Abstract
The human ether-a-go-go-related gene (hERG) encodes the pore-forming subunit of the rapidly activating delayed rectifier potassium channel (IKr), which is important for cardiac repolarization. Dysfunction of hERG causes long QT syndrome and sudden death, which occur in patients with cardiac ischemia. Cardiac ischemia is also associated with activation, up-regulation, and secretion of various proteolytic enzymes. Here, using whole-cell patch clamp and Western blotting analysis, we demonstrate that the hERG/IKr channel was selectively cleaved by the serine protease, proteinase K (PK). Using molecular biology techniques including making a chimeric channel between protease-sensitive hERG and insensitive human ether-a-go-go (hEAG), as well as application of the scorpion toxin BeKm-1, we identified that the S5-pore linker of hERG is the target domain for proteinase K cleavage. To investigate the physiological relevance of the unique susceptibility of hERG to proteases, we show that cardiac ischemia in a rabbit model was associated with a reduction in mature ERG expression and an increase in the expression of several proteases, including calpain. Using cell biology approaches, we found that calpain-1 was actively released into the extracellular milieu and cleaved hERG at the S5-pore linker. Using protease cleavage-predicting software and site-directed mutagenesis, we identified that calpain-1 cleaves hERG at position Gly-603 in the S5-pore linker of hERG. Clarification of protease-mediated damage of hERG extends our understanding of hERG regulation. Damage of hERG mediated by proteases such as calpain may contribute to ischemia-associated QT prolongation and sudden cardiac death.
Keywords: cell surface protein, electrophysiology, hERG, ion channel, patch clamp, potassium channel, protease
Introduction
Coordinated ion channel activity controls the rhythmic heartbeat, and dysfunction of ion channels causes cardiac arrhythmias and sudden death (1). The human ether-a-go-go-related gene (hERG)2 encodes the pore-forming subunit of the rapidly activating delayed rectifier K+ channel (IKr), which plays an important role in the repolarization of cardiac action potentials (2, 3). The ventricular action potential duration is reflected by the QT interval on an electrocardiogram. Reduction of hERG current (IhERG) prolongs the action potential and causes long QT syndrome (LQTS), a cardiac electrical disorder that predisposes individuals to the potentially fatal ventricular arrhythmia torsades de pointes and sudden death (1, 4). Many loss-of-function mutations in hERG have been identified in humans, which decrease IKr and cause type 2 inherited LQTS (5). Dysfunction of hERG also represents a primary cause of acquired LQTS. For example, hERG is preferentially targeted by a large number of drugs, giving rise to drug-induced LQTS (1). Moreover, we have shown that a reduction in the serum K+ concentration (hypokalemia) selectively decreases hERG function and expression, leading to LQTS (6). We demonstrated that the hERG channel requires extracellular K+ to maintain the function and membrane stability (7).
The S5-pore linker of the hERG channel is unusually long (43 amino acids) compared with the linkers of other voltage-gated K+ (Kv) channels (10–23 amino acids) (8, 9). It is exposed to the extracellular side of the membrane and contains the unique binding domain for scorpion toxins (e.g. BeKm-1) that selectively block hERG channels with an IC50 in low nanomolar concentrations (10–12). It was demonstrated that the S5-pore linker of hERG is the selective target domain for cleavage by experimental serine proteases, including PK and protease XIV and XXIV (13). However, it is not known whether hERG/IKr is also susceptible to proteases under physiological and/or pathophysiological conditions.
Ischemic heart disease is a leading cause of death, and it is the major cause of sudden cardiac death worldwide (14). Cardiac ischemia activates various proteases, such as the cysteine protease calpain (15, 16) (which are actively secreted into the extracellular milieu (17–19)), serine proteases such as membrane-anchored matriptase-2 (20, 21), and matrix metalloproteinases (MMPs) (22, 23). Protease-mediated cleavage of various cell surface proteins has been reported (24). Although many factors may contribute to sudden death in ischemic heart disease, the finding that QT prolongation occurs in many ischemic cases has changed the classical concept of the cardiac ischemia cascade (25). The QT interval is abnormally prolonged in patients with unstable angina (26) and acute myocardial infarction (27). The extent of QT prolongation was shown to be an independent predictor of sudden cardiac death (27, 28). It is currently unknown how cardiac ischemia causes QT prolongation.
In the present study, we demonstrate that hERG expressed in HEK cells and IKr in cardiomyocytes are uniquely susceptible to extracellular protease-mediated damage. We show that the cleavage site is located in the extracellular S5-pore linker. We show that replacing the S5-pore linker of hERG with that of human EAG (hEAG) completely eliminated the PK sensitivity of hERG channels. As well, application of the hERG S5-pore linker binding scorpion toxin BeKm-1 effectively protected ERG channels from protease-mediated cleavage. Furthermore, our data show that coronary ligation-induced cardiac ischemia in rabbits led to a selective reduction in mature ERG protein expression with a concomitant increase in the cysteine protease calpain, the serine protease matriptase-2, and the matrix metalloproteinase MMP-2 expression. In particular, we found that transfection of calpain-1 into HEK cells led to a drastic increase in the expression of calpain-1 in the culture medium, and transfer of this medium to hERG-expressing HEK cells resulted in a reduction in mature hERG channel expression and IhERG. Finally, we identified that calpain-1 cleaves mature hERG in the S5-pore linker. Using protease cleavage-predicting software (PROSPER) and site-directed mutagenesis in the S5-pore linker, we identified that calpain-1 cleaves mature hERG at amino acid position Gly-603. Recognition of the unique susceptibility of hERG channels to extracellular proteases extends our understanding of hERG regulation as well as the development of QT prolongation and arrhythmias in pathophysiological conditions such as cardiac ischemia.
Results
The hERG/IKr Channel Displays Unique Sensitivity to Proteolytic Degradation
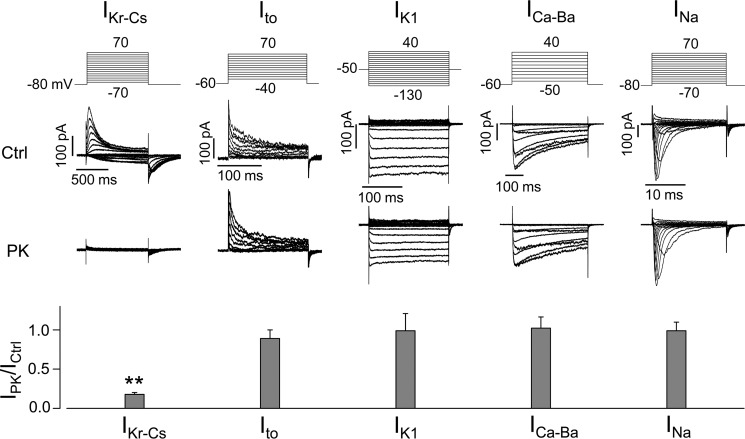
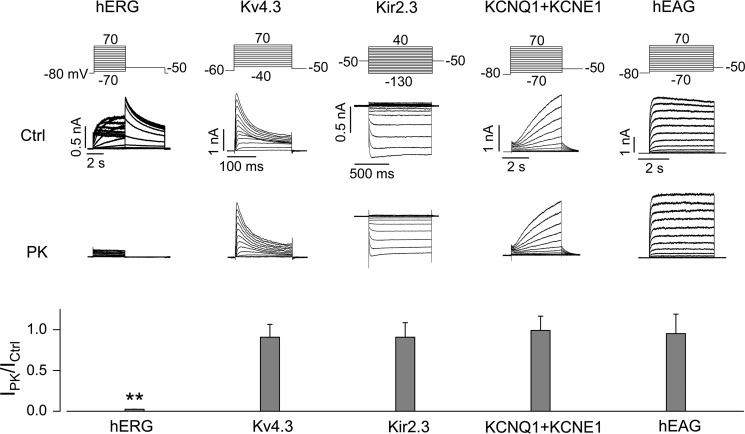
We examined the effects of PK treatment on IKr, Ito (transient outward K+ current), IK1 (inward rectifier K+ current), ICa-Ba (Ba2+-mediated L-type Ca2+ channel current), and INa (Na+ current) in ventricular myocytes from neonatal rats. Ventricular myocytes were isolated and cultured on glass coverslips for 24 h. Cells were treated with PK (200 μg/ml) in the culture medium at 37 °C for 20 min and then transferred to the recording chamber for current recordings using the whole-cell patch clamp method (6, 7, 29). Whereas PK treatment nearly eliminated IKr-Cs (Cs+-mediated IKr), it had no effect on Ito, IK1, ICa-Ba, or INa (Fig. 1). To further understand the specificity of proteases on K+ channels, we studied the effects of PK on hERG, Kv4.3 (encoding Ito), Kir2.3 (encoding IK1), KCNQ1 + KCNE1 (encoding IKs), and hEAG (hERG-related K+ channel) channels stably expressed in HEK 293 cells. K+ channel-expressing HEK cells were treated with PK (200 μg/ml) in the culture medium at 37 °C for 20 min. The cells were then collected for whole-cell patch clamp analysis. As shown in Fig. 2, PK treatment selectively eliminated IhERG without affecting other channels.
FIGURE 1.
Effects of PK on various currents of native channels in isolated neonatal rat cardiomyocytes. Families of Cs+-mediated IKr (IKr-Cs), Ito, IK1, Ba2+-mediated L-type Ca2+ channel current (ICa-Ba), and INa recorded in control (Ctrl) and PK (200 μg/ml, 20 min)-treated neonatal rat ventricular myocytes along with the voltage protocols are shown. Specific native ion channel currents recorded from each PK-treated cell are normalized to the mean value of the corresponding currents recorded from control cells and summarized in the bar graph below the representative currents. **, p < 0.01 versus Ctrl; n = 8–11 cells from three independent treatments for each channel. Error bars, S.E.
FIGURE 2.
Effects of PK on various currents of potassium channels stably expressed in HEK 293 cells. Families of hERG, Kv4.3, Kir2.3, KCNQ1 + KCNE1, and hEAG currents in control (Ctrl) and PK (200 μg/ml, 20 min)-treated cells along with the voltage protocols are shown. Specific channel currents recorded from each PK-treated cell are normalized to the mean value of corresponding currents recorded from control cells and summarized in the bar graph below the representative currents. **, p < 0.01 versus control. n = 7–16 cells from three independent treatments for each channel. Error bars, S.E.
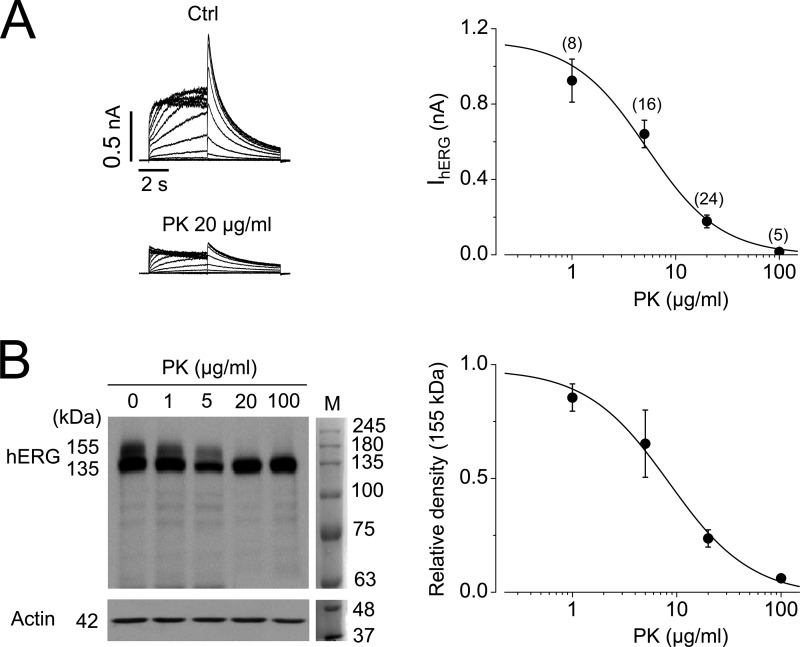
The use of PK (e.g. 200 μg/ml, 20 min) as an experimental tool to cleave cell surface hERG and Kv1.5 channels has been previously established by our laboratory and others (29–32). To investigate the potential physiological relevance of protease-mediated hERG damage, we cultured stably hERG-expressing HEK (hERG-HEK) cells with various concentrations of PK (1, 5, 20, and 100 μg/ml) in the culture medium (minimum essential medium (MEM) supplemented with 10% FBS) for 12 h. The cells were then collected for patch clamp or Western blotting analysis. As shown in Fig. 3A, PK reduced IhERG in a concentration-dependent manner with an EC50 of 5.9 μg/ml. To determine whether the PK-mediated reduction of IhERG was a result of cell surface cleavage of mature hERG channels, we performed Western blotting analysis of whole cell lysate in control and PK-treated cells. Normal hERG proteins on Western blots display two bands with molecular masses of 155 and 135 kDa. Using various techniques, including biotin-mediated cell surface protein analysis, we and others have established that, for hERG proteins extracted from cells under normal culture conditions, the 155 kDa band reflects the mature, fully glycosylated channels localized in the plasma membrane, and the 135 kDa band reflects the immature, core-glycosylated hERG channels localized intracellularly (6, 13, 29–31, 33, 34). As shown in Fig. 3B, PK treatment for 12 h led to a concentration-dependent reduction of the 155-kDa hERG band with an EC50 of 8.4 μg/ml. However, PK treatment did not affect the intracellularly localized 135-kDa (immature) hERG proteins. These data indicate that PK-mediated cleavage of mature hERG protein is responsible for the reduced IhERG.
FIGURE 3.
Concentration-dependent effects of 12-h treatment with PK on hERG channels expressed in HEK cells cultured in standard medium (MEM plus 10% FBS). A, concentration-dependent effects of PK for 12 h on IhERG. Left, hERG current traces in control (Ctrl) or 20 μg/ml PK-treated cells. Right, IhERG plotted against PK concentrations and fit to the Hill equation. EC50 = 5.9 μg/ml, Hill coefficient = 1.0. (Numbers above each dot represent number of cells examined from four independent treatments.) B, concentration-dependent effects of PK for 12 h on hERG expression. Left, representative Western blot demonstrating hERG expression after 12-h PK treatment at various concentrations. Actin is used as a loading control. M, molecular weight marker. Right, the 155 kDa band intensities normalized to the control (0) and fit to the Hill equation. EC50 = 8.4 μg/ml, Hill coefficient = 1.1 (n = 3). Error bars, S.E.
The Proteolytic Site Is in the S5-pore Linker of hERG Channels
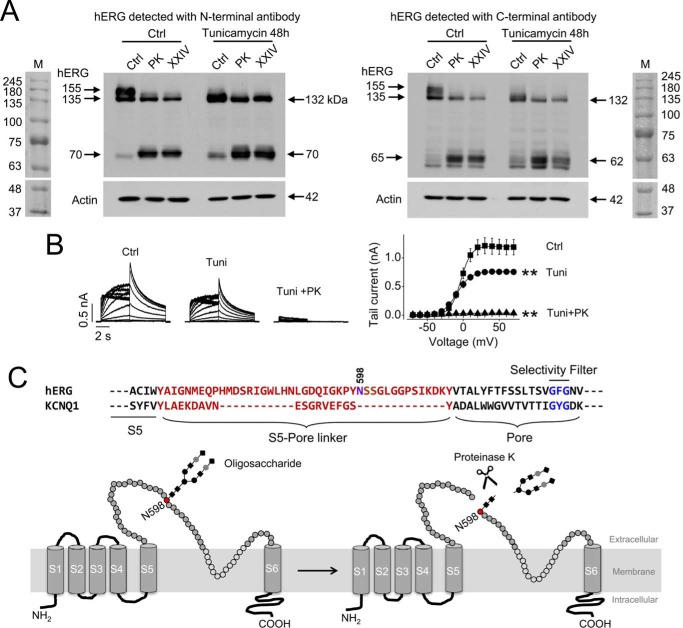
To investigate the PK cleavage site of hERG channels, we analyzed hERG degradation products from hERG-HEK cells treated with PK (200 μg/ml) at 37 °C for 20 min. Because proteinase K cannot permeate the cell membrane, it has been used as a tool to cleave cell surface protein to identify the plasma membrane-localized hERG channel proteins (29–31). As expected, PK treatment selectively abolished the 155-kDa hERG band without affecting the 135-kDa hERG band (Fig. 4A). The disappearance of the 155 kDa band was accompanied by the appearance of a 70 kDa band fragment when an N terminus-targeting hERG (N-20) antibody was used (Fig. 4A, left). When a C terminus-targeting hERG antibody (C-20) was used, the elimination of the 155 kDa band of hERG was accompanied by the appearance of a 65 kDa band fragment (Fig. 4A, right).
FIGURE 4.
PK cleavage of hERG channels. A, detection of hERG expression after PK (200 μg/ml, 20 min) or protease XXIV (XXIV; 200 μg/ml, 20 min) treatment in hERG-HEK cells in control (Ctrl) conditions or following culture with tunicamycin (10 μg/ml) for 48 h. hERG expression was detected using an N-terminal anti-hERG antibody (N-20, left) or a C-terminal anti-hERG antibody (C-20, right). M, molecular weight marker. B, families of hERG currents from control cells, tunicamycin-treated cells without or with PK treatment (left), and the summarized activation curves (right) in each condition. **, p < 0.01 versus control, maximal tail currents (n = 6 cells in each group). C, amino acid sequence alignment of S5-pore linker and pore region of hERG and KCNQ1. The S5-pore linkers of both channels are highlighted in red. The pore region and selectivity filter of each channel are highlighted in black and blue, respectively. The N-linked glycosylation site of hERG is marked at position 598. Below the sequences is an illustration depicting the potential site for PK-mediated cleavage. The S5-pore linker with N-linked oligosaccharide before and after PK cut is shown. Error bars, S.E.
Extracellularly applied proteases may cleave hERG channels by targeting the S1-S2 linker, the S3-S4 linker, the S5-pore linker, or the pore-S6 linker. Structurally, whereas the S5-pore linker contains 43 amino acid residues, the S1-S2, S3-S4, and pore-S6 linkers contain about 26, 4, and 6 amino acid residues, respectively (UniProt, Q12809). The long S5-pore linker is approximately in the middle of the channel protein sequence. Based on ExPASy-ProtParam Tool (software) analysis of cleavage products by PK, the cleavage site is predicted to be in the S5-pore linker (Fig. 4).
The combined molecular mass of the N-terminal and C-terminal fragments after PK digestion is 135 kDa (70 + 65 kDa), which is 20 kDa less than the mature 155-kDa hERG protein that has been cleaved. Two possibilities may account for the missing 20-kDa fragment. First, a small peptide fragment of 20 kDa could be digested from the channel during cleavage. Second, an N-linked oligosaccharide at position Asn-598 in the S5-pore linker, which results from full glycosylation during channel maturation, may be cleaved off during digestion. The second possibility was previously proposed by Rajamani et al. (13). Our data as described below also support the notion that N-linked oligosaccharide in the S5-pore linker is cleaved off during PK digestion.
Under normal culture/physiological conditions, the hERG subunit is synthesized as a 132-kDa protein and undergoes core glycosylation with the addition of a 3-kDa glycan to become the 135-kDa immature product in the endoplasmic reticulum. During maturation, the core-glycosylated (135-kDa) hERG undergoes complex glycosylation in the Golgi apparatus with the addition of a 20-kDa oligosaccharide to become the 155-kDa mature protein, which is transported to the plasma membrane and has a half-life of ∼11 h (35, 36). The glycosylation of hERG channels has been shown to be N-linked at position Asn-598 and can be prevented by tunicamycin treatment or by the N598Q mutation (13, 35). It has been shown that glycosylation is not required for the cell surface expression of functional hERG channels (35). Thus, after prevention of N-linked glycosylation by either tunicamycin treatment or N598Q mutation, there is neither mature protein at 155 kDa nor immature protein at 135 kDa; instead, there is mature protein at 132 kDa localized in the plasma membrane and immature protein also at 132 kDa localized intracellularly (13, 35). Consistently, our data show that treatment of hERG-HEK cells with tunicamycin (10 μg/ml) for 48 h resulted in hERG proteins that display a single band at 132 kDa (Fig. 4A, right side of each panel). Tunicamycin-treated cells with only 132-kDa hERG proteins still display hERG current, indicating that a portion of 132-kDa protein represents mature functional channels. Removal of glycosylation did not affect the half-activation voltage of IhERG (control, −3.5 ± 1.5 mV (n = 6); tunicamycin, −8.9 ± 3.4 mV (n = 6); p > 0.05). However, it increased the slope factor of the activation curve of IhERG (control, 6.8 ± 0.2 mV (n = 6); tunicamycin, 8.7 ± 0.4 mV (n = 6); p < 0.01) and reduced the IhERG amplitude by 37% (control, 1.21 ± 0.14 nA (n = 6); tunicamycin, 0.76 ± 0.05 nA (n = 6); p < 0.01). Cleavage of tunicamycin-treated cells with PK abolished IhERG (Fig. 4B) and decreased the intensity of the 132 kDa band, indicating that the mature cell surface 132-kDa hERG channels had been cleaved, and the immature intracellularly localized 132-kDa hERG channels were not affected (13). Cleavage of the cell surface 132-kDa hERG produced a 70-kDa N-terminal fragment and a 62-kDa C-terminal fragment (Fig. 4A). Because the fragments from normal (glycosylated) and tunicamycin-treated (unglycosylated) hERG channels are similar in size, we conclude that PK treatment removes the oligosaccharide at Asn-598 while cleaving hERG (Fig. 4C). The digestion site for protease XXIV in the hERG channel is similar to that of PK, as shown in Fig. 4A.
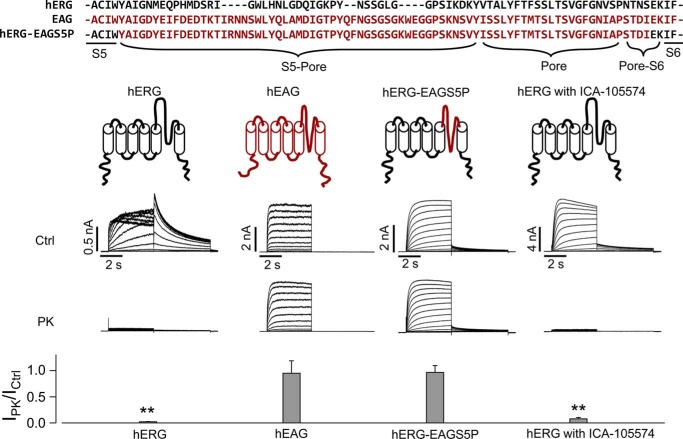
To directly address the notion that the S5-pore linker of hERG is the region cleaved by proteases, we used a hERG-EAG chimeric channel in which the S5-pore linker of hERG was replaced by the S5-pore linker of EAG (37) (Fig. 5). Our data show that neither hEAG (Fig. 2) nor bovine EAG (bEAG) (data not shown) was sensitive to PK. The whole amino acid sequence of bEAG shares a 96% homology with hEAG. Furthermore, the amino acid sequences of the S5-pore of hEAG and bEAG are 100% identical. We named the mutant hERG channel with the EAG S5-pore linker hERG-EAGS5P. As shown in Fig. 5, hERG-EAGS5P was completely resistant to PK. Unlike hERG, the mutant hERG-EAGS5P did not inactivate. However, the insensitivity of hERG-EAGS5P to proteases was not a result of deficient inactivation, because hERG channels with inactivation removed by ICA-105574 (38) were still sensitive to PK cleavage (Fig. 5).
FIGURE 5.
The proteolytic site for PK is in the S5-pore linker of hERG channels. Amino acid sequence alignment of the S5-pore linker and pore region of hERG, EAG, and hERG-EAGS5P channels along with the channel topologies are shown above the current traces. hERG and EAG are illustrated in black and red, respectively. Current traces of hERG, hEAG, hERG-EAGS5P, and hERG in the presence of 30 μm ICA-105574 in control (Ctrl) or PK-treated cells are shown. Specific currents recorded from each PK-treated cell are normalized to the mean value of the corresponding currents recorded from control cells and summarized in the bar graph below the current traces. **, p < 0.01. n = 7–16 cells from three independent treatments for each channel. Error bars, S.E.
The BeKm-1 Peptide Protects hERG from Protease Cleavage
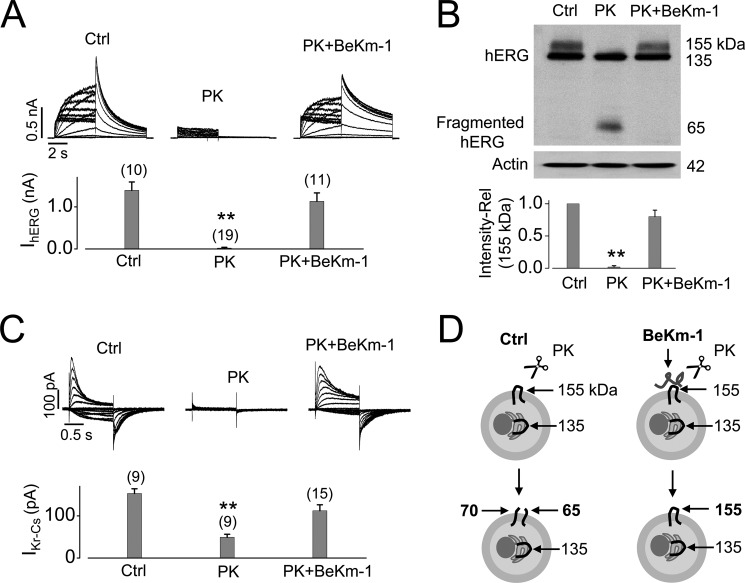
To further confirm that the S5-pore linker of hERG is the protease cleavage site and to explore means to protect hERG from protease cleavage, we examined the protective effects of the scorpion toxin BeKm-1 on PK-mediated hERG cleavage. BeKm-1 is a peptide that selectively binds to the S5-pore linker of hERG with a dissociation constant in low nanomolar concentrations (10–12). We discovered that BeKm-1 effectively prevented PK-mediated cleavage of hERG channels. As shown in Fig. 6A, whereas PK treatment of hERG-HEK cells completely eliminated IhERG, the same treatment in the presence of BeKm-1 (1 μm) did not affect IhERG. As shown in Fig. 6B, the BeKm-1 protection of hERG from PK cleavage was also confirmed by Western blotting analysis. Furthermore, in isolated cardiac myocytes, BeKm-1 also effectively protected IKr from PK-mediated cleavage (Fig. 6C). Because BeKm-1 selectively binds to the hERG S5-pore linker, we propose that such binding probably shields the linker from digestion by proteases such as PK (Fig. 6D).
FIGURE 6.
The scorpion toxin BeKm-1 protects ERG channels against PK-mediated cleavage. A, hERG current traces in control (Ctrl) or PK-treated (200 μg/ml, 20 min) hERG-HEK cells in the absence or presence of BeKm-1 (1 μm). IhERG is summarized in the bar graph below the current traces for each group. The numbers above the bar graphs indicate the number of cells examined from three independent experiments. B, Western blot demonstrating hERG expression in control or PK-treated hERG-HEK cells in the absence or presence of BeKm-1 (1 μm). hERG was detected using an anti-hERG antibody targeting the C terminus. The band intensities of the 155-kDa hERG in PK-treated cells are normalized to that of control and summarized below the Western blot image (n = 4). C, families of Cs+-mediated IKr in neonatal rat ventricular myocytes in control or in PK treatment with or without BeKm-1 (1 μm). The maximal tail currents upon repolarization to −80 mV are summarized below the current traces. The numbers above the bar graphs indicate the number of cells examined from three independent experiments. D, diagram illustrating that BeKm-1 protects the hERG S5-pore linker from being digested by PK. **, p < 0.01 versus control. Error bars, S.E.
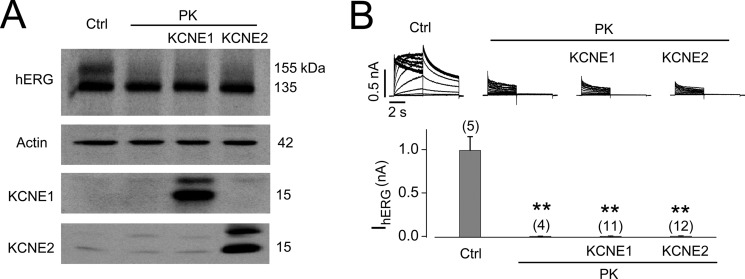
It has been reported that KCNE1 (minK) or KCNE2 (MiRP) serves as subunits that associate with hERG to form functional channels (39, 40). To determine whether KCNE1 or KCNE2 is protective against PK cleavage of hERG, we transfected empty pcDNA3 vector (control), KCNE1, or KCNE2 plasmid into hERG-HEK cells. 24 h after transfection, cells were treated with PK (200 μg/ml) for 20 min at 37 °C. Treated cells were subsequently subjected to Western blotting and patch clamp analyses. As shown in Fig. 7, neither KCNE1 nor KCNE2 prevented PK-mediated degradation of hERG channels. This finding is consistent with the observation that IKr in ventricular myocytes that may contain KCNE1 and KCNE2 is still susceptible to PK digestion, as shown in the present study (Fig. 1) as well as reported previously (13).
FIGURE 7.
Neither KCNE1 nor KCNE2 protects hERG channels against PK-mediated cleavage. A, Western blot demonstrating hERG expression in control (Ctrl) or PK-treated hERG-HEK cells without or with KCNE1 or KCNE2 expression (n = 3). B, IhERG recorded from control or PK-treated hERG-HEK cells without or with KCNE1 or KCNE2 expression. Summarized IhERG amplitude is shown below the current traces. The numbers above the bar graphs indicate the number of cells examined from two independent experiments. **, p < 0.01 versus control. Error bars, S.E.
Cardiac Ischemia Reduces Mature ERG Channel Expression and Up-regulates Proteases
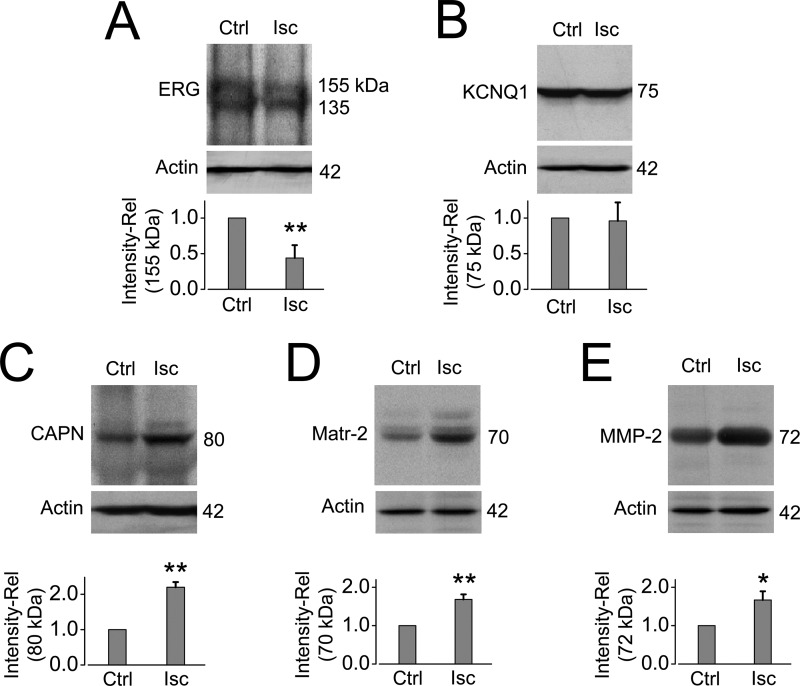
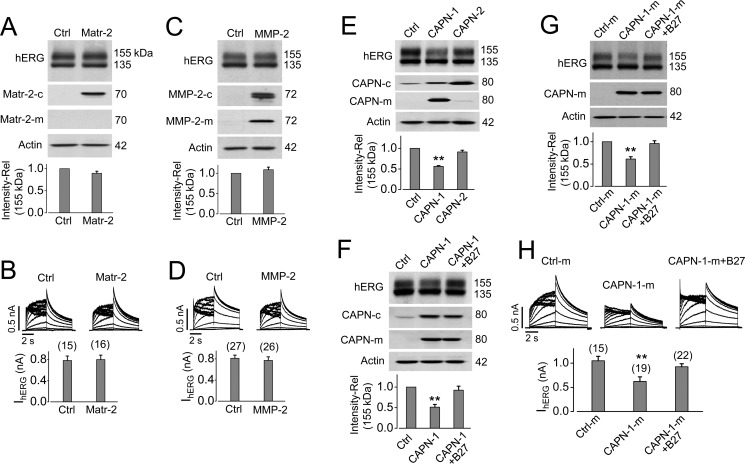
PK is frequently used as an experimental tool to determine the cell surface localization of ion channels and other proteins (29–32). To investigate whether hERG is also susceptible to proteases elevated under certain pathophysiological conditions, such as ischemia (41), we induced cardiac ischemia in rabbits via left circumflex coronary artery ligation and isolated the ventricular tissue for Western blotting analysis 24 h after ligation. Our data show that the mature ERG channel expression was significantly reduced in the ischemic ventricular tissues (Fig. 8A), and this reduction was selective to ERG, because the KCNQ1 channel was unaffected (Fig. 8B). Along with reduced ERG, cardiac ischemia led to a significant up-regulation of the cysteine protease calpain (Fig. 8C), the membrane-anchored serine protease matriptase-2 (Fig. 8D), and matrix metalloproteinase MMP-2 (Fig. 8E). In fact, the activation and up-regulation of these proteases in ischemic conditions have been previously reported (15, 21–23, 41). We chose to focus on these three proteases because calpain and MMP have been reported to secrete into the extracellular milieu (41), whereas matriptase-2 is located in the plasma membrane with its catalytic head functioning extracellularly (42).
FIGURE 8.
Cardiac ischemia in rabbits reduces mature ERG protein expression and increases the expression of calpain, matriptase-2, and MMP-2. A and B, Western blots of ventricular tissue from control (Ctrl) or ischemic (Isc) rabbit hearts showing that 24-h ischemia significantly decreases the 155-kDa ERG expression (n = 3) but not KCNQ1 expression (n = 3). C–E, Western blots demonstrating that ventricular tissue from ischemic rabbit hearts contains significantly elevated levels of calpain (CAPN; n = 4), matriptase-2 (Matr-2; n = 4), and MMP-2 (n = 6) compared with control. Actin was used as a loading control. The band intensities are normalized to their respective controls and shown as relative values below corresponding Western blot images. *, p < 0.05 versus control; **, p < 0.01 versus control. Error bars, S.E.
Calpain-1 Secretion Cleaves Mature hERG Extracellularly
To determine whether matriptase-2, MMP-2, and calpain cleave hERG channels, we transfected their respective plasmids into hERG-HEK cells. 24 h after transfection, whole-cell patch clamp and Western blotting analysis were performed to determine the effect of these proteases on hERG channels. Our data with Western blotting analysis show that matriptase-2 was primarily expressed in the cell lysate with undetectable expression levels in the culture medium (Fig. 9A). Neither hERG expression (Fig. 9A) nor IhERG (Fig. 9B) was affected by matriptase-2 overexpression. Transfection of MMP-2 plasmid led to a significant expression of MMP-2 in the cell lysate and culture medium (Fig. 9C). Overexpression of MMP-2 had no effect on either hERG expression (Fig. 9C) or IhERG (Fig. 9D).
FIGURE 9.
Extracellular calpain-1 decreases mature hERG expression and IhERG. A and B, Matriptase-2 does not affect hERG expression or IhERG. hERG-HEK cells were transfected with pcDNA3 (control (Ctrl)) or matriptase-2 (Matr-2). Expression of hERG and matriptase-2 from whole-cell lysate (Matr-2-c), and matriptase-2 from culture medium (Matr-2-m) are shown in A, whereas IhERG from cells transfected with pcDNA3 (control) or matriptase-2 plasmid are shown in B. C and D, MMP-2 does not affect hERG expression or IhERG. Expression of hERG and MMP-2 from whole-cell lysate (MMP-2-c) and MMP-2 from culture medium (MMP-2-m) are shown in C, whereas IhERG from cells transfected with pcDNA3 (control) or MMP-2 plasmid is shown in D. E, calpain-1 (CAPN-1), but not calpain-2 (CAPN-2), decreases mature hERG expression. Expression of hERG, CAPN-1, or CAPN-2 from whole-cell lysate (CAPN-c) and CAPN-1 and CAPN-2 from culture medium (CAPN-m) are shown. CAPN-1 but not CAPN-2 transfection led to its elevated expression in culture medium, which was associated with a decrease in the 155-kDa hERG band. F, cell membrane-impermeant calpain inhibitor peptide B27 (1 μm) abolishes CAPN-1-mediated hERG reduction. G and H, the culture medium of calpain-1-transfected cells decreases mature hERG expression and IhERG, and these effects are abolished by the calpain inhibitor peptide B27. For Western blotting analysis of hERG expression in A, C, E, F, and G, the intensities of the 155 kDa band are normalized to the control and expressed as relative values (n = 3–7). Actin was used as a loading control. For IhERG analyses in B, D, and H, tail current amplitudes under each treatment are summarized below representative current traces. The numbers above the bar graphs indicate the number of cells examined from 3–5 independent experiments. **, p < 0.01 versus control. Error bars, S.E.
There are two main isoforms of calpain, calpain-1 (μ-calpain) and calpain-2 (m-calpain) (43). The anti-calpain antibody that we used (sc-30064, Santa Cruz Biotechnology, Inc.) detects both calpain-1 and calpain-2. To investigate the effects of calpain-1 and calpain-2 on hERG channels, we transfected their respective plasmids (CAPN1, SC116897; CAPN2, SC119079 (OriGene); pcDNA3 empty vector as control) into hERG-HEK cells. 24 h after transfection, cells were collected for Western blotting and patch clamp analyses. As shown in Fig. 9E, calpain-1, but not calpain-2, significantly reduced the mature (155-kDa) hERG channel expression, whereas the immature (135-kDa) hERG expression was not affected. Interestingly, besides the elevated expression of calpain-1 and calpain-2 in the cell lysate, a significant level of calpain-1, but not calpain-2, was observed in the culture medium of the transfected cells (Fig. 9E). These observations prompted us to hypothesize that the secreted calpain-1 in the culture medium is responsible for the reduction in mature hERG expression. Consistent with this hypothesis, our data show that the treatment of membrane-impermeant calpain inhibitor peptide B27 (1 μm) completely prevented the calpain-1-induced hERG reduction (Fig. 9F). To further demonstrate that secreted calpain-1 is responsible for the mature hERG reduction, we transfected HEK cells with either pcDNA3 or calpain-1. 24 h after transfection, culture medium of transfected cells were collected and applied to non-transfected hERG-HEK cells for 12 h. Medium from calpain-1-transfected cells (CAPN-1-m) reduced the mature hERG protein expression (Fig. 9G) and IhERG (Fig. 9H). Again, the presence of the calpain inhibitor peptide B27 abolished the CAPN-1-m-mediated reduction in mature hERG expression (Fig. 9G) and IhERG (Fig. 9H).
Calpain-1 Cleaves hERG in the S5-pore Linker at Amino Acid Position Gly-603
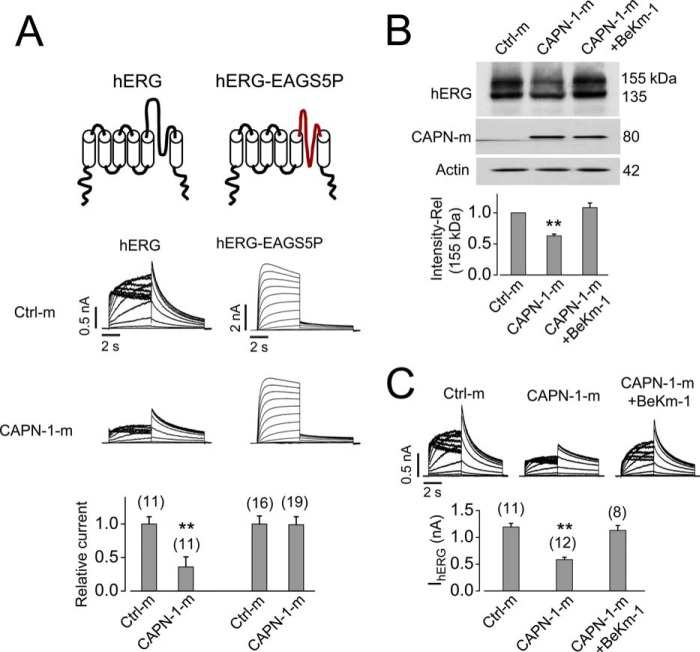
To determine whether the S5-pore linker is the region susceptible to calpain-1 cleavage, we examined the effects of calpain-1 on the mutant hERG-EAGS5P. We collected the culture medium from HEK cells 24 h after transfection with pcDNA3 (Ctrl-m) or CAPN-1 plasmid (CAPN-1-m) and applied it to HEK cells expressing WT hERG or mutant hERG-EAGS5P for 12 h. As shown in Fig. 10A, whereas calpain-1-m reduced IhERG, it had no effect on IhERG-EAGS5P. Furthermore, BeKm-1 (1 μm), which binds to the S5-pore linker, abolished the calpain-1-m-induced reduction in mature hERG expression (Fig. 10B) and IhERG (Fig. 10C).
FIGURE 10.
Calpain-1 decreases hERG through targeting the extracellular S5-pore linker. A, calpain-1 decreases hERG but not hERG-EAGS5P current. 24 h after transfection of pcDNA3- (control) or CAPN-1 into HEK cells, transfected cell medium (Ctrl-m or CAPN-1-m) was collected and applied to hERG-HEK cells for 12 h before patch clamp analyses. Topologies of a single subunit of hERG and hERG-EAGS5P are shown at the top. Representative currents of hERG or hERG-EAGS5P channels with Ctrl-m or CAPN-1-m are shown in the middle. The current of hERG or hERG-EAGS5P from each cell under all conditions is normalized to its mean value from cells cultured with Ctrl-m and summarized below the current traces. B and C, BeKm-1 (1 μm) abolishes CAPN-1-m-mediated reduction in mature hERG protein expression (B) and IhERG (C). Ctrl-m or CAPN-1-m were applied to hERG-HEK cells cultured in the absence or presence of BeKm-1 (1 μm) for 12 h. For Western blotting analysis, intensities of the 155-kDa hERG band from cells cultured with CAPN-1-m in the absence or presence of BeKm-1 (1 μm) were normalized to the control (Ctrl-m) in each gel and then summarized (n = 4). Actin was used as a loading control. For patch clamp analysis, summarized tail current amplitudes from each group of cells are shown below the representative current traces. The numbers above the bar graphs indicate the number of cells examined from three independent treatments. **, p < 0.01 versus control. Error bars, S.E.
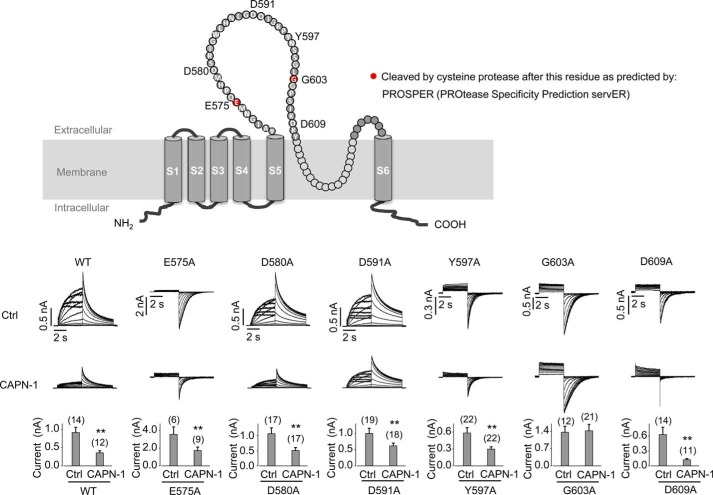
To determine the exact calpain-1 cleavage site, we analyzed the hERG S5-pore linker using PROSPER (Protease Specificity Prediction Server (44), an integrated feature-based tool for predicting substrate cleavage sites by proteases). Two cleavage sites for cysteine proteases were predicted: GNME↓QPHM and SGLG↓GPSI (Fig. 11, top). We thus constructed E575A and G603A mutant hERG channels to disrupt the predicted cleavage sites as indicated by PROSPER analysis. Additional random alanine-scanning mutations in the S5-pore linker were performed, and those expressing functional channels were included in the analysis as negative controls. Overall, WT, E575A, D580A, D591A, Y597A, G603A, or D609A mutant hERG plasmid was transfected to HEK cells along with empty pcDNA3 (control) or calpain-1 plasmid. GFP was also included in the transfection to identify transfected cells for electrophysiological recordings, which were performed 24 h after transfection. As shown in Fig. 11, WT as well as D580A, D591A, Y597A, and D609A mutant hERG channels remained sensitive to calpain-1 digestion. Between the two predicted calpain cleavage sites (E575A and G603A), whereas the E575A mutant remained sensitive to calpain-1 cleavage, the G603A completely abolished the calpain-1-mediated hERG reduction (Fig. 11, bottom). Thus, we conclude that calpain-1 cleaves hERG in the extracellular S5-pore linker specifically at the amino acid Gly-603.
FIGURE 11.
Extracellular calpain-1 cleaves hERG at amino acid position Gly-603 in the S5-pore linker. Top, topological illustration of the amino acid sequence of the hERG S5-pore linker and predicted cysteine protease cleavage sites generated by PROSPER. Bottom, representative current traces along with the summarized current amplitudes of WT and mutant hERG channels from pcDNA3 (Ctrl)- or calpain-1 (CAPN-1)-transfected cells. For WT, D580A, and D591A, the 5 mm K+ bath solution and the voltage protocol shown in Fig. 2 for hERG were used. The tail currents at −50 mV were used for measuring the current amplitudes. For E575A, Y597A, G603A, and D609A, due to their small outward currents in 5 mm K+-containing bath solution, their currents were recorded in a 135 mm K+-containing bath solution. The membrane was held at −80 mV, and 4-s depolarizing steps to voltages between −70 and 70 mV in 10-mV increments were applied at a pulse interval of 15 s. The inward tail currents on the repolarization steps to −80 mV after a 50-mV depolarization were used for analysis of the current amplitude. The numbers above the bar graphs indicate the number of cells examined from 3–5 independent experiments. **, p < 0.01 versus control. Error bars, S.E.
Discussion
hERG/IKr is important for human ventricular repolarization (1, 4). The unique susceptibility of hERG/IKr to extracellular proteases was indicated during cardiomyocyte isolation for many years. To record and analyze native IKr and other cardiac channel currents, isolation of single cardiac myocytes is commonly performed using enzymatic digestion and dissociation (45–47). An unusual proteolytic degradation of hERG has been suggested in some of these studies. Enzymes that are used for cardiac myocyte isolation include collagenase, protease XIV, protease XXIV, trypsin, etc. (45–47). Despite the fact that hERG is the predominant channel for human cardiac repolarization and its defects cause LQTS (1, 4), an absence of IKr in ventricular myocytes isolated from human tissues has been reported by Beuckelmann et al. (48). Similarly, Schaffer et al. (49) reported that IKr was absent in 32 of 34 ventricular myocytes isolated from human tissue. As well, Konarzewska et al. (50) reported that the delayed rectifier K+ current (IK) was not measurable, but inward rectifier K+ current IK1 and transient outward K+ current Ito were robust in human ventricular myocytes. They speculated that the use of protease XXIV during cardiomyocyte isolation might have decreased IK (50). The high susceptibility of hERG and native IKr to PK, protease XIV, and XXIV, but not to collagenase and trypsin, was directly demonstrated by Rajamani et al. (13). They studied hERG, KCNQ1 + KCNE1, hKir2.1 (KCNJ2), mEAG K+ channels, Na+ channels, and L-type Ca2+ channels heterologously expressed in HEK cells as well as native IKr, IKs, and IK1 in canine ventricular myocytes. They found that exogenous PK, protease XIV, and protease XXIV selectively degraded cell surface hERG/IKr channels without affecting other channel currents (13). Through analyzing the degradation products of normal glycosylated WT and unglycosylated N598Q mutant hERG channel proteins generated by PK, Rajamani et al. (13) also proposed the possibility that PK cleaves hERG in the S5-pore linker while trimming off the oligosaccharide at position Asn-598.
In the present study, using stable cell lines expressing various K+ channels and using neonatal rat ventricular myocytes, we confirmed the unique susceptibility of hERG/IKr to proteases. Our results shown in Figs. 1, 2, and 4 are consistent with data published previously by Rajamani et al. (13). However, the present study revealed several original findings from both clinical and molecular biology perspectives. To investigate the potential physiological relevance of protease susceptibility of hERG channels, we applied various concentrations of PK to the normal cell culture medium (MEM plus 10% FBS) during cell culture for 12 h. A concentration-dependent cleavage of cell surface hERG channels was observed (Fig. 3), indicating that extracellular proteases can chronically damage hERG function. In this regard, we found that calpain is up-regulated along with a decreased ERG expression in a rabbit cardiac ischemic model (Fig. 8). In the expression system of HEK cells, we discovered that overexpression of calpain-1, but not calpain-2, led to a significant protein expression level in the cell culture medium (Fig. 9E). Furthermore, we discovered that extracellular calpain-1 cleaved hERG at position Gly-603 in the S5-pore linker of the channel (Fig. 11). The protease (calpain)-mediated damage of IKr provides a novel mechanism for QT prolongation, arrhythmias, and sudden death in patients with ischemic heart disease.
Furthermore, using a chimeric channel made between PK-sensitive hERG and PK-insensitive EAG, we demonstrate that the S5-pore linker is the target region for both PK and calpain-1 (Figs. 5 and 10). Most interestingly, our data reveal that the scorpion toxin BeKm-1, a 36-amino acid peptide, which binds to the S5-pore linker, can effectively prevent PK- and calpain-1-mediated cleavage of hERG channels (Figs. 6 and 10). Although BeKm-1 itself may not be an appropriate agent to protect hERG from protease-mediated damage due to its channel blocking property, it nevertheless provides a novel concept for the development of compounds that selectively protect hERG without blocking the channel.
Because proteases such as PK and calpain-1 are relatively non-selective, it is interesting to find that among various cardiac channels, the hERG channel is selectively cleaved by these enzymes. On the other hand, extracellular proteases may only access cell surface protein ectodomains (domains that are exposed extracellularly), not domains buried within the lipid bilayer. Thus, their cleaving effectiveness on specific cell surface proteins would depend on the extent of exposure, the amino acid sequence, and the three-dimensional structure of the domains. hERG is distinctive from other cardiac K+ channels in several ways. For example, it displays extracellular K+ dependence, fast inactivation, and promiscuous interactions with a wide spectrum of drugs (6, 51). In particular, hERG possesses an unusually long S5-pore linker with 43 amino acids, whereas other voltage-gated potassium channels usually possess S5-pore linkers with 10–23 amino acid residues (e.g. KCNQ1; Fig. 4C) (8, 9). Using two novel approaches, replacing the S5-pore linker of hERG with the linker of EAG (hERG-EAGS5P) and shielding the S5-pore linker with the scorpion toxin BeKm-1, we directly demonstrated that the S5-pore linker is the cleavage site by PK and calpain-1 (Figs. 5 and 10). By analyzing PK-produced fragments of WT hERG and hERG without N-linked glycosylation, we conclude that PK removes the 20-kDa oligosaccharide while cleaving the hERG channel in the S5-pore linker (Fig. 4). N-Linked glycosylation takes place at position Asn-598, and GKPY↓NSSG is a PROSPER-predicted serine protease cleavage site. Cleavage at this site would release a 20-kDa oligosaccharide (Fig. 4C) and generate 65.5- and 61.2-kDa fragments (ExPASy-ProtParam Tool), which are close to the detected 70-kDa N-terminal and 65-kDa C-terminal fragments (Fig. 4A).
Whereas PK can cleave hERG in minutes (13), calpain-mediated cleavage of hERG occurs in hours. Our data show that whereas hERG fragments can be detected in cells treated with PK for 20 min (Fig. 4A), no hERG fragment was detected in cells treated with PK for 12 h (Fig. 3B). We believe that the hERG fragments are degraded to undetectable levels during prolonged exposure to PK. Given that calpain-1-mediated cleavage of hERG channels occurs in hours, detection of fragments was difficult. Indeed, among 12 experiments in which hERG detection was performed in proteins from hERG-HEK cells cultured with the calpain-1 medium for 12 h, a visible, faint hERG fragment of 70 kDa was observed in only two experiments using an N-terminal antibody (data not shown). The detected 70-kDa fragment is close to the 66.0-kDa N-fragment predicted by the ExPASy-ProtParam Tool at the cleavage site of Gly-603, which is 5 amino acids away from Asn-598. Despite the difficulty of detecting fragments after calpain-1 digestion, our data, obtained using the chimeric channel between hERG and EAG to replace the hERG S5-pore linker (Fig. 10A), using an hERG-specific toxin peptide to shield the S5-pore linker (Fig. 10, B and C), and using site-directed mutagenesis to disrupt the predicted cleavage sites (Fig. 11), consistently indicate that calpain-1 cleaves hERG in the S5-pore linker at position Gly-603.
The hERG-protease interaction described in the present study has important clinical implications. Constitutively active proteases in the extracellular environment are known to regulate various cellular processes, including the activity of ion channels (52). Because the S5-pore linker of hERG is localized extracellularly, secreted/released proteases as well as membrane-anchored proteases have the potential of cleaving cell surface hERG channels. In particular, protease activity is significantly enhanced in various diseased conditions, such as cardiac ischemia, which is associated with high incidences of QT prolongation, arrhythmias, and sudden cardiac death (25, 27, 28). Using a rabbit ischemia model, we demonstrated that a decrease in the mature ERG expression was accompanied by increases in calpain, matriptase-2, and MMP-2 expressions (Fig. 8). Our data show that, unlike PK and protease XXIV, membrane-anchored serine protease matriptase-2 did not cleave hERG channels (Fig. 9, A and B). As well, MMP-2 had no effect on hERG (Fig. 9, C and D). MMP-2 is a type IV collagenase that is secreted extracellularly to cleave type IV collagen, gelatin, and fibronectin (53). Data from our laboratory (not shown) and others indicate that collagenase II does not cleave hERG (13). One of the most significant findings in the present study is that calpain-1 is significantly secreted into the culture medium, which cleaves hERG at position Gly-603 in the S5-pore linker. Although the present study focused on the effects of matriptase-2, MMP-2, and calpain on hERG channels, other proteases may also damage hERG. Enhanced activities of various extracellular proteases in conditions such as ischemic injury have been documented (41). Further research would be required to identify other proteases that potentially cleave hERG channels. In animal experiments, the inhibition of proteases such as calpain and chymase during cardiac ischemia has been shown to be cardio-protective and antiarrhythmic (54, 55). It was reported that prolongation of the QTc interval is seen uniformly during early transmural ischemia (25). Consequently, protecting hERG channels from protease-induced damage could have important therapeutic potential for cardiac ischemic patients. Protease inhibitors are currently used clinically to treat various diseases (56). However, general protease inhibition would affect many cellular processes. Therefore, selective protection of hERG channels from protease damage would be desirable. In this regard, our finding that the 36-amino acid scorpion toxin BeKm-1 prevents PK- as well as calpain-mediated hERG damage is of importance. BeKm-1 selectively binds to the hERG S5-pore linker (10–12). We believe that such binding is likely to shield the linker from digestion by proteases, including PK and calpain. However, WT BeKm-1 may have limited practical use due to the fact that it blocks hERG channels. Nonetheless, this finding revealed the novel concept toward developing new peptides that can protect hERG from protease cleavage without blocking the channel. This is theoretically possible because, unlike most hERG blockers, which bind to the internal mouth of the channel pore and completely block the channel, scorpion toxin peptide BeKm-1 binds to the outer vestibule of hERG channels and does not completely block the channel (11). Further investigation of the molecular mechanisms of peptide-mediated protection of hERG would facilitate identification of new peptides that bind to the S5-pore linker without blocking the channel. Such peptides would have the potential to protect hERG against protease-mediated damage and thus prevent hERG damage-associated LQTS and ventricular arrhythmias during cardiac ischemia.
In summary, our study demonstrates, for the first time, that, in addition to the experimentally used serine protease PK, calpain-1, which is up-regulated in cardiac ischemia, cleaves hERG channels in the S5-pore linker, specifically at amino acid position Gly-603. Furthermore, the selective hERG S5-pore linker-binding peptide, BeKm-1, effectively protects hERG against cleavage by PK and calpain-1. These data extend our understanding of hERG regulation, provide novel mechanisms of arrhythmogenesis, and offer insights into innovative anti-arrhythmic strategies by selectively protecting hERG in conditions such as cardiac ischemia.
Experimental Procedures
Molecular Biology
hERG cDNA was obtained from Dr. Gail A. Robertson (University of Wisconsin, Madison, WI) (3). Point mutations of hERG were generated using an overlap extension PCR technique (7). bEAG and hERG-EAG S5-pore plasmids (hERG with its S5-pore linker replaced by the bEAG S5-pore linker, hERG-EAGS5P) were obtained from Dr. Eckhard Ficker (Rammelkamp Center for Education and Research, MetroHealth Medical Center, Cleveland, OH (37)). KCNQ1 and KCNE1 cDNAs were obtained from Dr. Michael Sanguinetti (University of Utah, Salt Lake City, UT). KCNE2 cDNA was obtained from Dr. Steve Goldstein (University of Chicago). Kir2.3 cDNA was obtained from Dr. Carol Vandenberg (University of California, Santa Barbara, CA). Kv4.3 cDNA was provided by Gui-Rong Li (University of Hong Kong). hEAG cDNA was obtained from Dr. Luis Pardo (Max-Planck Institute of Experimental Medicine, Göttingen, Germany). GFP cDNA (pIRES2-EGFP (6029-1), Clontech, Mountain View, CA) was co-transfected for cell selection for patch clamp experiments with transiently expressed cells. Stable HEK 293 cell lines that express various K+ channels were generated using G418 for selection and maintenance as described previously (6). The calpain-1 (SC116897), calpain-2 (SC119079), and MMP-2 (SC321560) plasmids were purchased from Origene Technologies Inc. (Rockville, MD). Myc-Matriptase-2 (TMPRSS6) (HG12317-G-M) was purchased from Sino Biological (Beijing, China).
Extracellular Cleavage of Cell Surface Proteins
For the extracellular calpain-1 cleavage of hERG channels, HEK cells were transfected with calpain-1 or empty pcDNA3 (control) plasmid. 24 h after transfection, the culture medium (Ctrl-m or CAPN-1-m) was collected and applied to hERG-HEK cells for 12 h. The cells were then collected for patch clamp or Western blotting analysis.
Patch Clamp Recording Method
The whole-cell patch clamp method was used. Details regarding procedures and solutions for recording various currents in cardiomyocytes as well as various K+ channel-expressing HEK stable cell lines have been described previously (6, 7). In particular, the 5 mm K+ (standard) bath solution contained 135 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 10 mm HEPES (pH 7.4 with NaOH). The 135 mm K+ bath solution contained 135 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 10 mm HEPES (pH 7.4 with KOH). All patch clamp experiments were performed at room temperature (22 ± 1 °C).
Western Blotting Analysis
Whole-cell lysates were extracted from hERG-HEK cells. Protein concentration was determined by using the Bio-Rad DC protein assay kit. 15 μg of protein in 50 μl of SDS-PAGE sample buffer was loaded and separated on 8% SDS-PAGE for 2 h and then electroblotted overnight at 4 °C onto PVDF membranes (Bio-Rad). Membranes were blocked using 5% skim milk in 0.1% Tween 20-containing TBS for 1 h. Membranes were immunoblotted for 1 h using appropriate primary antibodies and then incubated with corresponding horseradish peroxidase-conjugated secondary antibodies. The signals were detected using the ECL plus Western blotting detection kit (GE Healthcare). The BLUeye Prestained protein ladder (GeneDirex) was used to identify band sizes. For quantification of Western blotting data, band intensities of proteins of interest in each gel were first normalized to their respective actin intensities; the band intensities of treatment group(s) were then normalized to their respective control(s) in the same gel and expressed as relative values.
Rabbit Ischemia Model
Animal protocols were approved by the Queen's University Animal Care Committee, and coronary ligation experiments were performed in the animal operating suites at the Queen's University animal care center. New Zealand White male rabbits (2.5–3.5 kg) were divided into control and ischemic groups. The rabbits were sedated with ketamine and medetomidine (10 and 0.2 mg/kg, intramuscularly). Rabbits were endotracheally intubated and maintained on a small animal ventilator (Harvard Apparatus) with a respiratory rate of 60 breaths/min and a fraction of inspired oxygen of 40%. Anesthesia was maintained with isoflurane (5.0% in oxygen at 2 liters/min) via mask/ventilator. A left thoracotomy was performed, and the left circumflex artery ∼1 cm below the left atrial appendage was isolated. A 6.0 silk suture was placed proximally to the left circumflex coronary artery. Complete artery ligation was performed on ischemic rabbits, and the suture was placed but removed on sham-operated control rabbits. The chest was closed with layered sutures. 24 h after operation, rabbits were euthanized, and the hearts were excised. Left ventricular tissues in the affected regions were used for protein extraction and Western blotting analysis using protocols as described previously (6).
Neonatal Rat Ventricular Myocyte Isolation and Culture
Single ventricular myocytes were isolated from 1–2-day-old Sprague-Dawley rats of either sex by enzymatic dissociation, as described previously (29). Cells were cultured in DMEM/Ham's F-12 medium (Invitrogen) supplemented with 10% FBS. Myocytes were cultured on coverslips for 24 h and then treated with PK in the absence or presence of BeKm-1. IKr, Ito, IK1, ICa, and INa in treated and control cells were recorded using the whole-cell patch clamp method as described previously (29, 57).
Reagents and Antibodies
MEM, Opti-MEM, Lipofectamine 2000, and FBS were purchased from Invitrogen. PK (P6556), protease XXIV (P8038), G418 (A1720), tunicamycin (T7765), rabbit anti-KCNE2 (M3318), mouse anti-actin (A4700), and mouse anti-Myc (M4439) primary antibodies and all chemicals for patch clamp experiments were purchased from Sigma-Aldrich. Rabbit anti-matriptase-2 (ab56180) was purchased from Abcam. Goat anti-hERG (C-20 (sc-15968, C-terminal) and N-20 (sc-15966, N-terminal)), goat anti-matriptase-2 (sc-54240), goat anti-KCNE1 (sc-16796), rabbit anti-MMP-2 (sc-10736), and rabbit anti-calpain (sc-30064) primary antibodies and goat anti-rabbit (sc-2004), goat anti-mouse (sc-2005), and mouse anti-goat (sc-2354) IgG-HRP secondary antibodies were purchased from Santa Cruz Biotechnology. Trypsin (LS003707) and Collagenase II (LS004176) were purchased from Worthington. B27 calpain inhibitor peptide (AS-60844) was purchased from AnaSpec (Fremont, CA). BeKm-1 (13BEK001) was purchased from Smartox Biotechnology (France).
All data are expressed as the mean ± S.E. A one-way analysis of variance or two-tailed Student's t test was used to determine statistical significance between the control and test groups. A p value ≤0.05 was considered significant.
Author Contributions
S. Z. conceived the study, obtained funding, and coordinated and designed the study; S. L., J. G., W. L., and T. Y. were responsible for experimental design and execution and data analysis; S. L. and S. Z. wrote the paper; and S. L., J. G., W. L., T. Y., and S. Z. gave final approval.
This work was supported by Canadian Institutes of Health Research Grant MOP 72911 (to S. Z.). The authors declare that they have no conflicts of interest with the contents of this article.
- hERG
- human ether-a-go-go-related gene
- EAG
- ether-a-go-go
- hEAG and bEAG
- human and bovine EAG, respectively
- HEK
- human embryonic kidney
- IhERG
- hERG current
- IKr
- the rapidly activating delayed rectifier potassium channel
- LQTS
- long QT syndrome
- MMP
- matrix metalloproteinase; voltage-gated K+
- PK
- proteinase K
- MEM
- minimum essential medium.
References
- 1. Keating M. T., and Sanguinetti M. C. (2001) Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104, 569–580 [DOI] [PubMed] [Google Scholar]
- 2. Sanguinetti M. C., Jiang C., Curran M. E., and Keating M. T. (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81, 299–307 [DOI] [PubMed] [Google Scholar]
- 3. Trudeau M. C., Warmke J. W., Ganetzky B., and Robertson G. A. (1995) HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269, 92–95 [DOI] [PubMed] [Google Scholar]
- 4. Curran M. E., Splawski I., Timothy K. W., Vincent G. M., Green E. D., and Keating M. T. (1995) A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 80, 795–803 [DOI] [PubMed] [Google Scholar]
- 5. Anderson C. L., Delisle B. P., Anson B. D., Kilby J. A., Will M. L., Tester D. J., Gong Q., Zhou Z., Ackerman M. J., and January C. T. (2006) Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113, 365–373 [DOI] [PubMed] [Google Scholar]
- 6. Guo J., Massaeli H., Xu J., Jia Z., Wigle J. T., Mesaeli N., and Zhang S. (2009) Extracellular K+ concentration controls cell surface density of IKr in rabbit hearts and of the HERG channel in human cell lines. J. Clin. Invest. 119, 2745–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Massaeli H., Guo J., Xu J., and Zhang S. (2010) Extracellular K+ is a prerequisite for the function and plasma membrane stability of HERG channels. Circ. Res. 106, 1072–1082 [DOI] [PubMed] [Google Scholar]
- 8. Torres A. M., Bansal P. S., Sunde M., Clarke C. E., Bursill J. A., Smith D. J., Bauskin A., Breit S. N., Campbell T. J., Alewood P. F., Kuchel P. W., and Vandenberg J. I. (2003) Structure of the HERG K+ channel S5P extracellular linker: role of an amphipathic α-helix in C-type inactivation. J. Biol. Chem. 278, 42136–42148 [DOI] [PubMed] [Google Scholar]
- 9. Jiang M., Zhang M., Maslennikov I. V., Liu J., Wu D. M., Korolkova Y. V., Arseniev A. S., Grishin E. V., and Tseng G. N. (2005) Dynamic conformational changes of extracellular S5-P linkers in the hERG channel. J. Physiol 569, 75–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korolkova Y. V., Bocharov E. V., Angelo K., Maslennikov I. V., Grinenko O. V., Lipkin A. V., Nosyreva E. D., Pluzhnikov K. A., Olesen S. P., Arseniev A. S., and Grishin E. V. (2002) New binding site on common molecular scaffold provides HERG channel specificity of scorpion toxin BeKm-1. J. Biol. Chem. 277, 43104–43109 [DOI] [PubMed] [Google Scholar]
- 11. Zhang M., Korolkova Y. V., Liu J., Jiang M., Grishin E. V., and Tseng G. N. (2003) BeKm-1 is a HERG-specific toxin that shares the structure with ChTx but the mechanism of action with ErgTx1. Biophys. J. 84, 3022–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tseng G. N., Sonawane K. D., Korolkova Y. V., Zhang M., Liu J., Grishin E. V., and Guy H. R. (2007) Probing the outer mouth structure of the HERG channel with peptide toxin footprinting and molecular modeling. Biophys. J. 92, 3524–3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rajamani S., Anderson C. L., Valdivia C. R., Eckhardt L. L., Foell J. D., Robertson G. A., Kamp T. J., Makielski J. C., Anson B. D., and January C. T. (2006) Specific serine proteases selectively damage KCNH2 (hERG1) potassium channels and IKr. Am. J. Physiol. Heart Circ. Physiol. 290, H1278–H1288 [DOI] [PubMed] [Google Scholar]
- 14. Mehta D., Curwin J., Gomes J. A., and Fuster V. (1997) Sudden death in coronary artery disease: acute ischemia versus myocardial substrate. Circulation 96, 3215–3223 [DOI] [PubMed] [Google Scholar]
- 15. Chohan P. K., Singh R. B., Dhalla N. S., and Netticadan T. (2006) l-Arginine administration recovers sarcoplasmic reticulum function in ischemic reperfused hearts by preventing calpain activation. Cardiovasc. Res. 69, 152–163 [DOI] [PubMed] [Google Scholar]
- 16. Letavernier E., Zafrani L., Perez J., Letavernier B., Haymann J. P., and Baud L. (2012) The role of calpains in myocardial remodelling and heart failure. Cardiovasc. Res. 96, 38–45 [DOI] [PubMed] [Google Scholar]
- 17. Xu L., and Deng X. (2006) Protein kinase Cι promotes nicotine-induced migration and invasion of cancer cells via phosphorylation of micro- and m-calpains. J. Biol. Chem. 281, 4457–4466 [DOI] [PubMed] [Google Scholar]
- 18. Letavernier B., Zafrani L., Nassar D., Perez J., Levi C., Bellocq A., Mesnard L., Sachon E., Haymann J. P., Aractingi S., Faussat A. M., Baud L., and Letavernier E. (2012) Calpains contribute to vascular repair in rapidly progressive form of glomerulonephritis: potential role of their externalization. Arterioscler. Thromb. Vasc. Biol. 32, 335–342 [DOI] [PubMed] [Google Scholar]
- 19. Perez J., Dansou B., Hervé R., Levi C., Tamouza H., Vandermeersch S., Demey-Thomas E., Haymann J. P., Zafrani L., Klatzmann D., Boissier M. C., Letavernier E., and Baud L. (2016) Calpains released by T lymphocytes cleave TLR2 to control IL-17 expression. J. Immunol. 196, 168–181 [DOI] [PubMed] [Google Scholar]
- 20. Lakhal S., Schödel J., Townsend A. R., Pugh C. W., Ratcliffe P. J., and Mole D. R. (2011) Regulation of type II transmembrane serine proteinase TMPRSS6 by hypoxia-inducible factors: new link between hypoxia signaling and iron homeostasis. J. Biol. Chem. 286, 4090–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maurer E., Gütschow M., and Stirnberg M. (2012) Matriptase-2 (TMPRSS6) is directly up-regulated by hypoxia inducible factor-1: identification of a hypoxia-responsive element in the TMPRSS6 promoter region. Biol. Chem. 393, 535–540 [DOI] [PubMed] [Google Scholar]
- 22. Kai H., Ikeda H., Yasukawa H., Kai M., Seki Y., Kuwahara F., Ueno T., Sugi K., and Imaizumi T. (1998) Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 32, 368–372 [DOI] [PubMed] [Google Scholar]
- 23. Cheung P. Y., Sawicki G., Wozniak M., Wang W., Radomski M. W., and Schulz R. (2000) Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 101, 1833–1839 [DOI] [PubMed] [Google Scholar]
- 24. Szklarczyk A., Ewaleifoh O., Beique J. C., Wang Y., Knorr D., Haughey N., Malpica T., Mattson M. P., Huganir R., and Conant K. (2008) MMP-7 cleaves the NR1 NMDA receptor subunit and modifies NMDA receptor function. FASEB J. 22, 3757–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kenigsberg D. N., Khanal S., Kowalski M., and Krishnan S. C. (2007) Prolongation of the QTc interval is seen uniformly during early transmural ischemia. J. Am. Coll. Cardiol. 49, 1299–1305 [DOI] [PubMed] [Google Scholar]
- 26. Shawl F. A., Velasco C. E., Goldbaum T. S., and Forman M. B. (1990) Effect of coronary angioplasty on electrocardiographic changes in patients with unstable angina secondary to left anterior descending coronary artery disease. J. Am. Coll. Cardiol. 16, 325–331 [DOI] [PubMed] [Google Scholar]
- 27. Schwartz P. J., and Wolf S. (1978) QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation 57, 1074–1077 [DOI] [PubMed] [Google Scholar]
- 28. Gadaleta F. L., Llois S. C., Lapuente A. R., Batchvarov V. N., and Kaski J. C. (2003) Prognostic value of corrected QT-interval prolongation in patients with unstable angina pectoris. Am. J. Cardiol. 92, 203–205 [DOI] [PubMed] [Google Scholar]
- 29. Guo J., Massaeli H., Li W., Xu J., Luo T., Shaw J., Kirshenbaum L. A., and Zhang S. (2007) Identification of IKr and its trafficking disruption induced by probucol in cultured neonatal rat cardiomyocytes. J. Pharmacol. Exp. Ther. 321, 911–920 [DOI] [PubMed] [Google Scholar]
- 30. Zhou Z., Gong Q., Epstein M. L., and January C. T. (1998) HERG channel dysfunction in human long QT syndrome: intracellular transport and functional defects. J. Biol. Chem. 273, 21061–21066 [DOI] [PubMed] [Google Scholar]
- 31. Chen J., Guo J., Yang T., Li W., Lamothe S. M., Kang Y., Szendrey J. A., and Zhang S. (2015) Rab11-dependent recycling of the human ether-a-go-go-related gene (hERG) channel. J. Biol. Chem. 290, 21101–21113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hogan-Cann A., Li W., Guo J., Yang T., and Zhang S. (2016) Proteolytic cleavage in the S1-S2 linker of the Kv1.5 channel does not affect channel function. Biochim. Biophys. Acta 1858, 1082–1090 [DOI] [PubMed] [Google Scholar]
- 33. Gianulis E. C., and Trudeau M. C. (2011) Rescue of aberrant gating by a genetically encoded PAS (Per-Arnt-Sim) domain in several long QT syndrome mutant human ether-a-go-go-related gene potassium channels. J. Biol. Chem. 286, 22160–22169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang Y., Guo J., Yang T., Li W., and Zhang S. (2015) Regulation of the human ether-a-go-go-related gene (hERG) potassium channel by Nedd4 family interacting proteins (Ndfips). Biochem. J. 472, 71–82 [DOI] [PubMed] [Google Scholar]
- 35. Gong Q., Anderson C. L., January C. T., and Zhou Z. (2002) Role of glycosylation in cell surface expression and stability of HERG potassium channels. Am. J. Physiol. Heart Circ. Physiol. 283, H77–H84 [DOI] [PubMed] [Google Scholar]
- 36. Ficker E., Dennis A. T., Wang L., and Brown A. M. (2003) Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ. Res. 92, e87–e100 [DOI] [PubMed] [Google Scholar]
- 37. Ficker E., Jarolimek W., Kiehn J., Baumann A., and Brown A. M. (1998) Molecular determinants of dofetilide block of HERG K+ channels. Circ. Res. 82, 386–395 [DOI] [PubMed] [Google Scholar]
- 38. Gerlach A. C., Stoehr S. J., and Castle N. A. (2010) Pharmacological removal of human ether-a-go-go-related gene potassium channel inactivation by 3-nitro-N-(4-phenoxyphenyl) benzamide (ICA-105574). Mol. Pharmacol. 77, 58–68 [DOI] [PubMed] [Google Scholar]
- 39. McDonald T. V., Yu Z., Ming Z., Palma E., Meyers M. B., Wang K. W., Goldstein S. A. N., and Fishman G. I. (1997) A minK-HERG complex regulates the cardiac potassium current IKr. Nature 388, 289–292 [DOI] [PubMed] [Google Scholar]
- 40. Abbott G. W., Sesti F., Splawski I., Buck M. E., Lehmann M. H., Timothy K. W., Keating M. T., and Goldstein S. A. N. (1999) MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell 97, 175–187 [DOI] [PubMed] [Google Scholar]
- 41. Müller A. L., Hryshko L. V., and Dhalla N. S. (2013) Extracellular and intracellular proteases in cardiac dysfunction due to ischemia-reperfusion injury. Int. J. Cardiol. 164, 39–47 [DOI] [PubMed] [Google Scholar]
- 42. Velasco G., Cal S., Quesada V., Sánchez L. M., and López-Otín C. (2002) Matriptase-2, a membrane-bound mosaic serine proteinase predominantly expressed in human liver and showing degrading activity against extracellular matrix proteins. J. Biol. Chem. 277, 37637–37646 [DOI] [PubMed] [Google Scholar]
- 43. Goll D. E., Thompson V. F., Li H., Wei W., and Cong J. (2003) The calpain system. Physiol. Rev. 83, 731–801 [DOI] [PubMed] [Google Scholar]
- 44. Song J., Tan H., Perry A. J., Akutsu T., Webb G. I., Whisstock J. C., and Pike R. N. (2012) PROSPER: an integrated feature-based tool for predicting protease substrate cleavage sites. PLoS One 7, e50300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peeters G. A., Sanguinetti M. C., Eki Y., Konarzewska H., Renlund D. G., Karwande S. V., and Barry W. H. (1995) Method for isolation of human ventricular myocytes from single endocardial and epicardial biopsies. Am. J. Physiol. 268, H1757–H1764 [DOI] [PubMed] [Google Scholar]
- 46. Sheets M. F., January C. T., and Fozzard H. A. (1983) Isolation and characterization of single canine cardiac purkinje cells. Circ. Res. 53, 544–548 [DOI] [PubMed] [Google Scholar]
- 47. Vahouny G. V., Wei R., Starkweather R., and Davis C. (1970) Preparation of beating heart cells from adult rats. Science 167, 1616–1618 [DOI] [PubMed] [Google Scholar]
- 48. Beuckelmann D. J., Näbauer M., and Erdmann E. (1993) Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ. Res. 73, 379–385 [DOI] [PubMed] [Google Scholar]
- 49. Schaffer P., Pelzmann B., Bernhart E., Lang P., Mächler H., Rigler B., and Koidl B. (1999) Repolarizing currents in ventricular myocytes from young patients with tetralogy of Fallot. Cardiovasc. Res. 43, 332–343 [DOI] [PubMed] [Google Scholar]
- 50. Konarzewska H., Peeters G. A., and Sanguinetti M. C. (1995) Repolarizing K+ currents in nonfailing human hearts. Similarities between right septal subendocardial and left subepicardial ventricular myocytes. Circulation 92, 1179–1187 [DOI] [PubMed] [Google Scholar]
- 51. Sanguinetti M. C., and Tristani-Firouzi M. (2006) hERG potassium channels and cardiac arrhythmia. Nature 440, 463–469 [DOI] [PubMed] [Google Scholar]
- 52. Donaldson S. H., Hirsh A., Li D. C., Holloway G., Chao J., Boucher R. C., and Gabriel S. E. (2002) Regulation of the epithelial sodium channel by serine proteases in human airways. J. Biol. Chem. 277, 8338–8345 [DOI] [PubMed] [Google Scholar]
- 53. Aimes R. T., and Quigley J. P. (1995) Matrix metalloproteinase-2 is an interstitial collagenase: inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific ¾- and ¼-length fragments. J. Biol. Chem. 270, 5872–5876 [DOI] [PubMed] [Google Scholar]
- 54. Jin D., Takai S., Sakaguchi M., Okamoto Y., Muramatsu M., and Miyazaki M. (2004) An antiarrhythmic effect of a chymase inhibitor after myocardial infarction. J. Pharmacol. Exp. Ther. 309, 490–497 [DOI] [PubMed] [Google Scholar]
- 55. Neuhof C., Fabiunk V., Speth M., Möller A., Fritz F., Tillmanns H., Neuhof H., and Erdogan A. (2008) Reduction of myocardial infarction by postischemic administration of the calpain inhibitor A-705253 in comparison to the Na+/H+ exchange inhibitor Cariporide in isolated perfused rabbit hearts. Biol. Chem. 389, 1505–1512 [DOI] [PubMed] [Google Scholar]
- 56. Turk B. (2006) Targeting proteases: successes, failures and future prospects. Nat. Rev. Drug Discov. 5, 785–799 [DOI] [PubMed] [Google Scholar]
- 57. Zhao Y., Wang T., Guo J., Yang T., Li W., Koichopolos J., Lamothe S. M., Kang Y., Ma A., and Zhang S. (2016) Febrile temperature facilitates hERG/IKr degradation through an altered K+ dependence. Heart Rhythm 10.1016/j.hrthm.2016.06.019 [DOI] [PubMed] [Google Scholar]