Effect of ApoE4 on differentiation
ApoE is one of the major LRP1 ligands in the CNS. It has been shown that ApoE deficient NSPCs display reduced survival and impaired oligodendroglial differentiation capacities (Gan et al. 2011). According to the present study, LRP1 knockout NSPCs have similar characteristics. As LRP1 is not the only ApoE receptor, it is not yet clear whether the ApoE effect on NSPCs is mediated by LRP1. We have tested whether the addition of lipidated or lipid-free ApoE4 in the culture medium would have an effect on NSPC differentiation in the case of LRP1+/+ and LRP1−/− cells. For these experiments, recombinant ApoE4 was used, as it has the strongest promoting effect on murine NSPCs proliferation and survival according to the previous studies (Gan et al. 2011).
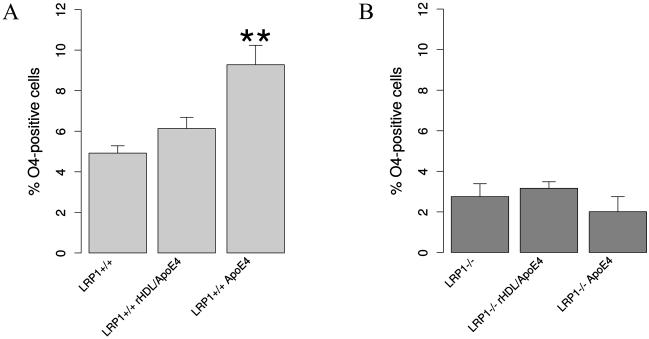
The addition of lipid-free ApoE4 to the differentiation medium had a significant supportive effect with regard to oligodendroglial differentiation. Upon ApoE4 stimulation, LRP1+/+ NSPCs generated nearly 50% more oligodendrocytes. There was a tendentious, but not significant increase in the percentage of generated oligodendrocytes from the wild type NSPCs treated with lipidated ApoE4 (Fig. 7 A). At the same time, ApoE4 did not influence oligodendroglial differentiation of LRP1 knockout NSPCs (Fig. 7 B). This indicates that the role of ApoE in the differentiation of NSPCs most likely depends on its binding to LRP1.
Fig. 7. ApoE promotes oligodendroglial differentiation via LRP1.
The results of the differentiation assay: A. illustrating that lipid-free ApoE4 addition to the differentiation medium increases the amount of O4-positive oligodendrocytes generated from LRP1+/+ (N = 4). This suggests that LRP1-dependent ApoE uptake promotes the differentiation of NSPCs towards oligodendrocytes. B. Oligodendroglial differentiation of LRP1−/− NSPCs is not altered upon ApoE4 treatment. Data are expressed as mean ± SE (* indicates P < 0.05, ** — P < 0.01).
Cholesterol promotes oligodendroglial differentiation independently of LRP1
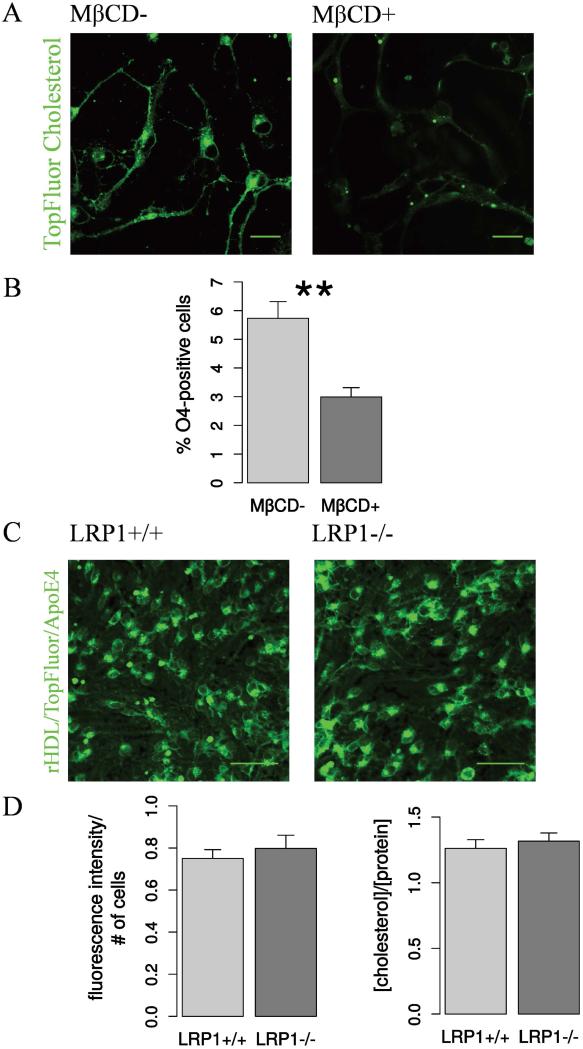
Unlike other tissues in the CNS ApoE is the major cholesterol carrier. LRP1 conditional deletion in mature neurons under the control of the CAMKII promoter led to a significant reduction of cholesterol concentration in the CNS tissue (Liu et al. 2010). Because oligodendroglial and neuronal differentiation presumably depend on the cholesterol concentration (Bieberich 2012; Orth and Bellosta 2012; Paintlia et al. 2010), we proposed that the deprivation of cholesterol might result in the impaired differentiation. In order to test whether the cholesterol concentration has an effect on NSPCs differentiation, we chemically depleted cholesterol from the wild type, not Cre-treated NSPCs using methyl-β-cyclodextrin (MβCD). MβCD is able to form inclusion complexes with cholesterol, thereby enhancing its water solubility and extracting cholesterol from the cell membrane (López et al. 2013). The reduction of cholesterol levels in the membrane of the wild type NSPCs pre-treated with TopFluor Cholesterol upon MβCD administration is illustrated in Fig. 8 A. MβCD treated NSPCs generated less oligodendrocytes, similar to the LRP1−/− condition Fig. 8 B. This result confirmed that normal cholesterol concentration is important for oligodendrogenesis.
Fig. 8. Cholesterol is important for oligodendroglial differentiation but cholesterol levels are not affected by LRP1 deletion.
A. Representative live confocal fluorescent images of NSPCs after TopFluor cholesterol accumulation and subsequent cholesterol extraction with MβCD demonstrate the effectiveness of the MβCD-treatment. Scale bar 20 μm. B. The results of the differentiation assay show that MβCD-treatment has a negative effect on oligodendroglia, decreasing the percentage of O4-positive oligodendrocytes generated from wild type NSPCs. This suggests that cholesterol is involved in oligodendrogenesis (N = 7). C. Representative live confocal fluorescent images of adherent proliferating NSPCs after rHDL/TopFluor/apoE4 cholesterol accumulation and the quantification of fluorescence intensity. D. Quantification of rHDL/TopFluor/apoE4 fluorescent intensity shows no difference in cholesterol uptake between LRP1−/− and LRP1+/+ cells (N = 4). Scale bar 50 μm. Total cholesterol concentration measured with the AmplexRed cholesterol kit does not differ between LRP1 and LRP1+/+ NSPCs (N = 16). Data are expressed as mean ± SE, (* indicates P < 0.05, ** — P < 0.01).
To test if the observed effect could indeed be due to cholesterol trafficking and total cholesterol concentration changes, we have estimated the rHDL/TopFluor/apoE4 uptake by LRP1+/+ and LRP1−/− cells by measuring the fluorescence intensity after a short treatment for 15 minutes (Fig. 8 C). We have also assessed the total cholesterol levels in LRP1+/+ and LRP1−/− NSPCs using the AmplexRed Cholesterol kit. Surprisingly, the measurements did not indicate any changes in either receptor mediated cholesterol uptake or total cholesterol concentration in the knockout cells compared to the wild type (Fig 8 D). We interpret this observation as indicating that the function of LRP1 in NSPCs differentiation depends rather on the other aspects of LRP1/ApoE interaction, such as the activation of downstream signaling.
NPXY motifs play role in oligodendrocyte differentiation
Besides its function as a cholesterol carrier, ApoE can activate a variety of signaling pathways (Zhou 2013). Thus, ApoE added to NSPCs stimulates survival and proliferation via the MAPK/ERK signaling pathway (Gan et al. 2011). However, it was unclear which ApoE-receptor mediates this function. LRP1 is also involved in the activation of signaling cascades, including MAPK/ERK, PI3K/Akt and Wnt-signaling, which are important for NSPCs differentiation. Ligand binding to LRP1 can lead to the activation of downstream signaling, which is mediated by the interaction of C-terminal NPXY LRP1 domains with adaptor proteins (Guttman et al. 2009; Martin et al. 2008; Terrand et al. 2009; Tsen et al. 2013). Tyrosine phosphorylation on the NPXY domains provides docking sites for signaling adaptor proteins, which in turn activate Ras/Raf/Mek/ERK signaling (Lin and Hu 2014). Alternatively, phosphorylated LRP1 can activate the PI3K/Akt/mTOR pathway (Fuentealba et al. 2009; Woldt et al. 2011).
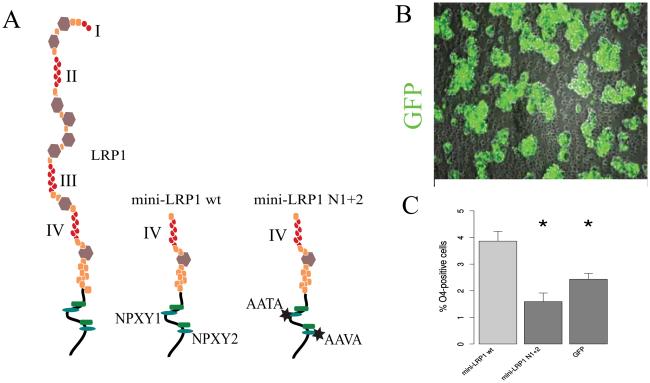
We questioned whether dysfunction of the LRP1 intracellular domain would cause a similar phenotype as the deletion of full-length LRP1. In order to answer this question, we transfected LRP1−/− NSPCs with two different N-terminal truncated constructs: a LRP1 wild type mini-receptor construct, a mutated LRP1 mini-receptor construct both expressing the N-terminal ligand binding domain IV and C-terminal intracellular domain (Fig 9 A) and a GFP-expressing plasmid as a control. The LRP1 wild type mini-receptor construct has one ApoE binding site, and a fully functional intracellular domain, therefore it is supposed to partially rescue the function of LRP1 with regard to ligand binding and signal transduction. The mutations introduced in the C-terminus of NPXY1+2 LRP1 mini-receptor prevent binding of most intracellular ligands and therefore can interfere with the activation of downstream signaling pathways. Electroporation of NSPCs with a wild type LRP1 mini-receptor construct could rescue the oligodendroglial differentiation, whereas NPXY1+2 mutant LRP1 mini-receptor did not have an effect on differentiation, which was not significantly different from the GFP-transfected knockout cells (Fig 9 B). This result supports the hypothesis, that the function of LRP1 in differentiation may depend on downstream signaling.
Fig. 9. Wild type but not NPXY mutant mini LRP1 is able to rescue oligodendroglial differentiation in LRP1 cells.
A. Schematic representation of full-length receptor LRP1, wild type and mutated mini-receptors. I-IV = the ligand binding domains. B. A representative fluorescent image of neurospheres electroporated with GFP, illustrating high transfection efficiency. The result of the differentiation assays shows that LRP1 knockout NSPCs transfected with wild type mini LRP1 generated significantly more O4-positive oligodendrocytes compared to NSPCs transfected with mini-LRP1 including the mutated intracellular domain, or GFP-transfected cells (N = 4). Data are expressed as mean ± SE, (* indicates P < 0.05, ** — P < 0.01).
LRP1 effect on NSPCs differentiation may depend on MAPK/ERK and Akt
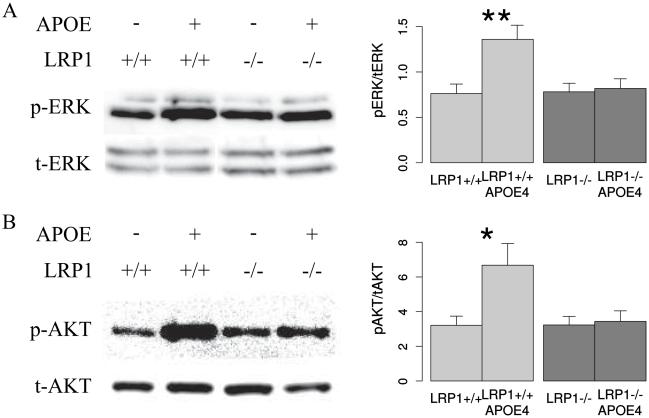
In order to investigate whether the LRP1-mediated effect of ApoE on NSPCs differentiation is a result of downstream MAPK/ERK or PI3K/Akt signaling, we have measured ERK and Akt phosphorylation upon lipid-free ApoE4 stimulation in LRP1+/+ and LRP1−/− cells. We could observe a significant increase of ERK1/2 phosphorylation upon ApoE4 stimulation in LRP1+/+, but not in LRP1−/− NSPCs (Fig 10 A). Similar to ERK1/2 in the case of Akt signaling we could also demonstrate an increase in Akt-phosphorylation upon ApoE4 stimulation in the LRP1+/+, but not in LRP1−/− cells (Fig. 10 B). These results suggest that the observed LRP1 role in differentiation could be due to the downstream MAPK/ERK and Akt-signaling activation upon ApoE binding.
Fig. 10. LRP1 promotes ERK and Akt phosphorylation upon ApoE stimulation.
Representative Western blot analysis and quantification, showing that ApoE can enhance ERK1/2 (A) and Akt phosphorylation (B) in LRP1+/+ but not LRP−/− cells (N = 6, Akt; N = 7, ERK). The band intensity of p-Akt and p-ERK is normalized to total Akt and ERK respectively. Data are expressed as mean ± SE (* indicates P < 0.05, ** — P < 0.01). p-ERK = phosphorylated ERK, p-Akt = phosphorylated Akt, t-ERK = total ERK, t-Akt = total Akt.
Discussion
Our previous study has identified LRP1 as a novel LeX carrier in the cortical ventricular zone (Hennen et al. 2013). The current results expand this finding also for the central channel of the spinal cord.
LeX is known to be present in the developing spinal cord (Dodd and Jessell 1986; Karus et al. 2013). However, LeX expression was previously undetectable at stage E12.5 but highly upregulated at stage E15.5 as shown by our Western blot experiments (Karus et al. 2013). This pattern corresponds to the upregulation of the level of the known LeX carrier-proteins: Tenascin-C and Phosphacan. In the current study we find a clear co-localization of LeX and LRP1 in the radial glia cell compartment of the spinal cord already at E12.5, when the other known LeX carrier-proteins are not expressed. Immunoprecipitation confirms the LeX glycosylation of fully mature LRP1 in the developing spinal cord.
Given our previous and current studies it can be concluded that LRP1 is highly expressed and specifically glycosylated in the NSPC compartment of the developing CNS, which may indicate its important role in NSPCs. To address this question we further studied the function of LRP1 in NSPC proliferation, survival and differentiation.
In general, the function of LRP1 in cell proliferation is controversial and depends on the cell types and conditions that are studied. On the one hand, LRP1 can stimulate proliferation of mouse embryonic fibroblasts upon treatment with LRP1 ligands by stimulating downstream pro-proliferative signaling. (Derocq et al. 2012; Kozlova et al. 2015; Muratoglu et al. 2010). On the other hand, LRP1 can suppress lung tumor cell, hepatic stellate cell and smooth muscle cell proliferation, most likely by endocytic uptake of pro-proliferative molecules (Boucher et al. 2003; Llorente-Cortes et al. 2012; Meng et al. 2011). LRP1 seems to have a promotive effect on NSPC proliferation in both examined CNS regions, the cortex and spinal cord, favoring the hypothesis of the downstream signaling activation.
The role of LRP1 in controlling cell survival is more consistent. LRP1 was shown to promote survival of Schwann cells upon injury via PI3K-Akt activation (Mantuano et al. 2011). It can also have a positive effect on survival and neurite outgrowth of developing sensory neurons by pERK1/2 activation (Yamauchi et al. 2013). Stimulation of LRP1 with its ligands, ApoE-containing lipoproteins and α2-macroglobulin had a pro-survival effect on the retinal ganglion neurons (Hayashi et al. 2007). In line with available studies on other CNS and PNS cell-types, we also observed that LRP1 knockout NSPCs from both, the cortex and spinal cord, are more susceptible to apoptosis, demonstrating a pro-survival effect of LRP1.
An observed promoting role of LRP1 in proliferation and survival of NSPCs agrees with a proliferative and pro-survival effect of ApoE on the same cell type (Gan et al. 2011). In the light of our results, LRP1 is likely to be a mediator of the ApoE effect.
Different from the situation concerning proliferation and survival, very little is known about the role of LRP1 in neural stem cell differentiation. Here we show that LRP1-deletion has a negative impact on different stages of oligodendroglial differentiation. These results suggest that LRP1 is important for oligodendroglial differentiation and is required already at the earliest stage of differentiation.
So far, the role of LRP1 in the CNS has been mainly studied in the adult neurons, where it promotes neurite outgrowth, axonal regeneration, and takes part in synaptic transmission (Liu et al. 2010; Martin et al. 2008; May et al. 2004; Nakajima et al. 2013; Yoon et al. 2013). Here we demonstrate that it is also required in early stages of neural development. Additionally, we could show the changes in neuronal polarity of LRP1 knockout neurons. Consistent with previous studies (Nakajima et al. 2013; Qiu et al. 2004), the dendritic length of LRP1 −/− neurons was reduced. However, the length of the longer neurites, which presumably correspond to axons, was increased in LRP1 deficient neurons compared to control. As suggested by Liu et al., (2013) alterations in neuronal polarity can lead to an overexcited phenotype. Our observations may also provide a link between neuronal polarity and over-excitation caused by LRP1 deletion, reported in the previous studies.
The role of LRP1 in NSPC differentiation is a newly observed phenomenon. We went further to uncover the underlying mechanism. LRP1 interacts with over 60 ligands (Gonias and Campana 2014; Lillis et al. 2008; Ling et al. 2004; Spijkers et al. 2008; Spuch et al. 2012; Vaillant et al. 2007) and depending on the ligand the downstream effect of binding can be drastically different. ApoE, abundantly expressed in the CNS (Xu et al. 2006), is one of the major LRP1 ligands. ApoE is involved in oligodendroglial differentiation (Gan et al. 2011). The ApoE-mimetic peptide (COG112) can stimulate axonal regeneration and remyelination after peripheral nerve injury (Gu et al. 2013; Li et al. 2010). Given that ApoE can interact with several other receptors, it was not clear whether these effects were mediated by LRP1. Here we show that lipid-free ApoE4 has a clear promotive effect on oligodendroglial differentiation in the LRP1+/+, but not in LRP1−/− NSPCs. These findings suggest that LRP1 promotes oligodendroglial differentiation upon ApoE4 binding. Interestingly, the rHDL/apoE4 had less influence on oligodendrogenesis compared to the lipid-free ApoE4. The difference we noted could be due to the fact that unlike most other ApoE receptors, LRP is able to bind a very lipid-poor ApoE (Narita et al. 2002). Thereby there might be more ApoE4 molecules binding LRP1 in the lipid-free than in the lipidated ApoE condition. Other studies have demonstrated that lipid-free ApoE can stimulate the neurite outgrowth in PC12 cells and proliferation and survival of NSPCs (Gan et al. 2011; Nathan et al. 1994). (Ruiz et al. 2005) claim that receptor-dependent specificity for lipid-bound versus lipid-free ApoE forms may have physiological relevance. Together with the previous observations our results raise the question of lipid-free (or lipid-poor) ApoE presence in the CNS, which requires further investigation.
Although the promotive effect of the lipid-free ApoE4 on oligodendrocyte differentiation was stronger compared to the lipid-bound ApoE4, the contribution of the cholesterol-transport function of apoE cannot be definitively excluded at this point. ApoE is a major source of cholesterol in the CNS (Han 2004). High cholesterol levels are essential for myelin membrane growth (Fünfschilling et al. 2012) and cholesterol biosynthesis impairment can lead to corpus callosum agenesis (Waterham 2002). Therefore, cholesterol could be important for oligodendrocyte differentiation and maturation. Cholesterol is also critical for neuronal membrane expansion in the embryonic brain and the inhibition of cholesterol synthesis in cortical neurons prenatally leads to neonatal lethality (Hayashi et al. 2007). Cholesterol levels are significantly reduced in the brain tissue of αCamKII-Cre LRP1 knockout mice lacking LRP1 in adult neurons (Liu et al. 2010).
We assumed that in the case of LRP1 knockout in NSPCs, the receptor-mediated cholesterol uptake and therefore the cholesterol concentration should also be reduced, which was, surprisingly, not the case. This could be due to the compensation by other LDL-receptors, which might take place in NSPCs, but not in adult neurons. It could also be explained by the down-regulation of the ATP-binding cassette transporter A1 (ABCA1), which mediates cholesterol transport out of the cell (Wang et al. 2001). ABCA1 downregulation has been observed consequent to LRP1 deletion (Zhou et al. 2009).
As cholesterol levels are not reduced in LRP1 knockout NSPCs, we have further tested whether LRP1 plays a role in NSPC differentiation due to the other consequence of LRP1/ApoE interaction, namely downstream signaling activation. In the current study we demonstrate that a LRP1 wild type mini-receptor containing the C-terminus, transmembrane domain and binding domain IV with intact NPXY1 and NPXY2 motifs was able to rescue oligodendroglial differentiation, whereas mutations in NPXY1 and NPXY2 motifs prevented the rescue effect. This illustrates a potential involvement of intracellular NPXY domains in oligodendroglial differentiation and supports a hypothesis of a signaling dependent effect of LRP1.
Therefore, we have further explored whether MAPK/ERK and PI3K/Akt signaling is affected by LRP1 deletion.
The basic rate of ERK1/2 and Akt phosphorylation was rather high and did not differ between LRP1−/− and LRP1+/+ unstimulated conditions. This may be due to the cell-cell- or cell-matrix-interactions and activation of MAPK/ERK and PI3K/Akt via other receptors (Prowse et al. 2011). However, the addition of ApoE could significantly enhance ERK1/2 and Akt phosphorylation in the LRP1+/+, but not in LRP1−/− NSPCs, suggesting that LRP1 is a direct mediator of ApoE-dependent MAPK/ERK and PI3K/Akt activation.
The effect of MAPK/ERK signaling on the development of oligodendrocytes has been extensively investigated. MAPK/ERK signaling is important for long-term survival and the proper timing of oligodendroglial differentiation (Ren et al. 2013). ERK2 has been specifically shown to regulate MBP expression and ERK2 deletion resulted in myelination delay (Fyffe-Maricich et al. 2011).
The PI3K/Akt pathway is also important for oligodendroglial differentiation. Activation of Akt via adrenal cortical hormones could promote oligodendrogenesis and the effect was abolished by inhibition of Akt (Cai et al. 2014; Maki et al. 2015). PDGFRα, transferrin and the thrombin receptor also can modulate the generation of OPCs through Akt signaling (Pérez et al. 2013; Pituch et al. 2015; Yoon et al. 2015).
(Guardiola-Diaz et al. 2012) examined in parallel the role of Ras/Raf/Mek/ERK and PI3K/Akt/mTOR pathways in oligodendrogenesis. This laboratory could demonstrate a sequential and non-overlapping developmental stage-specific requirement of both pathways during oligodendrocyte lineage progression. Specifically, for the transition of early to late OPCs ERK1/2 signaling was required, while the transition of immature to mature oligodendrocytes was dependent on the PI3K/Akt/mTOR signaling. Here we show that LRP1 deletion has a negative effect on all stages of oligodendrogenesis, which could be explained by the attenuation of both discussed signaling pathways.
Alternatively, the influence of LRP1 deletion on oligodendroglial differentiation could be a consequence of altered integrin expression and function. LRP1 was shown to be involved in β1-integrin maturation, transport to the cell surface and internalization (Rabiej et al. 2016; Salicioni et al. 2004). Moreover, LRP1 knockout may indirectly affect integrin expression by downregulating ERK phosphorylation, as has been recently demonstrated for hematopoietic stem/progenitor cells (Wang et al. 2015) At the same time different integrins are known to play role in oligodendrocyte differentiation and maturation (Baron et al. 2014; Blaschuk et al. 2000; Buttery and ffrench-Constant 1999; Terada et al. 2002). The potential link between LRP1 and different members of the integrin family in the context of NSPCs differentiation remains to be further investigated.
In summary, our results show that LRP1 has a positive effect on NSPC proliferation and survival, promotes oligodendroglial and neuronal differentiation, but negatively effects astrogliogenesis in vitro. According to our findings, the function of LRP1 in oligodendrogenesis is likely independent of the cholesterol content. We suggest that ApoE binding to LRP1 and consequent downstream MAPK/ERK and PI3K/Akt signaling activation could be involved in oligodendroglial lineage progression. Further studies are needed to uncover the exact intermediate components of LRP1-mediated ERK and Akt activation triggered by ApoE. The role of other LRP1 ligands, highly expressed in the CNS, such as α2-macroglobulin or tissue plasminogen activator in NSPC differentiation and self-renewal also remains to be elucidated. Additionally, the mechanism of LRP1 function in neurogenesis and astroglial differentiation has to be investigated in the future.
In conclusion, the findings presented here open new possibilities for manipulating NSPC fate in vitro, as we have identified LRP1 to be a modulator of NSPC properties. The novel functions of LRP1 described here may also be implicated in CNS development in vivo and maintenance of adult neural stem cells.
Main points.
LRP1 deletion compromises proliferation and survival of NSPCs.
LRP1 modulates differentiation of NSPCs.
ApoE promotes oligodendroglial differentiation via LRP1 mediated activation of the ERK and Akt-signaling pathways.
Acknowledgements
We acknowledge grant support by the Stem Cell Network North Rhine-Westphalia, the German Research Foundation (DFG: SFB 642; SPP 1109; GRK 736; GSC 98/1; SPP-1757, Fa 159/20-1 to A.F.; ED 79/4-1 and PI 379/8-1 to C.U.P.), the German Ministry of Education, Research and Technology (BMBF 01GN0503) and the Ruhr-University (President’s Special Programme Call 2008) to A.F., and the National Institutes of Health (NIH-GM105561) to V.N. for selected aspects of the project. We thank the International Graduate School of Neuroscience (IGSN) for financial support to D.S. We also greatly acknowledge Derya Cengiz for performing neurite outgrowth analysis and Western blots, Tuyen N. Tran for his assistance in preparing rHDL, Ewa Bres for critical reading of the manuscript and Andrey Rozenberg for his help with data analysis.
References
- Alvarez-Buylla A, García-Verdugo JM. Neurogenesis in adult subventricular zone. The Journal of Neuroscience. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron W, Bijlard M, Nomden A, de Jonge JC, Teunissen CE, Hoekstra D. Sulfatide-mediated control of extracellular matrix-dependent oligodendrocyte maturation. Glia. 2014;62:927–42. doi: 10.1002/glia.22650. [DOI] [PubMed] [Google Scholar]
- Bertram B, Wiese S, von Holst A. High-efficiency transfection and survival rates of embryonic and adult mouse neural stem cells achieved by electroporation. Journal of Neuroscience Methods. 2012;209:420–427. doi: 10.1016/j.jneumeth.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Bieberich E. It’s a lipid's world: Bioactive lipid metabolism and signaling in neural stem cell differentiation. Neurochemical Research. 2012;37:1208–1229. doi: 10.1007/s11064-011-0698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschuk KL, Frost EE, ffrench-Constant C. The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by alphaV integrins. Development. 2000;127:1961–9. doi: 10.1242/dev.127.9.1961. [DOI] [PubMed] [Google Scholar]
- Boucher P, Gotthardt M, Li W-P, Anderson RGW, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science (New York, NY) 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- Boucher P, Herz J. Signaling through LRP1: protection from atherosclerosis and beyond. Biochemical Pharmacology. 2011;81:1–5. doi: 10.1016/j.bcp.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- Cai Q, Yao Z, Li H. Catalpol promotes oligodendrocyte survival and oligodendrocyte progenitor differentiation via the Akt signaling pathway in rats with chronic cerebral hypoperfusion. Brain Research. 2014;1560:27–35. doi: 10.1016/j.brainres.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Campana WM, Li X, Dragojlovic N, Janes J, Gaultier A, Gonias SL. The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:11197–11207. doi: 10.1523/JNEUROSCI.2709-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Developmental Biology. 2006;291:300–313. doi: 10.1016/j.ydbio.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Choy N, Raussens V, Narayanaswami V. Inter-molecular coiled-coil formation in human apolipoprotein E C-terminal domain. J Mol Biol. 2003;334:527–39. doi: 10.1016/j.jmb.2003.09.059. [DOI] [PubMed] [Google Scholar]
- Derocq D, Prébois C, Beaujouin M, Laurent-Matha V, Pattingre S, Smith GK, Liaudet-Coopman E. Cathepsin D is partly endocytosed by the LRP1 receptor and inhibits LRP1-regulated intramembrane proteolysis. Oncogene. 2012;31:3202–3212. doi: 10.1038/onc.2011.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Jessell TM. Cell surface glycoconjugates and carbohydrate-binding proteins: possible recognition signals in sensory neurone development. The Journal of Experimental Biology. 1986;124:225–238. doi: 10.1242/jeb.124.1.225. [DOI] [PubMed] [Google Scholar]
- Faissner A, Reinhard J. The extracellular matrix compartment of neural stem and glial progenitor cells. Glia. 2015;63:1330–49. doi: 10.1002/glia.22839. [DOI] [PubMed] [Google Scholar]
- Fuentealba RA, Liu Q, Kanekiyo T, Zhang J, Bu G. Low Density Lipoprotein Receptor-related Protein 1 promotes anti-apoptotic signaling in neurons by activating Akt survival pathway. Journal of Biological Chemistry. 2009;284:34045–34053. doi: 10.1074/jbc.M109.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fünfschilling U, Jockusch WJ, Sivakumar N, Möbius W, Corthals K, Li S, Quintes S, Kim Y, Schaap IAT, Rhee J-S. Critical time window of neuronal cholesterol synthesis during neurite outgrowth. The Journal of Neuroscience. 2012;32:7632–7645. doi: 10.1523/JNEUROSCI.1352-11.2012. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Karlo JC, Landreth GE, Miller RH. The ERK2 mitogen-activated protein kinase regulates the timing of oligodendrocyte differentiation. The Journal of Neuroscience. 2011;31:843–850. doi: 10.1523/JNEUROSCI.3239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan HT, Tham M, Hariharan S, Ramasamy S, Yu YH, Ahmed S. Identification of ApoE as an autocrine/paracrine factor that stimulates neural stem cell survival via MAPK/ERK signaling pathway. Journal of Neurochemistry. 2011;117:565–578. doi: 10.1111/j.1471-4159.2011.07227.x. [DOI] [PubMed] [Google Scholar]
- Garwood J, Schnädelbach O, Clement A, Schütte K, Bach A, Faissner A. DSD-1-proteoglycan is the mouse homolog of phosphacan and displays opposing effects on neurite outgrowth dependent on neuronal lineage. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1999;19:3888–3899. doi: 10.1523/JNEUROSCI.19-10-03888.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonias SL, Campana WM. LDL receptor-related protein-1: a regulator of inflammation in atherosclerosis, cancer, and injury to the nervous system. The American Journal of Pathology. 2014;184:18–27. doi: 10.1016/j.ajpath.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M, Huttner WB. The cell biology of neurogenesis. Nature Reviews Molecular Cell Biology. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Grey A, Zhu Q, Watson M, Callon K, Cornish J. Lactoferrin potently inhibits osteoblast apoptosis, via an LRP1-independent pathway. Molecular and Cellular Endocrinology. 2006;251:96–102. doi: 10.1016/j.mce.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Gu Z, Li F, Zhang YP, Shields LBE, Hu X, Zheng Y, Yu P, Zhang Y, Cai J, Vitek MP. Apolipoprotein E mimetic promotes functional and histological recovery in lysolecithin-induced spinal cord demyelination in mice. Journal of Neurology & Neurophysiology. 20132014:10. doi: 10.4172/2155-9562.S12-010. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Ishii A, Bansal R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia. 2012;60:476–486. doi: 10.1002/glia.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, Betts GN, Barnes H, Ghassemian M, van der Geer P, Komives EA. Interactions of the NPXY microdomains of the LDL Receptor-Related Protein 1. Proteomics. 2009;9:5016–5028. doi: 10.1002/pmic.200900457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X. The role of apolipoprotein E in lipid metabolism in the central nervous system. Cellular and molecular life sciences: CMLS. 2004;61:1896–1906. doi: 10.1007/s00018-004-4009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. The Journal of Neuroscience. 2007;27:1933–1941. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennen E, Czopka T, Faissner A. Structurally distinct LewisX glycans distinguish subpopulations of neural stem/progenitor cells. The Journal of Biological Chemistry. 2011;286:16321–16331. doi: 10.1074/jbc.M110.201095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennen E, Faissner A. LewisX: a neural stem cell specific glycan? The International Journal of Biochemistry & Cell Biology. 2012;44:830–833. doi: 10.1016/j.biocel.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Hennen E, Safina D, Haussmann U, Wörsdörfer P, Edenhofer F, Poetsch A, Faissner A. A LewisX-glycoprotein screen identifies the low density lipoprotein receptor-related protein 1 (LRP1) as a modulator of oligodendrogenesis in mice. J Biol Chem. 2013;288:16538–16545. doi: 10.1074/jbc.M112.419812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger S, Pietrzik CU. Functional role of lipoprotein receptors in Alzheimer's disease. Current Alzheimer Research. 2008;5:15–25. doi: 10.2174/156720508783884675. [DOI] [PubMed] [Google Scholar]
- Karus M, Denecke B, ffrench-Constant C, Wiese S, Faissner A. The extracellular matrix molecule tenascin C modulates expression levels and territories of key patterning genes during spinal cord astrocyte specification. Development (Cambridge, England) 2011;138:5321–5331. doi: 10.1242/dev.067413. [DOI] [PubMed] [Google Scholar]
- Karus M, Hennen E, Safina D, Klausmeyer A, Wiese S, Faissner A. Differential expression of micro-heterogeneous LewisX-type glycans in the stem cell compartment of the developing mouse spinal cord. Neurochemical Research. 2013;38:1285–1294. doi: 10.1007/s11064-013-1048-6. [DOI] [PubMed] [Google Scholar]
- Kozlova N, Jensen JK, Chi TF, Samoylenko A, Kietzmann T. PAI-1 modulates cell migration in a LRP1-dependent manner via β-catenin and ERK1/2. Thrombosis and Haemostasis. 2015;113:988–998. doi: 10.1160/TH14-08-0678. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annual review of neuroscience. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F-Q, Fowler KA, Neil JE, Colton CA, Vitek MP. An apolipoprotein E-mimetic stimulates axonal regeneration and remyelination after peripheral nerve injury. The Journal of Pharmacology and Experimental Therapeutics. 2010;334:106–115. doi: 10.1124/jpet.110.167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiological Reviews. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Hu K. LRP-1: functions, signaling and implications in kidney and other diseases. International Journal of Molecular Sciences. 2014;15:22887–22901. doi: 10.3390/ijms151222887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling T-Y, Chen C-L, Huang Y-H, Liu I-H, Huang SS, Huang JS. Identification and characterization of the acidic pH binding sites for growth regulatory ligands of low density lipoprotein receptor-related protein-1. The Journal of Biological Chemistry. 2004;279:38736–38748. doi: 10.1074/jbc.M310537200. [DOI] [PubMed] [Google Scholar]
- Liu C, Lin C, Whitaker DT, Bakeri H, Bulgakov OV, Liu P, Lei J, Dong L, Li T, Swaroop A. Prickle1 is expressed in distinct cell populations of the central nervous system and contributes to neuronal morphogenesis. Hum Mol Genet. 2013;22:2234–46. doi: 10.1093/hmg/ddt075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Trotter J, Zhang J, Peters MM, Cheng H, Bao J, Han X, Weeber EJ, Bu G. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30:17068–17078. doi: 10.1523/JNEUROSCI.4067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Cortes V, Barbarigo V, Badimon L. Low density lipoprotein receptor-related protein 1 modulates the proliferation and migration of human hepatic stellate cells. Journal of Cellular Physiology. 2012 doi: 10.1002/jcp.24080. [DOI] [PubMed] [Google Scholar]
- López CA, de Vries AH, Marrink SJ. Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes. Scientific Reports. 2013:3. doi: 10.1038/srep02071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis SA, Reynolds BA. Generation and differentiation of neurospheres from murine embryonic day 14 central nervous system tissue. Methods in Molecular Biology (Clifton, NJ) 2005;290:265–280. doi: 10.1385/1-59259-838-2:265. [DOI] [PubMed] [Google Scholar]
- Maier W, Bednorz M, Meister S, Roebroek A, Weggen S, Schmitt U, Pietrzik CU. LRP1 is critical for the surface distribution and internalization of the NR2B NMDA receptor subtype. Molecular neurodegeneration. 2013;8:25. doi: 10.1186/1750-1326-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki T, Takahashi Y, Miyamoto N, Liang AC, Ihara M, Lo EH, Arai K. Adrenomedullin promotes differentiation of oligodendrocyte precursor cells into myelin-basic-protein expressing oligodendrocytes under pathological conditions in vitro. Stem Cell Research. 2015;15:68–74. doi: 10.1016/j.scr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Gotz M. Radial glia - from boring cables to stem cell stars. Development. 2013;140:483–6. doi: 10.1242/dev.085852. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–64. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Mantuano E, Henry K, Yamauchi T, Hiramatsu N, Yamauchi K, Orita S, Takahashi K, Lin JH, Gonias SL, Campana WM. The unfolded protein response is a major mechanism by which LRP1 regulates Schwann cell survival after injury. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31:13376–13385. doi: 10.1523/JNEUROSCI.2850-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantuano E, Jo M, Gonias SL, Campana WM. Low density lipoprotein receptor-related protein (LRP1) regulates Rac1 and RhoA reciprocally to control Schwann cell adhesion and migration. The Journal of Biological Chemistry. 2010;285:14259–14266. doi: 10.1074/jbc.M109.085126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AM, Kuhlmann C, Trossbach S, Jaeger S, Waldron E, Roebroek A, Luhmann HJ, Laatsch A, Weggen S, Lessmann V. The functional role of the second NPXY motif of the LRP1 beta-chain in tissue-type plasminogen activator-mediated activation of N-methyl-D-aspartate receptors. The Journal of Biological Chemistry. 2008;283:12004–12013. doi: 10.1074/jbc.M707607200. others. [DOI] [PubMed] [Google Scholar]
- May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, Noebels JL, Beffert U, Sweatt JD, Weeber EJ. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Molecular and Cellular Biology. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Chen G, Zhang X, Wang Z, Thomas DG, Giordano TJ, Beer DG, Wang MM. Stromal LRP1 in lung adenocarcinoma predicts clinical outcome. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2011;17:2426–2433. doi: 10.1158/1078-0432.CCR-10-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G-l, Song H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratoglu SC, Mikhailenko I, Newton C, Migliorini M, Strickland DK. Low density lipoprotein receptor-related protein 1 (LRP1) forms a signaling complex with platelet-derived growth factor receptor-beta in endosomes and regulates activation of the MAPK pathway. The Journal of Biological Chemistry. 2010;285:14308–14317. doi: 10.1074/jbc.M109.046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima C, Kulik A, Frotscher M, Herz J, Schäfer M, Bock HH, May P. Low Density Lipoprotein Receptor-related Protein 1 (LRP1) modulates N-Methyl-d-aspartate (NMDA) Receptor-dependent intracellular signaling and NMDA-induced regulation of postsynaptic protein complexes. Journal of Biological Chemistry. 2013;288:21909–21923. doi: 10.1074/jbc.M112.444364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Holtzman DM, Fagan AM, LaDu MJ, Yu L, Han X, Gross RW, Bu G, Schwartz AL. Cellular catabolism of lipid poor apolipoprotein E via cell surface LDL receptor-related protein. J Biochem. 2002;132:743–9. doi: 10.1093/oxfordjournals.jbchem.a003282. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science (New York, NY) 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Nichols AV, Gong EL, Blanche PJ, Forte TM, Shore VG. Pathways in the formation of human plasma high density lipoprotein subpopulations containing apolipoprotein A-I without apolipoprotein A-II. J Lipid Res. 1987;28:719–32. [PubMed] [Google Scholar]
- Nolden L, Edenhofer F, Haupt S, Koch P, Wunderlich FT, Siemen H, Brüstle O. Site-specific recombination in human embryonic stem cells induced by cell-permeant Cre recombinase. Nature Methods. 2006;3:461–467. doi: 10.1038/nmeth884. [DOI] [PubMed] [Google Scholar]
- Orth M, Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. 2012 doi: 10.1155/2012/292598. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintlia AS, Paintlia MK, Singh AK, Singh I. Activation of PPAR-γ and PTEN cascade participates in lovastatin-mediated accelerated differentiation of oligodendrocyte progenitor cells. Glia. 2010;58:1669–1685. doi: 10.1002/glia.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez MJ, Fernandez N, Pasquini JM. Oligodendrocyte differentiation and signaling after transferrin internalization: a mechanism of action. Experimental Neurology. 2013;248:262–274. doi: 10.1016/j.expneurol.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Petit A, Sanders AD, Kennedy TE, Tetzlaff W, Glattfelder KJ, Dalley RA, Puchalski RB, Jones AR, Roskams AJ. Adult spinal cord radial glia display a unique progenitor phenotype. PLoS One. 2011;6:e24538. doi: 10.1371/journal.pone.0024538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Götz M. Radial glial cell heterogeneity — the source of diverse progeny in the CNS. Progress in Neurobiology. 2007;83:2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Pituch KC, Moyano AL, Lopez-Rosas A, Marottoli FM, Li G, Hu C, van Breemen R, Månsson JE, Givogri MI. Dysfunction of platelet-derived growth factor receptor α (PDGFRα) represses the production of oligodendrocytes from arylsulfatase A-deficient multipotential neural precursor cells. The Journal of Biological Chemistry. 2015;290:7040–7053. doi: 10.1074/jbc.M115.636498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowse ABJ, Chong F, Gray PP, Munro TP. Stem cell integrins: Implications for ex-vivo culture and cellular therapies. Stem Cell Research. 2011;6:1–12. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Pruszak J, Sonntag K-C, Aung MH, Sanchez-Pernaute R, Isacson O. Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells (Dayton, Ohio) 2007;25:2257–2268. doi: 10.1634/stemcells.2006-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Hyman BT, Rebeck GW. Apolipoprotein E receptors mediate neurite outgrowth through activation of p44/42 mitogen-activated protein kinase in primary neurons. Journal of Biological Chemistry. 2004;279:34948–34956. doi: 10.1074/jbc.M401055200. [DOI] [PubMed] [Google Scholar]
- Rabiej VK, Pflanzner T, Wagner T, Goetze K, Storck SE, Eble JA, Weggen S, Mueller-Klieser W, Pietrzik CU. Low density lipoprotein receptor-related protein 1 mediated endocytosis of beta1-integrin influences cell adhesion and cell migration. Exp Cell Res. 2016;340:102–15. doi: 10.1016/j.yexcr.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? International Journal of Neuropsychopharmacology. 2013;16:691–700. doi: 10.1017/S1461145712001095. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres — re-evaluating the relationship. Nature Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Developmental Biology. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Roebroek AJM, Reekmans S, Lauwers A, Feyaerts N, Smeijers L, Hartmann D. Mutant Lrp1 knock-in mice generated by recombinase-mediated cassette exchange reveal differential importance of the NPXY motifs in the intracellular domain of LRP1 for normal fetal development. Molecular and Cellular Biology. 2006;26:605–616. doi: 10.1128/MCB.26.2.605-616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlmann A, Gotthardt M, Willnow TE, Hammer RE, Herz J. Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nature biotechnology. 1996;14:1562–1565. doi: 10.1038/nbt1196-1562. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Kouiavskaia D, Migliorini M, Robinson S, Saenko EL, Gorlatova N, Li D, Lawrence D, Hyman BT, Weisgraber KH. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res. 2005;46:1721–31. doi: 10.1194/jlr.M500114-JLR200. others. [DOI] [PubMed] [Google Scholar]
- Salicioni AM, Gaultier A, Brownlee C, Cheezum MK, Gonias SL. Low density lipoprotein receptor-related protein-1 promotes beta1 integrin maturation and transport to the cell surface. J Biol Chem. 2004;279:10005–12. doi: 10.1074/jbc.M306625200. [DOI] [PubMed] [Google Scholar]
- Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proceedings of the National Academy of Sciences of the United States of America. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Developmental Biology. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Spijkers PP, Denis CV, Blom AM, Lenting PJ. Cellular uptake of C4b-binding protein is mediated by heparan sulfate proteoglycans and CD91/LDL receptor-related protein. European Journal of Immunology. 2008;38:809–817. doi: 10.1002/eji.200737722. [DOI] [PubMed] [Google Scholar]
- Spuch C, Ortolano S, Navarro C. LRP-1 and LRP-2 receptors function in the membrane neuron. Trafficking mechanisms and proteolytic processing in Alzheimer's disease. Frontiers in Physiology. 2012:3. doi: 10.3389/fphys.2012.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck SE, Meister S, Nahrath J, Meissner JN, Schubert N, Di Spiezio A, Baches S, Vandenbroucke RE, Bouter Y, Prikulis I. Endothelial LRP1 transports amyloid-beta1-42 across the blood-brain barrier. J Clin Invest. 2016;126:123–36. doi: 10.1172/JCI81108. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Faissner A, Gehrig B, Schachner M. Isolation and biochemical characterization of a neural proteoglycan expressing the L5 carbohydrate epitope. Journal of Neurochemistry. 1990;55:1494–1506. doi: 10.1111/j.1471-4159.1990.tb04931.x. [DOI] [PubMed] [Google Scholar]
- Tang L, Wu JJ, Ma Q, Cui T, Andreopoulos FM, Gil J, Valdes J, Davis SC, Li J. Human lactoferrin stimulates skin keratinocyte function and wound re-epithelialization. The British Journal of Dermatology. 2010;163:38–47. doi: 10.1111/j.1365-2133.2010.09748.x. [DOI] [PubMed] [Google Scholar]
- Taverna E, Gotz M, Huttner WB. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annu Rev Cell Dev Biol. 2014;30:465–502. doi: 10.1146/annurev-cellbio-101011-155801. [DOI] [PubMed] [Google Scholar]
- Terada N, Baracskay K, Kinter M, Melrose S, Brophy PJ, Boucheix C, Bjartmar C, Kidd G, Trapp BD. The tetraspanin protein, CD9, is expressed by progenitor cells committed to oligodendrogenesis and is linked to beta1 integrin, CD81, and Tspan-2. Glia. 2002;40:350–9. doi: 10.1002/glia.10134. [DOI] [PubMed] [Google Scholar]
- Terrand J, Bruban V, Zhou L, Gong W, El Asmar Z, May P, Zurhove K, Haffner P, Philippe C, Woldt E. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. The Journal of Biological Chemistry. 2009;284:381–388. doi: 10.1074/jbc.M806538200. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharidis U, Long K, Ffrench-Constant C, Faissner A. Regulation of the neural stem cell compartment by extracellular matrix constituents. Prog Brain Res. 2014;214:3–28. doi: 10.1016/B978-0-444-63486-3.00001-3. [DOI] [PubMed] [Google Scholar]
- Tsen F, Bhatia A, O'Brien K, Cheng C-F, Chen M, Hay N, Stiles B, Woodley DT, Li W. Extracellular heat shock protein 90 signals through subdomain II and the NPVY motif of LRP-1 receptor to Akt1 and Akt2: a circuit essential for promoting skin cell migration in vitro and wound healing in vivo. Molecular and Cellular Biology. 2013;33:4947–4959. doi: 10.1128/MCB.00559-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant C, Michos O, Orolicki S, Brellier F, Taieb S, Moreno E, Té H, Zeller R, Monard D. Protease nexin 1 and its receptor LRP modulate SHH signalling during cerebellar development. Development (Cambridge, England) 2007;134:1745–1754. doi: 10.1242/dev.02840. [DOI] [PubMed] [Google Scholar]
- von Holst A, Sirko S, Faissner A. The unique 473HD-Chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J Neurosci. 2006;26:4082–94. doi: 10.1523/JNEUROSCI.0422-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Silver DL, Thiele C, Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. The Journal of Biological Chemistry. 2001;276:23742–23747. doi: 10.1074/jbc.M102348200. [DOI] [PubMed] [Google Scholar]
- Wang X, Gao M, Schouteden S, Roebroek A, Eggermont K, van Veldhoven PP, Liu G, Peters T, Scharffetter-Kochanek K, Verfaillie CM. Hematopoietic stem/progenitor cells directly contribute to arteriosclerotic progression via integrin beta2. Stem Cells. 2015;33:1230–40. doi: 10.1002/stem.1939. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham H. Inherited disorders of cholesterol biosynthesis. Clinical Genetics. 2002;61:393–403. doi: 10.1034/j.1399-0004.2002.610601.x. [DOI] [PubMed] [Google Scholar]
- Woldt E, Matz RL, Terrand J, Mlih M, Gracia C, Foppolo S, Martin S, Bruban V, Ji J, Velot E. Differential signaling by adaptor molecules LRP1 and ShcA regulates adipogenesis by the insulin-like growth factor-1 receptor. The Journal of Biological Chemistry. 2011;286:16775–16782. doi: 10.1074/jbc.M110.212878. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaji S, Manya H, Nakagawa N, Takematsu H, Endo T, Kannagi R, Yoshihara T, Asano M, Oka S. Major glycan structure underlying expression of the Lewis X epitope in the developing brain is O-mannose-linked glycans on phosphacan/RPTPβ. Glycobiology. 2015;25:376–385. doi: 10.1093/glycob/cwu118. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Boyer AM, Schwarting GA. Fucose-containing glycolipids are stage- and region-specific antigens in developing embryonic brain of rodents. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:3045–3049. doi: 10.1073/pnas.82.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Yamauchi T, Mantuano E, Murakami K, Henry K, Takahashi K, Campana WM. Low-density lipoprotein receptor related protein-1 (LRP1)-dependent cell signaling promotes neurotrophic activity in embryonic sensory neurons. PloS One. 2013;8:e75497. doi: 10.1371/journal.pone.0075497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Taga T, Nakamura K, Ariga T, Yu RK. Characterization of glycoconjugate antigens in mouse embryonic neural precursor cells. Journal of Neurochemistry. 2005;95:1311–1320. doi: 10.1111/j.1471-4159.2005.03452.x. [DOI] [PubMed] [Google Scholar]
- Yoon C, Van Niekerk EA, Henry K, Ishikawa T, Orita S, Tuszynski MH, Campana WM. Low-density lipoprotein receptor-related protein 1 (LRP1)-dependent cell signaling promotes axonal regeneration. The Journal of Biological Chemistry. 2013;288:26557–26568. doi: 10.1074/jbc.M113.478552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Radulovic M, Drucker KL, Wu J, Scarisbrick IA. The thrombin receptor is a critical extracellular switch controlling myelination. Glia. 2015;63:846–859. doi: 10.1002/glia.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RK, Suzuki Y, Yanagisawa M. Membrane glycolipids in stem cells. FEBS letters. 2010;584:1694–1699. doi: 10.1016/j.febslet.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Takayama Y, Boucher P, Tallquist MD, Herz J. LRP1 regulates architecture of the vascular wall by controlling PDGFRbeta-dependent phosphatidylinositol 3-kinase activation. PloS One. 2009;4:e6922. doi: 10.1371/journal.pone.0006922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T-B. Signaling pathways of apoE and its role of gene expression in glomerulus diseases. Journal of Receptor and Signal Transduction Research. 2013;33:73–78. doi: 10.3109/10799893.2013.765466. [DOI] [PubMed] [Google Scholar]