Abstract
The aim of the present study was to investigate the role of nitric oxide (NO) in the antifungal activity of Shikonin (SK) against Candida albicans (C. albicans) and to clarify the underlying mechanism. The results showed that the NO donors S-nitrosoglutathione (GSNO) and L-arginine could enhance the antifungal activity of SK, whereas the NO production inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) attenuated antifungal action. Using the fluorescent dye 3-amino,4-aminomethyl-2′, 7-difluorescein, diacetate (DAF-FM DA), we found that the accumulation of NO in C. albicans was increased markedly by SK in a time- and dose-dependent manner. In addition, the results of real-time reverse transcription-PCR (RT-PCR) demonstrated that the transcription level of YHB1 in C. albicans was greatly increased upon incubation of SK. Consistently, the YHB1-null mutant (yhb1Δ/Δ) exhibited a higher susceptibility to SK than wild-type cells. In addition, although the transcription level of CTA4 in C. albicans was not significantly changed when exposed to SK, the CTA4-null mutant (cta4Δ/Δ) was more susceptible to SK. Collectively, SK is the agent found to execute its antifungal activity directly via endogenous NO accumulation, and NO-mediated damage is related to the suppression of YHB1 and the function of CTA4.
Keywords: Candida albicans, CTA4, nitric oxide, Shikonin, YHB1
INTRODUCTION
Candida albicans (C. albicans) is the most important cause of fungal infection in humans, especially in immunocompromised patients.1 Available antifungals predominantly include azoles, echinocandins, polyenes and allylamines.2 Each of these classes of antifungals has its own distinct mode of action. Azoles target cytochrome P450 lanosterol 14α-demethylase, thereby impeding conversion of lanosterol to fecosterol and subsequently blocking ergosterol biosynthesis.3 Echinocandins interfere with cell wall synthesis by inhibiting β-1,-3-glucan synthase.4 Polyenes have an affinity to bind membrane sterols that results in the formation of aqueous pores, leading to leakage of crucial cellular components and subsequent cell death.5 Allylamines specifically target squalene epoxidase.6 However, the emergence of resistant strains of Candida because of prolonged use of drugs and that some pathogenic Candida species are intrinsically resistant to antifungals make candidiasis increasingly difficult to cure.7 The limited arsenal of antifungals constantly requires researchers to find new antifungals using novel mechanisms. In this context, herbal antifungals have acquired significance owing to their natural origin.8
Shikonin (SK), a molecule originally found in China, is the major constituent of the red pigment extracts from the roots of the plant Lithospermum erythrorhizon Sieb. et Zucc (LE). SK was reported to exhibit certain functions and was widely used to prepare an ointment to treat wounds, burns and hemorrhoids in Japan;9 to induce necrosis or apoptosis through generating reactive oxygen species to treat gastric cancers;10 and to serve possible therapeutic roles in brain disorders involving uncontrolled inflammatory responses.11 We recently found that SK exerted an antifungal effect on almost all C. albicans isolates tested. More importantly, the action of SK was shown to be >16 times higher than that of fluconazole against some fluconazole-resistant C. albicans isolates. However, although mitochondrial aerobic respiration and endogenous reactive oxygen species were identified as being involved in the antifungal activity of SK,12 the intrinsic mechanisms of the antifungal activity of SK have not been fully determined.
Nitric oxide (NO), an essential messenger involved in numerous physiological functions, has attracted much attention for its antimicrobial and antifungal activity and its synergistic effects when co-delivered with chemotherapeutic drugs.13, 14 S-nitrosoglutathione (GSNO), a potent NO donor formed by the interaction of NO with intracellular glutathione, exhibited antimicrobial activity against methicillin-resistant Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa,15 and the NO precursor L-arginine was used against Salmonella.16 In addition, the significant increase of fecal oocyst shedding after treatment with the NO synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) partly suggests that arginine-derived NO may reduce the parasite load in experimental cryptosporidiosis.17 Heilman et al.18 indicated that the hyphal form of the fungus was more susceptible to a biocompatible NO-donating material that delivers NO that is induced using light. However, the direct role of endogenous NO involved in the antifungal activity of antimycotics has not yet been studied. In this study, we demonstrated the endogenous accumulation of NO in C. albicans when treated with SK, and we clarified the intrinsic mechanisms. We used GSNO, L-arginine and L-NAME to show that the antifungal efficacy of SK was directly related to the intracellular levels of NO. Utilizing real-time reverse transcription-PCR (RT-PCR), the nitrosative stress was found to be mediated by YHB1, the only recognized gene that has been reported to encode the key NO-detoxifying enzyme, flavohemoglobin/nitric oxide dioxygenase,19 as well as CTA4, the first reported regulator of YHB1 in C. albicans.20
MATERIALS AND METHODS
Strains, media and compounds
All strains used in this study are listed in Table 1. Strains were routinely maintained on Sabouraud dextrose agar and propagated in liquid yeast extract peptone dextrose (YPD) medium (1% w/v yeast extract, 2% w/v peptone and 2% w/v dextrose), peptone, yeast extract and agar were supplied by Becton Dickinson & Co. (Sparks, MD, USA). Batches of media were inoculated from YPD agar plates containing freshly grown C. albicans and were incubated overnight in an orbital shaker at 30 °C. Before being used in the following experiments, blastospores were harvested and washed twice in sterile phosphate-buffered saline (PBS; 10 mM phosphate buffer, 2.7 mM potassium chloride and 137 mM sodium chloride (pH 7.4)). The cells were then suspended in a yeast nitrogen base (YNB; Difco, Becton Dickinson & Co.) medium supplemented with 50 mM glucose, counted in a hemocytometer and adjusted to the desired cell density. During the experiments, 6.4 mg/mL SK (National Institutes for Food and Drug Control, Peking, China) and 8 mg/mL GSNO (Sigma-Aldrich, St Louis, MO, USA) in dimethyl sulfoxide, 10 mg/mL L-arginine and 100 mg/mL L-NAME (Sigma-Aldrich) in ultrapure distilled water and 5 mM NO fluorescent probe (3-Amino,4-aminomethyl-2', 7-difluorescein, diacetate (DAF-FM DA); Beyotime Institute Biotechnology, Jiangsu, China) were used as stock solutions and added to the culture suspensions to obtain the required concentrations.
Table 1. Candida albicans strains used in this study.
| Strain | Phenotype | Genotype | Reference |
|---|---|---|---|
| SC5314 | Prototroph | 21 | |
| SN250 | Reference strain Arg- | his1Δ/his1Δ, leu2Δ::C. dubliniensis HIS1 /leu2Δ::C. maltosa LEU2, arg4Δ /arg4Δ, URA3/ura3Δ::imm434, IRO1/iro1Δ::imm434 | 22 |
| cta4Δ/Δ | orf19.7374 mutant Arg- | his1Δ/his1Δ, leu2Δ /leu2Δ, arg4Δ /arg4Δ, URA3/ura3Δ::imm434, IRO1/iro1Δ::imm434, orf19.7374Δ::C. dubliniensisHIS1/orf19.7374Δ::C. maltosaLEU2 | 22 |
| yhb1Δ/Δ | orf19.3707 mutant Arg- | his1Δ/his1Δ, leu2Δ /leu2Δ, arg4Δ /arg4Δ, URA3/ura3Δ::imm434, IRO1/iro1Δ::imm434, orf19.3707Δ::C. dubliniensisHIS1/orf19.3707Δ::C. maltosaLEU2 | 22 |
Survival rate test
The antifungal activities of SK in the absence or presence of GSNO, L-NAME or L-arginine were assessed as described previously.23 Briefly, after incubation for 1 h, samples were washed, resuspended in PBS and placed on YPD agar plates. The fraction of viable cells was determined by counting the colonies and by calculating the percentage of surviving C. albicans cells relative to the control treatment.
Endogenous nitric oxide measurement
Intracellular levels of NO and the accumulation of NO in C. albicans were measured with DAF-FM DA. Briefly, culture suspensions were treated with the compounds; at different times, the samples were taken and washed with PBS, and then DAF-FM DA was added to the final concentration of 5 μM. The samples were incubated for 30 min in the dark at 30 °C, and then washed three times with PBS. To detect intracellular levels of NO, 300 μL of cell suspension was analyzed using a flow cytometer with excitation at 495 nm and emission at 515 nm set for DAF-FM DA, after which the samples were resuspended in 1 mL of PBS. To measure the accumulation of NO in C. albicans, the samples were resuspended in 500 μL of PBS, and then 10 μL of cell suspension was dropped on a clean glass sheet and analyzed using a Confocal Laser Scanning Microscope with excitation at 495 nm and emission at 515 nm for DAF-FM DA. The cover glass was added and then sealed with nail polish.
Real-time reverse transcription-PCR
RNA isolation and real-time RT-PCR were performed as described previously,24 with some modifications. The isolated RNA was resuspended in diethyl pyrocarbonate-treated water. The OD260 and OD280 were measured. First-strand complementary DNAs (cDNAs) were synthesized from 3 μg of total RNA in a 60 μL reaction volume using the cDNA synthesis kit for RT-PCR (TaKaRa Biotechnology, Dalian, China). Independent quantitative real-time PCR was performed in triplicate using the Lightcycler system (Roche Diagnostics, GmbH, Mannheim, Germany). SYBR Green I (TaKaRa) was used to visualize and monitor the amplified product in real time according to the manufacturer's protocol. YHB1 was amplified with the forward primer 5′-TGT TAG ACA TGT CCC AGG TGG-3' and the reverse primer 5'-CCA ATA CCA CCA GCA ACG AA-3'. The PCR protocol consisted of denaturation (95 °C for 10 s), 40 cycles of amplification and quantification (95 °C for 5 s, 60 °C for 34 s with a single fluorescence measurement), a melting curve analysis (60 °C–95 °C with a heating rate of 0.1 °C per s and a continuous fluorescence measurement) and, finally, cooling to 40 °C. A standard curve for each primer set was performed with 1:10, 1:25, 1:50, 1:100, 1:250 and 1:500 dilutions of the cDNAs. The slopes of the standard curves were within 10% of 100% efficiency. The change in fluorescence of SYBR Green I dye in every cycle was monitored using the Lightcycler system software, and the threshold cycle (CT) above background for each reaction was calculated. The CT value of 18S rRNA (amplified with the forward primer 5′-TCT TTC TTG ATT TTG TGG GTG G-3′ and the reverse primer 5′-TCG ATA GTC CCT CTA AGA AGT G-3′) was subtracted from that of the tested genes to obtain a ΔCT value. The ΔCT value of an arbitrary calibrator was subtracted from the ΔCT value of each sample to obtain a ΔΔCT value. The gene expression level relative to the calibrator was expressed as 2−ΔΔCT.
Sensitivities of C. albicans mutants to Shikonin
The antifungal susceptibility test was performed using the Clinical and Laboratory Standards Institute (Document M27-A) method.25 Briefly, the initial concentrations of CTA4-null mutant (°C), YHB1-null mutant (yhb1Δ/Δ) and the wild type in RPMI-1640 medium were adjusted to 1 × 103 colony-forming units (CFUs)/ml, whereas the final concentration of SK was 0.125–64 μg/mL. After the plates had been incubated at 30 °C for 24 h, the optical density of each well was measured at 600 nm (OD600). Each isolate was tested in triplicate, and the MIC80 values were determined as the lowest concentration of the agent that inhibited growth by 80% compared with that of the drug-free wells.12 To obtain a representative growth curve, strains in the desired cell density (1 × 105 CFUs/mL) were treated with certain concentrations of SK in YPD liquid medium that were incubated overnight in an orbital shaker at 30 °C; the control group received only dimethyl sulfoxide. Meanwhile, a sample was taken to measure the OD600 value at different times.
Statistical criteria
Data are expressed as the mean±SD of the independent assays in triplicate. Student's t-test was employed to assess the significance of the differences. A P-value of <0.05 or <0.01 was considered statistically significant.
RESULTS
The effect of the nitric oxide donor on the activity of Shikonin
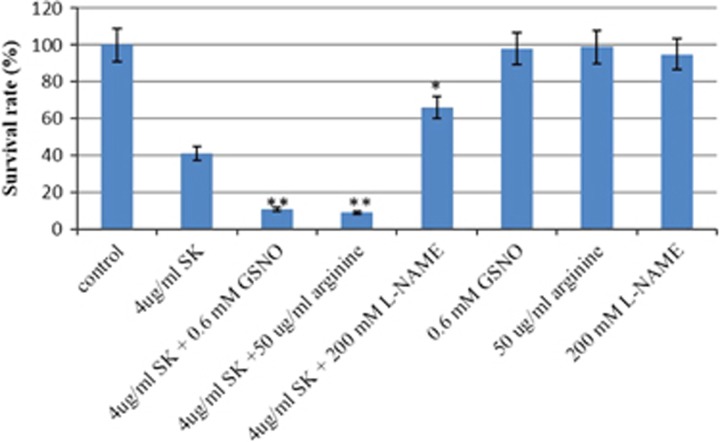
GSNO and L-arginine are both donors of NO in cells. We examined the effect of GSNO and L-arginine on the activity of SK against C. albicans SC5314. To this end, half the maximal inhibitory concentration of SK (IC50) was used. Treatment of C. albicans suspension with 4 μg/mL SK resulted in ~40% viable cells compared with the untreated group (Figure 1). We used this concentration of SK to further investigate the effect of GSNO and L-arginine on the activity of SK against C. albicans SC5314. As shown in Figure 1, the treatment of the combination of 4 μg/mL SK with 0.6 mM GSNO resulted in an ~11% viable rate of C. albicans, whereas an ~9% survival rate was obtained when treated with 4 μg/mL SK and 50 μg/mL L-arginine. Meanwhile, there was no significant effect of GSNO or L-arginine on the viabilities of C. albicans cells.
Figure 1.
The effect of NO on the antifungal activity of SK against C. albicans. Suspensions of C. albicans SC5314 were exposed to 4 μg/mL SK, 50 μg/mL arginine, 200 mM L-NAME, 0.6 mM GSNO or a combination for 2 h. The samples were then washed, resuspended in PBS and plated on YPD agar plates. The fraction of viable cells was determined by counting the colonies and calculating the percentage of surviving C. albicans cells relative to the control treatment. Data are expressed as the mean±SD of triplicate assays. *P<0.05; **P<0.01 compared with values from the treatment of 4 μg/mL SK.
The effect of nitric oxide scavenger on the activity of Shikonin
To further our investigation, we examined the effect of the NO scavenger L-NAME on the antifungal activity of SK. The result showed that when incubated with 200 mM L-NAME, there was no significant change in the survival rate of C. albicans cells, although the rate was a little lower than the untreated group. When treated with a combination of 4 μg/mL SK with 200 μg/mL L-NAME, the viable rate of C. albicans cells became ~66%, whereas 4 μg/mL SK treatment still resulted in ~40% viable cells (Figure 1).
Shikonin induced the accumulation of nitric oxide
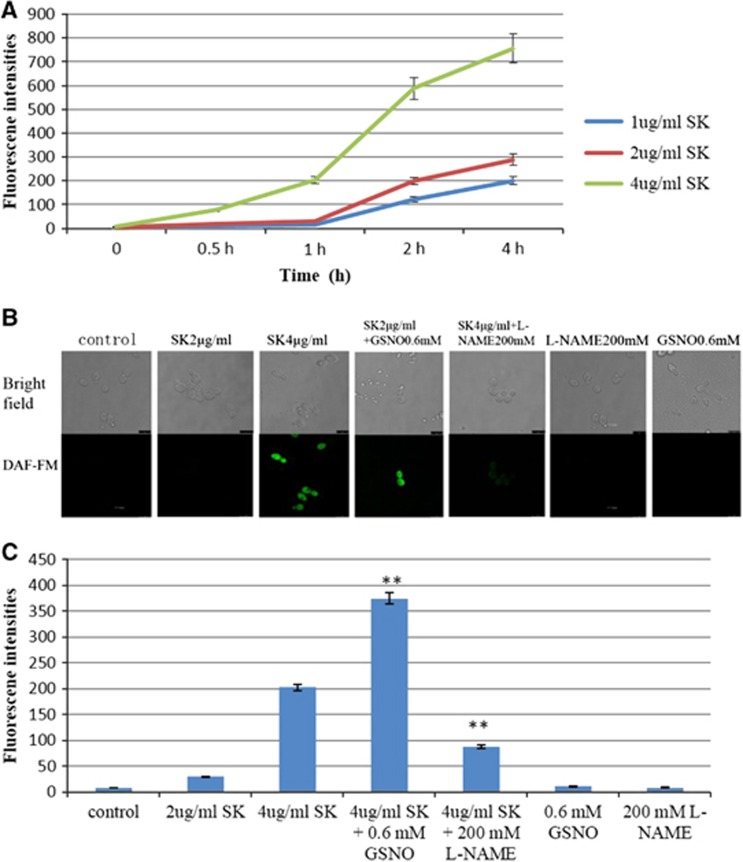
The survival rate indicated that the enhanced antifungal activity of SK might be related to NO. Thus, we detected the accumulation of endogenous NO in C. albicans cells using a confocal laser scanning microscope and flow cytometer. As is shown in our results, the incubation of SK resulted in an increased level of intracellular NO in a time- and dose-dependent manner (Figure 2A). Consistently, there was strong fluorescence in C. albicans cells when incubated with 4 μg/mL SK, and the fluorescence became weak when combined with 200 mM L-NAME. Conversely, there was no fluorescence in cells that were incubated with 2 μg/mL SK, but strong fluorescence was detected when combined with 0.6 mM GSNO. Meanwhile, cells treated with L-NAME and GSNO showed no NO accumulation (Figure 2B). Similar results were obtained when the intracellular levels of NO in C. albicans cells were measured using a flow cytometer (Figure 2C).
Figure 2.
Measurement of intracellular levels of nitric oxide (NO) in C. albicans induced by SK. (A) C. albicans cells were treated with different concentrations of SK; at different times, samples were washed with PBS and analyzed for the production of intracellular NO using a flow cytometer with excitation at 495 nm and emission at 515 nm after incubation with a fluorescent probe. (B) Suspension cells were incubated with SK, GSNO, L-NAME or a combination for 2 h. NO accumulation was analyzed by the use of a Confocal Laser Scanning Microscope with excitation at 495 nm and emission at 515 nm in the green area. Bar: 7.5 μM. (C) C. albicans cells were treated with SK, GSNO, L-NAME or a combination for 2 h, then intracellular NO production was measured by a flow cytometer with excitation at 495 nm and emission at 515 nm after incubation with a fluorescent probe. Data are expressed as the mean±SD of triplicate assays. **P<0.01 compared with values from the 4 μg/mL SK treatment.
Influence of Shikonin on the expression of YHB1
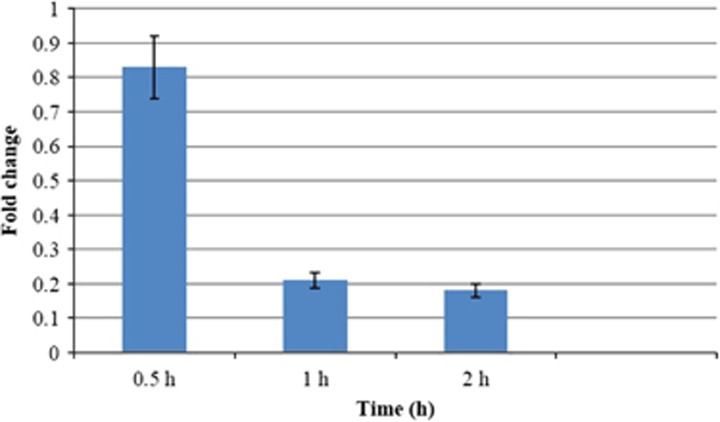
Because YHB1 is the only recognized gene to encode the key NO-detoxifying enzyme flavohemoglobin/nitric oxide dioxygenase, we investigated the putative role of YHB1 in the antifungal activity of SK against C. albicans. To this end, the effect of SK on the expression of YHB1 in C. albicans cells was assessed. The results showed a marked change in the transcript levels of YHB1 mRNA in a time-dependent manner; specifically, after exposure to 2 μg/mL SK for 0.5 h, there was no significant change in the transcript level. However, after 1 h of treatment, the YHB1 transcript level had declined from 83% to 21%, and the greatest amount of reduction was obtained after C. albicans cells had been exposed to SK for 2 h, at which time the transcript level was 18%. Furthermore, C. albicans cells exhibited no significant change in the YHB1 transcript level after being treated with 2 μg/mL SK for 4 h (Figure 3).
Figure 3.
The effect of SK on the transcription of YHB1. C. albicans cells were treated with 2 μg/mL SK; a sample was taken at different times. Total RNA was extracted and reversely transcribed to cDNA. cDNA was then used for real-time quantitative PCR to detect expression levels of YHB1. Values represent the mean±SD of three replicates.
The sensitivity of YHB1 or CTA4 mutant to Shikonin
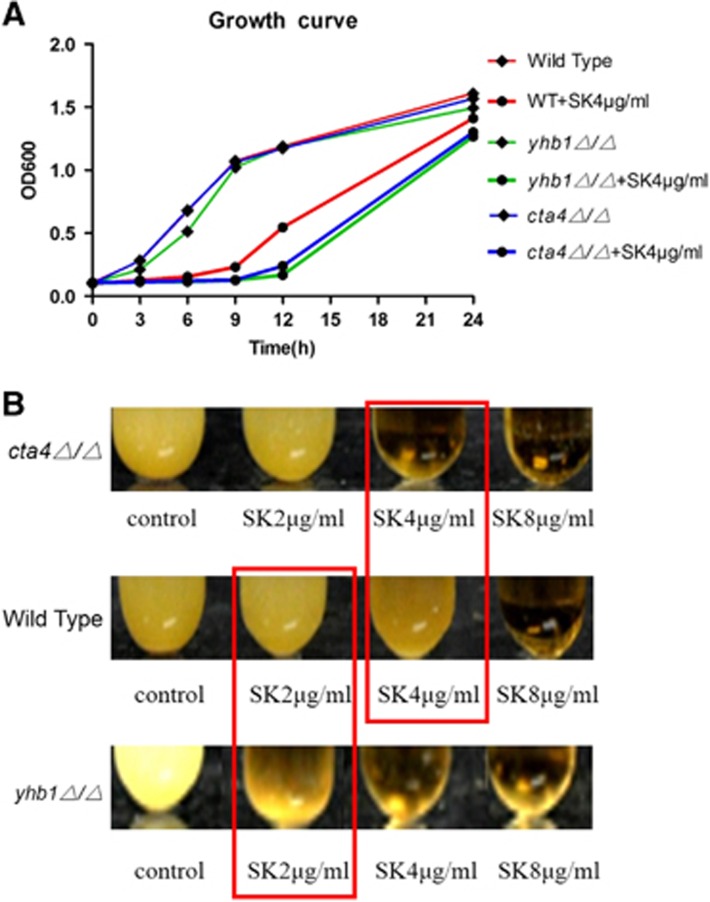
The special role of YHB1 in preventing C. albicans from NO-mediated damage implied that the antifungal activity of SK might be related to the suppression of YHB1. We examined the function of YHB1 in the antifungal activity of SK against C. albicans, using the YHB1-null mutant (yhb1Δ/Δ). As shown in the results, the deletion of YHB1 had no effect on the growth rate of C. albicans (Figure 4A), whereas the YHB1-null mutant (yhb1Δ/Δ) was more susceptible to SK than the wild type (Figure 4B and Table 2), and the growth rate of the YHB1-null mutant treated with 4 μg/mL SK was less than that of the wild type (Figure 4A).
Figure 4.
Sensitivity of C. albicans mutants to SK. Strains of the CTA4-null mutant (cta4Δ/Δ), YHB1-null mutant (yhb1Δ/Δ) and the wild type were treated with SK. All strains were incubated overnight in an orbital shaker at 30 °C. (A) At different times, a sample was taken to measure the OD600 value, from which a representative time-kill curve was obtained. (B) Photographs were taken after 24 h of incubation. Data are expressed as the mean±SD of triplicate assays.
Table 2. Minimum inhibitory concentrations (MIC80) for shikonin (SK; μg/mL) against Candida albicans.
|
Candida albicans | |||
|---|---|---|---|
| Wild type | cta4Δ/Δ | yhb1Δ/Δ | |
| SK | 8 | 4 | 4 |
Because CTA4 regulates YHB1 and Cta4p is the first protein reported to initiate a NO response in C. albicans, we investigated the role of CTA4 in the antifungal activity of SK. The results showed that the deletion of CTA4 increased the susceptibility of C. albicans to 4 μg/mL SK (Figure 4B and Table 2); the growth rate of the CTA4-null mutant (cta4Δ/Δ) was similar to that of the YHB1-null mutant (yhb1Δ/Δ) (Figure 4A), pointing to a clear relationship between the antifungal activity of SK and CTA4.
DISCUSSION
The naphthoquinone pigment SK from the root of L. erythrorhizon has been reported to exhibit antimicrobial, anticancer, antithrombotic, anti-inflammatory and anti-atherosclerosis functions.11 Our previous work has indicated that SK could function as an antifungal compound against C. albicans by shifting mitochondrial aerobic respiration and inducing endogenous reactive oxygen species augmentation.12 In this study, the role of NO in the antifungal activity of SK against C. albicans was tested. The antifungal armamentarium for the treatment or prophylaxis of invasive fungal infections is very limited. In addition, the increased evolvement of C. albicans resistant to antifungal therapy requires a high treatment dose, increasing the risk of toxicity and side effects.26 There has been an urgent need to develop effective drugs with novel mechanisms for combating fungal diseases. NO is a gaseous radical that has diverse roles in biological systems, including vasodilation, signaling, defense and the destruction of microbes. NO release is part of the defense of higher organisms against invading microbial pathogens.27 NO has potent antifungal activity when produced by macrophages within the host,13 and a stable NO-releasing nanoparticle platform can serve as an innovative therapeutic to which the fungi are unlikely to evolve resistance.28 Our results showed that both GSNO, a store of NO,15 and L-arginine, a producer of NO by the catalytic action of constitutive and inducible NO synthase isoforms in cells,16 could enhance the antifungal activity of SK, whereas L-NAME, the competitive inhibitor of NO synthase,17 attenuated the action. These results prompted us to hypothesize that the enhanced antifungal activity of SK might also be due to increased nitrosative damage. As expected, the incubation of C. albicans cells with SK induced changes in the intracellular levels of NO in a time- and dose-dependent manner: the increased level of NO by SK was enhanced by GSNO, whereas L-NAME attenuated the action. David et al. have reported that fluconazole could increase the expression of genes related to nitrosative damage.29 However, the antifungal role of NO accumulation directly induced using antifungal agents has not been reported and, to our knowledge, this is the first report demonstrating that the accumulation of NO is directly involved in the antifungal activity of antimycotic compounds against fungi. As NO is produced by macrophages to kill C. albicans and fungi treated with a NO-releasing nanoparticle platform are unlikely to evolve resistance,13, 28 SK may serve as a potential agent to induce mortality of C. albicans in vivo.
C. albicans utilizes several mechanisms to counteract nitrosative stresses, including the active detoxification of NO via flavohemoglobins, scavenging NO via the antioxidant system and the upregulation of repair systems to counteract damage.30 Flavohemoglobins could metabolize NO to nitrate and protect microbes from NO-mediated damage, growth inhibition and killing by NO-releasing immune cells.31 In C. albicans, the most highly induced gene is YHB1 that encodes the key NO-detoxifying enzyme flavohemoglobin/nitric oxide dioxygenase under nitrosative stresses.19 Our results showed that the transcription of YHB1 dramatically declined in a time-dependent manner after the treatment of SK, and the YHB1-null mutant (yhb1Δ/Δ) was more susceptible to SK treatment than the wild-type cells.
How yeasts detect NO and which signaling pathways mediate NO responses remains unclear. Nevertheless, Chiranand et al.20 reported that YHB1 expression was activated by the regulator CTA4, and the inactivation of CTA4 inhibits YHB1 induction in response to NO. Consistent with these findings, our investigation demonstrated that the CTA4-null mutant (cta4Δ/Δ) manifested a remarkable susceptibility to SK treatment, although the expression of CTA4 exhibited no significant change in C. albicans when treated with SK (data not shown). This contradiction strongly suggests that CTA4 may function in the post-translated level during the NO-mediated damage of SK, and SK may directly influence the proteins translated by CTA4, further regulating the function of YHB1 to encode the key enzyme-detoxifying nitrosative damage. In addition, other regulators may exist that influence the transcription of YHB1 in C. albicans to counteract nitrosative stresses. Sellam et al.32 have recently demonstrated that Cwt1p was a negative modulator of nitrosative stress resistance through direct transcriptional control of YHB1. Whether SK coordinates with the transcription factor Cwt1p that targets YHB1 or affects other pathways requires further investigation.
In conclusion, SK is able to induce the accumulation of intracellular NO in C. albicans, which subsequently manifests nitrosative stresses, and the NO-mediated damage in C. albicans by SK is related to the suppression of YHB1 and the function of CTA4. Our study sheds new light on the pathway in which C. albicans responds to nitrosative stresses.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) 2013CB531602 and National Natural Science Foundation of China (81271798, 81330083 and 81273474). We also thank Dr Suzanne M Noble (University of California San Francisco) for kindly providing C. albicans SN250, CTA4-null mutant (cta4Δ/Δ) and YHB1-null mutant (yhb1Δ/Δ).
References
- Kim J, Sudbery P. Candida albicans, a major human fungal pathogen. J Microbiol 2011; 49: 171–177. [DOI] [PubMed] [Google Scholar]
- Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol 2003; 11: 272–279. [DOI] [PubMed] [Google Scholar]
- Warrilow AG, Martel CM, Parker JE et al. Azole binding properties of Candida albicans sterol 14-alpha demethylase (CaCYP51). Antimicrob Agents Chemother 2010; 54: 4235–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher AJ, Chahine EB, Balcer HE. Echinocandins: the newest class of antifungals. Ann Pharmacother 2009; 43: 1647–1657. [DOI] [PubMed] [Google Scholar]
- Ellis D. Amphotericin B: spectrum and resistance. J Antimicrob Chemother 2002; 49: 7–10. [DOI] [PubMed] [Google Scholar]
- Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 1999; 12: 501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins RA. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med Mycol 2005; 43: 285–318. [DOI] [PubMed] [Google Scholar]
- Martins CV, da Silva DL, Neres AT et al. Curcumin as a promising antifungal of clinical interest. J Antimicrob Chemother 2009; 63: 337–339. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Abe H, Yoshizaki F. In vitro antifungal activity of naphthoquinone derivatives. Biol Pharm Bull 2002; 25: 669–670. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Kao SH, Hunag JE et al. Shikonin time-dependently induced necrosis or apoptosis in gastric cancer cells via generation of reactive oxygen species. Chem Biol Interact 2014; 211: 44–53. [DOI] [PubMed] [Google Scholar]
- Nam KN, Son MS, Park JH et al. Shikonins attenuate microglial inflammatory responses by inhibition of ERK, Akt, and NF-kappaB: neuroprotective implications. Neuropharmacology 2008; 55: 819–825. [DOI] [PubMed] [Google Scholar]
- Miao H, Zhao L, Li C et al. Inhibitory effect of Shikonin on Candida albicans growth. Biol Pharm Bull 2012; 35: 1956–1963. [DOI] [PubMed] [Google Scholar]
- Ullmann BD, Myers H, Chiranand W et al. Inducible defense mechanism against nitric oxide in Candida albicans. Eukaryot Cell 2004; 3: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemke AC, Shiva S, Burns JL et al. Nitrite modulates bacterial antibiotic susceptibility and biofilm formation in association with airway epithelial cells. Free Radic Biol Med 2014; 77: 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AJ, Blecher K, Schairer D et al. Improved antimicrobial efficacy with nitric oxide releasing nanoparticle generated S-nitrosoglutathione. Nitric Oxide 2011; 25: 381–386. [DOI] [PubMed] [Google Scholar]
- Haque SS. Impact of nitric oxide precursor L-arginine on oxidative stress against typhoid. Bratisl Lek Listy 2012; 113: 589–591. [DOI] [PubMed] [Google Scholar]
- Leitch GJ, He Q. Arginine-derived nitric oxide reduces fecal oocyst shedding in nude mice infected with Cryptosporidium parvum. Infect Immun 1994; 62: 5173–5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman BJ, Tadle AC, Pimentel LR et al. Selective damage to hyphal form through light-induced delivery of nitric oxide to Candida albicans colonies. J Inorg Biochem 2013; 123: 18–22. [DOI] [PubMed] [Google Scholar]
- Hromatka BS, Noble SM, Johnson AD. Transcriptional response of Candida albicans to nitric oxide and the role of the YHB1 gene in nitrosative stress and virulence. Mol Biol Cell 2005; 16: 4814–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiranand W, McLeod I, Zhou H et al. CTA4 transcription factor mediates induction of nitrosative stress response in Candida albicans. Eukaryot Cell 2008; 7: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993; 134: 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, French S, Kohn LA et al. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet 2010; 42: 590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AC, de Campos Rasteiro VM, Pereira CA et al. Susceptibility of Candida albicans and Candida dubliniensis to erythrosine- and LED-mediated photodynamic therapy. Arch Oral Biol 2011; 56: 1299–1305. [DOI] [PubMed] [Google Scholar]
- Cao Y, Zhu Z, Chen X et al. Effect of amphotericin B on the metabolic profiles of Candida albicans. J Proteome Res 2013; 12: 2921–2932. [DOI] [PubMed] [Google Scholar]
- Kagan S, Jabbour A, Sionov E et al. Anti-Candida albicans biofilm effect of novel heterocyclic compounds. J Antimicrob Chemother 2014; 69: 416–427. [DOI] [PubMed] [Google Scholar]
- Vincent BM, Lancaster AK, Scherz-Shouval R et al. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol 2013; 11: e1001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2004; 2: 820–832. [DOI] [PubMed] [Google Scholar]
- Macherla C, Sanchez DA, Ahmadi MS et al. Nitric oxide releasing nanoparticles for treatment of Candida albicans burn infections. Front Microbiol 2012; 3: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana DM, Nombela C, Pla J. Fluconazole at subinhibitory concentrations induces the oxidative- and nitrosative-responsive genes TRR1, GRE2 and YHB1, and enhances the resistance of Candida albicans to phagocytes. J Antimicrob Chemother 2010; 65: 54–62. [DOI] [PubMed] [Google Scholar]
- Tillmann A, Gow NA, Brown AJ. Nitric oxide and nitrosative stress tolerance in yeast. Biochem Soc Trans 2011; 39: 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmick RA, Fletcher AE, Gardner AM et al. Imidazole antibiotics inhibit the nitric oxide dioxygenase function of microbial flavohemoglobin. Antimicrob Agents Chemother 2005; 49: 1837–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam A, Tebbji F, Whiteway M et al. A novel role for the transcription factor Cwt1p as a negative regulator of nitrosative stress in Candida albicans. PLoS One 2012; 7: e43956. [DOI] [PMC free article] [PubMed] [Google Scholar]