Abstract
Gene editing in non-human primates may lead to valuable models for exploring the etiologies and therapeutic strategies of genetically based neurological disorders in humans. However, a monkey model of neurological disorders that closely mimics pathological and behavioral deficits in humans has not yet been successfully generated. Microcephalin 1 (MCPH1) is implicated in the evolution of the human brain, and MCPH1 mutation causes microcephaly accompanied by mental retardation. Here we generated a cynomolgus monkey (Macaca fascicularis) carrying biallelic MCPH1 mutations using transcription activator-like effector nucleases. The monkey recapitulated most of the important clinical features observed in patients, including marked reductions in head circumference, premature chromosome condensation (PCC), hypoplasia of the corpus callosum and upper limb spasticity. Moreover, overexpression of MCPH1 in mutated dermal fibroblasts rescued the PCC syndrome. This monkey model may help us elucidate the role of MCPH1 in the pathogenesis of human microcephaly and better understand the function of this protein in the evolution of primate brain size.
Keywords: MCPH1, microcephaly, TALEN, gene mutant, cynomolgus monkey
Introduction
Human pluripotent stem cells can be used to develop various in vitro organoid culture systems, including cerebral organoid, which may be used to understand the mechanisms involved in organogenesis and disease1,2. However, the human brain contains over 170 billion neurons and non-neuronal cells distributed throughout various regions3. In vitro organogenesis remains insufficient to model human neurological and mental disorders. In recent decades, gene-edited animal models, especially rodent models, have been generated and used to improve our understanding of pathogenic mechanisms and to test potential treatments for human neurological diseases. However, given the myriad behavioral, physiological and anatomical differences between humans and rodents, many rodent models fail to faithfully recapitulate the human condition4,5,6. Because non-human primates have perceptual, cognitive and behavioral repertoires very similar to those of humans, the development of non-human primate models that mimic human neurological diseases would critically contribute to our understanding of the mechanisms at work in the human brain7,8.
Autosomal recessive primary microcephaly (MCPH) is a neurodevelopmental disorder that is defined by the presence of a head circumference that is three standard deviations below the mean (−3 SD) for age and sex9,10,11. More than 12 causative genes have been identified; the first of which was designated as MCPH1 (microcephalin 1; also known as BRIT1 for BRCT-repeat inhibitor of human telomerase reverse transcriptase (hTERT) expression)12,13. Evolutionary studies have suggested that changes in the MCPH1 gene played an important role in the enlargement of the human brain. Moreover, this gene demonstrates evidence of positive selection during the evolution of humans and great apes14,15,16. Truncations in the MCPH1 gene are associated with significantly reduced brain size and short stature12,17,18. Some patients exhibit mental retardation and other neurological conditions, including spasticity18,19,20. Magnetic resonance imaging (MRI) revealed the presence of cerebral malformations, such as a simplified gyral pattern in the brain and hypoplasia of the corpus callosum18,21,22,23. Several elegant studies have demonstrated that MCPH1-deficient mice could recapitulate some key features of microcephaly and could thus help us understand the pathogenic mechanism. For example, Chen et al. reported MCPH1-deficient mice exhibited small skull sizes and hearing impairment24. Another group also found disruption of MCPH1 caused microcephaly due to a premature switching of neuroprogenitors from symmetric to asymmetric division25. So far, no neurologic symptoms have been found in mouse models and not all MCPH1 mutant mice recapitulate the severely reduced brain size observed in human patients26,27. Thus, a nonhuman primate model is desirable.
Genetically modified monkey models for human diseases, such as a transgenic Huntington's disease monkey model and an autism-related gene MECP2 transgenic monkey model, have been created and were thought to mimic human behavior and cognitive phenotypes better than rodents28,29. However, precise gene editing in monkeys is still a time-consuming and difficult undertaking. More recently, several studies have improved upon the techniques using transcription activator-like effector nucleases (TALENs) or clustered regularly interspaced short palindromic repeats/Cas930,31,32,33. Two groups reported the generation of MECP2 knockout monkey models using TALENs30,31. However, it is not clear whether the TALEN-mediated gene mutagenesis can effectively disrupt the targeted gene function and recapitulate disease phenotypes in non-human primates. Here we report a TALEN-generated MCPH1 mutant cynomolgus monkey and the phenotypic characterization of this potential monkey model of MCPH.
Results
Generation of the MCPH1 mutant monkey
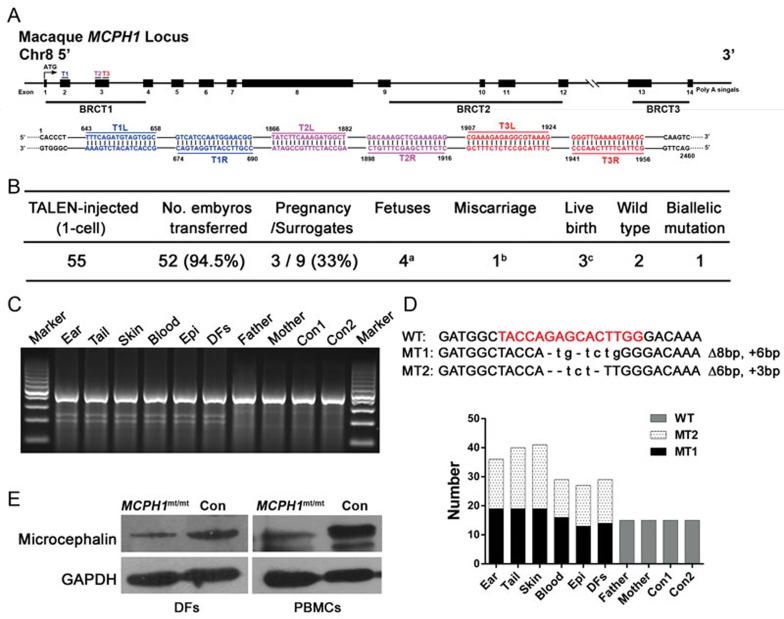
Similar to the human gene, the monkey MCPH1 gene comprises 14 exons and encodes an 842 amino acid protein, which contains three BRCT (BRCA1 C terminus) domains playing the diverse roles of MCPH1 by mediating its interactions with distinct partners9. We designed three TALEN pairs targeting different sites inside exons 2 and 3 (MCPH1-T1∼T3), which encode the N-terminal BRCT1 domain (Figure 1A).
Figure 1.
Generation and genotype analysis of the TALEN-targeted MCPH1 mutant monkey. (A) Schematic of TALEN-targeting sites within exons 2 and 3 of the monkey MCPH1 locus. The three BRCT (BRCA1 C terminus) domains are shown, and the TALEN-targeting sequences are labeled T1, T2 and T3 (L, R). (B) Summary of embryo transfer (ET) procedures undertaken after TALEN injection in cynomolgus monkeys. Notes: a, including one set of twins; b, miscarriage occurred on the 46th day after ET, and c, three cynomolgus monkeys were born live after 162 days of gestation. (C) T7EN1 assays were performed on six tissue samples obtained from the MCPH1mt/mt monkey. The PCR products were annealed with PCR products from wild-type monkeys, and digestion was performed. Epi, oral epithelium; DFs, dermal fibroblasts; Con1 and Con2, the two wild-type monkeys. (D) Sanger sequencing was performed on PCR products amplified from tissues of the MCPH1mt/mt monkey. The targeting site is highlighted in red. Targeted integration and the sizes of insertions (+, in lowercase) and/or deletions (Δ) are presented to the right of each allele. The columns indicate the read numbers. WT, wild-type; MT1 and MT2, the two mutations. (E) Western blot confirms that MCPH1 expression is reduced in MCPH1mt/mt monkey DFs and peripheral blood mononuclear cells (PBMCs). MCPH1mt/mt, MCPH1 mutant monkey; Con, wild-type monkey.
The targeting efficiency of TALEN pairs was examined in COS-7 cells using the T7 endonuclease 1 (T7EN1) assay; all three TALEN pairs could introduce mutations in the MCPH1 locus. The best indel rate approached 45.2%. Sanger sequencing revealed that MCPH1-T2 yielded the highest mutation rate (8 of 24 sequencing reads were mutated; 33%) in its target site, indicating that this TALEN pair could efficiently introduce genomic mutations in the MCPH1 gene. The targeting efficiencies of all three TALEN pairs are presented in Supplementary information, Figure S1A.
To further test the targeting efficiency of MCPH1-T2 in monkey embryos, we injected the MCPH1-T2 mRNA at two concentrations (20 ng/μl and 50 ng/μl) into the cytoplasm of 23 cynomolgus monkey zygotes obtained by intracytoplasmic sperm injection (ICSI). Although Sanger sequencing revealed that 85.7% of embryos injected with 50 ng/μl mRNA were mutant, only 50% of the embryos developed to the morula/blastocyst stage (Supplementary information, Figure S1B-S1D). In the lower concentration group, the mutation rate was 50%, and the embryos were of better quality, with 62.5% developing to the morula/blastocyst stage. As these results indicate that 20 ng/μl MCPH1-T2 could produce mutations in monkey embryos with acceptable efficiency and low toxicity, this was used for the subsequent experiments.
We injected 55 embryos with MCPH1-T2 mRNA and transferred 52 embryos into nine surrogate female monkeys (Figure 1B). Three surrogates became pregnant. One experienced a miscarriage at day 46 after embryo transfer. The aborted embryo was not collected. The remaining two females delivered three offspring upon the completion of pregnancy (∼165 days).
Genotype analysis of the MCPH1 mutant monkey
T7EN1 assays and Sanger sequencing both revealed that the twin offspring were wild type (Supplementary information, Figure S2A and S2B). In contrast, the singleton offspring (female) carried biallelic MCPH1 gene mutations at the targeting site and was designated as the MCPH1mt/mt monkey. Samples from the ear, tail, skin, blood, oral epithelium (Epi) and dermal fibroblast cells (DFs) of the MCPH1mt/mt monkey could be cleaved by T7EN1, whereas those of its parents and the controls could not be cleaved (Figure 1C). These results demonstrate that the MCPH1mt/mt monkey contains mutations in the MCPH1 sequence. Further sequencing of the TALEN-targeting site of the MCPH1mt/mt monkey revealed the presence of two different mutations in the MCPH1 gene (Figure 1D): an 8-bp deletion together with a 6-bp insertion that resulted in a nonsense mutation and a 6-bp deletion with a 3-bp insertion that resulted in a deletion/missense mutation. These two alterations were observed in all tested tissue samples at a ∼ 1:1 ratio. We selected > 200 colonies and failed to identify any wild-type colony upon sequencing. We performed PCR with a primer pair that could amplify the wild-type allele but not the mutant allele to detect more sensitively the biallelic mutations of the MCPH1 gene, and did not find the wild-type MCPH1 allele in the MCPH1mt/mt monkey (Supplementary information, Figure S2C). These results demonstrate that the MCPH1mt/mt monkey carries biallelic mutations in the MCPH1 gene. Parents of the MCPH1mt/mt monkey contain only the wild-type MCPH1 allele. This result confirms that the mutations in the offspring were generated by TALEN-mediated mutagenesis.
Because TALENs may also cause off-target mutations34,35, we searched for any potential off-target mutations in the MCPH1mt/mt monkey. TALENoffer computational prediction tool36 was used to predict the possible off-target sites (OTSs) in the Macaca fascicularis genome. Thirteen potential OTSs with TALENoffer score > −1.61 were chosen, and no mutation was detected by T7EN1 assays and Sanger sequencing in these sites in the MCPH1mt/mt monkey. Because these potential OTSs were all intronic loci, we next tested additional 18 potential OTSs located in exons with TALENoffer score > −1.98 and found no mutation in these sites (Supplementary information, Figure S2D and Table S1). The frame-shift and deletion/missense mutations in the MCPH1mt/mt monkey genome were predicted to generate a truncated protein (75 aa) and a protein missing one amino acid (p.57QST>HL), respectively (Supplementary information, Figure S2E). Western blot revealed that microcephalin was expressed at lower levels in the MCPH1mt/mt monkey DFs and peripheral blood mononuclear cells (PBMCs) compared with controls (Figure 1E).
MCPH1 mutant monkey exhibited a visibly smaller brain
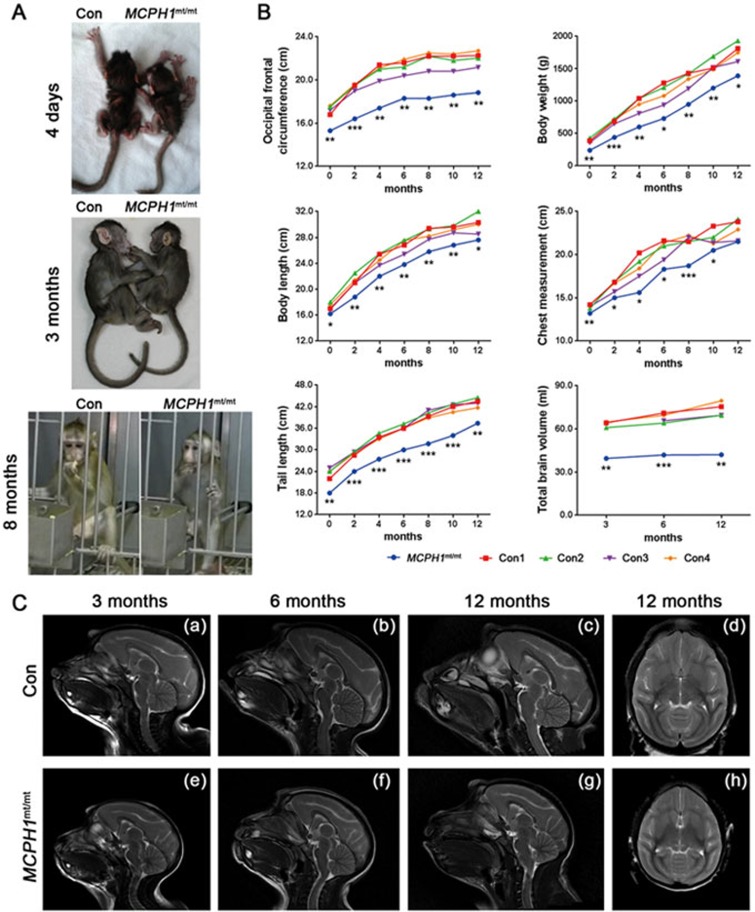
Humans with MCPH1 mutations are characterised by significant reductions in head circumference and may exhibit shortened height12,17,18. To assess the phenotype of the MCPH1mt/mt monkey, four age-matched wild-type female cynomolgus monkeys were selected as controls. The female MCPH1mt/mt monkey exhibited a visibly smaller brain (head) and a slightly shorter stature than the wild-type monkeys (Figure 2A). Noticeable microcephaly was observed at birth, with an occipital-frontal circumference (OFC) of 15.3 cm (−5 SD, mean ± SD of controls: 17.3 ± 0.4 cm). The monkey's body length at birth was average at 16.2 cm (−2.3 SD, mean ± SD of controls: 17.4 ± 0.5 cm), whereas the body weight at birth was only 0.241 kg (−5 SD), which was much lower than that of wild-type monkeys (mean ± SD: 0.390 ± 0.030 kg) of the same age and sex. Longitudinal measurement of OFC, body weight, body length, tail length and chest circumference revealed a slower growth trajectory of the MCPH1mt/mt monkey (Figure 2B). The OFC of the MCPH1mt/mt monkey was below normal (− 4 to −11 SD) during the first year of life. The weight of the MCPH1mt/mt monkey was only ∼65% compared with wild-type animals before 6 months of age and below normal (−2 to −8 SD) during the first year of life. The differences between the MCPH1mt/mt monkey and controls in body length, tail length and chest measurement were also significant. In contrast, monthly examinations of the teeth revealed no significant difference between the MCPH1mt/mt monkey and controls, with all teeth erupting before 6 months of age.
Figure 2.
Physical measurements and MRI-based phenotypes of the TALEN-targeted MCPH1 mutant monkey. (A) Images of the MCPH1mt/mt monkey and a wild-type monkey of the same age and sex. The MCPH1 mutant monkey exhibits a visibly smaller head and shorter stature. (B) Longitudinal measurements of the occipital frontal circumference, body weight, body length, tail length and chest circumference. Total brain volumes were calculated using MRI. Con 1-4, four wild-type monkeys of the same age and sex. All data are represented as the mean ± SD, n = 3 and *P < 0.05, **P < 0.01, ***P < 0.001 versus wild-type monkeys (Student's t-test). (C) MRI images show the typical reduction of brain volume of the MCPH1mt/mt monkey (e-h) in comparison to an age- and sex-matched control (a-d). Sagittal T2-weighted images indicating that the corpus callosum of the MCPH1mt/mt monkey (e-g) is thinner than that of a wild-type monkey (a-c). Axial T2-weighted images displaying most of the brain anatomy is normal except for the size in the MCPH1mt/mt monkey (h) compared with wild-type monkeys (d). Con, wild-type monkey; MCPH1mt/mt, MCPH1 mutant monkey.
Because previous studies demonstrated that MCPH1 mutant patients may exhibit a simplified gyral pattern, hypoplasia of the corpus callosum and frontal lobe18,21,37, we performed MRI to detect the brain size of the MCPH1mt/mt monkey. The total brain volume (TBVs) was 41.9 ml in the MCPH1mt/mt monkey compared with 67.6 ± 3.3 ml (mean ± SD) in wild-type monkeys at 6-month old (−8 SD; Figure 2B). The corpus callosum formed but appeared thin for the animal's age, most notably in the region of the body (Figure 2C (a-c, e-g)). In addition to hypoplasia of the corpus callosum, MRI did not detect any other obvious malformations in the brain of the MCPH1mt/mt monkey (Figure 2C (d, h)). These results suggest that the phenotype of the MCPH1mt/mt monkey is similar to the symptoms of MCPH1 patients.
Behavior and neurologic changes in the MCPH1 mutant monkey
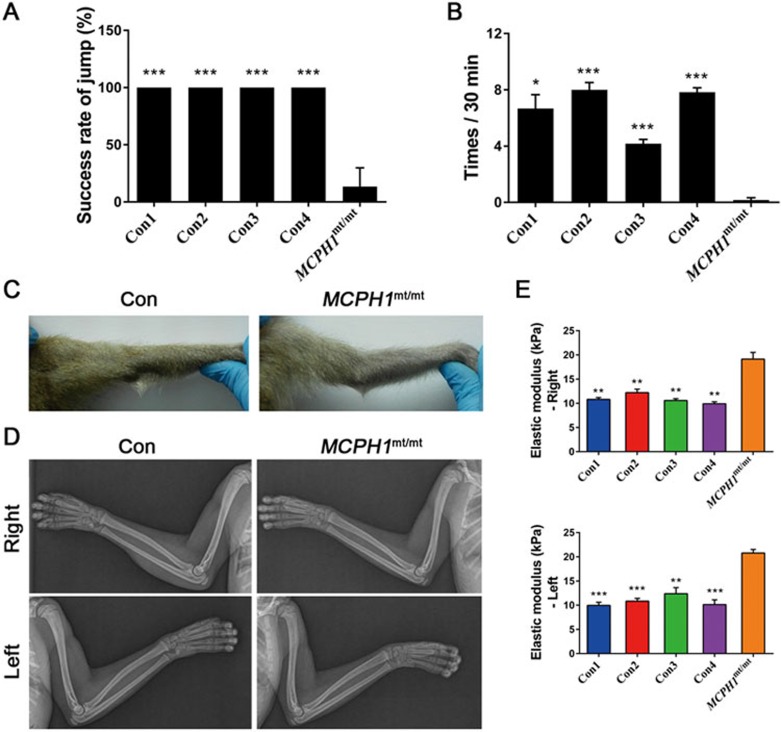
Neitzel et al. reported a MCPH1 mutant patient who was unable to walk without support until 4.75 years18, whereas another report described a patient with delayed acquisition of fine motor skills19. The MCPH1mt/mt monkey walked freely without any assistance similar to the controls; however, the MCPH1mt/mt monkey had occasional spasms (Supplementary information, Movie S1) and often could not jump or climb as flexibly as the controls (Supplementary information, Movie S2). We evaluated the videos at random and calculated the success rates of jumps made by wild-type and MCPH1mt/mt monkeys over 30 min. The total number of times the MCPH1mt/mt monkey attempted to jump did not differ from the wild-type monkeys. However, the former succeeded only one-seventh of the time, whereas the controls never failed (Figure 3A). Notably, the MCPH1mt/mt monkey rarely hung upside down, whereas the wild-type monkeys did so once approximately every 4 min. The wild-type monkeys hung upside down 3-13 times within 30 min, whereas the MCPH1mt/mt monkey did so only once within 180 min (from a total of six random videos; Figure 3B).
Figure 3.
Behavioral and neurological changes in MCPH1 gene mutant monkey. (A) Histogram depicting the jumping success rate of wild-type monkeys and the MCPH1mt/mt monkey in 30 min. The MCPH1 mutant monkey almost always failed to successfully jump up to a higher perch. n = 6. (B) Histogram depicting the instances of hanging upside down observed for wild-type monkeys and the MCPH1mt/mt monkey in 30 min. n = 6. (C) Images of the arms of wild-type monkeys and the MCPH1 mutant monkey. The latter arms are bent and could not be fully extended by external force. (D) X-ray films depicting both arms of wild-type monkeys and the MCPH1 mutant monkey. Radiological examination of the MCPH1mt/mt monkey did not find any bone deformity. (E) Histogram depicting the shear modulus of wild-type monkeys and the MCPH1mt/mt monkey. The biceps brachii of the MCPH1 mutant monkey exhibited significantly greater stiffness than that of the controls. n = 4. All data are represented as the mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 versus the MCPH1mt/mt monkey (one-way ANOVA). MCPH1mt/mt, MCPH1 mutant monkey; Con, wild-type monkey.
In addition to the above results, the MCPH1mt/mt monkey's arms could not be fully extended under external force, even when the animal was anesthetized (Figure 3C), which was similar to the previous observation that a MCPH1 patient demonstrated spasticity with hyperreflexia of the patellar and Achilles tendon reflexes and clonus18. Radiological examination of the MCPH1mt/mt monkey did not find any bone deformity (Figure 3D). Thus, we used shear-wave elastography to quantify biceps brachii stiffness at a 90° fixed position38. Among the control monkeys, the mean elasticity values of the right biceps brachii muscle ranged from 9.90 to 12.20 kPa, whereas those of the left ranged from 9.95 to 12.38 kPa. In contrast, in the MCPH1mt/mt monkey, these values averaged 19.12 ± 1.37 and 20.77 ± 0.75 kPa (mean ± SEM), respectively. The biceps brachii of the MCPH1mt/mt monkey had significantly greater stiffness than the controls, as confirmed by one-way ANOVA (Figure 3E). This result suggests that the introduced MCPH1 mutation may affect the motor skills of the mutant monkey in a manner similar to that observed in some patients.
Cellular phenotype of the MCPH1 mutant monkey
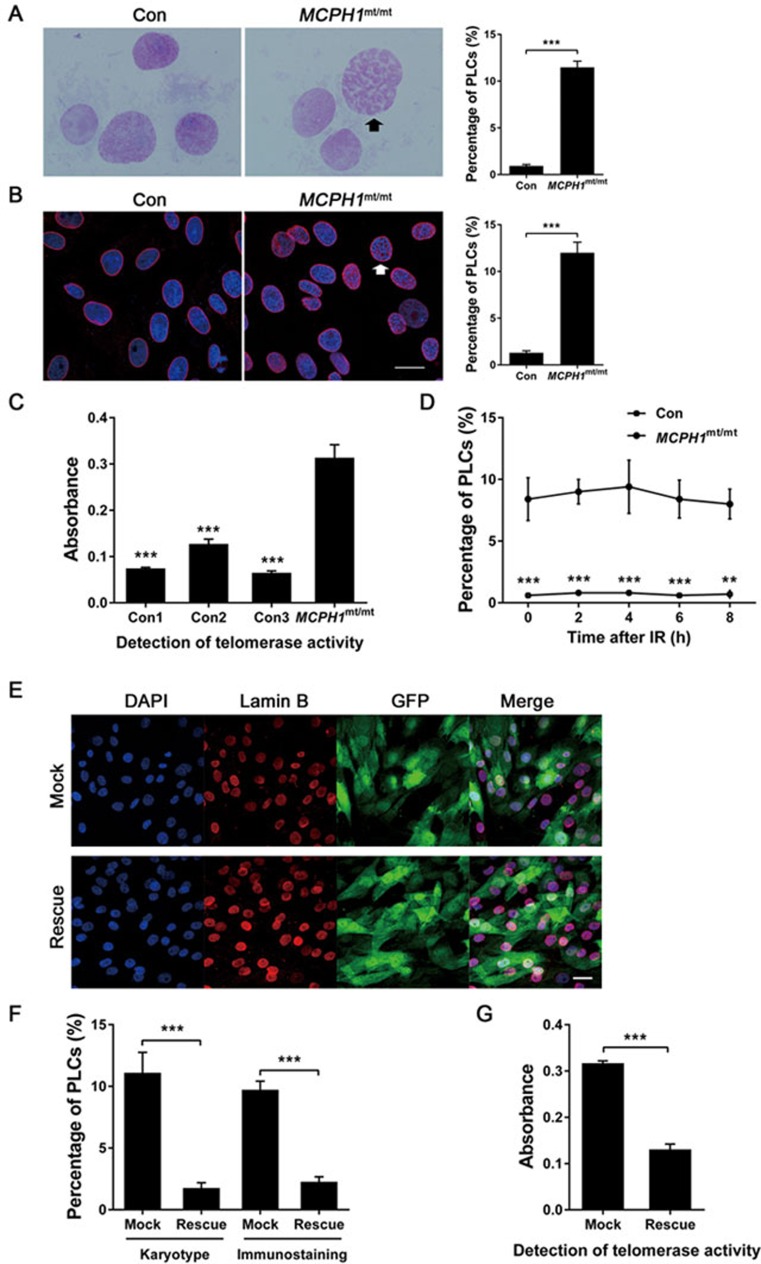
MCPH1-related microcephaly has also been associated with premature chromosome condensation (PCC) syndrome17,18,39,40, in which PCC occurs at interphase, resulting in a high number of cells with prophase-like chromatin. To investigate whether cells of the MCPH1mt/mt monkey exhibited evidence of PCC, we examined chromosome preparation made from DFs. Markedly more nuclei containing condensed prophase-like chromatin were found in the DFs of the MCPH1mt/mt monkey (mean ± SEM: 11.5% ± 0.7%) than in the wild-type cells (mean ± SEM: 0.9% ± 0.1%; Figure 4A). To investigate whether the nuclear envelope was retained or disassembled in PCC in the prophase-like cells (PLCs), the expression of Lamin B in DFs was detected by immunofluorescence staining, and the chromatin was stained with DAPI (Figure 4B). PLCs with intact nuclear envelopes were relatively common among the DFs of the MCPH1mt/mt monkey (mean ± SEM: 12.0% ± 1.1%), but not in the controls (mean ± SEM: 1.30% ± 0.2%). Collectively, these data demonstrate that the MCPH1mt/mt monkey presents typical cellular symptoms of MCPH1-mutant microcephalus patients.
Figure 4.
Cellular phenotype of TALEN-targeted MCPH1 gene mutant monkey. (A) Chromosome preparations of normal and MCPH1mt/mt DFs without prior colchicine treatment. A high proportion of prophase-like cells (black arrow) are present in the MCPH1mt/mt DFs. The histogram depicts the proportion of cells with a prophase-like appearance. (B) Immunofluorescence analysis of lamin B (red) and DAPI (blue) in normal and MCPH1mt/mt DFs. Many of the MCPH1mt/mt DFs exhibit intact nuclear membranes with prematurely condensed chromosomes. A cell (white arrow) shows a remarkable prophase-like appearance. The histogram shows the proportion of cells with a prophase-like appearance. Bar = 20 μm. (C) Telomerase enzymatic activity in DFs was assessed using the telomeric repeat amplification protocol (TRAP) versus MCPH1mt/mt monkey (one-way ANOVA). (D) The proportion of cells with a prophase-like appearance in MCPH1mt/mt DFs after ionizing irradiation. (E) Immunofluorescence analysis of lamin B (red) and DAPI (blue) in MCPH1mt/mt DFs. GFP fluorescence reflects the viral infection efficiency. Bar = 20 μm. (F) The histogram depicts the proportion of cells with a prophase-like appearance among MCPH1mt/mt DFs transfected with vectors encoding eGFP (Mock) or MCPH1-eGFP (Rescue). “Karyotype” indicates the chromosome preparation experiment, whereas “immunostaining” indicates the immunofluorescence analysis of lamin B. (G) Telomerase enzymatic activity was quantified by TRAP in MCPH1mt/mt DFs transfected with vectors encoding eGFP (Mock) or MCPH1-eGFP (Rescue). All data are represented as the mean ± SEM, n = 3, **P < 0.01, ***P < 0.001 (Student's t-test expect C). Con, wild-type monkey; MCPH1mt/mt, MCPH1 mutant monkey; Mock, eGFP expression vector; Rescue, MCPH1-eGFP expression vector; PLCs, prophase-like cells.
As MCPH1 is a BRCT-repeat protein capable of inhibiting hTERT expression41,42, we examined whether TERT was inhibited in MCPH1mt/mt cells. We used the telomeric repeat amplification protocol (TRAP) to assess telomerase enzymatic activity and found that telomerase activity was significantly activated in MCPH1mt/mt DFs compared with wild-type DFs (P < 0.001; Figure 4C).
A previous report revealed that normal prophase cells decondense their chromatin to allow repair following DNA damage, but MCPH1 patient cells do not43,44. We examined whether ionizing irradiation (IR) affected the chromosome condensation behavior of MCPH1mt/mt DFs compared with controls. Cultured DFs were subject to 1 Gy irradiation, and cytological preparations were made at 2-h intervals for 8 h following IR before chromatin morphology was analyzed. We found that MCPH1mt/mt DFs failed to completely decondense their chromatin following DNA damage. The number of PLCs remained relatively constant at all time points in MCPH1mt/mt DFs, whereas relatively fewer PLCs were found in control DFs (Figure 4D). Therefore, the chromosome condensation behavior of MCPH1-mutant DFs remained after DNA damage.
To confirm that the phenomena observed in vitro were due to MCPH1 mutations, an MCPH1-expressing lentiviral vector was established and introduced into MCPH1mt/mt monkey DFs to generate MCPH1rescue DFs (Supplementary information, Figure S3A). As a control, MCPH1mt/mt monkey DFs were transduced with a mock vector (encoding eGFP alone) to generate MCPH1mock DFs. After viral infection, > 90% of the DFs were infected, as reflected by GFP fluorescence (Figure 4E). Western blot revealed that microcephalin expression was significantly upregulated in MCPH1rescue DFs compared with the low level observed in MCPH1mock DFs (Supplementary information, Figure S3B). An analysis of chromosome morphology revealed that the proportion of PLCs in MCPH1rescue DFs (mean ± SEM: 1.8% ± 0.2%) was reduced markedly compared with MCPH1mock DFs (mean ± SEM: 11.1% ± 1.0%) to a level similar to that observed in wild-type cells (Figure 4E and 4F). The TRAP test also revealed that MCPH1rescue DFs could inhibit TERT expression to the same degree as in controls (Figure 4G). These data indicate that ectopic expression of MCPH1 could reverse the mutant phenotypes of MCPH1mt/mt monkey DFs, suggesting that the mutations in MCPH1 but no other potential OTS are primarily responsible for the phenotype observed in the MCPH1mt/mt monkey.
Discussion
Here we used TALEN technology to successfully generate the first MCPH1 mutant cynomolgus monkey. In monkey embryos, our TALEN pairs exhibited high targeting efficiency with low toxicity as previously reported30,31. Using these TALEN pairs, we obtained one live female monkey harboring biallelic MCPH1 mutations without detectable off-target effect. Our study suggest that TALEN technology can be successfully adopted to develop rapid, efficient and precise mutagenesis in cynomolgus monkeys.
The human MCPH1 gene is a recessive gene located on chromosome 8p23. The MCPH1 gene of the cynomolgus monkey is similarly mapped to chromosome 8. Our target sites are located in exon 2 or 3, and nearly all of the missense mutations shown to cause primary microcephaly are located in exon 2 or 39,17,19,20,40,45. These exons encode the evolutionarily conserved N-terminal BRCT1 domain39,46. Genotype analysis revealed that the TALEN-generated MCPH1 mutant cynomolgus monkey has two types of mutations. One is predicted to generate a truncated protein (75 aa) with impaired function, whereas the other a protein missing one amino acid (p.57QST>HL). The p.57QST>HL mutation is likely to have a functional consequence, as QST is highly conserved among different species (Supplementary information, Figure S4A). Structure modeling of the N-terminal domain of microcephalin suggests the QST to HL mutation may reduce the original three turns of α-helix to two turns of α-helix (Supplementary information, Figure S4B), thus affecting the protein stability or interaction with other proteins. Richards et al. reported that the α2 region containing QST residues (from p.57 to p.66; Supplementary information, Figure S4A) is one of the important sites for MCPH1 function in preventing abnormal metaphase chromosome condensation39. Taken together, p.57QST>HL mutation may significantly disrupt microcephalin structure and function.
Small head circumference is an important clinical index used to evaluate patients with MCPH1 gene defects47. The head circumference of patients is in the range of −3 to −11 SD below the means. The head circumference of the MCPH1mt/mt monkey was remarkably smaller (approximately −5 SD) than that of the wild-type controls until 12 months of age. Previous studies have reported that MCPH1 mutant patients always exhibit short stature, but the brain size is always significantly more reduced than height9,18. The MCPH1mt/mt monkey also exhibited a slower growth trajectory, and we then calculated the ratio of OFC to body length of the MCPH1 mutant monkey and control animals. The ratio was 68.2% in the MCPH1mt/mt monkey, but 73.1% ± 3.0% in the controls at 12 months of age (mean ± SD, P < 0.05, Student's t-test), suggesting the brain size is also significantly more reduced than the length in MCPH1 mutant monkey. MRI revealed that the MCPH1mt/mt monkey had a thin and hypoplastic corpus callosum but no other apparent cerebral malformation. Similarly, MRI scans of some MCPH1 mutant patients have identified malformations of the brain, such as hypoplasia of the frontal lobe and corpus callosum, reduction of the cerebral cortex, and a primitive, coarsened gyral pattern18,21. In contrast, in most MCPH1 mutant mice, MRI analyses did not reveal any obvious malformation in the brain26,27. Only Gruber and Zhou et al. reported that the cerebral cortex was reduced in their MCPH1 mutant mice24,25,48. These results indicate that MCPH1 is likely to have a more important role in the brain development of primates than that of mice.
As some MCPH1 mutant patients were reported to display spasticity and delayed motor skills, we hypothesized that the dysmyotonia and body cramping observed in the MCPH1mt/mt monkey were related to the gene mutation. Spasticity has been connected with a thin corpus callosum in various other diseases49,50. In a previous report, a MCPH1 mutant patient exhibited agenesis of the genu of the corpus callosum with other cerebral malformations, and she also showed slow developmental progress combined with profound mental retardation18. Because brain development continues after birth in primates and the MCPH1mt/mt monkey is in the early stage of life, the etiology of these symptoms remains unknown. To our knowledge, no neurological symptom has been reported in a MCPH1 mutant mouse. These prior and present observations provide additional support for our belief that MCPH1 has a more critical role during brain development in primates than in mice.
At the cellular level, PCC is a key discriminating feature presented in human MCPH1 patients18,39, and the percentage of PLCs reportedly ranges from 3.3% to 18%. Here we show that ∼11.5% of the MCPH1mt/mtDFs were PLCs, which is significantly higher than in the control. Consistent with the report of Gavvovidis et al.44, we observed that the DFs of the MCPH1mt/mt monkey did not decondense their chromatin following DNA damage (which normally occurs to allow repair). Moreover, cytological studies also verified that MCPH1 acts as a BRCT-repeat-containing inhibitor of TERT expression. The PCC phenotype is reportedly caused by the lack of a functional N-terminal BRCT domain of microcephalin39,40. Some patients have no significant increase in the fraction of prophase stage chromosomes; their mutations have been mapped in exons 4 and 551,52. In our study, the western blot analysis revealed an intermediate level of microcephalin protein expression. Because the antigen used to develop this antibody is located between amino acid 400 and 500 of the canonical sequence according to Uniprot data for microcephalin, a truncated protein (75 aa) cannot be detected, but a protein missing one amino acid (p.57QST>HL) can be detected by the antibody. As we did not observe any wild-type MCPH1 allele and we could observe PCC in this monkey, we presume that the observed protein may be translated from the deletion/missense mutant allele. To further confirm that the observed cellular phenomena were due to the mutation of MCPH1, we ectopically expressed MCPH1 in MCPH1mt/mt monkey DFs and found that this strategy could reverse the mutant phenotypes. Taken together, the in vitro findings demonstrate that the MCPH1 mutant monkey cells possess most of the features of MCPH1 mutant patient cells.
In summary, we have successfully established a MCPH1 mutant cynomolgus monkey model using TALENs. The phenotype of the MCPH1mt/mt monkey closely resembles that of MCPH1 mutant microcephaly in humans, indicating this animal model may provide a new strategy for studying the pathogenesis of microcephaly and the role microcephalin in the evolution of the brain size in primates.
Materials and Methods
Animals
Six healthy female cynomolgus monkeys (age 5-8 years) with regular menstrual cycles were selected as oocyte donors for superovulation, and nine multiparous monkeys were chosen as embryo recipients. Three healthy male cynomolgus monkeys (age 8 to 10 years) with body weights ∼ 8 kg were selected as sperm donors. All animals were housed at the Blooming Spring Biological Technology Development Co., LTD, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The use and care of animals were approved by the Ethics Committee of Sun Yat-sen University.
Sperm and oocyte collection, TALEN injection, and embryo transfer
Monkey semen was collected by rectal probe electro-ejaculation and washed with TL-HEPES. Embryo collection was performed as previously described53. Six healthy female cynomolgus monkeys were intramuscularly injected with rhFSH (recombinant human follitropin alfa; GONAL-F, Merck Serono) daily for 8 days, followed by rhCG (recombinant human chorionic gonadotropin alfa; OVIDREL, Merck Serono) on day 9. Oocytes were collected by laparoscopy 33-36 h after rhCG administration53,54. Metaphase II (MII, first polar body is present) oocytes were cultured in CMRL-1066 medium containing 0.1% Na-lactate (Sigma, L1375) and subjected to intracytoplasmic sperm injection (ICSI). At 8∼10 h after ICSI, the zygotes were injected with TALEN mRNAs at 20 or 50 ng/μl. The zygotes were transferred into surrogate females at the pronuclear stage or cultured in embryo culture medium-9 (HECM-9) containing 10% fetal bovine serum (FBS; Hyclone Laboratories, SH30088.02) at 37 °C in 5% CO2. Early pregnancy diagnosis was performed by ultrasonography ∼ 30 days after embryo transfer. Both clinical pregnancy and the number of fetuses were confirmed by fetal cardiac activity and the presence of a yolk sac, as detected by ultrasonography54.
Cell culture
DFs derived from the MCPH1mt/mt monkey and wild-type animals were established from monkey ears and propagated in standard condition. Cells were grown in Dulbecco's modified Eagle's medium (DMEM)/high glucose (Hyclone) containing 10% FBS and maintained at 37 °C in air containing 5% CO2.
COS-7 cells were cultured in DMEM/high glucose supplemented with 10% FBS and 1% (w/v) penicillin/streptomycin (Invitrogen/Gibco). TALEN expression plasmids were transfected into COS-7 cells by electroporation (BioRad Gene Pulser XL), and an eGFP-encoding plasmid was used as a control. Cells were collected for further analysis at 72 h post electroporation.
TALEN target site design and unit assembly
TALEN target sites were designed and constructed according to the instruction provided with the e-TALEN kit (Viewsolid Biotech). Briefly, units were assembled to construct the TALE repeats according to the target sequences, using several rounds of digestion/ligation. The constructed repetitive TALE fragments were then digested and inserted into the pCS2-eTALEN-T vector.
Production of TALEN mRNAs
TALEN plasmids (5 μg; pCS2-eTALEN-T) were linearized by NotI digestion in NEBuffer 2 (NEB) for 6 h at 37 °C, purified with a PCR purification kit (Qiagen), eluted with RNase-free water and diluted to a final concentration of ∼ 200 ng/μl. In vitro transcription was performed using an SP6 mMESSAGE mMACHINE Kit (Ambion) according to the manufacturer's instruction. The 10-μl reaction mixture, which contained 5 μl of 2× NTP/CAP, 1 μl of 10× reaction buffer, 1 μl of SP6 enzyme mix and 500 ng of linear template DNA, was incubated at 37 °C for 3 h. The TALENs were recovered by lithium chloride precipitation. Then, the left and right TALEN RNAs were mixed at a ratio of 1:1 (∼ 50 ng/μl each), and the mixture was stored at −80 °C.
T7EN1 cleavage assay and genomic DNA sequencing
Genomic DNA was isolated from cultured embryos using a GenomePlex Single Cell Whole Genome Amplification Kit (Sigma) according to the manufacturer's protocol. Genomic DNA was isolated from tissues using a DNA extraction kit (Qiagen) according to the manufacturer's instructions. PCR amplicons, including nuclease target sites or OTSs, were generated using ExTaq polymerase (Takara) and the primers listed in Supplementary information, Table S2. The obtained PCR products ranged in the size from 300 to 600 bp and were purified with a PCR purification kit. Briefly, the T7EN1 assay was performed as follows. Using a thermocycler, we denatured and annealed the PCR products in 1× NEBuffer 2 (NEB) to form heteroduplex DNA, which was then digested with T7EN1 (NEB) for 15∼25 min at 37 °C and analyzed by 2% agarose gel electrophoresis. The cleavage bands indicated modification of the target sites. Purified PCR products were also ligated into the pMD19-T vector (Takara), transformed into competent E. coli strain DH5α (Takara) and subjected to sequencing analysis.
Off-target analysis
Using TALENoffer, we selected 13 intronic loci and 18 exonic loci as potential OTS (Supplementary information, Table S1). The potential OTSs were amplified from monkey genomic DNA using specific primers (Supplementary information, Table S2). Then, ∼ 300 ng of each purified PCR product mixed with wild-type PCR product was digested with T7EN1 for 15∼25 min, and the treated products were recovered by 2% agarose gel electrophoresis.
Physical measurements
Four age-matched wild-type female cynomolgus monkeys were selected as normal controls. All monkeys were raised under the same condition in the same room. Physical measurements, including OFC, body weight, body length, tail length and chest circumference, were obtained monthly. Here we present data representing ∼ 2-month intervals.
MRI measurements and calculation of brain volume
Brain images of monkeys were acquired using a GE Discovery MR750 3.0T scanner (GE, Milwaukee, USA) with an HD wrist array coil. Monkeys were initially anesthetized with 3∼5 mg/kg of Telazol, and then intubated and maintained under anesthesia using 1.0%∼1.5% isoflurane mixed with O2. All major physiological parameters were monitored continuously and maintained within normal ranges. With respect to the detailed sequence parameters of the MRI scans, we obtained the following: SAG T2 Propeller FRFSE-weighted images (FRFSE, TE = 98 ms, TR = 9 578 ms, FOV = 120 mm, matrix = 384 × 384, thickness = 2 mm, spacing = 0.2 mm, FA = 142, BW = 83.3 KHz, ETL = 32, NEX = 1.5); Ax T2 Propeller FSE-weighted images (FSE, TE = 89 ms, TR = 10 219 ms, FOV = 120 mm, matrix = 320 × 320, thickness = 2 mm, spacing = 0.2 mm, FA = 142, BW = 62.5 KHz, ETL = 32, NEX = 2); and Ax 3D T1 BRAVO-weighted images (BRAVO, TE = 3.7 ms, TR = 8.7 ms, TI = 450, FOV = 128 mm, matrix = 256 × 256, thickness = 0.5 mm, FA = 12, BW = 31.25 KHz, NEX = 1). Regional and TBVs were calculated using the FSL Brain Extraction Tool (FSL5.0) and DPABI_V1.2_141101 software55,56.
X-ray
A dual-detector X-ray unit (DiDiEleva01; Philips, Holland) was used to acquire radiographs at 10 mA with 60 kV, using a grid and an exposure time of 0.2 s. An SRO 33100 ROT 360 X-ray tube (Philips) was used. The focal spot was 0.7 × 0.7 mm, and the focal distance for all images was 110 mm. The DigitalDiagnost (2.1.4 V22.13.567 2012) software program was used for radiology.
Behavioral assessment
For behavioral assessments, the MCPH1mt/mt monkey was caged with each of four wild-type monkeys in turn and recorded by a camera at a regular daily time when the monkeys were 8-month old. Videos were collected and divided into a period of 30 min and six random videos were selected for each monkey. They were next analyzed by two raters in a blinded manner. The following types of behavioral movements were scored: the times of each monkey jump (jump from the bottom to the top using only its legs) and success, as well as the times of each monkey hang upside down in 30 min.
Ultrasound elastography
The forearm of each anesthetized monkey was placed in a 90° flexed position, and ultrasound and shear-wave ultrasound elastographic measurements of the biceps brachii were performed using an Aixplorer ultrasound scanner (Supersonic Imagine; Aix-en-Provence, France) coupled with a linear transducer array (4-15 MHz, SuperLinear 15-4; Vermon, Tours, France). The biceps brachii tendon elasticity values were measured within a defined region of interest at the thickest point. All measurements were performed by two ultrasound specialist radiologists and reported in kilopascals.
Chromosome preparation and PLC assay
Metaphase spreads of DFs were performed using standard diagnostic laboratory procedures. Slides were stained with fresh 10% Giemsa stain for 10 min. Approximately 1 000 nuclei per experiment were counted in three independent experiments for each group, and the percentage of prophase-like nuclei was determined with respect to total nuclei.
Western blot analysis
Total proteins were extracted from DFs and PBMCs, and analyzed as previously described. Equal amounts of soluble protein were separated via sodium dodecyl sulphate-polyacrylamide gel electrophoresis using 10% Tris-glycine mini-gels and transferred onto a nitrocellulose membrane (Bio-Rad). Rabbit anti-BRIT1 (1:1 000, #4120; Cell Signaling) and rabbit anti-GAPDH (1:5 000, #2118; Cell Signaling) were used as the primary antibodies. Chemiluminescence (Amersham) was used to visualise the protein bands on X-ray films (Kodak). All of the western blot exposures were within the linear detection range.
Telomeric repeat amplification assay
Cellular telomerase activity was measured by telomerase repeat sequence amplification-enzyme linked immunosorbent assay using the TeloTAGGGTelomerase PCR ELISA kit (11854666910; Roche) according to the manufacturer's instructions. Sample absorbance was measured with a Model 550 Microplate Reader (Bio-Rad, USA) at 450/690 nm within 30 min after addition of the stop reagent.
IR and immunofluorescence staining
Logarithmically proliferating DFs were irradiated using an X-ray source (Ua = 160 kV; I = 25 mA; dose rate, 1.02 Gy/min; Rs2000; Rad Source). For immunofluorescence, DFs grown on coverslips were fixed with 4% (v/v) paraformaldehyde in phosphate-buffered saline (pH 7.4) for 15 min at room temperature, permeabilized in 0.1% (v/v) Triton X-100 and blocked using 2% (w/v) bovine serum albumin. The cells were then incubated at 4 °C overnight with anti-lamin B (sc-6216; Santa Cruz) in 2% (w/v) bovine serum albumin. The cells were washed and incubated with Alexa Fluor 488/Alexa Fluor 594-conjugated goat anti-mouse/anti-rabbit secondary antibody (Invitrogen) at room temperature for 1 h in the dark. Nuclei were counterstained with DAPI (Sigma). The results were analyzed by direct observation under laser confocal microscopy (LSM 780; Carl Zeiss) and using ZEN 2009 Light Edition software (Carl Zeiss).
Statistical analysis
Statistical analyses were performed with SPSS software (version 17.0; SPSS Institute). Significant differences between means were assessed by Student's t-test or one-way ANOVA. Statistical significance was assumed at P < 0.05, and all P-values are presented as two-tailed results.
Author Contributions
QK and WL designed experiments, performed research, interpreted data and wrote the manuscript. XL performed research and interpreted data and wrote the manuscript. CH, HZ, YM, DX, TW and FFM contributed to embryo manipulation. LH, XF, ZK and HX performed MRI and X-ray analysis. KL and JR performed ultrasound elastography. WH performed bioinformatics analysis. ZC, XS and XL contributed to chromosome preparation. MZ contributed to protein structure prediction and analysis. FZ and JX provided TALENs. BTL. edited the manuscript. QZ, SH and APX designed and supervised research, interpreted data and wrote the manuscript. QK, WL and XL contributed equally to this work.
Competing Financial Interests
The authors declare no competing financial interests.
Acknowledgments
This work was supported by National Basic Research Program of China (2012CBA01302), the National Natural Science Foundation of China (81425016, 31301219 and 81570593), the Fundamental Research Funds for the Central Universities (15ykjc01c), PhD Programs Foundation of Ministry of Education of China (20130171120061), the Key Scientific and Technological Projects of Guangdong Province (2014B020226002, 2014B020228003, 2014B020225007, 2015B020228002 and 2016B030229002), Key Scientific and Technological Program of Guangzhou City (201400000003-3, 201508020262, 201300000089 and 2010U1-E00551), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (GDUPS, 2013) and Pearl River S&T Nova Program of Guangzhou (2014J2200037).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Evaluation of TALEN-mediated mutagenesis in COS-7 cells and cynomolgus monkey embryos
Genotype analysis of the twins' monkey and the MCPH1-mutant monkey
Ectopic expression of MCPH1 in MCPH1mt/mt DFs
Cross species alignment and the crystal structure of microcephalin
OTS sequence information for the MCPH1-mutant monkey
The primers used in this work
The MCPH1 mutant monkey (without neck strap) had occasional spasms
The MCPH1 mutant monkey (without neck strap) jumped but failed
References
- Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 2012; 10:771–785. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, et al. Cerebral organoids model human brain development and microcephaly. Nature 2013; 501:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci 2009; 3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Rakic P. Development of layer I neurons in the primate cerebral cortex. J Neurosci 2001; 21:5607–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 1995; 373:523–527. [DOI] [PubMed] [Google Scholar]
- Kishi N, Sato K, Sasaki E, Okano H. Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev Growth Differ 2014; 56:53–62. [DOI] [PubMed] [Google Scholar]
- Chan AW. Transgenic nonhuman primates for neurodegenerative diseases. Reprod Biol Endocrinol 2004; 2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte JC, Callaway EM, Churchland P, et al. Brains, Genes, and Primates. Neuron 2015; 86:617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvers JN. MCPH1: a window into brain development and evolution. Front Cell Neurosci 2015; 9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods CG, Parker A. Investigating microcephaly. Arch Dis Child 2013; 98:707–713. [DOI] [PubMed] [Google Scholar]
- Woods CG, Bond J, Enard W. Autosomal recessive primary microcephaly (MCPH): a review of clinical, molecular, and evolutionary findings. Am J Hum Genet 2005; 76:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AP, McHale DP, Campbell DA, et al. Primary autosomal recessive microcephaly (MCPH1) maps to chromosome 8p22-pter. Am J Hum Genet 1998; 63:541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faheem M, Naseer MI, Rasool M, et al. Molecular genetics of human primary microcephaly: an overview. BMC Med Genomics 2015; 8 (Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PD, Anderson JR, Vallender EJ, Choi SS, Lahn BT. Reconstructing the evolutionary history of microcephalin, a gene controlling human brain size. Hum Mol Genet 2004; 13:1139–1145. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Su B. Molecular evolution of microcephalin, a gene determining human brain size. Hum Mol Genet 2004; 13:1131–1137. [DOI] [PubMed] [Google Scholar]
- Evans PD, Gilbert SL, Mekel-Bobrov N, et al. Microcephalin, a gene regulating brain size, continues to evolve adaptively in humans. Science 2005; 309:1717–1720. [DOI] [PubMed] [Google Scholar]
- Jackson AP, Eastwood H, Bell SM, et al. Identification of microcephalin, a protein implicated in determining the size of the human brain. Am J Hum Genet 2002; 71:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitzel H, Neumann LM, Schindler D, et al. Premature chromosome condensation in humans associated with microcephaly and mental retardation: a novel autosomal recessive condition. Am J Hum Genet 2002; 70:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimborn M, Richter R, Sternberg N, et al. The first missense alteration in the MCPH1 gene causes autosomal recessive microcephaly with an extremely mild cellular and clinical phenotype. Hum Mutat 2005; 26:496. [DOI] [PubMed] [Google Scholar]
- Darvish H, Esmaeeli-Nieh S, Monajemi GB, et al. A clinical and molecular genetic study of 112 Iranian families with primary microcephaly. J Med Genet 2010; 47:823–828. [DOI] [PubMed] [Google Scholar]
- Pfau RB, Thrush DL, Hamelberg E, et al. MCPH1 deletion in a newborn with severe microcephaly and premature chromosome condensation. Eur J Med Genet 2013; 56:609–613. [DOI] [PubMed] [Google Scholar]
- Ghani-Kakhki M, Robinson PN, Morlot S, et al. Two missense mutations in the primary autosomal recessive microcephaly gene MCPH1 disrupt the function of the highly conserved N-terminal BRCT domain of microcephalin. Mol Syndromol 2012; 3:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimborn M, Bell SM, Felix C, et al. Mutations in microcephalin cause aberrant regulation of chromosome condensation. Am J Hum Genet 2004; 75:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ingham N, Clare S, et al. Mcph1-deficient mice reveal a role for MCPH1 in otitis media. PLoS One 2013; 8:e58156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Zhou Z, Sukchev M, et al. MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat Cell Biol 2011; 13:1325–1334. [DOI] [PubMed] [Google Scholar]
- Trimborn M, Ghani M, Walther DJ, et al. Establishment of a mouse model with misregulated chromosome condensation due to defective Mcph1 function. PLoS One 2010; 5:e9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Gao H, Lin S, et al. BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA repair and maintaining genomic stability in mice. PLoS Genet 2010; 6:e1000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Cheng P, Banta H, et al. Towards a transgenic model of Huntington's disease in a non-human primate. Nature 2008; 453:921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li X, Zhang JT, et al. Autism-like behaviours and germline transmission in transgenic monkeys overexpressing MeCP2. Nature 2016; 530:98–102. [DOI] [PubMed] [Google Scholar]
- Liu H, Chen Y, Niu Y, et al. TALEN-mediated gene mutagenesis in rhesus and cynomolgus monkeys. Cell Stem Cell 2014; 14:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou X, Zhu Y, et al. Generation of a monkey with MECP2 mutations by TALEN-based gene targeting. Neurosci Bull 2014; 30:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Shen B, Cui Y, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 2014; 156:836–843. [DOI] [PubMed] [Google Scholar]
- Wan H, Feng C, Teng F, et al. One-step generation of p53 gene biallelic mutant cynomolgus monkey via the CRISPR/Cas system. Cell Res 2014; 25:258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussolino C, Morbitzer R, Lutge F, et al. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res 2011; 39:9283–9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Wang H, Kiani S, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol 2011; 29:731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau J, Boch J, Posch S. TALENoffer: genome-wide TALEN off-target prediction. Bioinformatics 2013; 29:2931–2932. [DOI] [PubMed] [Google Scholar]
- Ghani-Kakhki M, Robinson PN, Morlot S, et al. Two missense mutations in the primary autosomal recessive microcephaly gene MCPH1 disrupt the function of the highly conserved N-terminal BRCT domain of microcephalin. Mol Syndromol 2012; 3:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourpaille L, Hug F, Nordez A. Influence of passive muscle tension on electromechanical delay in humans. PLoS One 2013; 8:e53159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MW, Leung JWC, Roe SM, et al. A Pocket on the surface of the N-terminal BRCT domain of Mcph1 is required to prevent abnormal chromosome condensation. J Mol Biol 2010; 395:908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JW, Leitch A, Wood JL, et al. SET nuclear oncogene associates with microcephalin/MCPH1 and regulates chromosome condensation. J Biol Chem 2011; 286:21393–21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Elledge SJ. Multiple tumor suppressor pathways negatively regulate telomerase. Cell 2003; 113:881–889. [DOI] [PubMed] [Google Scholar]
- Shi L, Li M, Su B. MCPH1/BRIT1 represses transcription of the human telomerase reverse transcriptase gene. Gene 2012; 495:1–9. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Cole RW. Entry into mitosis in vertebrate somatic cells is guarded by a chromosome damage checkpoint that reverses the cell cycle when triggered during early but not late prophase. J Cell Biol 1998; 142:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavvovidis I, Pohlmann C, Marchal JA, et al. MCPH1 patient cells exhibit delayed release from DNA damage-induced G2/M checkpoint arrest. Cell Cycle 2010; 9:4893–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini MM, Tonekaboni SH, Papari E, et al. A novel mutation in MCPH1 gene in an Iranian family with primary microcephaly. J Pak Med Assoc 2012; 62:1244–1247. [PubMed] [Google Scholar]
- Shi L, Li M, Lin Q, Qi X, Su B. Functional divergence of the brain-size regulating gene MCPH1 during primate evolution and the origin of humans. BMC Biol 2013; 11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S, Ahmad W, Hassan MJ. Autosomal recessive primary microcephaly (MCPH): clinical manifestations, genetic heterogeneity and mutation continuum. Orphanet J Rare Dis 2011; 6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Tapias A, Bruhn C, et al. DNA damage response in microcephaly development of MCPH1 mouse model. DNA Repair 2013; 12:645–655. [DOI] [PubMed] [Google Scholar]
- Ma J, Xiong L, Chang Y, et al. Novel mutations c.[5121_5122insAG]+[6859C>T] of the SPG11 gene associated with cerebellum hypometabolism in a Chinese case of hereditary spastic paraplegia with thin corpus callosum. Parkinsonism Relat Disord 2014; 20:256–259. [DOI] [PubMed] [Google Scholar]
- Heimer G, Marek-Yagel D, Eyal E, et al. SLC1A4 mutations cause a novel disorder of intellectual disability, progressive microcephaly, spasticity and thin corpus callosum. Clin Genet 2015; 88:327–335. [DOI] [PubMed] [Google Scholar]
- Farooq M, Baig S, Tommerup N, Kjaer KW. Craniosynostosis-microcephaly with chromosomal breakage and other abnormalities is caused by a truncating MCPH1 mutation and is allelic to premature chromosomal condensation syndrome and primary autosomal recessive microcephaly type 1. Am J Med Genet Part A 2010; 152A:495–497. [DOI] [PubMed] [Google Scholar]
- Ghafouri-Fard S, Fardaei M, Gholami M, Miryounesi M. A case report: Autosomal recessive microcephaly caused by a novel mutation in MCPH1 gene. Gene 2015; 571:149–150. [DOI] [PubMed] [Google Scholar]
- Niu Y, Yu Y, Bernat A, et al. Transgenic rhesus monkeys produced by gene transfer into early-cleavage-stage embryos using a simian immunodeficiency virus-based vector. Proc Natl Acad Sci USA 2010; 107:17663–17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Niu Y, Yang S, et al. The available time window for embryo transfer in the rhesus monkey (Macaca mulatta). Am J Primatol 2012; 74:165–173. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23 (Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage 2012; 62:782–790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of TALEN-mediated mutagenesis in COS-7 cells and cynomolgus monkey embryos
Genotype analysis of the twins' monkey and the MCPH1-mutant monkey
Ectopic expression of MCPH1 in MCPH1mt/mt DFs
Cross species alignment and the crystal structure of microcephalin
OTS sequence information for the MCPH1-mutant monkey
The primers used in this work
The MCPH1 mutant monkey (without neck strap) had occasional spasms
The MCPH1 mutant monkey (without neck strap) jumped but failed