Abstract
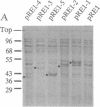
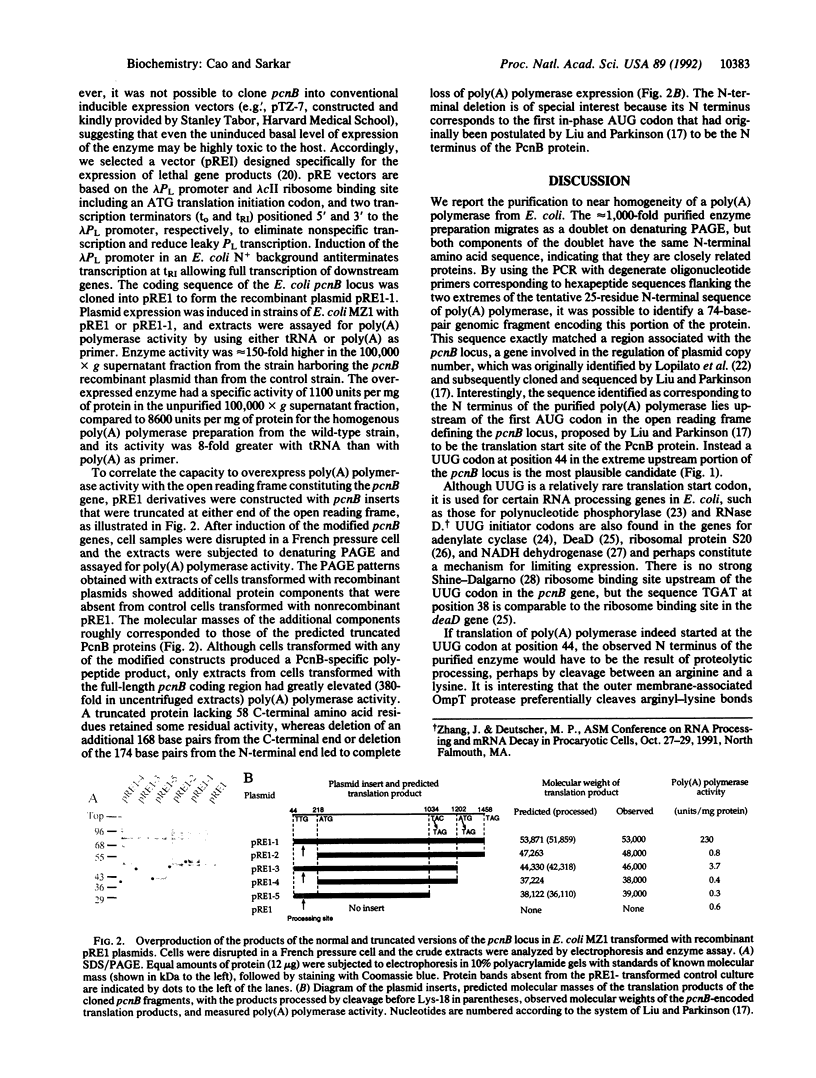
Many bacterial mRNAs, like those of eukaryotes, carry a polyadenylate sequence at their 3' termini, but neither the function of the bacterial poly(A) moieties nor their biosynthesis have been elucidated. To develop a genetic tool to approach the problem of bacterial poly(A) RNA, we have sought to identify the genes responsible for mRNA polyadenylylation. A poly(A) polymerase was purified to homogeneity from extracts of Escherichia coli and subjected to N-terminal sequence analysis. The 25-residue amino acid sequence obtained was used to design primers for the amplification of the corresponding coding region by the PCR from an E. coli DNA template. A 74-base-pair DNA segment was obtained that matched a region in the pcnB locus of E. coli, a gene that had originally been identified as controlling plasmid copy number [J. Lopilato, S. Bortner & J. Beckwith (1986) Mol. Gen. Genet. 205, 285-290] and was subsequently cloned and sequenced [J. Liu & J. S. Parkinson (1989) J. Bacteriol. 171, 1254-1261]. Direct evidence that the pcnB locus encodes poly(A) polymerase was provided by the observation that a bacterial strain transformed with an inducible expression vector carrying pcnB as a translational fusion produced 100-fold elevated levels of poly(A) polymerase upon induction. No increased poly(A) polymerase activity was observed in cells transformed with expression vectors carrying truncated forms of the pcnB gene. The identification of a gene encoding bacterial poly(A) polymerase opens the way for the study of the biosynthesis and function of bacterial polyadenylylated mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUGUST J. T., ORTIZ P. J., HURWITZ J. Ribonucleic acid-dependent ribonucleotide incorporation. I. Purification and properties of the enzyme. J Biol Chem. 1962 Dec;237:3786–3793. [PubMed] [Google Scholar]
- Gershon P. D., Ahn B. Y., Garfield M., Moss B. Poly(A) polymerase and a dissociable polyadenylation stimulatory factor encoded by vaccinia virus. Cell. 1991 Sep 20;66(6):1269–1278. doi: 10.1016/0092-8674(91)90048-4. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna Y., Langley D., Sarkar N. Detection of high levels of polyadenylate-containing RNA in bacteria by the use of a single-step RNA isolation procedure. Nucleic Acids Res. 1981 Jul 24;9(14):3545–3554. doi: 10.1093/nar/9.14.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna Y., Sarkar N. Characterization of polyadenylate-containing ribonucleic acid from Bacillus subtilis. Biochemistry. 1982 May 25;21(11):2724–2729. doi: 10.1021/bi00540a023. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna Y., Sarkar N. The synthesis of DNA complementary to polyadenylate-containing RNA from Bacillus subtilis. J Biol Chem. 1982 Mar 25;257(6):2747–2750. [PubMed] [Google Scholar]
- Karnik P., Gopalakrishna Y., Sarkar N. Construction of a cDNA library from polyadenylated RNA of Bacillus subtilis and the determination of some 3'-terminal sequences. Gene. 1986;49(1):161–165. doi: 10.1016/0378-1119(86)90397-5. [DOI] [PubMed] [Google Scholar]
- Karnik P., Taljanidisz J., Sasvari-Szekely M., Sarkar N. 3'-terminal polyadenylate sequences of Escherichia coli tryptophan synthetase alpha-subunit messenger RNA. J Mol Biol. 1987 Jul 20;196(2):347–354. doi: 10.1016/0022-2836(87)90695-4. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lingner J., Kellermann J., Keller W. Cloning and expression of the essential gene for poly(A) polymerase from S. cerevisiae. Nature. 1991 Dec 12;354(6353):496–498. doi: 10.1038/354496a0. [DOI] [PubMed] [Google Scholar]
- Liu J. D., Parkinson J. S. Genetic evidence for interaction between the CheW and Tsr proteins during chemoreceptor signaling by Escherichia coli. J Bacteriol. 1991 Aug;173(16):4941–4951. doi: 10.1128/jb.173.16.4941-4951.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. D., Parkinson J. S. Genetics and sequence analysis of the pcnB locus, an Escherichia coli gene involved in plasmid copy number control. J Bacteriol. 1989 Mar;171(3):1254–1261. doi: 10.1128/jb.171.3.1254-1261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopilato J., Bortner S., Beckwith J. Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduce plasmid copy number of pBR322 and its derivatives. Mol Gen Genet. 1986 Nov;205(2):285–290. doi: 10.1007/BF00430440. [DOI] [PubMed] [Google Scholar]
- Mackie G. A. Nucleotide sequence of the gene for ribosomal protein S20 and its flanking regions. J Biol Chem. 1981 Aug 10;256(15):8177–8182. [PubMed] [Google Scholar]
- Masters M., March J. B., Oliver I. R., Collins J. F. A possible role for the pcnB gene product of Escherichia coli in modulating RNA: RNA interactions. Mol Gen Genet. 1990 Jan;220(2):341–344. doi: 10.1007/BF00260507. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Raabe T., Bollum F. J., Manley J. L. Primary structure and expression of bovine poly(A) polymerase. Nature. 1991 Sep 19;353(6341):229–234. doi: 10.1038/353229a0. [DOI] [PubMed] [Google Scholar]
- Ramanarayanan M., Srinivasan P. R. Further studies on the isolation and properties of polyriboadenylate polymerase from Escherichia coli PR7 (RNase I-, pnp). J Biol Chem. 1976 Oct 25;251(20):6274–6286. [PubMed] [Google Scholar]
- Reddy P., Peterkofsky A., McKenney K. Hyperexpression and purification of Escherichia coli adenylate cyclase using a vector designed for expression of lethal gene products. Nucleic Acids Res. 1989 Dec 25;17(24):10473–10488. doi: 10.1093/nar/17.24.10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Haziza C., Danchin A. Regulation of adenylate cyclase synthesis in Escherichia coli: nucleotide sequence of the control region. EMBO J. 1983;2(5):791–797. doi: 10.1002/j.1460-2075.1983.tb01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier P., Grunberg-Manago M., Portier C. Nucleotide sequence of the pnp gene of Escherichia coli encoding polynucleotide phosphorylase. Homology of the primary structure of the protein with the RNA-binding domain of ribosomal protein S1. J Biol Chem. 1987 Jan 5;262(1):63–68. [PubMed] [Google Scholar]
- Sarkar N., Langley D., Paulus H. Isolation and characterization of polyadenylate-containing RNA from Bacillus brevis. Biochemistry. 1978 Aug 22;17(17):3468–3474. doi: 10.1021/bi00610a007. [DOI] [PubMed] [Google Scholar]
- Schäfer R., Zillig W., Priess H. A novel RNA-primed polynucleotidepyrophosphorylase from E. Coli. FEBS Lett. 1972 Sep 1;25(1):87–90. doi: 10.1016/0014-5793(72)80460-5. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A. E. Purification and characterization of adenosine triphosphate: ribonucleic acid adenyltransferase from Escherichia coli. Eur J Biochem. 1973 Aug 1;37(1):31–40. doi: 10.1111/j.1432-1033.1973.tb02953.x. [DOI] [PubMed] [Google Scholar]
- Sugimura K., Higashi N. A novel outer-membrane-associated protease in Escherichia coli. J Bacteriol. 1988 Aug;170(8):3650–3654. doi: 10.1128/jb.170.8.3650-3654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taljanidisz J., Karnik P., Sarkar N. Messenger ribonucleic acid for the lipoprotein of the Escherichia coli outer membrane is polyadenylated. J Mol Biol. 1987 Feb 5;193(3):507–515. doi: 10.1016/0022-2836(87)90263-4. [DOI] [PubMed] [Google Scholar]
- Toone W. M., Rudd K. E., Friesen J. D. deaD, a new Escherichia coli gene encoding a presumed ATP-dependent RNA helicase, can suppress a mutation in rpsB, the gene encoding ribosomal protein S2. J Bacteriol. 1991 Jun;173(11):3291–3302. doi: 10.1128/jb.173.11.3291-3302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E., Martin G., Schiltz E., Keller W. Isolation and expression of cDNA clones encoding mammalian poly(A) polymerase. EMBO J. 1991 Dec;10(13):4251–4257. doi: 10.1002/j.1460-2075.1991.tb05003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E. Purification and characterization of a mammalian polyadenylate polymerase involved in the 3' end processing of messenger RNA precursors. J Biol Chem. 1991 Feb 15;266(5):3131–3139. [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990 Jul;15(7):277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- Young I. G., Rogers B. L., Campbell H. D., Jaworowski A., Shaw D. C. Nucleotide sequence coding for the respiratory NADH dehydrogenase of Escherichia coli. UUG initiation codon. Eur J Biochem. 1981 May;116(1):165–170. doi: 10.1111/j.1432-1033.1981.tb05314.x. [DOI] [PubMed] [Google Scholar]
- Zuber M., Patterson T. A., Court D. L. Analysis of nutR, a site required for transcription antitermination in phage lambda. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4514–4518. doi: 10.1073/pnas.84.13.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]